INTRODUCTION
Pulmonary hypertension (PH) is characterized by progressive and abnormal proliferation of smooth muscle cells and vasoconstriction, leading to narrowing, obliteration, and remodeling of the pulmonary circulation,1 with the resultant increment in circulatory afterload, increased right ventricular (RV) wall stress,2 abnormal RV mechanics,3 and ultimately, right heart failure and death. PH is estimated to affect around 1% of the world population, and it can affect up to 10% of individuals older than 65 years worldwide.4,5 The higher prevalence of PH in the elderly is mostly due to PH associated with left heart disease, which is classified as World Symposium on PH (WSPH) Group 2 PH.6
PH is associated with impaired quality of life and increased cardiovascular morbidity and mortality, as well as with increased economic burden. Significant progress has been made in the understanding of the different forms of PH, especially pulmonary arterial hypertension or PAH (WSPH Group 1 PH). Different prognostic variables have been identified to predict treatment response and progression of disease, and several predictive models have been developed.7-10 Moreover, several therapeutic agents targeting different pathways are available, which are used in combination for control, and perhaps, even reverse remodeling of the disease.11,12 However, despite all progress made, a significant delay in diagnosis and referral to expert centers remains, which delays the initiation of proper care, and patients are diagnosed at advanced stages.13-15 Although effective therapies exist for PAH, the mean average cost per patient is estimated at 80,000 US dollars per year.16,17 Even among patients with health insurance coverage, deductibles and co-pays can lead to substantial out-of-pocket costs.18,19 Excessive costs have also been described in other WSPH groups such as chronic thromboembolic PH.20,21 Because of the economic burden and sometimes prohibitive cost of therapies, many patients may not receive standard-of-care therapies, a reality that is probably more dramatic in the developing world.4,5,17
Socioeconomic status (SES) refers to an individual’s social and economic standing, reflecting its social or economic rank in a given social group.22 Social determinants of health (SDOH) refer to conditions in which individuals are born, grow, live, work, and age,23 and are implicated in health inequalities and poor conditions of daily life, which disproportionately affect certain groups such as minorities. Recent years have brought renewed attention to racial and ethnic inequalities in health, especially since the events of sociopolitical violence that arose in the year 2020, including the coronavirus disease 2019 (COVID-19) pandemic.24-26 In the current review, we provide an overview of the current knowledge of racial, ethnic, and socioeconomic inequalities in patients with PAH (Fig. 1). We first review some of the concepts that are described in the article, and describe some of the intrinsic limitations to its current assessment. We then describe differences in race, ethnicity, and the socioeconomic profile of individuals with PAH. Finally, we describe gaps in knowledge and areas that need to be further explored.
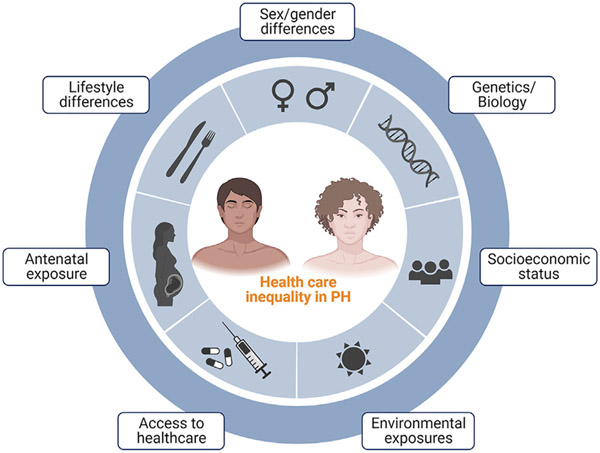
Fig. 1.
Central figure: Health care inequality in PH. Biological, environmental, and socioeconomic factors influence the course of the disease at different levels such as early antenatal exposure, lifestyle choices, social deprivation, and eventually, socioeconomic status and access to health care. (Created with BioRender.com.)
CONSIDERATIONS
The study of associations between race, ethnicity, and SES is complex, as we will proceed to describe. We consider important to characterize first potential barriers and pitfalls in the analysis of health inequality in PAH.
Racial segregation.
Despite important changes in the last decades, the United States remains a highly segregated country.27,28 Racial segregation leads to marked differences in environmental and social exposures among different racial groups, and differences in outcomes based on race or ethnicity may actually reflect differences in the type of social or environmental exposure.29 As such, it is important that studies analyzing differences based on race or ethnicity account for differences in exposures and socioeconomics. For example, some studies describing differences in survival rates in Hispanics with PAH could not adjust for differences in SES or SDOH,30,31 which warrant caution in the interpretation of these associations.32,33
Interaction of race, ethnicity, and SES.
Certain minority groups such as Hispanics and Blacks are more likely to live in economically-deprived communities.34 Although it is possible that some biological differences attributed to racial differences could explain different outcomes, the overlap between race/ethnicity and SES complicates the analysis if differences are due to “race and social class”, or “race or social class”.29 Going back to the example of survival in Hispanics with PAH, Bernardo and colleagues studied the association between Hispanic ethnicity and survival in patients with PAH, adjusting not only for age, sex, and PAH subtype but also for SDOH such as income, education level, and insurance status.3
Data source.
Proper analysis of differences in outcomes based on race/ethnicity requires that data sources are large, geographically diverse, and have sufficient representation of minorities.29 Moreover, these data sources have to include key socioeconomic variables for proper analysis and adjustment of covariates in multivariate analysis. Unfortunately, rarely a dataset will have these ideal characteristics, which limits the analysis. For instance, the largest registry of PH in the United States is the REVEAL registry (Registry to Evaluate Early and Long-term PAH Disease Management).7 Although REVEAL is rich in clinical and biological data pertaining to PH, overall it lacks information related to SES and SDOH; also, minorities are underrepresented in REVEAL.35-37
Measures of SES.
It is related to annual income, educational attainment, poverty level, and occupation, and no single metric can properly quantify SES. As such, it is common practice to report the individual components of SES and SDOH. Other attractive descriptors of SES are described next.
Minorities and people belonging to socially-disadvantaged communities frequently reside in different geographic locations than White or high-income individuals. As such, geographic metrics such as zone improvement (ZIP) codes can provide insight into differences in social and environmental exposures, and be used as a surrogate of SES. Unfortunately, not all PH databases routinely include ZIP codes or other geographic metrics in their datasets (with exception of REVEAL and the Pulmonary Vascular Disease Phenomics Program or PVDOMICS).38,39 Such an analysis has not been completed in the United States yet, but a study from Scotland used a geographic index (Scottish Index of Multiple Deprivation) to gather estimates of social deprivation in patients with PH.40
Net worth is an attractive metric of SES.29,41 In simple terms, net worth measures the accumulated value of all assets minus liabilities, and is related to income. However, net worth measures far more than income, as it also accounts for inter-generational transfer of wealth, capturing the accumulated socioeconomic advantage that certain racial groups may enjoy, compared with racial minorities. Net worth also measures the quality of financial decision-making.29 To the best of our knowledge, net worth is not included in current PH datasets.
We do not describe these pitfalls as a criticism of the existing literature on differences in race/ethnicity and SES in PH, but rather as an effort to emphasize the complexity of the question under study, and to standardize a common language. No single metric of SES will be accurate enough to fully characterize an individual’s profile. As such, we recommend using multiple measures of SES, and we favor describing individual SDOH components in the analysis of health inequality.
LIMITATIONS IN CURRENT UNDERSTANDING OF PULMONARY ARTERIAL HYPERTENSION DISPARITIES
In-depth understanding of PAH disparities depend on an analysis of population-level data that includes a diverse representation of race/ethnicity, geography, and SES.29 Unfortunately, in most PAH clinical trials and registries there is a noticeable under-representation of minorities and little data surrounding social and environmental risk factors.36
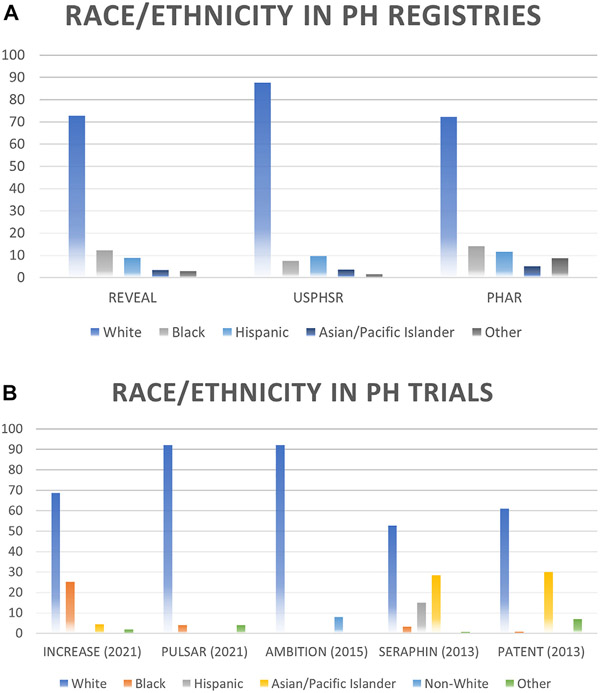
REVEAL (Registry to Evaluate Early and Long-term PAH Disease Management) is the largest registry of PAH in the United States.7 In REVEAL, the proportion of Hispanics was 8.9%, whereas the expected proportion of Hispanics should have been close to 11.5%, based on US census estimates (Fig. 2A). Similarly, the proportion of Asian/Pacific Islander participants was 3.3%, in comparison with an expected proportion of 6.1%.35,37,38 Overall, Black patients were accurately represented (12.2%, for an expected proportion of 10.9%). Under-representation of racial and ethnic minorities is also notable in United States Pulmonary Hypertension Scientific Registry (USPHSR). In USPHSR, Hispanics and Asians accounted for 9.6% and 3.2% of participants, respectively42 (see Fig. 2A). Owing to the limited diversity of participants in these registries, Asians and Pacific Islanders are grouped as a single category, although recent evidence suggests that Native Hawaiian and other Pacific Islanders have unique characteristics (eg, different exposures, comorbidities, and barriers to care) when compared with other individuals from Asia, and should be included as a different racial category.43
Fig. 2.
Representation of minorities in PH clinical trials and registries. (A) Data in US-based registries and (B) Data in PH clinical trials. PH, pulmonary hypertension; PHAR, Pulmonary Hypertension Association Registry; REVEAL, Registry to Evaluate Early and Long-term PAH disease management; USPHSR, United States Pulmonary Hypertension Scientific Registry.
Under-representation of racial and ethnic minorities is additionally notable in the two largest clinical trials in PAH. In SERAPHIN (Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome), out of 742 participants, only 14.7% of participants were of Hispanic ethnicity, and 2.6% were Black, compared with 54.5% of White participants (see Fig. 2B).44 As a reference, based on census estimates by the year 2013, the proportion of White, Hispanic, and Black individuals should be 62.4%, 17.4% and 12.3%.45 From the prostacyclin PGI2 receptor agonist in pulmonary arterial hypertension (GRIPHON) trial, a clinical trial of 1156 participants, no racial or ethnic data are available.46 A pooled cohort analysis of different clinical trials in PAH analyzed trends over time of participants in these trials. Information from 18 trials was extracted, for a total of 6599 participants, of which 66.5% were non-Hispanic White, 14.4% Asian, 10.5% Hispanic, and 3.9% Black.47 Most Asian and Hispanic participants were recruited from Asia and Latin America, respectively, and these participants may have different social and environmental exposures than patients in the United States, which limits the generalizability of these findings.
Although we mostly refer to PAH in the current review, a few points can be highlighted in other forms of PH. The INCREASE trial (Safety and Efficacy of Inhaled Treprostinil in Adult PH With ILD Including CPFE) was a randomized clinical trial of patients with PH in the setting of interstitial lung disease, where out of the 362 patients included, 8.3% were Hispanic, 21.8% Black, and 73% White.48 CHEST-1 (Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1) was a clinical trial in patients with chronic thromboembolic PH, and out of 261 randomized patients, 71% were White and 3% Black.49 Of note, Hispanic ethnicity was not reported. Less is known about other forms of PH and there is a lack of data regarding disparities in other forms of PH.
Current data on race/ethnicity in PAH are still limited and most studies are reported in non-Hispanic Black and Hispanic patients.37,38 Less is known about the profile of Asian patients,50 Native Americans,51,52 and Native Hawaiian and other Pacific Islanders. Moreover, very little is known about racial/ethnic and socioeconomic differences in outcomes outside the United States and Europe. Less is known about PH in low- and middle-income countries (LMIC). Although the global prevalence of PH is estimated to be between 20 million and 70 million people,4,53 the prevalence of PAH in LMIC is not known.
As most PAH prognostic and therapeutic models are derived from these datasets, patients of different backgrounds may get treated with data not representing the same diversity37 We agree with the recent American Thoracic Society statement on disparities in PAH which highlights the need for PAH trials and registries to increase the level of diversity of participants and to improve the collection of information related to race/ethnicity and socioeconomic factors. This information is needed to identify patients at increased risk for worse outcomes due to difficulty accessing health care, lack of or limited health care insurance, poor SES, and distrust of the health care system.37,54 Granular data on race/ethnicity, SES, SDOH, environmental exposures, and interaction among these factors is needed to understand and overcome barriers to care, and to offer personalized, evidence-based diagnostic, therapeutic, and prognostic interventions that ensure equality of care.37
PULMONARY ARTERIAL HYPERTENSION AND GENDER
PAH is a disease predominantly affecting female patients, although the disease in male patients tends to be more severe.55-57 More recently, Ventetuolo and colleagues described the role of sexual dimorphism, and its influence on exercise capacity and hemodynamics, with a differential relationship based on age or body mass index.58 The role of estrogen metabolites in the biology of the disease is extensively reviewed elsewhere55,59-61 and outside the scope of our review.
Overall, women are well-represented in most PAH trials and registries, which is expected given the female sex preponderance of PAH.7,42,44,46,62,63 There is very limited information on gender-based disparities in patients with PH, although a few points can be inferred. Analysis of participant-level data from different trials found a lower 6-minute walk distance (6MWD) in women than in men.58 Although there may be other explanations for these differences such as different disease phenotypes, one could speculate it also reflects delays in trial enrollment of women, and women are enrolled at more advanced stages with the associated impairment on exercise capacity. A study with PH patients from Cameroon found higher exposure to indoor cooking fumes in women; a history of tuberculosis was also more frequent in women.64
Unequal access to reproductive health care throughout the United States certainly affects women of reproductive age. Female patients with PAH are counseled against pregnancy based on international consensus guidelines,65,66 and pregnant patients with PAH are often counseled to pursue termination.67 With the recent Supreme Court decision to overturn Roe v. Wade, ending the right to abortion it established under the US Constitution, the authority to ban or otherwise restrict access to abortion is now determined at the state level. Pregnant patients in certain states with PH will face several challenges affecting their care,68 such as having to relocate to a different state to pursue termination, with its associated logistic costs, which is particularly challenging to all women, but particularly for minoritized women of lower socioeconomic status. Moreover, for women with PAH unable to pursue a medically-indicated pregnancy termination, fetal and maternal morbidity and mortality will further increase67 and broaden the already existing disparities.
PULMONARY ARTERIAL HYPERTENSION AND RACE/ETHNICITY
Non-Hispanic Black patients with PAH experience differences in disease etiology, disease presentations, treatment responses, and some clinical outcomes when compared with other ethnicities. As an example, non-Hispanic Black patients with PAH have a higher frequency of connective tissue disease such as scleroderma when compared with White patients. Furthermore, the disease profile of scleroderma-associated PAH may be more severe in Black patients: In a cohort analysis of 160 patients from the Johns Hopkins PH program, Black patients had a worse functional class on presentation, lower exercise capacity, higher levels of natriuretic peptides (reflecting higher right heart strain), more severe hemodynamic metrics, and a trend toward decreased survival.69 In addition to scleroderma, PH associated with sarcoidosis and sickle cell disease is also more frequent in Black individuals.70,71
Non-Hispanic Black patients have a different response pattern to endothelin receptor antagonists when compared with White patients.72 A pooled analysis of six randomized clinical trials of endothelin receptor antagonists in PAH showed that whereas the placebo-adjusted change in 6MWD in White patients was an increment of 41.5 m, there was a decrease of 3.5 m in Blacks, a treatment response difference of 45 m (95% CI, −2.4–92.4 m), a difference that persisted in adjusted models.72 It was hypothesized that these differences in treatment response could be related to higher ET-1 levels in Black patients, not sufficiently inhibited by the dose of the endothelin receptor antagonists used in the trials.72,73
The data related to differences in clinical outcomes between non-Hispanic Black patients and other races/ethnicities have been mixed.74,75 In the largest analysis using data from REVEAL, there were no differences in survival in Black patients when compared with other races/ethnicities.31 Parikh and colleagues used data from two large academic centers (Duke University Medical Center and the Cleveland Clinic Foundation), for a total of 250 patients.75 Black patients had decreased survival as compared with Whites (HR 2.06, 95% CI 1.18–3.44), even after adjusting for age and functional class. But after adding insurance status to the model, this survival difference was no longer present, which suggests that some of the outcome differences were related to proper access to care (or lack of). Medrek and colleagues used data from REVEAL to study the association between race/ethnicity and transplant-free survival in patients with PAH.31 Although in the unadjusted analysis, there was improved survival in Black individuals compared with Whites, this difference was no longer seen after adjusting for age and PAH subtype (HR 0.931, 95% CI 0.767–1.130). As previously mentioned, REVEAL does not allow for the assessment of socioeconomic factors.
PULMONARY ARTERIAL HYPERTENSION IN HISPANICS
Overall, Hispanic patients with PAH tend to be younger and have a higher frequency of congenital heart disease and portopulmonary hypertension, as compared with non-Hispanic Whites.38,54 Uncorrected congenital heart disease is more frequent in Hispanics, believed to be related to lack of access to proper care in early childhood, where a congenital defect would have been identified early on so corrective surgery be performed.38 The higher frequency of cirrhosis and PH is thought to be related to a higher frequency of hepatitis B and C infections that went untreated.76 What follows is a discussion of three of the largest studies that describe PAH in Hispanic populations.
Karnes and colleagues performed an elegant study using data from the National Biological Sample and Data Repository for PAH (PAH Biobank), and compared differences in outcomes between Hispanic and non-Hispanic White and Black patients with idiopathic/hereditary PAH.30 In this study, ethnicity was self-reported by individuals, but genomic data were also gathered to determine the genetic ancestry of study participants. The authors found that Hispanic patients had better survival than both non-Hispanic White and Black patients, after adjusting for age, sex, pulmonary vascular resistance, and use of prostacyclin analogs (HR 0.46, 95% CI 0.21–0.99), and similar results were obtained on a validation cohort from the Allegheny Health Network (HR 0.65, 95% CI 0.42–0.99). Limitations of the study included the data source, which may not be representative of the PAH population in the United States but only of a few specialized centers. Second, this study focused mainly on idiopathic and heritable PAH rather than other types of PH, which further limits the generalizability of these findings. Furthermore, combining different data sources (to increase sample size) comes with the risk of introducing phenotypic heterogeneity: cohorts of patients that differ in the time data were collected, duration of follow-up, and different subtypes of PAH (idiopathic/heritable for the PAH biobank and all forms of PAH for the other cohorts).77 The study did not account for differences in social, economic, or environmental determinants of health.33
Medrek and colleagues used data from REVEAL to compare differences in outcomes based on race/ethnicity.31 In this study, patients self-reported their race/ethnicity. Although the univariate analysis suggested better survival in Hispanics, no association was found in the multivariate analysis (which adjusted for age and PAH subtype). As previously mentioned, the study could not account for differences in SES as this information is not available in REVEAL. As the authors commented, “the role of SES and access to care on outcomes in PAH remains unclear and warrants further study”.31
We recently used data from the Pulmonary Hypertension Association Registry (PHAR) to describe the profile of Hispanic patients with PAH, and to characterize differences in SDOH and clinical outcomes.3 PHAR is a particularly unique dataset because it has a higher representation of minorities including Hispanics and Black individuals,37 but also because information on SDOH is routinely and prospectively collected.62,78 Socioeconomic metrics and SDOH were significantly different in the Hispanic population: Hispanic patients had less optimal health care insurance, lower annual income, lower education level, and were more likely to be unemployed, as compared with non-Hispanic White patients.3 For instance, 32% of Hispanics had an annual income of less than 20,000 US dollars, as compared with 17.4% of non-Hispanic Whites. Hispanic patients had a higher frequency of emergency visits and a higher number of hospitalizations, despite similar functional class, disease risk severity, exercise capacity, natriuretic peptide levels, or PAH-specific therapy. Although the unadjusted analysis showed better survival in Hispanic individuals, this association was no longer present after adjustment for age, sex, PAH subtype, and SDOH (annual income, health insurance, and education level). Limitations of the study included a smaller dataset than REVEAL although the SDOH data in PHAR was in fact richer. Second, and very important: Data from PHAR (as well as data from REVEAL) reflect data of patients referred to PAH expert centers, and may not reflect the reality of the vast majority of patients treated in the community.
Based on the results of these three studies, we believe that the better survival noticed in Hispanic patients in the unadjusted analysis is mostly related to the younger age of Hispanic patients. Hispanic patients are younger, and younger age by itself is associated with improved outcomes.79 Once adjusting for age, this survival benefit is no longer seen. Other factors accounting for a possible survival benefit include the predominant PAH subtype. Hispanic patients have a higher frequency of congenital heart disease, which also by itself is associated with longer survival (related to the pattern of RV adaptation and a persistent fetal phenotype).80 We do not deny that certain factors could offer a biological advantage in Hispanics, such as an increased RV mass (reflecting a more efficient RV adaptation),81 but we emphasize that race/ethnicity has to be put in the context of different social and environmental factors. Although some biological factors could offer a potential biological advantage, how much this matters under the effects of structural and SDOH has yet to be fully characterized.
PULMONARY HYPERTENSION IN LOW- AND MIDDLE-INCOME COUNTRIES
The prevalence of PAH in the United States and Europe is estimated at around 6.6 to 26 cases per million individuals.35,82 There are limited data about PAH in LMIC currently available. Available data are generally limited to prevalence and etiology with far less attention paid to differences in treatment and outcomes. A study from Uruguay (52 patients) reported a PAH prevalence of 15.1 cases per million individuals, and the more common etiologies were congenital heart disease (34%), followed by idiopathic PAH (32%), and connective tissue disease (26%).83 In a study from Saudi Arabia (107 patients), idiopathic PAH (51%), congenital heart disease (27%) and connective tissue disease (15%) were the most common etiologies.84 Thienemann and colleagues reported data from Cameroon, Mozambique, Nigeria, and South Africa as part of the Pan African Pulmonary Hypertension Cohort Registry Study.85 From 220 patients with all forms of PH (16% with PAH), the most common etiologies were human immunodeficiency virus (HIV) infection (50%), idiopathic PAH, and congenital heart disease (24% each). Tuberculosis was considered the etiology in 70% of cases of PH associated with chronic lung disease. Etiologies of PH such as schistosomiasis, HIV infection, uncorrected congenital heart disease, and chronic high altitude exposure are more common in LMIC.17 The exact prevalence of pulmonary vascular disease caused by Schistosoma infection is not known, although based on small reports from Brazil and Africa, the prevalence ranges from 7.7% to 33%.86 Etiologies such as schistosomiasis and HIV infection deserve special attention because they represent treatable and preventable conditions; if identified and treated before the development of PAH, a significant reduction in morbi-mortality could be expected.
The Pan African Pulmonary Hypertension Cohort Registry Study offers some insight into SDOH within PH patients in Africa.85 Up to 22% of participants never went to school, 38% completed only elementary school, and 35% had an income of less than 30 US dollars per month. A sub-study from the same registry described differences of interest in exposure based on gender. Although smoking and alcohol use were more frequent in men, exposure to indoor cooking fumes and history of tuberculosis were more frequent in women.64 How the SDOH in this population impacts disease presentation, access to care, morbidity, and mortality warrants further study.
The Global Burden of Disease study is a multinational effort to produce comparable, consistent measures of disease burden for national and subnational populations.87 In collaboration with the Pulmonary Vascular Research Institute (PVRI), the Global Burden of Disease investigators published the results of a systematic review to estimate the prevalence, incidence, and mortality of PAH globally.88 Reported prevalence ranged from 3.7 to 150 cases per million. Reported incidence ranged from 0.008 to 1.4 cases/100,000 person-years. The reported 1-year survival ranged from 72% to 91%.88
PULMONARY ARTERIAL HYPERTENSION IN RURAL POPULATIONS
The treatment of patients with PAH is largely limited to referral centers located in metropolitan areas. Optimal PAH care requires frequent in-person assessments. Transportation barriers limiting access to PAH referral centers in rural areas include the cost of gas, limited public transportation options, and lack of a driver or personal vehicle89,90 Patients from disadvantaged economic backgrounds in rural areas with limited access to PAH centers are more likely to miss clinic appointments, have challenges related to pharmacy access, and experience delays in medical care.19,89 The impact of these access-to-care challenges for PAH patients in rural areas are apparent: A recent study reported higher mortality among PH patients living in rural areas compared with urban/metropolitan areas.91 Macias and colleagues performed an analysis of adults with PH from a commercial health insurance/Medicare Advantage database. Rurality was estimated based on the ZIP codes of participants. From over 6000 participants, 90% had a residency in metropolitan counties, 4.1% in large urban counties, 5.1% in small urban counties, and 0.8% in rural counties. The population was predominantly White (62.3%), and 20.3% of participants had high poverty rates. Mortality risk was significantly higher among those living in small urban counties (HR 1.48; 95% CI 1.14–1.92) and rural counties (HR 2.01; 95% CI 1.13–3.57) after adjusting for age, sex, neighborhood poverty rate, race/ethnicity, and disease burden.91
Telemedicine could be a means of overcoming transportation barriers in rural areas, however, that depends on having access to proper technology, knowledge about the use of such technology, and having stable internet connectivity, which unfortunately is frequently lacking in rural locations.92,93
GAPS IN KNOWLEDGE AND FUTURE DIRECTIONS
PAH in low-income individuals needs to be better characterized, both from a the socioeconomic and a biological perspective. Datasets that include information on SDOH such as PHAR3,62,94 and PVDOMICS63 can provide better insight into this special patient population. Furthermore, it is important to better understand the significance of PH in populations not currently well-represented in PH databases or registries such as Pacific Islanders43 and Native Americans.51,52
As we mentioned above, more than one metric of SES is needed for a better visualization of health inequality. Analysis using environmental exposure and geographic differences (using ZIP code data or social vulnerability indices95) can provide additional insight.
The interaction between race/ethnicity and SES needs to be further delineated. The intrinsic biological factors of different populations could be further characterized, and datasets that include OMICS metrics such as PVDOMICS63 are ideal for this.
Given the complex interplay between race/ethnicity, SES/SDOH, and environmental exposures, we think there could be a potential role for machine learning and network analysis to better understand these interactions and how they relate to health care barriers and differences in disease outcomes.96
A better understanding of how to reduce the barriers to equitable access and implementation of PAH care is needed. Through collaboration between at-risk populations, patient advocacy groups (such as the Pulmonary Hypertension Association), and PAH specialists, researchers and professional societies, an improved understanding of barriers to PAH equity is needed to successfully implement PAH interventions that reduce the disparities discussed throughout this article. Organizations such as PVRI are leading the effort to equalize access to care in individuals suffering from PH worldwide, such as estimating the global prevalence of PH,88 and developing different task forces to improve access to PH health care worldwide.
CLINICS CARE POINTS.
There is significant under-representation of minorities in most PH clinical trials and registries.
Proper representation of minorities is key to ensuring proper care and identifying barriers to diagnosis or treatment.
Race/ethnicity are social concepts, influenced by racial segregation. A proper understanding of differences in racial/ethnic biological differences in PH is not possible unless data are accounted for a different socioeconomic profile.
KEY POINTS.
There is significant under-representation of minorities with pulmonary arterial hypertension (PAH) in most clinical trials and registries. An important mechanism of health inequality and disparities in care is the lack of inclusivity in national registries and clinical trials.
Proper representation of minorities is key to ensuring proper care and identifying barriers to diagnosis or treatment. Striving for accurate racial and ethnic representation is essential for individualization of treatment algorithms and generalizability of outcomes in PAH.
Race/ethnicity are social concepts, intrinsically associated with socioeconomic status, which is influenced by racial segregation. A proper understanding of race/ethnicity as biological variables in pulmonary hypertension is not possible unless also accounting for differences in socioeconomic factors.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES
Summary Conflict of interest statements: Dr R.J. Bernardo serves on the medical advisory board for Janssen Pharmaceuticals. Dr V.A. de Jesus Perez has no conflict of interest to declare.
REFERENCES
- 1.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019;53(1). 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its Load in pulmonary hypertension. J Am Coll Cardiol 2017;69(2):236–43. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo RJ, Lu D, Ramirez RL 3rd, et al. Hispanic ethnicity and social determinants of health in pulmonary arterial hypertension: the pulmonary hypertension association registry. Ann Am Thorac Soc 2022. 10.1513/AnnalsATS.202109-1051OC. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016;4(4):306–22. [DOI] [PubMed] [Google Scholar]
- 5.Hassoun PM. Pulmonary arterial hypertension. N Engl J Med 2021;385(25):2361–76. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension. Circulation 2010;122(2):164–72. [DOI] [PubMed] [Google Scholar]
- 8.Sitbon O, Benza RL, Badesch DB, et al. Validation of two predictive models for survival in pulmonary arterial hypertension. Eur Respir J 2015;46(1):152–64. [DOI] [PubMed] [Google Scholar]
- 9.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019;53(1):1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad F, Contrepois K, Amsallem M, et al. The right heart network and risk stratification in pulmonary arterial hypertension. Chest 2021;11. 10.1016/j.chest.2021.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galie N, Humbert M, Vachiery JL, et al. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of Cardiology (ESC) and the European Respiratory society (ERS): Endorsed by: association for European Paediatric and congenital Cardiology (AEPC), international society for heart and lung Transplantation (ISHLT). Eur Heart J 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 12.Vizza CD, Lang IM, Badagliacca R, et al. Aggressive afterload lowering to improve the right ventricle: a New Target for medical therapy in pulmonary arterial hypertension? Am J Respir Crit Care Med 2022;205(7):751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest 2011;140(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strange G, Gabbay E, Kermeen F, et al. Time from symptoms to definitive diagnosis of idiopathic pulmonary arterial hypertension: the delay study. Pulm Circ 2013;3(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandras SA, Ventura HO, Corris PA. Breaking down the barriers: why the delay in referral for pulmonary arterial hypertension? Ochsner J 2016;16(3):257–62. [PMC free article] [PubMed] [Google Scholar]
- 16.Sikirica M, Iorga SR, Bancroft T, et al. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res 2014;14:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich S, Haworth SG, Hassoun PM, et al. Pulmonary hypertension: the unaddressed global health burden. Lancet Respir Med 2018;6(8):577–9. [DOI] [PubMed] [Google Scholar]
- 18.Helgeson SA, Menon D, Helmi H, et al. Psychosocial and financial burden of therapy in USA patients with pulmonary arterial hypertension. Diseases 2020;8(2). 10.3390/diseases8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillmeyer KR, Rinne ST, Qian SX, et al. Socioeconomically disadvantaged Veterans experience treatment delays for pulmonary arterial hypertension. Pulm Circ 2022. 10.1002/pul2.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirson NY, Birnbaum HG, Ivanova JI, et al. Excess costs associated with patients with chronic thromboembolic pulmonary hypertension in a US privately insured population. Appl Health Econ Health Policy 2011;9(6):377–87. [DOI] [PubMed] [Google Scholar]
- 21.Boon G, van den Hout WB, Barco S, et al. A model for estimating the health economic impact of earlier diagnosis of chronic thromboembolic pulmonary hypertension. ERJ Open Res 2021;7(3). 10.1183/23120541.00719-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieger N. A glossary for social epidemiology. J Epidemiol Community Health 2001;55(10):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marmot M, Friel S, Bell R, et al. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet 2008;372(9650):1661–9. [DOI] [PubMed] [Google Scholar]
- 24.Thakur N, Lovinsky-Desir S, Bime C, et al. The structural and social determinants of the racial/ethnic disparities in the U.S. COVID-19 Pandemic. What’s our role? Am J Respir Crit Care Med 2020;202(7):943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merritt D. George Floyd’s death and COVID-19: inflection points in the Anthropocene Era? J Anal Psychol 2021;66(3):750–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger N. ENOUGH: COVID-19, structural racism, police brutality, plutocracy, climate change-and time for health justice, democratic governance, and an equitable, sustainable future. Am J Public Health 2020;110(11):1620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James SA. The strangest of all encounters: racial and ethnic discrimination in US health care. Cad Saúde Pública 2017;1(Suppl 1):e00104416, 33Suppl. [DOI] [PubMed] [Google Scholar]
- 28.Athey S, Ferguson B, Gentzkow M, et al. Estimating experienced racial segregation in US cities using large-scale GPS data. Proc Natl Acad Sci U S A 2021;16(46):118. 10.1073/pnas.2026160118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaVeist TA. Disentangling race and socioeconomic status: a key to understanding health inequalities. J Urban Health 2005;82(2 Suppl 3):iii26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karnes JH, Wiener HW, Schwantes-An TH, et al. Genetic admixture and survival in diverse populations with pulmonary arterial hypertension. Am J Respir Crit Care Med 2020;201(11):1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medrek S, Sahay S, Zhao C, et al. Impact of race on survival in pulmonary arterial hypertension: results from the REVEAL registry. J Heart Lung Transplant 2020;39(4):321–30. [DOI] [PubMed] [Google Scholar]
- 32.Minai OA, Yan T, Mascha E, et al. Race as an independent prognostic factor in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol 2005;96(5):740. [DOI] [PubMed] [Google Scholar]
- 33.Non AL, Chang SY. Challenging the role of genetic ancestry in explaining racial/ethnic health disparities. Am J Respir Crit Care Med 2021;203(3):397–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol 2016;35(4):407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 2011;139(1):128–37. [DOI] [PubMed] [Google Scholar]
- 36.Goel K, Hon SM, Farber HW, et al. Pulmonary arterial hypertension: what rare diseases tell us about disparities in disease registries, clinical trials, and treatment algorithms. Chest 2021;160(5):1981–3. [DOI] [PubMed] [Google Scholar]
- 37.Bernardo RJ, de Jesus Perez VA. Hispanic ethnicity and social determinants of health: Harnessing data from the pulmonary hypertension association registry. Advances in Pulmonary Hypertension 2022;21(2):44–8. [Google Scholar]
- 38.Medrek SK, Sahay S. Ethnicity in pulmonary arterial hypertension: possibilities for novel phenotypes in the age of personalized medicine. Chest 2018;153(2):310–20. [DOI] [PubMed] [Google Scholar]
- 39.Hemnes AR, Beck GJ, Newman JH, et al. Pvdomics: a multi-center study to improve understanding of pulmonary vascular disease through Phenomics. Circ Res 2017;121(10):1136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellino K, Kerridge S, Church C, et al. Social deprivation and prognosis in Scottish patients with pulmonary arterial hypertension. Eur Respir J 2018;51(2). 10.1183/13993003.00444-2017. [DOI] [PubMed] [Google Scholar]
- 41.South E, Venkataramani A, Dalembert G. Building Black wealth — the role of health Systems in closing the gap. N Engl J Med 2022;387(9):844–9. [DOI] [PubMed] [Google Scholar]
- 42.Badlam JB, Badesch DB, Austin ED, et al. United States pulmonary hypertension scientific registry: Baseline characteristics. Chest 2021;159(1):311–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taparra K, Qu V, Pollom E. Disparities in survival and comorbidity burden between Asian and native Hawaiian and other Pacific Islander patients with Cancer. JAMA Netw Open 2022;5(8):e2226327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369(9):809–18. [DOI] [PubMed] [Google Scholar]
- 45.Pew Research Center: Population, by Race and Ethnicity: 2013. Available at: https://www.pewresearch.org/hispanic/ph_2015-03_statistical-portrait-of-hispanics-in-the-united-states-2013_current-01/.
- 46.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015;373(26):2522–33. [DOI] [PubMed] [Google Scholar]
- 47.Min J, Appleby DH, McClelland RL, et al. Secular and regional trends among pulmonary arterial hypertension clinical trial participants. Ann Am Thorac Soc 2022;19(6):952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled Treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 2021;384(4):325–34. [DOI] [PubMed] [Google Scholar]
- 49.Ghofrani H-A, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369(4):319–29. [DOI] [PubMed] [Google Scholar]
- 50.Lim Y, Low TT, Chan SP, et al. Pulmonary arterial hypertension in a multi-ethnic Asian population: characteristics, survival and mortality predictors from a 14-year follow-up study. Respirology 2019;24(2):162–70. [DOI] [PubMed] [Google Scholar]
- 51.Dubroff J, Melendres L, Lin Y, et al. High geographic prevalence of pulmonary artery hypertension: associations with ethnicity, drug use, and altitude. Pulm Circ 2020;10(1). 2045894019894534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali T. Prevalence and correlates of pulmonary hypertension and right ventricular geometric abnormalities in an American Indian population. publisher not identified; 2000. Available at: http://gateway.proquest.com/openurl?url_ver=Z39.88-2004&rft_val_fmt=info:ofi/fmt:kev:mtx:dissertation&res_dat=xri:pqm&rft_dat=xri:pqdiss:9978709. [Google Scholar]
- 53.Gidwani S, Nair A. The burden of pulmonary hypertension in resource-limited settings. Glob Heart 2014;9(3):297–310. [DOI] [PubMed] [Google Scholar]
- 54.Talwar A, Garcia JGN, Tsai H, et al. Health disparities in patients with pulmonary arterial hypertension: a Blueprint for action. An official American thoracic society statement. Am J Respir Crit Care Med 2017;196(8):e32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keen J, Prisco SZ, Prins KW. Sex differences in right ventricular dysfunction: insights from the bench to bedside. Front Physiol 2020;11:623129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheron C, McBride SA, Antigny F, et al. Sex and gender in pulmonary arterial hypertension. Eur Respir Rev 2021;(162):30. 10.1183/16000617.0330-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lakshmanan S, Jankowich M, Wu WC, et al. Gender differences in risk factors associated with pulmonary artery Systolic Pressure, heart failure, and mortality in Blacks: Jackson heart study. J Am Heart Assoc 2020;9(1):e013034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ventetuolo CE, Moutchia J, Baird GL, et al. Baseline sex differences in pulmonary arterial hypertension randomized clinical trials. Ann Am Thorac Soc 2022. 10.1513/AnnalsATS.202203-207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Jesus Perez VA. Making sense of the estrogen paradox in pulmonary arterial hypertension. Am J Respir Crit Care Med 2011;184(6):629–30. [DOI] [PubMed] [Google Scholar]
- 60.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014;307(1):L7–26. [DOI] [PubMed] [Google Scholar]
- 61.Hester J, Ventetuolo C, Lahm T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr Physiol 2019;10(1):125–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gray MP, Kawut SM. The pulmonary hypertension association registry: rationale, design, and role in quality improvement. Advances in Pulmonary Hypertension 2018;16(4):185–8. [Google Scholar]
- 63.Hemnes AR, Leopold JA, Radeva MK, et al. Clinical characteristics and transplant-free survival across the Spectrum of pulmonary vascular disease. J Am Coll Cardiol 2022;80(7):697–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aminde LN, Dzudie A, Kengne AP, et al. Gender disparities in pulmonary hypertension at a tertiary centre in Cameroon. S Afr Med J 2017;107(10):892–9. [DOI] [PubMed] [Google Scholar]
- 65.Hemnes AR, Kiely DG, Cockrill BA, et al. Statement on pregnancy in pulmonary hypertension from the pulmonary vascular research Institute. Pulm Circ 2015;5(3):435–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Humbert M, Kovacs G, Hoeper MM, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2022. 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 67.Coursen J, Simpson CE, Mukherjee M, et al. Pregnancy considerations in the multidisciplinary care of patients with pulmonary arterial hypertension. J Cardiovasc Dev Dis 2022;(8):9. 10.3390/jcdd9080260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Londras F, Cleeve A, Rodriguez MI, et al. The impact of provider restrictions on abortion-related outcomes: a synthesis of legal and health evidence. Reprod Health 2022;19(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blanco I, Mathai S, Shafiq M, et al. Severity of systemic sclerosis-associated pulmonary arterial hypertension in African Americans. Medicine (Baltim) 2014;93(5):177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med 2018;139:72–8. [DOI] [PubMed] [Google Scholar]
- 71.Klings ES, Machado RF, Barst RJ, et al. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med 2014;189(6):727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gabler NB, French B, Strom BL, et al. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest 2012;141(1):20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ergul S, Parish DC, Puett D, et al. Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension. Hypertension 1996;28(4):652–5. [DOI] [PubMed] [Google Scholar]
- 74.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 2005;95(2):199–203. [DOI] [PubMed] [Google Scholar]
- 75.Parikh KS, Stackhouse KA, Hart SA, et al. Health insurance and racial disparities in pulmonary hypertension outcomes. Am J Manag Care 2017;23(8):474–80. [PubMed] [Google Scholar]
- 76.Diaz R, Ferrer G. Pulmonary arterial hypertension in Hispanics. Cureus 2019;11(10):e5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Austin ED, Hamid R. Deconstructing the melting pot in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020;201(11):1329–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DesJardin JT, Kolaitis NA, Kime N, et al. Age-related differences in hemodynamics and functional status in pulmonary arterial hypertension: Baseline results from the Pulmonary Hypertension Association Registry. J Heart Lung Transplant 2020;39(9):945–53. [DOI] [PubMed] [Google Scholar]
- 79.Hjalmarsson C, Radegran G, Kylhammar D, et al. Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur Respir J 2018;51(5). 10.1183/13993003.02310-2017. [DOI] [PubMed] [Google Scholar]
- 80.Guimaron S, Guihaire J, Amsallem M, et al. Current knowledge and recent advances of right ventricular Molecular biology and metabolism from congenital heart disease to chronic pulmonary hypertension. BioMed Res Int 2018;2018:1981568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawut SM, Lima JA, Barr RG, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation 2011;123(22):2542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoeper MM, Huscher D, Pittrow D. Incidence and prevalence of pulmonary arterial hypertension in Germany. Int J Cardiol 2016;203:612–3. [DOI] [PubMed] [Google Scholar]
- 83.Ana IG, Gabriela P, Cecilia C, et al. Diez años de experiencia de un centro de referencia e hipertensión arterial pulmonar en Uruguay. Rev Méd Urug 2019;35(3). 10.29193/RMU.35.3.3. [DOI] [Google Scholar]
- 84.Idrees M, Alnajashi K, Abdulhameed J, et al. Saudi experience in the management of pulmonary arterial hypertension; the outcome of PAH therapy with the exclusion of chronic parenteral prostacyclin. Ann Thorac Med 2015;10(3):204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thienemann F, Dzudie A, Mocumbi AO, et al. The causes, treatment, and outcome of pulmonary hypertension in Africa: Insights from the Pan African pulmonary hypertension cohort (PAPUCO) registry. Int J Cardiol 2016;221:205–11. [DOI] [PubMed] [Google Scholar]
- 86.Butrous G. Pulmonary vascular diseases Secondary to schistosomiasis. Advances in Pulmonary Hypertension 2017;15(3):144–8. [Google Scholar]
- 87.Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emmons-Bell S, Johnson C, Boon-Dooley A, et al. Prevalence, incidence, and survival of pulmonary arterial hypertension: a systematic review for the global burden of disease 2020 study. Pulm Circ 2022;12(1):e12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health 2013;38(5):976–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kotani K. Transportation Issues in rural healthcare. J Prev Med Public Health 2020;53(2):149–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Macias CG, Wharam JF, Maron BA, et al. Urban-rural disparities in pulmonary hypertension mortality. Ann Am Thorac Soc 2020. 10.1513/AnnalsATS.202003-234RL. [DOI] [PubMed] [Google Scholar]
- 92.Marcin JP, Shaikh U, Steinhorn RH. Addressing health disparities in rural communities using telehealth. Pediatr Res 2016;79(1–2):169–76. [DOI] [PubMed] [Google Scholar]
- 93.Kolluri S, Stead TS, Mangal RK, et al. Telehealth in response to the rural health disparity. Health Psychol Res 2022;10(3):37445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DuBrock HM, Burger CD, Bartolome SD, et al. Health disparities and treatment approaches in portopulmonary hypertension and idiopathic pulmonary arterial hypertension: an analysis of the Pulmonary Hypertension Association Registry. Pulm Circ 2021;11(3). 20458940211020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee YC, Chang KY, Mirsaeidi M. Association of County-level social vulnerability with chronic Respiratory diseases mortality in the United States. Ann Am Thorac Soc 2022. 10.1513/AnnalsATS.202202-136OC. [DOI] [PubMed] [Google Scholar]
- 96.Rhodes CJ, Sweatt AJ, Maron BA. Harnessing Big data to advance treatment and understanding of pulmonary hypertension. Circ Res 2022;130(9):1423–44. [DOI] [PMC free article] [PubMed] [Google Scholar]