Abstract
Purpose of review
The assessment of thyroid nodules is a common clinical problem, linked to the high incidence of thyroid nodules in the population and the low incidence of aggressive thyroid carcinoma. The screening is therefore one of the strengths of our patient care. Recently, the 2023 Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) and 2022 WHO classification of thyroid neoplasms have been released based on the definition of new entities and the growing impact of molecular testing. The aim of this review is to analyze how these upgrades can help us in the daily routine practice diagnosis of thyroid cancer.
Recent findings
Our review is focused on the most frequent thyroid tumors derived from thyroid follicular cell. Fine needle aspiration (FNA) is the gold standard for the screening of thyroid nodules with very high levels of sensitivity and specificity. These sensitivity and specificity are improved by molecular testing, which refines the risk of malignancy. The 2023 TBSRTC integrates molecular data and the upgrades integrated in the 2022 WHO classification such as the ‘low-risk neoplasms’ and the ‘high-grade follicular-cells derived carcinoma’. The morphological examination remains crucial since the capsular and/or vascular invasion are key features of malignancy in the follicular thyroid neoplasms. Low-risk neoplasms represent a clinical challenge since no specific guidelines are available. Challenges remain regarding oncocytic thyroid lesions, which are not associated with specific diagnostic molecular biomarkers. Molecular testing can help not only in deciphering the prognosis but also in the targeted therapeutic strategy.
Summary
While molecular testing has succeeded to substantially improve the pre and postoperative diagnosis and risk stratification of thyroid tumors, the morphological examination is still central in the daily routine diagnosis of thyroid pathology. Future is the integrated diagnosis of clinical, morphological, molecular and epigenetic features with the help of artificial intelligence algorithms.
Keywords: Bethesda system, fine needle aspiration, molecular testing, screening, thyroid cancer, WHO classification
INTRODUCTION
The assessment of thyroid nodules is a common clinical problem, reaching 60% in the adult population, with a female predominance [1]. By contrast, the majority of the thyroid nodules are benign with 8–16% of them being cancers [2–4]. According to the WHO, thyroid cancer is the ninth most frequent global cancer (∼1% of global cancer cases). In 2020, 586 000 new cases were reported [5–9]. Most of them are differentiated thyroid cancers (DTCs), mainly represented by papillary thyroid carcinomas (PTC; 85% prevalence) and follicular thyroid carcinoma (FTC; 10% prevalence) [4,10]. Poorly differentiated (PDTC) and anaplastic thyroid carcinomas (ATC) (1–2% prevalence) are less frequent but associated with a worse prognosis, including high risk of distant metastasis. However, the disease-specific mortality of thyroid cancer is very low and remains stable, though to be due, in part, to the earlier detection of small cancers leading to the actual ‘overdiagnosis’ [9–11]. The important clinical challenge remains the management of patients with thyroid nodules in order to propose the best therapeutic strategy (e.g. active surveillance, surgery, radioactive iodine, targeted therapy) and to avoid overtreatment mostly inappropriate surgical choice.
Box 1.
no caption available
Fine needle aspiration (FNA) is still the most accurate and cost-effective method to stratify patients with thyroid tumors according to their risk of malignancy (ROM) [12▪▪,13]. Recently, the new Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) has been released, based on the new entities and terminology included in the 2022 WHO classification, and the growing impact of molecular testing [13].
In the past several years, molecular testing has underlined the correlation between genotype and phenotype in thyroid disease such as BRAF V600E and RAS mutations are classically found in PTC and follicular-type thyroid tumors, respectively [14–16]. Moreover, molecular testing has also succeeded to refine the ROM of cytological diagnosis and, recently, to propose prognostic and predictive biomarkers [12▪▪]. At the same time, histopathology features are still needed since capsular and vascular invasions are the only cancer diagnosis markers for encapsulated follicular tumors [17].
This review aims to provide the most recent changes in the cytological and histological classification of thyroid tumors according to the 2023 TBSRTC and the 2022 WHO classification focusing on the new entities that have been introduced molecular data and quantitative criteria.
Fine needle aspiration of thyroid neoplasms
FNA is the most reliable, simple and cost-effective technique of distinguishing benign from suspicious or malignant thyroid nodules, resulting in stratification especially for surgery choice. FNA is the gold standard in the evaluation of thyroid nodules with a very high sensitivity and specificity, near to 100% in the diagnosis of thyroid cancer [18–20]. The TBSRTC provide a reporting system of FNA thyroid specimens leading to a tiered diagnostic classification (Bethesda I to IV) (see Table 1 and Fig. 1a-d-g), which implies ROM and recommended management strategies [12▪▪]. The recently 2023 update TBSRTC integrates the new 2022 WHO classification of Thyroid Neoplasms terminology, including all high-grade follicular-derived carcinomas, PDTC as well as differentiated high-grade thyroid carcinoma (DHGTC). The terms ‘Nondiagnostic/Unsatisfactory’ is simplified with ‘Nondiagnostic’ corresponding to Bethesda I, the ‘Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance (AUS/FLUS)’ is now termed as ‘Atypia of Undetermined Significance (AUS)’ (Bethesda III) and the ‘Follicular Neoplasm/Suspicious For a Follicular Neoplasm (FN/SFN)’ in ‘Follicular Neoplasm (FN)’ (Bethesda IV) [12▪▪]. Another important modification is the integration of adjusted ROM for pediatric population, in which higher ROM is observed, leading to more aggressive therapeutic management. Finally, the use of DNA and RNA molecular testing is widely described along their diagnostic, prognostic and predictive roles [12▪▪].
Table 1.
The 2023 TBSRTC for adult population associated with the risk of malignancy
| Diagnostic category | ROMa, mean% (range) | Usual management |
| Nondiagnostic | 13 (5–20) | Repeat FNA with ultrasound guidance |
| Benign | 4 (2–7) | Clinical and sonographic follow-up |
| Atypia of undetermined significance | 22 (13–30) | Repeat FNA, molecular testing, diagnostic lobectomy, or surveillance |
| Follicular neoplasm | 30 (23–34) | Molecular testing, diagnostic lobectomy |
| Suspicious for malignancy | 74 (67–83) | Molecular testing, lobectomy or near-total thyroidectomy |
| Malignant | 97 (97–100) | Lobectomy or near-total thyroidectomy |
TBSRTC, Bethesda System for Reporting Thyroid Cytopathology. Adapted from [12▪▪].
FIGURE 1.
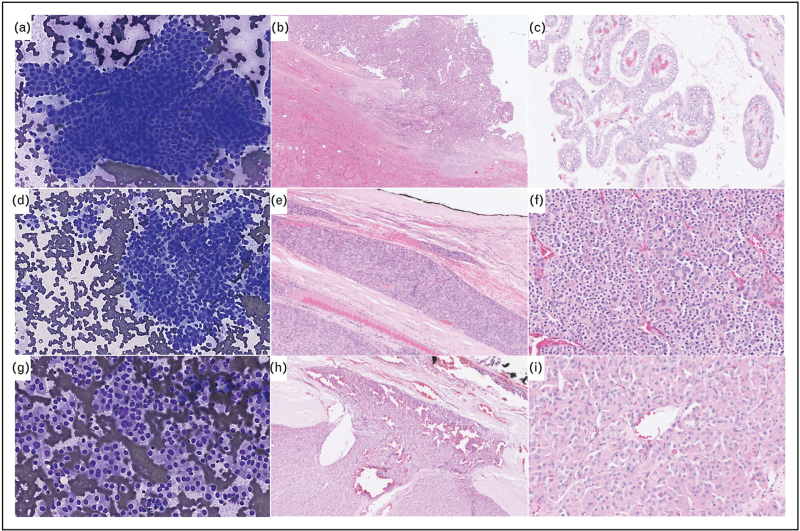
Cytological and histopathological features of follicular-derived thyroid neoplasms. (a–c) Invasive encapsulated papillary carcinoma. Cytological smear shows typical elongated nuclei with grooves and pseudoinclusions leading to Bethesda VI classification (a) (Diff-Quick staining, X200). Transcapsular invasion (b) and papillary architecture with nuclear score of 3. (c) [HE staining, X200 (b), X400 (c)]. (d–f) Angioinvasive poorly differentiated thyroid carcinoma. Cytological smear shows highly cellular crowded groups of uniform follicular cells arranged in microfollicles Bethesda IV (d) (Diff-Quik staining, X200). Vascular invasion (e) and solid/trabecular and microfollicular architecture (f) [HE staining, X200 (e), X400 (f)]. (g–i) Oncocytic follicular thyroid carcinoma. Cytological smear shows highly cellular groups of oncocytic cells Bethesda IV (g) (Diff-Quik staining, X200). Transcapsular invasion (h) and oncocytic cells arranged in a microfollicular architecture (i) [HE staining, X200 (h), X400 (i)].
Alone, the positive predictive value (PPV) of the FNA reaches 97–99%. FNA cytology succeed to diagnose benign or malignant nodule in 70–75% of cases [19]. In our series, we reported the accuracy of FNA to be 97.8% (benign vs. malignant) [1]. In a meta-analysis published in 2012, they reported a sensibility and specificity of 97 and 50.7%, respectively. The positive and negative predictive value (NPV) for adult population was 98.6 and 55.9%, respectively, and the accuracy of 68.8% using the TBSRTC [20] including 25% of ‘indeterminate cytology’ (Bethesda III, IV and V) with an expected ROM of 13–30% (Bethesda III), 23–34% (Bethesda IV), 67–83% (Bethesda V) and 97–100% (Bethesda VI) according to the 2023 TBSRTC [12▪▪,21]. It is important to precise that, if the ‘Noninvasive Follicular Thyroid Neoplasm with Papillary Like Nuclear Features’ (NIFTP) are excluded, the ROM is evaluated to 16% (Bethesda III), 23% (Bethesda IV), 65% (Bethesda V) and 94% (Bethesda VI) [12▪▪].
Many efforts have been done to refine the ROM of the thyroid cytological diagnosis, particularly for thyroid nodules with indeterminate (AUS or FN) cytology since most of these patients undergo diagnostic surgical resection for benign lesions [19,21]. Unnecessary surgery can be associated with per-operative complications such as hypoparathyroidism (transient and permanent hypoparathyroidism in 14–83 and 0.12–11%, respectively), recurrent laryngeal nerve paralysis and in some cases to life-long thyroid hormone replacement [21–23].
Molecular biomarkers for the diagnosis of thyroid neoplasms
In addition to the clinical and radiological data that can efficiently help to predict malignancy, molecular alterations are useful as preoperative biomarkers to precise the ROM. Postoperative molecular testing will provide prognostic information and potential targeted molecular alterations for therapy-resistant disease [14,24▪▪]. The prognostic and predictive molecular biomarkers will be discussed below. Which is quite remarkable in the thyroid molecular landscape is the correlation between histology and genetic changes, associated with a multistep tumorigenic process [14].
Thyroid carcinogenesis involved molecular alterations resulting in the overactivation of the mitogen activated kinase (MAPK) (RAS-RAF-MEK-ERK) and the phosphoinositide-3 kinase PI3K/AKT/mTOR signaling pathways [12▪▪,25]. Both of these pathways are associated with the regulation of proliferation, angiogenesis and migration of cells [24▪▪]. Molecular alterations of the MAPK pathway downstream signaling molecules such as RAS, BRAF and the single-pass transmembrane tyrosine kinase receptor RET (rearranged during transfection)/PTC and TRK are all mutually exclusive. These molecular alterations are considered to be early molecular events [25]. In FTC, RAS, PIK3CA and AKT1 activating mutations and mutations of PTEN are crucial to activate the PI3K/AKT pathway [26]. Based on mutational and transcriptomic profiles, the two molecular thyroid subtypes classically described, ‘BRAF-like’ and ‘RAS-like’, are both associated with different levels of MAPK signaling pathway activation [16,25,27]. In the BRAF-like tumors, robust activation of the MAPK pathway is observed, while in the RAS-like tumors, both MAPK (at a lesser degree) and PI3K/AKT pathway are activated [25].
BRAF-like tumors typically harbored papillary-type atypia, classically represented by the PTC with the exception of the invasive encapsulated follicular variant papillary carcinoma (IEFVPTC) which is associated with the RAS-like tumors [24▪▪]. Interestingly, PTC harbor one of the lowest tumor burdens among all cancer types (approximately 0.41 nonsilent mutations per mega base) [16,24▪▪,28]. BRAF-like molecular alterations include BRAF V600E mutation and ALK, BRAF, RET, NTRK1/3 and MET fusions [24▪▪]. BRAF V600E mutation and RET/BRAF fusions (1–2% of PTC) are highly specific of classical PTC with some morphological specificities associated with RET-fused PTC. Indeed, diffuse sclerosing PTC and postradiation solid/trabecular PTC are commonly associated with RET fusion [24▪▪,29]. Mutation of the proto-oncogene BRAF (BRAF V600E; 98–99% of all BRAF mutations found in PTC) is the most common molecular alteration found in PTC, especially high-risk PTC including tall cell, classical and hobnail [16,24▪▪,30] but have also been found in Warthin-like PTC [31,32]. NTRK1–3 (3–5% of PTC) and ALK (1–3% of PTC) fusions are found in classical PTC but with a predominant infiltrative follicular presentation [29,33–36]. To note, NTRK1–3 (ETV6-NTRK3; 22%) fusion is the most commonly found in BRAF wild-type PTC in the pediatric population with a frequency of 14.5% found in post-Chernobyl PTC cases [24▪▪,36,37]. The other less common mutations found in PTC are HRAS, KRAS, PPM1D, CHEK2, MEK1,PPARG, THADA, LTK, MET and FRFR2 genes mutations [16,24▪▪].
RAS-like tumors typically harbored a follicular pattern, classically represented by the FTC and the IEFVPTC [24▪▪]. RAS-like molecular alterations include RAS (NRAS, HRAS, KRAS), BRAF K601E, DICER1, EZH1, EIF1AX, PTEN mutations and PPARG, THADA gene fusions [24▪▪]. These molecular alterations are found in a broad spectrum of thyroid neoplasms including benign, indefinite for malignancy and malignant follicular-patterned tumors, considering thus these alterations as ‘indeterminate for malignancy’ [24▪▪].
In FTC, RAS mutations, and particularly NRAS (recurrent codon 61) mutations (followed by HRAS and KRAS mutations) are found in nearly 50% of cases [26]. PAX8-PPARG are less frequent (10–40%) than RAS mutations in FTC and reported in 0–30% of IEFVPTC, in benign lesions such as follicular thyroid adenomas (FTAs; 5–20%) and in up to 30% in NIFTP, less than 10% in follicular tumor of uncertain malignant potential (FT-UMP) and rare in well differentiated tumor of uncertain malignant potential (WDT-UMP) [14,24▪▪].
EIF1AX is described in various benign and malignant thyroid neoplasms such as FTAs (<10%) to FT-UMP (<10%), WT-UMP (<10%), NIFTP (<10%), IEFVPTC (<10%), FTC (5%) and even PDTC and ATC (11%) [14,24▪▪,38].
Molecular alterations in the PI3K/AKT pathway (PIK3CA, PTEN mutations) are more frequently found in FTC (approximatively 10%) than in FTA [39]; PTEN and PIK3CA mutations are also common in PDTC (0–20%) and ATC (5–25%) [14]. Even PTEN mutations is considered to be an early event in the tumorigenesis of thyroid cancers, the presence of this mutation is suggested in more advanced thyroid carcinomas such DHGTC, PDTC and ATC. Moreover, the loss of PTEN IHC staining in both benign and malignant neoplasms is indicative of Cowden syndrome [24▪▪,40].
Late molecular driver events suggesting tumor progression and dedifferenciation include TERT promoter mutations and TP53, AKT1 and PIK3CA mutations [24▪▪,38]. In DHGTC, PDTC and ATC, co-occurrence of early and late molecular driver events reflect the multistep progression from well to poorly differentiated tumors [24▪▪]. PDTC mainly harbor RAS mutations as an early event by contrast to DHGTC, which harbor BRAF V600E mutation [38]. TP53 mutations can also been found in PTC, especially in the hobnail, columnar and tall cell subtype and in oncocytic carcinoma (OTC) [41,42]. Interestingly, by contrast to OTC, FTC do not harbor TP53 mutations but 15% of TERT promoter mutations, associated with a worse prognosis [43,44]. DAXX, TP53, NRAS, NF1, CDKN1A, ARHGAP35 and the TERT promoter are other common mutations found in OTC [42].
Integrated diagnosis of fine needle aspiration and molecular biomarkers
These molecular profiles do not allow to provide binary decision but refine the ROM provided by the TBSRTC [13]. Molecular testing is particularly recommended for indeterminate cytological FNAs, helping to guide clinical management between clinical/sonographic surveillance and surgical resection [20]. The TBSRTC described three molecular diagnostic categories: low molecular probability of cancer (∼3% ROM), intermediate molecular probability of cancer and high molecular probability of cancer (97–99% ROM) [13]. The molecular testing has also been able to identify high-risk cancers. In the literature, different molecular platforms exist for thyroid FNAs ranging from laboratory-developed to commercialized testing such as Afirma, ThyroSeq, ThyGenX/ThyraMIR and RosettaGX Reveal [45] with various NPV (81–100%) and PPV (29–81%) described [12▪▪,46–48].
The 112-gene test ThyroSeq v3 Genomic Classifier (GC) demonstrated high sensitivity and NPV with high specificity/ PPV in a cohort of indeterminate cytology (n = 257 Bethesda III and IV) [49]. With a NPV of 97%, more than 60% of patients with indeterminate cytology may avoid surgery if GC was performed with more than 80% of benign nodules yielding a negative test result [49]. They reported three molecular groups: high-risk group (TERT, TP53 mutation; 100% cancer/NIFTP prevalence), BRAF-like group (BRAF V600E, NTRK3, RET and BRAF fusions; 100% cancer/NIFTP prevalence) and RAS-like group (NRAS, HRAS, KRAS, EIF1AX, BRAF K601E, PTEN, IDH2, DICER1 mutations and PPARG, THADA fusions; 62% cancer/NIFTP prevalence) [49]. Using the seven-gene panel on 1172 FNA samples, Bellecine et al.[50] underlined that the rate of mutation-positive FNAs is directly proportional to the prevalence of cancer.
Regarding FNA diagnosis for oncocytic neoplasms, copy number alterations (CNAs) have also been found to be predictive of malignancy, in conjunction of other DNA mutations (e.g. TERT promoter mutations) and nodule size [51].
Histological diagnosis of thyroid neoplasms: 2022 WHO classification
The fifth edition of the WHO classification of endocrine tumors has been released in 2022 (see Table 2) integrating thyroid tumors with the major group that derived from follicular epithelial cells and calcitonin-secreting C cells [24▪▪].
Table 2.
The 2022 WHO classification of follicular cell derived thyroid neoplasms
| 1. Benign tumors |
| Thyroid follicular nodular disease |
| Follicular adenoma |
| Follicular adenoma with papillary architecture |
| Oncocytic adenoma of the thyroid |
| 2. Low-risk neoplasms |
| Noninvasive follicular thyroid neoplasm with papillary-like nuclear features |
| Thyroid tumors of uncertain malignant potential |
| Hyalinizing trabecular tumor |
| 3. Malignant neoplasms |
| Follicular thyroid carcinoma |
| Invasive encapsulated follicular variant papillary carcinoma |
| Papillary thyroid carcinoma |
| Oncocytic carcinoma of the thyroid |
| Follicular-derived carcinomas, high-grade Differentiated high-grade thyroid carcinoma Poorly differentiated thyroid carcinoma |
| Anaplastic follicular cell–derived thyroid carcinoma |
Adapted from [24▪▪].
Follicular cells derived tumors are divided into benign neoplasms, low-risk neoplasms and cancers.
Regarding the benign thyroid nodular change, the new nomenclature simplifies the terms ‘Multifocal hyperplastic /multinodular goiter (MNG)’ in follicular nodular disease [24▪▪].
The benign neoplasms included FTAs that can harbor microfollicular or macrofollicular architecture, with a RAS-like molecular profile. Oncocytic FTA (>75% of oncocytic cells) of the thyroid is now considered to be a distinct entity in the WHO classification, with specific molecular alterations such as alterations in the mtDNA [42]. A new distinct form of FA is the ‘FA with papillary architecture’, predominantly found in women with subclinical or symptomatic (15–60%) hyperthyroidism. They are typically described to be ‘hot nodules’. At microscopic examination, intrafollicular papillary architecture (centripetal intrafollicular papillae) reflecting hyperfunctional status is found with no PTC nuclear features or capsular/vascular invasion [52,53].
Leading the introduction of the NIFTP entity in the 2017 WHO classification, a new category of tumors, the ‘low-risk neoplasms’, have been introduced in the 2022 WHO classification. These tumors comprised the ‘NIFTP’ entity, tumors with uncertain invasiveness (UMP) and tumors with striking nuclear features of papillary carcinoma (HTT) [24▪▪]. These entities are clinically and morphologically borderline with very low risk of distant metastasis [24▪▪]. In this group of follicular neoplasms, morphological examination is crucial to establish the diagnosis since the presence of capsular, vascular invasion and/or extrathyroidal extension (ETE) are key features of malignancy [54,55]. Indeed, since the 2017 WHO classification, the diagnosis of thyroid cancer has been fundamentally changed such as papillary nuclear features are not sufficient anymore to make the diagnosis of PTC. For encapsulated nodules the major diagnostic criteria are the vascular/capsular infiltrative pattern. However, many studies have underlined the poor interobserver concordance in the evaluation of these features [55]. Published guidelines provide precise definition for capsular invasion and vascular invasion criteria [54]. Angioinvasion has been quite controversial regarding its prognostic role, this aspect will be discussed in the FTC section [25].
NIFTP is more frequently described in women, and represent 10–20% of all the thyroid tumors diagnosed in Western countries with less frequency in Asia [24▪▪]. Papillary nuclear feature interobserver variability leads to significant variations of the incidence of NIFTP between institutions [56,57]. Therefore, the new 2022 WHO classification has integrated quantification criteria to diagnose thyroid cancers, including nuclear score (0–3) based on nuclear size and shape, membrane irregularities and chromatin characteristics. Other morphological features are quantified such as, among others, mitotic count, KI-67 labeling index or percentage of growth pattern (solid, trabecular, insular, papillary) [24▪▪,56]. For the NIFTP, in addition to the absence of capsular and vascular invasion and the presence of nuclear features of papillary carcinoma (nuclear score of 2–3), the other diagnosis criteria are related to the growth pattern with less than 1% true papillae, less than 30% solid/trabecular/insular growth pattern and no psammoma bodies neither tumor necrosis and a low mitotic count (<3 mitosis/2 mm2) [24▪▪].
At molecular level, 52% of NIFTP are categorized as RAS-like tumors. The presence of a BRAF V600E mutation is considered as evidence of PTC diagnosis (detected by immunochemistry or by molecular testing), and is an exclusion diagnostic criterion [58,59].
With a female predominance, the tumors of uncertain malignant potential (UMP) have an incidence of 0.5–3% The diagnosis of follicular tumor (FT)/well differentiated tumor (WDT)-UMP is based on questionable invasion (capsular and/or vascular) found in encapsulated or well circumscribed follicular neoplasms. WDT-UMP have more PTC nuclear features (nuclear score 2–3) than FT-UMP (no nuclear PTC features or nuclear score 0–1). These tumors are molecularly RAS-like since RAS mutations are found in nearly 40% of cases. Very few cases have been reported to have mutations in EIF1AX and TSHr genes. The presence of BRAF V600E mutation, TERT or TP53 mutations required extensive examination of the tumor to exclude carcinoma [60,61]. These challenging histological diagnoses remain a clinical challenge since no robust guidelines are actually available regarding their management.
HTT is estimated to be less than 1% of thyroid neoplasms [62]. The diagnostic criteria are the presence of pure trabecular architecture with intra-trabecular hyalinization and PTC nuclear features including prominent grooves, vacuoles and membrane irregularities. No BRAF or RAS-like molecular alterations are found, but PAX8-GLIS3 and PAX8-GLIS1 fusions are the molecular hallmarks [63].
Malignancies of thyroid follicular cells included FTC, IEFVPTC, PTC, OTC and follicular-derived carcinomas, high-grade [24▪▪]. The vast majority of thyroid cancers (80–85% in adults, 90% in pediatric population) are PTC, classically ‘BRAF-like’ tumors [64]. PTC is defined to have either papillary or solid/trabecular architecture, or invasive growth in follicular-patterned tumors [24▪▪] (Fig. 1b,c). The follicular variant of PTC is defined to have more than 90% of follicular architecture with very few or no papillae and can be encapsulated (with vascular and/or capsular invasion, named as IEFVPTC) or infiltrative. The IEFVPTC is now reported as a separate entity while the infiltrative follicular variant of PTC is described in the PTC chapter's subtypes. Interestingly, both have different molecular profiles such as IEFVPTC is a RAS-like tumor and infiltrative FVPTC is a BRAF-like tumor. IEFVPTC can be subclassified in different prognostic subgroups; minimally invasive (capsular invasion only), angioinvasive or widely invasive, such as reported in FTC. Other PTC's subtypes described in the 2022 WHO classification are the tall cell variant (>30% tall cell), columnar cell variant, hobnail (>30% hobnail), solid (>50% solid), diffuse sclerosing, whartin like and oncocytic (>75% oncocytes) subtype [24▪▪]. The columnar cell, hobnail and diffuse sclerosing subtypes are associated with poor prognosis in terms of disease-free survival and mortality [65–67].
FTC represent 5–15% of DTC with a female adult predominance, the pediatric presentation is rare. Interestingly, a higher incidence of FTC than PTC has been described in iodine-deficiency area [68]. The key diagnostic features of FTC are the vascular and/or capsular invasion with three subtypes described, in the same way then IEFVPTC: minimally invasive, angioinvasive and widely invasive. The disease-free survival is significantly different between these subtypes: 97, 81 and 46%, respectively [69]. For the angioinvasive FTC, the extent of vascular invasion is crucial for the prognostic such as extensive (≥4) vascular invasion is an independent predictor of DFS [70]. The oncocytic form of FTC, the OTC, is now reported as a separate entity in the 2022 WHO classification (Fig. 1h,i). With a female adult predisposition, the OTC is uncommon and represent less than 5% of all DTC [24▪▪]. Metastasis at diagnosis are found in more than 15–40% of cases for widely invasive tumors [71]. In the same way than for FTC, the number of mitosis per 2 mm2 and tumor necrosis have to be evaluated to exclude high-grade features. Classical thyroid molecular alterations such as RAS, BRAF or PAX8-PPARG are described at low frequency in OTC. As previously described, the recurrent molecular alterations found in OTC included mitochondrial DNA and chromatin regulatory genes alterations such as SETD2 gene [71,72].
In 2000, Akslen and LiVolsi [73] proposed morphological features that identified intermediate prognosis PTC, between DTC and ATC, based on the mitotic count and tumor necrosis. The grading of PTC was found to have a significant impact on prognosis, regardless of the histological differentiation (e.g. insular, trabecular, …) [73]. In 2007, the Turin consensus allowed to clearly identify criteria of PDTC, including presence of a solid/trabecular/insular pattern of growth, absence of the conventional nuclear features of papillary carcinoma and presence of at least one of the following features: convoluted nuclei; mitotic activity at least 3 × 10 HPF; and tumor necrosis [74]. Now, the 2022 WHO classification has introduced these two new categories of nonanaplastic high-grade follicular-derived carcinomas, which included PDTC and DHGTC [24▪▪]. The criteria to identify these tumors are based on the mitotic count (≥ 3 per 2 mm2 for PDTC, ≥ 5 mitoses per 2 mm2 for DHGTC), the necrosis and the morphological pattern (solid/trabecular/insular for PDTC and papillary, follicular or oncocytic for DHGTC) with invasion and no anaplastic features (Fig. 1e,f) [24▪▪]. These tumors represent 0.3–7% of thyroid cancer associated with long standing goiter and comprise 50% of FDG-PET positive/cold by scintigraphy tumors [75]. With a 5-year overall survival of 50–70%, 20–25% of these patients have metastasis at diagnosis [24▪▪].
High-grade follicular-derived nonanaplastic thyroid carcinomas have intermediate load of mutations between well differentiated carcinomas (e.g. BRAF and RAS mutations) and anaplastic carcinomas (e.g. TERT and TP53 mutations) [38].
Molecular biomarkers for the prognosis and therapeutic strategy management of thyroid neoplasms
As discussed above preoperative molecular analyses are used to guide the clinical management of patient with thyroid nodules, while postoperative testing refine the prognosis and potentially identified targeted molecular alterations [24▪▪].
Regarding PTC, the prognostic role of BRAF remains quite debatable since PTC microcarcinoma, which have an excellent long-term evolution, are frequently associated with BRAF mutation. However, many studies have underlined the poor outcome (e.g. recurrence and staging) of BRAF-mutated tumors as compared to wild-type tumors [30]. TERT promoter mutations have a synergic impact with BRAF and provide bad prognosis (e.g. distant metastasis, cancer dedifferentiation) mainly in older patients. TERT promoter mutations also have a predictive impact since resistance to radioactive treatment is described in these tumors [24▪▪,76]. In a morphological benign or low-risk thyroid neoplasms, the presence of BRAF V600E mutation with TP53, PIK3CA or TERT promoter mutations should excluded morphological features of malignancy [24▪▪]. Recently, PLEKHS1 promoter mutations (found in 13% of PTC) were reported in aggressive DTC. Moreover, by multivariate analysis, the presence of TERT promoter, PLEKHS1 promoter or TP53 mutations (in association with either BRAF or RAS mutations) were significantly associated with radioiodine refractory disease [77].
RET, NTRK1-3, BRAF, ALK, ROS and mTOR molecular testing is mandatory since these molecular alterations are targetable by specific drugs: Dabrafenib, Selumetinib and Trametinib for BRAF and RAS-mutated thyroid carcinomas, respectively. Larotrectinib, Repotrectinib and Entrectinib are used for NTRK/ALK/ROS-fused thyroid carcinoma [12▪▪,78].
Epigenetic alterations (e.g. hypomethylation) have been associated with worse prognosis (e.g. distant metastasis and dedifferentiation) for FTC and PTC [79,80]
CONCLUSION
Molecular testing has succeeded to substantially improve the pre and postoperative diagnosis and risk stratification of thyroid tumors, leading to a better screening of the patients and a more appropriate clinical management. However, challenges remain since the 2022 WHO classification has introduced new entities, which are ‘molecular indeterminate’ and require additional upgrades. The morphological examination is still central in the diagnosis of thyroid tumors but are limited by the lack of reproducibility of the criteria and the difficulty of quantification. The morphological assessment will benefit as in other areas of implementation from computational pathology. Future in thyroid disease is the integrated diagnosis of clinical, morphological, molecular and epigenetic features with the help of artificial intelligence algorithms.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Rorive S, D’Haene N, Fossion C, et al. Ultrasound-guided fine-needle aspiration of thyroid nodules: stratification of malignancy risk using follicular proliferation grading, clinical and ultrasonographic features. Eur J Endocrinol 2010; 162:1107–1115. [DOI] [PubMed] [Google Scholar]
- 2.Grani G, Sponziello M, Pecce V, et al. Contemporary thyroid nodule evaluation and management. J Clin Endocrinol Metab 2020; 105:2869–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burman KD, Wartofsky L. Thyroid nodules. Solomon CG, éditeur. N Engl J Med 2015; 373:2347–2356. [DOI] [PubMed] [Google Scholar]
- 4.Raman P, Koenig RJ. Pax-8-PPAR-γ fusion protein in thyroid carcinoma. Nat Rev Endocrinol 2014; 10:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017; 317:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Vecchia C, Negri E. Thyroid cancer: the thyroid cancer epidemic - overdiagnosis or a real increase? Nat Rev Endocrinol 2017; 13:318–319. [DOI] [PubMed] [Google Scholar]
- 7.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma LX, Espin-Garcia O, Bedard PL, et al. Clinical application of next-generation sequencing in advanced thyroid cancers. Thyroid 2022; 32:657–666. [DOI] [PubMed] [Google Scholar]
- 9.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. [DOI] [PubMed] [Google Scholar]
- 10.Soares P, Póvoa AA, Melo M, et al. Molecular pathology of nonfamilial follicular epithelial-derived thyroid cancer in adults: from RAS/BRAF-like tumor designations to molecular risk stratification. Endocr Pathol 2021; 32:44–62. [DOI] [PubMed] [Google Scholar]
- 11.Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am 2019; 48:23–35. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Ali SZ, VanderLaan PA. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria, and explanatory notes [Internet]. Cham: Springer International Publishing; 2023. [Google Scholar]; An extensive overview about the new Reporting Thyroid Cytopathology classification.
- 13.Han M, Fan F. Bethesda System for Reporting Thyroid Cytopathology: an updated review. J Clin Transl Pathol 2023; 3:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acquaviva G, Visani M, Repaci A, et al. Molecular pathology of thyroid tumours of follicular cells: a review of genetic alterations and their clinicopathological relevance. Histopathology 2018; 72:6–31. [DOI] [PubMed] [Google Scholar]
- 15.Le Mercier M, D’Haene N, Rorive S, et al. Next generation sequencing: a new technology to improve the diagnosis of thyroid cancers. Rev Med Bruxell 2016; 37:436–438. [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol 2011; 24:1545–1552. [DOI] [PubMed] [Google Scholar]
- 18.Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2017; 27:481–483. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforova MN, Mercurio S, Wald AI, et al. Analytical performance of ThyroSeq v3 Genomic Classifier for cancer diagnosis in thyroid nodules. Cancer 2018; 124:1682–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bongiovanni M, Spitale A, Faquin WC, et al. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol 2012; 56:333–339. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Prera JC, Valderrabano P, Creed JH, et al. Molecular determinants of thyroid nodules with indeterminate cytology and RAS mutations. Thyroid 2021; 31:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yazicioğlu MÖ, Yilmaz A, Kocaöz S, et al. Risks and prediction of postoperative hypoparathyroidism due to thyroid surgery. Sci Rep 2021; 11:11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brisebois S, Vahabzadeh-Hagh AM, Zubiaur F, Merati A. 43 - Management of recurrent laryngeal nerve paralysis. In: Randolph GW, editor. Surgery of the thyroid and parathyroid glands (3rd ed.) [Internet]. Elsevier; 2021. pp. 410–418.e2. [citéd 28 July 2023]. https://www.sciencedirect.com/science/article/pii/B9780323661270000430. [Google Scholar]
- 24▪▪.Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO Classification of thyroid neoplasms. Endocr Pathol 2022; 33:27–63. [DOI] [PubMed] [Google Scholar]; An extensive overview of the new WHO classification of thyroid neoplasms.
- 25.Papp S, Asa SL. When thyroid carcinoma goes bad: a morphological and molecular analysis. Head Neck Pathol 2015; 9:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prete A, Borges de Souza P, Censi S, et al. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol (Lausanne) 2020; 11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo SK, Lee S, Kim SJ, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLOS Genet 2016; 12:e1006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romei C, Elisei R. A narrative review of genetic alterations in primary thyroid epithelial cancer. Int J Mol Sci 2021; 22:1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu YH, Wirth LJ, Farahani AA, et al. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Mod Pathol 2020; 33:2458–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisapia P, Pepe F, Iaccarino A, et al. BRAF: a two-faced Janus. Cells 2020; 9:2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han F, Zhang L, Zhang S, et al. Occult oncocytic papillary thyroid carcinoma with lymphoid stroma (Warthin-like tumor): report of a case with concomitant mutations of BRAF V600E and V600K. Int J Clin Exp Pathol 2015; 8:5896–5901. [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo MK, Bae JS, Lee S, et al. The Warthin-like variant of papillary thyroid carcinoma: a comparison with classic type in the patients with coexisting Hashimoto's Thyroiditis. Int J Endocrinol 2015; 2015:456027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera M, Ricarte-Filho J, Knauf J, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol 2010; 23:1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panebianco F, Nikitski AV, Nikiforova MN, et al. Characterization of thyroid cancer driven by known and novel ALK fusions. Endocr Relat Cancer 2019; 26:803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pekova B, Sykorova V, Mastnikova K, et al. NTRK fusion genes in thyroid carcinomas: clinicopathological characteristics and their impacts on prognosis. Cancers (Basel) 2021; 13:1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakushina VD, Lerner LV, Lavrov AV. Gene fusions in thyroid cancer. Thyroid 2018; 28:158–167. [DOI] [PubMed] [Google Scholar]
- 37.Prasad ML, Vyas M, Horne MJ, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer 2016; 122:1097–1107. [DOI] [PubMed] [Google Scholar]
- 38.Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 2016; 126:1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Hou P, Yu H, et al. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/akt pathway in thyroid tumors. J Clin Endocrinol Metab 2007; 92:2387–2390. [DOI] [PubMed] [Google Scholar]
- 40.Barletta JA, Bellizzi AM, Hornick JL. Immunohistochemical staining of thyroidectomy specimens for PTEN can aid in the identification of patients with Cowden syndrome. Am J Surg Pathol 2011; 35:1505–1511. [DOI] [PubMed] [Google Scholar]
- 41.Ganly I, Makarov V, Deraje S, et al. Integrated genomic analysis of Hürthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell 2018; 34:256–270. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopal RK, Kübler K, Calvo SE, et al. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in Hürthle cell carcinoma. Cancer Cell 2018; 34:242–255. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim TH, Kim YE, Ahn S, et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr Relat Cancer 2016; 23:813–823. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer 2013; 20:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishino M, Nikiforova M. Update on molecular testing for cytologically indeterminate thyroid nodules. Arch Pathol Lab Med 2018; 142:446–457. [DOI] [PubMed] [Google Scholar]
- 46.Le Mercier M, D’Haene N, De Nève N, et al. Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology 2015; 66:215–224. [DOI] [PubMed] [Google Scholar]
- 47.Ma L, Ae W, Jw S, et al. Multiplatform molecular test performance in indeterminate thyroid nodules. Diagn Cytopathol 2020; 48:1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song Y, Xu G, Ma T, et al. Utility of a multigene testing for preoperative evaluation of indeterminate thyroid nodules: a prospective blinded single center study in China. Cancer Med 2020; 9:8397–8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steward DL, Carty SE, Sippel RS, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol 2019; 5:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellevicine C, Migliatico I, Sgariglia R, et al. Evaluation of BRAF, RAS, RET/PTC, and PAX8/PPARg alterations in different Bethesda diagnostic categories: a multicentric prospective study on the validity of the 7-gene panel test in 1172 thyroid FNAs deriving from different hospitals in South Italy. Cancer Cytopathol 2020; 128:107–118. [DOI] [PubMed] [Google Scholar]
- 51.Doerfler WR, Nikitski AV, Morariu EM, et al. Molecular alterations in Hürthle cell nodules and preoperative cancer risk. Endocr Relat Cancer 2021; 28:301–309. [DOI] [PubMed] [Google Scholar]
- 52.LiVolsi VA, Baloch ZW. The pathology of hyperthyroidism. Front Endocrinol (Lausanne) 2018; 9:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandiar D, Smitha T. Sanderson's polster. J Oral Maxillofac Pathol 2018; 22:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishino M, Jacob J. Invasion in thyroid cancer: controversies and best practices. Semin Diagn Pathol 2020; 37:219–227. [DOI] [PubMed] [Google Scholar]
- 55.Turk AT, Asa SL, Baloch ZW, et al. Interobserver variability in the histopathologic assessment of extrathyroidal extension of well differentiated thyroid carcinoma supports the New American Joint Committee on Cancer Eighth Edition Criteria for Tumor Staging. Thyroid 2019; 29:619–624. [DOI] [PubMed] [Google Scholar]
- 56.Thompson LDR, Poller DN, Kakudo K, et al. An international interobserver variability reporting of the Nuclear Scoring Criteria to diagnose noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a validation study. Endocr Pathol 2018; 29:242–249. [DOI] [PubMed] [Google Scholar]
- 57.Paja M, Zafón C, Iglesias C, et al. Rate of noninvasive follicular thyroid neoplasms with papillary-like nuclear features depends on pathologist's criteria: a multicentre retrospective Southern European study with prolonged follow-up. Endocrine 2021; 73:131–140. [DOI] [PubMed] [Google Scholar]
- 58.Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016; 2:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu B, Ghossein RA. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): an update. Head Neck Pathol 2020; 14:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hysek M, Paulsson JO, Jatta K, et al. Clinical routine TERT promoter mutational screening of follicular thyroid tumors of uncertain malignant potential (FT-UMPs): a useful predictor of metastatic disease. Cancers (Basel) 2019; 11:1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duan H, Liu X, Ren X, et al. Mutation profiles of follicular thyroid tumors by targeted sequencing. Diagn Pathol 2019; 14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carney JA, Hirokawa M, Lloyd RV, et al. Hyalinizing trabecular tumors of the thyroid gland are almost all benign. Am J Surg Pathol 2008; 32:1877–1889. [DOI] [PubMed] [Google Scholar]
- 63.Nikiforova MN, Nikitski AV, Panebianco F, et al. GLIS rearrangement is a genomic hallmark of hyalinizing trabecular tumor of the thyroid gland. Thyroid 2019; 29:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitahara CM, Sosa JA. Understanding the ever-changing incidence of thyroid cancer. Nat Rev Endocrinol 2020; 16:617–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong KS, Chen TY, Higgins SE, et al. A potential diagnostic pitfall for hobnail variant of papillary thyroid carcinoma. Histopathology 2020; 76:707–713. [DOI] [PubMed] [Google Scholar]
- 66.Ghossein R, Livolsi VA. Papillary thyroid carcinoma tall cell variant. Thyroid 2008; 18:1179–1181. [DOI] [PubMed] [Google Scholar]
- 67.Malandrino P, Russo M, Regalbuto C, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid 2016; 26:1285–1292. [DOI] [PubMed] [Google Scholar]
- 68.Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 2015; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Neill CJ, Vaughan L, Learoyd DL, et al. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol 2011; 37:181–185. [DOI] [PubMed] [Google Scholar]
- 70.Xu B, Wang L, Tuttle RM, et al. Prognostic impact of extent of vascular invasion in low-grade encapsulated follicular cell-derived thyroid carcinomas: a clinicopathologic study of 276 cases. Hum Pathol 2015; 46:1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kure S, Ohashi R. Thyroid Hürthle cell carcinoma: clinical, pathological, and molecular features. Cancers (Basel) 2020; 13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pecce V, Verrienti A, Abballe L, et al. Loss of function SETD2 mutations in poorly differentiated metastases from two Hürthle cell carcinomas of the thyroid. Cancers (Basel) 2020; 12:1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akslen LA, LiVolsi VA. Prognostic significance of histologic grading compared with subclassification of papillary thyroid carcinoma. Cancer 2000; 88:1902–1908. [PubMed] [Google Scholar]
- 74.Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 2007; 31:1256–1264. [DOI] [PubMed] [Google Scholar]
- 75.Rivera M, Ghossein RA, Schoder H, et al. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer 2008; 113:48–56. [DOI] [PubMed] [Google Scholar]
- 76.Melo M, da Rocha AG, Vinagre J, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab 2014; 99:E754–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung CK, Jung SH, Jeon S, et al. Risk stratification using a novel genetic classifier including PLEKHS1 promoter mutations for differentiated thyroid cancer with distant metastasis. Thyroid 2020; 30:1589–1600. [DOI] [PubMed] [Google Scholar]
- 78.Salvatore D, Santoro M, Schlumberger M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat Rev Endocrinol 2021; 17:296–306. [DOI] [PubMed] [Google Scholar]
- 79.Zafon C, Gil J, Pérez-González B, Jordà M. DNA methylation in thyroid cancer. Endocr Relat Cancer 2019; 26:R415–R439. [DOI] [PubMed] [Google Scholar]
- 80.Klein Hesselink EN, Zafon C, Villalmanzo N, et al. Increased global DNA hypomethylation in distant metastatic and dedifferentiated thyroid cancer. J Clin Endocrinol Metab 2018; 103:397–406. [DOI] [PubMed] [Google Scholar]