Abstract
Klinefelter syndrome (KS) is the most common genetic cause of human male infertility. However, the effect of the extra X chromosome on different testicular cell types remains poorly understood. Here, we profiled testicular single-cell transcriptomes from three KS patients and normal karyotype control individuals. Among the different somatic cells, Sertoli cells showed the greatest transcriptome changes in KS patients. Further analysis showed that X-inactive-specific transcript (XIST), a key factor that inactivates one X chromosome in female mammals, was widely expressed in each testicular somatic cell type but not in Sertoli cells. The loss of XIST in Sertoli cells leads to an increased level of X chromosome genes, and further disrupts their transcription pattern and cellular function. This phenomenon was not detected in other somatic cells such as Leydig cells and vascular endothelial cells. These results proposed a new mechanism to explain why testicular atrophy in KS patients is heterogeneous with loss of seminiferous tubules but interstitial hyperplasia. Our study provides a theoretical basis for subsequent research and related treatment of KS by identifying Sertoli cell-specific X chromosome inactivation failure.
Keywords: Klinefelter syndrome, nonobstructive azoospermia, Sertoli cell, spermatogenesis, X chromosome inactivation
INTRODUCTION
Klinefelter syndrome (KS), 47,XXY, is one of the most frequent genetic causes of infertility. It occurs in approximately 0.015% of newborn males in the world.1 KS patients exhibit persistent and irreversible degeneration of seminiferous tubules in the testes, which is manifested as a significantly reduced testicular volume since puberty, and the difference becomes even greater by adulthood.2 Within the testes of KS patients, Sertoli cells, as the scaffold of the seminiferous tubules, are completely absent in most areas. However, the remaining Sertoli cells can still construct a seminiferous tubule structure (although the structure exhibits obvious pathological morphology, including reduced cell volume, an increased nucleo-cytoplasmic ratio, and loss of intercellular space) with other somatic cells and even support complete spermatogenesis for mosaicism of XY germ cells.3 This indicates that the dose effect of the X chromosome genes greatly affects the cell fate and testicular morphology. Furthermore, compared with that in Sertoli cells, the damage caused by the extra X chromosome in other testicular cells (such as Leydig cells) and other systems (such as the cardiovascular, hepatobiliary, and respiratory systems) is so mild in KS patients that it is often neglected by doctors. Thus, different types of cells often show different susceptibilities to an extra X chromosome.
The X chromosome inactivation (XCI) and activation are regulated by completely different mechanisms in various biological processes. From embryonic day 7.5 (E 7.5) to E 12.5 of development, primordial germ cells undergo global removal of DNA methylation, leading to a global (including the X chromosome) increase in transcription.4 During meiosis, the unsynapsed X and Y chromosomes are transcriptionally silenced through meiotic sex chromosome inactivation and are sequestered in a cellular domain known as the sex body.5 After the initiation of spermiogenesis, the histone-to-protamine transition and high level of DNA methylation in spermatids also lead to global or independent repression of sex chromosome expression.6,7 In addition to the above mechanisms of X chromosome silencing during spermatogenesis, X-inactive-specific transcript (XIST)-dependent XCI, a more universal XCI mechanism, plays a critical role in balancing the gene dosage between XY and XX cells by silencing one of the two X chromosomes in XX cells.8,9
However, it is certain that some genes are not or are only slightly affected by the XCI process.10,11 These genes escape X inactivation. In women, approximately 15% of X-linked genes are biallelically expressed and most genes have a stable inactivation pattern.12 The inactive state of the X chromosome can be (partially) reversed, and this X chromosome reactivation (XCR) might be associated with disease.13,14 Therefore, we hypothesized that the heterogeneity of damage due to an additional X chromosome in different KS cells may occur because of the failure of the XCI process (a global increase in X gene expression) or the overexpression of some X genes that escape inactivation. Therefore, we profiled and analyzed over 37 000 single-cell transcriptomes from whole testes of patients with obstructive azoospermia (OA; patients with normal spermatogenesis) and KS patients and compared the X gene expression pattern among different testicular cells and within the same cell type. In addition, we also attempted to explain the etiological characteristics of several known XCI mechanisms to increase the understanding of the pathogenesis of azoospermia caused by KS.
PARTICIPANTS AND METHODS
Contact information for reagents and resource sharing
The RNA-seq matrix data can be obtained from the GEO database (GSE149512).15 The data also can be found in MHA database (http://malehealthatlas.cn/).16 Requests for further information, resources, and reagents should be directed to and will be fulfilled by the correspondence (ZL).
Experimental model and participants
The experiments performed in this study were approved by the Ethics Committee of Shanghai General Hospital, Shanghai, China (Approval No. 2016KY196). All patients underwent microdissection testicular sperm extraction (micro-TESE) at Shanghai General Hospital from October 2018 to October 2019. All participants signed consent forms after being fully informed of the goal and characteristics of our study. Fresh testicular tissues were obtained from abandoned tissues after testicular sperm extraction operations performed for the following indications: OA (n = 2), KS (n = 3), and Y chromosome microdeletions of the azoospermia factor a (AZFa) regions (n = 1). All samples were stored at 4°C after surgical excision and treated within 4 h to ensure cell activity. Each sample was equally divided into three parts, of which one part was transformed into single-cell suspension by enzymolysis for single-cell RNA sequencing (scRNA-seq), 4.0 mg ml−1 collagenase type IV (Gibco, New York, NY, USA), 2.5 mg ml−1 hyaluronidase (Sigma-Aldrich, Saint Louis, MO, USA), and 1.0 mg ml−1 trypsin (Sigma-Aldrich) at 37°C for 20 min (Figure 1a). One part was used to Sertoli cell isolation and culture, and the last part was fixed with paraformaldehyde and then embedded in paraffin for histological examination. The karyotypes, genotypes, sex hormone levels, and morphology of seminiferous tubules of OA donors were normal. The karyotypes of three KS samples were XXY without mosaic. The AZFa region microdeletions (AZFa-Del) sample was used as a negative control to exclude germ cell interference. This patient was diagnosed by quantitative polymerase chain reaction (qPCR) examination before hospitalization, which showed that in this sample, sY84 and sY86 were completely deleted. Among all donors, other abnormal genotypes related to spermatogenic disorders were excluded by whole-exome sequencing.
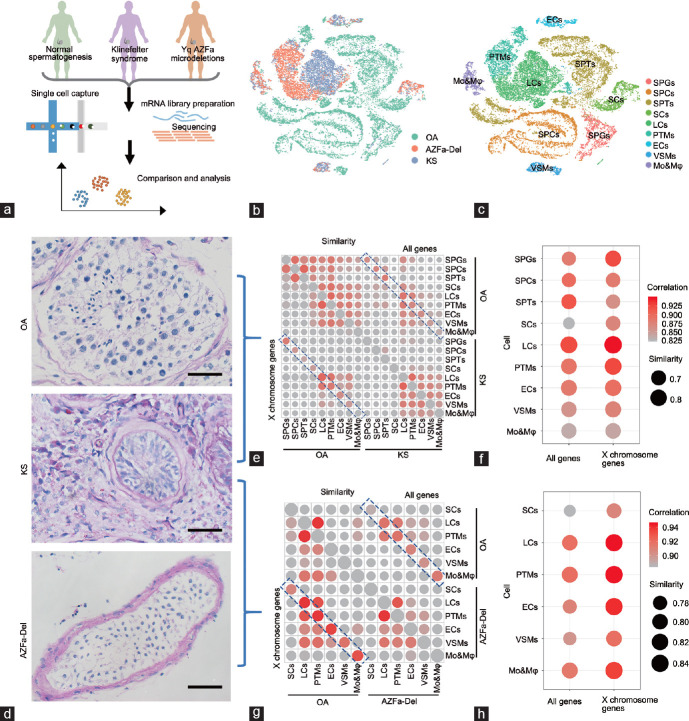
Figure 1.
The global transcriptome pattern of obstructive azoospermia, Klinefelter syndrome, and AZFa-Del testes. (a) Schematic illustration of the study workflow. (b) T-distributed stochastic neighbor embedding plots of all six testicular single-cell samples. Each cell is labeled with a different color according to its sample type. (c) T-distributed stochastic neighbor embedding plots of all six testicular single-cell samples. Each cell is labeled with a different color according to its cell cluster. (d) Glycogen periodic acid–Schiff staining of OA, KS, and AZFa-Del testes illustrated typical pathological changes in their histological morphology. The scale bars represent 50 µm. (e) A bubble diagram shows the similarity between KS and OA. The size of points represents the value of Jaccard distance. The upper right half represents the result when all expressed genes are considered, and the lower left half represents the results when only X chromosome genes are considered. The colors of the points represent the regression coefficients. The size of points represents the value of Jaccard distance. The colors of the points represent the regression coefficients. (f) A bubble diagram shows an enlarged view of the contents of the blue dotted rectangle, illustrating the similarities between the same types of KS and OA cells. The size of points represents the value of Jaccard distance. The colors of the points represent the regression coefficients. (g) A bubble diagram shows the similarity between KS and AZFa-Del. The size of points represents the value of Jaccard distance. The upper right half represents the result when all expressed genes are considered, and the lower left half represents the results when only X chromosome genes are considered. The colors of the points represent the regression coefficients. The size of points represents the value of Jaccard distance. The colors of the points represent the regression coefficients. (h) A bubble diagram shows an enlarged view of the contents of the blue dotted rectangle, illustrating the similarities between the same types of KS and AZFa-Del cells. The size of points represents the value of Jaccard distance. The colors of the points represent the regression coefficients. SPGs: spermatogonia; SPCs: spermatocytes; SPT: spermatids; SCs: Sertoli cells; LCs: Leydig cells; PTMs: peritubular myoid cells; ECs: endothelial cells; VSMs: vascular smooth muscle cells; Mo&Mφ: monocyte and macrophage; OA: obstructive azoospermia; KS: Klinefelter syndrome; AZFa-Del: Y chromosome microdeletions of the AZFa regions; AZFa: azoospermia factor a.
Histological examination
Fresh testicular tissues from donors were fixed in 4% paraformaldehyde for 12–24 h at 4°C, embedded in paraffin, and sectioned. Before staining, tissue sections were dewaxed in xylene, rehydrated using a gradient series of ethanol solutions, and washed in distilled water. Then, the sections were stained with periodic acid–Schiff (PAS)/hematoxylin (Servicebio, Wuhan, China) and dehydrated using increasing concentrations of ethanol and xylene. Sections were allowed to dry before applying neutral resin to the coverslips. The staining images were captured with a Nikon Eclipse Ti-S fluorescence microscope (Nikon, Tokyo, Japan).
DNA-fluorescence in situ hybridization (FISH) of KS Sertoli cells
KS Sertoli cells were isolated by sequential two-step enzyme digestion, as described in our previous study.17 Briefly, testicular tissues were enzymatically digested with 4.0 mg ml−1 collagenase type IV for 5 min to remove most interstitial cells (such as Leydig cells and PTM cells). Then, the tissues were digested with 4.0 mg ml−1 collagenase type IV, 2.5 mg ml−1 hyaluronidase, and 1.0 mg ml−1 trypsin at 37°C for 20 min to divide the remaining tissue into single cells. After adherent growth for 24 h, these cells were redigested with 4.0 mg ml−1 collagenase type IV for 1 min to remove germ cells. The remaining adherent Sertoli cells were digested with trypsin and fixed with 4% paraformaldehyde. DNA-FISH was performed according to the guidelines of the CEP X SpectrumOrange/Y SpectrumGreen DNA Probe Kit (Abbott, Chicago, IL, USA). In brief, after denaturation of the specimen DNA at 73°C for 5 min, 10 μl of the probe solution was incubated with the slide at 42°C for 30 min. After washing with SSC buffer (sodium chloride and sodium citrate), nuclei were labeled with 4’,6-diamidino-2-phenylindole (DAPI; Solarbio, Shanghai, China) by incubating tissue sections for 15 min. Images were captured with an OLYMPUS IX83 confocal microscope (OLYMPUS, Tokyo, Japan).
Mapping, sample quality control, and integration of scRNA-seq data analysis
Single-cell isolation and RNA-seq library preparation were described in our previous study.15 The raw data are labeled as GSE149512. After mapping, a Seurat object was created using the gene expression matrix through the Seurat package in R (Alcatel-Lucent, Jasmine Hill, NJ, USA). Cells were further filtered according to the following threshold parameters: total number of expressed genes, 500–9000; total unique molecular identifier (UMI) count, between negative infinity and 35 000; and proportion of mitochondrial genes expressed, <40%. Normalization was performed according to the software package manual (https://satijalab.org/seurat/v3.1/pbmc3k_tutorial.html). Batch correction was performed using the IntegrateData function in the Seurat package.
Cell identification and clustering analysis
After batch correction, the merged Seurat objects were scaled and analyzed by principal component analysis. Then, the first 20 principal components (PCs) were used to construct a K-nearest-neighbor (KNN) graph and refine the edge weights between any two cells. Based on all of the local cell neighborhoods, the FindClusters function with a resolution parameter of 0.1 was used to cluster the cells. In total, 17 clusters were identified, and these clusters were renamed according to accepted marker genes. The first 20 PCs were also used to perform nonlinear dimensional reduction by tSNE, and the dimension reduction plots were produced as output.
Similarity and correlation of testicular cells between KS, OA and AZFa-Del in pairs
The expression of each gene was treated as an independent variable. Similarities were represented with the Jaccard index, which was calculated with the vegan 2.5-6 R package. The result is shown as 1−Jaccard distance. The correlations were calculated with the function cor (object, method = spearman) in the stats 3.6.0 R package.
Identification of differentially expressed genes (DEGs)
The Seurat FindAllMarkers function (test.use = “wilcox”) is based on the normalized UMI count and was used to identify unique cluster-specific marker genes. Only the genes that were detected in at least 10% of the cells were tested, and the average log2(fold change) threshold was set as 0.25, 0.5, or 1 as needed.
Upstream regulators, canonical pathways, and bio-function analysis
Ingenuity pathway analysis (IPA) was used to analyze the upstream regulators, canonical pathways, and bio-function differences between KS and OA Sertoli cells. A total of 1333 DEGs with a change of over 0.25 log2(fold change) and with P < 0.05 were input into IPA. IPA terms with P < 0.05 and z > 2 or z <−2 were considered significant.
Prediction and analysis of candidate transcriptional regulators of XIST
The first 3000 bases at the transcription initiation site of XIST were considered the regulatory binding region, and 23 potential transcription regulators were predicted through the PROMO database (https://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3; last accessed on 05 December 2022). Then, the binding sites of these transcription factors were found and scored using the JASPAR database.
Transcription factor network construction
A total of 1469 human transcription factors in the AnimalTFDB data set and a gene expression matrix of OA and KS Sertoli cells were used as input for the ARACNe-AP software. The results of the ARACNe-AP analysis were input into the ssmarina (version 1.01) R package, which further calculated the marina objects containing the normalized enrichment scores, P values, and a specific set of regulators.
RESULTS
Global transcriptome profiling showed that Sertoli cells underwent the greatest change in KS patients
To understand the global changes in each type of testicular cell in KS patients, we merged the single-cell expression data of the KS (3 patients) group with those of the OA (2 patients with normal spermatogenesis) and Y chromosome AZFa-Del (1 patient) groups. The sex hormone levels of the two OA donors were within the normal range. All three KS donors and the AZFa-Del donor had higher levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), but their testosterone (T) and estradiol levels were within the normal ranges (Supplementary Figure 1a (170KB, tif) ). In total, 27 597 OA cells, 8267 KS cells, and 5434 AZFa-Del cells were profiled. On average, 9359 reads (UMIs), 2719 expressed genes, and 3.7% mitochondrial genes were detected per cell; however, different types of cells showed high heterogeneity. In general, germ cells exhibited more active transcription and a lower proportion of mitochondrial genes (Supplementary Figure 2a (478.5KB, tif) ). T-distributed stochastic neighbor embedding (tSNE) analysis showed that cells from the three types of patients had markedly different 2D spatial distributions, but good repeatability was demonstrated within the patient groups (Figures 1b and Supplementary Figure 2c (478.5KB, tif) ). In total, nine cell clusters were identified in the whole cell population based on the expression of known cell type-specific markers (Figure 1c, Supplementary Figure 2b (478.5KB, tif) , and Supplementary Table 1 (533.9KB, pdf) ).
The PAS/hematoxylin staining results showed that the OA testes exhibited normal morphology. In the AZFa-Del testis, seminiferous tubules showed Sertoli cell-only syndrome with a Johnsen score of 2 and exhibited vascular basement membrane thickening without interstitial proliferation. In addition, the morphology of these Sertoli cells was similar to that of OA testes. Unlike other types of nonobstructive azoospermia, the typical pathological changes in different regions of KS testes showed significant heterogeneity. In KS testes, most seminiferous tubules exhibited more severe atrophy, with Johnsen scores of 0–1 (germ cells were completely absent, and Sertoli cells were also reduced with abnormal morphology), and exhibited vascular basement membrane thickening with severe interstitial proliferation. However, a small number of larger and opaque seminiferous tubules with incomplete or even normal spermatogenesis were still present (Figure 1d and Supplementary Figure 1b (170KB, tif) ).
Then, to further assess the effects of an extra X chromosome on various cell types, we calculated the Jaccard distance and correlations among nine cell types between KS and OA patients and found that Sertoli cells had the least similarity (largest Jaccard distance) and lowest correlation (smallest r index) compared with other cell types (Figure 1e and 1f). When only the X chromosome genes were considered, Sertoli cells were still one of the most variable cell clusters (Figure 1f). In addition, to exclude the potential effects of germ cells on somatic cells, we also compared KS cells with AZFa-Del cells using the same method and obtained similar results (Figure 1g and 1h). These results indicated that Sertoli cells underwent the greatest change of any cell type in the presence of an extra X chromosome.
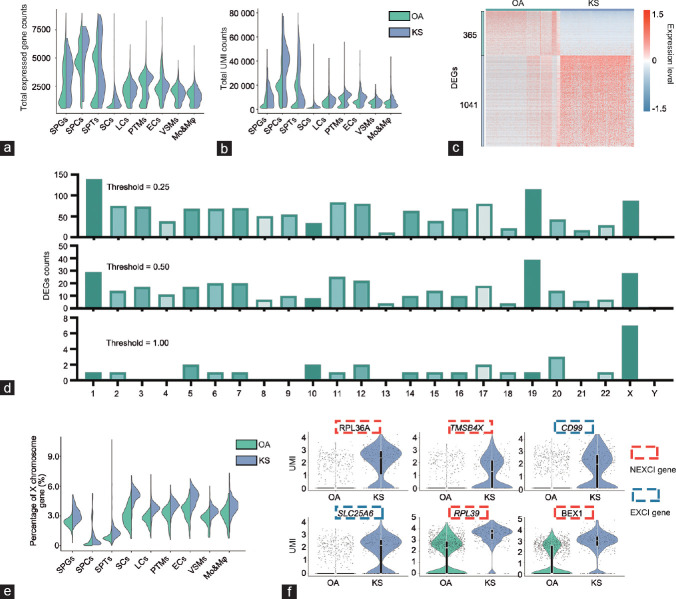
Transcriptional changes in KS Sertoli cells mainly occurred on the X chromosome genes
Because Sertoli cells underwent the greatest change among the nine cell clusters in KS patients, we then focused on these cells and further analyzed the changes that occurred. In total, we obtained 1062 OA Sertoli cells, 434 AZFa-Del Sertoli cells, and 262 KS Sertoli cells. The global transcriptional level of Sertoli cells was the lowest among other cell types. The total number of expressed genes and total UMI count per cell in KS Sertoli cells were higher than those in OA Sertoli cells. This result was also observed in germ cells but not in other somatic cells (Figure 2a and 2b). When the fold change threshold was set to 0.25, we observed 365 downregulated and 1041 upregulated DEGs in KS Sertoli cells compared with OA Sertoli cells (Supplementary Table 2 (286.8KB, pdf) ), and most of these DEGs were located on chromosome 1 (139 genes), chromosome 19 (115 genes), and the X chromosome (87 genes), as shown in Figure 2c. When the fold change threshold was increased to 0.5, most of the DEGs were still located on these chromosomes. However, when the fold change threshold was further increased to 1, only 29 DEGs were found, and seven were located on the X chromosome. This proportion was much higher than that observed for autosomes (Figure 2d). This result indicated that the extra X chromosome had a significant dose effect on gene expression levels; however, the dosage effect in other somatic cells was markedly weaker than that in Sertoli cells (Figure 2e). As expected, the expression levels of these seven X chromosome DEGs were higher in KS patients than those in OA patients. Interestingly, among these X chromosome DEGs, CD99 and solute carrier family 25 member 6 (SLC25A6) are encoded by the pseudo-autosomal region (PAR) and would be expected to escape XCI (EXCI genes), with expression proportional to sex chromosome number; however, ribosomal protein L36a (RPL36A), thymosin beta 4 X-linked (TMSB4X), ribosomal protein L39 (RPL39), brain expressed X-linked 1 (BEX1), and glucose-6-phosphate dehydrogenase (G6PD) did not escape from XCI (NEXCI genes) according to a previous study on females (Figure 2f).18 These results indicated that some NEXCI genes also escaped from XCI in KS Sertoli cells, leading to an increased X chromosome expression level in KS Sertoli cells.
Figure 2.
Comparison of global and X chromosome gene expression patterns between obstructive azoospermia and Klinefelter syndrome Sertoli cells. (a) Violin plot shows the total expressed gene counts of Sertoli cells; the column is divided according to cell type, including OA and KS cells. (b) Violin plot shows the total unique molecular identifier counts of Sertoli cells; the column is divided according to cell type, including OA and KS cells. (c) Heatmap shows DEGs between OA and KS Sertoli cells. DEG counts are shown on the left of the color bar, which indicates the cell type annotation. (d) The number of DEGs between OA and KS Sertoli cells located on each chromosome under different thresholds are shown. (e) A violin plot shows the percentage of X chromosome gene expression in Sertoli cells; the column is divided into OA and KS cells. (f) Violin plot shows the expression of the top six DEGs located on the X chromosome of Sertoli cells. The color of the rectangular box represents whether this gene can escape from XCI according to a previous study on females. SPGs: spermatogonia; SPCs: spermatocytes; SPT: spermatids; SCs: Sertoli cells; LCs: Leydig cells; PTMs: peritubular myoid cells; ECs: endothelial cells; VSMs: vascular smooth muscle cells; Mo&Mφ: monocyte and macrophage; OA: obstructive azoospermia; KS: Klinefelter syndrome; XCI: X chromosome inactivation; EXCI genes: genes that escape from XCI; NEXCI genes: genes that not escape from XCI; UMI: unique molecular identifier; DEG: differentially expressed genes.
Failure of XCI in Sertoli cells
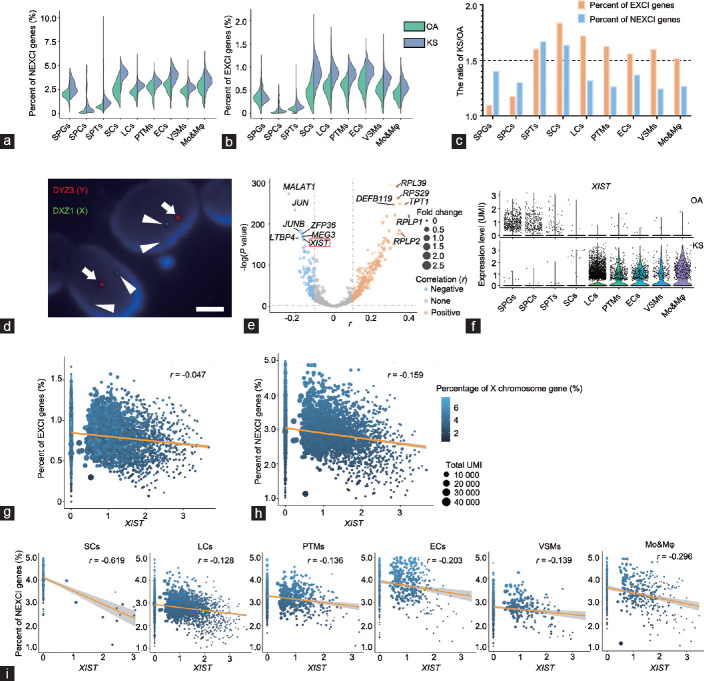
To further understand why KS Sertoli cells underwent a greater increase in the X gene level than OA Sertoli cells among somatic cells, we calculated the percent of EXCI gene and NEXCI gene expression in nine clusters of cells. As expected, the percentage of EXCI genes in all six types of KS somatic cells increased at least 1.5-fold but less than 2-fold compared with OA cells, indicating that the X chromosome gene expression level and X chromosome number were not strictly linearly correlated. However, the percentage of NEXCI gene expression in KS Sertoli cells was also 1.5 times higher than that in OA cells, and this result was not found in the other five somatic cell types, even though the percentage of NEXCI gene expression in these cells was also increased in KS cells. All of these results indicated that Sertoli cells have different levels of ability to silence the extra X chromosome compared with other somatic cells (Figure 3a–3c).
Figure 3.
The relationship between X chromosome gene expression patterns and XIST expression levels in Klinefelter syndrome. (a) Violin plot shows the percent of NEXCI genes in Sertoli cells. The column is divided according to cell type, comparing between OA and KS samples. (b) Violin plot shows the percent of EXCI genes in Sertoli cells. The column is divided according to cell type, comparing between OA and KS samples. (c) The ratio of NEXCI gene or EXCI gene expression levels between KS and OA cells. (d) DNA-fluorescence in situ hybridization shows the karyotype of sex chromosomes of KS Sertoli cells. The long arrow indicates the DYZ3 region of the Y chromosome (labeled by red fluorescence), and the triangular arrow indicates the DXZ3 region of the X chromosome (labeled by green fluorescence). The scale bar represents 0.5 µm. (e) Volcano plot shows the correlation between NEXCI gene expression and that of each gene in all KS testicular cells. The X-axis represents the r value of the correlation coefficient. The Y-axis represents the P value. The sizes of the points represent the fold change in the gene expression level in KS cells compared with OA cells. (f) Violin plot shows the expression level of XIST in each cell cluster of OA (upper panel) or KS (lower panel) cells. (g) Regression analysis shows the correlation between XIST expression and the EXCI gene expression level in total KS somatic cells. The sizes of the points represent the total unique molecular identifier count of the cells, and the color represents the percentage of X chromosome gene expression. (h) Regression analysis shows the correlation between XIST expression and the NEXCI gene expression level in total KS somatic cells. The sizes of the points represent the total unique molecular identifier count of the cells, and the color represents the percentage of X chromosome gene expression. (i) Regression analysis shows the correlation between XIST expression and NEXCI gene expression level in different clusters of KS somatic cells. The sizes of the points represent the total unique molecular identifier count of the cells, and the color represents the percentage of X chromosome gene expression. XIST: X-inactive-specific transcript; SPGs: spermatogonia; SPCs: spermatocytes; SPT: spermatids; SCs: Sertoli cells; LCs: Leydig cells; PTMs: peritubular myoid cells; ECs: endothelial cells; VSMs: vascular smooth muscle cells; Mo&Mφ: monocyte and macrophage; OA: obstructive azoospermia; KS: Klinefelter syndrome; XCI: X chromosome inactivation; EXCI genes: genes that escape from XCI; NEXCI genes: genes that not escape from XCI; UMI: unique molecular identifier; DYZ: DYZ3 alpha satellite DNA probe corresponding to Yp11.1-q11.1 labeled with spectrumOrange; DXZ: DXZ1 alpha satellite DNA probe corresponding to Xp11.1-g11.1 labeled with spectrumGreen.
Next, we identified the karyotype of KS Sertoli cells to determine whether the reason for this phenomenon was that KS Sertoli cells contained more than two X chromosomes. However, the DNA-FISH results showed that the karyotype of all Sertoli cells of all three patients (59, 37, and 41 Sertoli cells, respectively) in this study was 47,XXY (Figure 3d), indicating that the overexpression of X chromosome genes was not because of multiple chromosomes (48,aja202315Y or other karyotypes). Next, we calculated the correlation between the expression level of each gene and the percentage of NEXCI gene expression in KS somatic cells (including Sertoli cells) to identify the universal upstream or downstream regulatory genes that are most closely related to XCI. As a result, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), Jun proto-oncogene (JUN), JunB proto-oncogene (JUNB), latent transforming growth factor beta-binding protein 4 (LTBP4), ZFP36 ring finger protein (ZFP36), maternally expressed 3 (MEG3), and XIST showed the highest negative correlation with NEXCI gene expression (Figure 3e). XIST was the first noncoding gene identified within the X inactivation center (XIC). It is expressed exclusively from the XIC of the inactive X chromosome, and is essential for the initiation and spread of XCI. Except for Sertoli cells, KS somatic cells had a significantly higher level of XIST than OA cells (Figure 3f). In somatic cells (including Sertoli cells), the XIST expression level was negatively correlated with the percentage of NEXCI gene expression (r = −0.159, P = 1.24×10−4; Figure 3h); however, its correlation with the percentage of EXCI genes was not strong (r = −0.047, P = 0.015; Figure 3g). The expression level of XIST was low in KS Sertoli cells. However, to answer the question of whether XIST affects NEXCI gene expression in these cells, we isolated KS Sertoli cells and found that XIST expression was strongly negatively correlated with the percentage of NEXCI gene expression (r = −0.619, P = 1.24 × 10−6), and this negative correlation was also observed in other somatic cells (Figure 3i). Furthermore, we also calculated the expression percent of the X chromosome gene and the expression level of XIST in each patient, and found a similar expression pattern in different KS patients (Supplementary Figure 3a (137.6KB, tif) and 3b (137.6KB, tif) ). These results indicate that XIST is a universal silencing factor for NEXCI gene expression in KS somatic cells, including Sertoli cells, and the abnormal increase in NEXCI gene expression in KS Sertoli cells may be caused by low XIST expression.
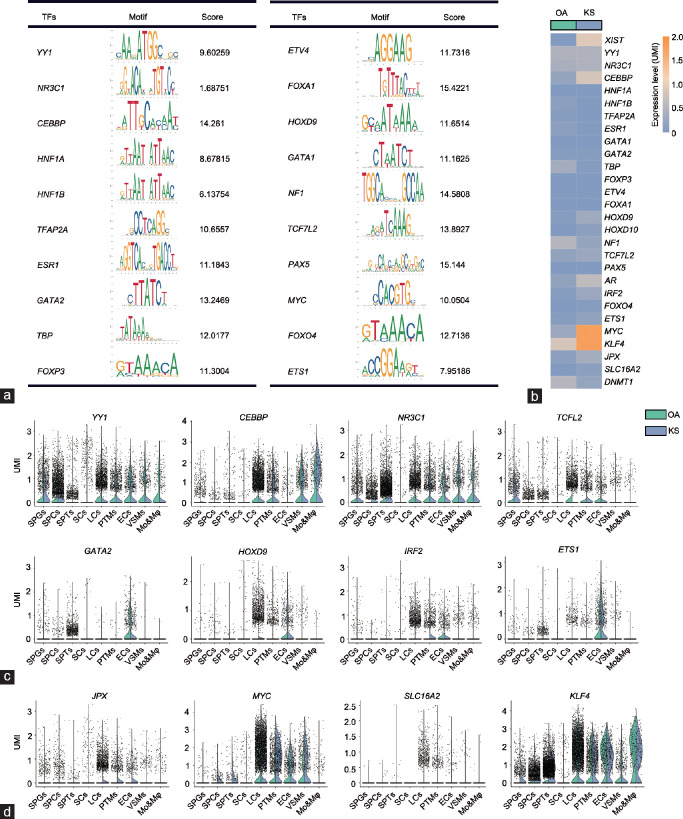
Identification of known and novel candidate regulators of XIST expression in KS testicular cells
To explore why KS Sertoli cells expressed the lowest level of XIST among the nine clusters, we further predicted and analyzed the regulators of XIST. In total, 20 candidate regulators were found through the PROMO dataset, and forkhead box A1 (FOXA1), paired box 5 (PAX5), neurofibromin 1 (NF1), CCAAT enhancer-binding protein beta (CEBPB), and GATA-binding protein 2 (GATA2) showed the top binding scores for the promoter region of XIST (Figure 4a). Among these new candidates and other known regulators such as MYC proto-oncogene (MYC), JPX transcript (JPX), and KLF transcription factor 4 (KLF4), CEBPB, homeobox D9 (HOXD9), androgen receptor (AR), and MYC were upregulated in KS cells, but TATA-box-binding protein (TBP), NF1, and DNA methyltransferase 1 (DNMT1) were downregulated in KS Sertoli cells (Figure 4b). After regulators with low expression levels were filtered out, we found eight new candidate regulators that showed a cell type-specific expression pattern. Specifically, YY1 transcription factor (YY1), nuclear receptor subfamily 3 group C member 1 (NR3C1), and transcription factor 7 like 2 (TCF7L2) were expressed in both germ cells and somatic cells, and CREB-binding protein (CREBBP), GATA2, HOXD9, interferon regulatory factor 2 (IRF2), and ETS proto-oncogene 1 (ETS1) were only expressed in one or some types of somatic cells. Interestingly, all of the predicted regulators showed no or extremely low expression in Sertoli cells (Figure 4c). In addition, some reported regulators of XIST also showed an “absent in Sertoli cells” expression pattern (Figure 4d). However, the expression of most of these regulators was higher in somatic cells than that in germ cells. The cell type-specific pattern of these XIST regulators may be related to the loss of XIST expression in Sertoli cells and germ cells.
Figure 4.
Candidate regulators of XIST. (a) Twenty candidate transcription regulators and their binding motifs within −3 kb of the transcriptional start site of the XIST gene. The score was calculated using the JASPAR dataset. (b) Heatmap showing the expression level of XIST and its regulators in OA and KS Sertoli cells. (c) A violin plot shows the expression level of XIST regulators predicted by the PROMO dataset in each cell cluster of OA and KS cells. (d) A violin plot shows the expression level of XIST regulators validated by previous studies in each cell cluster of OA and KS cells. XIST: X-inactive-specific transcript; SPGs: spermatogonia; SPCs: spermatocytes; SPT: spermatids; SCs: Sertoli cells; LCs: Leydig cells; PTMs: peritubular myoid cells; ECs: endothelial cells; VSMs: vascular smooth muscle cells; Mo&Mφ: monocyte and macrophage; OA: obstructive azoospermia; KS: Klinefelter syndrome; UMI: unique molecular identifier.
Pathological changes in biological processes in KS Sertoli cells
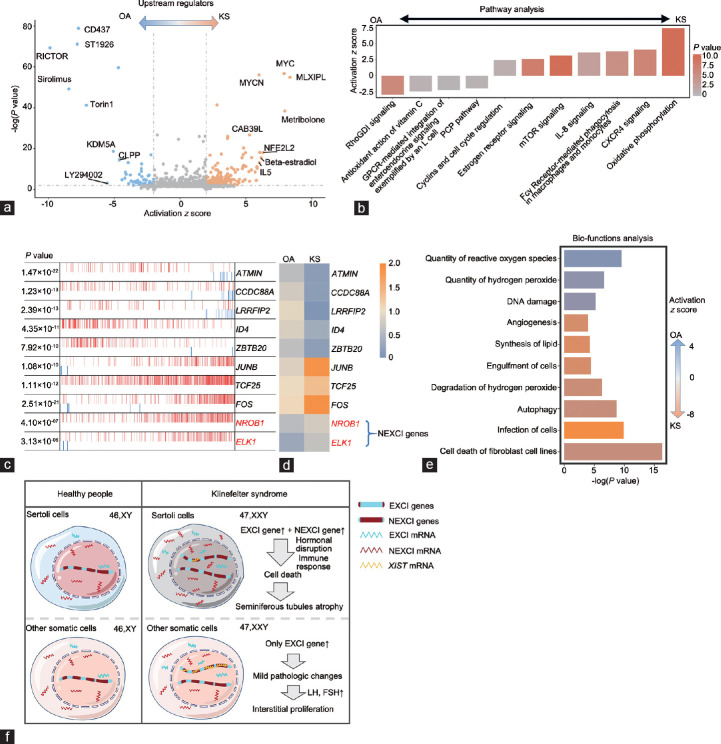
Sertoli cells function as scaffolds of seminiferous tubules and “nurse” cells of spermatogenesis and therefore are one of the most important components of the spermatic environment. The pathological changes in KS Sertoli cells were directly related to the phenotype of KS testes (seminiferous tubule atrophy and failure of spermatogenesis). To understand these changes, we first predicted the upstream signaling with IPA based on the DEGs between OA and KS Sertoli cells. Among these signaling pathways, we found that metribolone (a synthetic nonaromatizable androgen and anabolic steroid), β-estradiol, and interleukin (IL)-5 contributed to a strong activation effect (activation z score >2) in KS Sertoli cells, indicating that an abnormal inflammatory response and hormone disruption occurred in these cells. Consistent with signaling activation in the KS phenotype, immunosuppressant-associated drugs such as sirolimus19 and CLPP20 produced negative signaling with regard to the KS phenotype in Sertoli cells (Figure 5a). Next, we analyzed the changes in pathways in KS Sertoli cells, and IPA results showed that “estrogen receptor signaling” and inflammatory response terms such as “CXCR4 signaling”, “Fcγ receptor-mediated phagocytosis in macrophage and monocytes”, and “IL-8 signaling” were activated in KS Sertoli cells (Figure 5b). Considering that some KS patients have symptoms of androgen deficiency (the androgen levels of all three KS patients were in the low-to-normal range, but the gonadotrophic hormone levels were higher than the normal range), abnormal enrichment of “estrogen receptor signaling” in KS Sertoli cells may also be caused by Leydig cell dysfunction. Therefore, we also examined changes in the androgen synthesis pathway in KS Leydig cells. Interestingly, most genes related to testosterone and dihydrotestosterone synthesis were upregulated in KS cells compared with normal adult cells (Supplementary Figure 4 (149.7KB, tif) ), which could be caused by the high gonadotropin levels in KS patients.
Figure 5.
Changes in the regulatory pathway and bio-function in Klinefelter syndrome Sertoli cells. (a) Volcano plot shows upstream signaling, which may induce the differences between OA and KS Sertoli cells. (b) Ingenuity pathway analysis (IPA) pathway terms that are enriched and decreased in KS Sertoli cells are shown as a bar plot. The Y-axis represents the z score, and the red gradient indicates low-to-high P values. (c) The top 10 candidate master regulators that may induce the differences between OA and KS Sertoli cells were identified by the Master Regulator Inference Algorithm (MARINa). In the MARINa plots, activated targets are colored red, and repressed targets are colored blue for each potential master regulator (vertical lines on the X-axis). On the X-axis, genes were rank-sorted by their differential expression in OA and KS Sertoli cells from left to right. The P values on the left indicate the significance of enrichment. (d) Heatmap showing the expression levels of top 10 regulators in OA and KS Sertoli cells. (e) IPA bio-function terms enriched or decreased in KS Sertoli cells are shown as a bar plot. The X-axis represents the P value, and the gradient from orange to blue indicates KS to OA bias activation. (f) The schematic diagram shows the main pathologic changes and associated mechanism in KS somatic cells. OA: obstructive azoospermia; KS: Klinefelter syndrome; XCI: X chromosome inactivation; EXCI genes: genes that escape from XCI; NEXCI genes: genes that not escape from XCI; LH: luteinizing hormone; FSH: follicle-stimulating hormone.
To investigate the master regulators and construct the transcriptional regulatory network during the pathological state of KS, we utilized the Algorithm for the Reconstruction of Accurate Cellular Networks (ARACNe) method to analyze all 1469 known transcription factors from the Animal Transcription Factor Database. Compared with those in OA Sertoli cells, the expression levels of ATMIN, CCDC88A, LRRFIP2, ID4, and ZBTB20 decreased, and the expression levels of JUNB, TCF25, FOS, NR0B1, and ELK1 increased in KS Sertoli cells (Figure 5c and 5d), indicating possible regulatory roles in the pathological process.
When specific bio-function changes were examined, we noticed that terms such as “cell death of fibroblast cell lines”, “infection of cells”, “autophagy”, and “synthesis of lipid” were enriched in KS Sertoli cells, and terms such as “quantity of reactive oxygen species”, “quantity of hydrogen peroxide”, and “DNA damage” were enriched in OA Sertoli cells (Figure 5e). In general, these results described the changes in upstream signaling, pathways, regulators, and bio-functions in KS Sertoli cells (Figure 5f).
KS germ cells with low XIST expression were in the maintenance phase of XCI
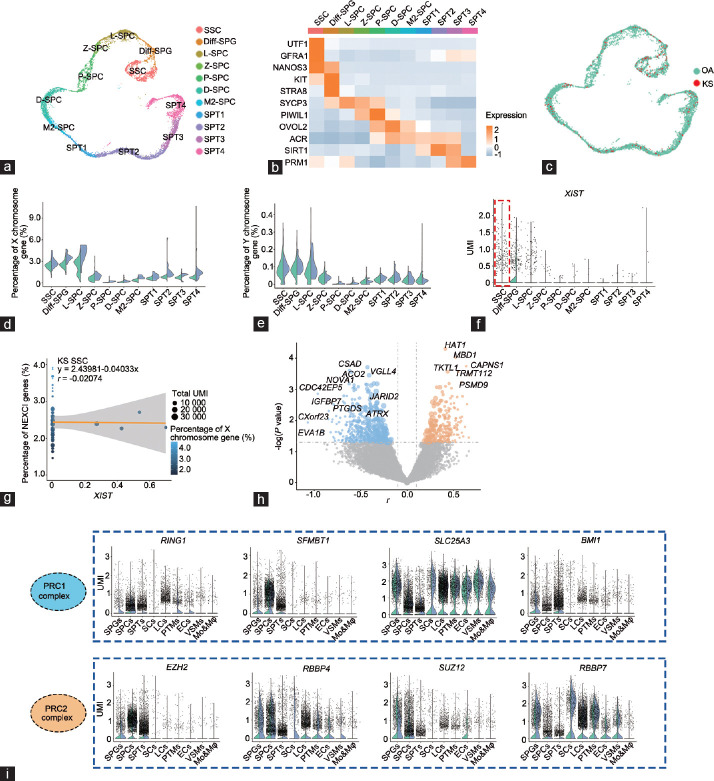
In the above analysis, we noticed that both Sertoli cells and germ cells expressed XIST at low levels (Figure 3f); however, the percentage of X chromosome genes in spermatogonia (SPGs) and spermatocytes (SPCs) in KS patients were not as high as those in KS Sertoli cells. To explain this paradox, we further analyzed other mechanisms in germ cells that may affect X chromosome activation. Germ cells were further divided into 11 clusters according to known markers (Figure 6a and 6b), and each cluster contained KS cells, which showed little difference from OA cells (Figure 6c). In addition, the change in the expression level of sex chromosome genes during germ cell development was far greater than the difference between KS and OA cells. During meiosis, the expression of sex chromosome genes was extremely limited in both OA and KS cells (Figure 6d and 6e), indicating that the meiotic silencing of the unsynapsed chromatin (MSUC) mechanism has an important and effective function in both X chromosomes in KS germ cells. Interestingly, XIST was mainly expressed in spermatogonial stem cells (SSCs) and SPGs; and in SSCs, the XIST expression level in KS cells was not higher than that in OA cells (Figure 6f). Unlike KS somatic cells, no significant correlation was found between the XIST expression level and the percentage of NEXCI gene expression (r = −0.04033, P = 0.63), indicating that XIST may not play a key role in XCI in KS SSCs (Figure 6g). In addition, we found no difference in NEXCI gene expression levels between OA and KS cells, indicating that there are other mechanisms that regulate the expression of X gene in KS SSCs. Therefore, we assessed the correlations between the percentage of X chromosome gene expression and the expression level of each gene in KS SSCs and found that CXorf23, CSAD, ACO2, PTGDS, NOVA1, and IGFBP7 showed negative correlations (Figure 6h). In addition, the expression levels of CSAD, PTGDS, NOVA1, and IGFBP7 in KS SSCs were higher than those in OA SSCs, indicating that these genes may be candidate regulators of the X chromosome in KS SSCs. In addition to these candidate regulators, we also noticed that JARID2, which is implicated in the initial XIST-induced targeting of PRC2 to the inactive X chromosome,21 also showed a significant correlation with NEXCI gene expression levels in KS SSCs (Figure 6h). During the initiation and maintenance phases of XCI, the PRC1 and PRC2 complexes are recruited and bind to the X chromosome and help to lock in the inactive state of the X chromosome.22 Interestingly, nearly all parts of the PRC1 and PRC2 complexes showed a germ cell-enriched expression pattern, and SFMBT1, BMI1, EZH2, and SUZ12 exhibited low expression levels in somatic cells (Figure 6i). In addition, SLC25A3 and RBBP4 were highly expressed in KS Sertoli cells but not in normal Sertoli cells (Figure 6i). Based on these results, we speculated that even though XIST was expressed at low levels in both KS Sertoli cells and germ cells, the latter still showed a relatively low level of X gene expression, which may be associated with a germ cell-specific PRC1/2-dependent XCI mechanism.
Figure 6.
The regulation of X chromosome gene expression patterns in Klinefelter syndrome germ cells. (a) T-distributed stochastic neighbor embedding plots of OA and KS germ cells. Each cell is labeled with a different color according to its cell cluster. (b) Heatmap showing the expression level of known stage-specific markers during human spermatogenesis in OA and KS germ cells. (c) T-distributed stochastic neighbor embedding plots of OA and KS germ cells. Each cell is labeled with a different color according to its sample type. (d) Violin plot shows the percentage of X chromosome gene expression levels in each germ cell subpopulation of OA and KS cells. (e) Violin plot shows the percentage of Y chromosome gene expression levels in each germ cell subpopulation of OA and KS cells. (f) Violin plot shows the XIST gene expression levels in each germ cell subpopulation of OA and KS cells. (g) Regression analysis shows the correlation between XIST expression and NEXCI gene expression levels in KS SSCs. (h) Volcano plot shows the correlation between NEXCI genes and each gene expression level in all KS SSCs. The X-axis represents the r value of the correlation coefficient. The Y-axis represents the P value. The sizes of the points represent the fold change of the gene expression level in KS cells compared with OA cells. (i) A violin plot shows the expression of the PRC1 and PRC2 complex in OA and KS germ cells. SSC: spermatogonial stem cells; Diff-SPG: differentiating or differentiated spermatogonia; L-SPC: leptotene spermatocytes; Z-SPC: zygotene spermatocytes; P-SPC: pachytene spermatocytes; D-SPC: diplotene spermatocytes; M2-SPC: secondary spermatocytes; SPT1–4: four stages of spermatids; OA: obstructive azoospermia; KS: Klinefelter syndrome; XCI: X chromosome inactivation; NEXCI genes: genes that not escape from XCI; UMI: unique molecular identifier; PRC1: polycomb repressor complex 1; XIST: X-inactive-specific transcript.
DISCUSSION
Klinefelter syndrome is a common cause of male infertility and affects up to 3.1% of the infertile male population and approximately 10% of azoospermia patients.23,24 Testosterone replacement can improve the quality of life but has no positive effect on infertility, indicating that androgen deficiency may not be the main cause of spermatogenesis failure in KS patients. The low androgen levels in KS patients may be caused by a decreased number or dysfunction of Leydig cells. However, approximately 75% of adult males with KS are misdiagnosed because of normal fertility and a mild disease phenotype. In addition, most patients with azoospermia caused by KS (72%) have the possibility of sperm retrieval through microdissection and testicular sperm extraction.23 These findings indicate that the dosage effect of the X chromosome may affect the progression and prognosis of KS. The dosage effect of the X chromosome in KS includes at least three aspects: (1) high-grade chromosome aneuploidies (48,aja202315Y; 48,XXYY; or 49,aja202315XY) or 46,XY/47,XXY mosaicism; (2) differences in sensitivities to X chromosome genes; and (3) differences in the ability to silence the extra X chromosome. Among these factors, the karyotype is arguably the most critical. It has been reported that only 46,XY SSCs can enter meiosis.3 In this study, all three of our patients underwent successful sperm extraction, suggesting possible germ cell mosaicism. However, the DNA-FISH results showed that nearly all somatic cells in the seminiferous tubules, as well as those in vitreous degeneration lesions, had a karyotype of 47,XXY. With traditional RNA sequencing (RNA-seq), it is difficult to clearly describe the heterogeneous phenotype and explore the potential mechanism within KS testicular cells. Here, we profiled over 35 000 testicular single-cell transcriptomes from OA and KS patients and observed the heterogeneity of susceptibility to an extra X chromosome and the ability to counter X gene overload within KS testicular cells.
Except in cells undergoing meiosis, XCI triggered by XIST is the most important regulatory mechanism of X chromosome silencing in XX cells. XIST RNA is transcribed in the XIC and then spreads to spatially close sites and eventually to the entire X chromosome.8 During the silencing maintenance phase, XIST RNA recruits silencing complexes, such as polycomb repressor complex 1 (PRC1), PRC2, and Jarid2 to lock the inactive state of the post-XCI chromosome.8,25 Previous studies have mainly focused on females, and researchers have found and identified approximately 15% of X chromosome genes that may escape from XCI.12 Our study obtained a similar result that most EXCI genes were upregulated in KS somatic cells; however, the percentage of NEXCI gene expression in KS Sertoli cells was also increased over 1.5-fold. Furthermore, XIST showed low expression levels in KS Sertoli cells; however, in the few KS Sertoli cells that expressed XIST, a significant negative correlation was found between the XIST expression level and the percentage of NEXCI gene expression, indicating that the decreased XCI ability in KS Sertoli cells was caused by the quantity of XIST expression but not the dysfunction of the XIST mechanism. In addition, premeiotic germ cells also expressed XIST at low levels, similar to those in Sertoli cells, but they also expressed high levels of PRC1/2 complexes. In these cells, XIST expression was not negatively correlated with the percentage of NEXCI gene expression. Although the recruitment of PRC2 to the X chromosome is directly or indirectly dependent on XIST, a significant spatial separation of PRC2 proteins and XIST was observed.26 Furthermore, no PRC2 factors were found in a recent study that purified XIST interacting partners.27 Therefore, we speculated that premeiotic germ cells were in an XIST-independent XCI maintenance phase. We found that XCI was completely dominated by a different mechanism in meiotic cells, and MSUC played a critical role in both OA and KS cells.
XIST is tightly controlled by a series of pluripotency factors, trans-acting factors, and cis-regulators in an X dosage-sensitive manner. For example, YY1, which has been implicated as a trans-acting activator of XIST expression and helps to tether XIST RNA to the X chromosome during XCI,28 was widely expressed in different types of testicular cells but not in Sertoli cells. JPX, which enhances XIST expression in cis,29 was expressed in KS somatic cells but not in KS Sertoli cells and normal somatic cells. In addition, we found that most XIST regulators showed a “low expression in Sertoli cells” pattern, indicating the presence of one or more common upstream regulatory signaling disorders in KS Sertoli cells. Overexpression of XIST in Sertoli cells is a potential treatment for KS patients; however, XIST RNA is too long (19 kbp) to embed in a vector. Therefore, a more feasible treatment may be to target the upstream signaling of XIST. In addition, although KS Sertoli cells lack XIST, the NEXCI genes were not increased by twofold in these cells, indicating the presence of other potential X-silencing mechanisms such as the PRC1/2 complex.
The pathological changes in KS Sertoli cells may be caused by abnormal estrogen receptor signaling and the immune response, as shown by pathway analysis. Furthermore, according to the MGI database (http://www.informatics.jax.org/), we found 14 NEXCI DEGs between OA and KS Sertoli cells with fertility-associated phenotypes in a mouse model (Supplementary Table 3). For example, TIMP-1 functions as a coregulator of basal testicular steroidogenesis, and mice with mutations in this gene have higher testis weights than age-matched wild-type males.30 Ubiquitously transcribed tetratricopeptide repeat protein X-linked (UTX; H3K27me3 demethylase) may cause downregulation of lactate and cholesterol metabolism, thus affecting affects the nursing functions of XX/Sry Sertoli cells.31 HPRT1 plays a central role in the generation of purine nucleotides through the purine salvage pathway, and its mutant male mice at 34–38 weeks displayed complete testicular atrophy.32 The abnormally high expression of these NEXCI DEGs may directly damage Sertoli cells alone or coordinately, and cause spermatogenesis failure.
Supplementary Table 3.
Fertility-associated X-differentially expressed genes between obstructive azoospermia and Klinefelter syndrome Sertoli cells
| p_val | avg_logFC | KO mouse showed a fertility-associated phenotype | |
|---|---|---|---|
| TIMP1 | 1.96E-27 | 0.92397 | MGI: 3512125 |
| UXT | 1.20E-24 | 0.645173 | MGI: 6159372 |
| HPRT1 | 1.12E-17 | 0.529665 | MGI: 3831277 |
| PDHA1 | 1.32E-20 | 0.49432 | MGI: 2386703 |
| SAT1 | 2.05E-15 | 0.454061 | MGI: 3521903 |
| CETN2 | 1.68E-16 | 0.432882 | MGI: 5634782 |
| CDK16 | 6.25E-09 | 0.341665 | MGI: 5319210 |
| PGRMC1 | 1.42E-09 | 0.335683 | MGI: 5775088 |
| ABCD1 | 4.50E-05 | 0.287123 | MGI: 1861771 |
| USP11 | 2.50E-08 | 0.284873 | MGI: 6363163 |
| SMS | 5.64E-11 | 0.27472 | MGI: 4431547 |
| NXF3 | 1.11E-10 | 0.264091 | MGI: 4950382 |
| ELK1 | 1.03E-11 | 0.259978 | MGI: 3028871 |
| UBE2A | 1.74E-06 | 0.250153 | MGI: 5488492 |
MGI: mouse genome informatics; KO: knockout
Although we performed a transcriptional analysis to uncover the pathological changes and potential mechanisms of the KS spermatogenic microenvironment, additional evidence is still needed, such as immunohistochemistry and RNA-FISH in testis tissue sections. Some candidate regulators also need to be identified by functional experiments. In addition, some important regulators of XIST such as its antisense version, TSIX, were not detected in this study because its RNA lacks 3’ poly-A signaling to be captured and undergoes reverse transcription. We observed abnormal hormonal and immune responses in KS Sertoli cells and predicted that some small molecule drugs could potentially have therapeutic effects, such as ST1962, sirolimus, and CD437. In the future, the spermatogenic microenvironment in KS patients may be improved through endocrine regulation and suppression of abnormal immune responses so that genetic safety issues caused by gene editing can be avoided.
In general, based on the single-cell transcriptome data from normal adults and KS patients, we propose a novel mechanism in which the weak XCI ability of Sertoli cells causes their high susceptibility to the extra X chromosome, and this increased damage to Sertoli cells leads to atrophy of seminiferous tubules and failure of spermatogenesis.
AUTHOR CONTRIBUTIONS
LYZ, PL, ZL, and ZZ designed the experiments. PL, RHT, and CCY collected the samples and clinical information. LYZ isolated cells for scRNA-seq and performed bioinformatics analyses. YZC drew the schematic illustration. LYZ and YXT wrote the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
Clinical information and heterogeneous testicular atrophy in Klinefelter syndrome. (a) The clinical information of two OA and three KS patients. (b) Photograph of a typical KS testis during microdissection and testicular sperm extraction revealing heterogeneous atrophy in different regions. Hematoxylin and eosin staining shows the histological changes in each region. Region I, larger and opaque seminiferous tubules with normal spermatogenesis; Region II, thin and transparent seminiferous tubules that only contain pathological Sertoli cells; Region III, severely atrophic seminiferous tubules with almost all Sertoli cells and lacking germ cells. The scale bar represents 40 µm. OA: obstructive azoospermia; KS: Klinefelter syndrome.
Single-cell RNA sequencing quality information and known marker expression in each cluster. (a) The single-cell RNA sequencing quality information includes the total gene expression counts, unique molecular identifier counts, and the percent of mitochondrial gene expression projected on the tSNE plot. (b) Expression patterns of selected markers projected on the tSNE plot. (c) tSNE plots of testicular single-cell samples. Each cell is labeled with a different color according to patient ID (left and right panels) or cell cluster (middle panel). tSNE: T-distributed stochastic neighbor embedding.
Expression percent of X chromosome gene and expression level of XIST in each sample. (a and b) Violin plot shows the expression percent of X chromosome gene (a) and expression level of XIST (b) in each patient. XIST: X-inactive-specific transcript.
Change in the androgen synthesis pathway in Klinefelter syndrome Leydig cells. Violin plot shows the expression levels of androgen synthesis pathway-related genes in Klinefelter syndrome Leydig cells.
DEGs of each cell cluster in normal state.
DEGs between KS and OA SCs.
ACKNOWLEDGMENT
This work was supported by grants from the National Key R&D Program of China (2022YFC2702700), National Natural Science Foundation of China (82201756 and 82171597), China Postdoctoral Science Foundation (2021M703747), and GuangDong Basic and Applied Basic Research Foundation (2021A1515111109). We thank Dr. Jian-Ming Zeng and his bioinformatics team for their reference codes.
REFERENCES
- 1.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome:a national registry study. J Clin Endocrinol Metab. 2003;88:622–6. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- 2.Aksglaede L, Skakkebaek NE, Almstrup K, Juul A. Clinical and biological parameters in 166 boys, adolescents and adults with nonmosaic Klinefelter syndrome: a Copenhagen experience. Acta Paediatr. 2011;100:793–806. doi: 10.1111/j.1651-2227.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 3.Bergère M, Wainer R, Nataf V, Bailly M, Gombault M, et al. Biopsied testis cells of four 47,XXY patients: fluorescence in-situ hybridization and ICSI results. Hum Reprod. 2002;17:32–7. doi: 10.1093/humrep/17.1.32. [DOI] [PubMed] [Google Scholar]
- 4.Popp C, Dean W, Feng S, Cokus SJ, Andrews S, et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–5. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handel MA. The XY body: a specialized meiotic chromatin domain. Exp Cell Res. 2004;296:57–63. doi: 10.1016/j.yexcr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Namekawa SH, Park PJ, Zhang LF, Shima JE, McCarrey JR, et al. Postmeiotic sex chromatin in the male germline of mice. Cur Biol. 2006;16:660–7. doi: 10.1016/j.cub.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 7.Sin HS, Ichijima Y, Koh E, Namiki M, Namekawa SH. Human postmeiotic sex chromatin and its impact on sex chromosome evolution. Genome Res. 2012;22:827–36. doi: 10.1101/gr.135046.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galupa R, Heard E. X-chromosome inactivation: new insights into cis and trans regulation. Curr Opin Genet Dev. 2015;31:57–66. doi: 10.1016/j.gde.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–4. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 10.Sin HS, Namekawa SH. The great escape: active genes on inactive sex chromosomes and their evolutionary implications. Epigenetics. 2013;8:887–92. doi: 10.4161/epi.25672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller JL, Skaletsky H, Brown LG, Zaghlul S, Rock S, et al. Independent specialization of the human and mouse X chromosomes for the male germ line. Nat Genet. 2013;45:1083–7. doi: 10.1038/ng.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Hum Genet. 2011;130:237–45. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naumova N, Imakaev M, Fudenberg G, Zhan Y, Lajoie BR, et al. Organization of the mitotic chromosome. Science. 2013;342:948–53. doi: 10.1126/science.1236083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert Finestra T, Gribnau J. X chromosome inactivation: silencing, topology and reactivation. Curr Opin Cell Biol. 2017;46:54–61. doi: 10.1016/j.ceb.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Yao C, Xing X, Jing T, Li P, et al. Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat Commun. 2020;11:3949. doi: 10.1038/s41467-020-19414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Zhao Y, Yao C, Dai Y, Li Z, et al. MHA, an interactive website for scRNA-seq data of male genitourinary development and disease. Andrology. 2023 doi: 10.1111/andr.13402. Doi: 10.1111/ andr.13402. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Yao C, Tian R, Zhu Z, Zhao L, et al. miR-202-3p regulates sertoli cell proliferation, synthesis function, and apoptosis by targeting LRP6 and Cyclin D1 of Wnt/β-catenin signaling. Mol Ther Nucleic Acids. 2019;14:1–19. doi: 10.1016/j.omtn.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dossin F, Pinheiro I, Zylicz JJ, Roensch J, Collombet S, et al. SPEN integrates transcriptional and epigenetic control of X-inactivation. Nature. 2020;578:455–60. doi: 10.1038/s41586-020-1974-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 20.Bhandari V, Wong KS, Zhou JL, Mabanglo MF, Batey RA, et al. The role of ClpP protease in bacterial pathogenesis and human diseases. ACS Chem Biol. 2018;13:1413–25. doi: 10.1021/acschembio.8b00124. [DOI] [PubMed] [Google Scholar]
- 21.da Rocha ST, Boeva V, Escamilla-Del-Arenal M, Ancelin K, Granier C, et al. Jarid2 is implicated in the initial Xist-induced targeting of PRC2 to the inactive X chromosome. Mol Cell. 2014;53:301–16. doi: 10.1016/j.molcel.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Sarma K, Cifuentes-Rojas C, Ergun A, Del Rosario A, Jeon Y, et al. ATRX directs binding of PRC2 to Xist RNA and polycomb targets. Cell. 2014;159:1228. doi: 10.1016/j.cell.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Dabaja AA, Schlegel PN. Microdissection testicular sperm extraction: an update. Asian J Androl. 2012;15:35–9. doi: 10.1038/aja.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet. 2004;364:273–83. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- 25.Vidal M, Starowicz K. Polycomb complexes PRC1 and their function in hematopoiesis. Exp Hematol. 2017;48:12–31. doi: 10.1016/j.exphem.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Cerase A, Smeets D, Tang YA, Gdula M, Kraus F, et al. Spatial separation of Xist RNA and polycomb proteins revealed by superresolution microscopy. Proc Natl Acad Sci U S A. 2014;111:2235–40. doi: 10.1073/pnas.1312951111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu C, Zhang QC, da Rocha ST, Flynn RA, Bharadwaj M, et al. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161:404–16. doi: 10.1016/j.cell.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon Y, Lee JT. YY1 tethers Xist RNA to the inactive X nucleation center. Cell. 2011;146:119–33. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian D, Sun S, Lee JT. The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell. 2010;143:390–403. doi: 10.1016/j.cell.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nothnick WB, Soloway PD, Curry TE., Jr Pattern of messenger ribonucleic acid expression of tissue inhibitors of metalloproteinases (TIMPs) during testicular maturation in male mice lacking a functional TIMP-1 gene. Biol Reprod. 1998;59:364–70. doi: 10.1095/biolreprod59.2.364. [DOI] [PubMed] [Google Scholar]
- 31.Shishido Y, Baba T, Sato T, Shima Y, Miyabayashi K, et al. Differential lactate and cholesterol synthetic activities in XY and XX Sertoli cells. Sci Rep. 2017;7:41912. doi: 10.1038/srep41912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ordway JM, Tallaksen-Greene S, Gutekunst CA, Bernstein EM, Cearley JA, et al. Ectopically expressed CAG repeats cause intranuclear inclusions and a progressive late onset neurological phenotype in the mouse. Cell. 1997;91:753–63. doi: 10.1016/s0092-8674(00)80464-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical information and heterogeneous testicular atrophy in Klinefelter syndrome. (a) The clinical information of two OA and three KS patients. (b) Photograph of a typical KS testis during microdissection and testicular sperm extraction revealing heterogeneous atrophy in different regions. Hematoxylin and eosin staining shows the histological changes in each region. Region I, larger and opaque seminiferous tubules with normal spermatogenesis; Region II, thin and transparent seminiferous tubules that only contain pathological Sertoli cells; Region III, severely atrophic seminiferous tubules with almost all Sertoli cells and lacking germ cells. The scale bar represents 40 µm. OA: obstructive azoospermia; KS: Klinefelter syndrome.
Single-cell RNA sequencing quality information and known marker expression in each cluster. (a) The single-cell RNA sequencing quality information includes the total gene expression counts, unique molecular identifier counts, and the percent of mitochondrial gene expression projected on the tSNE plot. (b) Expression patterns of selected markers projected on the tSNE plot. (c) tSNE plots of testicular single-cell samples. Each cell is labeled with a different color according to patient ID (left and right panels) or cell cluster (middle panel). tSNE: T-distributed stochastic neighbor embedding.
Expression percent of X chromosome gene and expression level of XIST in each sample. (a and b) Violin plot shows the expression percent of X chromosome gene (a) and expression level of XIST (b) in each patient. XIST: X-inactive-specific transcript.
Change in the androgen synthesis pathway in Klinefelter syndrome Leydig cells. Violin plot shows the expression levels of androgen synthesis pathway-related genes in Klinefelter syndrome Leydig cells.
DEGs of each cell cluster in normal state.
DEGs between KS and OA SCs.