Abstract
Background/aims
The English Diabetic Eye Screening Programme (DESP) offers people living with diabetes (PLD) annual screening. Less frequent screening has been advocated among PLD without diabetic retinopathy (DR), but evidence for each ethnic group is limited. We examined the potential effect of biennial versus annual screening on the detection of sight-threatening diabetic retinopathy (STDR) and proliferative diabetic retinopathy (PDR) among PLD without DR from a large urban multi-ethnic English DESP.
Methods
PLD in North-East London DESP (January 2012 to December 2021) with no DR on two prior consecutive screening visits with up to 8 years of follow-up were examined. Annual STDR and PDR incidence rates, overall and by ethnicity, were quantified. Delays in identification of STDR and PDR events had 2-year screening intervals been used were determined.
Findings
Among 82 782 PLD (37% white, 36% South Asian, and 16% black people), there were 1788 incident STDR cases over mean (SD) 4.3 (2.4) years (STDR rate 0.51, 95% CI 0.47 to 0.55 per 100-person-years). STDR incidence rates per 100-person-years by ethnicity were 0.55 (95% CI 0.48 to 0.62) for South Asian, 0.34 (95% CI 0.29 to 0.40) for white, and 0.77 (95% CI 0.65 to 0.90) for black people. Biennial screening would have delayed diagnosis by 1 year for 56.3% (1007/1788) with STDR and 43.6% (45/103) with PDR. Standardised cumulative rates of delayed STDR per 100 000 persons for each ethnic group were 1904 (95% CI 1683 to 2154) for black people, 1276 (95% CI 1153 to 1412) for South Asian people, and 844 (95% CI 745 to 955) for white people.
Interpretation
Biennial screening would have delayed detection of some STDR and PDR by 1 year, especially among those of black ethnic origin, leading to healthcare inequalities.
Keywords: Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
The UK National Screening Committee currently recommends annual eye screening for diabetic retinopathy among people living with diabetes at high risk of sight loss, but biennial screening among those at low risk of sight loss.
Ethnic differences in diabetes and the development of sight-threatening diabetes complications have been reported.
The effect of biennial versus annual diabetic eye screening among different ethnic groups at low risk of complications has not been quantified in large multi-ethnic diabetic eye screening programmes in the UK.
WHAT THIS STUDY ADDS
We provide incidence rates for the development of new sight-threatening diabetic retinopathy (STDR) and proliferative diabetic retinopathy in a low-risk group, overall and by different ethnic and age groups, in this diverse sociodemographic population without previous diabetic retinopathy.
Implementation of biennial screening in this population would have delayed referral to hospital eye services by a year in nearly half of those with sight-threatening diabetes (56%) and proliferative retinopathy (44%), but higher absolute rates of delay were observed among the youngest and oldest compared with middle aged and pre-retirement age groups, and those of black ethnic origin compared with other ethnic groups.
While the absolute number delayed is small relative to the size of the overall cohort, age and ethnic inequalities in delayed identification of complications were apparent.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
National implementation of a 2-year diabetic eye screening interval for people with low-risk diabetic retinopathy grades does not affect all population sub-groups equally with respect to delays in the detection and referral of the most serious eye disease. Younger people and people of black and Asian ethnicities are affected more than other groups with potential effects on vision and treatment outcomes.
Introduction
Diabetic retinopathy is a major microvascular complication of diabetes which can result in sight loss, presenting a major global challenge.1 However, early detection and treatment can prevent or delay sight loss. The National Health Service (NHS) Diabetic Eye Screening Programme (DESP) was introduced in 2003 to identify those with diabetic retinopathy so early treatment can be used.2 The English NHS DESP currently performs 2.3 million eye screening appointments each year, generating approximately 13 million retinal images, and the number of appointments and images has increased over time.3 Retinal images from the DESP are assessed by up to three trained human graders for the presence and severity of diabetic retinopathy (DR), and those with potentially sight-threatening diabetic retinopathy (STDR) are referred to hospital eye services for further assessment and potential treatment. This represents a major challenge to healthcare providers, given increasing patient numbers and finite resources within a publicly funded healthcare system.
Evidence has suggested that biennial rather than annual screening among those at low risk would be safe and cost-effective, potentially reducing the number of appointments and workload.4–6 However, not all evidence has been as supportive, concluding that there is insufficient evidence to recommend screening beyond 1 year.7 The UK National Screening Committee (NSC) recommended change in 2016 to biennial screening for those at low risk of sight loss.8 The rationale for change was predominantly based on an audit commissioned by the NSC of nearly 350 000 patients from seven geographically dispersed UK DESPs. This showed progression to STDR (and more serious proliferative diabetic retinopathy (PDR)) among those without DR at two successive screening episodes at least 12 months apart was low (with approximately 0.7% developing referable DR over 2 years).9 While this number was considered low, a number of limitations were raised, including the use of retrospective audit data (as opposed to use of preferred randomised controlled trial data), and whether extending follow-up to 2 years could have an adverse impact on attendance once introduced, especially among some sociodemographic groups.9 Moreover, while use of the geographically dispersed UK DESP centres would incorporate different age, sex and ethnicity profiles, effects of biennial screening by ethnicity and different age groups were not explicitly quantified. This is highly relevant for ethnicity, given ethnic differences in both diabetes and complications of diabetes, particularly in a UK setting, where those of South Asian ethnicity are at higher risk of diabetes, severe diabetic retinopathy and associated sight-loss, compared with white people.10–12 While biennial screening among those at low risk of sight loss has been approved, uptake thus far has been limited (despite the potential resource and cost savings). Hence, it remains unclear whether this extended screening frequency could lead to inequalities in healthcare.
Using one of the largest, most ethnically diverse DESP in North-East London (NELDESP), we examined progression to STDR and PDR among those without DR on two consecutive annual screens to determine incidence rates by sociodemographic groups, and the potential for delay in the detection of STDR and more serious PDR if biennial screening was introduced, rather than the current annual screening interval.
Methods
The study population comprised people living with diabetes (PLD) registered in the NELDESP, who were offered screening appointments from 3 January 2012 to 31 December 2021.
Setting
North-East London is an ethnically diverse region with higher than national average levels of deprivation and mortality.13 The NELDESP is provided by the Homerton Healthcare NHS Foundation Trust, and serves people with diabetes living in inner-city areas with multi-ethnic populations. The NELDESP is run according to English NHS DESP standards.14 People with diabetes aged ≥12 years are identified through the electronic ‘General Practice to Diabetic Retinopathy Screening’ patient identification system. This notifies DESPs about all people with diabetes in their catchment area. All new eligible people are invited for screening within 3 months of notification and the list of PLD eligible for screening by NELDESP is actively maintained.15
Screening visit
A screening visit entails history taking by specialist staff, visual acuity assessment, and capture under pupil dilation of two 45° digital retinal images, centred on the fovea and optic nerve for each eye, respectively. Up to three qualified graders assess the images for the presence and severity of diabetic retinopathy following a multilevel internally and externally quality-assured process.14 The UK NSC classification system for diabetic retinopathy grades in order of increasing severity: no retinopathy (R0), mild non-proliferative diabetic retinopathy (R1), moderate to severe non-proliferative diabetic retinopathy (R2), referable diabetic maculopathy (M1), and PDR (R3).16 STDR comprises retinopathy grades R2, M1, and R3 and referred according to NSC timescales to hospital eye services for assessment and potential treatment; PDR is urgently referred. Images which were not able to be graded (U) were excluded from the analysis.
Data extraction
We identified people registered in the NELDESP during the study period, calculated postcode-derived Index of Multiple Deprivation (IMD) rank scores for each episode, and carried out an anonymised data extraction for all appointments using structured query language searches. An anonymised database was created and stored within the Homerton Trust’s network for analysis. The cohort went through a staged exclusion process, illustrated in online supplemental figure 1, to identify a study cohort of PLD with two consecutive annual screening episodes grades of no DR (ie, R0M0) in both eyes. People with stable treated PDR (R3S) and STDR were excluded at baseline.
bjo-2023-324097supp001.pdf (58.2KB, pdf)
Variables
Routinely collected data from the NELDESP included age at first appointment (categorised as <45 years, 45 to <55, 55 to <65, and 65 years and older), sex, self-defined ethnicity (coded according to Office for National Statistics (ONS) standards as: white, black, South Asian, Chinese, any other Asian, mixed, other, and unknown categories for the purpose of these analyses),17 type of diabetes (type 2, type 1, other, and unknown), self-defined duration of diabetes or from date of diagnosis as registered on the screening database, baseline retinopathy severity (to identify those with no diabetic retinopathy (R0M0) in either eye on two consecutive screening visits),18 and IMD. The IMD combines and weights indicators of deprivation and is the nationally recognised measure of relative deprivation in England.19 IMD scores were split into quintiles (where first and fifth are the most and least deprived, respectively) following data of the 2019 English indices of deprivation.19
Statistical analysis
We calculated annual incidence rates of STDR (defined as the presence of any R2, R3, or M1) in either eye,16 18 among those with two consecutive annual screening visits without DR (R0M0). Rates were reported by age group, sex, and ethnic group. Note, the median follow-up period between appointments was 1.0 (0.9–1.1) years, providing annual rates. Mutually adjusted hazard ratios for the development of STDR were calculated using Cox regression by age group, sex, ethnicity, IMD groups and by duration of diabetes. The proportionality assumption was assessed by graphical inspection of Schoenfeld residuals. To examine the impact of biennial screening intervals, PLD who were R0M0 on two consecutive annual screens were assigned to a virtual biennial screening schedule. Fourteen-month time breaks were used to mirror the annual cycle uptake observed in this cohort. The number of STDR and PDR occurring between biennial screening intervals was quantified. People who developed DR (grades ≥R1 M0), or had U grade, were right censored at the time of diagnosis. All analyses were undertaken with R (version 4.2.2).20 The Survival package was used for survival analyses.21
Results
A total of 82 782 PLD from an identified cohort of 200 304 PLD in the NELDESP remained with all relevant demographic data, eyes which could be adequately assessed using fundus photography, and who had no prior diabetic retinopathy on two consecutive screening occasions (online supplemental figure 1). Table 1 summarises baseline characteristics of the cohort where the mean (SD) age at baseline was 56.7 (14.4) years and 52% (42 846/82 782) were male. Eleven percent (10.8%, 21 662/200 064) of PLD never attended their diabetic eye screening during the study period despite being offered a median (IQR) of 3.0 (1.0–7.0) appointments. The characteristics of PLD who never attended are summarised in online supplemental table 1. The proportion of episodes with U grades in people with recorded grades was 0.1% (205/176 972).
Table 1.
Baseline characteristics of the cohort among those without diabetic retinopathy on two consecutive annual screening visits
| Characteristic | n=82 782 (%) |
| Follow-up (SD) years | 4.3 (2.4) |
| Age at baseline (SD) years | 56.7 (14.4) |
| Age categories | |
| <45 years | 16 488 (20%) |
| 45 to <55 years | 20 207 (24%) |
| 55 to <65 years | 20 762 (25%) |
| 65 years and over | 25 325 (31%) |
| Sex | |
| Female | 39 936 (48%) |
| Male | 42 846 (52%) |
| Type of diabetes | |
| Type 2 | 78 992 (95%) |
| Type 1 | 2125 (2.6%) |
| Other | 137 (0.2%) |
| Missing | 1528 (1.8%) |
| Ethnicity | |
| White | 30 350 (37%) |
| South Asian | 29 703 (36%) |
| Black | 13 391 (16%) |
| Any other Asian | 4786 (5.8%) |
| Other | 2319 (2.8%) |
| Mixed | 1006 (1.2%) |
| Chinese | 577 (0.7%) |
| Unknown | 650 (0.8%) |
| Duration of diabetes | 4.0 (5.3) |
| Index of Multiple Deprivation | |
| 1 | 8855 (11%) |
| 2 | 26 255 (32%) |
| 3 | 23 956 (29%) |
| 4 | 15 510 (19%) |
| 5 | 8192 (9.9%) |
| Missing | 14 (<0.1%) |
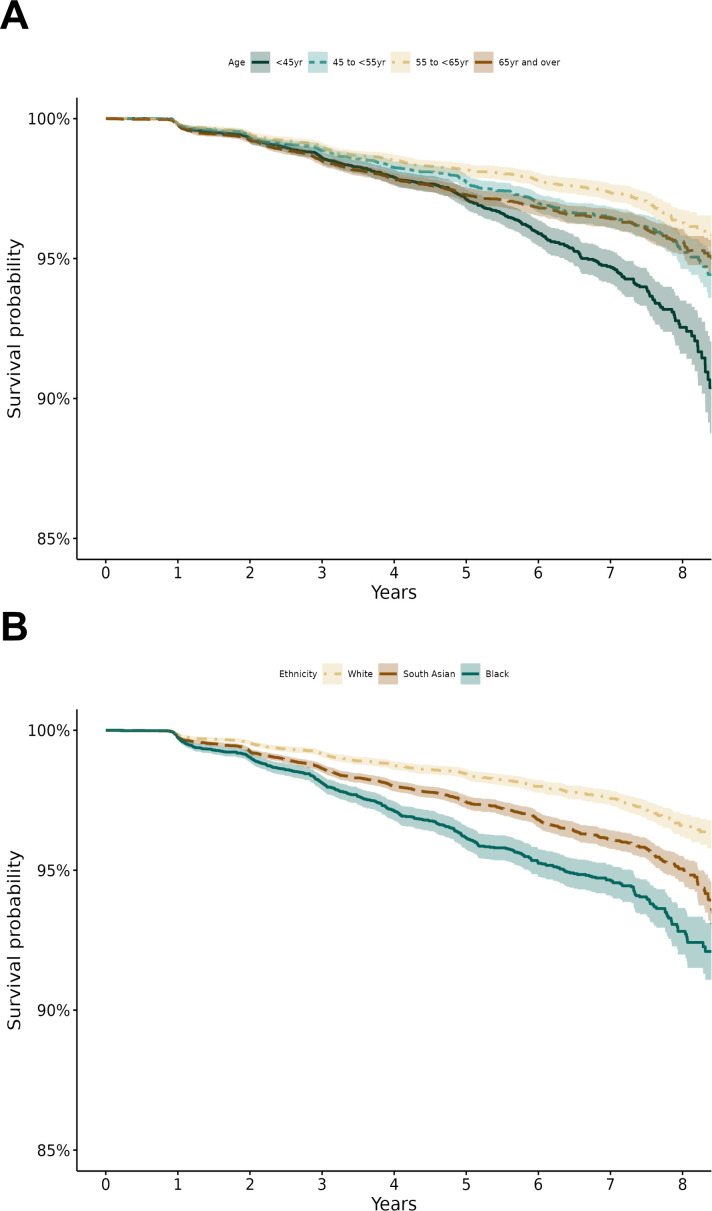
Cumulative incidence rates of STDR over the follow-up period are shown in online supplemental table 2, overall, by age, sex, ethnicity, type of diabetes and IMD group. Progression to STDR with advancing yearly intervals showed a graded increase in cumulative rates over time, which was more pronounced in the youngest and oldest age groups when compared with lower rates in middle age and pre-retirement age groups. Males had consistently lower STDR rates compared with females, and those with type 1 diabetes consistently had higher rates compared with type 2 diabetes, reflective of diabetes duration. There was no clear pattern across levels of deprivation (IMD). The most striking differences in STDR rates over time were with ethnicity, where PLD of black ethnicity had the highest STDR rates, with South Asian and any other Asian having higher rates compared with white. Those categorised as ‘mixed’ or ‘other’ ethnicity also showed higher rates over the study period. These sociodemographic differences in STDR rates were confirmed by hazard ratios (table 2) showing notably higher risk of STDR among black people (121% higher, 95% confidence interval (95% CI) 93% to 153%) and modestly higher risk among South Asian individuals (54% higher, 95% CI 35% to 74%) compared with white people. The decreased risk of STDR with increasing age (with the lowest risk among those of pre-retirement age compared with the youngest age group) is also apparent. Sex was not associated. Figure 1 displays Kaplan-Meier plots of STDR survival which shows that the probability of remaining STDR-free over the 8-year study period is lowest among the youngest age group and highest among the pre-retirement age group (figure 1A), lowest among black individuals, intermediate among South Asian people, and highest among white people (figure 1B).
Table 2.
Mutually adjusted hazard ratios of STDR by age groups, sex, ethnic group and IMD in those with two consecutive screening appointments with no retinopathy (R0M0)
| Characteristic | HR (95% CI) | P value |
| Age (per 5 year increase) | 0.96 (0.94 to 0.98) | 1.5e-4 |
| Age categories | ||
| <45 years | 1.00 | |
| 45 to <55 years | 0.70 (0.61 to 0.80) | 2.6e-07 |
| 55 to <65 years | 0.54 (0.47 to 0.63) | 2.8e-16 |
| 65 years and over | 0.70 (0.61 to 0.81) | 1.2e-06 |
| Sex | ||
| Female | 1.00 | |
| Male | 0.96 (0.88 to 1.06) | 0.449 |
| Ethnicity | ||
| White | 1.00 | |
| South Asian | 1.54 (1.35 to 1.74) | 2.4e-11 |
| Black | 2.21 (1.93 to 2.53) | 8.5e-31 |
| Any other Asian | 1.39 (1.12 to 1.72) | 0.003 |
| Other | 1.86 (1.42 to 2.44) | 6.1e-06 |
| Mixed | 2.07 (1.39 to 3.07) | 3.3e-04 |
| Chinese | 0.63 (0.26 to 1.52) | 0.306 |
| Unknown | 1.91 (0.85 to 4.28) | 0.118 |
| Duration of diabetes (per 5 year increase) | 1.26 (1.21 to 1.30) | 1.1e-38 |
| Type of diabetes | ||
| Type 2 | 1.00 | |
| Type 1 | 1.41 (1.08 to 1.83) | 0.011 |
| Other | 1.83 (0.68 to 4.88) | 0.229 |
| Missing | 1.35 (0.91 to 2.01) | 0.134 |
| Deprivation (IMD quintiles) | ||
| 1 | 1.00 | |
| 2 | 1.16 (0.98 to 1.37) | 0.081 |
| 3 | 1.06 (0.90 to 1.26) | 0.487 |
| 4 | 1.08 (0.90 to 1.30) | 0.393 |
| 5 | 1.12 (0.91 to 1.39) | 0.288 |
IMD, Index of Multiple Deprivation; STDR, sight-threatening diabetic retinopathy.
Figure 1.
Kaplan Meier plots showing probability of STDR survival over time by (A) age and (B) ethnic group.
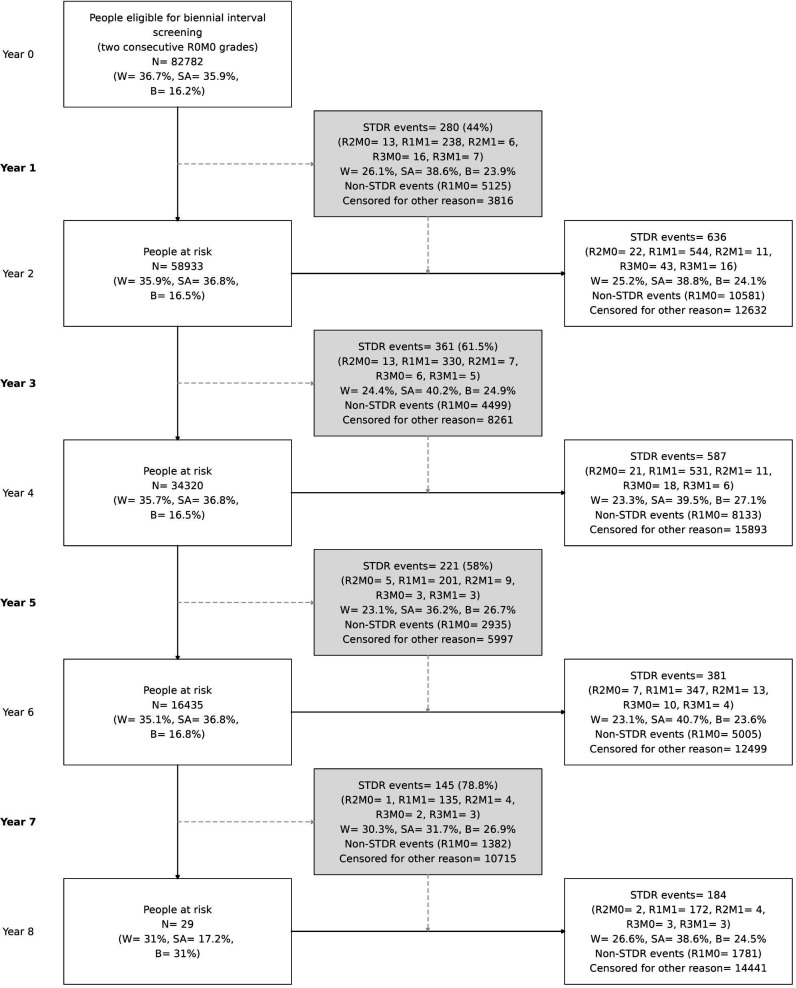
We examined the potential impact of a biennial screening pathway. The numbers that developed STDR and PDR in the intervening years overall and by ethnic group are shown in figure 2. Among the 82 782 PLD, STDR was present in 1788 and PDR in 103 over the study period. However, if the cohort had undergone biennial screening, STDR and PDR would have been present in 56.3% (1007/1788) and 43.6% (45/103) at the 1 year interval, respectively (figure 2). Hence, there would have been a 1 year delay in the diagnosis of these cases with biennial screening. The near 50% with a 1 year delay in STDR and PDR diagnosis remained consistent over the study period (figure 2). The delayed STDR cases by ethnic group were 256/30 350 for white, 379/29 730 for South Asian, and 256/13 391 for black individuals (table 3)—equivalent to 844, 1276, and 1904 per 100 000 screened biennially, for each ethnic group, respectively (table 3). For PDR, numbers were much lower, but rates were still higher among black people (90 per 100 000), compared with white (46 per 100 000) and South Asian individuals (44 per 100 000). By age group, delayed STDR events per 100 000 persons were highest (1504 events, 95% CI 1327 to 1705) among those aged <45 years, 1178 (95% CI 1036 to 1339) for those aged 45 to <55 years, lowest (987 events, 95% CI 859 to 1134) among those aged 55 to <65 years, and 1248 (95% CI 1116 to 1394) in the oldest age group aged 65 years and over (table 3). For PDR, there were fewer PDR events among the youngest age groups (36 per 100 000), but notably more among the oldest age group (95 per 100 000).
Figure 2.
Model of biennial screening in the NELDESP cohort. Grey boxes in column 2 show the number of STDR and PDR events that would have been diagnosed and referred in routine annual screening during the biennial interval but diagnosed at least one year later in two-yearly interval screening. A breakdown by 3 major ethnic groups is presented in each box. Percentages in grey boxes relative to the total STDR and PDR (R3) events from a 2-year interval. W: white, SA: South Asian, B: black. B, black; SA, South Asian; STDR, sight-threatening diabetic retinopathy; W, white.
Table 3.
Numbers with delayed PDR and STDR events associated with biennial screening by ethnic and age group
| Characteristic | Total eligible | Delayed PDR events | Delayed PDR events per 100 000 persons (95% CI) | Delayed STDR events | Delayed STDR events per 100 000 persons (95% CI) |
| Ethnicity | |||||
| White | 30 350 | 14 | 46 (26 to 80) | 256 | 844 (745 to 955) |
| South Asian | 29 703 | 13 | 44 (24 to 77) | 379 | 1276 (1153 to 1412) |
| Black | 13 391 | 12 | 90 (49 to 161) | 255 | 1904 (1683 to 2154) |
| Age group | |||||
| <45 years | 16 488 | 6 | 36 (15 to 84) | 248 | 1504 (1327 to 1705) |
| 45 to <55 years | 20 207 | 7 | 35 (15 to 75) | 238 | 1178 (1036 to 1339) |
| 55 to <65 years | 20 762 | 8 | 39 (18 to 79) | 205 | 987 (859 to 1134) |
| 65 years and over | 25 325 | 24 | 95 (62 to 143) | 316 | 1248 (1116 to 1394) |
PDR, proliferative diabetic retinopathy; STDR, sight-threatening diabetic retinopathy.
Discussion
Using real-world data from one of the largest most ethnically diverse UK DESP we have shown pronounced sociodemographic differences in the development of STDR and PDR among PLD at low risk of diabetes-related sight loss. Younger age groups (<45 years) compared with older age groups (especially those aged 55 to <65 years), and those of black and South Asian ethnic origin compared with whites, were at greater risk of developing STDR. Higher STDR among the youngest age group is of particular concern, given their trajectory for longer exposure to disease. Given these sociodemographic differences, we have shown that introducing biennial as opposed to annual diabetic eye screening could worsen sight loss among certain sociodemographic groups because of delayed detection of STDR and PDR, potentially adding to healthcare inequalities.
The UK NSC has recommended biennial screening among PLD with no DR on two consecutive screening visits, as the risk of progression to referable was considered low (~0.7% per year) and cases would still be treatable if delayed.8 This recommendation was underpinned by a large study of 354 549 PLD from seven nationally dispersed UK DESP,9 which showed confidence intervals of study estimates of referable diabetic eye disease ranging from 0–1.6%, and a calculated p value for heterogeneity of <0.001. While heterogeneity was attributed to potential differences in age, sex, ethnicity and glycaemic control of screened populations, sociodemographic characteristics of the seven studies were not outlined, and effects of sociodemographic factors were not explicitly quantified. While percentages of referable retinopathy were considered low, numbers will be considerable both in terms of delayed diagnosis and potentially irrecoverable sight loss when scaled up to the national screening programme.3 The current study explicitly quantified the potential impact of a biennial screening frequency by ethnic and age group, and identified those who would be more adversely affected. Moreover, we have previously shown that these high-risk sociodemographic groups, especially younger age groups, are less likely to attend screening appointments among this screened population.15 The introduction of biennial screening could plausibly disenfranchise PLD with no retinopathy from the programme, especially among the more disadvantaged or high risk sociodemographic groups, leading to further delays in diagnosis. A key issue is the potential adverse consequences of delayed diagnosis as a result of biennial screening. While those with referable retinopathy could still be treated later, as acknowledged within NSC recommendations,8 inevitably there would be more extreme cases (ie, with PDR) who would experience irrecoverable sight loss. While we have shown that PDR occurrence is small, ethnic and age group disparities in the numbers are apparent and would still be appreciable within such a large screening programme, particularly among the oldest and black ethnic groups.
With increasing numbers being seen by the DESP3 within a publicly funded healthcare system, the need for cost effectiveness while maintaining patient safety is paramount. The DESP could adopt the NSC recommendation for biennial screening among those at low risk, accepting that this would lead to age and ethnic inequality, or consider a more nuanced screening interval by sociodemographic factors to avoid inequality as suggested by others.18 However, tailored screening intervals would need to be decided and resources made available to administer such screening appointments. Alternatively, artificial intelligence (AI) technologies could be used to assist in maintaining the current status quo in screening frequency. Automated diabetic eye screening has been used in Scotland for over a decade, and is used or being considered for use elsewhere.22 However, automated screening is not currently licensed for use in the English NHS DESP, although a recent evidence synthesis review recommended staged implementation of one AI commercial system,22 which has been extensively validated to show adequate levels of screening performance and could halve the workload of human graders.22–25 Using AI to filter out images without DR has been shown to be safe and cost-effective,24 25 especially as humans could take longer to grade retinal images to ensure absence of retinopathy if a 2-year screening interval were to be adopted. While the effectiveness of AI has been demonstrated,22–25 quantifying equity of AI performance across different ethnic and age groups is needed—akin to formally assessing the potential impact of a biennial screening programme in different sociodemographic groups carried out in this study. Previous work has shown the potential cost-effectiveness of these different screening approaches, but further economic modelling is needed to compare directly the cost effectiveness of these different approaches, particularly among less privileged sociodemographic groups.5 6 18 26
Our study has several strengths. First is the use of a large, multi-ethnic DESP to determine the incidence of STDR and PDR among PLD without retinopathy in different sociodemographic groups, particularly by ethnicity where there were high levels of recording (~99%). While 41% of the cohort who had two consecutive screening episodes without DR were used, prevalence of DR in the entire cohort was reassuringly similar to previous reports and is representative of the UK.6 12 27 DR classification was carried out by trained assessors within the NELDESP, following a multilevel internally and externally quality-assured grading protocol that meets national recommendations. Limitations include the use of annual screening data to simulate biennial screening. These findings may give an over optimistic indication of compliance as implementing biennial screening may worsen adherence to an extended screening regimen. However, these findings using real-world data reflect clinical practice. A randomised controlled clinical trial would be the gold standard of assessing the impact of biennial screening, but such a study would need to be large to compare impact across different age and ethnic groups. More importantly, technologies to assist in screening are evolving so rapidly (both in terms of instrumentation, including standard 45° and wide field retinal imaging cameras, as well as AI automated retinal image analysis systems), findings could well be outdated before completion. Hence, we believe using ‘real-world’ large scale NHS data to assess the impact is important.
The incentive of biennial screening is to release capacity in the NHS and lessen the inconvenience for PLD at low risk of sight loss of attending eye screening appointments every year,8 but there is a need to address the potential to amplify ethnic and age inequalities in healthcare.28 This study is unique in providing the comprehensive high-quality data needed to inform policymakers and healthcare professionals about potential age and ethnic ramifications of introducing a change in screening frequency, particularly in deprived populations both in the UK and other international settings which provide diabetic eye screening.29 We would urge replication of these findings in other multi-ethnic DESP. Our findings suggest that ethnic and age inequalities in care could worsen with the introduction of biennial screening among PLD at low risk of diabetes-related sight loss. Moving forward either alternative technologies which could allow annual screening of PLD at low risk to continue, or more nuanced screening intervals among different sociodemographic groups, warrant further consideration in providing more equitable healthcare.
Footnotes
Twitter: @abraham_olvera1, @SGUL_PHRI
Collaborators: The Artificial Intelligence & Automated Retinal Image Analysis Systems (ARIAS) Research Group: John Anderson, Sarah Barman, Louis Bolter, Ryan Chambers, Lakshmi Chandrasekaran, Umar Chaudhry, Emily Y Chew, Catherine Egan, Jiri Fajtl, Frederick L Ferris, Aroon D Hingorani, Aaron Y Lee, Abraham Olvera-Barrios, Christopher G Owen, Paolo Remagnino, Alicja R Rudnicka, Royce Shakespeare, Reecha Sofat, Adnan Tufail, Alasdair N Warwick, Charlotte Wahlich, Roshan Welikala, Kathryn Willis, Yue Wu.
Contributors: AO-B, CGO, ARR, JA, AT, and CE designed the study. JA, LB, and RC acquired the data. RC, JA, and AO-B curated the data. AO-B, CGO, and ARR undertook statistical analyses. AO-B, CGO, ARR, JA, and RC had direct access and verified the underlying data. AO-B and CGO wrote the first version of the manuscript. All authors, interpreted results, critically reviewed, and edited the manuscript. AO-B and CGO had the final responsibility for the decision to submit for publication and are guarantors of the work.
Funding: This work is funded by (i) Wellcome Trust Collaborative Award (224390/Z/21/Z), and (ii) NHS Transformation Directorate and The Health Foundation, managed by the National Institute for Health and Social Care Research (AI_HI200008). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS Transformation Directorate, The Health Foundation, National Institute for Health Research, or the Department of Health and Social Care.
Competing interests: AYL: has received fees from Santen, Genetech, FDA, Johnson and Johnson, Carl Zeiss Meditec, Gyroscope, Regeneron, and has a non-remunerative relation with Microsoft. CE: has received fees from Heidelberg Engineering, Inozyme Pharma. AT: has received fees from Annexon, Apellis, Bayer, Genentech, Iveric Bio, Novartis, Oxurion, and Roche.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: On behalf of the ARIAS Research Group, John Anderson, Sarah Barman, Louis Bolter, Ryan Chambers, Lakshmi Chandrasekaran, Umar Chaudhry, Emily Y Chew, Catherine Egan, Jiri Fajtl, Frederick L Ferris, Aroon D Hingorani, Aaron Y Lee, Abraham Olvera-Barrios, Christopher G Owen, Paolo Remagnino, Alicja R Rudnicka, Royce Shakespeare, Reecha Sofat, Adnan Tufail, Alasdair N Warwick, Charlotte Wahlich, Roshan Welikala, Kathryn Willis, and Yue Wu
Data availability statement
Data are available upon reasonable request. The North-East London Diabetic Eye Screening Programme data are not publicly available because of restrictions on data sharing. A fully anonymised dataset is available from the Programme upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by NHS Health Research Authority and Health and Care Research Wales (IRAS No. 265637). Participants gave informed consent to participate in the study before taking part. The study was carried out in accordance with the Declaration of Helsinki.
References
- 1. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scanlon PH. The English national screening programme for diabetic retinopathy 2003-2016. Acta Diabetol 2017;54:515–25. 10.1007/s00592-017-0974-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Office for Health Improvement & Disparities . 6. NHS diabetic eye screening (DES) programme. NHS screening programmes in England: 2019 to 2020. 2023. Available: https://www.gov.uk/government/publications/nhs-screening-programmes-annual-report/nhs-screening-programmes-in-england-2019-to-2020#nhs-diabetic-eye-screening-des-programme
- 4. Echouffo-Tcheugui JB, Ali MK, Roglic G, et al. Screening intervals for diabetic retinopathy and incidence of visual loss: a systematic review. Diabet Med 2013;30:1272–92. 10.1111/dme.12274 [DOI] [PubMed] [Google Scholar]
- 5. Thomas RL, Winfield TG, Prettyjohns M, et al. Cost-effectiveness of biennial screening for diabetes related retinopathy in people with type 1 and type 2 diabetes compared to annual screening. Eur J Health Econ 2020;21:993–1002. 10.1007/s10198-020-01191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scanlon PH, Aldington SJ, Leal J, et al. Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Health Technol Assess 2015;19:1–116. 10.3310/hta19740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor-Phillips S, Mistry H, Leslie R, et al. Extending the diabetic retinopathy screening interval beyond 1 year: systematic review. Br J Ophthalmol 2016;100:105–14. 10.1136/bjophthalmol-2014-305938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. UK National Screening Committee . Adult screening programme: Diabetic retinopathy. 2016. Available: https://view-health-screening-recommendations.service.gov.uk/diabetic-retinopathy/#:~:text=In%202016%20the%20UK%20NSC,2%20years%20rather%20than%20annually
- 9. Leese GP, Stratton IM, Land M, et al. Progression of diabetes retinal status within community screening programs and potential implications for screening intervals. Diabetes Care 2015;38:488–94. 10.2337/dc14-1778 [DOI] [PubMed] [Google Scholar]
- 10. Goff LM. Ethnicity and type 2 diabetes in the UK. Diabet Med 2019;36:927–38. 10.1111/dme.13895 [DOI] [PubMed] [Google Scholar]
- 11. Sivaprasad S, Gupta B, Gulliford MC, et al. Ethnic variation in the prevalence of visual impairment in people attending diabetic retinopathy screening in the United Kingdom (DRIVE UK). PLoS One 2012;7:e39608. 10.1371/journal.pone.0039608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathur R, Bhaskaran K, Edwards E, et al. Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: a cohort study in the clinical practice research Datalink 2004-2014. BMJ Open 2017;7:e014444. 10.1136/bmjopen-2016-014444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Public Health England . Screening Quality Assurance visit report: NHS Diabetic Eye Screening Service North East London Diabetic Eye Screening Programme. 2017. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/657800/North_east_london_qa_visit_diabetic_eye_executive_summary_may_2017.pdf
- 14. Office for Health Improvement and Disparities . Diabetic eye screening programme: standards. 2019. Available: https://www.gov.uk/government/publications/diabetic-eye-screening-programme-standards
- 15. Olvera-Barrios A, Seltene M, Heeren TFC, et al. Effect of ethnicity and other sociodemographic factors on attendance at diabetic eye screening: a 12-month retrospective cohort study. BMJ Open 2021;11:e046264. 10.1136/bmjopen-2020-046264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harding S, Greenwood R, Aldington S, et al. Grading and disease management in national screening for diabetic retinopathy in England and Wales. Diabet Med 2003;20:965–71. 10.1111/j.1464-5491.2003.01077.x [DOI] [PubMed] [Google Scholar]
- 17. Census 2021 . Ethnicity, national identity and religion in the UK and non-UK born population. 2022. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity
- 18. Stratton IM, Aldington SJ, Taylor DJ, et al. A simple risk stratification for time to development of sight-threatening diabetic retinopathy. Diabetes Care 2013;36:580–5. 10.2337/dc12-0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ministry of Housing CLG . National statistics: English indices of deprivation 2019. 2019. Available: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019
- 20. Team . The R Project for Statistical Computing. 2022. Available: https://www.r-project.org/
- 21. Therneau TM, Lumley T, Crowson C. Survival: survival analysis. 2023. Available: https://cran.r-project.org/web/packages/survival/index.html
- 22. Zhelev Z, Peters J, Rogers M, et al. Automated grading in the Diabetic Eye Screening Programme: external review against programme appraisal criteria for the UK National Screening Committee. 2021. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1035903/Evidence_summary_AI_in_DESP_2021.pdf
- 23. Heydon P, Egan C, Bolter L, et al. Prospective evaluation of an artificial intelligence-enabled algorithm for automated diabetic retinopathy screening of 30 000 patients. Br J Ophthalmol 2021;105:723–8. 10.1136/bjophthalmol-2020-316594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tufail A, Rudisill C, Egan C, et al. Automated diabetic retinopathy image assessment software: diagnostic accuracy and cost-effectiveness compared with human graders. Ophthalmology 2017;124:343–51. 10.1016/j.ophtha.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 25. Tufail A, Kapetanakis VV, Salas-Vega S, et al. An observational study to assess if automated diabetic retinopathy image assessment software can replace one or more steps of manual imaging grading and to determine their cost-effectiveness. Health Technol Assess 2016;20:1–72. 10.3310/hta20920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Broadbent DM, Wang A, Cheyne CP, et al. Safety and cost-effectiveness of Individualised screening for diabetic retinopathy: the ISDR open-label, equivalence RCT. Diabetologia 2021;64:56–69. 10.1007/s00125-020-05313-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas RL, Dunstan FD, Luzio SD, et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol 2015;99:64–8. 10.1136/bjophthalmol-2013-304017 [DOI] [PubMed] [Google Scholar]
- 28. Kapadla D, Zhang J, Salaway S, et al. Ethnic inequalities in healthcare: a rapid evidence review. 2022. Available: https://www.nhsrho.org/wp-content/uploads/2022/02/RHO-Rapid-Review-Final-Report_Summary_v.4.pdf
- 29. Raleigh V. The health of people from ethnic minority groups in England: the Kings Fund, Available: https://www.kingsfund.org.uk/publications/health-people-ethnic-minority-groups-england#conclusion
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjo-2023-324097supp001.pdf (58.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The North-East London Diabetic Eye Screening Programme data are not publicly available because of restrictions on data sharing. A fully anonymised dataset is available from the Programme upon reasonable request.