Abstract
Objective
We aimed to test the hypothesis that automated fibrosis score calculation and electronic reminder messages could increase the detection of advanced liver disease in patients with type 2 diabetes.
Design
In this pragmatic randomised controlled trial at five general medical or diabetes clinics in Hong Kong and Malaysia, we randomly assigned patients in a 1:1 ratio to the intervention group with Fibrosis-4 index and aspartate aminotransferase-to-platelet ratio index automatically calculated based on routine blood tests, followed by electronic reminder messages to alert clinicians of abnormal results, or the control group with usual care. The primary outcome was the proportion of patients with increased fibrosis scores who received appropriate care (referred for hepatology care or specific fibrosis assessment) within 1 year.
Results
Between May 2020 and Oct 2021, 1379 patients were screened, of whom 533 and 528 were assigned to the intervention and control groups, respectively. A total of 55 out of 165 (33.3%) patients with increased fibrosis scores in the intervention group received appropriate care, compared with 4 of 131 (3.1%) patients in the control group (difference 30.2% (95% CI 22.4% to 38%); p<0.001). Overall, 11 out of 533 (2.1%) patients in the intervention group and 1 out of 528 (0.2%) patients in the control group were confirmed to have advanced liver disease (difference 1.9% (95% CI 0.61% to 3.5%); p=0.006).
Conclusion
Automated fibrosis score calculation and electronic reminders can increase referral of patients with type 2 diabetes and abnormal fibrosis scores at non-hepatology settings.
Trial registration number
Keywords: FATTY LIVER, CIRRHOSIS, HEPATIC FIBROSIS, DIABETES MELLITUS, PRIMARY CARE
WHAT IS ALREADY KNOWN ON THIS TOPIC
Type 2 diabetes is strongly associated with non-alcoholic fatty liver disease (NAFLD) and its severity.
The majority of patients with NAFLD and metabolic risk factors are seen in primary care and non-hepatology settings.
Although clinical care pathways have been proposed and tested in primary care settings, it is unclear how the message can reach healthcare providers who are less aware of the importance of NAFLD.
WHAT THIS STUDY ADDS
In this randomised controlled trial, we found that automated calculation of simple fibrosis scores, followed by reminder messages in the electronic clinical management system, could substantially increase appropriate referral to hepatologists and arrangement of specific fibrosis tests for patients with type 2 diabetes and abnormal fibrosis scores.
However, less than 20% of patients with abnormal fibrosis scores were confirmed to have advanced liver fibrosis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The results of our clinical trial confirm the feasibility and efficacy of a care model based on automated fibrosis score calculation and electronic reminder messages to increase identification of advanced liver disease among patients with type 2 diabetes. Non-invasive tests in the care model should be further refined to improve the overall accuracy in detecting advanced liver disease and minimise false-positive results.
Introduction
Non-alcoholic fatty liver disease (NAFLD), recently renamed as metabolic dysfunction-associated steatotic liver disease or metabolic-associated fatty liver disease, affects around 30% of the global adult population and is an important cause of cirrhosis and hepatocellular carcinoma.1 Unlike other chronic liver diseases, the vast majority of patients with NAFLD are seen in primary care and non-hepatology settings.2 Besides, only a fraction of patients will eventually develop liver-related complications. Among the various histological features of NAFLD, fibrosis stage has the strongest correlation with future liver-related morbidity and mortality.3 Therefore, it is important to build a clinical care pathway focusing on the liaison between primary and specialist care and effective use of non-invasive tests of liver fibrosis.
Several professional societies have recommended a two-step approach for case identification and assessment in patients with NAFLD or risk factors of NAFLD.4–6 The idea is to use a simple fibrosis score such as the Fibrosis-4 index (FIB-4) for initial assessment. Such scores typically comprise routine laboratory tests and can thus be calculated in primary care with almost no additional cost.7 Though crude, these scores have high negative predictive values for ruling out advanced liver fibrosis due to NAFLD and other chronic liver diseases. Patients with abnormal scores can then undergo more specific non-invasive tests of fibrosis, either in primary care or specialist settings depending on test availability. In prospective studies in primary care settings, such a two-step approach has been shown to increase the detection of advanced fibrosis while reducing the number of unnecessary referrals of patients with mild liver disease.8
Nonetheless, unless structured as part of the routine, such clinical care pathways depend heavily on clinicians interested in NAFLD and may not be generalisable. Therefore, we proposed and tested a care model based on automated fibrosis score calculation and electronic reminder messages in patients with type 2 diabetes.
Methods
Study design and participants
This randomised, parallel group, pragmatic trial was done at three general medical and two diabetes clinics in Hong Kong and Malaysia. There were 10–20 family doctors, general medical clinicians, endocrinologists or trainees at each clinic. The aim of the trial was to test the hypothesis that automated fibrosis score calculation and electronic reminder messages could increase the proportion of patients with type 2 diabetes and abnormal simple fibrosis scores who received appropriate management, defined as referral to hepatologists or further fibrosis assessments including vibration controlled transient elastography (VCTE), other ultrasound elastography, magnetic resonance elastography, liver biopsy or specific blood biomarkers of liver fibrosis such as FibroTest and FibroMeter.7
Eligible patients were 18–70 years of age with a history of type 2 diabetes. We selected this patient population because over 50% of patients with type 2 diabetes have NAFLD, and type 2 diabetes is an important risk factor for cirrhosis and hepatocellular carcinoma.9 Current European multisociety guidelines also recommend case finding of advanced liver disease in this population.10 Patients were excluded if they had type 1 diabetes, history of hepatocellular carcinoma or hepatic decompensation, other active malignancies or were already receiving care by gastroenterologists or hepatologists. Notably, although the primary focus of this study was to identify patients with NAFLD and advanced liver fibrosis, we did not exclude patients with other chronic liver diseases such as chronic viral hepatitis and alcohol-related liver disease because patients in real life can have other liver diseases, and it would be meaningful to detect those diseases, especially if advanced fibrosis has already developed.
The trial was conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation.
Randomisation and blinding
Eligible patients were randomly assigned in a 1:1 ratio (permuted block size of 4–10) to the intervention group and control group. Concealment of group allocation was achieved through putting the group allocation cards in consecutively numbered and sealed envelopes. After each clinic session, the central investigator (VW-SW) would send the group allocation of the newly recruited patients to the local investigator, who would then handle the fibrosis scores and reminder messages. Neither the central nor local investigators had direct patient contact. In contrast, patients and clinicians seeing the patients were partially blinded to the group assignment. A clinician would know that a patient was in the intervention group when he/she saw a reminder message regarding abnormal fibrosis scores. Otherwise, the system would not alert a clinician to study recruitment, group assignment or fibrosis score results.
Procedures
For patients in the intervention group, FIB-4 and aspartate aminotransferase (AST)-to-platelet ratio index (APRI) were measured at the next scheduled blood tests according to published formulae.11 12 If the scores were abnormal (ie, FIB-4≥1.3 in patients younger than 65 years or ≥2.0 in patients older than 65 years and/or APRI>0.5),13 the following pop-up message would appear in the electronic clinical management system every time a clinician logged into the patient file for the coming 1 year:
“This patient has high FIB-4 (and/or AST-to-platelet ratio index) of xxx suggestive of significant liver fibrosis. Please consider referring the patient to the hepatology clinic or arranging further test such as FibroScan.”
Patients in the control group underwent the same blood tests. Clinicians had access to the raw liver function test and complete blood count, but the fibrosis score results would not be specifically shown, and there were no reminder messages regardless of the test results. This was to mimic usual care when there was no dedicated care model for case identification.
Regardless of group assignment and fibrosis score results, patients referred to the hepatology clinic were seen within 12 weeks. Assessments at the hepatology clinic included history taking and physical examination to identify risk factors of chronic liver diseases, blood tests for hepatitis B surface antigen and antihepatitis C virus antibody, and other diagnostic workup as clinically indicated. VCTE (FibroScan, Echosens, Paris, France) was then performed by experienced operators for liver stiffness assessment according to the instructions and training by the manufacturer.14 The M or XL probe was chosen according to the machine’s automatic probe selection tool. The median of 10 measurements was used to reflect the severity of liver fibrosis.
Outcomes
The primary outcome was the proportion of patients with high fibrosis scores who received appropriate care (referral to hepatologists or further specific liver assessments for liver fibrosis) within 1 year of the baseline visit. The choice of the follow-up interval was based on the fact that some patients with type 2 diabetes might be seen at multiple clinics, and clinicians who do not provide prescriptions typically review patients annually. Considering the potential variability of fibrosis scores over time, we examined a separate cohort of 8700 patients with NAFLD with serial FIB-4 at a median interval of 1 year (online supplemental table 1). Among 5002 patients with FIB-4<1.3 at baseline, approximately 4231 patients (84.6%) continued to have FIB-4<1.3 at follow-up. Among 3698 patients with FIB-4≥1.3 at baseline, 82.0% continued to have FIB-4≥1.3 at follow-up. We calculated the weighted Kappa of the two FIB-4 measurements using linear weights. The weighted Kappa was 0.632 (95% CI 0.618 to 0.646). This suggests substantial agreement between the two measurements of FIB-4, indicating a good reliability of the two FIB-4 measurements. Secondary outcomes included the proportion of patients in the overall population (regardless of fibrosis scores) referred to hepatologists, inappropriate referrals (proportion of patients with low fibrosis scores who were referred to hepatologists) and the proportion of patients confirmed to have advanced fibrosis, defined by one of the following: (1) liver stiffness measurement by VCTE≥10 kPa, (2) fibrosis stages 3–4 by liver biopsy, (3) unequivocal radiological features of cirrhosis (cirrhosis with nodular appearance, splenomegaly, ascites or varices) or (4) clinical, radiological or endoscopic evidence of portal hypertension.
gutjnl-2023-330269supp001.pdf (90.3KB, pdf)
Statistical analysis
There was a mistake in the original sample size calculation in the protocol V.1.0 dated on 28 February 2019, which was then rectified and approved by the ethics committee in an amended protocol V.1.1 dated on 27 March 2023. The sample size remained unchanged in this amendment, but the calculations were corrected. According to preliminary data from our previous study, 33% and 10% of patients with type 2 diabetes had increased FIB-4 and APRI, respectively, and around 10% of patients with advanced fibrosis were under hepatology care.9 15 In another study at the primary care, reminders increased adequate surveillance for hepatocellular carcinoma from 18.2% to 27.6%.16 To be conservative, we assumed that up to 20% of patients with high fibrosis scores in the control group would be referred to hepatologists or receive further liver assessments. We also decided that the care model would be clinically meaningful if the referral rate for patients with high fibrosis scores in the intervention group was at least 37%. To detect this difference at a two-sided 5% significance level and 80% power, we needed 107 patients with high fibrosis scores per group. Using a more conservative estimation of 25% of patients having increased fibrosis scores and a dropout rate of 10%, a sample size of 952 patients (476 patients per arm) was needed.
All analyses were done in the intention-to-treat population, which included all patients who underwent randomisation. Patients who were lost to follow-up were considered not to have hepatology referral or further liver assessments. Continuous variables were expressed in mean (SD) or median (25th percentile, 75th percentile or minimum to maximum) as appropriate, while categorical variables were presented as number (percentage). Chi-square test or Fisher exact test was performed to compare the primary and secondary outcomes between the intervention and control groups, and the 95% CIs of the difference in proportions between groups were calculated by the Newcombe-Wilson hybrid score method; continuity correction for CI calculation was used when the proportion was equal or close to zero. Binary logistic regression model was performed to identify factors associated with hepatology referral or further fibrosis assessment. The data were analysed using IBM SPSS Statistics V.27 software and R (V.4.2.2; R Core Team, 2022). This trial was registered with ClinicalTrials.gov, NCT04241575 and was completed.
Results
Patient’s characteristics
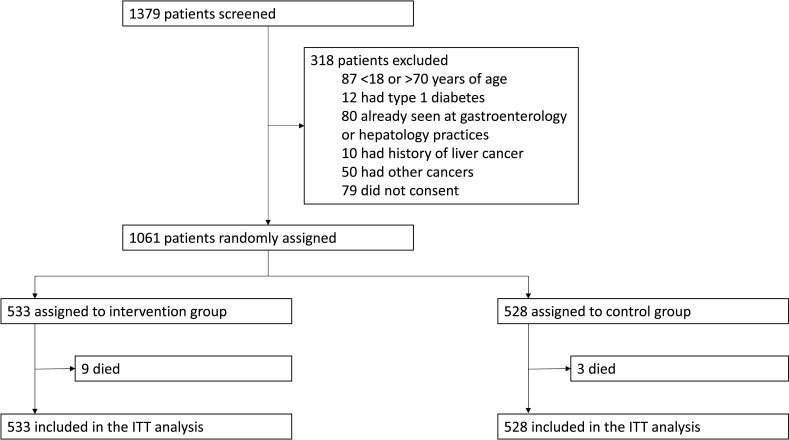
Between 19 May 2020 and 14 October 2021, 1379 patients with type 2 diabetes from five general medical or diabetes clinics were screened, of whom 1061 were randomly assigned at a ratio of 1:1 to the intervention (n=533) and control (n=528) groups. The intention-to-treat population included all 1061 randomly assigned patients (figure 1).
Figure 1.
Trial profile. ITT, intention-to-treat.
Demographics and baseline characteristics were similar between the study groups (table 1). The mean age was 59.4 years (SD 8.3), body mass index of 26.6 kg/m2 (SD 5.0) and waist circumference of 94 cm (SD 12). In total, 503 (47.4%) patients were women. A total of 185 (17.4%) patients reported current alcohol consumption, whereas 127 (12.0%) were current smokers. The vast majority of patients (95.3%) were on metformin, followed by sulphonylurea (61.2%), dipeptidyl peptidase-4 inhibitors (57.5%), sodium–glucose cotransporter-2 inhibitors (43.9%) and thiazolidinediones (16.2%). In total, 526 (49.6%) and 53 (5.0%) patients were on insulin and glucagon-like peptide-1 receptor agonists, respectively. Overall, 44 (4.1%) patients had positive hepatitis B surface antigen. No patient had chronic hepatitis C.
Table 1.
Baseline characteristics
| Intervention group (n=533) |
Control group (n=528) |
|
| Age, years | 59.5 (8.2) | 59.3 (8.3) |
| Female sex, n (%) | 246 (46.2) | 257 (48.7) |
| Smoking, n (%) | ||
| Current smoker | 64 (12.0) | 63 (11.9) |
| Ex-smoker | 77 (14.4) | 67 (12.7) |
| Non-smoker | 392 (73.5) | 398 (75.4) |
| Alcohol, n (%) | ||
| Current drinker | 91 (17.1) | 94 (17.8) |
| Ex-drinker | 32 (6.0) | 27 (5.1) |
| Non-drinker | 410 (76.9) | 407 (77.1) |
| Setting, n (%) | ||
| General medical clinic | 367 (68.9) | 362 (68.6) |
| Diabetes clinic | 166 (31.1) | 166 (31.4) |
| Country/region, n (%) | ||
| Hong Kong, China | 468 (87.8) | 465 (88.1) |
| Malaysia | 65 (12.2) | 63 (11.9) |
| Body mass index, kg/m2 | 26.4 (4.8) | 26.7 (5.2) |
| Waist circumference, cm | 94 (12) | 95 (13) |
| Laboratory parameters | ||
| Creatinine, μmol/L | 80 (63–99) | 80 (63–98) |
| Estimated glomerular filtration rate, mL/min per 1.73 m2* | 79 (24) | 80 (23) |
| Albumin, g/L | 39 (4) | 39 (4) |
| Bilirubin, μmol/L | 10 (7–13) | 9 (7–12) |
| Alanine aminotransferase, U/L | 24 (18–34) | 22 (17–31) |
| Aspartate aminotransferase, U/L | 24 (19–29) | 22 (19–27) |
| Gamma-glutamyl transpeptidase, U/L | 28 (20–44) | 27 (19–39) |
| Fasting plasma glucose, mmol/L | 7.4 (2.4) | 7.4 (2.7) |
| Haemoglobin A1c, % | 7.4 (1.4) | 7.3 (1.3) |
| Total cholesterol, mmol/L | 4.1 (0.9) | 4.1 (1.0) |
| HDL cholesterol, mmol/L | 1.3 (0.3) | 1.2 (0.3) |
| LDL cholesterol, mmol/L | 2.1 (0.7) | 2.1 (0.8) |
| Triglycerides, mmol/L | 1.4 (1.0–1.9) | 1.4 (1.0–2.0) |
| Platelet count, ×109 /L | 248 (69) | 258 (67) |
| Positive hepatitis B surface antigen, n (%) | 24 (4.5) | 20 (3.8) |
| Positive antihepatitis C virus antibody, n (%) | 0 | 0 |
| Fibrosis-4 index | 1.22 (0.89–1.61) | 1.13 (0.85–1.45) |
| ≥1.3 in patients <65 years and ≥2.0 in patients ≥65 years, n (%) | 159 (29.8) | 126 (23.9) |
| Aspartate aminotransferase-to-platelet ratio index | 0.25 (0.19–0.35) | 0.23 (0.17–0.30) |
| >0.5, n (%) | 60 (11.3) | 17 (3.2) |
| Increased Fibrosis-4 index or aspartate aminotransferase-to-platelet ratio index, n (%) | 165 (31.0) | 131 (24.8) |
| Treatment for diabetes, n (%) | ||
| Metformin | 508 (95.3) | 503 (95.3) |
| Sulphonylurea | 327 (61.4) | 322 (61.0) |
| Dipeptidyl peptidase-4 inhibitor | 310 (58.2) | 300 (56.8) |
| Sodium–glucose cotransporter-2 inhibitor | 231 (43.3) | 235 (44.5) |
| Thiazolidinediones | 88 (16.5) | 84 (15.9) |
| Glucagon-like peptide-1 receptor agonist | 24 (4.5) | 29 (5.5) |
| Insulin | 277 (52.0) | 249 (47.2) |
Data are mean (SD), median (25th percentile, 75th percentile) or number (%).
*Calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The median FIB-4 was 1.22 and 1.13 and the median APRI was 0.25 and 0.23 in the intervention and control groups, respectively (table 1). Overall, 165 (31.0%) patients in the intervention group and 131 (24.8%) patients in the control group had increased FIB-4 and/or APRI, among whom the majority had increased FIB-4, and the use of APRI only identified an additional 1% of patients with abnormal fibrosis scores. The higher proportion of patients with high fibrosis scores in the intervention group was largely explained by the clustering of scores around the predefined cut-offs and outliers in the intervention group with high AST level due to harmful drinking (online supplemental figure 1).
Key study outcomes
The primary outcome, referral to hepatologists or further fibrosis assessment within 1 year among patients with increased fibrosis scores, was achieved in 55 (33.3%) of 165 patients in the intervention group and 4 (3.1%) of 131 patients in the control group (difference 30.2% (95% CI 22.4% to 38.0%); p<0.001) (table 2). Among 332 patients seen at the diabetes clinics, 78 (23.5%) had increased fibrosis scores, and 18 of 38 (47%) patients in the intervention group versus 3 of 40 (8%) patients in the control group were referred to hepatology clinic and/or underwent further fibrosis assessment (p<0.001). Among 729 patients seen at the general medical clinics, 218 (29.9%) had increased fibrosis scores, and 37 of 127 (29%) patients in the intervention group versus 1 of 91 (1%) patients in the control group were referred to hepatology clinic and/or underwent further fibrosis assessment (p<0.001). Among patients with abnormal alanine aminotransferase (ALT) (≥30 U/L in men and ≥19 U/L in women) and/or AST (≥40 U/L), 156 had increased fibrosis scores, and 37 of 96 (39%) patients in the intervention group and 3 of 60 (5%) patients in the control group achieved the primary outcome (p<0.001). Among patients with normal ALT and AST, 140 had increased fibrosis scores, and 18 of 69 (26%) patients in the intervention group and 1 of 71 (1%) patients in the control group achieved the primary outcome (p<0.001).
Table 2.
Study outcomes by intention-to-treat analysis
| Intervention group | Control group | Difference between groups (95% CI) | P value | Number needed to screen (95% CI) | |
| Primary outcome | |||||
| Proportion of patients with high fibrosis scores who were referred for hepatology care or further fibrosis assessment within 1 year | 55/165 (33.3%) | 4/131 (3.1%) | 30.2% (22.2% to 38.0%) | <0.001 | 4 (3 to 5) |
| Secondary outcomes | |||||
| Proportion of patients in the entire cohort who were referred for hepatology care or further fibrosis assessment within 1 year* | 57/533 (10.7%) | 6/528 (1.1%) | 9.6% (6.9% to 12.5%) | <0.001 | 11 (9 to 15) |
| Proportion of patients with low fibrosis scores who were referred for hepatology care within 1 year | 1/368 (0.3%) | 2/397 (0.5%) | −0.2% (−1.8% to 1.3%) | >0.999 | – |
| Liver stiffness measurement≥10 kPa† | 11/56 (19.6%) | 1/5 (20.0%) | −0.4% (−51.3% to 22.7%) | >0.999 | – |
| Liver stiffness measurement≥15 kPa† | 6/56 (10.7%) | 0/5 (0%) | 10.7% (−43.4% to 22.6%) | >0.999 | – |
| Hepatic decompensation within 1 year‡ | 1/533 (0.2%) | 0/528 (0%) | 0.2% (−0.7% to 1.2%) | >0.999 | 533 (181 to ∞) |
| Proportion of patients confirmed to have advanced liver disease within 1 year‡ | 11/533 (2.1%) | 1/528 (0.2%) | 1.9% (0.61% to 3.5%) | 0.006 | 54 (32 to 164) |
*The numerators are different from those for the primary outcome because one patient with low fibrosis scores was referred to hepatology clinic and one patient underwent vibration controlled transient elastography directly in the intervention group, and two patients with low fibrosis scores in the control group were referred to hepatology clinic.
†Among patients who had undergone vibration controlled transient elastography examination.
‡Confirmed advanced liver disease was defined as liver stiffness measurement at or above 10 kPa by vibration controlled transient elastography, fibrosis stage 3 or 4 by liver biopsy, unequivocal radiological features of cirrhosis and/or manifestations or portal hypertension. In this study, only one patient in the intervention group developed jaundice, acute variceal haemorrhage and hepatic encephalopathy. Advanced liver disease was confirmed by vibration controlled transient elastography in all the remaining patients.
The number needed to screen to increase one case of appropriate management was 11 (95% CI 9 to 15). In the entire cohort, 57 (10.7%) of 533 patients in the intervention group and 6 (1.1%) of 528 patients in the control group were referred to hepatologists or received further fibrosis assessment (difference 9.6% (95% CI 6.8% to 12.4%); p<0.001), among whom one patient underwent VCTE without referral to hepatology care. Conversely, among those with low fibrosis scores, only 1 (0.3%) of 368 patients in the intervention group and 2 (0.5%) of 397 patients in the control group were referred to hepatologists (difference −0.2% (95% CI −1.1% to 0.7%); p>0.999).
Findings on further workup
In the intervention group, 56 patients were referred to the hepatology clinic with a median of 2 extra clinic visits, 56 patients underwent VCTE examination and 18 patients underwent liver biopsy. In the control group, six patients were referred to the hepatology clinic with a median of two extra clinic visits, five patients underwent VCTE examination and none had liver biopsy.
Among 61 patients who underwent further liver fibrosis assessment, all had VCTE examination. The median liver stiffness was 5.7 kPa (range 0.4–47.2) in the intervention group and 4.9 kPa (3.9–13.2) in the control group, and the median controlled attenuation parameter was 273 dB/m (150–400) in the intervention group and 314 dB/m (216–355) in the control group. Eleven patients in the intervention group and one patient in the control group had liver stiffness measurement at or above 10 kPa, and six patients in the intervention group and no patient in the control group had liver stiffness measurement at or above 15 kPa (table 2). Only one patient in the intervention group developed clinical hepatic decompensation with jaundice, acute variceal haemorrhage and hepatic encephalopathy 4 months after baseline. He was a 70-year-old man with FIB-4 of 5.6 and APRI of 1.6. His liver stiffness measurement by VCTE was 24.6 kPa prior to the development of hepatic decompensation. He was subsequently diagnosed with alcohol-related liver cirrhosis. Overall, 11 (2.1%) of 533 patients in the intervention group and 1 (0.2%) of 528 patients in the control group were confirmed to have advanced liver disease (difference 2.1% (95% CI 0.61% to 3.5%); p=0.006).
Among patients who had attended hepatology care, 30 (53%) of 57 patients in the intervention group were diagnosed with NAFLD, 5 (9%) had alcohol-related liver disease and 3 (5%) had chronic hepatitis B (table 3). No liver disease was identified in 19 (33%) patients. Among six patients in the control group who had attended hepatology care, three had NAFLD and three were not found to have any liver disease.
Table 3.
Final diagnosis of the patients attending hepatology care
| Aetiology of liver disease | Intervention group (n=57) |
Control group (n=6) |
| Non-alcoholic fatty liver disease | 30 | 3 |
| Alcohol-related liver disease | 5 | 0 |
| Chronic hepatitis B | 3 | 0 |
| No liver disease identified | 19 | 3 |
Data are number of patients.
Factors associated with appropriate care
By multivariable analysis, abnormal fibrosis scores were the only factor associated with the primary outcome in the intervention group (online supplemental table 2). Among patients with abnormal fibrosis scores in the intervention group, female sex, current alcohol consumption, being seen at the diabetes clinic and high serum triglycerides were independent factors associated with the primary outcome (online supplemental table 3).
Safety
During the study period, 9 (1.7%) of 533 patients in the intervention group died. The causes of death included COVID-19 (n=2), pneumonia (n=2), cardiovascular disease (n=2), renal failure (n=2) and intracranial haemorrhage (n=1). In total, 3 (0.6%) of 528 patients in the control group died. The causes of death were COVID-19 (n=1), diabetes (n=1) and sepsis (n=1). None of the deaths were related to study interventions. There were also no serious adverse events related to study interventions.
Discussion
In 2022, the EASL-Lancet Liver Commission highlighted the deficiencies in liver disease management, with an emphasis on the need to detect liver disease at its early and reversible disease stages, and develop clinical pathways and models of care to address the dissociation between primary and secondary care.17 As such, our randomised controlled trial clearly demonstrates that it is possible to use automated fibrosis score calculation and electronic reminders to improve the detection of patients with advanced liver disease in primary care and non-hepatology settings. It also highlights deficiencies in the model that need to be addressed in future studies.
Type 2 diabetes increases the risk of cirrhosis, cirrhotic complications and liver-related mortality.18 A number of prospective studies have used VCTE and found that 10%–20% of patients with type 2 diabetes may have advanced liver fibrosis.9 19 20 In another study of 3012 NAFLD patients seen in primary care in the UK, the use of FIB-4 as initial assessment, followed by the enhanced liver fibrosis score in patients with indeterminate FIB-4 results, increased the detection of advanced fibrosis and cirrhosis by fivefold and threefold, respectively, while at the same time reducing unnecessary referrals.8 Although these studies highlighted the feasibility of applying non-invasive tests of liver fibrosis in primary care settings, we argue that such approaches rely on the cooperation of colleagues who are aware of and interested in NAFLD. The pathway would fall apart if fibrosis scores are not ordered or calculated in the first place. In the real world, awareness of NAFLD among clinicians remains an issue,21 and a recent survey showed that no country had a national or subnational strategy for NAFLD.22 For these reasons, we proposed a clinical care pathway to streamline the process. Instead of relying on active assessment by clinicians, automated fibrosis score calculation using routine blood results followed by reminder messages in the electronic clinical management system increased appropriate referral for hepatology assessment or further fibrosis tests in patients with increased fibrosis scores from 3.1% to 33.3%. In comparison, another study using an automated algorithm called intelligent liver function testing demonstrated a 43% increase in the diagnosis of liver disease in 64 patients with abnormal liver blood tests in primary care setting.23 In Germany, ‘Check-up 35’ involved APRI calculations in individuals with elevated AST and/or ALT to identify patients with cirrhosis.24 Using a different approach, a cluster randomised trial at 10 primary care practices in Southampton showed that a nurse-led clinic based on serum fibrosis biomarkers and VCTE increased the identification of liver disease.25
Nonetheless, clinicians at the general medical and diabetes clinics failed to respond to abnormal fibrosis score results and electronic reminders in two-thirds of cases. We therefore performed logistic regression analyses to identify factors associated with hepatology referral or further fibrosis assessment. In the overall population, assignment in the intervention group and increased fibrosis scores were the only independent factors associated with the primary outcome. When the analysis was restricted to patients with increased fibrosis scores in the intervention group, female sex, current drinker, being seen at the diabetes clinic and high serum triglycerides were associated with hepatology referral or further fibrosis assessment. Serum triglycerides are strongly associated with intrahepatic triglyceride content, a defining feature of NAFLD.26 As the primary outcome was not associated with other metabolic factors, we suspect that some of the referrals were triggered by radiological diagnosis of NAFLD. The reason behind the association between female sex and appropriate referral is unclear, though it may reflect a difference in healthcare-seeking behaviour. In contrast, referral was not prompted by patient age (a risk factor for advanced liver disease),27 28 anthropometric factors, glycaemic control, elevated liver enzymes, diagnosis of viral hepatitis or thrombocytopenia (a proxy of cirrhosis). In Malaysia and Hong Kong, a patient needs to pay around US$6 and US$13 to see a hepatologist at a public hospital, respectively, so the cost should not be a major determinant of patient behaviour. As the primary aim of this study was to determine whether clinicians and patients at non-hepatology settings would respond to reminder messages of abnormal fibrosis score results, we refrained from asking the clinicians about their decisions on referral and management in order to avoid artificially interfering with their decisions. Future studies should determine the reasons for not acting on abnormal fibrosis scores and identify areas for improvement.
In a systematic review and meta-analysis of 80 studies, the pooled prevalence of advanced fibrosis among patients with type 2 diabetes was 17%.29 In the current study, 2.1% of the patients in the intervention group were confirmed to have advanced liver disease (table 2). This can be explained by the fact that two-thirds of patients with high fibrosis scores were not referred for further assessment, and that a fraction of patients with normal fibrosis scores might nonetheless harbour advanced liver disease. From this estimation, up to 15% of patients with type 2 diabetes and advanced fibrosis might remain undiagnosed. Clearly, further optimisation of the clinical care pathway is needed.
When implementing any screening programme, the resource implications should be considered. In the current study, we needed to use automated fibrosis score calculation and electronic reminders on 11 patients to increase appropriate referral of 1 patient. APRI added little to FIB-4 in terms of case identification. For ease of implementation, we agree with recent guidelines to use one fibrosis score such as FIB-4 instead of a mixture of non-invasive tests as the first step.4–6 FIB-4 has a high negative predictive value to exclude advanced fibrosis30; people with persistently normal FIB-4 also have a very low risk to develop cirrhosis and hepatocellular carcinoma in the long run.31 However, FIB-4 has modest positive predictive value to confirm advanced liver fibrosis, especially when the test is applied in the non-hepatology setting where the pretest probability of advanced fibrosis is low.32 In the current study, among patients who eventually underwent VCTE, 80% had liver stiffness below 10 kPa. Similarly, in a recent secondary analysis of the 2017–2018 US National Health and Nutrition Examination Survey, 90% of the participants with FIB-4 below 1.3 had liver stiffness below 8 kPa, thus supporting its high negative predictive value.33 However, 18% of the participants from the general population had increased FIB-4. Among those with FIB-4 1.3–2.67 and >2.67, only 13% and 33% had liver stiffness above 8 kPa, respectively. Thus, unless a second test is accessible in non-hepatology settings, the vast number of patients with abnormal fibrosis scores may exceed the capacity of hepatology service in most countries.
Because the intervention in this study involved both fibrosis score calculation and reminder messages, it is difficult to distinguish the effects of each component, though we speculate that the reminder messages were the main driver of clinician actions given that non-hepatologists might not be familiar with the fibrosis scores. It is possible to test this theory by conducting a study using reminder messages delinked from non-invasive tests. However, we believe that initial screening by fibrosis scores would provide a better reason to deliver the reminder messages. Besides, the number of patients with type 2 diabetes should exceed the capacity of hepatologists in most if not all countries, and it is reasonable to have initial screening at non-hepatology settings, in line with current guideline recommendations.4–6
Our study has the strengths of a randomised controlled trial design, multicentre involvement and broad inclusion criteria. It also has a few limitations. First, all participating clinics were in Asia. Because healthcare systems and healthcare-seeking behaviours differ across countries, our findings should be replicated in future studies. Second, fibrosis score calculation was based on a single assessment. In contrast, current guidelines recommend periodic assessments in patients with risk factors of NAFLD, typically every 1–3 years.4–6 It is possible that clinicians would more likely respond to repeatedly abnormal test results and reminder messages, though this remains to be proven. Third, the true difference between groups (3.1% vs 33.3%) deviated from our assumptions during sample size calculation (20% vs 37%). This could reflect the impact on referral pattern during the COVID-19 pandemic, but the study confirmed the efficacy of the approach nonetheless. Finally, our study is underpowered for determining the impact of the intervention on ‘hard’ clinical outcomes such as cirrhotic complications, hepatocellular carcinoma and liver-related death. Nevertheless, the primary aim of this study was to demonstrate whether automated fibrosis score calculation and reminder messages could improve the identification of advanced liver disease.
In conclusion, automated fibrosis score calculation and electronic reminders can increase referral of patients with type 2 diabetes and abnormal fibrosis scores at non-hepatology settings. However, over half of the patients with increased fibrosis scores did not receive appropriate care, and only a minority of referred patients actually had advanced liver disease. The findings of this trial shed light on how to refine the clinical care pathway.
Footnotes
Twitter: @TerryYip12, @wonglaihung, @LeeLingLim1
Contributors: WKC and VW-SW are the guarantors of this study. VW-SW designed the study. XZ, TC-FY, GL-HW, W-XL, LYL, L-LL, GL, LI, HL, JCTL, AM-LC, AP-SK, WKC and VW-SW were responsible for data collection. XZ, TC-FY and VW-SW participated in data analysis. XZ, TC-FY and VW-SW verified the data; all authors participated in data interpretation, manuscript review and writing and made the decision to submit the manuscript. XZ and VW-SW were responsible for preparation of the tables and figure. All authors vouch for the completeness and accuracy of the data and analyses and for the fidelity of the trial to the protocol and statistical analysis plan.
Funding: This study was supported in part by a direct grant from The Chinese University of Hong Kong (project reference 2022.031). The authors thank all of the patients who participated in this study, as well as study coordinators and staff of the participating centres for their support and assistance. The funder of the study did not have a role in study design, data collection, data analysis, data interpretation or manuscript preparation.
Competing interests: TC-FY has served as an advisory committee member and a speaker for Gilead Sciences. GL-HW has served as an advisory committee member for Gilead Sciences and Janssen, and as a speaker for Abbott, Abbvie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen and Roche. She has also received a research grant from Gilead Sciences. L-LL has served as an advisory committee member for Boehringer Ingelheim, Novo Nordisk and Viatris; and as a speaker for Abbott, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk, Roche, Sanofi, Servier and Zuellig Pharma. She has also received research grants from Abbott, AstraZeneca and Boehringer Ingelheim. HLYC has served as an Independent Non-Executive Director for Shanghai Henlius Biotech; as an advisory board member for Aligos, Aptorum, Arbutus, Janssen, Gilead, Glaxo-Smith-Kline, Roche, Vaccitech, Vir Biotechnology and Virion Therapeutics; and as a speaker for Gilead, Roche and Viatris. AP-SK has received honorarium for consultancy or giving lectures from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli-Lilly, Kyowa Kirin, Merck Serono, Nestle, Novo Nordisk, Pfizer and Sanofi. WKC has served as an advisory committee member for Roche, AbbVie, Boehringer Ingelheim and Novo Nordisk; and a speaker for Hisky Medical and Viatris. VW-SW has served as an advisory committee member for AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Merck, Novo Nordisk, Pfizer, Sagimet Biosciences and TARGET Pharma Solutions; and a speaker for Abbott, AbbVie, Gilead Sciences, Novo Nordisk and Unilab. He has also received a research grant from Gilead Sciences and is a cofounder of Illuminatio Medical Technology. All other authors declare no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (2019.139-T). Medical Research Ethics Committee, University of Malaya Medical Centre (NMRR-21-204-58535). Participants gave informed consent to participate in the study before taking part.
References
- 1. Powell EE, Wong V-S, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021;397:2212–24. 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 2. Wong VWS, Zelber-Sagi S, Cusi K, et al. Management of NAFLD in primary care settings. Liver Int 2022;42:2377–89. 10.1111/liv.15404 [DOI] [PubMed] [Google Scholar]
- 3. Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611–25. 10.1053/j.gastro.2020.01.043 [DOI] [PubMed] [Google Scholar]
- 4. Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657–69. 10.1053/j.gastro.2021.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cusi K, Isaacs S, Barb D, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings. Endocrine Practice 2022;28:528–62. 10.1016/j.eprac.2022.03.010 [DOI] [PubMed] [Google Scholar]
- 6. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77:1797–835. 10.1097/HEP.0000000000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong VW-S, Adams LA, de Lédinghen V, et al. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol 2018;15:461–78. 10.1038/s41575-018-0014-9 [DOI] [PubMed] [Google Scholar]
- 8. Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol 2019;71:371–8. 10.1016/j.jhep.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 9. Kwok R, Choi KC, Wong GL-H, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016;65:1359–68. 10.1136/gutjnl-2015-309265 [DOI] [PubMed] [Google Scholar]
- 10. European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 11. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV Coinfection. Hepatology 2006;43:1317–25. 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 12. Wai C-T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. 10.1053/jhep.2003.50346 [DOI] [PubMed] [Google Scholar]
- 13. McPherson S, Hardy T, Dufour J-F, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740–51. 10.1038/ajg.2016.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong VW-S, Irles M, Wong GL-H, et al. Unified interpretation of liver stiffness measurement by M and XL probes in non-alcoholic fatty liver disease. Gut 2019;68:2057–64. 10.1136/gutjnl-2018-317334 [DOI] [PubMed] [Google Scholar]
- 15. Lee HW, Wong GL-H, Kwok R, et al. Serial transient elastography examinations to monitor patients with type 2 diabetes: a prospective cohort study. Hepatology 2020;72:1230–41. 10.1002/hep.31142 [DOI] [PubMed] [Google Scholar]
- 16. Beste LA, Ioannou GN, Yang Y, et al. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol 2015;13:172–9. 10.1016/j.cgh.2014.04.033 [DOI] [PubMed] [Google Scholar]
- 17. Karlsen TH, Sheron N, Zelber-Sagi S, et al. The EASL-lancet liver commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022;399:61–116. 10.1016/S0140-6736(21)01701-3 [DOI] [PubMed] [Google Scholar]
- 18. Castera L, Cusi K. Diabetes and cirrhosis: current concepts on diagnosis and management. Hepatology 2023;77:2128–46. 10.1097/HEP.0000000000000263 [DOI] [PubMed] [Google Scholar]
- 19. Lai L-L, Wan Yusoff WNI, Vethakkan SR, et al. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol 2019;34:1396–403. 10.1111/jgh.14577 [DOI] [PubMed] [Google Scholar]
- 20. Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2021;44:399–406. 10.2337/dc20-1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Younossi ZM, Ong JP, Takahashi H, et al. A global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2022;20:e1456–68. 10.1016/j.cgh.2021.06.048 [DOI] [PubMed] [Google Scholar]
- 22. Lazarus JV, Mark HE, Villota-Rivas M, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol 2022;76:771–80. 10.1016/j.jhep.2021.10.025 [DOI] [PubMed] [Google Scholar]
- 23. Dillon JF, Miller MH, Robinson EM, et al. Intelligent liver function testing (iLFT): a trial of automated diagnosis and staging of liver disease in primary care. J Hepatol 2019;71:699–706. 10.1016/j.jhep.2019.05.033 [DOI] [PubMed] [Google Scholar]
- 24. Labenz C, Arslanow A, Nguyen-Tat M, et al. Structured early detection of asymptomatic liver cirrhosis: results of the population-based liver screening program SEAL. J Hepatol 2022;77:695–701. 10.1016/j.jhep.2022.04.009 [DOI] [PubMed] [Google Scholar]
- 25. El-Gohary M, Moore M, Roderick P, et al. Local care and treatment of liver disease (LOCATE) - A cluster-randomized feasibility study to discover, assess and manage early liver disease in primary care. PLoS One 2018;13:e0208798. 10.1371/journal.pone.0208798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wong VW-S, Chu WC-W, Wong GL-H, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012;61:409–15. 10.1136/gutjnl-2011-300342 [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Wong GL-H, Yip TC-F, et al. Risk of liver-related events by age and diabetes duration in patients with diabetes and nonalcoholic fatty liver disease. Hepatology 2022;76:1409–22. 10.1002/hep.32476 [DOI] [PubMed] [Google Scholar]
- 28. Lin H, Yip TC-F, Zhang X, et al. Age and the relative importance of liver-related deaths in nonalcoholic fatty liver disease. Hepatology 2023;77:573–84. 10.1002/hep.32633 [DOI] [PubMed] [Google Scholar]
- 29. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019;71:793–801. 10.1016/j.jhep.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 30. Mózes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006–19. 10.1136/gutjnl-2021-324243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagström H, Talbäck M, Andreasson A, et al. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol 2020;73:1023–9. 10.1016/j.jhep.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 32. Mahady SE, Macaskill P, Craig JC, et al. Diagnostic accuracy of noninvasive fibrosis scores in a population of individuals with a low prevalence of fibrosis. Clin Gastroenterol Hepatol 2017;15:1453–60. 10.1016/j.cgh.2017.02.031 [DOI] [PubMed] [Google Scholar]
- 33. Udompap P, Therneau TM, Canning RE, et al. Performance of American gastroenterological association clinical care pathway for the risk stratification of patients with nonalcoholic fatty liver disease in the US population. Hepatology 2023;77:931–41. 10.1002/hep.32739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2023-330269supp001.pdf (90.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request.