Abstract
Objective:
The objective of this study was to assess the pharmacokinetics, safety, and efficacy and confirm the dose of once-daily bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF; B/F/TAF) during pregnancy.
Design:
An open-label, multicenter, single-arm, phase 1b study (NCT03960645) was conducted in 33 virologically suppressed pregnant women with HIV-1.
Methods:
Participants received B/F/TAF (50/200/25 mg) from the second or third trimester through ∼16 weeks postpartum. Steady-state maternal plasma pharmacokinetic samples were collected at the second and third trimesters and 6 and 12 weeks postpartum for BIC, FTC, and TAF. Neonates (n = 29) were followed from birth to 4–8 weeks with sparse washout pharmacokinetic sampling for BIC and TAF. The proportion of participants with HIV-1 RNA less than 50 copies/ml at delivery (missing = excluded) was evaluated.
Results:
Mean areas under the concentration–time curve over the dosing interval (AUCtau) for BIC, FTC, and TAF were lower during pregnancy versus postpartum but were closer to AUCtau values for nonpregnant adults with HIV reported in other studies. Geometric least-squares mean ratios for BIC, FTC, and TAF AUCtau during pregnancy versus postpartum ranged from 41 to 45%, 64 to 69% and 57 to 78%, respectively. Mean BIC trough concentrations during pregnancy were more than 6.5-fold greater than the protein-adjusted 95% effective concentration. In neonates, the median BIC half-life was 43 h. Virologic suppression was maintained in all adult participants throughout the study, with no virologic failure or treatment-emergent resistance to HIV-1, no discontinuations because of adverse events, and no perinatal transmission.
Conclusion:
Exposures to BIC, FTC, and TAF were lower during pregnancy than postpartum. However, mean BIC trough concentrations were maintained at levels indicative of efficacious exposure, and FTC/TAF data were concordant with published literature in this population. Pharmacokinetic and safety data, combined with maintenance of robust virologic suppression, suggest that once-daily B/F/TAF without dose adjustment is appropriate during pregnancy.
Keywords: efficacy, neonate, pharmacokinetics, pregnancy, protein binding, safety
Introduction
Approximately 1.2 million women with HIV infection were pregnant in 2022 worldwide [1]. Guidelines recommend that pregnant women with HIV receive effective antiretroviral therapy (ART) for maternal health and to avoid perinatal transmission [2,3]; however, limited treatment options exist because of toxicity, low drug exposure, or teratogenicity [2]. Therefore, therapies that are well tolerated, effective, and convenient for pregnant women with HIV are needed.
The combination of bictegravir (BIC; B), emtricitabine (FTC; F), and tenofovir alafenamide (TAF) (B/F/TAF) is approved as a guideline-recommended, once-daily, single-tablet, complete regimen for the treatment of HIV type 1 (HIV-1) infection [4,5]. B/F/TAF treatment leads to high rates of virologic suppression in treatment-naive people with HIV and maintains virologic suppression among people switching from other ART regimens [6–10]. However, limited data exist regarding B/F/TAF use during pregnancy [4]; therefore, use in pregnant women is not currently included in the product label.
Pregnancy is associated with physiological changes that can influence drug disposition [11]. BIC is metabolized by uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) and cytochrome P450 (CYP) 3A [4], which both show increased activity during pregnancy [11]. BIC is also highly protein bound [4], and decreased plasma protein binding of drugs has been reported during pregnancy [11].
This open-label study evaluated the pharmacokinetics, safety, and efficacy of B/F/TAF in virologically suppressed pregnant women with HIV-1. The primary objective was to evaluate the steady-state pharmacokinetics of BIC during pregnancy and to confirm whether once-daily B/F/TAF is appropriate. The secondary objectives included evaluating the steady-state pharmacokinetics of FTC and TAF and assessing efficacy during pregnancy.
Methods
Ethics statement
The study protocol (Supplemental Digital Content Study Protocol) was approved by central or site-specific review boards and ethics committees (Supplemental Digital Content Table 1). Pregnant women provided written informed consent, and parents/legal guardians provided written informed consent for the participation of neonates born to women in the study.
Study design and participants
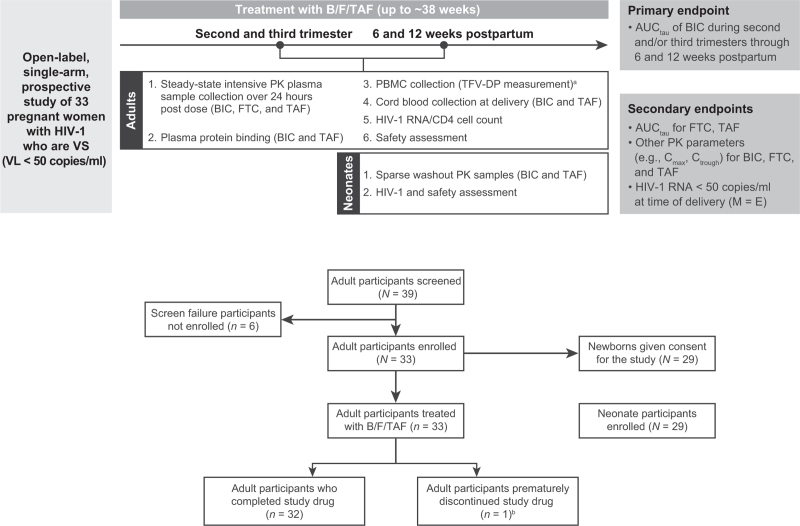
This was an open-label, multicenter, single-arm, prospective phase 1b study in pregnant women with HIV-1 (NCT03960645) (Fig. 1). Screening began on 28 June 2019 with final follow-up of the last participant on 18 August 2022. Details of study locations and full eligibility criteria are available (see Supplemental Digital Content Extended Methodology). Briefly, participants were aged at least 18 to less than 40 years, were pregnant for at least 12 and at most 31 weeks at screening, had HIV-1 RNA less than 50 copies/ml for at least 6 months, and were on stable ART (any regimen) for at least 6 months. Chronic hepatitis B virus and active hepatitis C virus infection were exclusions.
Fig. 1.
Study design and disposition of participants.
Neonates born to women participating in the study were followed from birth to 4–8 weeks of age if consent had been obtained from the parent/legal guardian. No neonatal participant in the study was treated with the study drug. aExploratory endpoint. bProtocol violation: M184V and other mutations at baseline, resulting in resistance to nucleoside/nucleotide reverse transcriptase inhibitor and nonnucleoside reverse transcriptase inhibitor treatment (n = 1). AUCtau, area under the plasma drug concentration–time curve over the dosing interval; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; BIC, bictegravir; Cmax, maximum observed plasma drug concentration; Ctrough, trough concentration; FTC, emtricitabine; HIV-1, HIV type 1; M = E, missing = excluded; PBMC, peripheral blood mononuclear cell; PK, pharmacokinetic; TAF, tenofovir alafenamide; TFV-DP, tenofovir diphosphate (active metabolite); VL, viral load; VS, virologically suppressed.
Adult participants were treated with B/F/TAF (50/200/25 mg) orally once daily without regard to food. Pharmacokinetic assessments were performed during the second and/or third trimester of pregnancy, at delivery, and 6 and 12 weeks postpartum. Efficacy and safety assessments were conducted throughout the study period.
Eligible neonates were followed from birth to 4–8 weeks. Neonates were not dosed with B/F/TAF.
Study outcomes
Study outcomes included measures of pharmacokinetics [BIC, FTC, TAF, tenofovir diphosphate (TFV-DP)], efficacy (HIV-1 RNA < 50 copies/ml, CD4 cell count) and safety assessments. Details of these assessments, including primary and secondary endpoints, are provided in Fig. 1. Additional details regarding pharmacokinetic assessments and statistical analyses are provided in Supplemental Digital Content Extended Methodology. All analyses were prespecified as per the study protocol.
Pharmacokinetic assessments
Adult steady-state plasma pharmacokinetic samples were collected for BIC, FTC, and TAF over 24 h post dose across four periods (second and/or third trimester and 6 and 12 weeks postpartum). Additionally, optional trough peripheral blood mononuclear cell (PBMC) samples for TFV-DP and protein binding samples for BIC and TAF were collected.
For BIC and TAF, maternal and umbilical cord samples were collected at delivery, and sparse washout pharmacokinetic samples were collected for up to 7 days after birth in enrolled neonates.
Concentrations of BIC, FTC, TAF, and TFV-DP and protein binding were quantified using validated assays. Pharmacokinetic parameters were generated using noncompartmental analyses (Phoenix WinNonlin v8.3.5).
Efficacy assessments
Adult plasma HIV-1 RNA levels were assessed at all visits. The proportion of adult participants with plasma HIV-1 RNA less than 50 copies/ml at each visit was summarized using the missing = excluded (M = E) approach. CD4 cell count was assessed at baseline and 12 weeks postpartum. Neonatal HIV-1 RNA levels were determined as available at birth and 4–8 weeks postbirth, and qualitative HIV-1 RNA or DNA PCR was performed according to local standard of care.
Safety assessments
Safety for adult and neonate participants was evaluated based on adverse event monitoring, clinical laboratory analyses, vital sign measurement, and physical examinations.
Resistance analysis
Resistance testing was to be performed for any participant who received at least one dose of study drug, maintained the study drug regimen, and met the criteria for virologic failure as described in the protocol.
Statistical analysis
A mixed-effects analysis of variance model was used for pharmacokinetic parameters, and ratios of geometric least-squares means (GLSMs) between pregnancy (second or third trimester) and postpartum (6 or 12 weeks postpartum) were calculated. The cross-trial comparison with data from nonpregnant adults with HIV was a qualitative numeric comparison to contextualize the findings.
Results
Participant disposition
In total, 33 pregnant women were enrolled from Dominican Republic (n = 3), Thailand (n = 25), and USA (n = 5) (Fig. 1); 32 participants completed the study. One participant prematurely discontinued B/F/TAF treatment after three doses because of a violation of key eligibility criteria. Participants received B/F/TAF treatment for up to approximately 38 weeks from pregnancy through a median of 16.1 (range, 15.7–18.0) weeks postpartum.
All 33 participants were included in the pharmacokinetics, efficacy, and safety analyses. Parental consent was provided to enroll 29 neonates in the study. Pregnant women were predominantly Asian (76%) with a median age of 30 years (Supplemental Digital Content Table 2).
Pharmacokinetics of bictegravir, tenofovir alafenamide, and emtricitabine
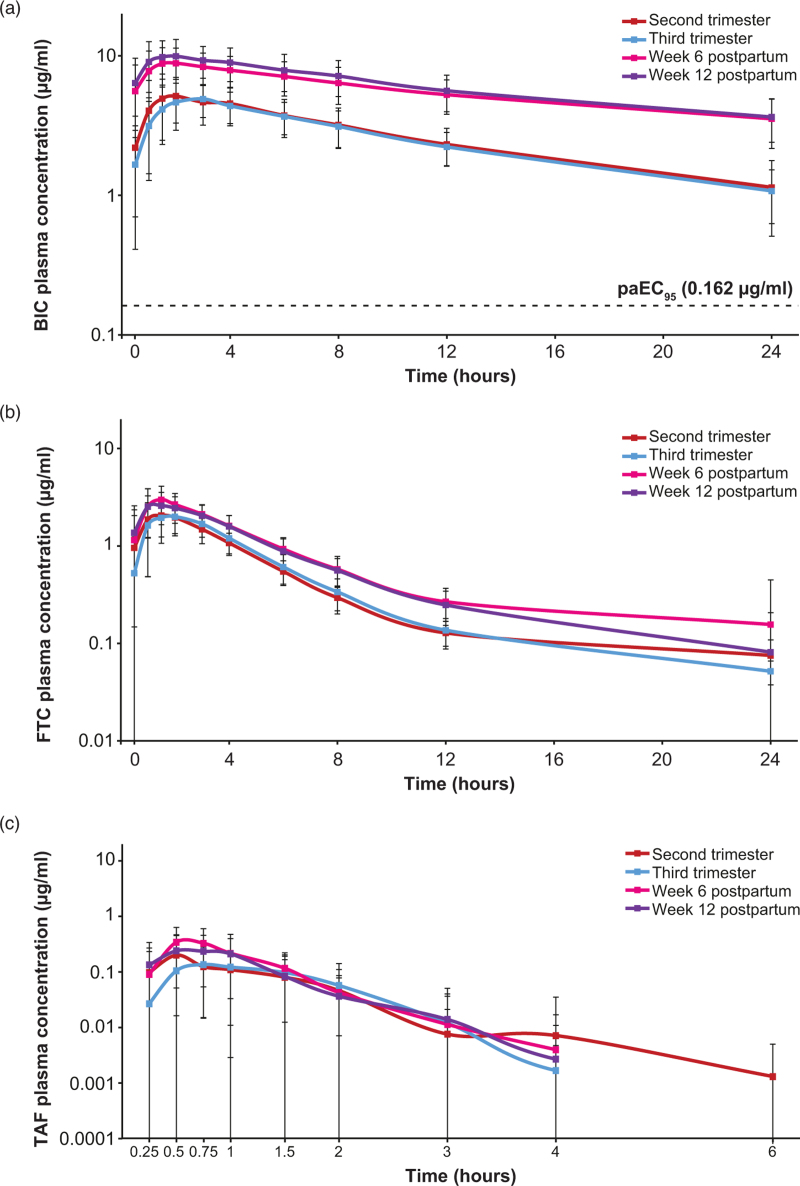
Plasma concentration–time profiles of BIC showed that exposure was similar between the second and third trimester, and between 6 and 12 weeks postpartum, but lower during pregnancy than postpartum (Fig. 2).
Fig. 2.
Mean (SD) steady-state plasma concentration–time profiles following B/F/TAF administration (semi-log scale) for (a) BIC, (b) FTC, and (c) TAF.
Mean postdose concentration values below the lower limit of quantitation (LLOQ) were not displayed; values below the limit of quantification were treated as 0 for predose and one-half the LLOQ for postdose summaries. LLOQ was defined as 0.02 μg/ml for BIC, 0.005 μg/ml for FTC, and 0.001 μg/ml for TAF. B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; BIC, bictegravir; FTC, emtricitabine; paEC95, inhibitory quotient at protein-adjusted 95% effective concentration; SD, standard deviation; TAF, tenofovir alafenamide.
Mean area under the concentration–time curve over the dosing interval (AUCtau) for BIC was lower during the second and third trimesters than at 6 and 12 weeks postpartum (Table 1). Percentage GLSM (%GLSM) ratios of BIC AUCtau during pregnancy versus postpartum (primary endpoint) ranged from 40.6 to 44.7% (Supplemental Digital Content Table 3). Mean trough concentration (Ctrough) for BIC during both the second and third trimesters was more than 6.5-fold greater than the protein-adjusted 95% effective concentration (paEC95) for BIC (0.162 μg/ml [4]) (Table 1), and individually greater than the paEC95 during pregnancy in all participants, except for one participant in the second trimester who also maintained virologic suppression (Supplemental Digital Content Figure 1).
Table 1.
Pharmacokinetic parameters for bictegravir.
| Second trimester (n = 21) | Third trimester (n = 30) | Week 6 postpartum (n = 31) | Week 12 postpartum (n = 32) | Nonpregnant adults with HIV (n = 1193)a | |
| AUCtau (h·μg/ml) [mean (%CV)] | 62.8 (32.2) | 60.2 (29.1) | 135 (26.9) | 148 (28.5) | 102 (26.9) |
| Unbound AUCtau (h·μg/ml [mean (%CV)]b | 0.224 (42.0) | 0.219 (33.9) | 0.354 (34.2) | 0.374 (32.2) | – |
| Cmax (μg/ml) [mean (%CV)] | 5.82 (30.1) | 5.37 (25.9) | 9.77 (23.3) | 11.0 (24.9) | 6.15 (22.9) |
| Ctrough (μg/ml) [mean (%CV)] | 1.05 (45.2) | 1.07 (41.7) | 3.53 (38.4) | 3.64 (34.1) | 2.61 (35.2) |
| CLss/F (ml/h) [mean (%CV)] | 912 (47.5) | 902 (31.8) | 399 (28.4) | 362 (26.5) | – |
| VZ/F (ml) [mean (%CV)] | 11,900 (37.1) | 13,400 (32.4) | 10,300 (35.9) | 8690 (27.6) | – |
| Tmax (h) [median (Q1, Q3)] | 2.00 (1.50, 3.00) | 2.00 (1.50, 3.00) | 1.50 (1.00, 3.00) | 1.50 (1.00, 2.00) | – |
| t½ (h) [median (Q1, Q3)] | 9.09 (8.24, 11.5) | 9.91 (9.10, 11.4) | 18.2 (14.4, 21.5) | 17.4 (14.3, 19.4) | – |
The PK parameter values for nonpregnant adults with HIV-1 are based on population PK analysis in Studies GS-US-380-1489, GS-US-380-1490, GS-US-380-1844, and GS-US-380-1878 for BIC (n = 1193) [4,13].
Unbound values were calculated by correcting the individual AUCtau estimates by the percentage unbound fraction for BIC.
%CV, percentage coefficient of variation; AUCtau, area under the plasma drug concentration–time curve over the dosing interval; BIC, bictegravir; CLss/F, apparent oral clearance of the drug at steady state; Cmax, maximum observed plasma concentration of drug; Ctrough, trough concentration; PK, pharmacokinetic; Q, quartile; t1/2, terminal elimination half-life; TAF, tenofovir alafenamide; Tmax, observed time point of Cmax; Vz/F, apparent volume of distribution.
BIC exposures during pregnancy were contextualized with those of nonpregnant adults with HIV, by utilizing pooled data from four phase 3 registrational trials (n = 1193; Table 1). BIC exposures in pregnancy were closer to those in nonpregnant adults than postpartum, with mean BIC AUCtau in the third trimester approximately 40% lower than in nonpregnant adults with HIV.
Plasma protein binding for BIC was lower during pregnancy than postpartum, with higher mean unbound fractions during the second (0.351%) and third (0.365%) trimesters of pregnancy compared with 6 weeks (0.261%) and 12 weeks (0.252%) postpartum (Supplemental Digital Content Table 4). When adjusted for protein binding, the %GLSM ratios for unbound BIC AUCtau during pregnancy versus postpartum ranged from 58.8 to 62.4%.
Plasma concentration–time profiles for FTC and TAF are shown in Fig. 2, and pharmacokinetic parameters are shown in Supplemental Digital Content Table 5. FTC and TAF exposures were generally lower during pregnancy than postpartum, with a less pronounced decrease seen in TAF exposure when corrected for the unbound fraction. However, the reduction in FTC and TAF exposures observed during pregnancy in this study was smaller than in a cross-study comparison with nonpregnant adults with HIV-1 (Supplementary Digital Content Table 5). The %GLSM ratios for exposure during pregnancy versus postpartum ranged from 64.3 to 69.2% for FTC AUCtau, 56.5 to 77.6% for TAF AUCtau, and 83.6 to 89.3% for unbound TAF AUCtau (Supplemental Digital Content Table 3).
Plasma protein binding of TAF was lower during pregnancy compared with postpartum (Supplemental Digital Content Table 4). Plasma protein binding for FTC was not evaluated, as previous data have shown that FTC exhibits low protein binding [12].
Trough TFV-DP levels in PBMCs were generally similar across pregnancy and postpartum periods with substantial inter-individual variability (Supplemental Digital Content Table 6).
The mean (percentage coefficient of variation) ratio of cord blood to maternal blood plasma concentration for BIC was 1.4 (34.5; n = 29), indicating that BIC crosses the placental barrier. TAF levels were below the limit of quantification in most samples (21 of 31 maternal; 26 of 28 cord blood); only two paired ratios for TAF were calculable (ratios of 0.09 and 1.12).
In available washout pharmacokinetic samples collected from enrolled neonates (n = 10), the median (interquartile range) BIC half-life was 43.1 (38.4–57.6) hours. TAF concentrations in all neonate blood samples were below the limit of quantification.
Efficacy of bictegravir/emtricitabine/tenofovir alafenamide
Virologic suppression (HIV-1 RNA <50 copies/ml) was maintained in 100% of participants during pregnancy, at delivery, and up to median (range) of 16.1 (15.7–18.0) weeks postpartum (analysis visit; Table 2). No virologic failure or treatment-emergent resistance was observed. CD4 cell count and CD4% among maternal participants remained stable throughout the study (Table 2).
Table 2.
Virologic suppression, CD4 cell count, and CD4% by visit during bictegravir/emtricitabine/tenofovir alafenamide treatment.
| B/F/TAF (N = 33) | |
| HIV-1 RNA <50 copies/ml [n/N (%)]a | |
| Baseline | 33/33 (100) |
| Week 12b | 23/23 (100) |
| Delivery | 32/32 (100) |
| Week 6 postpartum | 32/32 (100) |
| Week 12 postpartum | 32/32 (100) |
| Week 18 postpartumc | 32/32 (100) |
| CD4 cell count (cells/μl) [median (Q1, Q3)] | |
| Baseline | 558 (409, 720) |
| Week 12 postpartumc | 636 (491, 1026) |
| Change from baseline to week 12 postpartumd | 159 (27, 296) |
| CD4% [median (Q1, Q3)] | |
| Baseline | 32.3 (27.0, 40.2) |
| Week 12 postpartumc | 32.5 (29.2, 37.9) |
| Change from baseline to week 12 postpartumd | 0.1 (−2.3, 4.2) |
Missing = excluded analysis. The denominator for the percentages is the number of adult participants with nonmissing HIV-1 RNA value at each visit.
Visit performed 12 weeks after baseline, which could have been in the second or third trimester depending on the participant's enrollment date.
While prespecified windows for reporting advanced in 6-week intervals, actual data in week 18 postpartum identifier consist of 15–17 weeks postpregnancy follow-up, in alignment with protocol plan of 16 weeks of follow-up.
n = 31.
B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; Q, quartile.
Overall, 100% (29/29) of neonates had a negative/undetectable PCR test for HIV, indicating an absence of intrauterine or peripartum transmission. Quantitative or qualitative HIV-1 RNA PCR results were available for two neonates at both birth and 4–8 weeks of age. Qualitative HIV-1 DNA PCR results according to local standard of care were available for the remaining 27 neonates (6 at both birth and 4–8 weeks of age; 21 at 4–8 weeks of age).
Safety
Adverse events and laboratory abnormalities are shown in Table 3. Among adult participants, most adverse events were grade 1 or grade 2. Adverse events of grade 3 or higher were reported in two participants (6%) (gestational diabetes and pyrexia) and were not considered drug related. In total, six participants (18%) had serious adverse events (SAEs), with one SAE of false labor considered to be drug related (this participant subsequently had an uncomplicated pregnancy and delivered a healthy neonate). No other drug-related adverse events and no discontinuations because of adverse events were reported. Grade 3 laboratory abnormalities were reported in six participants: hematuria was reported in five participants (15%) (all postpartum) and glycosuria was reported in one participant (3%) who had gestational diabetes.
Table 3.
Adverse events and laboratory abnormalities.
| Maternal (N = 33) | Neonate (N = 29) | |
| AEs | ||
| Any AE | 26 (79) | 12 (41) |
| Most frequent AEs (occurring in ≥9% of participants in either group) | ||
| Back pain | 4 (12) | – |
| Gestational diabetes | 4 (12) | – |
| Anemia | 3 (9) | – |
| False labor | 3 (9) | – |
| Preeclampsia | 3 (9) | – |
| Neonatal jaundice | – | 3 (10) |
| Respiratory distress | – | 3 (10) |
| Drug-related AEs | 1 (3)a | 0 |
| SAEs | 6 (18)b | 5 (17)c |
| Drug-related SAEs | 1 (3)a | 0 |
| Grade ≥3 AEs | 2 (6) | 1 (3) |
| Drug-related grade ≥3 AEs | 0 | 0 |
| AEs leading to premature discontinuation of study drug | 0 | 0 |
| Death | 0 | 0 |
| Laboratory abnormalities | ||
| Any grade | 24 (73) | 5 (17) |
| Grade ≥3 | 6 (18) | 0 |
Data represent number (%) of participants. Median (Q1, Q3) duration of B/F/TAF exposure was 27 (23, 32) weeks.
False labor.
False labor (n = 3) and COVID-19, nonreassuring fetal heart rate pattern, preeclampsia, preterm premature rupture of membranes, and pyrexia (n = 1 for each).
Accessory auricle, atrial septal defect, sepsis neonatal, jaundice neonatal, neonatal asphyxia, and transient tachypnea of the newborn (n = 1 for each).
AE, adverse event; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; Q, quartile; SAE, serious adverse event.
Among neonate participants, most adverse events were grade 1 or 2. Adverse events of grade 3 or higher were reported in one neonate (3%): a grade 4 adverse event of neonatal asphyxia at birth that was not considered to be study drug-related and resolved on day 9. SAEs were reported for five neonates (17%). No adverse events considered related to study drug were reported and no neonatal deaths occurred. All laboratory abnormalities in neonates were grade 1.
Resistance
No participants in the study met the criteria for resistance testing.
Discussion
In this study, pregnant women with HIV-1 who received B/F/TAF had total BIC plasma exposures (AUCtau) that were ∼59% lower than week 12 postpartum (with unbound BIC being lower by ∼41%), and ∼40% lower than nonpregnant adults with HIV-1 [4,13]. Despite this reduction, mean BIC Ctrough levels during pregnancy remained approximately 6.5-fold greater than the paEC95[4], and all individual Ctrough values but one were above the paEC95. This individual was taking calcium and iron supplements during pregnancy, which may account for the lower BIC Ctrough observed during the second trimester because of a possible chelation interaction [4]; notably, the participant's BIC Ctrough levels were nine-fold higher during the third trimester (4.8-fold higher than the paEC95) and they remained virologically suppressed throughout the study.
Clinical data showing robust antiviral activity with 10-fold lower BIC doses and exposures close to paEC95 also support that BIC exposure in the present study was efficacious [14]. Furthermore, virologic suppression was maintained throughout the study, and there was no observed virologic failure, treatment-emergent resistance, or indication of intrauterine and peripartum transmission. CD4 cell count and CD4% remained stable throughout the study. B/F/TAF was generally well tolerated, with adverse events mostly grade 1 or 2 and no discontinuations due to adverse events.
Increased CYP3A4 and UGT1A1 activity and reduced protein binding of BIC likely contributed to the lower BIC exposures observed during pregnancy. The present study findings are concordant with data from the IMPAACT phase 4 study in pregnant women with HIV-1 administered B/F/TAF, which showed that total BIC exposure was lower in pregnancy versus postpartum (by 49–56%), and concentrations at 24 h post dose remained greater than the paEC95 at all time points [15]. Similar findings have been observed with dolutegravir, another integrase strand transfer inhibitor, where exposure during pregnancy was lower compared with postpartum [16].
The BIC half-life in neonates was longer than in adults. This was expected given the ontogeny of CYP3A4 and UGT1A1 [17].
FTC and TAF exposures were lower during pregnancy compared with postpartum in our study. Changes of similar magnitude in FTC and TAF exposure during pregnancy have been reported previously [18,19], and were not associated with virologic failure or perinatal transmission. Current US clinical guidelines state that both FTC and TAF are preferred nucleoside reverse transcriptase inhibitors in pregnancy, with no dose adjustments required for either [2].
B/F/TAF maintained virologic suppression throughout the study, and there was no observed virologic failure or perinatal transmission. This is consistent with the IMPAACT study findings of 90% of pregnant participants virologically suppressed at delivery, and no perinatal transmission [15].
Lastly, as done here, we believe it is pragmatic to contextualize pharmacokinetic observations during pregnancy with those in the nonpregnant population, where rich information on efficacy and safety implications for a range of exposures is generally available in development programs [4].
Our study did not identify any new safety or tolerability concerns for mothers who use B/F/TAF during pregnancy and postpartum, or for their neonates. The overall incidence and types of adverse events observed were consistent with those expected for the population studied.
Limitations of this study include the open-label uncontrolled nature of the design and the relatively small sample size. However, results of this study are in agreement with the independent findings of the IMPAACT study (n = 27) [15]. It is also common for smaller sample sizes to be used to guide dosing recommendations among sub-populations.
Due to disruption caused by the coronavirus disease 2019 (COVID-19) pandemic, some assessments were not conducted; these were handled as protocol deviations but did not affect the overall quality or interpretation of the study.
Conclusion
This study demonstrated that pregnant women with HIV-1 treated with once-daily B/F/TAF at the standard dose maintained virologic suppression into the postpartum follow-up period with no treatment-emergent resistance or indication of intrauterine and peripartum transmission. Although BIC exposure was lower during pregnancy than postpartum, mean BIC trough levels remained higher than the paEC95 value. Furthermore, B/F/TAF was generally well tolerated in pregnant women and neonates. In summary, the findings indicate that once-daily B/F/TAF without dose adjustment is a suitable treatment option throughout pregnancy.
Acknowledgements
We thank all participants and investigators involved in the study. We also thank Hal Martin, MD, MPH, for contributions to the protocol design, execution, analysis, and reporting; Hailin Huang, PhD, for contributions as the lead study statistician; Polina German, PharmD, for contributions to the study design and conception; as well as Anuja Raut, MS, Associate Director, Clinical Pharmacology, and Paige Daniel, PhD, Associate Scientist, Clinical Pharmacology (Certara USA, Inc.), for performing final pharmacokinetic noncompartmental analyses. Medical writing support was provided by Olivia Morris, PhD, at Aspire Scientific Limited (Bollington, UK). Funding for medical writing support for this article was provided by Gilead Sciences (Foster City, CA, USA).
Author contributions – Conceptualization: H.Z. Data curation: J.S., M.D. Formal analysis: H.Z., D.X., J.S., D.D.M. Funding acquisition: none. Investigation: H.Z., J.T.H., L.L., J.S., D.X., A.A., P.A., R.P., S.G., D.D.M. Methodology: H.Z., D.X., M.D., J.S. Project administration: none. Resources: J.T.H., R.P., S.G., D.D.M. Software: H.Z., J.S. Supervision: J.T.H., R.P., S.G., D.D.M. Validation: H.Z., D.X., M.D., J.S. Visualization: H.Z., J.S. Writing – original draft: H.Z., D.D.M. Writing – review and editing: H.Z., J.T.H., L.L., M.D., J.S., D.X., A.A., P.A., S.G., D.D.M.
All authors reviewed and critically revised the manuscript, approved the final version for submission, and agree to be accountable for the accuracy and integrity of the manuscript.
Data availability statement: Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting nonconflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Sciences’ discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
Funding: this study was funded by Gilead Sciences (Foster City, California, USA). The study sponsor, Gilead Sciences (Foster City, California, USA), played a role in the study design, data collection and analysis, decision to publish, and preparation of the manuscript.
Conflicts of interest
H.Z., J.T.H., L.L., M.D., J.S., D.X., P.A., R.P., S.G., and D.D.M. declare employment and restricted stocks from Gilead Sciences. A.A. declares research grants from Gilead Sciences, GSK, and MSD.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.World Health Organization (WHO). Estimated percentage of pregnant women living with HIV who received antiretrovirals for preventing mother-to-child transmission. 2023. Available at: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/estimated-percentage-of-pregnant-women-living-with-hiv-who-received-antiretrovirals-for-preventing-mother-to-child-transmission. [Accessed 9 August 2023] [Google Scholar]
- 2. U.S. Department of Health and Human Services. Recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the United States. 2023. Available at: https://clinicalinfo.hiv.gov/en/guidelines/perinatal. [Accessed 9 August 2023] [Google Scholar]
- 3.Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA Panel. JAMA 2023; 329:63–84. [DOI] [PubMed] [Google Scholar]
- 4. Gilead Sciences. Biktarvy prescribing information. 2022. Available at: https://www.gilead.com/-/media/files/pdfs/medicines/hiv/biktarvy/biktarvy_pi.pdf. [Accessed 19 July 2023] [Google Scholar]
- 5. Clinicalinfo.HIV.gov. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2023. Available at: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv. [Accessed 17 August 2023] [Google Scholar]
- 6.Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, Lopez-Cortes L, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, noninferiority trial. Lancet HIV 2018; 5:e357–e365. [DOI] [PubMed] [Google Scholar]
- 7.Daar ES, DeJesus E, Ruane P, Crofoot G, Oguchi G, Creticos C, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, noninferiority trial. Lancet HIV 2018; 5:e347–e356. [DOI] [PubMed] [Google Scholar]
- 8.Sax PE, Rockstroh JK, Luetkemeyer AF, Yazdanpanah Y, Ward D, Trottier B, et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with human immunodeficiency virus. Clin Infect Dis 2021; 73:e485–e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stellbrink HJ, Arribas JR, Stephens JL, Albrecht H, Sax PE, Maggiolo F, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, noninferiority trial. Lancet HIV 2019; 6:e364–e372. [DOI] [PubMed] [Google Scholar]
- 10.Wohl DA, Yazdanpanah Y, Baumgarten A, Clarke A, Thompson MA, Brinson C, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, noninferiority trial. Lancet HIV 2019; 6:e355–e363. [DOI] [PubMed] [Google Scholar]
- 11.Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seneviratne HK, Hamlin AN, Heck CJS, Bumpus NN. Spatial distribution profiles of emtricitabine, tenofovir, efavirenz, and rilpivirine in murine tissues following in vivo dosing correlate with their safety profiles in humans. ACS Pharmacol Transl Sci 2020; 3:655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Center for Drug Evaluation and Research. Bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) FDC - BIKTARVY® NDA 210251 Uni-Review. 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210251Orig1s000MultidisciplineR.pdf. [Accessed 22 June 2023] [Google Scholar]
- 14.Gallant JE, Thompson M, DeJesus E, Voskuhl GW, Wei X, Zhang H, et al. Antiviral activity, safety, and pharmacokinetics of bictegravir as 10-day monotherapy in HIV-1-infected adults. J Acquir Immune Defic Syndr 2017; 75:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powis KM, Pinilla M, Bergam L, Stek A, Brooks KM, Shapiro D, et al. Pharmacokinetics and virologic outcomes of bictegravir in pregnancy and postpartum. Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, Washington. 2023. [Google Scholar]
- 16.Hill A, Clayden P, Thorne C, Christie R, Zash R. Safety and pharmacokinetics of dolutegravir in HIV-positive pregnant women: a systematic review. J Virus Erad 2018; 4:66–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther 2014; 19:262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colbers AP, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, et al. PANNA network. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS 2013; 27:739–748. [DOI] [PubMed] [Google Scholar]
- 19.Brooks KM, Momper JD, Pinilla M, Stek AM, Barr E, Weinberg A, et al. Pharmacokinetics of tenofovir alafenamide with and without cobicistat in pregnant and postpartum women living with HIV. AIDS 2021; 35:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]