Abstract
This study aimed to evaluate the ability of rete testis thickness (RTT) and testicular shear wave elastography (SWE) to differentiate obstructive azoospermia (OA) from nonobstructive azoospermia (NOA). We assessed 290 testes of 145 infertile males with azoospermia and 94 testes of 47 healthy volunteers at Shanghai General Hospital (Shanghai, China) between August 2019 and October 2021. The testicular volume (TV), SWE, and RTT were compared among patients with OA and NOA and healthy controls. The diagnostic performances of the three variables were evaluated using the receiver operating characteristic curve. The TV, SWE, and RTT in OA differed significantly from those in NOA (all P ≤ 0.001) but were similar to those in healthy controls. Males with OA and NOA were similar at TVs of 9–11 cm3 (P = 0.838), with sensitivity, specificity, Youden index, and area under the curve of 50.0%, 84.2%, 0.34, and 0.662 (95% confidence interval [CI]: 0.502–0.799), respectively, for SWE cut-off of 3.1 kPa; and 94.1%, 79.2%, 0.74, and 0.904 (95% CI: 0.811–0.996), respectively, for RTT cut-off of 1.6 mm. The results showed that RTT performed significantly better than SWE in differentiating OA from NOA in the TV overlap range. In conclusion, ultrasonographic RTT evaluation proved a promising diagnostic approach to differentiate OA from NOA, particularly in the TV overlap range.
Keywords: azoospermia, differential diagnosis, rete testis thickness, shear wave elastography
INTRODUCTION
Male infertility prevalence is increasing, accounting for 10%–15% of the male population. Approximately, 5%–10% of infertile males are azoospermic.1 Azoospermia can be divided into obstructive azoospermia (OA) and nonobstructive azoospermia (NOA). The surgical treatment strategies for OA depend on the etiology and location of the obstruction. For example, vasoepididymostomy can be performed for epididymal obstruction.2,3 The most effective way to obtain sperm from males with NOA due to primary testicular failure is microdissection testicular sperm extraction (micro-TESE). When sperm is present, intracytoplasmic sperm injection can be carried out to achieve fertility.1,4 Therefore, precise identification of the azoospermia etiology is of great importance for patient management and treatment outcomes.
There are several modalities to evaluate males with azoospermia, including hormone level testing, semen analysis, vasography, testicular biopsy, and ultrasonography (US). At present, scrotal US is the preferred imaging modality when evaluating male infertility because of its noninvasiveness, low cost, safety, and ability to visualize the morphological characteristics of male reproductive organs.5 Du et al.6 showed that differentiation of OA from NOA based on testicular volume (TV) measurement through scrotal US had high sensitivity and specificity; however, there was considerable overlap in TV distribution between OA and NOA. Consequently, OA and NOA were statistically similar when TV was in the range of 10.1–13.0 cm3. It is important to find a way to identify the azoospermia etiology in this overlapping range.
Shear wave elastography (SWE) technology quantitatively evaluates tissue hardness in the region of interest by generating shear waves. In recent years, SWE has been gradually applied to quantify testicular elasticity and explore its usefulness in differentiating between the testes of healthy and infertile males and predicting infertility, with encouraging results.7–9 Furthermore, Abdelaal et al.10 suggested that SWE could help predict sperm retrieval from patients with NOA. Therefore, we used SWE to evaluate the performance of TV in the differential diagnosis of azoospermia in the overlap range. The long-neglected rete testis11 was revalued when several studies showed that spermatogonial stem cell transplantation could be performed through it.12,13 The rete testis is a seminiferous tubule that merges with the tubuli recti at the apex of the testicular lobule. The tubuli recti enter the testicular mediastinum to form an irregular anastomotic cavity.14 The typical sonographic appearance of the rete testis is a strip-shaped hyperechoic structure on the posterolateral aspect of the testis,15 connecting the mediastinum testis with the epididymal head in a longitudinal section. Waters et al.16 showed that some cases of OA showed rete testis ectasia. Moreover, it was shown that rete testis development was affected by the endocrine system17,18 and several diseases that cause NOA.7 Therefore, we investigated the differential diagnosis of azoospermia by evaluating rete testis thickness (RTT) using US.
This study aimed to evaluate the performance of SWE and RTT in differentiating OA from NOA in their TV overlap range.
PARTICIPANTS AND METHODS
Participants
This study included 162 infertile patients with azoospermia (study group) and 47 healthy volunteers (control group), recruited from Shanghai General Hospital (Shanghai, China) between August 2019 and October 2021. Of them, 145 patients were finally enrolled in our study. Patients with Klinefelter syndrome with the small TV were excluded because of resulting in inaccurate diagnosis, missing testicular biopsy, or missing a final clinical diagnosis. The control group had normal clinical examinations, hormonal levels, semen analysis, US examinations, and no history of relevant urological illnesses. A biopsy was not performed in this group. All clinical, laboratory examinations and surgical procedures were performed after the participants provided their written informed consent. This retrospective study was approved by the ethics committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine (Apprvoal No. 2020SQ041) and followed the tenets of the Declaration of Helsinki.
Ultrasonographic technique
Conventional scrotal US examination and SWE were performed using an Aplio i900 scanner (Canon Medical Systems Corp., Tochigi-ken, Japan) with a 5–18 MHz linear-array transducer. The bilateral TV (in cm3) was calculated by the Lambert formula:19 length × height × width × 0.71, because Hsieh et al.20 compared three commonly used formulas and showed that the Lambert formula was the most accurate. For the maximum RTT (in mm) measurement, we selected a longitudinal section with a clear display of the rete testis. If the rete testis could not be displayed, we marked its thickness as 0 mm. Stiffness was measured five times in the center of both testes at the same depth, and the average was taken as the testicular SWE value (in kPa).
All examinations were performed by the same skilled radiologist (XL) with 15 years of experience in conventional US and 4 years of experience in SWE and recorded on a hard disk incorporated in the scanner.
Image acquisition and data analysis
The participants were placed supine with the penis pulled up close to the abdomen. The bilateral testes were fully exposed and relatively stationary. The patients were advised to breathe calmly and not move during the examination to prevent artifacts and ensure optimal image quality. The probe was coated with adequate couplant to ensure full contact with the testes without signal loss. The couplant can also help avoid the influence of excessive pressure during the quantitative measurement of testicular SWE. The testicular images were divided into five subgroups based on the TV to further evaluate TV performance in azoospermia differential diagnosis; these included ≤7.0 cm3, 7.1–9.0 cm3, 9.1–11.0 cm3, 11.1–13.0 cm3, and ≥13.1 cm3.
We measured the upper, lower, anterior, and posterior diameters in longitudinal sections of the rete testis. The measurement error of the upper and lower diameters was larger than that of the anterior and posterior diameters because they could be fully displayed on the longitudinal sections. The anterior and posterior diameters were measured at their thickest place and recorded as the RTT (Figure 1).
Figure 1.
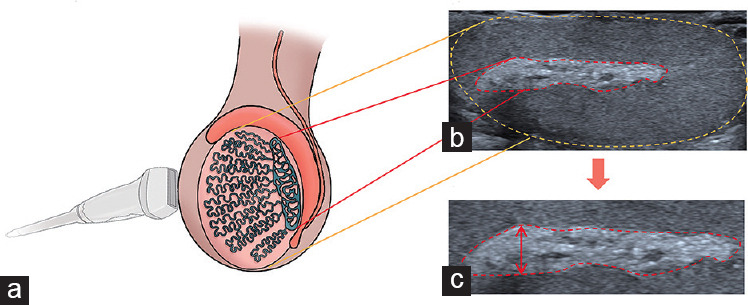
Schematic illustration of US rete testis measurement. (a) Schematic diagram of longitudinal testis scanning with a high-frequency probe. (b) US image, with the testis and rete testis shown in a schematic diagram. (c) RTT measurement (arrow). US: ultrasonographic; RTT: rete testis thickness.
The SWE image information was displayed in dual mode, with one-half of the screen displaying the color-coded stiffness box (red = hard, blue = soft) and the other half displaying propagation graph pattern information. The SWE examination systematically inspected the tissue in the maximum longitudinal plane. We carefully avoided mechanical pressure application during the examination to prevent modifying the stiffness value. The SWE sampling frame was placed at the center of the image by default, tangent to the testis outer edge and covering half of the middle testicular area or as close to it as possible. After the image was stable for several seconds, filled 90% of the frame, and the testicular center was relatively homogeneous with no apparent artifacts, we froze the image and measured the SWE value.
Reference standard
Azoospermia was demonstrated by standard semen analysis. Sex hormone measurement and testicular biopsy were used to differentiate OA and NOA in all patients. For NOA, chromosome and gene analyses were needed to examine chromosomal abnormalities. Testicular biopsy was performed after the SWE measurement to avoid altering testis stiffness by an invasive operation with bleeding or the start of scar formation. Histology assessments were performed by two pathologists (XBW and LRJ) with 15 years and 6 years of experience, respectively. In case of disagreement, a consensus was reached by discussion. The histology criteria used in this study followed those previously reported.19 The formal diagnoses were normal spermatogenesis, mild hypospermatogenesis, severe hypospermatogenesis, maturation arrest, and germ cell aplasia. The testicular biopsies in this study confirmed that no patient had OA on one side and NOA on the other.
Interoperator SWE and RTT reproducibility
Thirty consecutive patients (60 testes) were enrolled to evaluate interoperator SWE and RTT reproducibility. Measurements were made by an experienced radiologist (XL) with 4 years of experience in elastography and 15 years of experience in conventional US, and a beginner radiologist with 3 months of experience in elastography and 1 year of experience in conventional US. The experienced radiologist trained the beginner to perform SWE and RTT on a different cohort of 30 patients before performing the interoperator reproducibility assessment. The radiologists were blinded to each other’s measurements while performing SWE and RTT with a 10- to 20-min interval between measurements.
Statistical methods
Age and TV are expressed as mean ± standard deviation (s.d.) and were compared among groups using the analysis of variance (ANOVA) after ensuring homogeneity of variances for the data. The Kruskal–Wallis test compared the groups for hormonal levels, SWE, and RTT. When the Kruskal–Wallis result was significant (P < 0.05), the Wilcoxon signed-rank test compared every two-group combination (a total of three combinations). The hormonal levels, SWE, and RTT are expressed as median (interquartile range [IQR]). The volume subgroup distribution between the OA and NOA groups was tested using Pearson’s Chi-squared test. The follicle-stimulating hormone (FSH), luteinizing hormone (LH), SWE, and RTT in the TV subgroups, expressed as mean ± s.d., were compared in the OA and NOA groups by ANOVA or Wilcoxon signed-rank test according to the data homogeneity of variances. Receiver operating characteristic (ROC) curve analysis estimated the performance of TV, SWE, and RTT in differentiating OA from NOA; the area under the curve (AUC) and its 95% confidence interval (CI) were also calculated. The ROC curve analysis was also used to estimate the diagnostic performance of SWE and RTT in the range of overlapping TV for patients with OA and NOA. Differences with P < 0.05 were considered statistically significant. The intraclass correlation coefficient assessed the interoperator SWE and RTT reproducibility. The intraclass correlation coefficient agreement was interpreted as poor (0–0.2), fair (0.2–0.4), moderate (0.4–0.6), strong (0.6–0.8), or excellent (>0.8). IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA) was used to perform the statistical analysis. The diagnostic performance of the ROC curves was compared using the MedCalc 19.2.0 software program (MedCalc Software Ltd., Ostend, Belgium).
RESULTS
Enrolled patients and baseline characteristics
Of the 162 screened patients with azoospermia, we excluded 11 because of missing testicular biopsy and a final clinical diagnosis, four with Klinefelter syndrome with the small TV resulting in inaccurate measurement of RTT, and two with retrograde ejaculation based on postejaculation urine analysis. Finally, 145 patients were enrolled (Figure 2), among which 62 were with OA, and 83 were with NOA. The mean age of the OA group was 32 (s.d.: 5; range: 21–49) years, and pathology confirmed they all had normal spermatogenesis. The mean age of the NOA group was 30 (s.d.: 4; range: 19–48) years. This group included 68 patients with Sertoli cell-only syndrome, ten with maturation arrest, and five with hypospermatogenesis. The control group (n = 47) had a mean age of 31 (s.d.: 8; range: 19–50) years. The OA, NOA, and control groups were of similar age and had similar testosterone (T) levels (both P > 0.05). The FSH and LH levels were significantly higher in the NOA group than those in the OA group (both P < 0.001), while they were similar in the OA and control groups (both P > 0.05; Table 1).
Figure 2.
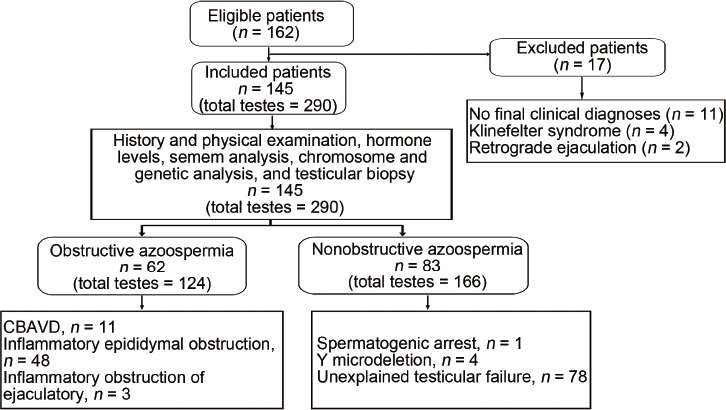
Patient enrollment and classification flowchart. CBAVD: congenital bilateral absence of the vas deferens.
Table 1.
Comparison of patient characteristics, hormones, testicular volume, shear wave elastography and rete testis thickness and patient distribution among the testicular volume subgroups
| Variable | OA (n=62) | NOA (n=83) | Control (n=47) | P |
|---|---|---|---|---|
| Age (year), mean±s.d. | 32±5 | 30±4 | 31±8 | 0.072 |
| Serum hormone, median (IQR) | ||||
| FSH (IU l−1) | 4.4 (3.5–5.6)a,c | 26.5 (19.8–32.1)a,b | 3.9 (3.6–6.2)b,c | <0.001 |
| LH (IU l−1) | 3.8 (3.0–5.2)a,c | 9.5 (6.2–15.5)a,b | 3.5 (3.1–6.2)b,c | <0.001 |
| Testosterone (µg l−1) | 3.6 (2.7–5.0) | 4.0 (2.8–5.6) | 3.7 (3.0–4.9) | 0.498 |
| TV (cm3), mean±s.d. | 14.5±3.5a | 7.2±3.6a,b | 13.7±2.9b | <0.001 |
| SWE value (kPa), median (IQR) | 2.6 (2.3–2.9)a | 2.9 (2.4–3.5)a,b | 2.5 (2.3–2.9)b | <0.001 |
| RTT (mm), median (IQR) | 2.1 (1.8–2.6)a | 1.6 (1.2–2.0)a,b | 2.0 (1.6–2.4)b | <0.001 |
| TV subgroups (cm3), n (%) | ||||
| ≤7.0 | 0 (0) | 81 (48.8) | - | <0.001 |
| 7.1–9.0 | 4 (3.2) | 40 (24.1) | - | <0.001 |
| 9.1–11.0 | 19 (15.3) | 24 (14.5) | - | 0.838 |
| 11.1–13.0 | 20 (16.1) | 12 (7.2) | - | 0.017 |
| ≥13.1 | 81 (65.3) | 9 (5.4) | - | <0.001 |
aP<0.001, significant difference between OA and NOA groups; bP<0.001, significant difference between NOA and control groups; cP>0.05, similar between OA and control groups. TV: testicular volume; SWE: shear wave elastography; RTT: rete testis thickness; OA: obstructive azoospermia; NOA: nonobstructive azoospermia; s.d.: standard deviation; IQR: interquartile range; FSH: follicle-stimulating hormone; LH: luteinizing hormone; - : not applicable
Comparison and diagnostic performance of TV, SWE, and RTT
The TV and RTT were significantly higher (both P < 0.001) and SWE significantly lower (P = 0.001) in the OA group than those in the NOA group (Figure 3), but the OA and control groups were similar in all three endpoints (TV: P = 0.071; RTT: P = 0.060; SWE: P > 0.05). The sensitivity, specificity, Youden index, and AUC values for differentiating OA from NOA were 91.9%, 81.9%, 0.74, and 0.932 (95% CI: 0.904–0.960), respectively, for TV with a cut-off of 10.5 cm3; 92.7%, 49.4%, 0.42, and 0.765 (95% CI: 0.711–0.818), respectively, for RTT with a cut-off of 1.6 mm; and 39.2%, 87.1%, 0.26, and 0.637 (95% CI: 0.574–0.700), respectively, for SWE with a cut-off of 3.1 kPa (Figure 4a). The diagnostic performance of TV was higher than that of RTT (P < 0.001) and the diagnostic performance of RTT was higher than that of SWE (P = 0.001).
Figure 3.
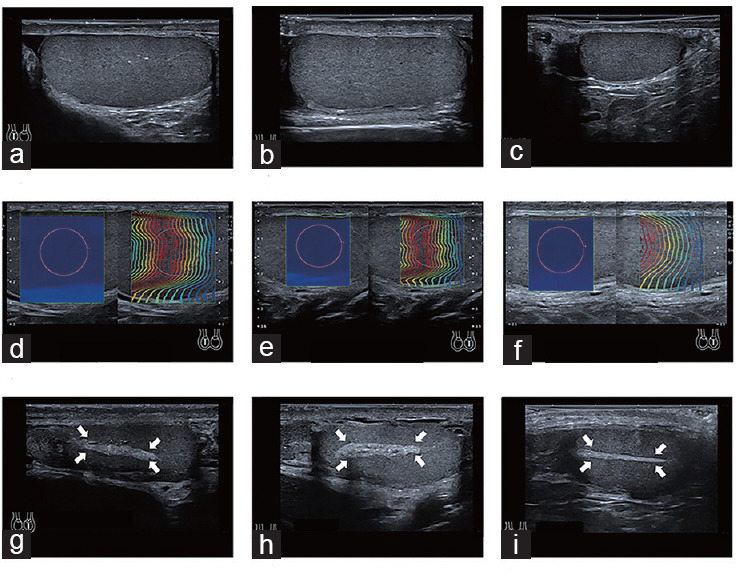
Comparison of TV, SWE, and RTT among the control, OA, and NOA groups. (a) A 30-year-old normally fertile male with a right TV of 12.0 cm3. (b) A 27-year-old male with OA and a right TV of 13.9 cm3. (c) A 27-year-old male with NOA and a right TV of 3.5 cm3, which was lower than the control and OA groups. (d) The SWE of the right testis in a 35-year-old male with normal fertility was 2.2 kPa. (e) The SWE of the left testis in a 27-year-old male with OA was 2.2 kPa. (f) The SWE of the left testis in a 29-year-old male with NOA was 3.0 kPa, higher than that of the control and OA groups. (g) The RTT of the left testis in a 30-year-old male with normal fertility male was 2.3 mm (arrow: rete testis). (h) The RTT of the left testis in a 27-year-old male with OA was 3.3 mm (arrow: rete testis). (i) The RTT of the right testis in a 34-year-old male with NOA was 1.5 mm, lower than that of the control and OA groups (arrow: rete testis). TV: testicular volume; SWE: shear wave elastography; RTT: rete testis thickness; OA: obstructive azoospermia; NOA: nonobstructive azoospermia.
Figure 4.
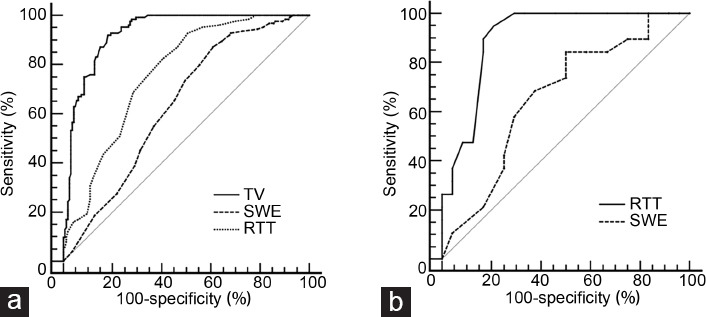
ROC curve. (a) ROC curve evaluating the diagnostic performance of TV, RTT, and SWE. The AUCs of TV, RTT, and SWE were 0.932 (95% CI: 0.904–0.960), 0.765 (95% CI: 0.711–0.818), and 0.637 (95% CI: 0.574–0.700), respectively. (b) ROC curve evaluating the diagnostic performance of RTT and SWE in the TV range of 9–11 cm3. The AUCs of RTT and SWE were 0.904 (95% CI: 0.811–0.996) and 0.662 (95% CI: 0.502–0.799), respectively. ROC: receiver operating characteristic; TV: testicular volume; RTT: rete testis thickness; SWE: shear wave elastography; AUC: area under the curve; CI: confidence interval.
Distribution of the TV subgroups and diagnostic performance of SWE and RTT
Although the diagnostic performance of TV was high, the OA and NOA groups overlapped in the TV range of 9.1–11.0 cm3, with no difference between them (P = 0.838; Table 1). In this TV range, azoospermia was identified by SWE and RTT (Table 2). The sensitivity, specificity, Youden index, and AUC for differentiating OA from NOA were 50.0%, 84.2%, 0.34, and 0.662 (95% CI: 0.502–0.799), respectively, for SWE with a cut-off of 3.1 kPa; and 94.1%, 79.2%, 0.74, and 0.904 (95% CI: 0.811–0.996), respectively, for RTT with a cut-off of 1.6 mm (Figure 4b).
Table 2.
Distribution of follicle-stimulating hormone, luteinizing hormone, shear wave elastography and rete testis thickness by testicular volume subgroup
| Variable | TV ≤7.0 cm3 | TV 7.1–9.0 cm3 | TV 9.1–11.0 cm3 | TV 11.1–13.0 cm3 | TV ≥13.1 cm3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| OA (n=0) | NOA (n=81) | OA (n=4) | NOA (n=40) | OA (n=19) | NOA (n=24) | OA (n=20) | NOA (n=12) | OA (n=81) | NOA (n=9) | |
| FSH (IU l−1) | NA | 29.3±16.1 | 5.6±1.7a | 27.1±7.5a | 5.1±2.1a | 22.5±13.9a | 4.3±1.9a | 18.7±10.8a | 4.9±2.3a | 20.3±17.3a |
| LH (IU l−1) | NA | 13.8±12.9 | 4.0±3.0 | 9.9±5.3 | 5.2±2.8a | 11.8±9.1a | 3.9±1.7a | 13.4±10.9a | 4.3±2.3a | 14.6±13.5a |
| SWE (kPa) | NA | 3.2±0.9 | 2.5±0.2 | 2.8±0.7 | 2.8±0.5 | 3.2±0.9 | 2.7±0.5 | 2.9±1.0 | 2.6±0.4a | 4.0±1.7a |
| RTT (mm) | NA | 1.5±0.8 | 2.1±0.4 | 1.5±0.9 | 2.3±0.6a | 1.2±0.6a | 2.4±0.7 | 1.9±0.8 | 2.2±0.6 | 1.9±0.5 |
aP<0.05, significant difference between OA and NOA groups. Values are presented as mean±s.d. FSH: follicle-stimulating hormone; LH: luteinizing hormone; SWE: shear wave elastography; RTT: rete testis thickness; OA: obstructive azoospermia; NOA: nonobstructive azoospermia; s.d.: standard deviation; TV: testicular volume; NA: not available
SWE and RTT reproducibility
The intraclass correlation coefficient values for SWE and RTT measurements were 0.885 (95% CI: 0.690–0.946) and 0.959 (95% CI: 0.932–0.975), respectively (both > 0.800), indicating excellent interoperator consistency.
DISCUSSION
Results of our study indicated that the TV of patients with OA was greater than that of patients with NOA but similar to that of the controls, consistent with those TVs in previous studies.6,19,21,22 Some investigators20,23 have reported that testicular size was directly related to testicular function and could help assess patients’ hormone status. In our study, the NOA group had a smaller TV and higher FSH and LH levels than the OA group. These results were consistent with a previous report.23 A TV of 10.5 cm3 was identified as the threshold between OA and NOA based on ROC curve analysis, with high sensitivity, specificity, and AUC. Du et al.6 used a TV of 13.0 cm3 as the threshold for differential diagnosis of azoospermia. Our study suggested that the overlapping range of TV was 9.1–11.0 cm3, somewhat different from the 10.1–13.0 cm3 reported by Du et al.6 We believed that differences in sample selection may explain the differences between these studies. However, there was no doubt that an overlapping TV range existed, making distinguishing azoospermia subtypes a challenge. We further assessed the ability of SWE and RTT to differentiate OA from NOA in this subgroup.
In the present study, testicular stiffness was higher in the NOA group than that in the OA group, which was similar to controls. Unlike our results, Rocher et al.7 found that testicular stiffness was lower in patients with non-Klinefelter syndrome NOA than that in patients with OA, while the testicular stiffness was lower in patients with OA than that of patients with Klinefelter syndrome NOA. Patients with Klinefelter syndrome were excluded from the present study, because we only collected four patients with Klinefelter syndrome, and the sample size was too small to be separately divided into different groups for research. To prevent bias, we excluded patients with Klinefelter syndrome in the NOA group. The main reason for the difference between the studies could be the patients’ age distribution and serum testosterone levels. In the present study, the mean age of the subjects in the NOA group was 30 years and serum T levels were similar to those of the OA and control groups. The T levels of the OA and control groups were in the normal range. The mean age of patients in the study by Rocher et al.7 was 37 years. Serum testosterone levels decrease with age, and testicles undergo age-related Leydig cell loss,24,25 which affects sperm production and results in testicular softening. Although SWE can distinguish between OA and NOA, its sensitivity was only 39.2%, indicating a high missed diagnosis rate. Although its specificity reached 87.1%, the AUC was still small (0.637). These results showed that the overall diagnostic performance of SWE was relatively low. Nevertheless, we evaluated the diagnostic capability of SWE in patients in the TV overlap range. The results showed little change in its specificity, but the sensitivity increased to 50.0%, and the Youden index and AUC value improved; however, its overall diagnostic performance remained low. Although the current study excluded patients with Klinefelter syndrome, these patients have a small TV with a distribution outside the TV overlap range.7,26 Evidently, SWE did not affect the diagnostic efficacy in the TV overlap range, irrespective of Klinefelter syndrome. Therefore, SWE has little value for azoospermia differential diagnosis in the TV overlap range.
To the best of our knowledge, there were few studies to evaluate the efficiency of RTT in azoospermia differential diagnosis before our study. We found that the RTT was higher in patients with OA than that in patients with NOA. This is because obstruction can cause dilation of the rete testis, resulting in an increase in its thickness. Although the RTT was higher in patients with OA than that in the control group, the difference was insignificant, possibly because of the structural composition of the rete testis. In humans, the rete testis has a mediastinal reticulum system that penetrates the testicular body and is surrounded by collagen bundles;17 consequently, the rete testis texture is relatively stiff, as confirmed by Rocher et al.7 using SWE. Although OA can dilate the rete testis, the current findings suggested it occurred only when the obstruction was confined to the testis or epididymal head. When the obstruction site was in the epididymal body or tail, or the vas deferens, the expansion of the rete testis was less obvious because of its relatively stiff texture. In the present study, the obstruction in most patients with OA was in the testicular body or tail. Therefore, patients in the OA and control groups had similar RTT. The ROC curve analysis showed that when the RTT cut-off was 1.6 mm, the sensitivity for azoospermia differential diagnosis was very high (92.7%), the specificity was 49.4%, and the AUC was 0.765. The overall diagnostic performance was still good. However, the RTT showed excellent diagnostic performance in the overlapping TV range, with a sensitivity of 94.1%, specificity of 79.2%, and AUC of 0.904. The reason RTT achieved such a good diagnostic performance may be related to the way NOA develops. NOA is affected by many pathogenic factors such as genetic, endocrine, and other congenital factors, and acquired factors, such as hypothalamic and pituitary diseases.27,28 Such diseases lead to primary testicular function failure or spermatogenesis dysfunction secondary to hypothalamic/pituitary dysfunction.1 Rete testis development is also regulated by endocrine and molecular pathways.17,18,29,30 Therefore, its development could be affected in NOA. The current study found that the RTT was smaller in patients with NOA than that in patients with OA and the control group, further demonstrating that the development of the rete testis was affected. From the perspective of azoospermia diagnostic performance, particularly in the TV overlap range, the RTT appeared to be a very valuable noninvasive diagnostic method.
It should be noted that other examination approaches, including semen analysis, sex hormone measurements, and genetic tests, have been applied to evaluate azoospermia. Low semen volume, low pH value, and very low fructose have been noted in cases with obstructed ejaculatory ducts. An abnormal karyotype (e.g., 47,XXY) and Y-chromosome microdeletions usually lead to NOA.1,2 However, the cause of azoospermia cannot be determined in some cases with normal testicular volume, semen volume, fructose values, sex hormones, and karyotype, and without Y-chromosome microdeletions. Ultrasonographic RTT evaluation seems promising, especially for differentiating OA from NOA in patients with TV in the overlapping range.
Our study had some limitations. First, this was a retrospective study. Second, screening the cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations would help to better define the RTT between genetically based and nongenetically based obstructions. We used ANOVA to compare the RTT between patients with and without the congenital bilateral absence of the vas deferens. The mean RTT values in the two groups were statistically similar. No statistical difference may also be caused by the small sample size of congenital bilateral absence of the vas deferens, which also indicates congenital bilateral absence of the vas deferens sample size in further research.
CONCLUSIONS
The presented preliminary data showed that SWE had a limited value in azoospermia differential diagnosis, while ultrasonographic RTT evaluation seemed a promising imaging diagnostic method, especially for azoospermia differential diagnosis in the TV overlapping range.
AUTHOR CONTRIBUTIONS
XL contributed to the conception of the study, performed ultrasound examination and analysis, and drafted the manuscript. RHT participated in the design of the study and performing surgery, and assisted in drafting the manuscript. PL performed surgery and participated in the statistical analysis. CXL and MHY participated in the data collection and analysis. CCY developed the original concept of this study. XBW and LRJ contributed to the histopathological analysis. RW and ZL participated in the design of the study and directed the related work. All authors read and approved the final manuscript and agree with the order of presentation of authors.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We are grateful to all the participants who took part in this study. This work was supported by the National Natural Science Foundation of China (No. 82071931 and No. 82130057), the Program for Shanghai Outstanding Medical Academic Leaders (2019LJ18), the Interdisciplinary Program of Shanghai Jiao Tong University (ZH2018ZDA17), the Program from Science and Technology Commission of Shanghai Municipality (20Y11912400), and the Clinical Research Innovation Team of Shanghai General Hospital (No. CTCCR-2019B05).
REFERENCES
- 1.Practice Committee of the American Society for Reproductive Medicine. Management of nonobstructive azoospermia:a committee opinion. Fertil Steril. 2018;110:1239–45. doi: 10.1016/j.fertnstert.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Male Reproduction and Urology. The management of obstructive azoospermia:a committee opinion. Fertil Steril. 2019;111:873–80. doi: 10.1016/j.fertnstert.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Chan PT, Brandell RA, Goldstein M. Prospective analysis of outcomes after microsurgical intussusception vasoepididymostomy. BJU Int. 2005;96:598–601. doi: 10.1111/j.1464-410X.2005.05691.x. [DOI] [PubMed] [Google Scholar]
- 4.Najari BB. Ultrasound evaluation of seminiferous tubules:a promising prognostic tool for men with nonobstructive azoospermia undergoing microsurgical testicular sperm extraction. Fertil Steril. 2020;113:73–4. doi: 10.1016/j.fertnstert.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, et al. Male infertility. Lancet. 2021;397:319–33. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 6.Du J, Li FH, Guo YF, Yang LM, Zheng JF, et al. Differential diagnosis of azoospermia and etiologic classification of obstructive azoospermia:role of scrotal and transrectal US. Radiology. 2010;256:493–503. doi: 10.1148/radiol.10091578. [DOI] [PubMed] [Google Scholar]
- 7.Rocher L, Criton A, Gennisson JL, Izard V, Ferlicot S, et al. Testicular shear wave elastography in normal and infertile men:a prospective study on 601 patients. Ultrasound Med Biol. 2017;43:782–9. doi: 10.1016/j.ultrasmedbio.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Trottmann M, Marcon J, D’Anastasi M, Bruce MF, Stief CG, et al. Shear-wave elastography of the testis in the healthy man –determination of standard values. Clin Hemorheol Microcirc. 2016;62:273–81. doi: 10.3233/CH-162046. [DOI] [PubMed] [Google Scholar]
- 9.Yavuz A, Yokus A, Taken K, Batur A, Ozgokce M, et al. Reliability of testicular stiffness quantification using shear wave elastography in predicting male fertility:a preliminary prospective study. Med Ultrason. 2018;20:141–7. doi: 10.11152/mu-1278. [DOI] [PubMed] [Google Scholar]
- 10.Abdelaal AM, El-Azizi HM, GamalEl Din SF, Abdulsalam Mohammad Azzazi O, Shokr Mohamed M. Evaluation of the potential role of shear wave elastography as a promising predictor of sperm retrieval in non-obstructive azoospermic patients:a prospective study. Andrology. 2021;9:1481–9. doi: 10.1111/andr.13005. [DOI] [PubMed] [Google Scholar]
- 11.Gul M, Hildorf S, Dong L, Thorup J, Hoffmann ER, et al. Review of injection techniques for spermatogonial stem cell transplantation. Hum Reprod Update. 2020;26:368–91. doi: 10.1093/humupd/dmaa003. [DOI] [PubMed] [Google Scholar]
- 12.Ning L, Meng J, Goossens E, Lahoutte T, Marichal M, et al. In search of an efficient injection technique for future clinical application of spermatogonial stem cell transplantation:infusion of contrast dyes in isolated cadaveric human testes. Fertil Steril. 2012;98:1443–8.e1. doi: 10.1016/j.fertnstert.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Faes K, Lahoutte T, Hoorens A, Tournaye H, Goossens E. In search of an improved injection technique for the clinical application of spermatogonial stem cell transplantation. Reprod Biomed Online. 2017;34:291–7. doi: 10.1016/j.rbmo.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Burrus JK, Lockhart ME, Kenney PJ, Kolettis PN. Cystic ectasia of the rete testis:clinical and radiographic features. J Urol. 2002;168:1436–8. doi: 10.1016/S0022-5347(05)64468-0. [DOI] [PubMed] [Google Scholar]
- 15.Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–26. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters K, Schnuelle JG, Cofield LG, Rush J, Boakari Y, et al. Tubular ectasia of the rete testis in an Angus bull. Reprod Domest Anim. 2021;56:1261–4. doi: 10.1111/rda.13985. [DOI] [PubMed] [Google Scholar]
- 17.Major AT, Estermann MA, Smith CA. Anatomy, endocrine regulation, and embryonic development of the rete testis. Endocrinology. 2021;162:bqab046. doi: 10.1210/endocr/bqab046. [DOI] [PubMed] [Google Scholar]
- 18.Hess RA, Sharpe RM, Hinton BT. Estrogens and development of the rete testis, efferent ductules, epididymis and vas deferens. Differentiation. 2021;118:41–71. doi: 10.1016/j.diff.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Du J, Wang ZQ, Li FH. The value of sonoelastography scores and the strain ratio in differential diagnosis of azoospermia. J Urol. 2012;188:1861–6. doi: 10.1016/j.juro.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh ML, Huang ST, Huang HC, Chen Y, Hsu YC. The reliability of ultrasonographic measurements for testicular volume assessment:comparison of three common formulas with true testicular volume. Asian J Androl. 2009;11:261–5. doi: 10.1038/aja.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boeri L, Capogrosso P, Ventimiglia E, Cazzaniga W, Pozzi E, et al. Testicular volume in infertile versus fertile white-European men:a case-control investigation in the real-life setting. Asian J Androl. 2021;23:501–9. doi: 10.4103/aja.aja_93_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han BH, Park SB, Seo JT, Chun YK. Usefulness of testicular volume, apparent diffusion coefficient, and normalized apparent diffusion coefficient in the MRI evaluation of infertile men with azoospermia. AJR Am J Roentgenol. 2018;210:543–8. doi: 10.2214/AJR.17.18276. [DOI] [PubMed] [Google Scholar]
- 23.Kavoussi PK, Hudson K, Machen GL, Barsky M, Lebovic DI, et al. FSH levels and testicular volumes are associated with the severity of testicular histopathology in men with non-obstructive azoospermia. J Assist Reprod Genet. 2021;38:3015–8. doi: 10.1007/s10815-021-02313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors:the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 25.Neaves WB, Johnson L, Porter JC, Parker CR, Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59:756–63. doi: 10.1210/jcem-59-4-756. [DOI] [PubMed] [Google Scholar]
- 26.Selice R, Caretta N, Di Mambro A, Torino M, Palego P, et al. Prostate volume and growth during testosterone replacement therapy is related to visceral obesity in Klinefelter syndrome. Eur J Endocrinol. 2013;169:743–9. doi: 10.1530/EJE-13-0488. [DOI] [PubMed] [Google Scholar]
- 27.Busch AS, Tüttelmann F, Cremers JF, Schubert M, Nordhoff V, et al. FSHB -211 G>T polymorphism as predictor for TESE success in patients with unexplained azoospermia. J Clin Endocrinol Metab. 2019;104:2315–24. doi: 10.1210/jc.2018-02249. [DOI] [PubMed] [Google Scholar]
- 28.Boissonnas CC, Jouannet P, Jammes H. Epigenetic disorders and male subfertility. Fertil Steril. 2013;99:624–31. doi: 10.1016/j.fertnstert.2013.01.124. [DOI] [PubMed] [Google Scholar]
- 29.de Mello Santos T, Hinton BT. We, the developing rete testis, efferent ducts, and Wolffian duct, all hereby agree that we need to connect. Andrology. 2019;7:581–7. doi: 10.1111/andr.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivas A, Fisher JS, McKinnell C, Atanassova N, Sharpe RM. Induction of reproductive tract developmental abnormalities in the male rat by lowering androgen production or action in combination with a low dose of diethylstilbestrol:evidence for importance of the androgen-estrogen balance. Endocrinology. 2002;143:4797–808. doi: 10.1210/en.2002-220531. [DOI] [PubMed] [Google Scholar]


