Abstract
The vaginal microbial community (“microbiota”) is a key component of the reproductive health of women, providing protection against urogenital infections. In sub-Saharan Africa, there is a high prevalence of bacterial vaginosis, a condition defined by bacterial overgrowth and a shift away from a Lactobacillus-dominated profile toward increased percentages of strict anaerobic species. Bacterial vaginosis is associated with an increased risk of HIV acquisition and transmission, as well as an increased risk of acquiring other sexually transmitted infections, preterm births, and pelvic inflammatory disease. Vaginal microbiota, rich in taxa of strict anaerobic species, disrupts the mucosal epithelial barrier through secretion of metabolites and enzymes that mediate inflammation. Advancements in next-generation sequencing technologies such as whole-genome sequencing have led to deeper profiling of the vaginal microbiome and further study of its potential role in HIV pathogenesis and treatment. Until recently data on the composition of the vaginal microbiome in sub-Saharan Africa have been limited; however, a number of studies have been published that highlight the critical role of vaginal microbiota in disease and health in African women. This article reviews these recent findings and identifies gaps in knowledge about variations in female genital commensal bacteria that could provide vital information to improve the effectiveness of interventions to prevent HIV and other sexually transmitted infections. In addition, we review the effects of pregnancy, contraception, and sexual practices on vaginal microbiome and the potential of vaginal microbiota on HIV transmission and prevention. A better understanding of the role of vaginal microbiota in host susceptibility to HIV infection and its prevention among African women could inform the development of novel local and systemic interventions to minimize new HIV infections among high-risk women.
Keywords: bacterial vaginosis, HIV, microbiome, pregnancy, sub-Saharan Africa, vaginal microbiota
Introduction
In 2016, females aged between 15–24 years in eastern and southern Africa accounted for 26% of the 1.8 million new HIV infections worldwide. The HIV incidence in these girls and women is 44% higher than that in African boys and men of the same age.1,2 In view of the evidence that new HIV infections are disproportionately higher among young women than men in sub-Saharan Africa,3,4 there is a need to understand the biological factors that increase host susceptibility, and in particular the role of the female genital microbiota that affects mucosal integrity. Normal vaginal microbiota provides host protection against invading pathogens including HIV and other sexually transmitted infections (STI).5,6 Commonly, the commensal bacterial species within the vaginal tract are predominantly Lactobacillus species, which play a homeostatic role of maintaining the acidic pH of the vaginal microenvironment through production of lactic acid.7 Lactic acid is a potent microbicide that can inactivate HIV and herpes simplex virus (HSV) type 2,8–10 and possibly other sexually transmitted pathogens. Lactobacillus species also competitively exclude genitourinary pathogens from establishing infection.11,12 Healthy vaginal microbiota modulates immune responses to invading pathogens13–15 and contributes to host immunity through the production of factors such as bacteriocins,16 hydrogen peroxide, and biosurfactants,17,18 as illustrated in the Figure. Variations in composition and function of vaginal microbiota can lead to increased susceptibility to infection.5,6 For example, Atopobium vaginae infection induced increased production of inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α, which may interfere with the innate mucosal barrier function.13 Other studies have shown that other specific bacterial species associated with bacterial vaginosis (BV), including Gardnerella vaginalis and Prevotella bivia, were strongly associated with vaginal inflammatory cytokines.19 Similarly, depletion of normal commensal microbiota increases the risk of damage to the vaginal epithelium, which subsequently secretes IL-33, a cytokine that blocks migration of effector T cells to vaginal mucosal tissue, thereby blocking the production of interferon gamma, a major cytokine in mucosal antiviral defense.14 Therefore, dysbiosis (abnormal microbiota due to derangement in its composition and function) can suppress the local host antiviral defense mechanisms and increase susceptibility to invading viral pathogens.
FIGURE 1. Diversity of vaginal microbiota increases risk of HIV acquisition.
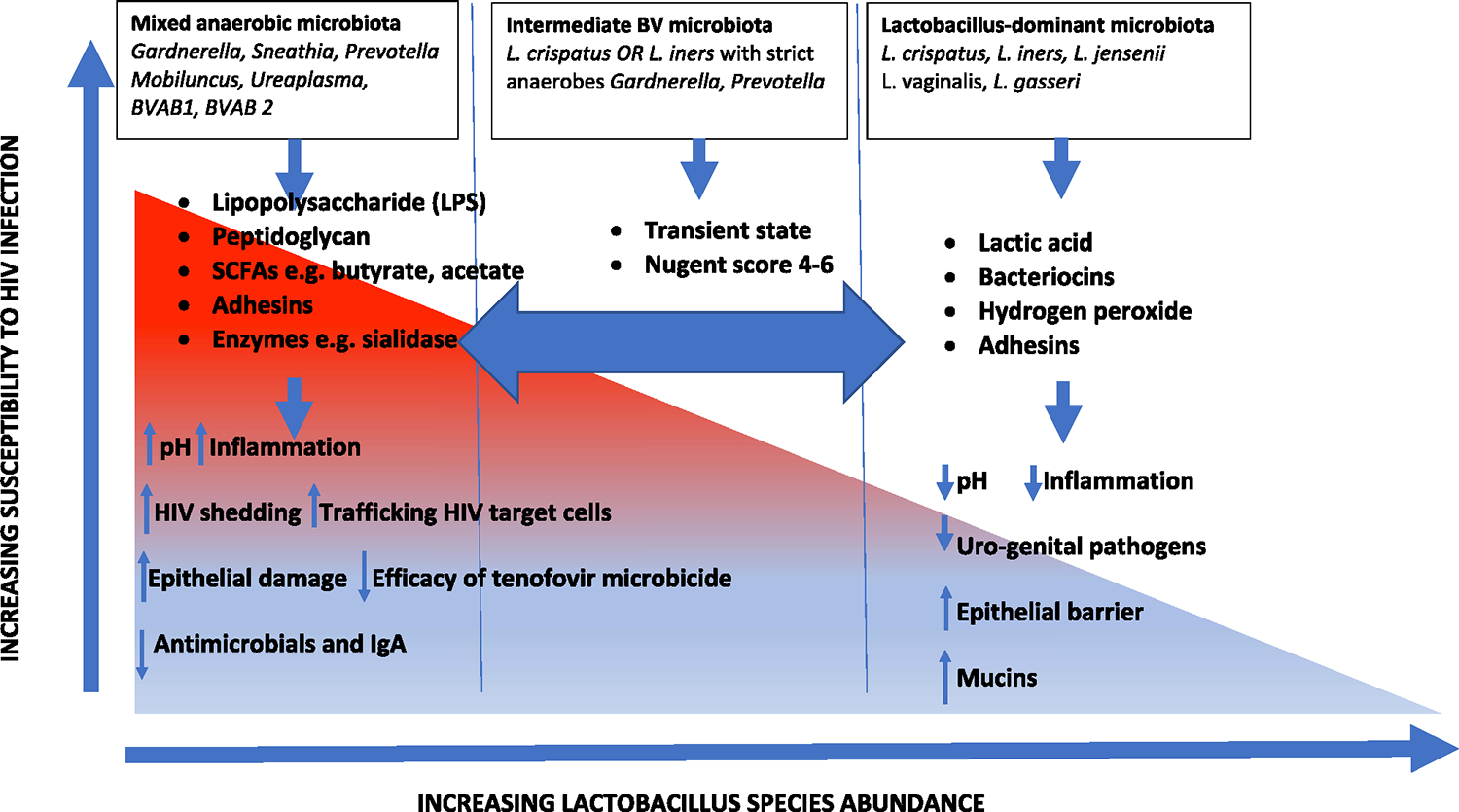
Summary of factors of vaginal microbiota associated to risk of HIV acquisition. Vaginal microbiota composed of diverse strict anaerobic bacteria contributes to weakened epithelial barrier, degrades host responses, and leads to increased frequency of HIV target cells.
BV, bacterial vaginosis; BVAB, BV–associated bacterium; SCFA, short chain fatty acids.
Bayigga. Diversity of vaginal microbiota in sub-Saharan Africa and its effects on HIV transmission and prevention. Am J Obstet Gynecol 2019.
A better understanding of the effects of physiological variations such as menstruation, pregnancy, and sexual activity on the composition and stability of vaginal microbiota and its effects on mucosal integrity and immune defense could inform innovations to protectsusceptible females from infection by sexually transmitted pathogens, including HIV. Alteration in the vaginal bacterial composition may lead to BV, a condition that is microbiologically characterized by lesser abundance of Lactobacillus species and/or overgrowth of anaerobic bacteria.20,21 BV is associated with higher HIV acquisition risk9 and shedding of HIV in cervicovaginal fluids.22 BV is diagnosed clinically as having 3 of the following criteria: alkaline vaginal pH (pH >4.5), presence of clue cells (sluffed vaginal epithelial cells laden with bacteria), a milky homogenous vaginal discharge, and release of a fishy odor upon addition of 10% potassium hydroxide to cervicovaginal fluid.23 BV-associated bacteria produce short chain fatty acids (SCFAs)24,25 that contribute to the increased risk of STIs.26–28 SCFAs such as acetic and butyric acids at a concentration of 20 mmol/L can increase the secretion of proinflammatory cytokines IL-6, IL-8, and IL-1β.29 Acetic acid enhances HIV-1 integration in CD4+ cells,30 and SCFAs have been implicated in the reactivation of latent HIV through inhibition of histone deacetylases.31 Prevalence of BV among women in sub-Saharan Africa is approximately 40%32,33 and the condition is persistent despite treatment. Differences in the lactobacilli concentrations in the microbiome of African vs non-African women may explain the high abundance of BV observed in African populations.
Numerous obstetric and gynecological complications such as pelvic inflammatory disease and preterm delivery are associated with BV. Women with BV are twice as likely to acquire Chlamydia trachomatis and Trichomonas vaginalis than women without BV34 and there is a significant association between HSV-2 and BV.28,35,36 Studies in a Ugandan population of women at high risk of acquiring HIV/STI showed that young age, alcohol use, and the number of previous partners is also associated with a high prevalence of BV.37
Newer molecular techniques such as high throughput sequencing have aided finer profiling of the vaginal microbiome, although well-characterized data are still limited. Due to the limited deployment of accurate techniques, vaginal bacterial communities in the variable clinical and physiological settings of high-risk women in sub-Saharan Africa are still underexplored. This article highlights specific gaps in knowledge about the female genital tract microbiome in sub-Saharan Africa, home to >70% of the annual new HIV infections among women and 90% of perinatal HIV infections.2,11 We also provide insight into the effects of the genital microbiome on host susceptibility to genital infections including HIV. In addition, we review the effect of pregnancy on the genital microbiome and susceptibility to HIV infection, as well as the effect of genital dysbiosis on preexposure prophylaxis and antiretroviral therapy for HIV prevention. Insight from this review could guide strategic development of the much needed biological interventions toward 0 HIV transmission among women in sub-Saharan Africa.
Differences in vaginal microbiota profiles among North American women representing different races and ethnicities
Profiling vaginal microbiota has mainly been done in populations in Europe and North America, including the first study to highlight differences in profiles of vaginal microbiota associated with ethnicity/race. This pioneering study, conducted by Ravel and colleagues,38 described the profile of the vaginal microbiome in 396 healthy, asymptomatic North American women representing 4 racial/ethnic groups: Asian, Black Hispanic, and Caucasian. Vaginal bacteria were characterized by pyrosequencing of the variable regions 1 and 2 of the 16S ribosomal RNA (rRNA) gene. Five distinct microbial communities were identified: community types I, II, III, and V, characterized by predominant populations of L crispatus, L gasseri, L iners, and L jensenii, respectively, and community type IV, which contained a greater diversity of taxa and a predominance of strict anaerobes.38 Interestingly, L iners and L crispatus were also detected in a majority of the group IV communities. In fact, all communities contained a subpopulation of lactic acid–producing bacteria, indicating that this characteristic is conserved across vaginal community groups irrespective of composition. Prevotella species were detected in 68% of all samples with an abundance of up to 45%. Lactobacillus-dominated communities were found in 90% of Caucasian, 80% of Asian, 65% of Hispanic, and 61% of Black women. Hispanic and Black women had a higher prevalence of BV (Nugent score of 7–10), and a higher vaginal pH than Caucasian and Asian women. Hispanic (34%) and Black (39%) women had a significantly higher prevalence of group IV community vaginal microbiota than did Asian (18%) and Caucasian North American (9%) women. Given that this study was conducted among healthy, asymptomatic women, this study suggests that non-Lactobacillus-dominant vaginal microbiota are normal and common in Black and Hispanic women. The variations in vaginal bacterial communities among North American women of various ethnicities and races revealed by this study underscored the need for more research on the composition of vaginal microbiota in women across the globe, and targeted risk assessment and risk management to prevent STIs including HIV in populations of women with high-risk vaginal bacterial community types.
Characteristics of the vaginal microbiota in African women
In a South African cohort of 146 healthy, asymptomatic women aged 18–23 years, Anahtar and colleagues19 showed that the vaginal microbiota contained fewer Lactobacillus species and a more diverse bacterial profile. Based on sequencing of the variable region 4 of the bacterial 16S rRNA gene, the bacterial communities were grouped into 4 community types (cervicotypes [CT]). CT1 had a higher abundance of L crispatus; CT2 was dominated by L iners; CT3 had predominantly Gardnerella; and CT4 was a heterogenous group without any dominant species. Prevotella species were detected in all communities, which agrees with the study by Ravel et al,38 where approximately 70% of the samples had Prevotella species. Only 37% of the participants had a Lactobacillus-dominant cervicovaginal community, with 77% of these having CT2, which was dominantly L iners. Among the 63% of women who did not have Lactobacillus dominance, 45% had CT3, which was Gardnerella-dominant community and 55% had the mixed CT4 community. Of the women in CT4, 50% had BV based on their Nugent scores despite being asymptomatic. This study also showed that Prevotella amnii, Mobiluncus mulieris, Sneathia amnii, and S sanguinegens (members of the CT4) elicited a proinflammatory response characterized by elevated levels of IL-1α, IL-1β, and IL-8 from vaginal epithelial cells unlike L crispatus. The high microbial diversity observed in women with CT4 correlated to higher concentrations of inflammatory cytokines and increased numbers of activated cervical CD4+ T cells highlighting the link between vaginal microbiota diversity and HIV acquisition risk.
In another South African study, Lennard and colleagues39 described 3 vaginal community groups: CT1, which had Gardnerella, Prevotella, and other anaerobes; CT2, which was lactobacilli (other than L iners) dominant; and CT3, which was L iners dominant. In this cohort of young South African HIV-negative women, only 8% had Gardnerella-dominant microbiota and 40 bacterial taxa were associated with genital inflammation including those previously reported by Anahtar et al.19 Genital inflammation, high Nugent score, and abundance of BV-associated bacterium-1 were observed in women with a diverse vaginal microbiota. However, no difference in number of HIV target cells between women with different vaginal microbiota composition was observed.
To describe the longitudinal changes in diversity of vaginal microbiota and inflammation, Jespers and colleagues40 quantitated specific vaginal microbiota bacteria and immune mediators in 80 African women over an 8-week period. Among HIV-negative adult women (adolescents and sex workers), vaginal microbiota profiles were relatively stable throughout the 5 visits. L iners was consistently present in 75% of the women and it occurred together with L crispatus in 35% of the women. P bivia and Escherichia coli were present in 90% of the women. Variations in vaginal microbiota bacteria concentrations were more marked within women than between women during the course of the study. Women who were amenorrhoeic (using progestin-injectable contraception) had significantly lower concentrations of Lactobacillus species, especially L crispatus, than women who had a menstrual cycle. Recent sex was also associated with lower concentrations of Lactobacillus species and P bivia. Approximately 50% of the women did not have Candida albicans, L jensenii, or L gasseri at any of the visits. Incident BV was associated with increased concentrations of proinflammatory cytokines.
In a study of 132 HIV-positive Tanzanian women, the Illumina platform (Illumina Inc, San Diego, CA) was used to sequence the variable region 6 of the 16SrRNA gene and microbiota profiles were categorized into 8 clusters: 2 dominated by either L iners or L crispatus and 4 BV-associated profiles, dominated by P bivia or Lachnospiraceae and a mixture of different species. L iners and G vaginalis were detected in all the samples. Interestingly, the Tanzanian women with a BV profile had a high abundance of P bivia, unlike the Caucasian and Black women in North America who had A vaginae and Clostridium species as the dominant species in BV microbiota profiles.41 P bivia triggers an inflammatory response within the genital tract due to its secretion of lipopolysaccharide (LPS), a potent activator of proinflammatory cytokines42 thereby increasing the risk of HIV acquisition and transmission. Similarly, A vaginae also elicits an inflammatory response from vaginal epithelial cells through the up-regulation of nuclear factor kappa-light-chain enhancer of activated B cells (NF-kB) transcription factor6 and increased secretion of chemokines.13 Both G vaginalis and A vaginae are found in biofilms, which render them resistant to antibiotic treatment and host immune factors.43 Treatment of BV patients with metronidazole led to a decreased diversity of their vaginal microbiota and increased abundances of L iners. However, antibiotic intervention did not result in a shift from BV-associated profile to a Lactobacillus-dominant profile. This African population also had relatively lower abundance of L crispatus and an absence of L jensenii unlike non-African populations.38,44 Table 1 summarizes studies that have examined the relationship between vaginal microbiota and the associated effects on host protection against infections. It is important to note that studies in sub-Saharan Africa differ considerably in terms of patient populations and experimental techniques used to evaluate microbiota; therefore, direct comparisons are limited. Hence there remains a need for well-designed studies to understand the different physiological and clinical contexts contributing to the diversity of vaginal bacterial in African women at high-risk for HIV acquisition.
TABLE 1.
Diversity of genital microbiome in sub-Sahara Africa and associated effects on risk of genital infections
| Author and year | Country | Study setting, participants’ description, and methods | Main findings |
|---|---|---|---|
| Anahtar et al,19 2015 | South Africa | HIV-negative women, age range 18–23 y Black women with no symptoms of BV 16S rRNA sequencing | Only 37% of participants had Lactobacillus-dominant microbiome; 45% of those who did not have Lactobacillus-dominant microbiome had Gardnerella-dominant bacterial communities. Higher diversity of microbial community was strongly associated with inflammation in absence of STIs and higher frequency of HIV target cells. |
| Borgdorff et al,45 2014 | Rwanda | Sex workers, median age 27 y, cross-sectional study 13% of Study participants were pregnant at time of sampling Phylogenetic microarray | 6 Clusters of microbial communities were identified with 2 dominated by L crispatus and L iners and another was mixture of anaerobic bacteria. Participants with L crispatus-dominant microbiome were less likely to have HIV, HSV-2, and HPV, and had less HIV shedding in cervicovaginal fluid. |
| Jespers et al,46 2015 | Kenya, Rwanda, South Africa | Sex workers, HIV positive (at least 6 mo on ART), pregnant women (<14 wk of amenorrhea), nonpregnant adolescents (16–17 y), women practicing intravaginal practices HIV-negative compared with nonpregnant women; quantitative PCR | HIV-positive women and sex workers had higher abundance of G vaginalis compared to reference groups. Pregnant women had higher abundance of Lactobacillus species. Having multiple partners was associated with higher prevalence of G vaginalis. Recent sexual exposure was associated with less concentrations of Lactobacillus species. |
| Gosmann et al,51 2017 | South Africa | 236 HIV-seronegative women Longitudinal study 16S rRNA Illumina sequencing | 58% of Women had high diversity vaginal microbiota communities dominated by G vaginalis, Prevotella, Megasphaera, Sneathia, and Shuttleworthia. Women with diverse microbiota and low Lactobacillus abundance had 4-fold increase in HIV acquisition compared to women with L crispatus dominance. Women with diverse microbiota had 17-fold increase in HIV target cells in genital tract when compared to women with L crispatus-dominant microbiota. |
| Lokken et al,85 2017 | Kenya | Female sex workers, media age 35 (31–39)y Gram staining and Nugent score Mycoplasma genitalium target amplification nucleic acid probe test Longitudinal study | At baseline, 40% of women had BV and 16.1% had Mycoplasma genitalium. Women with BV or Mycoplasma genitalium infection were more likely to be HIV infected at baseline. Having BV (Nugent score >7) at baseline was associated with 3.5-fold increase in odds of incident Mycoplasma genitalium infection. |
| Frank et al,53 2012 | Burkina Faso | HIV-1-infected pregnant women and their live-born children 36–38 wk of gestation Case-control study (nested in cohort) Cases: HIV-positive women whose live born babies were perinatally infected with HIV (n = 10) Controls: HIV-positive women whose live-born babies did not get HIV-infected (n = 54) 16S rRNA pyrosequencing | 30 of 64 had Lactobacillus-dominant microbiota; 77% L iners, 11% L crispatus, 3.9% L fornicalis, 3.2% L gasseri, and 0.5% L vaginalis. Another cluster of subjects had coagulase-negative staphylococci with lesser abundance of lactobacilli. Third group had mixture of genera, dominated by Gardnerella species. G vaginalis and Actinobacteria were twice more abundant in antepartum transmitters compared to nontransmitters. Brevibacterium casei and L gasseri were significantly associated with intrapartum transmission. Firmicutes were in lower abundances among antepartum transmitters compared to nontransmitting mothers. Diverse vaginal microbiota was associated with increased HIV transmission from mother to child. |
| Lennard et al,39 2017 | South Africa | HIV-negative, black women, 16–22 y 16S /RNA Illumina sequencing | 44% were BV positive, 13% BV intermediate, and 43 were BV negative by Nugent score. 3 Vaginal microbiota communities were identified; L iners-and L crispatus-dominant communities plus mixed anaerobe community. Having diverse microbiota was predictive of genital inflammation. High BVAB1 abundance was associated with inflammation and persistent BV, which are risk factors for HIV. |
| Myer et al,86 2005 | South Africa | Nested case-control study HIV-negative at enrollment, 35–65 y Amsel criteria and Gram stain for BV diagnosis | 74% of HIV seroconverters had BV Nugent score (7 –10). HIV seroconverters were more likely to have intermediate BV score. Women with BV were more likely to get HIV infected than those with normal vaginal microbiota (adjusted OR, 2.01; 95% CI, 1.12–3.62). |
| Mitchell et al,70 2012 | United States, Kenya | HIV-positive women No genital infections at enrollment Nonpregnant, 18–50 y of age Taxon-directed quantitative PCR | Presence of L crispatus was associated with 35% lower risk of HIV-1 RNA shedding in ART-naїve women. In women with suppressed HIV replication, L jensenii was associated with decreased HIV shedding. BVAB3 (IRR, 3.16; 95% CI, 1.36–7.32), Leptotrichia and Sneathia were associated with greater HIV shedding despite antiretroviral therapy. |
Available data in sub-Saharan Africa difficult to compare because of variable clinical and physiological characteristics of patients and different experimental methods used; hence need of well-designed studies to understand specific female populations to inform targeted preventive and therapeutic interventions.
ART, antiretroviral therapy; BV, bacterial vaginosis; BVAB, BV-associated bacterium; CI, confidence interval; cpn60, chaperonin 60; HPV, human papillomavirus; HSV, herpes simplex virus; IRR, incidence rate ratio; OR, odds ratio; PCR, polymerase chain reaction; rRNA, ribosomal RNA; STI, sexually transmitted infection.
Vaginal microbiota profiles in Tanzanian HIV-positive women differed from those observed in a cohort of Rwandese female sex workers. Borgdorff and colleagues45 used phylogenetic microarray to characterize the vaginal microbiome of 174 female sex workers in Rwanda. Results from this study indicated that there were 6 clusters: L crispatus and L iners dominating 2 of the clusters, R-I and R-II, respectively, and 4 clusters that had a high diversity and were dominated by Gardnerella, Atopobium, and Prevotella species. Clusters R-IV and R-VI had the aforementioned anaerobes and L iners. Cluster R-VI had the highest abundance of Prevotella species yet cluster R-III had the least abundance of L iners among the mixed clusters. Most of the samples had lactobacilli, with L iners and L crispatus detected in 74% and 16% of the studied samples. Prevotella, Corynebacterium, and Gardnerella species were also found in >80% of the samples. BV-associated bacteria Atopobium, Sneathia, Mobiluncus, Megasphaera, BV-associated bacterium-1, and Dialister were detected in approximately 50% of the samples though they were in low abundance in the Lactobacillus-dominant bacterial communities. Women in L crispatus-dominant cluster R-I were less likely to have HIV, HSV-2, and human papillomavirus than women in other clusters. STI prevalence was highest in women with the mixed anaerobe vaginal microbiota. Among the HIV-positive women, amounts of HIV-1 RNA in cervicovaginal lavage increased with increasing diversity of the vaginal microbiota community. Clusters dominated by L jensenii and L gasseri were not detected in this study; this agrees with the study by Hummelen et al,41 where there was no community dominated by either species. Both Atopobium and Prevotella species are known to trigger inflammation within the genital tract and alter innate immune responses of vaginal epithelium13; a condition that increases shedding of HIV and risk of STI such as Neisseria gonorrhoeae. In another study conducted at 3 sites in Kenya, Rwanda, and South Africa, the vaginal microbiota among women with an average risk of HIV infection was compared to that of women at high risk of HIV infection. The reference group that was categorized as average-risk HIV group had HIV-negative women who were not pregnant and did not engage in transactional sex or any intravaginal practices. Being a sex worker, being pregnant, or use of intravaginal substances was categorized as high-risk HIV group. Vaginal swabs were collected, and the presence of specific genus and species including Lactobacillus, L crispatus, L iners, L jensenii, L gasseri, L vaginalis, Gadnerella vaginalis, Atopobium vaginae, Prevotella bivia, Escherichia coli, and Candida albicans was detected using quantitative polymerase chain reaction. Lactobacillus genus was detected in 80% or more of the study population regardless of pregnancy, use of intravaginal practices, HIV status, or being a sex worker.46 L iners was the most abundant Lactobacillus species across all groups followed by L crispatus, L jensenii, and L vaginalis. Surprisingly, L jensenii and L gasseri were detected in this African population in contrast with the aforementioned studies by Hummelen et al41 and Borgdorff and colleagues.45 Jespers and colleagues46 showed that L vaginalis and L crispatus were associated with a healthy Nugent score (0–3) among adult nonpregnant, non-sex workers. A high presence of G vaginalis and E coli was observed in sex workers in addition to a low presence of L jensenii. BV is associated with formation of a biofilm by G vaginalis that favors growth of other anaerobes such as P bivia and Atopobium.47 Therefore, high presence of G vaginalis is a probable indicator of BV. Pregnant women had higher Lactobacillus concentrations (7.01 genome equivalents/mL) relative to nonpregnant HIV-negative women –6.08 genome equivalents/mL.48
In summary, vaginal microbiota communities in sub-Sahara African women have lesser abundances of Lactobacillus species with lower frequency of L gasseri and L jensenii in comparison to women from other geographical regions. There is also variability between the African populations studied, in particular the taxa that comprise the diverse microbiota profile. The presence of high diversity vaginal microbiota in healthy African women is in agreement with the high prevalence of BV previously determined by Gram staining and Nugent scores.33
Noteworthy is the fact that the method of assessment could contribute to the variability described; for example, pyrosequencing or the 454 platform makes more errors especially when sequencing ≥2 identical nucleotides.49 On the other hand, Illumina technology, eg, the MiSeq platform gives more reads than pyrosequencing allowing for more in-depth sequencing and multiplexing many samples in 1 run.41 Furthermore, when different bacteria have very similar 16S rRNA variable regions, whole-genome shotgun sequencing gives species-level taxonomic resolution.50 Variable region 4 of the 16S rRNA gene gives better resolution of BV-associated bacteria yet variable regions 1–3 give better resolution of Lactobacillus species.39 Differences in sample size, sample collection methods, and laboratory methods may also account for the differences in the vaginal microbiota profiles. Therefore, there is a need for well-designed studies aimed at understanding the inherent differences in genetics, parity, sexual practices, and socioeconomic factors between African and European and/or North American women that should not be disregarded.
Vaginal microbiota and risk of HIV transmission
Gosmann and colleagues51 characterized the vaginal microbiota profile of healthy, asymptomatic South African black women aged 18–23 years by sequencing the variable region 4 of the 16S rRNA bacterial gene. Four CT were identified. CT1 was L crispatus dominant with low diversity and was found in 10% of the women. CT2 was L iners dominant and was found in 32% of the women. Collectively, CT3 (n = 68) and CT4 (n = 70) made up the rest of the cervicovaginal bacterial microbiota with CT3 being G vaginalis dominant and CT4 dominated by a bacterial genus other than Lactobacillus or Gardnerella. They reported that individuals with diverse vaginal microbiota dominated by anaerobes except Gardnerella had a 4 times higher risk of HIV acquisition than women with L crispatus-dominated microbiota. Furthermore, women with diverse anaerobe-dominated microbiota had a 17-fold increase in the number of activated CD4+ T cells in the endocervix, which are primary target cells for HIV infection, and increased secretion of chemokines macrophage inflammatory protein-1beta (MIP-1β) and MIP-1α, which attract C-C chemokine receptor 5 (CCR5)-expressing cells. HIV infection is established via viral replication in CCR5+CD4+ T cells at the mucosa.52 These data imply that diverse vaginal microbiota increases risk of HIV acquisition through increased activation and trafficking of HIV target cells. L crispatus and L iners were associated with lower levels of inflammation, as illustrated in the Figure.
Diverse vaginal microbiota profiles have also been associated with an increased risk of mother-to-child HIV transmission. Among pregnant women in Burkina Faso, vaginal microbiota of HIV-positive women whose babies were infected with HIV antepartum had twice the abundance of G vaginalis and Actinobacteria than those that did not transmit HIV.53 About half (30 out of 64) of the women in the study had Lactobacillus-dominant microbiota: 77% L iners, 11% L crispatus, 3.9% L fornicalis, 3.2% L gasseri, and 0.5% L vaginalis.
Vaginal microbial profile and HIV pathogenesis
Diverse vaginal microbiota profiles are associated with BV38,43 and are typically composed of higher abundances of anaerobic bacteria such as G vaginalis, A vaginae, P bivia, Mobiluncus, and lesser concentrations of Lactobacillus species.20 It has been postulated that vaginal microbiota mediates increased risk of HIV acquisition through: (1) degradation of the epithelial barrier, (2) activation of inflammatory responses, (3) decrease in antimicrobial factors, and (4) increased activation of HIV target cells, as illustrated in the Figure. The abundance of G vaginalis increases with increase in the Nugent score and BV-positive status. Due to its secretion of adhesins, G vaginalis forms a biofilm on the epithelial surface in the cervicovaginal tract43 and provides a conducive environment for anaerobic bacteria such as A vaginae and BV-associated bacterium-1 to flourish. Persistent and recurrent BV has been attributed to the biofilm that facilitates evasion of microbes from both host immune factors and antibiotics.47 Recurrent BV leads to chronic inflammation, which is a driver of increased HIV risk. G vaginalis also produces vaginolysin, a pore-forming toxin that lyses vaginal epithelial cells and degrades the epithelial barrier.14 G vaginalis also produces sialidase and prolidase enzymes that degrade mucins and increase the sloughing off of vaginal epithelial cells.5 The direct damage of vaginal epithelium mediated by G vaginalis increases the risk of HIV acquisition. Shedding of vaginal epithelial cells in women with both HIV and BV could explain the higher HIV viral load in the cervicovaginal fluids of these individuals. Lysates of G vaginalis have also been shown to activate transcription of HIV long terminal repeat and increased NF-αB binding activity.54 Both Avaginae and P bivia activate inflammatory pathway through the transcription factor NF-κB.6 A vaginae has been shown to increase secretion of chemokines such as C-C motif chemokine ligand 20 (CCL-20), tumor necrosis factor,13 Regulated on Activation, Normal T cell Expressed and Secreted (RANTES),6 and IL-8;55,56 and both P bivia and Mobiluncus are associated with increased secretion of proinflammatory cytokines. Infection of vaginal epithelial cells by Avaginae and P bivia elicit increased expression of Mucin-1 (MUC1), MUC3, and MUC4.13 The inflammatory processes lead to increased frequency, trafficking, and activation of HIV target cells,5 hence increasing the chances of establishment of infection. Ureaplasma urealyticum, another BV-associated bacteria, produces elastase and IgA protease that degrades the antibodies at the vaginal mucosa,21 hence lowering host immunity and increasing susceptibility to HIV infection. P bivia and P disiens produce fibrinolysins and collagenase that degrade the mucosa barrier and increase sloughing off of vaginal epithelial cells.21 On the other hand, Lactobacillus-dominated vaginal bacterial communities have lower levels of IL-1α, IL-1β, and IL-8 in comparison to communities dominated by anaerobic bacteria.19 This is attributed to the lactic acid produced by the Lactobacillus species, which dampens inflammatory responses associated with increased risk of HIV acquisition.57 Lactic acid is also virucidal against HIV58 so a greater abundance of lactobacilli within the vaginal milieu should provide better protection against HIV infection.
Factors contributing to diversity of female genital microbiota
Hormonal changes
Vaginal microbiota composition is greatly affected by hormonal changes especially estrogen. Increase in estrogen levels allows greater adherence of lactobacilli to the epithelium of the female genital tract59 and subsequently a higher stability of the microbiome composition. Vaginal pH and glycogen content also affect the adherence of lactobacilli to the vaginal epithelium. Microbial species present at the vaginal mucosa differ between premenopausal and postmenopausal women. This is due to the associated drop in estrogen levels during menopause resulting in low amounts of lactobacilli in postmenopausal women.60,61 Hormonal replacement therapy leads to an increase in lactobacilli colonization of the vaginal epithelium in postmenopausal women,62 and is therefore a potential intervention to modify vaginal tract milieu. Spear et al63 showed that Lactobacillus are not able to utilize glycogen but rely on α-amylase within the vaginal fluids to digest glycogen into maltose, maltotriose, malpentaose, and other smaller polymers to aid its colonization. Pregnancy is associated with a stable microbiome with low alpha-diversity and Lactobacillus-dominance64 that shifts to a BV-associated profile during the postpartum period following a decrease in estrogen (Table 2). The stability of the vaginal microbiota during pregnancy seems to be increased secondary to high secretion of circulating estrogen levels that promote glycogen deposition in vaginal epithelial cells to favor colonization by lactobacilli. On the other hand, it has been observed that there are increases in G vaginalis and L iners while L crispatus and Ljensenii concentrations decrease during menses.65 This can be attributed to the presence of iron, which is an essential growth factor for G vaginalis. Similarly, the flow of menstrual blood may impede adherence of Lactobacillus species giving BV-associated microbiota an advantage at the onset of menses. Other factors that influence shift in vaginal bacterial composition include hormonal contraceptives, condom use, and intrinsic factors (innate and adaptive immunity) among others.66,67 Use of contraceptive vaginal rings containing estrogen has also been shown to significantly lower concentrations of BV-associated bacteria G vaginalis and A vaginae while promoting lactobacilli colonization.68 On the other hand, consistent condom use increases the abundance of L crispatus within the vaginal tract and a healthy Nugent score of 0–3 is more common in condom users than women who use in-trauterine device for contraception.66
TABLE 2.
Microbiome during pregnancy and its effects on susceptibility to genital infections
| Author and year | Country | Study setting, participants’ description, and methods | Main findings |
|---|---|---|---|
| Fettweis et al,87 2014 | United States | Healthy women; pregnant and nonpregnant Women diagnosed with BV; pregnant and nonpregnant Age: 18–44 y Black vs women of European ancestry | Differences in vaginal microbial profiles due to ethnicity, pregnancy, and alcohol use were observed and correlated to presence of BV-associated species. Black women had greater vaginal microbial diversity than women of European descent. |
| MacIntyre et al,64 2015 | United Kingdom | Healthy pregnant women Longitudinal study (pregnancy and at 6 wk postpartum) 16S rRNA sequencing using MiSeq | During pregnancy, vaginal microbiome was dominated by Lactobacillus species with low diversity. In this population, significant number of women had L jensenii-dominated microbiome profile and L gasseri was absent in samples from black women. Postpartum, microbiome shifts and there is less Lactobacillus species and higher diversity. |
| Freitas et al,88 2017 | Canada | Healthy pregnant women at gestation age of 11–16 wk Nonpregnant healthy women Pyrosequencing of cpn60 universal target region | Pregnant women had higher bacterial load, less rich and diverse microbiome profile, and less prevalence of Mollicutes compared to nonpregnant women. |
| Frank et al,53 2012 | Burkina Faso | HIV-positive women at 36–38 wk of gestation | Mother-to-child transmission of HIV was associated with diverse microbiota, notably G vaginalis. |
BV, bacterial vaginosis; rRNA, ribosomal RNA.
Sexual practices
Sexual intercourse and the use of lubricants are associated with rapid changes in vaginal microbiota and a higher incidence of BV.69 Women who have multiple partners and sexual debut at an early age are more likely to have BV than women with a single partner and later sexual debut.70 In addition, vaginal cleansing practices such as intravaginal cleansing have been associated with higher risk of BV and changes in composition of vaginal bacterial communities.71,72 Intravaginal cleaning with soap leads to alterations in the vaginal bacterial microbiota and BV.73 Birse et al74 reported that in a cohort of HIV-negative Kenyan women, those who practiced vaginal drying using a cloth or towel had vaginal microbiota characterized by increased bacterial diversity and lower abundances of Lactobacillus species than those who did not. In addition, douching with bactericidal agents is detrimental to vaginal health due to the depletion of bacterial species that are protective against urogenital pathogens.
Ethnicity/race
The composition of vaginal microbiome may also be influenced by genetic factors as evidenced by differences in bacterial communities observed by Ravel et al38 in their study of North American women of Asian, Caucasian, African, and Hispanic backgrounds. Caucasian women had a higher prevalence of Lactobacillus-dominant microbiome whereas the Black and Hispanic women had a more diverse, non-Lactobacillus-dominant microbiota.44 The vaginal microbiota can be inherited from mothers,75 suggesting that race is a contributor to composition of the vaginal microbiota profile. Differences due to polymorphisms in immune responses such as reduced cytokine and chemokine secretion may lead to increased risk of BV. Interestingly, black women have more polymorphisms that affect IL-6 responses potentially making them more susceptible to BV due to their inability to respond to LPS.76
Antibiotics
Antibiotics such as metronidazole and clindamycin that are used in the syndromic management of BV have been shown to alter the vaginal microbiome profile although they do not necessarily lead to the establishment of a Lactobacillus-dominated microbiota.15 Antibiotic-mediated dysbiosis has been shown to impair antiviral immunity at the vaginal mucosa. Upon depletion of commensal bacteria, the vaginal epithelium releases IL-33, which blocks migration of effector T cells to vaginal tissues,14 thereby inhibiting production of interferon-gamma, a key antiviral cytokine.
STIs and vaginal microbiota
Approximately 70% of the reported HIV cases are in sub-Saharan Africa11 and this region also has a considerable burden of other STIs including HSV-2, syphilis, and gonorrhea. Prevalence of gonorrhea, Chlamydia, and syphilis is higher among 15– to 24-year-old girls and women, yet BV and HSV-2 is higher among older women.32 Acquisition of STIs and HIV likely depends on the composition of vaginal microbiota. Women with BV are twice as likely to acquire Chlamydia and trichomoniasis than women without BV34 and there is a significant association between HSV-2 and BV.28,35,36 This implies that vaginal microbiota plays a role in the establishment of infection, and probably modulates immune responses at the vaginal mucosa. Successful treatment of BV would most likely reduce the incident rate of STIs, particularly during pregnancy when both the mother and baby are at a higher risk of HIV infection.
Effect of microbiota on preexposure prophylaxis, microbicides
Recent advances in antiretroviral therapy for HIV prevention include clinical trials on oral tenofovir (TFV)-based preexposure prophylaxis and 1% TFV gel. The efficacy of 1% TFV gel is lower among women with BV than among those women without BV, despite high adherence in both groups.77 This has been attributed to the rapid metabolism of TFV by G vaginalis, a key constituent of the BV microbiota community thus hindering the local dissemination of the drug when applied locally.78 This highlights the critical need to assess the impact of vaginal microbiota on the efficacy of novel microbicides such as the ring and film-based preexposure prophylaxis products. The dynamics of the vaginal microbiome, which are influenced by hormonal contraception use, pregnancy, menstrual cycle, and perimenopause, should be considered in the development of microbicides. Given that there is increased prevalence of BV in areas with high HIV incidence, there is need to reevaluate the role of microbiota in the success of locally applied microbicides.
Potential genital microbiota-based interventions to modulate host susceptibility to infection
Despite rigorous efforts to determine the composition of the vaginal microbiome in various populations, the functions of the different bacterial communities and their interactions within the vaginal ecosystem are underexplored. There is still a gap in identifying the contribution of the specific bacterial species to host immunity and efficacy of preexposure and postexposure prophylaxis for HIV infections. In the treatment of BV, probiotics have been developed to provide the lactic-acid producing bacteria such as L acidophilus and L rhamnosus GR-1, which led to cure, restoration of normal microbiota, and reduced recurrence of BV.79 Lactobacillus species can also be engineered to deliver drugs that are protective against STIs as has been evidenced in the successful delivery of cyanovirin-N, an HIV-1 inhibitor, to the mucosal surfaces of the genital tract in macaques.80 Lactic acid, a metabolite of the Lactobacillus species, is an effective microbicide against HIV58 and N gonorrhoeae.81 Lactic acid modulates immune responses at the vaginal mucosa, eliciting an antiinflammatory response that lowers the risk of HIV acquisition.57 Use of lactic acid gel, in combination with metronidazole, leads to increased lactobacilli colonization and reduces chances of BV recurrence.82 It is therefore likely that in the right dosing, lactic acid can be used as a microbicide against HIV. Bacteriocins, hydrogen peroxide, and biosurfactants are all products of the resident vaginal bacteria with the potential to protect against sexually transmitted diseases.
Use of microbicides or probiotics has been greatly hindered by lack of validated dosing strategies, strain-specific effects, and issues of product stability.83 Most studies that evaluate the potential of probiotics utilize small sample sizes so the effects may not be generalized to larger populations. Furthermore, there is need to consider the effect of genetic polymorphisms in immune response genes on efficacy of microbiota-based interventions; for example, the polymorphisms in IL-6 associated with lowered immune response to IL-1 and LPS is highly prevalent in black women.84 Single nucleotide polymorphisms in toll-like receptors are linked to decreased cytokine and chemokine secretions.76 Variations in vaginal microbiota composition within and between women make it almost impossible to have similar baseline characteristics that will guide development of microbiome-based interventions. Therefore, the success of a microbiota-based intervention would have to account for such specific physiological, social, and genetic differences.
Conclusion
Given the diversity of genital microbiome in sub-Saharan Africa populations, there could be untapped microbiome-based interventions to improve the stability of the female microbiome and reduce host susceptibility to genital infections including HIV. Such interventions should be tested to reduce the risk of STI acquisition in women, and also transmission of genital infections particularly from pregnant mothers to their unborn babies. In addition, there is a need to study the functional roles of the various bacterial species in relation to immunity and pathogenesis of STIs in well-characterized cohorts to inform effective treatment and prevention interventions. ■
Acknowledgments
L.B. and D.N. is supported through the DELTAS Africa Initiative (grant no. 107743). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences, Alliance for Accelerating Excellence in Science in Africa, and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency with funding from the Wellcome Trust (grant no. 107743) and the United Kingdom Government. The authors also acknowledge support from the Training of Ugandans in Basic Research on Tuberculosis and Emerging Infectious Diseases program funded by Fogarty International/National Institutes of Health D43 (grant D43TW009093-03). This funding supported L.B.’s training on vaginal microbiota and HIV pathogenesis.
Footnotes
The authors report no conflict of interest.
Contributor Information
Lois Bayigga, Departments of Immunology and Molecular Biology, Makerere University College of Health Sciences, Kampala, Uganda.
David P. Kateete, Departments of Immunology and Molecular Biology, Makerere University College of Health Sciences, Kampala, Uganda.
Deborah J. Anderson, Departments of Obstetrics and Gynecology, Microbiology, and Medicine, Boston University School of Medicine, Boston, MA.
Musa Sekikubo, Obstetrics and Gynecology, Makerere University College of Health Sciences, Kampala, Uganda.
Damalie Nakanjako, Internal Medicine, School of Medicine, Makerere University College of Health Sciences, Kampala, Uganda; Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS. UNAIDS Data 2017. Reference report UNAIDS/JC2910E. 2017. Available at: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf. Accessed December 20, 2018.
- 2.Joint United Nations Programme on HIV/AIDS. Global Plan towards the Elimination of New HIV Infections among Children by 2015 and Keeping Their Mothers Alive. UNAIDS/JC2137E. ISBN: 978–92-9173–897-7. Appia, 1211 Geneva 27, Switzerland. Available at: http://www.unaids.org/sites/default/files/media_asset/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en_0.pdf. [Google Scholar]
- 3.Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc 2015;18:19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magadi MA. Understanding the gender disparity in HIV infection across countries in sub-Saharan Africa: evidence from the demographic and health surveys. Sociol Health Illn 2011;33:522–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buve A, Jespers V, Crucitti T, Fichorova RN. The vaginal microbiota and susceptibility to HIV. AIDS 2014;28:2333–44. [DOI] [PubMed] [Google Scholar]
- 6.van de Wijgert J The vaginal microbiome and sexually transmitted infections are interlinked: consequences for treatment and prevention. PLoS Med 2017;14:e1002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun 1999;67:5170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti C, Malacrino C, Mastromarino P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol 2009;60(Suppl):19–26. [PubMed] [Google Scholar]
- 9.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008;22:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osset J, Bartolome RM, Garcia E, Andreu A. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis 2001;183:485–91. [DOI] [PubMed] [Google Scholar]
- 11.Atassi F, Brassart D, Grob P, Graf F, Servin AL. Vaginal Lactobacillus isolates inhibit uropathogenic Escherichia coli. FEMS Microbiol Lett 2006;257:132–8. [DOI] [PubMed] [Google Scholar]
- 12.Boris S, Suarez JE, Vazquez F, Barbes C. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun 1998;66:1985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis 2014;209:1989–99. [DOI] [PubMed] [Google Scholar]
- 14.Oh JE, Kim BC, Chang DH, et al. Dysbiosis-induced IL-33 contributes to impaired antiviral immunity in the genital mucosa. Proc Natl Acad Sci U S A 2016;113:E762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014;157:121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selle K, Klaenhammer TR. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol Rev 2013;37:915–35. [DOI] [PubMed] [Google Scholar]
- 17.Reid G, Heinemann C, Velraeds M, van der Mei HC, Busscher HJ. Biosurfactants produced by Lactobacillus. Methods Enzymol 1999;310:426–33. [DOI] [PubMed] [Google Scholar]
- 18.Walencka E, Rozalska S, Sadowska B, Rozalska B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol (Praha) 2008;53:61–6. [DOI] [PubMed] [Google Scholar]
- 19.Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015;42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev 2016;29:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Africa CW, Nel J, Stemmet M. Anaerobes and bacterial vaginosis in pregnancy: virulence factors contributing to vaginal colonization. Int J Environ Res Public Health 2014;11:6979–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman JS, Hitti J, Bukusi EA, et al. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS 2007;21:755–9. [DOI] [PubMed] [Google Scholar]
- 23.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14–22. [DOI] [PubMed] [Google Scholar]
- 24.Yeoman CJ, Thomas SM, Miller ME, et al. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS One 2013;8:e56111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aroutcheva A, Gariti D, Simon M, et al. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 2001;185:375–9. [DOI] [PubMed] [Google Scholar]
- 26.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 2012;9:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007;109:114–20. [DOI] [PubMed] [Google Scholar]
- 28.Cherpes TL, Meyn LA, Krohn MA, Hillier SL. Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora. Sex Transm Dis 2003;30:405–10. [DOI] [PubMed] [Google Scholar]
- 29.Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL, Spear GT. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am J Reprod Immunol 2012;67:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolduc JF, Hany L, Barat C, Ouellet M, Tremblay MJ. Epigenetic metabolite acetate inhibits class I/II histone deacetylases, promotes histone acetylation, and increases HIV-1 integration in CD4(+) T cells. J Virol 2017;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das B, Dobrowolski C, Shahir AM, et al. Short chain fatty acids potently induce latent HIV-1 in T-cells by activating P-TEFb and multiple histone modifications. Virology 2015;474:65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrone EA, Morrison CS, Chen PL, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018;15:e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jespers V, Crucitti T, Menten J, et al. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in sub-Saharan Africa: a cross-sectional study. PLoS One 2014;9:e109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brotman RM, Bradford LL, Conrad M, et al. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis 2012;39:807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 2003;37:319–25. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb SL, Douglas JM Jr, Foster M, et al. Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk-reduction counseling. J Infect Dis 2004;190:1059–67. [DOI] [PubMed] [Google Scholar]
- 37.Francis SC, Looker C, Vandepitte J, et al. Bacterial vaginosis among women at high risk for HIV in Uganda: high rate of recurrent diagnosis despite treatment. Sex Transm Infect 2016;92:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011;108(Suppl):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lennard K, Dabee S, Barnabas SL, et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect Immun 2017;86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jespers V, Kyongo J, Joseph S, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep 2017;7:11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hummelen R, Fernandes AD, Macklaim JM, et al. Deep sequencing of the vaginal microbiota of women with HIV. PLoS One 2010;5:e12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aroutcheva A, Ling Z, Faro S. Prevotella bivia as a source of lipopolysaccharide in the vagina. Anaerobe 2008;14:256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danielsson D, Teigen PK, Moi H. The genital econiche: focus on microbiota and bacterial vaginosis. Ann N Y Acad Sci 2011;1230:48–58. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 2007;1:121–33. [DOI] [PubMed] [Google Scholar]
- 45.Borgdorff H, Tsivtsivadze E, Verhelst R, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J 2014;8:1781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jespers V, van de Wijgert J, Cools P, et al. The significance of Lactobacillus crispatus and L vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. BMC Infect Dis 2015;15:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 2008;198:97.e1–6. [DOI] [PubMed] [Google Scholar]
- 48.Romero R, Hassan SS, Gajer P, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2014;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith DR, Quinlan AR, Peckham HE, et al. Rapid whole-genome mutational profiling using next-generation sequencing technologies. Genome Res 2008;18:1638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods 2012;9:811–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gosmann C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017;46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 2011;62:127–39. [DOI] [PubMed] [Google Scholar]
- 53.Frank DN, Manigart O, Leroy V, et al. Altered vaginal microbiota are associated with perinatal mother-to-child transmission of HIV in African women from Burkina Faso. J Acquir Immune Defic Syndr 2012;60:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashemi FB, Ghassemi M, Roebuck KA, Spear GT. Activation of human immunodeficiency virus type 1 expression by Gardnerella vaginalis. J Infect Dis 1999;179:924–30. [DOI] [PubMed] [Google Scholar]
- 55.Eade CR, Diaz C, Wood MP, et al. Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One 2012;7:e50106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Libby EK, Pascal KE, Mordechai E, Adelson ME, Trama JP. Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect 2008;10:439–46. [DOI] [PubMed] [Google Scholar]
- 57.Heinemann C, Reid G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Can J Microbiol 2005;51:777–81. [DOI] [PubMed] [Google Scholar]
- 58.Aldunate M, Tyssen D, Johnson A, et al. Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother 2013;68:2015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan RC, Bruce AW, Reid G. Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of gram-negative uropathogens by competitive exclusion. J Urol 1984;131:596–601. [DOI] [PubMed] [Google Scholar]
- 60.Devillard E, Burton JP, Hammond JA, Lam D, Reid G. Novel insight into the vaginal microflora in postmenopausal women under hormone replacement therapy as analyzed by PCR-denaturing gradient gel electrophoresis. Eur J Obstet Gynecol Reprod Biol 2004;117:76–81. [DOI] [PubMed] [Google Scholar]
- 61.Burton JP, Cadieux PA, Reid G. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl Environ Microbiol 2003;69:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cauci S, Driussi S, De Santo D, et al. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol 2002;40:2147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spear GT, French AL, Gilbert D, et al. Human alpha-amylase present in lower-genitaltract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis 2014;210:1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacIntyre DA, Chandiramani M, Lee YS, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 2015;5:8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santiago GL, Tency I, Verstraelen H, et al. Longitudinal qPCR study of the dynamics of L crispatus, L iners, A vaginae, (sialidase positive) G vaginalis, and P bivia in the vagina. PLoS One 2012;7:e45281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma L, Lv Z, Su J, et al. Consistent condom use increases the colonization of Lactobacillus crispatus in the vagina. PLoS One 2013;8:e70716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen CR, Cheng SC, Shiboski S, et al. Diaphragm used with Replens gel and risk of bacterial vaginosis: results from a randomized controlled trial. Infect Dis Obstet Gynecol 2012;2012:921519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crucitti T, Hardy L, van de Wijgert J, et al. Contraceptive rings promote vaginal lactobacilli in a high bacterial vaginosis prevalence population: a randomized, open-label longitudinal study in Rwandan women. PLoS One 2018;13:e0201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect 2010;86:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitchell CM, Fredricks DN, Winer RL, Koutsky L. Effect of sexual debut on vaginal microbiota in a cohort of young women. Obstet Gynecol 2012;120:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ness RB, Hillier SL, Richter HE, et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet Gynecol 2002;100:765. [DOI] [PubMed] [Google Scholar]
- 72.Schwebke JR, Desmond RA, Oh MK. Predictors of bacterial vaginosis in adolescent women who douche. Sex Transm Dis 2004;31:433–6. [DOI] [PubMed] [Google Scholar]
- 73.Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011;8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birse KD, Romas LM, Guthrie BL, et al. Genital injury signatures and microbiome alterations associated with depot medroxyprogesterone acetate usage and intravaginal drying practices. J Infect Dis 2016;215:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Si J, You HJ, Yu J, Sung J, Ko G. Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell Host Microbe 2017;21:97–105. [DOI] [PubMed] [Google Scholar]
- 76.Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis 2016;214(Suppl):S29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Velloza J, Heffron R. The vaginal microbiome and its potential to impact efficacy of HIV pre-exposure prophylaxis for women. Curr HIV/AIDS Rep 2017;14:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017;356:938–45. [DOI] [PubMed] [Google Scholar]
- 79.Falagas M, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect 2007;13:657–64. [DOI] [PubMed] [Google Scholar]
- 80.Lagenaur LA, Sanders-Beer BE, Brichacek B, et al. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol 2011;4:648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graver MA, Wade JJ. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob 2011;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Decena DC, Co JT, Manalastas RM Jr, et al. Metronidazole with Lactacyd vaginal gel in bacterial vaginosis. J Obstet Gynaecol Res 2006;32:243–51. [DOI] [PubMed] [Google Scholar]
- 83.Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther 2008;30:453–68. [DOI] [PubMed] [Google Scholar]
- 84.Genc MR, Onderdonk A. Endogenous bacterial flora in pregnant women and the influence of maternal genetic variation. BJOG 2011;118:154–63. [DOI] [PubMed] [Google Scholar]
- 85.Lokken EM, Balkus JE, Kiarie J, et al. Association of recent bacterial vaginosis with acquisition of mycoplasma genitalium. Am J Epidemiol 2017;186:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freitas AC, Bocking A, Hill JE, Money DM; VOGUE Research Group. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018;6:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fettweis JM, Brooks JP, Serrano MG, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014;160(Pt 10):2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Myer L, Denny L, Telerant R, de Souza M, Wright TC, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: A nested case-control study. J Infecti Dis 2005;192(8):1372–80. [DOI] [PubMed] [Google Scholar]


