Abstract
OBJECTIVES:
Critically ill women may receive less vital organ support than men but the mortality impact of this differential treatment remains unclear. We aimed to quantify sex differences in vital organ support provided to adult ICU patients and describe the relationship between sex, vital organ support, and mortality.
DESIGN:
In this retrospective observational study, we examined the provision of invasive ventilation (primary outcome), noninvasive ventilation, vasoactive medication, renal replacement therapy, extracorporeal membrane oxygenation (ECMO), or any one of these five vital organ supports in women compared with men. We performed logistic regression investigating the association of sex with each vital organ support, adjusted for illness severity, diagnosis, preexisting treatment limitation, year, and hospital. We performed logistic regression for hospital mortality adjusted for the same variables, stratified by vital organ support (secondary outcome).
SETTING AND PATIENTS:
ICU admissions in the Australia and New Zealand Intensive Care Society Adult Patient Database 2018–2021. This registry records admissions from 90% of ICUs in the two nations.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
We examined 699,535 ICU admissions (43.7% women) to 199 ICUs. After adjustment, women were less likely than men to receive invasive ventilation (odds ratio [OR], 0.64; 99% CI, 0.63–0.65) and each other organ support except ECMO. Women had lower adjusted hospital mortality overall (OR, 0.94; 99% CI, 0.91–0.97). Among patients who did not receive any organ support, women had significantly lower adjusted hospital mortality (OR, 0.82; 99% CI, 0.76–0.88); among patients who received any organ support women and men were equally likely to die (OR, 1.01; 99% CI, 0.97–1.04).
CONCLUSIONS:
Women received significantly less vital organ support than men in ICUs in Australia and New Zealand. However, our findings suggest that women may not be harmed by this conservative approach to treatment.
Keywords: gender, intensive care unit, invasive ventilation, organ support, sex differences
KEY POINTS.
Question: Do critically ill women and men receive the same amount of vital organ support, and how does this relate to the mortality of women compared with men?
Findings: In this large retrospective study of nearly 700,000 admissions to most ICUs across Australia and New Zealand, women were significantly less likely to receive invasive ventilation than men after adjustment for important confounders including illness severity. However, women had lower adjusted hospital mortality than men overall, this was related to significantly lower mortality among women who did not receive any organ support.
Meaning: Women were less likely to receive mechanical ventilation or any single vital organ support in ICU than men, yet they did not appear to be harmed by this conservative approach to treatment.
The provision of vital organ support in the ICU should be based upon the patient’s physiologic derangement and potential to benefit from such treatment. However, there is increasing evidence that a patient’s sex, race, and socioeconomic status impact upon treatment they receive in ICU (1–4).
Regarding patient sex, women appear less likely to receive invasive ventilation, vasoactive medication, renal replacement therapy (RRT), extracorporeal membrane oxygenation (ECMO), and tracheostomy than men (1, 5–7). However, a recent meta-analysis identified significant heterogeneity and risk of bias among existing studies of sex differences in ICU treatment (7). Many studies did not consider important confounders such as patient illness severity, admission diagnosis, and predefined limitations of medical treatment (LoMTs). To understand if the observed sex differences in ICU management represent equitable levels of support, it is important to confirm whether such differences reflect underlying variation in illness severity and treatment limitations, or sex itself.
Accordingly, in this study we aimed to examine sex differences in vital organ support provided to adult ICU patients in Australia and New Zealand. Specifically, we describe sex differences in the use of invasive ventilation, noninvasive ventilation (NIV), vasoactive medication, RRT, ECMO, and any one of these five organ supports. Our primary objective was to test the hypothesis that women would be less likely to receive invasive ventilation than men both before and after adjusting for important confounders. Our secondary objective was to test the hypothesis that there would be a relationship between sexes, all forms of vital organ support, and hospital mortality.
MATERIALS AND METHODS
Ethics Approval
The Alfred Health Human Research Ethics committee granted ethical approval for this study on April 1, 2021 (project number 200/21; “Sex differences in the outcomes, illness severity and resource use of intensive care patients in Australia and New Zealand”). The project was designed and conducted in accordance with the amended Declaration of Helsinki.
Study Design
This is a retrospective observational study of ICU admissions prospectively recorded in the Australia and New Zealand Intensive Care Society’s Adult Patient Database (APD). The APD is a clinical registry used for benchmarking of ICUs in Australia and New Zealand. There were 199 contributing ICUs during the study period, representing 90% of all ICUs in the two nations including all tertiary ICUs (8). Data collectors receive regular training and quality assurance review and data are collected using a standardized data dictionary. In addition, regular data checks further ensure the validity of recorded data (8).
Study Population
We included ICU admissions recorded in the APD between January 1, 2018, and December 31, 2021. We included patients 18 years old and over with complete data on sex, use of invasive ventilation, and LoMT. If a patient had multiple ICU admissions during the study period only the first ICU admission was included. We excluded patients admitted to ICU for palliative care or consideration of organ donation. We also excluded those classified as intersex, as this group represents less than 0.1% of ICU admissions in Australia and New Zealand (9).
Explanatory Variable: Sex
The APD data dictionary defines sex as the biological distinction between men and women; sex data are obtained from medical records. The registry does not record patient gender (10). This reflects current data recording practice in Australian hospital records: patient sex or gender is recorded and it is often unclear which is the intended focus (11). For consistency and readability, in this study we use the term “sex difference” and compare “women” and “men,” while acknowledging these limitations.
Outcomes: Organ Support
The primary outcome was sex differences in the provision of invasive ventilation through an artificial airway. Our secondary outcomes were sex differences in the provision of NIV via a mask, vasoactive medication (including inotropes and vasopressors), RRT (including continuous modes and intermittent hemodialysis), ECMO, or the provision of any one of these five vital organ supports.
Statistical Analysis
We report counts with percentage (n [%]) for categorical variables. We report normally distributed data using means (sd) and compared groups using the Student t test. For nonparametric data, we report median (interquartile range) and compared groups using the Wilcoxon rank-sum test. We took p value of less than 0.01 to indicate statistical significance and report 99% CIs throughout to increase the robustness of our findings.
We performed logistic regression analysis investigating the effect of sex on the provision of invasive ventilation adjusting for Acute Physiology and Chronic Health Evaluation (APACHE) III score, ICU admission diagnosis, preexisting LoMT, admission year, and hospital site. The APACHE III score is an illness severity score that incorporates age; chronic comorbidities including major organ failures, immune disorders, and hematological or metastatic malignancy; and acute physiologic derangement. The APACHE III score does not adjust for sex or gender. We adjusted for ICU admission diagnosis based on the Australian and New Zealand Intensive Care Society modification of the APACHE IV diagnosis list, which includes 117 individual diagnoses across both operative and nonoperative conditions (Table E1, http://links.lww.com/CCM/H424) (10). We repeated this logistic regression analysis for each individual vital organ support, and finally for the provision of any one of the five vital organ supports. Men were the reference sex group, therefore, odds ratios (ORs) greater than one indicates women were more likely to receive treatment than men. We performed complete case analysis, reporting the total number of patients included in each regression.
To mitigate the survival bias associated with receiving vital organ support at any point during ICU stay, the relationship between mortality and sex was determined separately according to vital organ support status. Therefore, we performed logistic regression for hospital mortality of those who received a vital organ support, and separately, for those who did not receive a vital organ support, adjusted for sex, APACHE III score, admission diagnosis, LoMT, admission year, and hospital site.
To further explore the relationship between illness severity, sex, and vital organ support, we performed subgroup analysis examining two strata of illness severity (below and above the median APACHE III score). We performed a sensitivity analysis excluding patients who received invasive ventilation within 24 hours of ICU admission, to examine patients who deteriorated later in their ICU admission. Similarly, we performed a sensitivity analysis excluding the COVID-19 years 2020 and 2021 as ICU case mix changed in this period. Finally, to estimate the potential impact of unmeasured confounders in this observational study, we calculated E-values for sex differences in the provision of each vital organ support (12).
We used STATA/BE 17 (StataCorp, College Station, TX) for all statistical analysis.
RESULTS
Study Population
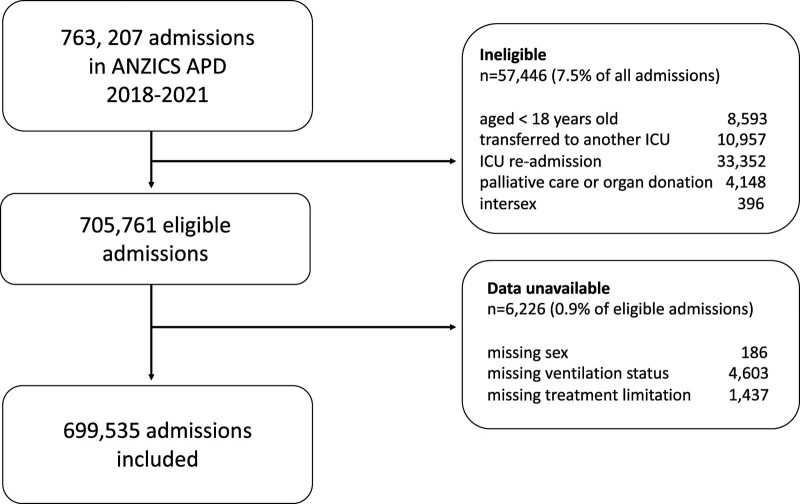
There were 763,207 ICU admissions recorded in the binational database during study period. Of these, 57,446 were excluded according to eligibility criteria (Fig. 1). Data pertaining to patient sex, invasive ventilation, or LoMT were unavailable in 6,226 (0.9%) of eligible admissions. The remaining 699,535 ICU patients were included in our study of whom 305,849 (43.7%) were women.
Figure 1.
Patient inclusion diagram. ANZICS APD = Australian and New Zealand Intensive Care Society Adult Patient Database.
Women were younger and had lower illness severity than men (Table 1). Despite this, more women than men had a LoMT order at ICU admission. Case mix varied between the sexes: women were less likely than men to be admitted due to cardiovascular illness, cardiac surgery, or trauma and more likely to be admitted with a gastrointestinal, hematological, metabolic, or renal disorder.
TABLE 1.
Characteristics of Women and Men Admitted to the ICU
| Characteristics | All Patients, n = 699,535 | Womena, n = 305,849 (43.7% of All Patients) | Mena, n = 393,686 (56.3% of All Patients) |
|---|---|---|---|
| Age, yr | 62.4 (17.4) | 61.4 (18.3) | 63.2 (16.6) |
| Acute Physiology and Chronic Health Evaluation III score | 50.1 (23.3) | 48.7 (23) | 51.2 (23.4) |
| Admission type | |||
| Elective | 275,852 (39.6%) | 115,818 (38%) | 160,034 (40.8%) |
| Emergency | 420,588 (60.4%) | 188,706 (62%) | 231,882 (59.2%) |
| Limitation of medical treatment | 53,789 (7.7%) | 25,456 (8.3%) | 28,333 (7.2%) |
| Length of ICU stay, hr | 41.1 (21.75–74) | 38.4 (21.3–70.8) | 42.8 (22.1–78.1) |
| Diagnostic category | |||
| Cardiovascular (excludes cardiac surgery) | 92,276 (13.2%) | 34,640 (11.4%) | 57,636 (14.7%) |
| Cardiac surgery | 70,783 (10.1%) | 16,858 (5.5%) | 53,925 (13.7%) |
| Respiratory | 99,794 (14.4%) | 44,645 (14.6%) | 55,149(14%) |
| Gastrointestinal | 116,561(16.7%) | 55,979 (18.3%) | 60,582 (15.4%) |
| Neurologic | 88,014 (12.6%) | 42,679 (14%) | 45,335 (11.5%) |
| Trauma | 32,155 (4.6%) | 9,761 (3.2%) | 22,394 (5.7%) |
| Sepsis | 53,285 (7.6%) | 23,798 (7.8%) | 29,487 (7.5%) |
| Metabolic, hematological, renal, and genitourinary | 95,934 (13.7%) | 52,099 (17.1%) | 43,835 (11.2%) |
| Musculoskeletal, soft tissue and skin | 49,419 (7.1%) | 24,732 (8.1%) | 24,687 (6.3%) |
| ICU mortality | 30,891 (4.4%) | 12,287 (4%) | 18,604 (4.7%) |
| Hospital mortality | 48,890 (7%) | 19,679 (6.5%) | 29,211 (7.5%) |
All variables were significantly different between women and men (p < 0.001); using t test or Wilcoxon rank-sum test as appropriate.
Data are presented as number and percentage of patients, mean and sd, or median and interquartile range.
Use of Vital Organ Support
A lower percentage of women than men received each vital organ support (Tables 2 and 3). This sex difference was largest for the provision of invasive ventilation (25.7% women vs 37.3% men). Furthermore, women who received invasive ventilation and vasoactive medication had significantly higher mean APACHE III scores than men who received these treatments. In contrast, women who received NIV or ECMO had lower illness severity scores than men.
Table 2.
Number of Patients Who Received Organ Support
| Vital Organ Support | Patients Receiving Vital Organ Support | Women Receiving Vital Organ Support |
Men Receiving Vital Organ Support |
|---|---|---|---|
| Invasive ventilation (n = 697,785) | 225,306 (32.2%) | 78,510 (25.7%) | 146,796 (37.3%) |
| Noninvasive ventilation (n = 583,077) | 62,849 (10.8%) | 27,221 (10.6%) | 35,628 (10.9%) |
| Vasoactive medication (n = 587,709) | 214,398 (36.3%) | 81,940 (31.7%) | 132,458 (39.8%) |
| Renal replacement therapy (n = 575,376) | 23,399 (4.1%) | 8,688 (3.4%) | 14,711 (4.6%) |
| Extracorporeal membrane oxygenation (n = 574,751) | 1,580 (0.3%) | 549 (0.2%) | 1,031 (0.3%) |
| Any vital organ support (n = 609,329) | 339,722 (55.1%) | 131,493 (49.4%) | 208,229 (59.5%) |
APACHE = Acute Physiology and Chronic Health Evaluation, n = patients with complete data for each organ support.
Data are presented as number and percentage of patients, mean with sd, absolute difference in means (99% CI), or odds ratios (99% CI) (see Table E2, http://links.lww.com/CCM/H424, for further details).
Table 3.
Mean Acute Physiology and Chronic Health Evaluation III Scores of Patients Who Received Organ Supports
| Vital Organ Support | Mean APACHE III of Women Receiving Vital Organ Support | Mean APACHE III of Men Receiving Organ Support | Absolute Difference in Mean APACHE III Score, Women Compared to Men (99% CI) |
|---|---|---|---|
| Invasive ventilation (n = 697,785) | 60.8 (27.3) | 58.6 (26.6) | 2.2 (1.9-2.5) |
| Noninvasive ventilation (n = 583,077) | 59 (23.2) | 60.1 (23.7) | -1.1 (-1.6 to -0.6) |
| Vasoactive medication (n = 587,709) | 62.9 (25.9) | 62.5 (25.6) | 0.4 (0.05-0.6) |
| Renal replacement therapy (n = 575,376) | 87 (28.6) | 86.9 (27.9) | 0.1 (-0.9-1.1) |
| Extracorporeal membrane oxygenation (n = 574,751) | 78.7 (32.5) | 84.3 (33.7) | -5.6 (-10.1 to -1) |
| Any vital organ support (n = 609,329) | 59.5 (25.2) | 59.1 (25.2) | 0.4 (0.2-0.7) |
APACHE = Acute Physiology and Chronic Health Evaluation, n = patients with complete data for each organ support.
Data are presented as number and percentage of patients, mean with sd, absolute difference in means (99% CI), or odds ratios (99% CI) (see Table E2, http://links.lww.com/CCM/H424, for further details).
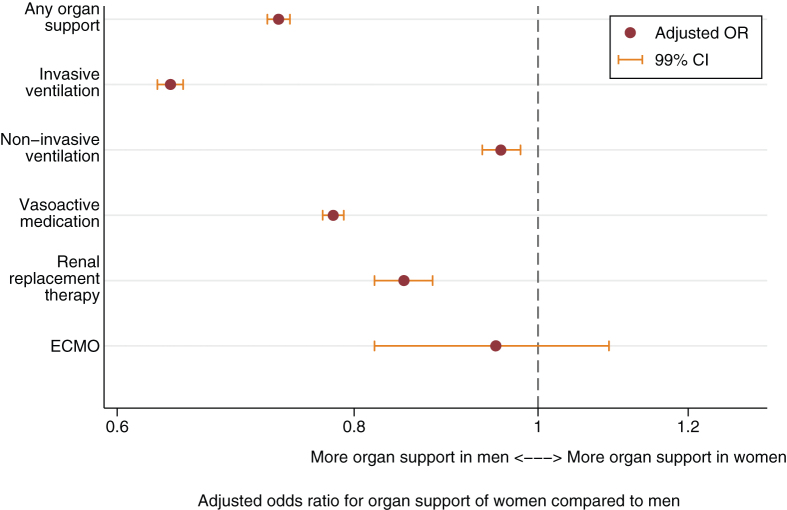
After adjustment for illness severity, diagnosis, LoMT, year, and hospital site, women were significantly less likely to receive each vital organ support except for ECMO (Fig. 2). This sex difference was most pronounced in the provision of invasive ventilation (OR, 0.64; 99% CI, 0.63–0.65; Fig. 3; and Table E2, http://links.lww.com/CCM/H424). After adjustment for confounders, ECMO was the only vital organ support that women and men were equally likely to receive.
Figure 2.
Vital organ support provided to women compared with men, adjusted for confounders. Vital organ support provided to women compared with men, adjusted for Acute Physiology and Chronic Health Evaluation III score, diagnosis, limitation of medical treatment, hospital site, and admission year in addition to sex in a logistic regression model. See Table E3 (http://links.lww.com/CCM/H424) for complete data included in each regression model. ECMO = extracorporeal membrane oxygenation, OR = odds ratio.
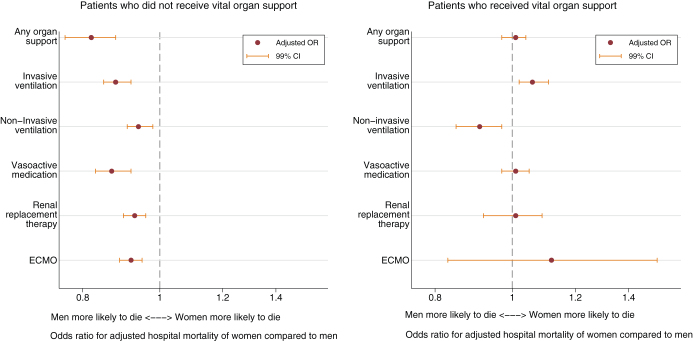
Figure 3.
Adjusted hospital mortality of women compared with men, stratified by vital organ support provided. The x-axis plots the adjusted hospital mortality of women compared with men, adjusted for Acute Physiology and Chronic Health Evaluation III score, admission diagnosis, limitation of medical treatment, admission year, and hospital site. The total study population is stratified according to the provision of each organ support: the left-hand graph plots mortality of patients who did not receive the organ support and the right-hand graph plots mortality patients who received the organ support. ECMO = extracorporeal membrane oxygenation, OR = odds ratio.
Sex Differences in Hospital Mortality and Their Relation to Vital Organ Support
The unadjusted hospital mortality was lower among women than men (6.5% vs 7.5%; p < 0.001). Women also had lower hospital mortality (OR, 0.94; 99% CI, 0.91–0.97) after adjustment for APACHE III score, admission diagnosis, LoMT, admission year, and hospital site.
The lower mortality among women, however, was related to the subgroup of patients who did not receive any vital organ support (Fig. 3). In contrast, among patients who received one or more vital organ supports, women and men were equally likely to die. Among patients who did not receive invasive ventilation, women were less likely to die than men; among patients who received invasive ventilation, women were more likely to die than men (Fig. 3).
Subgroup and Sensitivity Analyses
In subgroup analysis according to illness severity (Table E4, http://links.lww.com/CCM/H424), the higher-risk group showed no overall sex difference in adjusted hospital mortality yet women were still significantly less likely to receive invasive ventilation after adjustment for confounders. In contrast, in the lower risk group, women had a lower adjusted hospital mortality than men and were much less likely to receive invasive ventilation (Table E4, http://links.lww.com/CCM/H424). Again, this survival advantage for women was related to patients who did not receive invasive ventilation. Among lower-risk patients who received invasive ventilation, women were more likely to die than men.
On sensitivity analysis that excluded patients ventilated on their first day in ICU, women were still less likely than men to subsequently receive invasive ventilation (adjusted OR, 0.68; 99% CI, 0.65–0.71). Further, during the pre-COVID-19 period (2018 and 2019) women were again less likely to receive invasive ventilation (adjusted OR, 0.65; 99% CI, 0.64–0.67).
Regarding potential unmeasured confounding, the E-value for the association of sex with invasive ventilation was larger in magnitude than the point estimate OR (E-value, 1.81 vs OR 0.64 [inverse OR, 1.56]; E-value for CI, 1.79). Therefore, any unmeasured confounder/s would need a stronger association than that observed between sex and vital organ support to explain away this primary finding. We observed the same pattern in E-values for the association between sex and each other organ support except ECMO (Table E5, http://links.lww.com/CCM/H424).
DISCUSSION
Key Findings
In this large retrospective study of patients treated in 199 Australian and New Zealand ICUs, women received less vital organ support than men before and after adjustment for important confounders including diagnosis, illness severity score, treatment limitation (LoMT), year, and hospital site. Compared with men, women were less likely to receive invasive ventilation, NIV, vasoactive medication, and RRT, but equally likely to receive ECMO.
Despite receiving less vital organ support, women were less likely to die than men, even after adjustment for illness severity and other key confounders. This survival advantage was related to lower mortality among women in the subgroup of patients who did not receive any vital organ support. In contrast, among patients who received one or more vital organ supports, women and men were equally likely to die.
Comparison to Other Studies
Our finding that women were less likely than men to receive invasive ventilation and other vital organ supports except ECMO is consistent with many previous reports including our 2022 meta-analysis (5–7, 13–18). Of note, few previous studies have examined sex differences in the provision of ECMO. These had small case cohorts; a shortcoming which also applied to our ECMO cohort (6, 19).
In contrast to previous studies, we examined a comprehensive population of adult ICU patients across two nations with broadly consistent findings across multiple organ supports. Furthermore, we adjusted for predefined LoMTs, so the observed sex differences cannot be attributed to LoMTs. This is important because women were more likely than men to have a LoMT recorded prior to ICU admission. This disparity in LoMT is consistent with previous studies and may represent another systemic difference in the treatment of women and men (20, 21).
Unlike most previous studies, the women in our study population were younger, had lower illness severity scores and lower adjusted mortality than the men (1, 5, 7, 22). Therefore, we must consider whether the women in our study simply had less need for organ support—or even ICU admission—than men. However, we adjusted for both illness severity and ICU admission diagnosis in our logistic regression model for the provision of each organ support. Furthermore, in the highest-illness severity subgroup of patients, women had equivalent hospital mortality to men yet were significantly less likely to receive invasive ventilation. These observations support a true disparity in treatment provided to women and men.
Implications
There are several possible reasons why women received less vital organ support than men in our study. Clinicians may have underestimated illness severity in women or conversely overestimated illness severity in men. Such disparities in illness recognition are described elsewhere, for example, women are more likely to have delayed diagnosis of myocardial infarction and delayed revascularization (23). However, women were admitted to ICU at lower illness severity than men which suggests that clinicians did not systematically underestimate their illness severity. Admitting women to ICUs at lower illness severity may have facilitated observation and timely noninvasive treatment, averting the need for vital organ support.
Clinicians may believe that women prefer a more conservative approach to treatment than men, or women may choose more conservative treatment for themselves (1). In our study, women had more limitations to treatment “despite” having lower illness severity than men overall. We do not know what proportion of these LoMTs reflected the patients’ own advanced directives or were based on the clinician’s assessment of prognosis. Previous studies suggest that a minority of LoMTs are based on the patient’s directives. Instead, most LoMTs are defined by clinicians based on perceived prognosis and potential to benefit from treatment (20, 24).
Finally, the observed sex differences in vital organ support may be related to the use of standardized rather than sex-adjusted physiologic treatment thresholds. For example, women have lower baseline serum urea levels than men, so a standardized urea threshold for commencing RRT would lead to more men receiving RRT than women (7). However, this does not readily explain the observed sex differences in the provision of vasoactive medication or ventilation.
In our second key finding, among those patients who did not receive vital organ support, women had lower hospital mortality than men, and, in contrast, equivalent hospital mortality to men among those who did receive vital organ support. This suggests that either the conservative approach to treatment was particularly beneficial for women (or injurious to men) or that conservative treatment could potentially benefit all patients but was applied to women more frequently.
Avoiding vital organ support may be particularly beneficial to women because these treatments may be poorly tailored for female patients. For example, women intubated for respiratory failure are less likely to receive lung protective ventilation, instead receiving higher weight-adjusted tidal volumes than men (25). This leads to another possible explanation for the sex difference in vital organ support: clinicians may have correctly perceived the relative advantage to women of avoiding vital organ support and deliberately adopted a more conservative approach.
Alternatively, our findings may represent a natural experiment in which a higher treatment threshold was applied to one group of patients—women—with favorable outcomes. This leads to the hypothesis that all patients, including men, could benefit from a more conservative approach to treatment. While this hypothesis cannot be confirmed based on our observational study, there is some evidence from randomized clinical trials supporting a “less is more” approach to treating critical illness (26–28).
Our study demonstrates that we cannot assume that critical care interventions are applied equally between the sexes, nor that women and men have similar outcomes from these interventions. Therefore, it is vital that critical care trials present sex-disaggregated data with sex-based subgroup analyses.
Strengths and Limitations
This study has several key strengths. It is the largest study of sex differences in the treatment of ICU patients to date, including most adult ICU patients in Australia and New Zealand during the study period. Our results are robust across several types of vital organ support, novel in scope, and carry important implications.
We also acknowledge limitations to our study. As an observational study, we cannot determine the underlying cause for the observed sex differences in vital organ support, nor the direction of the relationship between sex, vital organ support, and hospital mortality. We examined patients admitted to ICU rather than all critically unwell patients; therefore, we cannot exclude the possibility that ICU admission collides with the relationship between patient sex and vital organ support, introducing bias. We could not test counterfactuals—for example, what if more men were admitted for observation only? What if more women received vital organ support?—and this necessarily limits our conclusions. Additionally, we did not have data on the time to initiation of vital organ support, which may be an important determinant of outcome following such support.
While we had complete data on provision of invasive ventilation, data on other organ supports was missing for a minority of patients. The impact of data missingness on our findings may be mitigated by the fact that the sex balance among patients with missing data reflected the study population overall.
Another limitation concerns the sex data, which is recorded in the APD from medical records and may have been self-reported or determined by clinicians or clerical staff. In the absence of an accompanying gender variable, the observed differences between “women” and “men” likely represent aspects of both biologically affected sex and socially grounded gender. The terms sex and gender are often used interchangeably in critical care research and health research more broadly (11, 22, 29, 30). Sex and gender also interact: the social context of men and women affects their biological differences and vice versa (29). Therefore, the “sex differences” observed here may be more accurately characterized as “sex/gender” differences. Furthermore, we considered only a binary definition of sex, so we are unable to comment upon the treatment and outcomes of intersex or nonbinary ICU patients. Understanding the characteristics of critical illness in nonbinary people is essential to ensuring equitable healthcare.
There is a relatively low illness severity and mortality in our study population compared with other similar studies, which could limit the generalizability of our findings (13, 16). However, our key finding of sex differences in the use of invasive ventilation persisted in the subgroup at highest risk of death. Furthermore, the use of invasive ventilation in our study population (approximately one in three patients) is in keeping with recent large studies of sex differences in ICU treatment (1, 17, 31). Finally, our findings may reflect the sociocultural context of Australia and New Zealand; though they are consistent with previous studies from high-income countries (7).
CONCLUSIONS
Among adult patients admitted to Australian and New Zealand ICUs, women received less vital organ support than men even after adjustment for important confounders including diagnosis, illness severity, and LoMT. Despite receiving less invasive therapy, women were also less likely to die than men. This survival advantage for women was confined to the subgroup of patients who did not receive any vital organ support. In contrast, among patients who received one or more vital organ supports, women and men were equally likely to die.
These observations suggest that critically ill women received more conservative treatment than men, and that, paradoxically, they may not have been harmed by this approach.
ACKNOWLEDGMENTS
We and the Australian and New Zealand Intensive Care Society Centre for Outcome and Resource Evaluation management committee would like to thank clinicians, data collectors, and researchers at the following contributing sites listed in E6 (http://links.lww.com/CCM/H424). In addition, we would like to acknowledge the helpful comments provided by Daryl Jones during the revision of this article.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Modra received funding from the Graeme Clark Institute of the University of Melbourne-Women in Science Technology and Mathematics Award, and the Australia and New Zealand Intensive Care Society-Peter Hicks Fellowship Award; she disclosed that she is a member of the Women in Intensive Care Medicine Network of the Australia and New Zealand Intensive Care Society. Dr Higgins received funding from a National Health and Medical Research Council Emerging Leader Fellowship (Grant #2008447). The remaining authors have disclosed that they do not have any potential conflicts of interest.
*See also p. 136.
Dr. Modra (0000-0002-0062-3705), Dr. Higgins (0000-0001-8295-7559), Dr. Pilcher (0000-0002-8939-7985), Dr. Bailey (0000-0002-5551-1401), and Dr. Bellomo (0000-0002-1650-8939).
REFERENCES
- 1.Todorov A, Kaufmann F, Arslani K, et al. ; Swiss Society of Intensive Care Medicine: Gender differences in the provision of intensive care: A Bayesian approach. Intensive Care Med. 2021; 47:577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danziger J, Angel Armengol de la Hoz M, Li W, et al. : Temporal trends in critical care outcomes in U.S. minority-serving hospitals. Am J Respir Crit Care Med. 2020; 201:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGowan SK, Sarigiannis KA, Fox SC, et al. : Racial disparities in ICU outcomes: A systematic review. Crit Care Med. 2022; 50:1–20 [DOI] [PubMed] [Google Scholar]
- 4.Garland A, Olafson K, Ramsey CD, et al. : Reassessing access to intensive care using an estimate of the population incidence of critical illness. Crit Care. 2018; 22:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zettersten E, Jaderling G, Bell M, et al. : A cohort study investigating the occurrence of differences in care provided to men and women in an intensive care unit. Sci Rep. 2021; 11:23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blecha S, Zeman F, Specht S, et al. : Invasiveness of treatment is gender dependent in intensive care. Anesth Analg. 2021; 132:1677–1683 [DOI] [PubMed] [Google Scholar]
- 7.Modra LJ, Higgins AM, Abeygunawardana VS, et al. : Sex differences in treatment of adult intensive care patients: A systematic review and meta-analysis. Crit Care Med. 2022; 50:913–923 [DOI] [PubMed] [Google Scholar]
- 8.ANZICS Centre for Outcomes and Resource Evaluation: Centre for Resource and Outcomes Evaluation 2020 Report. 2021. Available at: https://www.anzics.com.au/wp-content/uploads/2021/09/2020-ANZICS-CORE-Report.pdf. Accessed January 10, 2023
- 9.Modra LJ, Pilcher D, Bailey M, et al. : Sex differences in intensive care unit admissions in Australia and New Zealand. Crit Care Resusc. 2021; 23:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ANZICS Centre for Outcomes and Resource Evaluation: Adult Patient Database Data Dictionary. Version 6.1. 2022. Melbourne, VIC, Australia, ANZICS CORE. Available at: https://www.anzics.com.au/wp-content/uploads/2021/03/ANZICS-APD-Data-Dictionary.pdf. Accessed January 10, 2023 [Google Scholar]
- 11.Australian Government: Australian Institute of Health and Welfare (AIHW) Data by Sex and Gender. Version 10.0. 2023. Available at: https://www.aihw.gov.au/about-our-data/aihw-data-by-sex-and-gender. Accessed July 4, 2023
- 12.Haneuse S, VanderWeele TJ, Arterburn D: Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019; 321:602–603 [DOI] [PubMed] [Google Scholar]
- 13.Fowler RA, Sabur N, Li P, et al. : Sex- and age-based differences in the delivery and outcomes of critical care. CMAJ. 2007; 177:1513–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hessey E, Montgomery C, Zuege DJ, et al. : Sex-specific prevalence and outcomes of frailty in critically ill patients. J Intensive Care. 2020; 8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen HL, Lu CL, Yang HH: Women receive more trials of noninvasive ventilation for acute respiratory failure than men: A nationwide population-based study. Crit Care. 2011; 15:R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valentin A, Jordan B, Lang T, et al. : Gender-related differences in intensive care: A multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med. 2003; 31:1901–1907 [DOI] [PubMed] [Google Scholar]
- 17.Wernly B, Bruno RR, Kelm M, et al. : Sex-specific outcome disparities in very old patients admitted to intensive care medicine: A propensity matched analysis. Sci Rep. 2020; 10:18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuelsson C, Sjoberg F, Karlstrom G, et al. : Gender differences in outcome and use of resources do exist in Swedish intensive care, but to no advantage for women of premenopausal age. Crit Care. 2015; 19:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song J, Ahn S, Kim J, et al. : Sex-related disparities in the in-hospital management of patients with out-of-hospital cardiac arrest. Resuscitation. 2022; 173:47–55 [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann M, Perren A, Cerutti B, et al. ; Swiss Society of Intensive Care Medicine: Severity-adjusted ICU mortality only tells half the truth-the impact of treatment limitation in a nationwide database. Crit Care Med. 2020; 48:e1242–e1250 [DOI] [PubMed] [Google Scholar]
- 21.McPherson K, Carlos WG, 3rd, Emmett TW, et al. : Limitation of life-sustaining care in the critically ill: A systematic review of the literature. J Hosp Med. 2019; 14:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modra L, Higgins A, Vithanage R, et al. : Sex differences in illness severity and mortality among adult intensive care patients: A systematic review and meta-analysis. J Crit Care. 2021; 65:116–123 [DOI] [PubMed] [Google Scholar]
- 23.Vogel B, Acevedo M, Appelman Y, et al. : The lancet women and cardiovascular disease commission: Reducing the global burden by 2030. Lancet. 2021; 397:2385–2438 [DOI] [PubMed] [Google Scholar]
- 24.Rubio O, Arnau A, Cano S, et al. : Limitation of life support techniques at admission to the intensive care unit: A multicenter prospective cohort study. J Intensive Care. 2018; 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swart P, Deliberato RO, Johnson AEW, et al. : Impact of sex on use of low tidal volume ventilation in invasively ventilated ICU patients-a mediation analysis using two observational cohorts. PLoS One. 2021; 16:e0253933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auriemma CL, Van den Berghe G, Halpern SD: Less is more in critical care is supported by evidence-based medicine. Intensive Care Med. 2019; 45:1806–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagshaw SM, Wald R, Adhikari NKJ, et al. ; STARRT-AKI Investigators: Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020; 383:240–251 [DOI] [PubMed] [Google Scholar]
- 28.Lamontagne F, Richards-Belle A, Thomas K, et al. ; 65 trial investigators: The effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension. JAMA. 2020; 323:938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Springer KW, Stellman JM, Jordan-Young RM: Beyond a catalogue of differences: A theoretical frame and good practice guidelines for researching sex/gender in human health. Soc Sci Med. 2012; 74:1817–1824 [DOI] [PubMed] [Google Scholar]
- 30.Bewley S, McCartney M, Meads C, et al. : Sex, gender, and medical data. BMJ. 2021; 372:n735. [DOI] [PubMed] [Google Scholar]
- 31.Mahmood K, Eldeirawi K, Wahidi MM: Association of gender with outcomes in critically ill patients. Crit Care. 2012; 16:R92. [DOI] [PMC free article] [PubMed] [Google Scholar]