Abstract
Background
KBG syndrome is caused by haploinsufficiency of ANKRD11 and is characterised by macrodontia of upper central incisors, distinctive facial features, short stature, skeletal anomalies, developmental delay, brain malformations and seizures. The central nervous system (CNS) and skeletal features remain poorly defined.
Methods
CNS and/or skeletal imaging were collected from molecularly confirmed individuals with KBG syndrome through an international network. We evaluated the original imaging and compared our results with data in the literature.
Results
We identified 53 individuals, 44 with CNS and 40 with skeletal imaging. Common CNS findings included incomplete hippocampal inversion and posterior fossa malformations; these were significantly more common than previously reported (63.4% and 65.9% vs 1.1% and 24.7%, respectively). Additional features included patulous internal auditory canal, never described before in KBG syndrome, and the recurrence of ventriculomegaly, encephalic cysts, empty sella and low-lying conus medullaris. We found no correlation between these structural anomalies and epilepsy or intellectual disability. Prevalent skeletal findings comprised abnormalities of the spine including scoliosis, coccygeal anomalies and cervical ribs. Hand X-rays revealed frequent abnormalities of carpal bone morphology and maturation, including a greater delay in ossification compared with metacarpal/phalanx bones.
Conclusion
This cohort enabled us to describe the prevalence of very heterogeneous neuroradiological and skeletal anomalies in KBG syndrome. Knowledge of the spectrum of such anomalies will aid diagnostic accuracy, improve patient care and provide a reference for future research on the effects of ANKRD11 variants in skeletal and brain development.
Keywords: Congenital, Hereditary, and Neonatal Diseases and Abnormalities; Genetic Research; Patient Care; Pathological Conditions, Signs and Symptoms; Radiology
WHAT IS ALREADY KNOWN ON THIS TOPIC.
KBG syndrome is a well-characterised neurodevelopmental disorder caused by ANKRD11 haploinsufficiency, but the neuroimaging and skeletal features remain poorly defined and often overlooked.
WHAT THIS STUDY ADDS
Systematic evaluation of diagnostic imaging in a large cohort of patients highlighted the deep phenotype and natural history of KBG-related brain, spine and skeletal abnormalities, and allowed us to define their prevalence.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
This qualitative and quantitative description of the radiological and neuroradiological features will aid clinicians to better evaluate and manage individuals with KBG syndrome.
Our data provide a reference for future research on animal models concerning the cerebral and skeletal consequences of ANKRD11 variants.
Introduction
KBG syndrome (MIM #148050) is an autosomal dominant disorder caused by heterozygous variants in ANKRD11 (locus 16q24.3).1 It is characterised by neurological involvement with global developmental delay or mild/moderate intellectual disability (ID), EEG abnormalities with or without seizures, macrodontia of permanent upper central incisors, postnatal short stature, typical craniofacial phenotype, conductive hearing loss, hand anomalies, delayed skeletal maturation and costovertebral anomalies.2 3 It was first described in 1975 in three unrelated families with surname initials K, B and G.4 Around 400 patients have been described to date, but the syndrome is likely underdiagnosed due to affected individuals often presenting with mild and non-specific features.
ANKRD11 encodes the chromatin co-regulator Ankyrin Repeat Domain-Containing Protein 11.1 The vast majority of pathogenic variants are loss-of-function, with single nucleotide variants and small delins accounting for approximately 83% of pathogenic variants, while larger copy number variants (mostly deletions) account for approximately 17%.5 No genotype-phenotype correlations have been reported. ANKRD11 variants appear to be fully penetrant, but intrafamilial variability is commonly reported.1 5
ANKRD11 is highly expressed in the human brain and localises to the nuclei of neurons and glial cells, where it modulates transcription by binding nuclear receptor complexes and epigenetic regulators.1 6 Ankrd11 knockdown in the developing mouse brain results in decreased innervation and arborisation of dendrites in the cerebral cortex, possibly explaining ID as well as other neurodevelopmental and psychiatric comorbidities of KBG syndrome.7 Various non-specific brain anomalies have been reported in individuals with KBG syndrome, most notably enlarged cisterna magna, ventriculomegaly and arachnoid cysts,5 8–11 but also rare occurrences of abnormal corpus callosum, optic nerve hypoplasia, pericallosal lipoma, pituitary gland hypoplasia, periventricular nodular heterotopia and Chiari 1 malformation.3 8 9 12–15 Skeletal anomalies have been reported in 75% of affected individuals.2 5 16 The most frequent are costovertebral anomalies (kyphosis/scoliosis, cervical ribs, abnormal vertebral shape, end plate abnormalities) and hand anomalies (brachydactyly, clinodactyly of the fifth finger).2 3 5 8 9 12 14 17–19 Other abnormalities include a wide and persistent anterior fontanel, delayed skeletal maturation (>2 SD below the mean), sternal anomalies, hip dysplasia and rare or isolated findings such as craniosynostosis and osteopenia.5 9 16 18 Still, the phenotypical spectrum of neuroimaging and skeletal imaging, as well as the prevalence of brain and spine malformations, remains to be explored on large cohorts of patients with KBG syndrome.
We performed a systematic evaluation of the original neuroradiologiocal and skeletal imaging data from 53 individuals with genetically confirmed KBG syndrome. The aim was to deep-phenotype the spectrum of brain, spine and skeletal malformations, compare results with the existing literature and explore potential genotype-phenotype correlations.
Methods
Study cohort
Individuals with KBG syndrome were enrolled in a multicentric, international retrospective study coordinated by the institutions AUSL-IRCCS of Reggio Emilia and Danish Epilepsy Centre. Inclusion criteria were the diagnosis of KBG syndrome with a molecularly confirmed ANKRD11 variant or 16q24.3 deletion, and availability of diagnostic imaging of the brain and/or full or partial skeletal survey. An integral copy of the original imaging data was collected through the treating physician; diagnostic imaging was reviewed by two independent groups consisting of neuroradiologists, radiologists, paediatricians and geneticists. We primarily included MRI; if only brain CT scans were available, we exclusively reported data for cysts, enlarged posterior fossa and internal auditory canal (IAC) anomalies. Skeletal maturation was determined using the Greulich and Pyle method.20 Clinical data were collected through the treating physician using a customised clinical table including, but not limited to, birth and pregnancy information, developmental and cognitive milestones, brain images, physical examinations, behavioural and psychiatric comorbidities, epilepsy and congenital malformations. Written informed consent was obtained from each study participant (or, for minors or disabled cases, from parents or legal guardians).
Genetic analysis
Probands were investigated by either single gene, panels or whole exome sequencing (WES) requested by the treating physician. ANKRD11 variants were annotated using NCBI transcript NM_013275.5 (GRCh38/hg38), and classified according to the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) recommendations.21 22
Review of the literature
We searched MEDLINE (PubMed) with the keywords ‘KBG’, ‘ANKRD11’, limiting for articles written in English and published after 1990 to ensure optimal data collection. Last search was done in September 2022. Relevant references in the acquired articles, which were not found in the MEDLINE search, were further investigated. We retrieved 44 publications, from 1994 to 2022, describing a total of 373 individuals with KBG syndrome; of these, 89 had performed brain MRI, 182 spinal column X-rays, 141 hand X-rays, and 43 limb X-rays and were considered for a comparison with our cohort. We did not include reports from the ClinVar or DECIPHER databases because the clinical information was incomplete.
Statistical analysis
Analysis of our cohort is mainly descriptive. Statistical analysis was performed using R V.4.1.2. Fisher’s exact test (with Freeman-Halton extension for tables larger than 2×2) and Pearson’s χ2 test were used to investigate possible correlations between genotype and phenotype or between neuroradiological features and clinical presentations, and to compare the frequency of findings in our cohort and in the literature. One-way analysis of variance was used to describe the possible correlations of auxologic measurements with age and of MRI findings with seizures age of onset. P values <0.05 were considered statistically significant.
Results
We describe 51 novel and 2 previously reported individuals with KBG syndrome,8 19 comprising 29 females and 24 males with a median age of 11 years (range 2–32 years). For a detailed overview of clinical, genetic and imaging features in each participant, see online supplemental tables 1–5.
jmg-2023-109141supp003.xlsx (58.9KB, xlsx)
Neuroradiological features
Neuroradiological imaging comprised T1-/T2-weighted (3D-)MRI with coronal, axial and sagittal reconstruction of the brain from 41 participants and of the spine from 7. In addition, CT was available in three participants. Eleven individuals had two or more examinations (average 3) at different ages, the earliest at 2 months of life. On average, each series spanned 4.5 years, with the longest consisting of 10 MRIs over the course of 16 years (online supplemental table 1). See table 1 for an in-depth overview and frequency of neuroradiological features in the present cohort. Visualisation of the most significant anomalies is available in figure 1.
Table 1.
In-depth overview of intracranial features of the study cohort based on available imaging
| Feature | Participants, n/n evaluated | (%) |
| Incomplete hippocampal inversion | 26/41 | (63.4) |
| Bilateral | 19/41 | (46.3) |
| Monolateral | 7/41 | (17.1) |
| Posterior fossa malformations* | 29/44 | (65.9) |
| Large cisterna magna | 25/44 | (56.8) |
| Dandy-Walker continuum | 1/44 | (2.3) |
| Large cisterna magna with low attachment of tentorium and low torcula | 2/44 | (4.5) |
| Inferior cerebellar vermis hypoplasia | 1/44 | (2.3) |
| Lateral ventricle morphology abnormalities | 15/41 | (36.6) |
| Dilation of lateral ventricles | 10/41 | (24.4) |
| Dysmorphic atria | 5/41 | (12.2) |
| Temporal horn enlargement | 4/41 | (9.8) |
| Other ventricular dysmorphisms | 5/41 | (12.2) |
| Cysts* | 5/44 | (11.4) |
| Arachnoid cyst | 4/44 | (9.1) |
| Choroid plexus cyst | 1/44 | (2.3) |
| Subependymal cyst | 1/44 | (2.3) |
| Pars intermedia cyst | 1/44 | (2.3) |
| White matter abnormalities | 8/41 | (19.5) |
| Generalised reduction of white matter thickness | 2/41 | (4.9) |
| Peritrigonal reduction of white matter thickness | 2/41 | (4.9) |
| Localised signal alterations of white matter | 4/41 | (9.8) |
| Periventricular leukomalacia | 1/41 | (2.4) |
| Gliosis of the peritrigonal regions | 1/41 | (2.4) |
| Left cerebellar hemisphere porencephaly | 1/41 | (2.4) |
| Patulous internal auditory canal* | 8/12 | (66.7)† |
| Empty sella | 4/39 | (10.3) |
| Vascular abnormalities | 3/6 | (50.0)† |
| Other brain imaging abnormalities | 11/41 | (26.8) |
| Widened subarachnoid space | 2/41 | (4.9) |
| Dilation of perivascular space | 3/41 | (7.3) |
| Abnormal corpus callosum morphology | 2/41 | (4.9) |
| Olfactory nerve/bulb abnormalities | 2/41 | (4.9) |
| Ocular/optic nerve abnormalities | 2/41 | (4.9) |
| Dilation of Meckel’s cave | 1/41 | (2.4) |
| Agenesis of septum pellucidum | 1/41 | (2.4) |
| Lipoma tuber cinereum | 1/41 | (2.4) |
*Evaluated on both MRI and CT scan.
†Unlikely to be representative of the entire population (sample size too small).
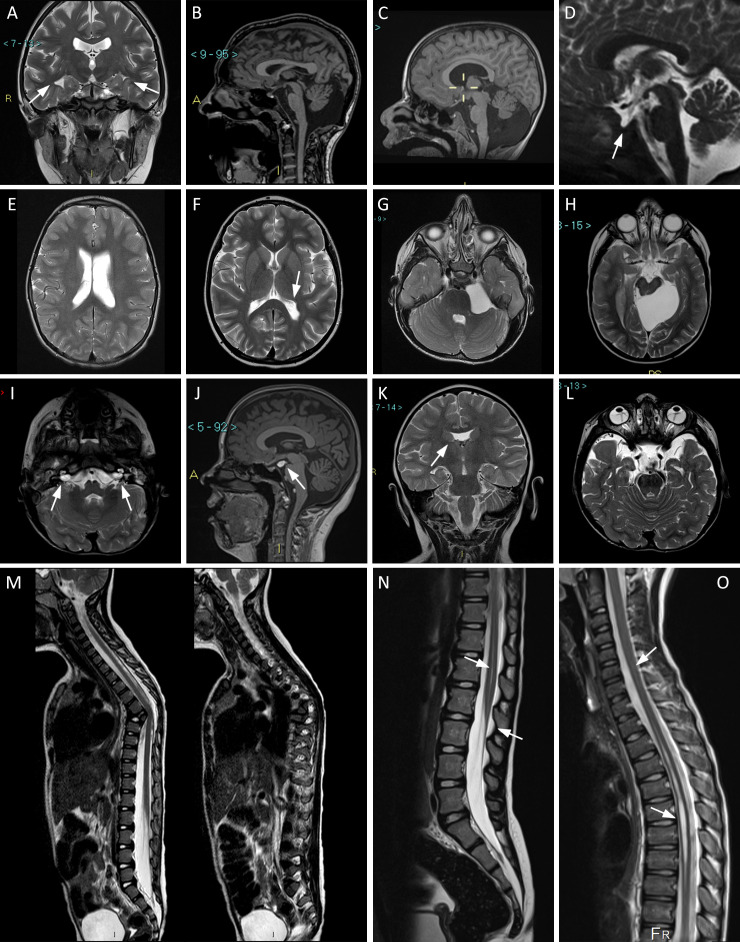
Figure 1.
Representative MRI images in our cohort. Arrows indicate the relevant details. (A) Pt. 16, coronal T2-weighted view: incomplete hippocampal inversion, bilateral. (B) Pt. 7, sagittal T1: large cisterna magna. (C) Pt. 4, sagittal T1: Dandy-Walker variant. (D) Pt. 25, sagittal T2: empty sella. (E) Pt. 23, axial T2 TSE: dilated and asymmetric lateral ventricles. (F) Pt. 10, axial T2 TSE: left trigonal enlargement. (G) Pt. 5, axial T2: arachnoid cyst in left cerebellopontine angle. (H) Pt. 31, axial T2: arachnoid cyst in left ambient cistern. (I) Pt. 12, axial T2: bilateral patulous internal auditory canal. (J) Pt. 16, sagittal T1: lipoma tuber cinereum. (K) Pt. 3, coronal T2: partial agenesis of septum pellucidum. (L) Pt. 12, axial T2: bilateral persistent hyperplastic primary vitreous. (M) Pt. 52, sagittal T2: focal dorsal kyphosis due to anomalous thoracic T6-T7 vertebral differentiation. (N) Pt. 51, sagittal T2 TSE: hydromyelia and low-lying Conus medullaris. (O) Pt. 38, sagittal T2 TSE: hydrosyringomyelia. Pt., participant.
Skeletal features
Skeletal features were studied based on the radiological imaging that were available in 40 participants. This comprised spine X-rays (29 individuals), hand X-rays (27 individuals) and jaw/tooth imaging (16 individuals, including orthopantomographies in 7). Hip scans were available in 14 participants, either as isolated imaging or as part of a spinal column evaluation series. Figure 2 shows an overview of relevant skeletal findings and table 2 summarises their frequencies.
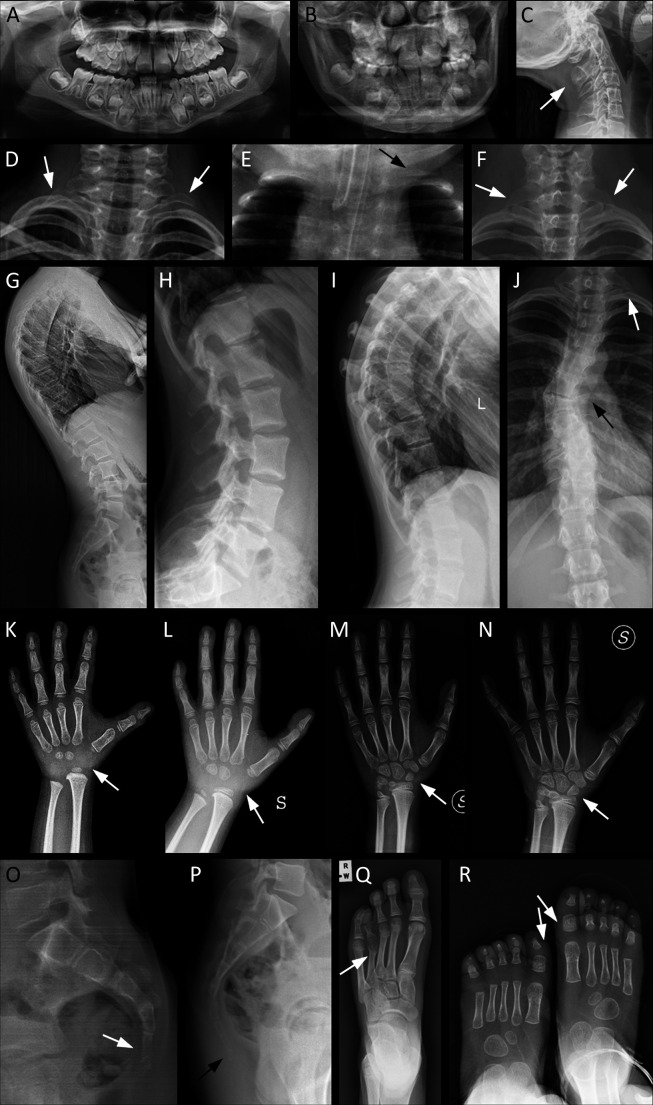
Figure 2.
Main radiological characteristics of selected patients from our cohort, representative of the skeletal features of KBG syndrome. Arrows indicate the most relevant details. (A) Pt. 10: orthopantomography showing macrodontia of the permanent upper incisors and dental crowding. (B) Pt. 32: macrodontia of the permanent upper incisors, dental crowding. (C) Pt. 1: cervical C2/C3 vertebral fusion. (D) Pt. 21: bilateral cervical ribs. (E) Pt. 12: supernumerary cervical rib on the left side. (F) Pt. 10: bilateral C7 hypertrophic transverse process. (G) Pt. 26: thoracic hyperkyphosis. (H–J) Spinal column anomalies of Pt. 39: tall lumbar vertebral bodies (H), kyphosis (I), left cervical rib and scoliosis due to thoracic hemivertebrae (J). (K–N) Evolution of the main anomalies of hand bones over time: delayed carpal ossification with absence of the proximal row at about 3 years (K) and 6 years of age (L); partial fusion of the lunate and triquetral carpal bones at 9 years (M); complete fusion of the lunate and triquetral bones at 10 years (N). (O) Pt. 32: agenesis of the coccyx: only the outline of the first coccygeal vertebra is present. (P) Pt. 26: supernumerary coccygeal vertebrae. (Q) Pt. 13: short and dysmorphic metatarsal and phalanges of fourth ray, bilaterally (right foot not shown). (R) Pt. 12: broad and short metatarsal and phalanges of first ray, bilaterally. Pt., participant.
Table 2.
In-depth overview of the skeletal and spinal features of the study cohort
| Feature | Participants, n/n evaluated | (%) | Evaluation type |
| Skull abnormalities | 19/43 | (44.2) | |
| Wide anterior fontanel | 13/43 | (30.2) | XR, CT, PE |
| Frontal bossing | 3/43 | (7.0) | XR, MRI, PE |
| Craniosynostosis | 2/16 | (12.5) | XR, CT, MRI |
| Other abnormalities of calvaria morphology | 6/16 | (37.5) | XR, CT, MRI |
| Jaw and tooth abnormalities | 44/51 | (86.3) | |
| Permanent upper incisors macrodontia | 30/35 | (85.7) | XR, PE |
| Deciduous incisors macrodontia | 9/16 | (56.3) | XR, PE |
| Tooth malposition/dental crowding | 21/51 | (41.2) | XR, PE |
| Oligodontia | 7/51 | (13.7) | XR, PE |
| Spine abnormalities | 17/32 | (62.5) | |
| Scoliosis | 9/32 | (40) | XR, MRI |
| Hyperkyphosis | 4/32 | (20) | XR, MRI |
| Hyperlordosis | 3/32 | (10) | XR, MRI |
| Abnormal vertebral morphology | 4/32 | (12.5) | XR, MRI |
| Coccygeal anomalies | 4/32 | (12.5) | XR, MRI |
| Low-lying conus medullaris | 4/7 | (57.1) | MRI |
| Hydromyelia/hydrosyringomyelia/ventriculus terminalis | 4/7 | (57.1) | MRI |
| Rib abnormalities | 18/29 | (62.1) | |
| Cervical rib | 14/29 | (48.3) | XR |
| C7 hypertrophic transverse process | 4/29 | (13.8) | XR |
| Hand abnormalities | 15/27 | (55.6) | |
| Carpal bone aplasia | 1/27 | (3.7) | XR |
| Carpal synostosis | 3/27 | (11.1) | XR |
| Other abnormalities of carpal morphology | 5/27 | (18.5) | XR |
| Abnormalities of metacarpal morphology | 2/27 | (7.4) | |
| Short middle phalanx of the fifth finger | 12/27 | (44.4) | XR |
| Short middle phalanx of the second and fifth finger | 3/27 | (11.1) | XR |
| Cone-shaped epiphyses of the phalanges | 1/27 | (3.7) | XR |
| Altered skeletal maturation | 15/27 | (55.6) | |
| Delayed maturation of the hand bones | 14/27 | (51.9) | XR |
| Advanced maturation of the hand bones | 1/27 | (3.7) | XR |
| Discordant maturation of carpals and phalanges | 6/27 | (22.2) | XR |
| Short upper limbs | 2/6 | (33.3) | XR |
| Short lower limbs | 1/5 | (20.0) | XR |
| Hip abnormalities | 8/14 | (57.1) | |
| Hip asymmetry | 3/14 | (21.4) | XR |
| Hip dysplasia | 3/14 | (21.4) | XR |
| Legg-Calvè-Perthes disease | 1/14 | (7.1) | XR |
| Absent femoral head ossific nucleus | 1/14 | (7.1) | XR |
| Foot abnormalities | 6/7 | (85.7) | |
| Broad and short tubular bones in first ray | 3/7 | (42.9) | XR |
| Short/dysmorphic tubular bones in other rays | 2/7 | (28.6) | XR |
| Cone-shaped epiphyses of the toes | 2/7 | (28.6) | XR |
| Calcaneal alteration | 1/7 | (14.3) | XR |
PE, physical examination; XR, X-ray.
Genetic analysis and further clinical information
Non-radiological clinical data were collected to complement the neurological and skeletal information, and are summarised in online supplemental table 4 and further discussed in online supplemental file 1. Online supplemental figure 2 offers an overview of the distinguishing facial features of some of the participants at different ages.
jmg-2023-109141supp002.pdf (165.5KB, pdf)
jmg-2023-109141supp001.pdf (589.2KB, pdf)
Figure 3 and online supplemental table 5 show the ANKRD11 variants in our cohort. Seventeen are novel, while all others have been reported before in the literature or in databases (LOVD, ClinVar, DECIPHER). Most variants occurred de novo; of 45 participants with available segregation analysis, 2 siblings and 4 unrelated individuals inherited the defective allele from a mildly affected parent. Participants 4, 31 and 44, who had large chromosome deletions involving multiple genes besides ANKRD11, did not show a more severe clinical presentation than those with intragenic variants. The recurring variant p.(Lys635Glnfs*26), found in five participants (8, 9, 12, 38 and 0), was associated with heterogeneous clinical features ranging from normal cognition to severe ID, and with absence or presence of skeletal and cerebral anomalies. We cannot exclude a different genetic origin for the rarest or isolated anomalies as the majority of participants were analysed through single gene sequencing or targeted Next Generation Sequencing (NGS) panels due to a strong clinical suspicion.
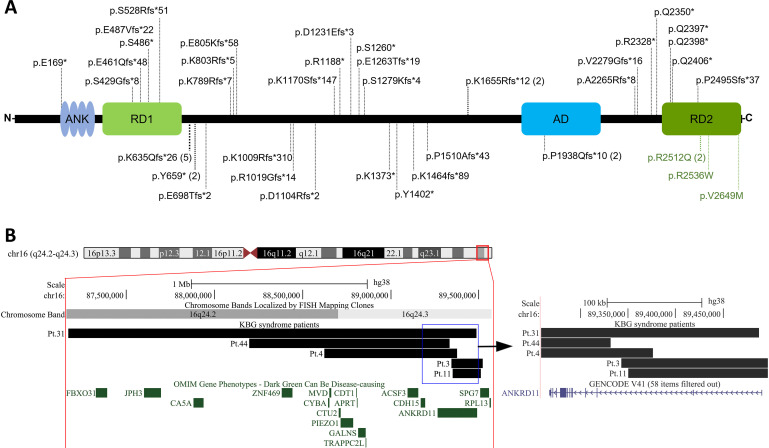
Figure 3.
Intragenic and structural variants in our cohort of patients with KBG. (A) ANKRD11 intragenic variants plotted on the protein diagram; numbers in parentheses indicate recurrence of a variant. (B) Large deletions in chromosome 16q24 visualised in UCSC Genome Browser (http://genome.ucsc.edu), assembly hg38. Zoom-in on the right shows the position of breakpoints relative to ANKRD11 exons. AD, activation domain; ANK, ankyrin repeats; RD, repressor domain.
Discussion
We performed an in-depth, systematic evaluation of original neuroradiological and skeletal imaging data from 53 individuals with genetically confirmed KBG syndrome, and compared results with the existing literature (figure 4). We found that both neuroradiological and skeletal abnormalities in KBG syndrome are very frequent as well as heterogeneous. Evaluation by two independent groups supports the accuracy of the description and prevalence of these findings.
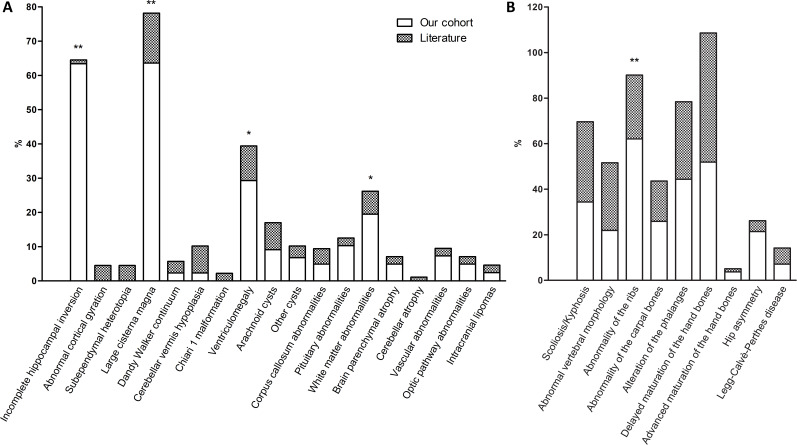
Figure 4.
Stacked histograms comparing the imaging findings in our cohort with those reported in the literature. (A) Brain abnormalities at diagnostic imaging. Cortical gyration anomalies consist solely of the four mild alterations reported in a volumetric study, and no macroscopic gyration defects were detected by standard MRI in any of the other publications. (B) Skeletal abnormalities at diagnostic imaging. Skeletal features mainly determined through physical examination rather than X-ray imaging, such as wide anterior fontanel and hip dysplasia, were not included because of the difficulty of establishing accurate ratios of evaluated patients. *Significant difference at p<0.05. **Significant difference at p<0.001.
We also compared imaging findings showing a frequency greater than 20% with the type of variant identified in each participant. No genotype-phenotype correlations were noted (online supplemental table 6) which is consistent with previous reports.3 8 23
We could rarely determine the original indication for requesting diagnostic imaging. In those cases, imaging was often performed as part of the clinical follow-up second to epilepsy or other neurodevelopmental signs. This may constitute a limitation in our study, possibly leading to an overestimate of these features. However, if we considered the prevalence across the 400 individuals with KBG reported to date rather than just the individuals with MRI, some findings (eg, large cisterna magna) would still show a marked increase compared with the general population. This limitation does not affect the comparison between our cohort and other retrospective reports, since the literature on brain MRIs is usually based on the same assumptions. Furthermore, defining these prevalences is a useful secondary endpoint. The main strength of this study consists of the in-depth description of the imaging findings in a large cohort of individuals with KBG syndrome, which may be useful to researchers, clinicians and families alike.
Neuroradiological anomalies
Brain MRI showed normal results in a few cases only (3/41); the most common abnormalities included incomplete hippocampal inversion (IHI), large cisterna magna and the identification of patulous IAC as a novel report in KBG syndrome. Based on participants with available serial MRIs, all features could be considered constitutional. We found no signs of neurodegeneration including atrophy or myelination defects.
Enlarged cisterna magna is an anatomical variant of the posterior fossa consisting in a focal enlargement of the subarachnoid space. It is a known feature of KBG syndrome (11.2% of cases with available imaging data), but was observed at a higher frequency in our cohort (28/44 patients, ie, 63.6%, p<0.001; figures 1B and 4A). This prevalence is significantly higher than in the general population, where it is considered to occur in about 3–10/1000 individuals.24 25 Two of our participants with enlarged cisterna magna also had low attachment of the tentorium and low torcula, presenting as benign features that were not associated with Chiari malformation. One participant had a posterior fossa abnormality at the mild end of the Dandy-Walker continuum, with hypoplasia and anticlockwise rotation of the cerebellar vermis and with dilated fourth ventricle (figure 1C). A similar anomaly was reported in at least three individuals with KBG in the literature.11 26 However, the non-specific association of this feature with various syndromes and the single report in a cohort as large as ours argue against its clinical utility in differential diagnosis, as previously proposed.26
MRI detected various forms of ventricular anomalies in 15 out of 41 (36.6%) participants, including enlarged lateral ventricles and dysmorphic trigone (figure 1E,F). The overall prevalence is significantly higher than in the literature (p=0.009), although most reports only mention ventriculomegaly and provide no further details.
IHI, one of the most common findings in our cohort (26/41, 63.4%) and mostly bilateral (19/26; figure 1A), has been previously reported in a single individual with KBG,10 probably because of different methodologies in diagnostic practice. It arises from a subtle defect of the enfolding process of the brain, and although the clinical significance is still debated, IHI is often considered an anatomical variant. IHI can be found in 6%–24% of healthy individuals with brain MRI data, usually monolateral on the left side and less frequently bilateral, but it is more commonly reported in individuals with other subclinical cortical malformations, with heterogeneous epilepsy syndromes or with inherited neurodevelopmental conditions.27 28 The high incidence of bilateral IHI in KBG syndrome may indicate a modest effect of ANKRD11 on cortical development, possibly unrelated to clinical manifestations. Notably, we did not observe subependymal heterotopia or diffuse abnormalities of cortical gyration. In the literature there is a single study describing four individuals with thin uncinate fascicles and localised sulcation anomalies, mild and without overt polymicrogiria.29 In our retrospective cohort, we could not perform a detailed analysis since volumetric MRI was not part of the diagnostic protocols, and MRI tractography was available for only one participant.
We observed white matter (WM) abnormalities less frequently (8/41) and mostly in the periventricular region, although we observed generalised thickness reduction in two cases. Two participants showed cerebellar involvement, one with porencephaly, the other with small rounded hypointensities on T2-weighted imaging representing a possible sign of calcification. Only eight individuals were described in the literature with heterogeneous WM abnormalities such as atrophy, delayed myelination, periventricular leukomalacia and cerebral calcifications,2 5 8–10 indicating a lack of specificity for KBG syndrome.
Abnormalities of the sellar region were noted in five participants and included empty sella (figure 1D), shallow pituitary fossa and cystic lesion of the pars intermedia. One participant was treated with somatotropin due to growth hormone deficiency. Another had normal hormonal levels, while referring physicians had not investigated the rest; all four had auxological parameters within normal ranges (online supplemental table 2). Only two individuals with sellar anomalies were described in the literature.13 30 Empty sella in children is usually an indication of pituitary gland hypolasia or aplasia and is considered one of the main risk factors of hormone deficiencies.31
We observed vascular anomalies in three participants, all benign anatomical variants reported with variable frequency in the general population.32 Since only six individuals were investigated specifically for vascular anomalies, these data do not fully represent our retrospective cohort.
We noted cystic lesions in six participants, with arachnoid cysts being the most frequent type (figure 1G,H), in accordance with the literature. We also confirmed the rarity of corpus callosum anomalies, hypoplasia of the optic nerve and intracranial lipomas (figure 1J), while Chiari malformation and cerebellar atrophy10 11 were absent in our cohort.
Novel features included a patulous IAC observed in 8 participants out of 12 with appropriate MRI or CT cross sections available (figure 1I). This rare anatomical variant, found in less than 0.5% of the general population, is characterised by a bilateral and usually symmetrical height increase at the midportion of the IAC.33 It differs from dilatation due to acoustic neuromas because the cortical margins and falciform crest of the canal are preserved. Its significance in the pathogenesis of congenital hearing impairment is controversial.33 34 In our cohort, hearing loss was reported in only two of the eight participants with patulous IAC, but in all four with confirmed normal IAC, suggesting that an association seems unlikely. Nevertheless, IAC evaluation may be considered during the follow-up of individuals with KBG syndrome and hearing defects, since patulous IAC was proposed as a risk of complications during ear surgery.34
Additional novel findings included partial agenesis of septum pellucidum (figure 1K), dilation of Meckel’s cave, persistent hyperplastic primary vitreous (figure 1L), and hypoplasia of olfactory bulb and olfactory nerve. Interestingly, it has been suggested that Ankrd11-deficient mice may show defects in olfactory bulb development (A. Voronova, personal communication at KBG Scientific Meeting, 7 June 2022).
Some features, most notably IHI and arachnoid cysts, have been proposed as non-specific markers of a genetic predisposition to seizures.27 35 In our cohort, statistical analysis using Fisher’s exact test revealed that neither epilepsy presence or age of onset, nor the severity of ID, was significantly associated with any specific MRI finding, or with the burden of brain anomalies (online supplemental table 7, online supplemental figure 2). This supports the genetic aetiology of seizures in individuals with KBG syndrome, arising from the role of ANKRD11 in neuronal plasticity1 rather than from structural abnormalities.
Skeletal and spine anomalies
Skeletal features have been studied more extensively in the past compared with neuroradiological features, and their prevalences match well with those of our cohort (figure 4B).
In 2018, we described an individual (participant 1 in this study) with a long and prominent coccyx, and postulated that this atypical anomaly should be considered in the differential diagnosis.19 A second individual in the present cohort had an elongated coccyx, while two more showed rare coccygeal anomalies (figure 2O,P), reinforcing the notion that ANKRD11 may be important—although not essential—for coccyx development. Anomalies in the development of the axial skeleton in general seem to be recurrent in KBG syndrome. Our cohort shows rare instances of cervical C2/C3 vertebral fusion, C1/occipital bone fusion, retroflexed odontoid process, dysmorphic dorsal vertebrae and tall bodies of the lumbar vertebrae in a total of four participants (figure 2C,H). Previous reports found abnormal vertebral morphology or vertebral fusion in about 30% of individuals with KBG syndrome.2 5 8 9 17 23 Overall, the occurrence of these features as isolated defects in the general population is thought to be much lower, about 1 in 2000–5000 live births.36 37 Most anomalies are benign, but some may progress to clinical relevance due to spinal cord compression, while C1/occiput fusion usually requires surgical intervention.36
Abnormal curvature of the vertebral column consisting of kyphosis, lordosis or scoliosis was reported for 24.6% individuals with KBG in the literature, and if we consider both imaging and clinical examinations, a similar rate emerged in our cohort (26.9%; online supplemental table 4, figure 1M and figure 2G–J).
Spine MRI in seven participants revealed low-lying conus medullaris ending below the L2 vertebra (n=4), hydromyelia (n=2), ventriculus terminalis (n=1) and hydro-syringomyelia (n=1) (figure 1N–O). While most of these findings are benign, syringomyelia can slowly evolve and cause paralysis, sensory loss and chronic pain starting from the third or fourth decade of life.38 Six reports of tethered cord in the literature17 30 39–42 further suggest that particular attention should be given to these findings. Importantly, a combination of medullary features with scoliosis and other vertebral abnormalities may contribute to an unfavourable clinical progression by spinal cord compression or bending. One of our participants with hydromyelia also had partial C1 vertebra/occiput fusion while another had hyperkyphosis. No specific symptoms were reported at the time of data collection, but since they were all younger than 20 years, we cannot exclude a later evolution. We suggest checking regularly for symptoms such as lower back pain, sensory or motor deficit of the legs, bladder or bowel dysfunction, and performing spine MRI at the earliest suspicion of a progressive anomaly of the spine. A prospective study and long-term follow-up in a larger cohort would be required to establish the exact risks associated with spinal anomalies.
Cervical ribs are known to be more frequent in KBG syndrome2 than in the general population, occurring in less than 1% of individuals and usually monolaterally.43 In the present cohort, cervical ribs and hypertrophy of C7 cervical vertebra transverse processes were bilateral in all but two cases and had a significantly higher frequency than previously reported (62.1% vs 28%, p<0.001). This is likely due to a more inclusive characterisation of C7 transverse process hypertrophy. In the context of other conditions, cervical ribs have been occasionally known to cause blood vessel or nerve compression.43 Thoracic outlet syndrome has yet to be reported in KBG syndrome, but should be considered in individuals experiencing numbness or pain of the arm, pain of the neck/upper back/upper chest, discolouration of the hand or dizziness.43
Another known feature of KBG syndrome is brachydactyly, often associated with different bone anomalies. Twelve of our participants with hand X-rays showed short middle phalanx of the fifth finger, with concomitant short middle phalanx of the second finger in three cases, which represents a type 4A brachydactyly.
Altered skeletal maturation is also common in KBG syndrome.2 16 18 In our cohort, one participant showed accelerated skeletal maturation while 14 had an ossification delay more than 2 SD below the sex-matched and age-matched population mean. Four additional individuals had a delay of at least −1 SD. Across all hand X-rays from 27 participants, we could observe a general trend of delayed maturation (average −1.96±2.06 SD), especially at younger ages (online supplemental table 3). We noted a greater ossification delay of the carpal bones compared with metacarpals and phalanges in nine participants, with a discrepancy >2 SD in six. We also observed recurrent abnormalities of carpal bone morphology, including aplasia, dysmorphic shape and lunate-triquetral fusion; the latter noted in three cases and never reported before in KBG syndrome. Carpal fusion can occur as an isolated finding in the paediatric population, but is usually infrequent, about 1:200.44 A longitudinal evaluation of hand radiographs from one participant allowed us to study the onset and evolution of carpal anomalies (figure 2K–N). At 3 years 5 months of age, the participant showed delayed ossification of the tubular bones and especially of the carpals, with absence of the proximal row up to 6 years of age. Maturation rate improved over time, and at 9 years of age, the tubular bones were adequate, while the carpals were still delayed; the lunate and triquetral bones showed partial synostosis and were completely fused together by 10 years of age. These data suggest that the marked ossification delay and morphological anomalies of the carpal bones may share a common pathogenetic mechanism, and support the crucial role of ANKRD11 in osteogenesis indicated by mouse models.45 46 These uncommon anomalies may also provide a useful contribution in suspecting KBG syndrome.
Foot X-rays showed a range of rare or isolated anomalies including cone-shaped epiphyses of toes, and dysmorphic or hypomorphic metatarsal bones and phalanges (figure 2Q,R). Hip scans revealed only two participants with asymmetry in the height of the femural heads and one participant with Legg-Calvè-Perthes disease of the right femur, which consists of femoral head degeneration secondary to blood supply deprivation due to unknown reasons. This anomaly has been observed in only one other case in the literature11 and could be an incidental finding.
Conclusion
Skeletal, spinal and brain anomalies are quite common among individuals with KBG syndrome. Combinations of these heterogeneous findings can be valuable in guiding the diagnostic suspicion. Because of the phenotypic variability of KBG syndrome, some individuals may display a single feature such as ID or short stature as the predominant clinical aspect. This is especially true of young children with deciduous dentition, who may not display macrodontia yet. Even when WES or a gene panel is employed to seek a molecular diagnosis, it may result in a variant of uncertain significance that needs to be addressed. Brain MRI or X-ray detection of the congenital features described in this study, such as patulous IAC or costovertebral and hand bone anomalies, can provide further clues for solving these cases. Brain MRI is not recommended as a routine first-tier diagnostic test in the absence of focal neurological deficits, especially because of concerns regarding the sedation of children. However, available imaging obtained while investigating ID or seizures may be reviewed to look for suggestive features, even if benign.
This knowledge can also be useful for the management of affected individuals. Some anatomical variants may be considered benign when isolated in the general population, but deserve proper attention in the follow-up of individuals with KBG syndrome. In fact, they can occur more frequently and in combination, with symptoms that may be difficult to detect in people with ID or autistic features. Spine abnormalities in particular, even if clinically silent at the time of detection, should be monitored over time and investigated for signs of a possible evolution.
Lastly, the spectrum of abnormalities presented here can be valuable for future research on the effects of ANKRD11 variants on skeletal and neural development, by providing a reference for cellular and animal models of KBG syndrome.
Acknowledgments
The authors of this publication are members of the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability ERN-ITHACA (EU Framework Partnership Agreement ID: 3HP-HP-FPA ERN-01-2016/739516). The authors wish to thank the patients and their family members for their cooperation in providing the medical data and photographs necessary for this publication, as well as the photographers Marco Bonazzi and Luca Valcavi. The authors are grateful for the contribution made by the Fondazione Cassa di Risparmio Manodori di Reggio Emilia.
Footnotes
Deceased: after contributing to the manuscript
Contributors: Conceptualisation: AB, LG. Data curation: FP, G Contrò, SGC, MN, G Carboni, AS, RZ, EC, AB. Formal analysis: SGC, RZ. Investigation: FP, LV, MN, G Carboni, AS, AMB, MG, SWG, CWO, RP, IR, DB. Methodology: AS, RP, LG, AB. Resources: GA, AMB, II, SM, EBB, MTC, MLD, KD, MB, MCD, AD, DD, SF, CRF, LW, WAG, MG, HG, SWG, TBH, LI, TK, DAK, FRL, GL, PL, GM, SFM, AM, RM, JEKN, AN, CWO, TP, IR, SPR, SLS, MFS, APAS, CTRMS, AG, JT, DB, ASo, MFB, RB, AC, CF, MI, LVM, SV, SSV, LG, AB. Supervision: LG, AB. Guarantor: LG. Writing—original draft: FP, G Contrò, LV, LG. Writing—review and editing: FP, G Contrò, SGC, MN, AMB, CRF, AC, WAG, HG, TBH, OZ, AB.
Funding: Sequencing and analysis for patients 49–51 partially supported by grant-RC Linea 1 “Studio fenotipo-genotipo delle malattie genetiche rare ad espressione neuropsichiatrica in età evolutiva”, Italian Ministry of Health for IRCCS Fondazione Stella Maris (RB, RM, GA). Sequencing and analysis for patient 46 was performed by Care4Rare Canada Consortium. Sequencing and analysis for patient 8 supported in part by the Intramural Research Program of the National Human Genome Research Institute.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data, except any confidential information about the study participants, are available on reasonable request by contacting the corresponding author. Novel variants have been submitted to the ClinVar database (accession numbers SCV003927975 to SCV003927993).
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
Ethics approval
The study was approved by the Research Ethics Committee of Area Vasta Emilia Nord (CE-AVEN 22/09/2020, protocol no. 2020/0109814) and by the competent local institutional review boards. Participants gave informed consent to participate in the study before taking part.
References
- 1. Sirmaci A, Spiliopoulos M, Brancati F, et al. Mutations in Ankrd11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am J Hum Genet 2011;89:289–94. 10.1016/j.ajhg.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skjei KL, Martin MM, Slavotinek AM. KBG syndrome: report of twins, neurological characteristics, and delineation of diagnostic criteria. Am J Med Genet A 2007;143A:292–300. 10.1002/ajmg.a.31597 [DOI] [PubMed] [Google Scholar]
- 3. Gnazzo M, Lepri FR, Dentici ML, et al. KBG syndrome: common and uncommon clinical features based on 31 new patients. Am J Med Genet A 2020;182:1073–83. 10.1002/ajmg.a.61524 [DOI] [PubMed] [Google Scholar]
- 4. Herrmann J, Pallister PD, Tiddy W, et al. The KBG syndrome-a syndrome of short stature, characteristic Facies, mental retardation, macrodontia and skeletal anomalies. Birth Defects Orig Artic Ser 1975;11:7–18. [PubMed] [Google Scholar]
- 5. Low K, Ashraf T, Canham N, et al. Clinical and genetic aspects of KBG syndrome. Am J Med Genet A 2016;170:2835–46. 10.1002/ajmg.a.37842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang A, Li C-W, Chen JD. Characterization of transcriptional regulatory domains of ankyrin repeat cofactor-1. Biochem Biophys Res Commun 2007;358:1034–40. 10.1016/j.bbrc.2007.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ka M, Kim W-Y. Ankrd11 associated with intellectual disability and autism regulates dendrite differentiation via the BDNF/Trkb signaling pathway. Neurobiol Dis 2018;111:138–52. 10.1016/j.nbd.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ockeloen CW, Willemsen MH, de Munnik S, et al. Further delineation of the KBG syndrome phenotype caused by Ankrd11 aberrations. Eur J Hum Genet 2015;23:1176–85. 10.1038/ejhg.2014.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murray N, Burgess B, Hay R, et al. KBG syndrome: an Australian experience. Am J Med Genet A 2017;173:1866–77. 10.1002/ajmg.a.38121 [DOI] [PubMed] [Google Scholar]
- 10. Novara F, Rinaldi B, Sisodiya SM, et al. Haploinsufficiency for Ankrd11-flanking genes makes the difference between KBG and 16Q24.3 microdeletion syndromes: 12 new cases. Eur J Hum Genet 2017;25:694–701. 10.1038/ejhg.2017.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scarano E, Tassone M, Graziano C, et al. Novel mutations and unreported clinical features in KBG syndrome. Mol Syndromol 2019;10:130–8. 10.1159/000496172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brancati F, D’Avanzo MG, Digilio MC, et al. KBG syndrome in a cohort of Italian patients. Am J Med Genet A 2004;131:144–9. 10.1002/ajmg.a.30292 [DOI] [PubMed] [Google Scholar]
- 13. Mattei D, Cavarzere P, Gaudino R, et al. Dysmorphic features and adult short stature: possible clinical markers of KBG syndrome. Ital J Pediatr 2021;47:15. 10.1186/s13052-021-00961-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyatake S, Okamoto N, Stark Z, et al. Ankrd11 variants cause variable clinical features associated with KBG syndrome and coffin-siris-like syndrome. J Hum Genet 2017;62:741–6. 10.1038/jhg.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willemsen MH, Fernandez BA, Bacino CA, et al. Identification of Ankrd11 and Znf778 as candidate genes for autism and variable cognitive impairment in the novel 16Q24.3 microdeletion syndrome. Eur J Hum Genet 2010;18:429–35. 10.1038/ejhg.2009.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morel Swols D, Foster J, Tekin M. KBG syndrome. Orphanet J Rare Dis 2017;12:183. 10.1186/s13023-017-0736-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zollino M, Battaglia A, D’Avanzo MG, et al. Six additional cases of the KBG syndrome: clinical reports and outline of the diagnostic criteria. Am J Med Genet 1994;52:302–7. 10.1002/ajmg.1320520310 [DOI] [PubMed] [Google Scholar]
- 18. Brancati F, Sarkozy A, Dallapiccola B. KBG syndrome. Orphanet J Rare Dis 2006;1:50. 10.1186/1750-1172-1-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Bernardi ML, Ivanovski I, Caraffi SG, et al. Prominent and elongated coccyx, a new manifestation of KBG syndrome associated with novel mutation in Ankrd11. Am J Med Genet A 2018;176:1991–5. 10.1002/ajmg.a.40386 [DOI] [PubMed] [Google Scholar]
- 20. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Am J Med Sci 1959;238:393. 10.1097/00000441-195909000-00030 [DOI] [Google Scholar]
- 21. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genetics in Medicine 2015;17:405–24. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riggs ER, Andersen EF, Cherry AM, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American college of medical genetics and genomics (ACMG) and the clinical genome resource (Clingen). Genetics in Medicine 2020;22:245–57. 10.1038/s41436-019-0686-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Boer E, Ockeloen CW, Kampen RA, et al. Missense variants in Ankrd11 cause KBG syndrome by impairment of stability or transcriptional activity of the encoded protein. Genet Med 2022;24:2051–64. 10.1016/j.gim.2022.06.007 [DOI] [PubMed] [Google Scholar]
- 24. Zimmer EZ, Lowenstein L, Bronshtein M, et al. Clinical significance of isolated Mega cisterna magna. Arch Gynecol Obstet 2007;276:487–90. 10.1007/s00404-007-0369-6 [DOI] [PubMed] [Google Scholar]
- 25. Gaillard F, Weerakkody Y, Niknejad M, et al. Mega cisterna magna. Reference Article, RadiopaediaOrg Radiopaedia 2023. 10.53347/rID-4585 [DOI] [Google Scholar]
- 26. Tago T, Suzuki T, Kashimada A, et al. Two case reports of KBG syndrome with dandy-walker variant. Pediatr Int 2021;63:1530–2. 10.1111/ped.14648 [DOI] [PubMed] [Google Scholar]
- 27. Mutti C, Riccò M, Bartolini Y, et al. Incomplete hippocampal inversion and epilepsy: a systematic review and meta-analysis. Epilepsia 2021;62:383–96. 10.1111/epi.16787 [DOI] [PubMed] [Google Scholar]
- 28. Cury C, Toro R, Cohen F, et al. Incomplete hippocampal inversion: a comprehensive MRI study of over 2000 subjects. Front Neuroanat 2015;9:160. 10.3389/fnana.2015.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiménez de la Peña M, Fernández-Mayoralas DM, López-Martín S, et al. Abnormal frontal gyrification pattern and uncinate development in patients with KBG syndrome caused by Ankrd11 aberrations. European Journal of Paediatric Neurology 2021;35:8–15. 10.1016/j.ejpn.2021.09.008 [DOI] [PubMed] [Google Scholar]
- 30. Gao F, Zhao X, Cao B, et al. Genetic and phenotypic spectrum of KBG syndrome: a report of 13 new Chinese cases and a review of the literature. J Pers Med 2022;12:407. 10.3390/jpm12030407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cerbone M, Dattani MT. Progression from isolated growth hormone deficiency to combined pituitary hormone deficiency. Growth Hormone & IGF Research 2017;37:19–25. 10.1016/j.ghir.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 32. Shiozaki E, Kawahara I, Uchida D, et al. Unruptured cerebral aneurysms with the segmental duplicated middle cerebral artery formed a fenestrated structure at origin. Surg Neurol Int 2022;13:33. 10.25259/SNI_1108_2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Migirov L. Patulous internal auditory canal. Arch Otolaryngol Head Neck Surg 2003;129:992–3. 10.1001/archotol.129.9.992 [DOI] [PubMed] [Google Scholar]
- 34. Stimmer H, Niedermeyer HP, Kehl V, et al. Nontumorous enlargement of the internal auditory canal: a risk factor for sensorineural hearing loss? A high resolution CT-study. Rofo 2015;187:450–8. 10.1055/s-0034-1399009 [DOI] [PubMed] [Google Scholar]
- 35. Orduna Martínez J, López Pisón J, Curto Simón B, et al. Intracranial arachnoid cysts and epilepsy in children: should this be treated surgically? our 29-year experience and review of the literature. Neurocirugía 2022;33:157–64. 10.1016/j.neucir.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 36. Giampietro PF, Dunwoodie SL, Kusumi K, et al. Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann N Y Acad Sci 2009;1151:38–67. 10.1111/j.1749-6632.2008.03452.x [DOI] [PubMed] [Google Scholar]
- 37. Heiskanen S, Syvänen J, Helenius I, et al. Increasing prevalence and high risk of associated anomalies in congenital vertebral defects: a population-based study. J Pediatr Orthop 2022;42:e538–43. 10.1097/BPO.0000000000002124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leclerc A, Matveeff L, Emery E. Syringomyelia and hydromyelia: current understanding and neurosurgical management. Rev Neurol (Paris) 2021;177:498–507. 10.1016/j.neurol.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 39. Tekin M, Kavaz A, Berberoğlu M, et al. The KBG syndrome: confirmation of autosomal dominant inheritance and further delineation of the phenotype. Am J Med Genet A 2004;130A:284–7. 10.1002/ajmg.a.30291 [DOI] [PubMed] [Google Scholar]
- 40. Maegawa GHB, Leite JCL, Félix TM, et al. Clinical variability in KBG syndrome: report of three unrelated families. Am J Med Genet A 2004;131:150–4. 10.1002/ajmg.a.30293 [DOI] [PubMed] [Google Scholar]
- 41. Isrie M, Hendriks Y, Gielissen N, et al. Haploinsufficiency of Ankrd11 causes mild cognitive impairment, short stature and minor dysmorphisms. Eur J Hum Genet 2012;20:131–3. 10.1038/ejhg.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alves RM, Uva P, Veiga MF, et al. Novel Ankrd11 gene mutation in an individual with a mild phenotype of KBG syndrome associated to a GEFS+ phenotypic spectrum: a case report. BMC Med Genet 2019;20:16. 10.1186/s12881-019-0745-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spadliński Ł, Cecot T, Majos A, et al. The epidemiological, morphological, and clinical aspects of the cervical ribs in humans. Biomed Res Int 2016;2016:8034613. 10.1155/2016/8034613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pruszczynski B, Saller J, Rogers KJ, et al. Incidence of carpal coalition in the pediatric population. J Pediatr Orthop 2016;36:e106–10. 10.1097/BPO.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 45. Barbaric I, Perry MJ, Dear TN, et al. An ENU-induced Mutation in the Ankrd11 gene results in an osteopenia-like phenotype in the mouse mutant yoda. Physiol Genomics 2008;32:311–21. 10.1152/physiolgenomics.00116.2007 [DOI] [PubMed] [Google Scholar]
- 46. Roth DM, Baddam P, Lin H, et al. The Chromatin regulator Ankrd11 controls palate and cranial bone development. Front Cell Dev Biol 2021;9:645386. 10.3389/fcell.2021.645386 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jmg-2023-109141supp003.xlsx (58.9KB, xlsx)
jmg-2023-109141supp002.pdf (165.5KB, pdf)
jmg-2023-109141supp001.pdf (589.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data, except any confidential information about the study participants, are available on reasonable request by contacting the corresponding author. Novel variants have been submitted to the ClinVar database (accession numbers SCV003927975 to SCV003927993).