Executive summary
Stroke is the second leading cause of death worldwide. The burden of disability after a stroke is also large, and is increasing at a faster pace in low-income and middle-income countries than in high-income countries. Alarmingly, the incidence of stroke is increasing in young and middle-aged people (ie, age <55 years) globally. Should these trends continue, Sustainable Development Goal 3.4 (reducing the burden of stroke as part of the general target to reduce the burden of non-communicable diseases by a third by 2030) will not be met.
In this Commission, we forecast the burden of stroke from 2020 to 2050. We project that stroke mortality will increase by 50%—from 6·6 million (95% uncertainty interval [UI] 6·0 million–7·1 million) in 2020, to 9·7 million (8·0 million–11·6 million) in 2050—with disability-adjusted life-years (DALYs) growing over the same period from 144·8 million (133·9 million–156·9 million) in 2020, to 189·3 million (161·8 million–224·9 million) in 2050. These projections prompted us to do a situational analysis across the four pillars of the stroke quadrangle: surveillance, prevention, acute care, and rehabilitation. We have also identified the barriers to, and facilitators for, the achievement of these four pillars.
Disability-adjusted life-years (DALYs).
The sum of the years of life lost as a result of premature mortality from a disease and the years lived with a disability associated with prevalent cases of the disease in a population. One DALY represents the loss of the equivalent of one year of full health
On the basis of our assessment, we have identified and prioritised several recommendations. For each of the four pillars (surveillance, prevention, acute care, and rehabilitation), we propose pragmatic solutions for the implementation of evidence-based interventions to reduce the global burden of stroke. The estimated direct (ie, treatment and rehabilitation) and indirect (considering productivity loss) costs of stroke globally are in excess of US$891 billion annually. The pragmatic solutions we put forwards for urgent implementation should help to mitigate these losses, reduce the global burden of stroke, and contribute to achievement of Sustainable Development Goal 3.4, the WHO Intersectoral Global Action Plan on epilepsy and other neurological disorders (2022–2031), and the WHO Global Action Plan for prevention and control of non-communicable diseases.
Reduction of the global burden of stroke, particularly in low-income and middle-income countries, by implementing primary and secondary stroke prevention strategies and evidence-based acute care and rehabilitation services is urgently required. Measures to facilitate this goal include: the establishment of a framework to monitor and assess the burden of stroke (and its risk factors) and stroke services at a national level; the implementation of integrated population-level and individual-level prevention strategies for people at any increased risk of cerebrovascular disease, with emphasis on early detection and control of hypertension; planning and delivery of acute stroke care services, including the establishment of stroke units with access to reperfusion therapies for ischaemic stroke and workforce training and capacity building (and monitoring of quality indicators for these services nationally, regionally, and globally); the promotion of interdisciplinary stroke care services, training for caregivers, and capacity building for community health workers and other health-care providers working in stroke rehabilitation; and the creation of a stroke advocacy and implementation ecosystem that includes all relevant communities, organisations, and stakeholders.
Editorial note:
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Introduction
The global burden of stroke is huge: in 2020, stroke was the second leading cause of death (6·6 million deaths) and the third leading cause of disability (responsible for 143 million disability-adjusted life-years [DALYs]) after neonatal disorders (in children) and ischaemic heart disease (in adults).1,2 Alarmingly, evidence suggests that the incidence of stroke in younger individuals (ie, people younger than 55 years) is increasing worldwide.3 The absolute number of people affected by stroke, which includes those who die or remain disabled, has almost doubled in the past 30 years.1 Most of the contemporary stroke burden—86% of global deaths and 89% of global DALYs lost because of stroke in 2020—is in low-income and middle-income countries (LMICs),1 and the burden of stroke is increasing faster in LMICs than in high-income countries (HICs).1 Stroke is also a leading cause of depression and dementia, which are other common non-communicable diseases (NCDs).4,5
Little progress has been made by most countries towards Sustainable Development Goal (SDG) 3.4—reducing premature mortality from NCDs by a third between 2015 and 2030.6 Achieving SDG 3.4 worldwide, which would in turn facilitate the achievement of nine other SDGs,7 would require an additional US$140 billion of spending on NCD interventions from 2023–30, but could help to avert 39 million deaths and generate $2·7 trillion in net economic benefits (with benefits outweighing costs by a factor of 19:1).6
Given that the incidence of stroke rises with age, the combination of growing populations and ageing demographics is likely to result in large increases in global deaths and disability in the future unless major improvements occur in population prevention programmes that reduce the risk of stroke.8 Thus, pragmatic solutions to reduce the burden of stroke and related NCDs are urgently needed to save lives and improve brain health, quality of life, and socioeconomic productivity globally.8–11
To proffer solutions for reducing the global burden of stroke, we established the World Stroke Organization–Lancet Neurology Commission on Stroke8,12–14 in collaboration with WHO. In this Commission, we estimate the current and future global burden of stroke, review evidence-based interventions, and prioritise pragmatic solutions for reducing disease burden across the four pillars of the stroke quadrangle (surveillance, prevention, acute care, and rehabilitation; figure 1).15 We also do a situational and gap analysis by reviewing stroke-related literature, guidelines, and findings from surveys.8,13,14 Our pathways for implementation of the evidence-based solutions (based on implementation science theories16) were created on the basis of thematic analysis of barriers and facilitators (appendix pp 4–16, 50). We believe that the implementation of the pragmatic solutions of this Commission will be crucial for the realisation of the WHO global action plan for the prevention and control of NCDs 2013–2030.17,18
Figure 1: The four pillars of the quadrangle to tackle the burden of stroke: surveillance, prevention, acute care, and rehabilitation.
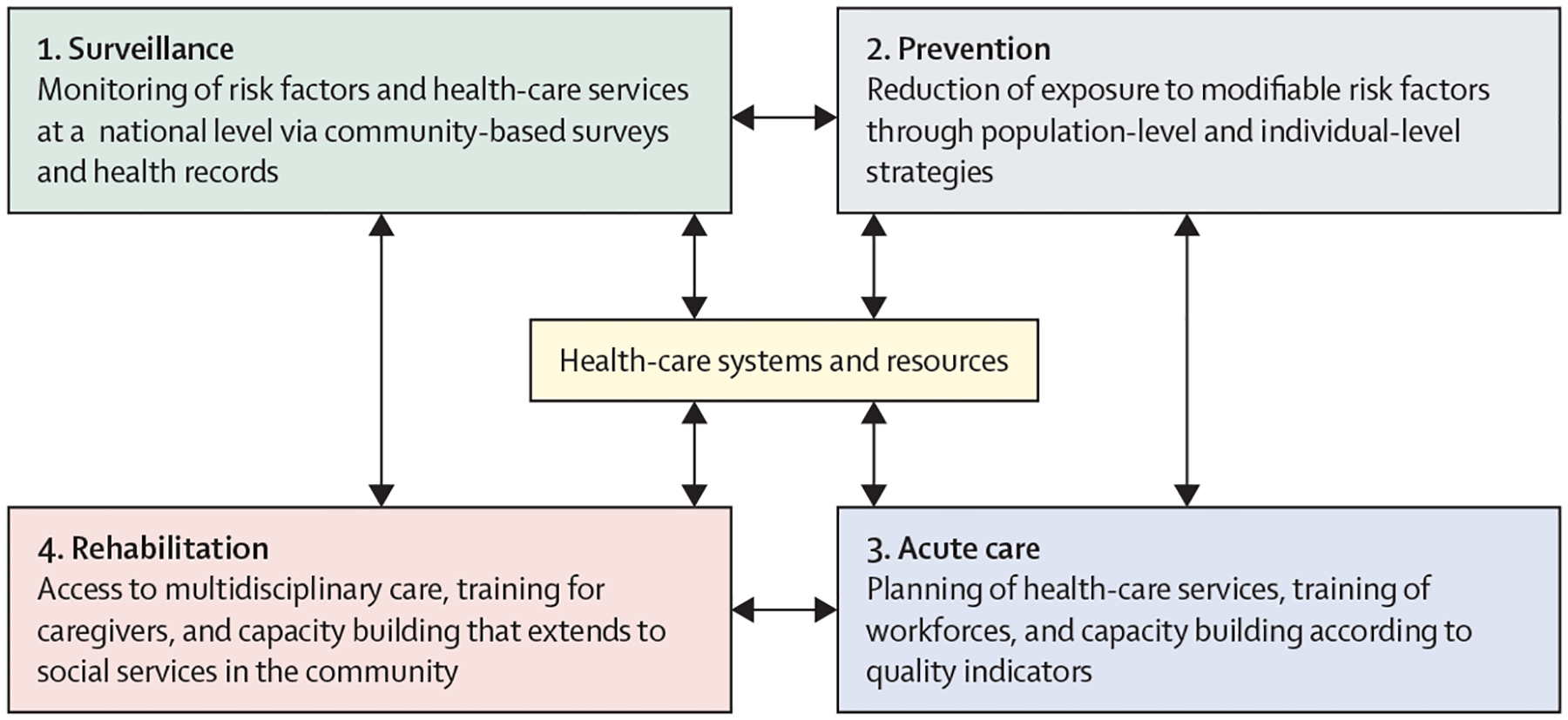
Surveillance strategies include establishing a framework for regular monitoring and assessment of the burden of stroke and its risk factors, and of health-care services at a national level via community-based surveys, data linkage, and electronic health records. These strategies provide the necessary evidence for planning and monitoring prevention, acute care, and rehabilitation interventions. Primordial, primary, and secondary prevention involve implementation of integrated population-wide strategies to reduce modifiable risk factors, such as hypertension and diabetes. Prevention strategies can reduce the incidence, mortality, and prevalence of stroke, and people who develop stroke benefit from secondary prevention (in addition to acute care). Acute stroke care should result in early diagnosis and involves evidence-based management that reduces mortality and improves functional outcomes. Finally, rehabilitation services provide interdisciplinary care for stroke survivors, with the aim of reducing disability-adjusted life-years and improving quality of life. Adapted with permission from Owolabi et al, 2023.11
Implementation science theories.
Scientific conceptual tools that enable researchers and practitioners to identify, describe, and explain important elements required for uptake of evidence-based practice and research into regular use by practitioners and policy makers
An enormous burden of disease can be averted
Previous estimates of the burden of stroke (measured as deaths and disability caused by a stroke) by the Global Burden of Disease (GBD) study were limited to 1990–2019,1 and the study also forecasted life expectancy, years of life lost, and mortality to 2040.19 However, for the long-term planning of health care, priority setting, and resource allocation, policy makers and national health services require projections of disease burden for at least the next 30 years. We used the same methods as the GBD study to estimate stroke burden for 2020 to 2050 overall and by age group (<60 years vs ≥60 years), World Bank country classification by income level (HICs vs LMICs), stroke type (ischaemic stroke vs intracerebral haemorrhage vs subarachnoid haemorrhage), and GBD super-regions. Our forecasts of stroke burden are based on estimates of mortality, incidence, and prevalence from the GBD 2019 study, and assume that medical procedures and prevention will be the same in 2050 as in 2019.1,2 Methods for establishing stroke types have been reported elsewhere,1 and further details of our methods are in the appendix (pp 6–7). Mortality rates for each individual cause were forecasted with a three-component model comprising the underlying (or risk deleted) mortality, modelled as a function of the Socio-demographic Index2 and time; a risk factor scalar that captures cause-specific combined risk-factor effects based upon the GBD comparative risk assessment, which quantifies risk–outcome associations accounting for risk factor mediation; and unexplained residual mortality. Forecasts of DALYs lost were produced from forecasts of years of life lost (via mortality) and years lived with disabilities (via prevalence and incidence).
Ischaemic stroke.
A neurological disease that occurs when the blood supply to part of the brain is blocked, with resulting death of the affected brain cells
Intracerebral haemorrhage.
A neurological disease that occurs when there is a bleeding from a blood vessel into the brain tissue, resulting in death of the affected tissue
Subarachnoid haemorrhage.
A neurological disease that occurs when there is a bleeding into the space surrounding the brain
Projections of mortality and disability
We estimate that the absolute number of people who will die from stroke worldwide will increase by 50% by 2050: from 6·6 million (95% uncertainty interval [UI] 6·0 million–7·1 million) in 2020, to 9·7 million (8·0 million–11·6 million) in 2050 (table 1). The burden of disability will also increase. The total number of DALYs from stroke is projected to increase by 31%: from 144·8 million (133·9 million–156·9 million) in 2020, to 189·4 million (161·8 million–224·9 million) in 2050 (figure 2, table 1; appendix pp 54, 63–67).
Table 1:
Estimates of the global stroke burden, 2020 and 2050, by World Bank income group
| Deaths per 100 000 person-years (95% UI) | Millions of deaths (95% UI) | DALYS per 100 000 person-years (95% UI) | Millions of DALYs (95% UI) | |||||
|---|---|---|---|---|---|---|---|---|
| 2020 | 2050 | 2020 | 2050 | 2020 | 2050 | 2020 | 2050 | |
| Global | ||||||||
| Overall | 84·89 (77·14–91·57) | 105·18 (87·28–125·75) | 6·62 (6·02–7·14) | 9·72 (8·07–11·62) | 1856·63 (1716·88–2010·86) | 2048·32 (1750·41–2432·64) | 144·84 (133·94–156·88) | 189·36 (161·82–224·88) |
| Overall (age-standardised) | 83·00 (75·32–89·61) | 54·31 (44·39–66·34) | ·· | ·· | 1747·70 (1612·34–1892·47) | 1229·16 (1029·90–1477·44) | ·· | ·· |
| <60 years* | 13·31 (12·10–14·47) | 9·93 (7·16–13·65) | 1·00 (0·91–1·09) | 0·98 (0·71–1·34) | 700·94 (638·28–767·75) | 649·85 (511·34–825·96) | 47·24 (43·01–51·54) | 46·40 (36·51–58·98) |
| ≥60 years* | 565·65 (508·59–613·37) | 361·69 (299·07–431·89) | 5·62 (5·08–6·09) | 8·75 (7·29–10·37) | 9184·71 (8392·76–9959·58) | 6795·05 (5890·94–7887·75) | 97·61 (89·20–105·85) | 142·95 (123·93–165·94) |
| High-income countries | ||||||||
| Overall | 75·97 (64·96–83·79) | 73·80 (60·26–85·93) | 0·92 (0·79–1·02) | 0·91 (0·74–1·06) | 1316·64 (1181·93–1435·60) | 1260·12 (1075·75–1438·24) | 15·95 (14·32–17·39) | 15·56 (13·28–17·76) |
| Overall (age-standardised) | 33·65 (29·10–36·88) | 18·86 (15·71–22·24) | ·· | ·· | 699·00 (629·87–761·14) | 488·27 (415·02–570·08) | ·· | ·· |
| <60 years* | 4·20 (3·92–4·48) | 3·15 (2·46–4·13) | 0·06 (0·05–0·06) | 0·04 (0·03–0·05) | 248·23 (224·59–275·23) | 200·28 (166·50–245·64) | 3·22 (2·93–3·55) | 2·43 (2·04–2·97) |
| ≥60 years* | 237·65 (203·36–262·05 | 127·68 (104·70–148·94) | 0·86 (0·73–0·95) | 0·87 (0·71–1·01) | 3820·45 (3413·56–4172·29) | 2482·53 (2125·54–2827·26) | 12·74 (11·27–13·95) | 13·12 (11·18–14·84) |
| Low-income and middle-income countries | ||||||||
| Overall | 86·50 (78·04 –93·82) | 109·95 (90·71–132·50) | 5·70 (5·14–6·18) | 8·81 (7·27–10·61) | 1954·66 (1798·69–2120·66) | 2168·44 (1832·77–2593·39) | 128·81 (118·53–139·75) | 173·68 (146·80–207·72) |
| Overall (age-standardised) | 105·82 (95·25–115·00) | 64·00 (52·48–77·90) | ·· | ·· | 2131·91 (1964·62–2315·49) | 1391·95 (1161·26–1678·44) | ·· | ·· |
| <60 years* | 15·37 (13·89–16·78) | 10·95 (7·88–15·15) | 0·94 (0·85–1·03) | 0·93 (0·67–1·29) | 754·92 (684·66–824·59) | 533·28 (414·75–682·72) | 43·98 (39·89–48·04) | 43·93 (34·29–56·03) |
| ≥60 years* | 732·10 (658·72–798·25) | 431·31 (358·28–514·82) | 4·76 (4·28–5·18) | 7·87 (6·53–9·37) | 11 896·98 (10 830·18–12 957·13) | 7337·88 (6301·95–8588·15) | 84·83 (77·36–92·39) | 129·75 (111·89–151·42) |
Global stroke deaths and DALYs (both absolute counts and rates per 100 000 person-years) are shown. DALYs=disability-adjusted life-years. 95% UI=95% uncertainty interval.
Rates per 100 000 person-years are age-standardised, whereas absolute counts are not.
Figure 2: Estimates of DALYs due to stroke, 2020 and 2050, by GBD super-region.
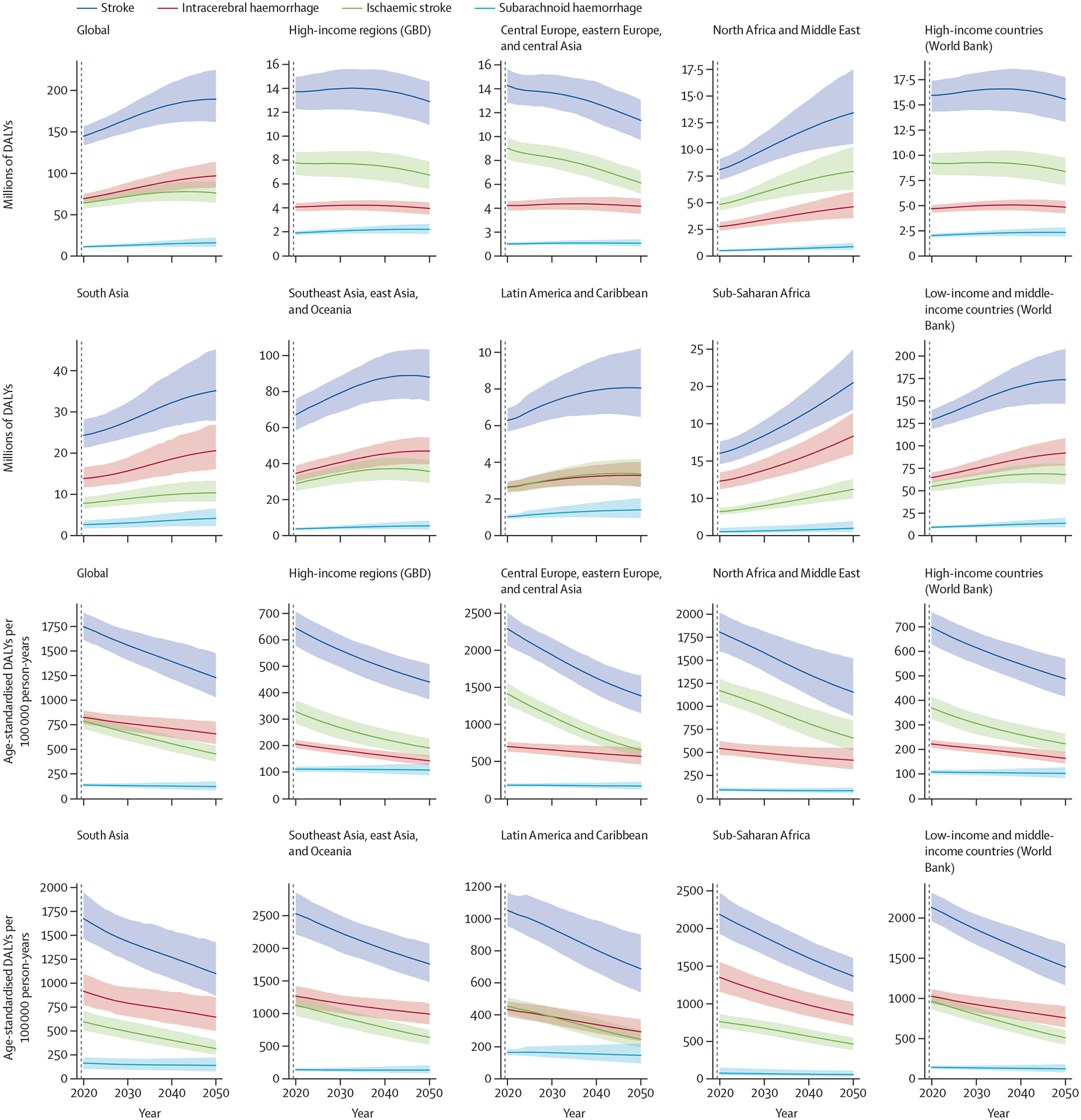
Data are absolute counts of DALYs and rates per 100 000 person-years. For comparison, the data are also represented by World Bank income group. Shaded areas represent 95% uncertainty intervals. GBD=Global Burden of Disease. DALYs=disability-adjusted life-years.
Although population growth and ageing are key drivers in forecasting estimates of stroke deaths and DALY counts, our survey analysis also suggests that an insufficient level of, and inequity in access to, high-quality prevention and acute and rehabilitation services will drive stroke-related deaths and disability, particularly in LMICs.
Mortality and disability according to age
We project an increase in the absolute number of stroke deaths in people aged 60 years or older (from 5·6 million [95% UI 5·1 million–6·1 million] in 2020, to 8·8 million [7·3 million–10·4 million] in 2050; table 1), probably due to ageing of the population. By contrast, the number of deaths in people younger than 60 years in 2050 is predicted to be roughly the same as that in 2020 (table 1). We also forecast a decrease in the age-standardised rate of stroke per 100 000 person-years in both people younger than 60 years (from 13·3 [12·1–14·5] in 2020, to 9·9 [7·2–13·7] in 2050) and in those aged 60 years or older (from 565·7 [508·6–613·4] in 2020, to 361·7 [299·1–431·9] in 2050; table 1; appendix pp 50–53, 63–64). The smaller proportional decrease in people younger than 60 years compared with those aged 60 years or older could be related to the reported increase in the prevalence of diabetes and overweight in the younger age group.20,21
Age-standardisation.
A statistical method used to compare disease rates or other health indicators (eg, risk factors, outcomes) between populations while accounting for differences in their age structure
In HICs, for people of all ages, age-standardised DALYs per 100 000 person-years are expected to decrease substantially, from 699·0 (95% UI 629·9–761·1) in 2020, to 488·3 (415·0–571·1) in 2050, but the absolute number of DALYs overall is projected to remain largely static (figure 2, table 1), probably as a result of population growth and ageing populations.
We project a non-significant decrease in DALYs in people younger than 60 years (from 47·2 million [43·0 million–51·5 million] in 2020, to 46·4 million [36·5 million–59·0 million] in 2050); in older people, we project a significant increase in DALYs from 97·6 million (89·2 million–105·9 million) in 2020, to 143·0 million (124·0 million–165·9 million) in 2050, probably due to continuous ageing of the population.
Mortality and disability by World Bank group
Our projections of stroke deaths in 2020 and 2050 by World Bank income group (table 2, figure 3) show that, although age-standardised stroke mortality will decline in both HICs (from 33·7 deaths per 100 000 person-years [95% UI 29·1–36·9] in 2020, to 18·9 per 100 000 person-years [15·7–22·2] in 2050) and LMICs (from 105·8 per 100 000 person-years [95·3–115·0] in 2020, to 64·0 per 100 000 person-years [52·5–77·9] in 2050), the absolute number of people who are projected to die from stroke will only slightly decrease in HICs and will sharply increase in LMICs. In these poorer countries, the proportion of global stroke deaths is projected to increase from 86% in 2020, to 91% in 2050. In other words, more than 90% of the deaths caused by stroke worldwide will occur in LMICs by 2050, if circumstances remain the same as today.
Table 2:
Estimates of mortality due to stroke, 2020 and 2050, by Global Burden of Disease super-region
| Deaths per 100 000 person-years (95% UI) | Millions of deaths (95% UI) | ||||
|---|---|---|---|---|---|
| 2020 | 2050 | 2020 | 2050 | ||
| Southeast Asia, east Asia, and Oceania | |||||
| Overall | 141·27 (121·11–159·81) | 232·25 (192·44–277·01) | 3·06 (2·62–3·46) | 4·94 (4·09–5·90) | |
| Overall (age-standardised) | 127·68 (108·49–144·44) | 82·54 (68·37–98·42) | ·· | ·· | |
| <60 years* | 16·85 (14·51–19·24) | 12·39 (9·35–15·59) | 0·44 (0·38–0·50) | 0·30 (0·22–0·37) | |
| ≥60 years* | 895·16 (761·93–1014·07) | 568·34 (467·50–677·61) | 2·62 (2·23–2·96) | 4·65 (3·83–5·51) | |
| Central Europe, eastern Europe, and central Asia | |||||
| Overall | 180·57 (162·50–198·46) | 164·32 (140·17–192·41) | 0·75 (0·68–0·83) | 0·66 (0·56–0·77) | |
| Overall (age-standardised) | 119·26 (107·35–131·04) | 62·53 (51·83–75·21) | ·· | ·· | |
| <60 years* | 15·71 (13·89–17·55) | 10·98 (6·89–16·45) | 0·07 (0·07–0·08) | 0·04 (0·03–0·07) | |
| ≥60 years* | 836·25 (751·08–917·84) | 419·53 (357·46–492·06) | 0·68 (0·61–0·75) | 0·61 (0·52–0·71) | |
| High-income | |||||
| Overall | 74·69 (63·41–83·43) | 69·50 (56·73–80·64) | 0·81 (0·69–0·91) | 0·78 (0·64–0·86) | |
| Overall (age-standardised) | 31·49 (27·14–34·92) | 17·21 (14·17–20·20) | ·· | ·· | |
| <60 years* | 3·66 (3·45–3·93) | 2·80 (2·18–3·61) | 0·05 (0·04–0·05) | 0·03 (0·03–0·04) | |
| ≥60 years* | 224·15 (190·90–250·25) | 116·96 (96·01–136·27) | 0·77 (0·64–0·86) | 0·75 (0·61–0·86) | |
| Latin America and Caribbean | |||||
| Overall | 48·58 (43·00–53·88) | 61·80 (46·78–80·77) | 0·29 (0·25–0·32) | 0·42 (0·32–0·55) | |
| Overall (age-standardised) | 49·61 (43·68–54·98) | 29·26 (21·48–39·93) | ·· | ·· | |
| <60 years* | 9·07 (8·07–10·24) | 6·49 (4·08–10·31) | 0·05 (0·05–0·07) | 0·05 (0·03–0·08) | |
| ≥60 years* | 330·39 (285·51–367·33) | 186·95 (140·68–246·18) | 0·23 (0·20–0·26) | 0·37 (0·28–0·48) | |
| North Africa and Middle East | |||||
| Overall | 51·42 (45·70–57·70) | 71·37 (52·93–98·35) | 0·32 (0·28–0·36) | 0·57 (0·43–0·80) | |
| Overall (age-standardised) | 86·57 (77·13–96·40) | 48·23 (36·12–65·80) | ·· | ·· | |
| <60 years* | 12·94 (10·83–15·52) | 9·34 (6·05–14·37) | 0·07 (0·06–0·08) | 0·08 (0·05–0·12) | |
| ≥60 years* | 596·41 (531·95–660·90) | 317·51 (243·27–418·31) | 0·25 (0·22–0·28) | 0·50 (0·38–0·67) | |
| Sub-Saharan Africa | |||||
| Overall | 36·80 (32·30–41·65) | 38·62 (31·51–46·47) | 0·41 (0·36–0·46) | 0·78 (0·64–0·94) | |
| Overall (age-standardised) | 105·19 (92·64–118·00) | 63·06 (52·63–73·21) | ·· | ·· | |
| <60 years* | 17·20 (14·15–20·43) | 11·07 (8·01–15·37) | 0·11 (0·09–0·13) | 0·18 (0·13–0·26) | |
| ≥60 years* | 714·50 (625·28–798·08) | 423·04 (357·14–484·48) | 0·30 (0·26–0·33) | 0·60 (0·50–0·69) | |
| South Asia | |||||
| Overall | 54·30 (46·78–63·19) | 75·24 (56·80–98·17) | 0·99 (0·85–1·15) | 1·56 (1·18–2·04) | |
| Overall (age-standardised) | 78·65 (67·53–91·29) | 46·82 (35·04–61·58) | ·· | ·· | |
| <60 years* | 14·49 (12·34–17·01) | 11·14 (7·30–17·00) | 0·21 (0·18–0·25) | 0·29 (0·19–0·44) | |
| ≥60 years* | 522·96 (447·36–605·91) | 293·92 (222·43–374·88) | 0·78 (0·67–0·90) | 1·27 (0·97–1·62) | |
Data are absolute counts of deaths and rates per 100 000 person-years. 95% UI=95% uncertainty interval.
Rates per 100 000 person-years are age-standardised, whereas absolute counts are not.
Figure 3: Estimates of mortality due to stroke, 2020 and 2050, by GBD super-region.
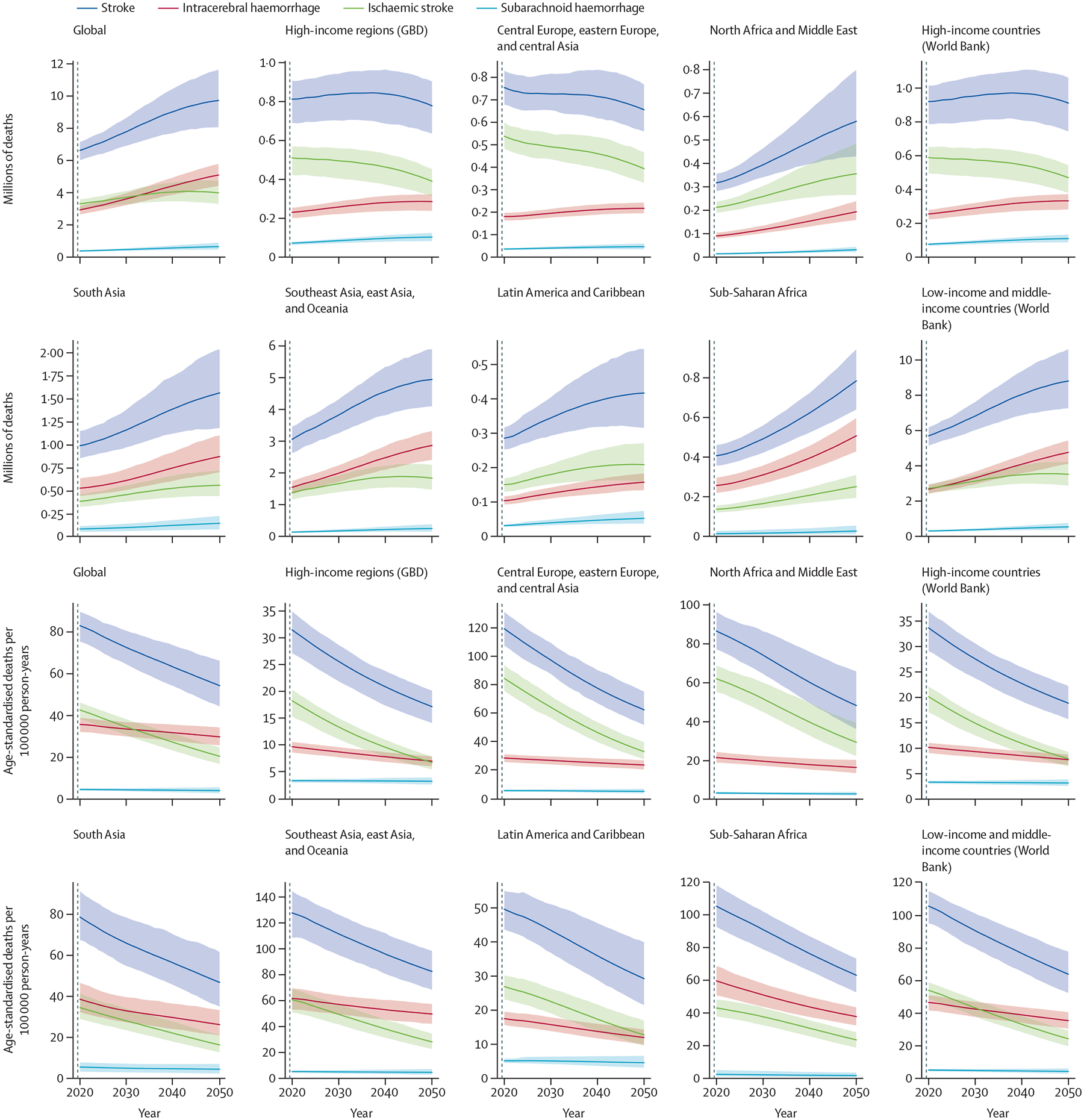
Data are absolute counts of deaths and rates per 100 000 person-years. For comparison, the data are also represented by World Bank country income group. Shaded areas represent 95% uncertainty intervals. GBD=Global Burden of Disease.
In LMICs, even though the age-standardised DALYs associated with stroke are expected to decrease from 2131·9 per 100 000 person-years (95% UI 1964·6–2315·5) in 2020, to 1392·0 per 100 000 person-years (1161·3–1678·4) in 2050, the absolute number of DALYs will increase over the same period (table 1), largely because of the growth and ageing of populations in these countries.
Mortality and disability by GBD super-region
In 2050, 4·9 million (95% UI 4·1 million–5·9 million) of the estimated 9·7 million stroke deaths are projected to occur in the Southeast Asia, east Asia, and Oceania super-region, accounting for 51% of all projected deaths, and 1·6 million (1·2 million–2·0 million) are projected to occur in the South Asia super-region (table 3). Age-standardised death rates are projected to fall between 2020 and 2050 across all GBD regions (figure 3), with the most substantial reductions in the high-income super-region and the Central Europe, eastern Europe, and central Asia super-region; however, the absolute number of deaths is projected to increase in the Southeast Asia, east Asia, and Oceania, Latin America and Caribbean, North Africa and Middle East, South Asia, and Sub-Saharan Africa super-regions (table 3). Similar geographical differences in stroke deaths and age-standardised mortality rates were noted for 1990–2019.1
Table 3:
Estimates of disability due to stroke, 2020 and 2050, by Global Burden of Disease super-region
| DALYs per 100 000 person-years (95% UI) | Millions of DALYs (95% UI) | |||
|---|---|---|---|---|
| 2020 | 2050 | 2020 | 2050 | |
| Southeast Asia, east Asia, and Oceania | ||||
| Overall | 3098·72 (2698·22–3503·00) | 4133·29 (3505·36–4851·04) | 87·98 (74·61–103·26) | 67·10 (58·43–75·85) |
| Overall (age-standardised) | 2531·07 (2210·74–2852·43) | 1760·79 (1481·49–2070·58) | ·· | ·· |
| <60 years* | 800·34 (695·10–911·04) | 616·17 (486·88–760·68) | 20·14 (17·56–22·99) | 13·93 (11·08–17·13) |
| ≥60 years* | 14 515·87 (12 576·37–16 386·97) | 9686·91 (8255·21–11 348·06) | 46·95 (40·65–53·10) | 74·06 (62·82–86·76) |
| Central Europe, eastern Europe, and central Asia | ||||
| Overall | 3415·23 (3075·35–3742·91) | 2844·59 (2435·30–3283·44) | 14·27 (12·85–15·64) | 11·35 (9·72–13·10) |
| Overall (age-standardised) | 2292·02 (2064·62–2511·35) | 1384·40 (1148·25–1660·84) | ·· | ·· |
| <60 years* | 739·83 (655·81–829·48) | 548·22 (401·81–739·17) | 3·35 (2·98–3·76) | 2·15 (1·59–2·86) |
| ≥60 years* | 13 040·44 (11771·67–14273·44) | 7174·71 (6260·53–8178·47) | 10·92 (9·86–11·94) | 9·20 (8·09–10·40) |
| High-income | ||||
| Overall | 1261·84 (1129·63–1375·59) | 1150·90 (976·25–1300·84) | 13·72 (12·28–14·96) | 12·89 (10·93–14·57) |
| Overall (age-standardised) | 644·58 (578·00–706·37) | 440·32 (375·18–507·88) | ·· | ·· |
| <60 years* | 220·88 (199·20–247·14) | 181·82 (150·95–222·68) | 2·54 (2·30–2·83) | 1·99 (1·67–2·41) |
| ≥60 years* | 3578·56 (3178·25–3923·08) | 2230·38 (1893·56–2519·85) | 11·17 (9·89–12·27) | 10·89 (9·31–12·32) |
| Latin America and Caribbean | ||||
| Overall | 1069·91 (951·33–1166·16) | 1193·46 (958·46–1511·55) | 6·29 (5·69–6·96) | 8·05 (6·47–10·20) |
| Overall (age-standardised) | 1053·01 (951·34–1166·16) | 687·61 (539·05–900·33) | ·· | ·· |
| <60 years* | 432·15 (383·19–484·38) | 319·56 (231·30–460·02) | 2·40 (2·13–2·69) | 2·26 (1·66–3·20) |
| ≥60 years* | 5352·31 (4787·79–5951·93) | 3236·32 (2639·65–4016·78) | 3·89 (3·49–4·32) | 5·79 (4·71–7·09) |
| North Africa and Middle East | ||||
| Overall | 1313·40 (1159·24–1476·81) | 1653·14 (1294·32–2154·86) | 8·10 (7·15–9·10) | 13·42 (10·51–17·50) |
| Overall (age-standardised) | 1807·75 (1596·44–2017·88) | 1154·33 (895·49–1521·04) | ·· | ·· |
| <60 years* | 666·19 (561·98–781·43) | 501·50 (358·19–710·24) | 3·60 (3·03–4·23) | 4·11 (2·95–5·78) |
| ≥60 years* | 9712·70 (8694·54–10 725·79) | 5674·98 (4573·65–7234·60) | 4·50 (4·02–4·93) | 9·31 (7·45–11·96) |
| Sub-Saharan Africa | ||||
| Overall | 998·46 (865·76–1138·49) | 1009·79 (831·57–1229·82) | 11·04 (9·57–12·59) | 20·51 (16·89–24·98) |
| Overall (age-standardised) | 2187·52 (1927·11–2473·86) | 1367·36 (1151·33–1606·96) | ·· | ·· |
| <60 years* | 788·34 (666·08–924·39) | 533·78 (407·41–709·48) | 5·39 (4·56–6·33) | 9·07 (6·90–12·08) |
| ≥60 years* | 11 876·43 (10 522·85–13 271·60) | 6576·47 (5670·19–7498·59) | 5·64 (4·99–6·31) | 11·43 (9·86–13·04) |
| South Asia | ||||
| Overall | 1336·09 (1174·54–1547·30) | 1690·59 (1338·26–2175·00) | 24·33 (21·40–28·18) | 35·16 (27·82–45·23) |
| Overall (age-standardised) | 1674·76 (1467·14–1945·97) | 1100·40 (865·51–1425·27) | ·· | ·· |
| <60 years* | 649·37 (562·23–758·80) | 515·61 (379·82–711·18) | 9·80 (8·50–11·43) | 12·89 (9·50–17·72) |
| ≥60 years* | 8775·28 (7640·72–10 189·38) | 5149·84 (4149·83–6403·86) | 14·53 (12·65–16·89) | 22·26 (17·97–27·69) |
Data are absolute counts of DALYs and rates per 100 000 person-years. DALYs=disability-adjusted life-years. 95% UI=95% uncertainty interval.
Rates per 100 000 person-years are age-standardised, whereas absolute counts are not.
The decreases in age-standardised DALYs per 100 000 person-years associated with stroke are projected to be similar across all GBD super-regions in 2050, with the largest decrease in the Central Europe, eastern Europe, and central Asia super-region (table 3). In the North Africa and Middle East and South Asia super-regions, we project a non-significant increase in age-standardised DALYs per 100 000 person-years associated with stroke in people younger than 60 years (table 3; appendix pp 56–59).
Death and disability by stroke type
We predict that an increasing proportion of stroke deaths globally would be caused by intracerebral haemorrhage (44·3% in 2020 vs 52·4% in 2050). This increase is largely attributable to the proportional increase of deaths from intracerebral haemorrhages in LMICs (from 2·7 million [95% UI 2·4 million–2·9 million] in 2020, to 4·8 million [4·1 million–5·4 million] in 2050), especially among people aged 60 years or older (from 2·0 million [1·8 million–2·2 million] in 2020, to 4·1 million [3·5 million–4·6 million] in 2050). The proportion of intracerebral haemorrhage in LMICs countries in 2050 is projected to be 1·5 times greater than that in HICs (accounting for 54% vs 37% of all stroke deaths), probably because of the higher prevalence and poorer control of hypertension in LMICs.22
Deaths from intracerebral haemorrhage among people younger than 60 years are projected to decrease in HICs (from 3·3 per 100 000 person-years [95% UI 3·0–3·5] in 2020, to 2·5 per 100 000 person-years [2·0–3·1] in 2050), probably because of better control of hypertension. Among GBD super-regions, the highest absolute number of deaths from intracerebral haemorrhage in 2050 is projected to be in the Southeast Asia, east Asia, and Oceania super-region (2·9 million [2·4 million–3·3 million]), but the fastest growth of deaths between 2020 and 2050 is projected to be in the Sub-Saharan Africa super-region (figure 3). Similar patterns in deaths from intracerebral haemorrhage were noted for 1990–2019.1
Worldwide, we forecast that age-standardised mortality rates will fall for each type of stroke in people of all ages, with the largest reductions for mortality from ischaemic stroke (from 42·6 per 100 000 person-years [95% UI 38·2–46·2] in 2020, to 20·5 per 100 000 person-years [16·8–24·7] in 2050), especially in HICs (table 4; appendix pp 52–55). The least reduced age-standardised mortality rates were projected for subarachnoid haemorrhage (table 4), a finding which is similar to previous observations.1
Table 4:
Estimates of global burden of death and disability, 2020 and 2050, by stroke type
| Age-standardised deaths per 100 000 person-years (95% UI) | Millions of deaths (95% UI) | Age-standardised DALYs per 100 000 person-years (95% UI) | Millions of DALYs (95% UI) | |||||
|---|---|---|---|---|---|---|---|---|
| 2020 | 2050 | 2020 | 2050 | 2020 | 2050 | 2020 | 2050 | |
| Ischaemic stroke | ||||||||
| Overall | 42·62 (38·23–46·20) | 20·50 (16·81–24·68) | 3·31 (2·98–3·58) | 4·00 (3·29–4·75) | 786·92 (706·13–864·10) | 452·26 (376·72–535·54) | 64·11 (57·47–70·40) | 76·33 (64·25–89·78) |
| <60 years | 2·45 (2·17–2·74) | 1·16 (0·77–1·72) | 0·18 (0·16–0·21) | 0·11 (0·08–0·17) | 153·88 (132·32–176·66) | 101·42 (78·77–127·76) | 11·42 (9·81–13·09) | 9·42 (7·33–11·81) |
| ≥60 years | 320·77 (286·71–347·95) | 154·46 (127·28–184·80) | 3·12 (2·81–3·38) | 3·87 (3·21–4·60) | 5170·52 (4665·88–5662·86) | 2881·74 (2423·50–3389·98) | 52·70 (47·57–57·67) | 66·91 (56·56–78·28) |
| Intracerebral haemorrhage | ||||||||
| Overall | 35·75 (32·20–38·85) | 29·78 (35·70–34·29) | 2·93 (2·65–3·19) | 5·10 (4·42–5·78) | 824·99 (756·20–895·45) | 656·14 (551·13–778·87) | 69·40 (63·54–75·35) | 96·90 (82·66–113·95) |
| <60 years | 10·08 (9·06–10·97) | 9·86 (7·78–12·53) | 0·68 (0·61–0·74) | 0·70 (0·56–0·89) | 393·75 (355·16–430·37) | 308·55 (241·47–397·86) | 29·25 (26·41–31·99) | 29·57 (23·32–37·67) |
| ≥60 years | 212·06 (189·37–230·59) | 208·80 (181·83–235·46) | 2·25 (2·01–2·45) | 4·39 (3·82–4·95) | 3811·23 (3462·76–4133·93) | 3063·13 (2636·05–3545·24) | 40·15 (36·52–43·54) | 67·32 (58·24–77·27) |
| Subarachnoid haemorrhage | ||||||||
| Overall | 4·63 (4·07–5·15) | 4·09 (2·87–5·68) | 0·38 (0·33–0·42) | 0·65 (0·47–0·89) | 135·79 (119·63–153·19) | 120·75 (81·48–172·88) | 11·33 (9·96–12·78) | 16·12 (11·19–22·66) |
| <60 years | 1·81 (1·55–2·17) | 1·63 (1·03–2·46) | 0·13 (0·11–0·16) | 0·16 (0·10–0·23) | 90·00 (77·50–106·87) | 80·32 (50·56–118·35) | 6·57 (5·66–7·78) | 7·41 (4·69–10·86) |
| ≥60 years | 24·16 (20·90–26·72) | 21·15 (15·03–29·17) | 0·25 (0·21–0·27) | 0·49 (0·35–0·68) | 452·89 (400·11–499·95) | 400·71 (280·68–555·91) | 4·76 (4·20–5·26) | 8·71 (6·18–12·03) |
Deaths and DALYs (both absolute counts and rates per 100 000 person-years) by stroke type are shown. DALYs=disability-adjusted life-years. 95% UI=95% uncertainty interval.
Intracerebral haemorrhage was projected to be the largest contributor of stroke-related DALYs in 2020 (48%) and 2050 (51%; tables 2, 5). Intracerebral haemorrhage accounted for a greater proportion of DALYs in people younger than 60 years (64%) than in those aged 60 years or older in 2050, probably because of more life-years lost and higher mortality in these patients.
Table 5:
Estimates of global economic burden of stroke, 2017 and 2050
| Low estimate 2017 (US$ billions) | High estimate 2017 (US$ billions) | Low estimate 2050 (US$ billions) | High estimate 2050 (US$ billions) | |
|---|---|---|---|---|
| High-income countries | 417·0 | 597·6 | 436·6–655·9 | 578·3–997·0 |
| Upper-middle-income countries | 279·1 | 410·0 | 273·5–743·6 | 334·2–1026·4 |
| Lower-middle-income countries | 48·3 | 64·8 | 159·5–200·4 | 170·4–252·5 |
| Low-income countries | 2·6 | 4·9 | 11·2–22·0 | 13·5–34·1 |
| Global | 745·9 | 1077·2 | 880·8–1621·9 | 1096·4–2310·0 |
The total cost includes both direct costs (associated with providing care for incident stroke cases and deaths) and indirect cost (ie, loss of income). Direct costs were calculated using previously described methods.8 The 2017 estimates comprise the low-cost and high-cost scenario estimates from Owolabi et al.8 Low estimates for 2050 assume that costs of treatment and rehabilitation grow at a rate 1% above the rate of non-medical inflation. High estimates for 2050 assume that costs of treatment and rehabilitation grow at 3% above the rate of non-medical inflation. For both low and high 2050 estimates, the range reflects the low-cost and high-cost estimates for acute and post-acute care in Owolabi et al.8
Age-standardised DALYs are projected to decrease from 2020 to 2050 for all types of stroke and age groups. We forecast that the highest proportion of age-standardised DALYs will be DALYs associated with intracerebral haemorrhage in the Southeast Asia, east Asia, and Oceania super-region, followed by intracerebral-haemorrhage-related DALYs in the Sub-Saharan Africa super-region, and ischaemic-stroke-related DALYs in the North Africa and Middle East and Central Europe, eastern Europe, and central Asia super-regions (figure 2).
Implications of these findings
In summary, our projections show that the absolute number of people who will die from stroke and will get disability due to stroke will continue to increase to 2050, although the pace of the overall increase in the stroke burden from 2020 to 2050 is slower than that in 1990–2019. However, we also project that age-standardised mortality and DALY rates will decrease. We project widening gaps in stroke burden (both in terms of deaths and disability) between rich and poor countries. A projected failure to meet the Sustainable Development Goals 3.4, and our projections of further increases in stroke burden, particularly in the Southeast Asia, east Asia, and Oceania and Sub-Saharan Africa super-regions, call for urgent action to improve stroke prevention and treatment across the globe (panel 1).
Panel 1: Key messages about the growing burden of stroke.
Because of population growth and ageing, the absolute number of people who will die from stroke worldwide will increase by 50%, compared with 2020, to 9·7 million in 2050. We also estimate that disability-adjusted life-years will grow by 31%, to 189·3 million in 2050. Our projections suggest that age-standardised mortality rates will decrease. However, the proportion of global stroke deaths accounted for by low-income and middle-income countries (LMICs) is projected to rise from 86% in 2020 to 91% by 2050. Similarly, the proportion of disability-adjusted life-years accounted for by LMICs will increase from 88% to 92% by 2050. In light of these estimates, by 2050, the substantial increases in the costs associated with stroke will cause distressing financial circumstances for many communities and national health systems. These unsustainable trends in burden and costs underline the importance of identifying interventions to prevent and manage stroke.
Implications
The burden of stroke will continue to increase worldwide and will disproportionally affect LMICs. The disparities in stroke burden between high-income countries and LMICs are projected to increase even further.
Current prevention strategies are insufficient, and the Sustainable Development Goals related to reducing the global burden of stroke will not be met.
Urgent measures to reduce stroke burden worldwide are needed, with an emphasis on LMICs, to increase a trained health-care workforce that can implement effective primary prevention strategies, including the early detection and adequate management of hypertension.
Effective interventions could result in substantial economic gains (because of reduced treatment and rehabilitation expenses). Evidence suggests that achieving the Sustainable Development Goals23 and WHO health targets17 with low-cost interventions—eg, early detection and adequate control of hypertension, reduction of salt content in processed foods, and smoking cessation campaigns—that cost less than US$1 per person per day in low-income countries and less than $3 a day in middle-income countries24 could reduce mortality from stroke and ischaemic heart disease by about 10%.25
Another promising strategy to reduce stroke incidence and mortality is population-wide primary prevention across the lifespan. It has been estimated that, for every $1 spent on the prevention of stroke and cardiovascular disease, there is a more than $10 return on investment.26 Additionally, primary prevention efforts directed at stroke would probably yield large gains because of the secondary effects of reducing the risk of heart disease, type 2 diabetes, dementia, and some types of cancer that share common risk factors, thus supporting achievements for a range of Sustainable Development Goals.
Scaling up of prevention of neurological disorders, including stroke, in 11 countries (Brazil, China, Colombia, Germany, Italy, Japan, Kenya, Lebanon, Romania, the UK, and the USA) to adequate levels by 2030 could save $2·4 trillion according to a 2022 estimate,27 while scaling up of interventions for treatment and rehabilitation to the required levels could save $911 billion and $727 billion, respectively.27
Research priorities
Monitoring and forecasting of the global burden of stroke, also at regional, national, and sub-national levels.
Developing interactive tools (including maps and data plots) showing the expected short-term and long-term effects of stroke prevention, treatment, and rehabilitation interventions on global, regional, and national burden (incidence, prevalence, deaths, years of live lost, years lived with disability, disability-adjusted life-years, and economic benefits).
Calculation of the effect of the burden of stroke on brain-health burden at global, regional, and national levels.
Our findings also suggest an increase (compared with 1990–2019) in the contribution of intracerebral haemorrhage to the overall increase in stroke burden in the world, especially in southeast Asia, east Asia, and Oceania. However, the fastest growth in deaths from intracerebral haemorrhage in 2050 is projected to occur in sub-Saharan Africa.
The relevance of hypertension control
The main risk factor for stroke—and particularly for intracerebral haemorrhage—is hypertension,1,28 and thus our projections support a call for improving prevention and treatment of hypertension.29 The number of people aged 30–79 years with hypertension roughly doubled between 1990 (331 million [95% CI 306 million–359 million] women and 317 million [292 million–344 million] men) and 2019 (626 million [584 million–668 million] women and 652 million [604 million–698 million] men), despite a stable age-standardised prevalence of the disorder.29 Among those with hypertension, only 23% (95% CI 20–27) of women and 18% (16–21) of men had their blood pressure under control. This increase in the overall prevalence of hypertension and poor control could be related to the widespread use of a high cardiovascular risk threshold (eg, a 5-year risk of a cardiovascular event of 15% or greater) for initiation of pharmacological treatment of increased blood pressure.30–34 Targeting individuals with high risk of cardiovascular disease and applying treatment thresholds for primary prevention of cardiovascular disease might be effective in an identifiable minority of patients,35,36 but this approach precludes access to prevention and effective treatment for a large proportion of the population, including individuals whose cardiovascular risk is below the treatment threshold but who have hypertension that requires pharmacological treatment.37–40 Pharmacological reduction of blood pressure lowers the risk of stroke and cardiovascular disease, even in patients with baseline systolic blood pressures of as low as 115 mm Hg, across all age groups.41 The World Stroke Organization and the World Federation of Neurology have called for the revision of primary prevention guidelines for stroke and cardiovascular disease42 to recommend primary prevention strategies in adults with any level of increased stroke or cardiovascular disease risk, with a focus on early detection and adequate management of hypertension.
Primary prevention.
Strategies to prevent stroke in persons with no previous history of stroke or transient ischemic attack
The situation in LMICs
Prevention and treatment of stroke are crucial for containing mortality and disability. Current detection and control of risk factors is suboptimal and inequitable, and the infrastructure for acute stroke care and rehabilitation is scarce, particularly in LMICs. Roughly half of these countries have implemented less than half of the recommended elements for acute care,43 meeting only about 30% of the acute care recommendations. Europe and central Asia have the stroke services that best align with evidence-based guidelines, whereas health-care systems in sub-Saharan Africa have implemented the fewest recommendations. Among LMICs, availability of physicians with expertise in stroke management is best in north Africa, the Middle East, eastern Europe, and central Asia and worst in sub-Saharan Africa. Many of the countries in these regions have low doctor-to-population ratios and inadequate numbers of multidisciplinary health-care professionals with training in acute and chronic care of patients with transient ischaemic attacks or stroke. Deficiencies in prevention and treatment of stroke due to workforce and resource shortages combine to account for an increased burden of stroke in LMICs.
Transient ischaemic attack.
A brief (usually only minutes or hours but less than 24 h) episode of brain dysfunction from an interruption in the blood supply to the brain or the eye without neuroimaging (CT or MRI) evidence of acute ischaemic brain injury
A huge economic burden
Health-care expenses associated with stroke were estimated to be as high as $315 billion in 2017 (the most recent year for which such data are available), with an additional loss in income of $576 billion: $891 billion in total.8 The incidence of stroke rises with age. As our projections show, because the world population keeps growing and the proportion of older people increasing, mortality and disability from stroke will grow too. As a result, the health-care costs associated with stroke might become unsustainable, unless prevention programmes that reduce the risk of stroke get implemented. Our forecasts also suggest that Asia’s share of global stroke deaths would rise from 61·3% of the global total in 2020 (about 4·1 million) to about 68·9% of global deaths from stroke in 2050 (around 6·6 million deaths).8 The proportion of annual global stroke deaths that sub-Saharan African countries will contribute, although smaller than that contributed by Asia, will also rise, from 6·2% in 2020, to 8·0% in 2050. Therefore, by 2050, the economic implications of stroke will be considerable, and they are more likely to be felt in Asia and Africa than elsewhere.8
Our methods to calculate the global stroke economic burden estimates for 2050 are supplied in the appendix (pp 33–35). Although our projections show that income losses because of stroke will rise by 2050, the rise is less sharp than that in direct expenses for two main reasons. First, the working-age population (ie, the proportion of the population aged 15–64 years), the primary driver of income losses from stroke in our projection model, is expected to increase only slightly from 2020 to 2050, but is expected to fall sharply in China and in countries currently classified as HICs per UN projections.44 Second, in our model we assumed that people would retire aged 65 years, which was the retirement age used to estimate income losses from stroke in 2017.8 If the proportion of older people (and particularly people older than 65 years) participating in work were to increase over time, our projections will underestimate income losses from stroke in 2050 (appendix pp 68–69).
The estimated aggregate economic costs of stroke, including direct costs and income losses, range from $746 billion to $1·08 trillion in 2017 prices. But by 2050, these costs are projected to rise to between $880 billion and US$2·31 trillion in 2017 prices (table 5).
Figure 4 shows estimated direct and indirect costs from stroke between 2017 and 2050. We predict large increases in direct costs and income losses from stroke in middle-income countries and increases in direct costs in HICs (appendix pp 68–69). The projections also suggest increased economic impacts of stroke in low-income countries in 2050.
Figure 4: Estimated direct costs and income losses associated with stroke, 2017 and 2050, by World Bank region.
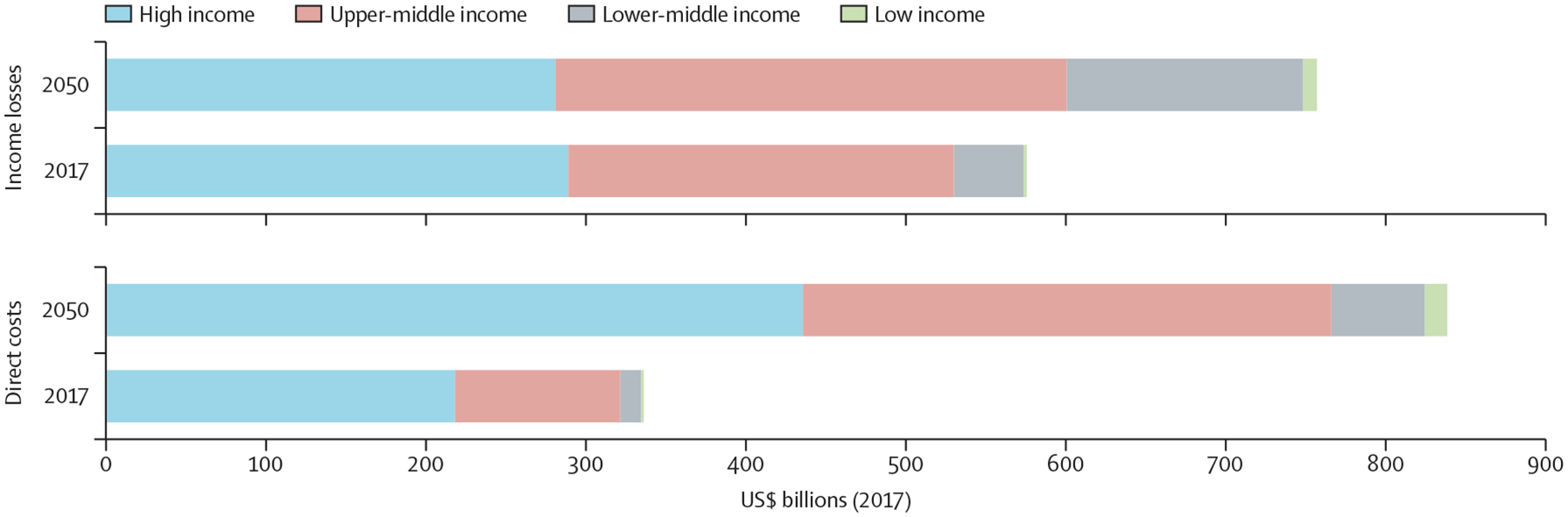
Our comparison is based on the forecast of regional means in table 1.
These projections of the economic consequences of stroke should be thought of as indicative, owing to the considerable uncertainties involved in predicting future direct costs and income losses. The accuracy of our projections of the economic implications of stroke in 2050 is crucially dependent on the economic forecasting model used, and we used a particularly simple model, which relied solely on demographics, regional characteristics, and starting gross domestic product per person for each country. We could not account for inflation from 2017 to 2050 (which is unknown), and our baseline cost estimates were for 2017 because no more recent data were available. Although our simple model captures key elements of standard empirical growth models, richer models of economic growth would also have accounted for projected changes in educational attainment, capital investments, changes in the policy environment, and climate implications. However, incorporation of these variables could have further contributed to the potential imprecision of our projected costs. Furthermore, our estimates of the direct costs of stroke are dependent on assumptions about the relative rate of price increase of non-medical goods and stroke treatment. The prices of these goods and treatment will in turn depend on treatment practices, the emergence of new technologies, and application of stroke treatment practices from HICs to the rest of the world, some of which will raise treatment costs. Conversely, economies of scale might lower treatment costs. Our strategy was to assume two different rates of expenditure growth in stroke treatment to try to account for this uncertainty: 1% above non-medical expenditure inflation, and 3% above non-medical expenditure inflation. The former has been used for health expenditure projections in Australia and is about the same as recent estimates for the period 2001–20 for the USA.8 The latter figure assumes that rapid increases in demand in middle-income and upper-middle-income countries for health services will put further pressures on prices.
Part 1: The importance of stroke surveillance
Epidemiological surveillance systems are fundamental for evidence-based planning, resource allocation, and determination of priorities to reduce the burden of stroke.45 These systems can provide comprehensive and contemporary information about incidence, prevalence, and outcomes of stroke, and also about risk factors. Data from these systems can be then used to develop, implement, and assess prevention, acute care, and rehabilitation programmes.46
Epidemiological surveillance.
The systematic collection, analysis, and dissemination of health data for the planning, implementation, and assessment of public health initiatives
Stroke surveillance is a key element recommended by the World Stroke Organization’s Global Stroke Care Guidelines43 and by several other international initiatives, including WHO’s Intersectoral global action plan on epilepsy and other neurological disorders 2022–2031 (IGAP).47 The IGAP acknowledges the role of surveillance data in informing evidence-based actions to improve policies, programmes, and services for the prevention and management of neurological diseases. It sets a target of 80% of countries regularly collecting and reporting on core indicators for neurological diseases by 2031.48 In the context of stroke surveillance, there is a need for countries to regularly collect data for, and report on, core indicators, such as incidence, recurrence, and mortality rates, outcomes, implementation of evidence-based care, participation in clinical registries, and prevalence of risk factors.43
World Stroke Organization’s Global Stroke Care Guidelines.
A roadmap that is intended to guide local health-care officials and stroke care clinical groups in establishing stroke systems of care and implementing as many of the defined components as possible throughout the continuum of care
In this section, we review advances towards these stroke surveillance goals. We also highlight gaps in stroke surveillance systems and propose pragmatic strategies for improving surveillance systems globally.
A call for national stroke registries
An ideal stroke surveillance system would include nationally representative data for the incidence, prevalence, and outcomes (eg, death, disability) of, and quality of care for, stroke, and for the prevalence of risk factors. These indicators are essential to inform strategies to reduce the burden of stroke. Data for stroke surveillance systems can come from incidence studies, registries, or population-level data for stroke-related hospitalisations and outcomes.49,50 The incidence and case fatality of stroke are traditionally monitored with so-called ideal incidence studies.51 However, these studies are resource-intensive and often impractical to undertake. For example, in the 2022 review of global stroke statistics,50 the authors identified only five ideal incidence studies that have been done worldwide in the past decade. By contrast, the number of national stroke registries has grown.50
Ideal incidence studies.
Incidence is a measure of how commonly or frequently new cases of a disease occurs in a specified population over a period. Ideal incidence studies follow criteria for so-called ideal population-based studies to ensure maximal ascertainment of stoke cases in the population concerned
National stroke registries can be a relatively inexpensive supplement or substitute for monitoring the data for fatal and non-fatal strokes. We have reviewed the data collected for the global stroke statistics50 and found that only 31 (14%) of 216 WHO member countries and territories have optimal stroke surveillance through their national stroke registries. All but seven of these 31 countries were HICs. There is only one national registry in the Central Europe, eastern Europe, and central Asia GBD super-region, one in the South Asia super-region, and three in the Southeast Asia, east Asia, and Oceania super-region (one in each region). No national registries were identified in the North Africa and Middle East, and Sub-Saharan Africa super-regions, showing that fewer resources are available for surveillance in these regions, a situation that is likely to continue unless there is substantial investment from governments. Only five countries (Bahrain, Finland, Israel, Russia, and Singapore) have stroke registries with the optimal nationwide coverage required for evidence-based health-care planning and resource allocation for stroke care.
Despite the value of these registries, they also have several limitations that restrict their use for optimal stroke surveillance. These limitations include errors in data entry (selection bias) or coding (classification bias),52 and lack of precision in hospital diagnostic coding of the cause of stroke53—eg, use of the International Classification of Diseases code I64 (stroke—not specified as ischaemic or haemorrhagic), or incorrectly coding transient ischaemic attacks or haemorrhagic stroke as ischaemic strokes. Another major challenge for these registries is nationwide coverage.
Data on mortality due to stroke are usually collected in national civil registration and vital statistics systems and submitted annually to WHO. Although these data are not without limitations—eg, data submitted could be incomplete data or not up to date50—they remain the most robust source of statistics on stroke mortality. However, only 138 (64%) WHO member countries and territories have submitted stroke-specific mortality data at least once to WHO in the past three decades.50 Most of the 78 countries that did not provide data were in the Sub-Saharan Africa (42 [54%]) or Southeast Asia, east Asia, and Oceania (18 [23%]) super-regions, potentially reflecting the lack of capacity for surveillance of stroke mortality in these regions. In the absence of data from national registration systems, mortality estimates are often extrapolated from non-representative studies or international demographic and epidemiological statistical models,54 which might not be as reliable. Given population growth in these regions (estimated to become home to about 45% of the total global population by 2030), ongoing demographic and epidemiological transitions leading to increases in NCDs, and the huge estimated burden of stroke (roughly 46% of the global burden of stroke1 as of 2019) in these regions, reliable data on stroke events and outcomes are urgently needed to inform public health policy and actions.55
Surveillance of risk factors
To reduce the burden of stroke, population-wide monitoring of its risk factors is needed. In settings where resources are limited, efforts should be focused on collecting high-quality data for risk factors that strongly predict stroke (eg, blood pressure, physical activity, lipid profile, diet, bodyweight, psychosocial factors, smoking, diabetes),28 are highly prevalent,56 are amenable to individual-level or population-level interventions,8,57 and are relatively easy and cheap to monitor. Ideal comprehensive surveillance of risk factors for stroke would include monitoring of blood pressure, anthropometrics (eg, weight, waist circumference), biochemical measures (eg, lipid profiles, blood glucose), lifestyle factors (eg, smoking, physical activity, alcohol intake, illicit drug use, stress), clinical factors (eg, atrial fibrillation), and environmental factors (eg, air pollution).
We found information about national health surveys for 196 countries or territories (figure 5). In 84 (43%) countries, the last survey was done before 2018 (appendix pp 70–80). In 37 countries, surveys were done solely by national agencies (eg, the US National Center for Health Statistics)58 to collect information about the health and wellbeing of residents. In most other countries, national agencies were supported by international organisations or development partners, including WHO (ie, the STEPwise approach to NCD Surveillance programme [WHO STEPS]; in 75 countries), the EU (31 countries), or the United States Agency for International Development (Demographic Health Survey [DHS]; in 54 countries). Information was unavailable for four HICs or territories (French Guiana, Macau, San Marino, and Monaco), eight countries in the Latin America and Caribbean super-region, six in the Sub-Saharan Africa super-region, Montenegro (in the Central Europe, eastern Europe, and central Asia super-region), and Ryukyu Islands (in the Southeast Asia, east Asia, and Oceania super-region).
Figure 5: Availability of national surveillance systems for risk factors of stroke.
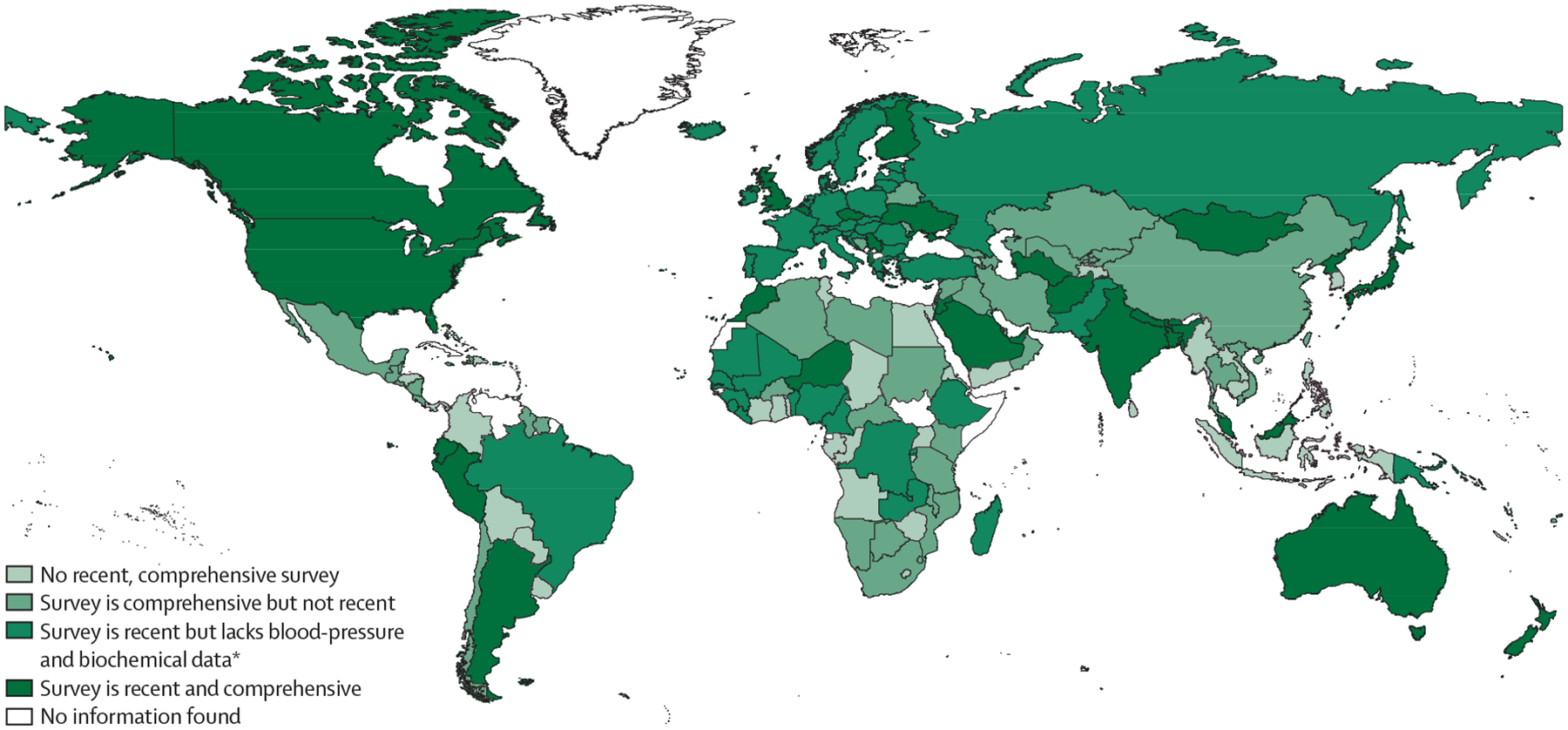
Surveys were considered recent if they were done in or after 2018, and comprehensive if they included measurements of blood pressure, anthropometrics (eg, weight, waist circumference), biochemical measures (eg, blood lipid profile, blood glucose), lifestyle factors (eg, smoking, diet, physical activity, alcohol intake), and diagnosis of metabolic conditions (eg, hypertension, overweight or obesity, dyslipidaemia, diabetes, atrial fibrillation). *Biochemical measurements refers to measurement of blood lipid profiles and blood glucose concentrations.
WHO STEPS.
WHO’s approach to surveillance of stroke and other non-communicable diseases, based on a simple, standardised method for collecting, analysing, and disseminating data for key metrics of disease burden (incidence and mortality) and risk factors
Lifestyle factors were measured in national health surveys in 181 (92%) of 196 countries and anthropometrics in 164 (84%), and diagnosis of metabolic conditions was recorded in 159 (81%). Most surveys were not comprehensive or recent (ie, were done before 2018). Five countries (Cameroon, DR Congo, São Tomé and Príncipe, Senegal, and Tunisia) did not measure any risk factor for stroke in their last national surveys (as of September, 2022). Although LMICs assessed more risk factors in their last surveys than HICs, their surveys were often not recent (ie, they were done before 2018). Compared with poorer countries, HICs had more often done recent and comprehensive surveys of risk factors for stroke. Overall, of the 112 countries in which surveys have not been done since before 2018, 109 were LMICs. Of the 84 countries with a survey done in or after 2018, only 32 (38%) included measures of blood pressure, and only 27 (32%) included comprehensive surveillance of risk factors for stroke. These gaps in the collection of comprehensive and contemporary information about important risk factors for stroke across the world, but particularly in LMICs, need to be addressed.
Barriers to, and facilitators of, surveillance
We have identified several themes (appendix pp 81–82), including both barriers to, and facilitators of, the creation of high-quality stroke surveillance services—eg, surveillance capacity, information technology, and governance of surveillance activities. These themes were identified in semi-structured interviews with 12 stroke surveillance experts, one from a HIC and one from an LMIC in each of the six WHO regions (appendix pp 7–16).
Surveillance capacity refers to the activities and availability of a trained workforce to capture stroke events or risk factors. Countries with good stroke surveillance have well-funded and trained workforces, can establish nationwide registries to monitor treatment and outcomes, and have regular risk factors surveys linked with population-based approaches, such as integration into censuses. By contrast, countries with poor surveillance capacity do not have nationally representative or standardised data available for stroke treatment or outcomes, generally because efforts are not nationally coordinated at the government level, with stroke surveillance activities instead managed by individual institutions or academic research networks.
Information technology affects the ability to deliver high-quality stroke surveillance services. Countries with strong health information systems can have good stroke surveillance. Internet-based systems allow data collection in real-time, which improves data quality. Digitalisation of medical records, mortality data, and other health databases enables and enhances analysis of stroke and risk factor data to inform policies. Electronic databases also increase access to data and enable data linkage to do complex studies on the epidemiology of stroke. Countries with good stroke surveillance can use data from surveillance systems to raise awareness of stroke and its risk factors in the community and with government stakeholders, leading to strategic investment. Countries with poorer surveillance have fragmented, incomprehensive health databases, limiting the use of data for decision making.
Strong governance facilitates the successful operation of high-quality national stroke surveillance systems. Robust governance requires political commitment, adequate funding, and independent advisory bodies. Countries with strong governance often have strong health information systems to enable data-driven decision making. Stroke surveillance activities are often government-led, with technical support from experts. A lack of government commitment to stroke surveillance hinders establishment of strong stroke surveillance systems. Poor availability of data for stroke hampers the ability to set priorities and secure funding for stroke services. However, even countries with good data availability and strong political commitment to stroke surveillance still report insufficient funding.
Pragmatic solutions to improve stroke surveillance
WHO recommends that each country establishes a programme that achieves near-universal monitoring of the important indicators of the burden of stroke—ie, incidence, recurrence, and mortality rates, prevalence of risk factors, stroke outcomes, implementation of evidence-based care for stroke, and participation in stroke clinical trials.59 A good example of such a programme is the Stroke Service Tracker in Europe, a survey developed as part of the action plan for stroke in Europe 2018–2030.60 This survey collects data for the quality of stroke care and stroke outcomes all over Europe to inform policies, programmes, and services for management and secondary prevention of stroke and to facilitate comparison across European countries.
For regions with limited resources, WHO has developed a three-step standardised surveillance system for stroke in 2002—STEPS.59,61 In a review62 of seven studies from nine LMICs that incorporated the WHO STEPS protocol, their adherence to the surveillance methods was variable. There were particular challenges with collecting neuroimaging data and data for non-fatal events in the community.62 Better training to improve capacity to undertake surveys, feasible surveillance protocols that are codesigned with local experts, electronic data collection, and the inclusion of data on stroke in existing monitoring systems for NCDs could facilitate identification of stroke cases in the community.62
One of the most popular and commonly used epidemiological methods for stroke surveillance in LMICs are door-to-door surveys. If a stroke prevalence survey is done with a sufficiently large sample size (ie, 25 000–30 000 people) and findings are combined with data from death certificates (eg, by using verbal autopsy procedures) collected in the same community over at least 3 years, fairly accurate incidence and mortality estimates can be derived. The central tenet of this approach is that non-fatal first-ever stroke events within the preceding 3 years are identified through a prevalence survey and then combined in the analysis with fatal first-ever stroke events, in the same population for the same study period, to calculate cumulative stroke incidence rates.63 This approach was first used in Italy64 and China in the 1980s and 1990s.65 It has more recently been used to update stroke incidence and mortality data in a large nationwide study66 in China in the late 2010s. Another epidemiological approach for stroke surveillance in LMICs is to do repeated community-based studies in the same population at different times67—an approach that has previously been used in Brazil.68 However, this approach is resource-intensive.
Verbal autopsy.
A WHO-standardised method of data collection to establish a possible cause of death based on oral interviews with a close relative or caregiver who witnessed the death of the deceased
Key recommendations for improving stroke surveillance are summarised in panel 2 and the appendix (pp 82–83). National stroke registries can be used to monitor hospitalisations for stroke.69 Registries with nationwide coverage can be coupled with national death registries to give a near complete picture of the burden of stroke.70 However, in countries with less access to hospital services, registries should be supplemented by case ascertainment of at least fatal (and if possible non-fatal) stroke occurring in the community. WHO standard’s verbal autopsy instrument71 could be used for case ascertainment, an approach that has been validated for use electronically in LMICs.72
Panel 2: Key messages about stroke surveillance.
Governments need to establish nationwide systems for monitoring the burden of stroke, through registries, electronic health records, and vital statistics systems. These systems must achieve near-universal surveillance of indicators of stroke burden and risk factors, to reliably inform the development of programmes for stroke prevention, acute care, and rehabilitation. Surveillance systems must become part of national stroke plans and monitoring systems for non-communicable diseases, which should be able to identify stroke cases in the community. Countries with limited capacity or resources could benefit from assistance, collaboration, and funding from international development agencies—eg, WHO, and the United States Agency for International Development.
Surveillance systems should assess the incidence, prevalence, management, and control of cardiovascular risk factors at the population level, and should be based on reliable measurements—eg, measurement of blood pressure rather than self-reported hypertension. Surveillance systems should include capacity building of personnel to ensure enough adequately trained people to collect and analyse the data. Career pathways for health-care professionals with training in epidemiology and biostatistics should be considered.
Data on risk factors for stroke should also be gathered as part of national censuses. All countries should have surveys with identified priorities and clearly defined cycles and data-collection. These surveys should be done regularly to detect changes over time. Countries should also invest on research capacity to regularly analyse the data collected and generate high-quality evidence to support decision making.
Governments should establish national stroke registries of hospitalised, non-hospitalised, fatal, and non-fatal strokes and transient ischaemic attacks. Such registries should be facilitated by linkage of population data for risk factors to hospitalisation and national death registries, which would enable clarification of the relationship between the burden of risk factors and stroke burden at a population level, and could provide a near-complete overview of the burden of stroke.
Every country should have electronic health-information systems, with interoperability between systems, to prevent duplication of data. In countries with established electronic or web-based platforms for data collection, the collection of data for stroke and its risk factors should be encouraged and incentivised to increase coverage. Large-scale collection of data via electronic systems that enable the ready exchange of health information could, in turn, facilitate the use of these data for surveillance purposes. For such platforms to be valuable and sustainable, staff training is needed to ensure appropriate documentation and coding, and the secure handling of electronic health data.
Irrespective of the surveillance system, consultation with communities, health-care providers, policy makers, health insurers, and implementation partners at each stage of development is essential. This engagement will ensure that legal, ethical, and socioeconomic considerations are taken into account, and that the system will meet the needs of the local community.
Research priorities
Mixed-methods research—research yielding both quantitative and qualitative data—focused on models to improve stroke surveillance should be prioritised, particularly in countries with limited resources. Such research should explore how to develop feasible surveillance protocols that address local needs, while ensuring collection of standardised data. Standardisation of data collection could involve adapting well established surveillance protocols (eg, WHO STEPS, Demographic and Health Surveys, European Health Interview Survey) or use of less costly but successful surveillance methods, such as repeated cross-sectional and community-based studies done in strategic locations.
Validation studies should be done to assess the quality and coverage of data for risk factors available in administrative datasets (eg, hospital records, primary care records).
Surveillance systems should be able to incorporate data on stroke genetics to facilitate the development of novel prognostic biomarkers and prevention strategies.
Novel digital tools for population-wide surveillance of stroke and its risk factors should be developed and validated.
There is increasing interest in using other large datasets of routinely collected, administrative data (eg, records of hospital admissions for acute stroke care) to monitor hospitalisations, quality of care, and outcomes after stroke.49 However, standardised methods and systems are needed to enable use of these data to provide timely and reliable estimates of stroke burden. Countries should establish monitoring systems that facilitate increased coverage of electronic medical records and interoperability between systems (to prevent duplication of data capture). Potential barriers to the use of routinely collected administrative data for stroke surveillance include the lack of the resources required to establish and maintain this infrastructure, particularly in poor settings—where access to electricity might be unreliable, for instance, or health workers might have inadequate technological literacy. Concerns have also been raised about the validity of data for diagnoses of stroke and comorbidities recorded in hospital data-collection systems,73 and about the privacy of electronic health records.74,75 These concerns can be overcome through appropriate training of staff on data coding, entry, and handling, regular review and audit of the quality of data collected, validation of data entry and coding methods, and appropriate data encryption.45,76–78
Irrespective of the monitoring system used, consultation with community groups, physicians and other health-care providers, policy makers, and implementation partners at each stage of their development and implementation is paramount. This engagement will ensure that ethical, legal, and social considerations are met,8,79 and that the system meets the needs of the community.
National monitoring systems for risk factors
There is a need for countries to establish national, geographically and ethnically representative surveillance frameworks and capacity, including funding and reporting mechanisms, for regular monitoring of important risk factors for stroke.61 Countries without frameworks can implement one of the three main types of surveys widely used to assess risk factors for stroke: WHO STEPS and the Demographic and Health Survey (DHS), both of which can be adapted by all countries, and the European Health Interview Survey, which can be used in the EU.
The WHO STEPS is a valid and reliable system for the surveillance of risk factors,61 which emphasises high-quality collection of a few variables, rather than large amounts of poor-quality data. The DHS is largely funded by the United States Agency for International Development, with support from host countries and other agencies (eg, the DHS Program). A major benefit of the DHS is the concurrent capacity building of workers through training and support tools to maximise the collection of high-quality data. The European Health Interview Survey is a collaborative effort between all EU member states in which validated instruments are used to collect standardised self-reported health data. It is complemented by the European Health Examination Survey, which collects data on blood pressure and biochemical measures (eg, lipid profiles, blood glucose concentrations).
Comprehensive surveillance of risk factors for stroke requires additional resources for staff, training, and data management. Use of digital tools for remote, large-scale collection of data for NCD risk factors is a potential novel approach. However, both the sampling methods (to ensure representativeness and ease of sharing of data) and the tools themselves should be validated before their use in national risk factor surveillance systems. A comprehensive, fully digital national survey of risk factors was recently done in India.80 It was adapted from the WHO STEPS system and was part of the Indian national NCD monitoring framework. The validated questionnaires used in the survey were digitised for data collection via an offline Android-based app, with standard definitions used for estimation of behavioural and biological risks (tobacco use, alcohol use, diet, physical activity, BMI, raised blood glucose concentrations, and increased blood pressure). Although it was a large (10 659 adults) and nationally representative survey that established sustainable mechanisms for doing further state or district surveys, the usual survey-related limitations applied: the risk factor data gathered were cross-sectional, and there might have been information and sampling biases.
To elucidate the genetics and pathobiology of stroke (and associated risk factors) across diverse ancestries, surveillance systems should incorporate population-based large-scale collection of data and biospecimens for multiomic research. Genomic and multiomic research could facilitate the development of novel predictive, diagnostic, and prognostic biomarkers, and better prophylactic, diagnostic, therapeutic, and restorative interventions. The inclusion of people from LMICs in such research is crucial to ensure that biomarkers and interventions are applicable to high-risk populations worldwide.81
Research priorities
As we have shown, stroke surveillance systems are essential for developing, implementing, and evaluating national programs to reduce the burden of stroke. Future research should therefore focus on the development, implementation, and assessment of national stroke surveillance systems, including measures to monitor health-care quality. There is a need to develop and validate methods for calculation of incidence, prevalence, and outcomes of stroke based on administrative data. Mixed-methods research focused on the implementation and assessment of models to improve stroke surveillance is necessary, and should also explore how these data for stroke and its risk factors can be used to improve stroke prevention and management.
The members of the World Stroke Organization participating in this Commission, in collaboration with other stakeholders, will work towards the implementation of the pragmatic solutions that we recommend. Commissioners and national stroke societies will champion co-implementation activities at the national level. A taskforce will leverage global partners to undertake several activities actions to improve stroke surveillance, including implementation of national strategies for stroke surveillance (via digital technologies, when possible), particularly for countries with little available data, and building capacity and resources (governance, training, and infrastructure) in LMICs to enable use of electronic medical records systems that could facilitate surveillance and data linkage.
To implement the recommendations of this Commission, countries should establish sustainable programmes for regular country-wide monitoring and assessment of stroke burden, modifiable risk factors, and stroke-related health services, all of which should be integrated with national stroke plans. Irrespective of the surveillance method used, collaboration is necessary49 to ensure that stroke surveillance systems sustainably meet the needs of all stakeholders.
Part 2: Strokes can be prevented: the strategies
Globally, one in four individuals older than 25 years will have a stroke, which means that the lifetime risk of stroke is 25%.82 Therefore, effective primordial, primary, and secondary prevention programmes are crucial to reduce lifetime risk and the effects of the disease.
Primordial prevention is aimed at preventing the emergence of stroke risk factors. Primary prevention involves early detection and control of risk factors, such as hypertension, dyslipidaemia, obesity, and diabetes, to avoid a first stroke event. Members of the World Stroke Organization–Lancet Neurology Commission have addressed primordial and primary prevention in a previous paper.8 Thus, in this Commission, we focus mainly on strategies for secondary prevention.
Secondary stroke prevention is commonly defined as the prevention of stroke in people who already had a stroke or a transient ischaemic attack. Recurrent strokes and transient ischaemic attacks contribute substantially to the overall burden of cerebrovascular disease83—about 20–30% of strokes occur in people who previously had a stroke or a transient ischaemic attack.83–85 People who had a stroke or a transient ischaemic attack are at increased risk of recurrent stroke particularly within the first few days of the index event.84,86 These recurrent strokes tend to be more disabling and to have poorer outcomes than the first stroke.87 Evidence suggests that 45–80% of recurrent strokes and transient ischaemic attacks could be prevented.88–90
An international cohort study91 showed that, with proper management, the risk of subsequent disabling or fatal stroke within 5 years of a minor ischaemic stroke or a transient ischaemic attack is only 10%. However, even though the probability of survival after a stroke has improved substantially over the past two decades, stroke recurrence has not been consistently reduced in many countries85,92,93 (although reductions have been noted in some countries94,95).
Prevention of recurrent strokes
The principles of secondary stroke prevention are to identify and treat the underlying causal pathologies of the first stroke to minimise the risk of recurrent stroke and other major vascular events. The evidence-based components of secondary stroke prevention comprise both medical and lifestyle interventions that are targeted to the cause of the first stroke and are aligned to the risk of recurrent stroke.83,90 Examples of medical interventions include the use of antihypertensives in people with hypertension (the leading modifiable risk factor for stroke) and the use of anticoagulants and anti-arrhythmics in patients with atrial fibrillation, whenever indicated.8 Furthermore, carotid interventions (endarterectomy or stenting) might be indicated in some patients with severe stenosis ipsilateral to a non-disabling ischaemic stroke or transient ischaemic attack.83 Examples of lifestyle interventions include consumption of a healthy diet, smoking cessation, and regular physical exercise.8
We have previously reviewed, by country income level, the available guidelines for secondary stroke prevention that address identification and management of risk factors in people after a stroke or a transient ischaemic attack. In our review, we found that secondary stroke prevention was very uncommon in low-income countries, and substantially less in common in middle-income countries than in HICs.14 However, even in HICs, the frequency of the assessment of risk factors for secondary stroke prevention was suboptimal.96 Routine secondary stroke prevention activities that were uncommonly used across all country income levels include risk assessment scores (eg, the congestive heart failure, hypertension, age ≥75 years [doubled], diabetes mellitus, previous stroke or transient ischemic attack [doubled], vascular disease, age 65–74 years, and female sex [CHA2DS2VASCc] score in patients with atrial fibrillation), assessment of biomedical and lifestyle risk factors, and patient education about management of risk factors.14
Atrial fibrillation.
An arrythmia (ie, irregular heart rhythm) originating from the atrium of the heart with a characteristic absent P wave on electrocardiograms
Carotid interventions.
Surgical interventions to restore or improve blood flow to the carotid vessels—eg, carotid endarectomy, stenting
Secondary stroke prevention has conventionally been delivered at the individual level and has been established around the doctor–patient relationship (ie, the stroke and its causes would have been diagnosed by a physician, who then would generate and manage a secondary prevention programme in conjunction with the patient). Other complementary inputs can come from family members, community services for education and monitoring (eg, nursing services, allied health care), and public education campaigns, and also from primary prevention strategies at the population level. Primary prevention is targeted at the population as a whole and, therefore, at individuals without clinically established stroke. At the population level, for instance, a 2 mm Hg decrease in systolic blood pressure would result in about a 10–24% decrease in the incidence of a first stroke.97 Similar interventions can be used in secondary prevention. The management of vascular risk factors at the individual level, with the prioritisation of early detection and control of hypertension, must be part of both primary and secondary stroke prevention strategies8 (figure 6; appendix pp 93–97).
Figure 6: Strategies for stroke prevention.
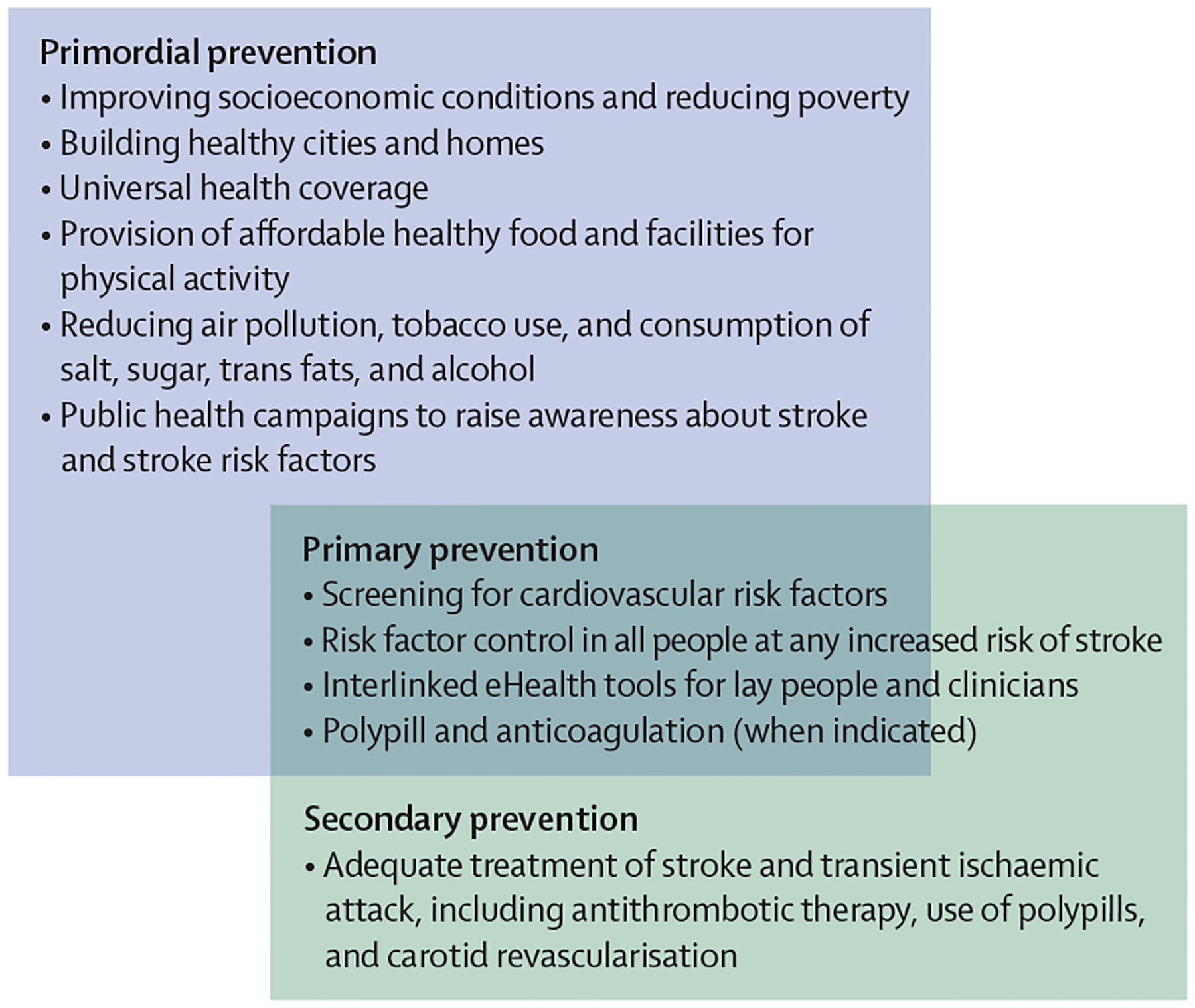
Major strategies for control of stroke risk factors for stroke at the population and individual levels are shown.
Primary stroke prevention interventions (eg, limitations on salt content in processed food, smoking cessation campaigns, air pollution reduction, incentives for purchasing healthy foods and disincentives for alcohol and other less healthy products) can be implemented by governments to reduce exposure to risk factors across the lifespan for the entire population, irrespective of the level of risk of stroke or cardiovascular disease. At an individual level, use of mobile technology could be implemented to facilitate control of risk factors (so-called motivational mass individual strategies for stroke prevention)37 along with task shifting (or sharing) of preventive tasks from physicians and nurses to trained and supervised health-care workers, particularly community-based health workers.98,99 Primary and secondary stroke prevention strategies in people with hypertension, diabetes, or dyslipidaemia can be further improved by using polypills.39,41,100,101 Polypills for primary stroke prevention commonly include a combination of low, fixed doses of blood-pressure-lowering and lipid-lowering drugs in one medication (polypills for secondary prevention of ischaemic stroke could also include fixed doses of antiplatelet drugs).100,102,103
Motivational mass individual strategies.
Motivational techniques delivered via smartphones that could be used in most of the adult population
Polypills.
Pills containing fixed doses of at least two drugs. Polypills containing blood-pressure-lowering and blood-lipid-lowering drugs can be prescribed for prevention of stroke and transient ischaemic attack
Despite these efforts, stroke recurrence remains unacceptably high (20–30%) in many countries. The failure of secondary prevention strategies to tackle stroke recurrence could be exacerbated by several barriers, including insufficient specialised training of physicians; lack of education of patients about the risk factors associated with their first stroke, the long-term risk of recurrence, or the importance of long-term monitoring of risk factors; maintenance of healthy lifestyle behaviours, and adherence to medical therapy; insufficient numbers of medical personnel; low affordability of preventive medications (especially in poor settings); and insufficient use of evidence-informed digital tools for developing person-specific and motivational recommendations (eg, the free Stroke Riskometer app for the general public and PreventS-MD for health professionals).8,37,98,104–110 Physicians’ inability to appreciate that these barriers (panel 3) can negatively affect motivation and effective self-management could be compounded by patients’ post-stroke sedentary behaviour, mood disorders, and speech and cognitive impairments, which are likely to lead to inadequate adherence to recommendations.111
Panel 3: Facilitators of, and barriers to, high-quality stroke prevention services.
Factors that affect the availability of high-quality stroke prevention services include system capacity (ie, the distribution of services across urban and rural areas, the continuum of these services, and the availability of a trained health workforce), universal health coverage, and governance.
Lack of trained health-care staff is a major barrier to providing primary and secondary stroke prevention services. The unequal distribution of health-care staff and socioeconomic differences between urban and rural areas creates inequalities in access to services, and negatively affects health literacy. By contrast, countries with a wide network of service delivery by a mixture of staff (eg, doctors, nurses, community health workers) with stroke-specific training can provide a wide range of stroke prevention services.
Universal health coverage influences access to stroke prevention services. Countries with universal health coverage can provide a wide range of stroke prevention services (eg, regular health checks, lifestyle programmes, pharmacological treatment) free or at low cost. The absence of universal health care exacerbates inequalities in access to stroke prevention services, and people tend to prioritise out-of-pocket costs for treatment rather than prevention, with few or no regular health checks for stroke risk factors.
An absence of government-led primary stroke prevention activities is linked to low population health literacy about stroke prevention. Strong governance in stroke prevention facilitates high-quality services. A political commitment to stroke prevention and a national strategy to control risk factors (eg, reduction in smoking prevalence, blood pressure control) enhances both primary and secondary stroke prevention.
Active participation of stroke organisations enhances governance. Countries with strong governance use health data for decision making, whereas a lack of government commitment to prevention of cerebrovascular diseases or clear national prevention guidelines hinders prevention.
Our survey of specialists of stroke services13 from 84 countries showed that, overall, only 40–46% of secondary stroke prevention activities were delivered to an acceptable level in participating hospitals (appendix pp 84–91). The strategies most often adopted were the use of antiplatelet drugs, anticoagulants, antihypertensives, and lipid-lowering drugs. The most widely available of these medicines were aspirin (available in 76 [90%] countries), metformin (73 [87%]), and thiazide diuretics (73 [87%]). The survey also showed disparities between regions: whereas stroke services in 31 (91%) of the 34 included countries in the WHO European region reported availability of angiotensin II receptor blockers, services in only four (33%) of the 12 included countries in the African region reported availability of these drugs. Importantly, availability of outpatient stroke or transient ischaemic attack clinics or stroke prevention clinics is better in upper-middle-income countries and HICs than in poorer countries. Education for patients and their families about stroke and lifestyle management was not available in many countries. There was also a lack of digital tools to support clinicians in the implementation and monitoring of secondary stroke prevention.105,112–114 Use of guidelines for assessment and management of risk factors for secondary prevention in people with stroke or transient ischaemic attack is also very uncommon in low-income countries, and sub stantially less in common in middle-income countries than in HICs.13 However, even in HICs, the level of assessment of risk factors for secondary stroke prevention is suboptimum.96
Previous findings115 and those from our survey of specialists in stroke services on the low use of secondary stroke prevention strategies are in line with results from surveys of national scientific societies and stroke experts in Europe116 and China,117 and those from a systematic review118 of ischaemic stroke prevention guidelines. We also noted poor follow-up of patients after stroke or a transient ischaemic attack. Follow-up should be multimodal and structured, and include not only assessment of modifiable risk factors but also other key components identified in the post-stroke checklist endorsed by the World Stroke Organization.119 Scientific evidence is emerging in support of such a systematic integrated approach that addresses not only secondary prevention, but also post-stroke rehabilitation, and community reintegration.120
World Stroke Organization’s post-stroke checklist.
A checklist to help health-care professionals identify post-stroke problems amenable to treatment or referral
Pragmatic solutions to improve secondary prevention
Successful implementation of secondary prevention strategies in people who had a stroke or a transient ischaemic attack could reduce the burden of stroke by as much as 25%,84 and effective primary stroke prevention strategies at the individual level could reduce the burden of stroke by as much as 50%.98 Thus, the need for adequate prevention services has been emphasised in several international publications and initiatives, such as IGAP,47 the Brain Health Initiative,121 OneNeurology,122 the Heart and Brain Initiative,123 the NCD Alliance, the Global Coalition for Circulatory Health,124 and the WHO Strategic Technical Advisory Group on NCD-related Research and Innovation.125
As the risk factors for stroke are also risk factors for other major NCDs, such as dementia, ischaemic heart disease, peripheral vascular disease, diabetes, chronic obstructive pulmonary disease, and cancer (appendix p 58), reducing exposure to these risk factors should help to prevent not only stroke but also other major NCDs.
The most recent World Stroke Organization guideline and action plan for secondary prevention43 emphasises the importance of setting up stroke prevention clinics staffed with health-care professionals with expertise in stroke care, risk reduction programmes, and educational tools. A good example of transferring or sharing tasks from highly trained health-care professionals to paramedical health-care workers was reported in India in 2020.99,126,127 A scalable public health intervention to strengthen control of hypertension in primary care settings yielded substantial improvements in blood pressure control (48·1% increase in the number of patients with their blood pressure under control compared with baseline) compared with that achieved in hospitals (22·9% increase).128
Digital technologies have been proposed as a new strategy for improving both primary and secondary stroke prevention. A large randomised controlled trial129 in India showed the feasibility and acceptability of a mobile health intervention (comprising text messages, educational videos, and a workbook accessed via mobile phones) for secondary stroke prevention. The intervention also led to significant reductions in alcohol intake and smoking and significantly improved medication adherence. Other good examples of the practical use of digital technologies are the validated (including cross-cultural validation) and free Stroke Riskometer app,106,107,109,130 which is available in 19 languages in 78 countries, and the PreventS-MD webapp for clinicians (appendix pp 36–49).104,105,110 PreventS-MD has been tested for usability (as measured by the validated System Usability Scale)35 in 27 countries (12 [44%] of which were LMICs) and was judged to have excellent usability by health-care professionals.104,110 In primary and secondary stroke and cardiovascular disease prevention, PreventS-MD was also rated consistently highly by both health-care professionals and patients in terms of acceptability and usefulness. Patients and health-care professionals expressed a high level of understanding of, and adherence to, the tool’s recommendations, including readiness to change their behaviour and to adopt a healthier lifestyle. At 1 month follow-up, 100% of patients reported adherence to the recommendations generated by PreventS-MD.110 Educational campaigns implemented both digitally and face to face can increase awareness about stroke symptoms and the necessary steps that need to be followed to seek medical help.131
The effective integration of governmental policies and public-health campaigns with the multidisciplinary health care needed to provide evidence-based individual management would enable successful implementation of primordial, primary, and secondary prevention strategies worldwide.8,130,132 A good example of systematic follow-up after stroke is the model applied in the STROKE-CARD study120 in Austria, in which prevention of secondary stroke and cardiovascular disease were integrated into both a standardised 3-month follow-up visit and a web-based patient portal, which enabled patients to review post-stroke complications and comorbidities and express needs for further rehabilitation, and provided education, counselling, and information on life after stroke.
A tripartite approach comprising behavioural, pharmacological, and health-system-level and societal interventions (based on the socioecological model), could minimise the fragmentation and inefficiency of primary stroke prevention.57 Health-care system-level interventions would include screening for stroke risk factors at every clinical encounter, while societal interventions should include ensuring availability of affordable healthy foods, clean air, and spaces for physical activity.
Socioecological model.
A model that conceptualises health in the context of the complex interplay between individual, family, community, and societal factors
Action plans for primordial, primary, and secondary stroke prevention should align with WHO targets for reducing NCDs.133 These country-specific and financially sustainable action plans should be developed by local experts on the basis of the best available evidence and informed by national, culturally appropriate, and up-to-date stroke prevention guidelines. Unfortunately, there is a shortage of operational national plans that align with the WHO’s Global Action Plan for NCDs.133 Furthermore, although national guidelines for primary and secondary stroke prevention are available in various HICs,83,133–136 there is a paucity of evidence-based, context-appropriate, pragmatic guidelines in LMICs.132 The only available digital tool to support clinicians in evidence-based primary and secondary stroke prevention interventions in both hospital and outpatient settings is the PreventS-MD webapp.104,105 Although most preventive strategies should be similar in all countries, differences in the population-attributable risks and lifetime risk of stroke, and in the availability and affordability of resources, should be considered when setting realistic and culturally appropriate local goals and priorities.
Guidelines and digital tools
We recommend the use of the most recent high-quality evidence-based guidelines by the American Heart Association, the European Society of Cardiology, and the European Stroke Organisation for primary prevention of stroke and cardiovascular disease137,138 and secondary prevention in patients after a stroke or a transient ischaemic attack,83,139–141 which can be adapted to the local context. The traditional approach to management of risk factors (which targets people at high risk of cardiovascular disease) is complex, expensive, and only moderately successful.37,39,142 The results of a 2021 WHO systematic review143 based on several high-quality randomised controlled trials that included a large number of participants clearly showed that screening for cardiovascular risk and risk factors has not reduced cardiovascular morbidity and mortality in the general population. Moreover, serious adverse effects were identified (eg, increased mortality). Absolute-risk thresholds should not be the main and sole criterion for selecting people for pharmacological management of hypertension, diabetes, or dyslipidaemia,38,42,144 which was the approach used in the recent WHO guidelines for the treatment of hypertension.145 WHO’s cardiovascular disease risk charts146,147 can be used, when feasible, to target individuals at high risk of cardiovascular disease for primary prevention among an identified minority of the population, but use of this strategy alone means that people at low or moderate risk of cardiovascular disease are overlooked, although up to 90% of all cardiovascular and stroke events occur in these people.35,36,148 The gap between prevention strategies targeted at people at high-risk of cardiovascular and stroke events and population-wide prevention strategies could be bridged via the wide use of digital tools such as the Stroke Riskometer app8,37,98,104 and PreventS-MD.104–109
We suggest that all countries should have government-endorsed policies, guidelines, and stroke awareness campaigns (delivered via the media, schools, churches, etc). Realistic, culturally appropriate, and financially sustainable action plans for secondary stroke prevention can be integrated with corresponding primary prevention strategies and stroke services. Specific plans should also be established for upskilling of staff, including community health workers. Countries should develop a plan for prioritisation of multisectoral and accessible interventions, including telemedicine and other digital technologies.
Monitoring prevention
As stated previously, all countries should have a nationwide, surveillance system for measuring and monitoring the effects of primary and secondary stroke prevention activities. This system should first identify hospitalisation events (step 1), then identify fatal stroke events in the community (step 2), and then non-fatal stroke events in the same community (step 3), according to the WHO STEPwise approach149 or similar approaches.51,63,150
Generally, the key performance indicators to monitor and assess primary prevention are stroke incidence and prevalence, and the prevalence of risk factors (particularly high blood pressure, atrial fibrillation, dyslipidaemia, diabetes, overweight and obesity, and smoking) over time. Key performance indicators for secondary stroke prevention after a stroke or a transient ischaemic attack should include the proportion of patients whose comorbidities (such as hypertension and diabetes) are under control during follow-up, the proportion without atrial fibrillation who are prescribed an antiplatelet agent, the proportion with atrial fibrillation who are prescribed an anticoagulant, and the proportion who are prescribed a statin. Other key indicators include the proportion of people who had a carotid territory ischaemic stroke or a transient ischaemic attack with symptomatically relevant extracranial carotid artery disease who undergo carotid revascularisation (when this procedure is indicated), the proportion of people who had either ischaemic or haemorrhagic strokes who are on antihypertensives and who have their blood pressure controlled, and the time from stroke onset to carotid revascularisation. Gaps in secondary stroke prevention can be addressed through national and international policy initiatives and clinical guidelines.116
To implement, monitor, and assess the pragmatic solutions recommended by this Commission (panel 4), we have established the World Stroke Organization implementation task force and implementation framework. This framework will leverage global resources, including the WHO and UN non-communicable disease control plan and the WHO intersectoral global action plan on epilepsy and other neurological disorders to address key environmental factors via changes in social policy (ie, social determinants of health) by making default choices healthy (eg, healthy cities, healthy food value chain); enhance stroke literacy through key community influencers who can deliver culturally tailored messages via social media and the media; address motivation and self-management skills; facilitate inclusion of the primary and secondary prevention recommendations outlined in this section in national stroke and cardiovascular disease management guidelines; and empower stroke Commissioners (country ambassadors for stroke prevention) to advocate for their rigorous implementation and assessment worldwide. The task force and framework are planned to operate at regional, national, and international levels in collaboration with relevant policy makers and implementation partners, including the World Hypertension League, the World Federation for Neurorehabilitation, NCD Alliance, WHO, Resolve To Save Lives, ministries of health, national and regional stroke organisations (including the African Stroke Organization, European Stroke Organisation, Middle East and North Africa Stroke Organization, Asian Pacific Stroke Organization, American Heart Association, and Registry of Stroke Care Quality), and neurological, cardiovascular, and NCD organisations.
Panel 4: Recommendations for stroke prevention.
Access to affordable medications for primary and secondary stroke prevention should be improved, with a focus on essential medications (such as polypills including blood-pressure and lipid-lowering drugs on the WHO list of essential medications)151,152 and tools (including the free Stroke Riskometer app for the general population and PreventS-MD for health professionals).104,110 Essential drugs for primary and secondary prevention should be subsidised and made available at all hospitals and outpatient clinics.
Protocol-based and regulated shifting or sharing of tasks from highly trained health-care professionals such as physicians and nurses, to supervised and trained paramedical health-care workers, particularly community health workers, should be emphasised to facilitate primary stroke prevention interventions at the individual level.98,99 Incentives for health-care staff in rural areas should be improved to encourage relocation and retention.98,99
A continuum of care for stroke prevention should be established. Preventative strategies, with emphasis on lifestyle modification, should be implemented for people at any increased risk of stroke or cardiovascular disease.98,153 Primary and secondary stroke prevention services should be freely accessible and thus require the introduction of universal health coverage. Governments need to devote a fixed proportion of the annual health budget to stroke prevention. Funding could come from taxation on tobacco, salt, sugar, and alcohol.37
Population health literacy on stroke prevention should be improved.
Research priorities
Motivational strategies to improve adherence to medication and lifestyle interventions should be investigated.
There is an urgent need to develop and update national guidelines for stroke prevention42 and increase the involvement of key stakeholders, including stroke organisations.8
The best balance of population-wide and individual risk-targeted primary prevention strategies for stroke and cardiovascular disease should be identified to maximise cost-effectiveness and minimise inequalities.37
Validation studies should be done to establish the effectiveness of the four primary stroke and dementia prevention strategies recommended by the World Stroke Organization in different populations—ie, population-wide prevention, motivational mobile or digital technologies, provision of low-dose combinations of generic antihypertensives and lipid-lowering drugs in a polypill for middle-aged and older adults with at least two behavioural or clinical stroke risk factors, and facilitation of the implementation of primary prevention strategies on the individual level by community health workers.
Implementation research is crucial to discover and test novel lifestyle interventions, drugs, and other interventions for primordial, primary, and secondary prevention of stroke and related cardiovascular and other non-communicable diseases.
The causes (including socioeconomic causes) of ethnic and racial disparities in stroke risk (including pathological types and causative subtypes) should be investigated, and culturally appropriate primary and secondary prevention strategies should be developed to mitigate these disparities.
High-quality population-based epidemiological studies are needed to measure global, national, and regional changes in the burden and distribution of risk factors for stroke.
Key performance indicators with specific, time-bound, measurable targets and timelines nationally, regionally, and internationally will be co-created and monitored according to the UN Development Assistance Framework theory-of-change approach.154 A theory of change is a method to explain how a given intervention, or set of interventions, is expected to lead to a specific change, drawing on a causal analysis of available evidence.154 In the UN Development Assistance Framework, a thorough theory of change helps to guide evidence-based strategies, with needs, assumptions, and risks clearly analysed and spelled out. Key principles for developing a theory of change are that the intervention should be developed consultatively to reflect the understanding of all relevant stakeholders; should be grounded in, tested with, and revised on the basis of robust evidence at all stages; and should support continuous learning and improvement from programme design to programme conclusion.154
Research priorities
Further research is needed to identify the best balance between population-wide and individually targeted prevention strategies for stroke and cardiovascular disease, to maximise cost-effectiveness and minimise inequalities (panel 4). Efforts are required to continue research into pragmatic, scalable, and cost-effective primary and secondary prevention interventions. Implementation research is also needed to scale up evidence-informed primordial, primary, and secondary stroke prevention strategies in different populations, including testing of low-risk preventive interventions in routine clinical care. Basic science and translational research leveraging genomics, transomics, and precision medicine to develop prophylactic interventions is also needed.155 These approaches should include participants from overlooked populations in LMICs and of different ethnicities, to ensure that the interventions are globally applicable.
Part 3: Acute care for all patients with stroke
Acute care refers to the health-care of patients within the 24–72 h after the onset of stroke symptoms.18 There is robust evidence to support two acute interventions in people with ischaemic stroke: admission to an inpatient stroke unit and use of reperfusion treatments (either intravenous thrombolysis or mechanical thrombectomy).
The main clinical components of acute stroke care (reperfusion treatments for ischaemic stroke, general management for acute stroke, and secondary prevention and treatment of complications in all patients) must be delivered as soon as possible after symptoms onset by a multidisciplinary team in a dedicated stroke unit (figure 7; panel 5). Rehabilitation is started in most cases within 24–48 h of stroke onset. General care for all patients with stroke is fundamental and includes management of blood pressure (with specific protocols for ischaemic and haemorrhagic stroke), blood glucose, body temperature, and oxygen levels. It is crucial that secondary stroke prevention and prevention and management of complications begin immediately after symptoms onset, and that rehabilitation is included in acute care. The implementation of such coordinated acute stroke management through a multidisciplinary team is key to improving long-term outcomes.
Figure 7: Overview of the services offered by minimal, essential, and advanced stroke services.
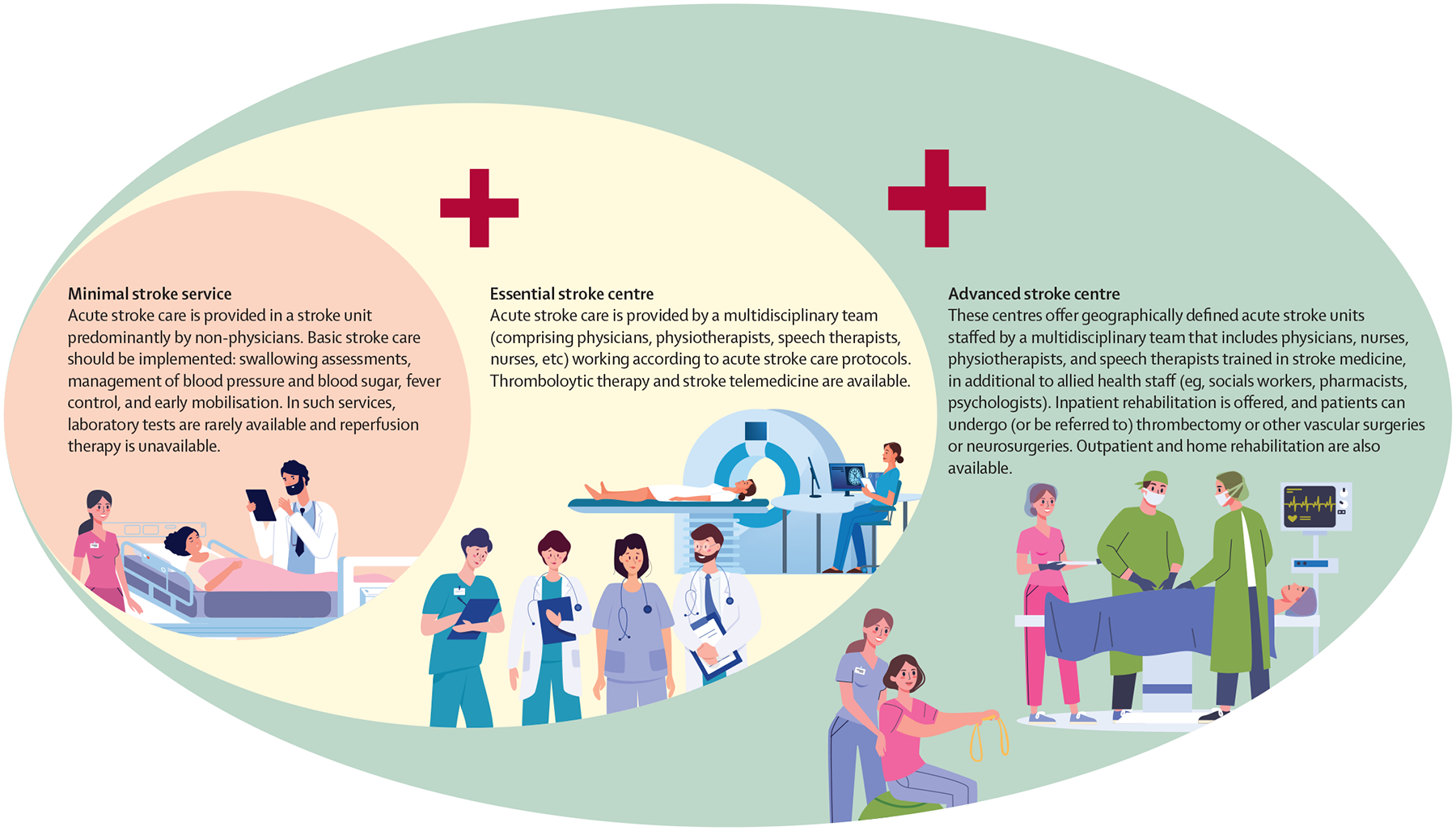
Advanced stroke centres offer all the services provided at essential stroke centres in addition to thrombectomy or referral for thrombectomy, and essential stroke centers offer multidisciplinary care and thrombolytic therapy in addition to services offered by minimal stroke service centres.
Panel 5: Stroke units and stroke centres.
The Stroke Unit Trialists’ Collaboration and Cochrane Stroke Group define a stroke unit as a dedicated, clearly defined area or ward in a hospital with beds allocated to patients with stroke where care is provided by nurses, physicians, and therapists with specialised training. This multidisciplinary team coordinates care through regular meetings.156–158 There are three types of stroke units: acute stroke units with monitored beds for patients in the first 72 h after symptoms onset that can administer intravenous thrombolysis (when indicated); rehabilitation stroke units for stabilised patients 48–72 h after symptoms onset; and comprehensive stroke units that provide acute stroke care (including intravenous thrombolysis), oversee basic interventions such as swallowing assessments, manage also other treatments (such as those for secondary prevention), provide early treatment of complications, and start rehabilitation.
The European Stroke Organisation defines a stroke centre as a hospital with infrastructure and related processes of care that provides a care pathway for patients with stroke.158 In this definition, patients are admitted to the stroke unit—a key component of stroke centres—which can be clustered on the same ward or scattered throughout the hospital.158 A stroke centre will provide different levels of institutional care depending on its structure, personnel, and resources.158
Reperfusion treatments.
An umbrella term for treatment that seeks to restore blood flow to the brain—eg, intravenous thrombolysis, mechanical thrombectomy
Intravenous thrombolysis.
A reperfusion treatment in which drugs are used to dissolve blood clots blocking an artery
Mechanical thrombectomy.
An endovascular technique for physically removing blood clots from the brain after an ischaemic stroke
Evidence-based acute care
In randomised controlled trials done mainly in well resourced middle-income countries and HICs, organised inpatient (ie, stroke unit) care was associated with reduced odds of poor outcomes (odds ratio [OR] 0·77 [95% CI 0·69–0·87]), death (0·76 [0·66–0·88]), death or long-term residence in institutional care facilities (0·76 [0·67–0·85]), and death or dependency (0·75 [0·66–0·85]) compared with alternative services (eg, treatment on general wards) at the end of scheduled follow-up (median 1 year).157 Although care on a formal stroke unit is more costly than conventional hospital care, it is cost-effective, at least in high-income countries.159–161 Despite more than 30 years of evidence showing the benefit of stroke units,162 many countries (mainly LMICs) do not have any stroke units,13 and even in HICs the number of beds available in stroke units is often insufficient to provide adequate acute care for all patients with stroke.157,163,164
Reperfusion treatments (either intravenous thrombolysis or mechanical thrombectomy) are also supported by robust evidence. The benefits of reperfusion therapy are highly time-dependent, and systems need to be well coordinated to minimise time from onset of symptoms to treatment. Thrombolysis reduces disability in a third of patients and increases the absolute proportion of patients with little to no disability by 10% if administered within 4·5 h of symptom onset.165 Some benefit can be derived in patients in whom mechanical thrombectomy is not indicated or planned if thrombolysis is administered up to 9 h after symptom onset.166 Despite the benefits of thrombolysis, the procedure is less effective in strokes caused by large vessel occlusion; in these patients, thrombolysis can recanalise only about 4% of intracranial carotid occlusions and 32% of proximal middle cerebral artery occlusions.167 To overcome this poor efficacy, several studies168–171 assessed the safety and efficacy of mechanical thrombectomy in patients with large vessel occlusion. Addition of mechanical thrombectomy to thrombolysis in patients with ischaemic stroke due to large vessel occlusion within 6 h of stroke onset increases functional independence (defined as a modified Rankin score of 0–2) at 90 days (OR 2·35 [95% CI 1·85–2·98]), but does not significantly reduce mortality (0·77 [0·54–1·10]).172
Large vessel occlusion.
A blockage of the internal carotid artery, or proximal segments of the middle cerebral, basilar, or vertebral arteries
Modified Rankin scale.
A standard scale used to measure disability, with scores ranging from 0 (no symptoms) to 6 (death)
Evidence suggests that the addition of endovascular thrombectomy to standard medical therapy (compared with standard therapy alone) 6–16 h after a patient was last known to be well results in significantly better functional outcomes (ie, a modified Rankin score 0–2) at 90 days (OR 2·67 [95% CI 1·60–4·48]) but does not significantly reduce mortality at 90 days (0·55 [0·30–1·02]) in patients with proximal occlusion of the anterior circulation.173 The DAWN trial170 showed that, in patients with stroke due to occlusion of the intracranial internal carotid artery or the proximal middle cerebral artery who had last been known to be well 6–24 h previously and who had a mismatch between the severity of the clinical deficit and infarct volume, outcomes for disability and functional independence at 90 days were better in those who underwent thrombectomy plus standard medical care than among those who received standard medical care alone, but there was no significant mortality difference between groups at 90 days (1·0 [1·0–2·0]). However, implementation of intravenous thrombolysis requires trained physicians and nurses, neuroimaging by use of CT or MRI, availability of thrombolytic drugs, and a monitored bed for the treatment. Mechanical thrombectomy requires angiographic imaging by CT and digital subtraction angiography, neurointerventionists, anaesthetists, neuro surgeons, specialised equipment, and, in most cases, a bed in an intensive care unit. Despite its enormous beneficial effects, thrombectomy is not yet available in several countries.13
Endovascular thrombectomy.
A surgical technique for removing a blood clot from the artery. A small incision is made in the groin, and then thin tubes (catheters) are threaded through the blood vessels to remove the clot
Digital subtraction angiography.
An imaging technique that uses fluoroscopy to visualise blood vessels. Structures such as bones are eliminated (or subtracted) digitally from the image, thus allowing for an accurate depiction of the blood vessels
Angiographic imaging.
An imaging technique used to visualise the lumen (ie, interior) of blood vessels and organs
As recommended by medical societies (eg, the European Stroke Organisation, the American Heart Association, the American Stroke Association), the organisation of regional acute care networks is fundamental to enable access to stroke centres for more patients, thereby increasing the impact that these centres can have.158,174,175 Stroke care networks connect hospitals with specialised stroke centres, or with other hospitals with stroke units or hospitals that are less specialised, and their benefits are well documented.176 We suggest that, because of their expertise, the team at the stroke centre (ie, the hub) is best placed to organise effective regional stroke networks. They should also work with health-care managers, other hospitals, and ambulance services to educate the population to recognise the symptoms of acute stroke and contact emergency medical services, to train pre-hospital staff and implement pre-hospital pathways that are fundamental to speed up access to the right hospital, to create a coordinated pathway to transfer patients from less specialised hospitals to those with stroke units or centres, and to ensure that secondary prevention and rehabilitation are started as early as possible. Stroke centre specialists could also support health-care managers in the creation of regional strategic plans for stroke care and prevention, as occurred in Brazil, Chile, and Egypt.177,178
Disparities in acute stroke care worldwide
The World Stroke Organization’s roadmap provides a framework for implementation, assessment, and monitoring of stroke services.43 According to this framework, stroke services are divided into minimal, essential, and advanced relative to a set of resources required for hospitals to attain each category. All hospitals that receive stroke patients in the acute phase but do not offer reperfusion treatments are classed as having minimal stroke services. In hospitals with minimal stroke services, low-cost protocols for general stroke care, which can be implemented even in low-income settings, can be used to improve the outcomes of patients with stroke—eg, swallowing assessments by trained health-care professionals, management of fever, blood pressure, and blood glucose concentrations, and early mobilisation.
Hospitals that provide access to acute stroke care, have an emergency service for stroke, can provide CT and intravenous thrombolysis, and have specialists trained to assist patients either on site or via telemedicine are classed as having essential stroke services. In Canada, the USA, and some other countries, these hospitals are called primary stroke centres. Most stroke centres worldwide fall into the essential services category, which does not require a high level of technology. To be classed as advanced, centres also need to have a stroke specialist, operating theatres, and a neurosurgeon who can provide stroke care, and to be able to provide endovascular thrombectomy and early inpatient rehabilitation. In the continuum of care, rehabilitation should begin within 24–48 h of hospitalisation.
The effectiveness of organised stroke care specifically adapted to low-resource settings has been shown in Conakry, Guinea, where a facility was set up that consisted of a dedicated area with three beds, and equipment for monitoring of heart rate, blood pressure, and blood oxygen saturation. Implementation of this minimal stroke unit without reperfusion therapy was associated with lower in-hospital mortality (7·2% vs 22·3%; p<0·0001), fewer clinical complications (4·1% vs 27·7%; p<0·001), and less pneumonia (3·3 vs 14·5%; p<0·001) compared with the period before the unit was implemented.179 Additionally, an epidemiological population-based study180 sponsored by the Brazilian Ministry of Health showed that some cities in Brazil that did not have any stroke centres had 90-day mortality rates of 40% (Sertãozinho) and 49% (Sobral), whereas in Joinville, a city with full implementation of a stroke network, 90-day mortality was 18%.
Availability of, and access to, acute care
Substantial efforts have been made to establish acute stroke units worldwide.135–138 However, despite the well-established benefit of acute interventions, implementation has been very slow and mostly partial, especially in LMICs,13 but also in Europe.181 A WHO review182 of the state of global stroke services (194 countries) showed that acute stroke care was available in roughly two-thirds or more of countries in all WHO regions, except for the African region.183 Disparities across income groups were substantial, with around a third of low-income countries having organised acute care services, compared with around half of lower-middle-income countries, roughly two-thirds of upper-middle-income countries, and four-fifths of HICs.13
Our survey of stroke services,13 with data from 314 hospitals in 84 countries, showed that only 111 (35%) hospitals had the minimum structure required to be considered an organised stroke centre offering reperfusion therapy (appendix pp 98–105).164,177,184,185 Stroke units were available in 117 (91%) of 129 hospitals in HICs, compared with three (18%) of 17 hospitals in low-income countries. Reperfusion treatments were available in 142 (60%) of 238 hospitals in HICs and upper-middle-income countries and only four (27%) of 15 hospitals in low-income countries. Protocolised swallowing assessments and strategies to minimise aspiration pneumonia (eg, appropriate diet and fluids or tube feeding)—cheap and simple interventions that can reduce the morbidity of stroke—were available in only 117 (34%) hospitals wordwide. Inequalities were substantial, with the availability of recommended services increasing with country income level. Guidelines and protocols were more commonly implemented in upper-middle-income countries (50 [46%] of 109) and HICs (54 [42%] of 129) than in lower-middle-income countries (17 [27%] of 63) and low-income countries (one [11%] of nine). In general, the lowest availability of stroke centres and the worst-structured centres (30 [29%] of 104 met the recommended minimal structure of the World Stroke Organization’s roadmap) were in the African region.
Aspiration pneumonia.
An infection of the lungs caused by inhaling saliva, food, liquid, vomit, or small foreign objects
Europe has the best structured hospitals for acute stroke care (59 [57%] of 104 met the recommended minimal structure),13 with more than 50% of the recommended elements of acute stroke care implemented in hospitals of all levels. However, a 2017 assessment of the burden of stroke in Europe164 showed that only about 30% of patients received care in a stroke unit and the proportion of patients treated in a stroke unit varied widely, from 10% in Romania to 80% in Sweden.164,181 Additionally, most European hospitals do not collect data to monitor stroke care, which makes improvement of the quality of services difficult.
Several quality indicators for acute care are suggested by different guidelines.43,158,186 It is essential to implement a minimum number of these indicators to monitor stroke care—at least at the individual hospital level, but ideally at a national level.43 Collection of data from all patients in a stroke registry to monitor the quality of stroke care is always challenging, particularly in LMICs. Experts from the European Stroke Organisation and the World Stroke Organization devised several main quality indicators (panel 6) that can be collected easily and that address the process of care (eg, time from arriving at a hospital to treatment, swallowing assessment), the use of treatments for reperfusion and prevention, and the main outcomes after stroke. These indicators were created to be used in the international Registry of Stroke Care Quality, which was followed by the Safety Implementation of Treatments in Stroke Quality Registry. These two registries now have data from a large number of patients from several countries, and have been used by the European Stroke Organisation and the World Stroke Organization Angels Award to recognise centres with good quality indicators (appendix pp 21–24), for the certification of stroke centres by the World Stroke Organization, and for the MT2020 programme (a worldwide campaign implemented by a global peer network of stroke experts since 2016 to accelerate access to thrombectomy). Stroke centre certification could be a helpful framework to encourage and monitor improvements to drive the quality of acute stroke care.
Panel 6: Suggested quality indicators for acute stroke care.
Proportion of patients with stroke who attend a stroke unit
Proportion of patients with stroke who undergo a swallowing assessment before feeding
Proportion of patients with suspected stroke examined with neuroimaging (ie, CT or MRI)
Proportion of patients with ischaemic stroke who undergo thrombolysis
Median door-to-needle time (ie, time from arrival at the hospital to administration of intravenous thrombolysis), and proportion of patients with door-to-needle time <30 min
Proportion of patients with ischaemic stroke treated with endovascular thrombectomy
Proportion of patients with symptomatic intracranial haemorrhage after reperfusion therapy
Proportion of patients with non-cardioembolic ischaemic stroke discharged on antiplatelet agents
Proportion of patients with ischaemic stroke and atrial fibrillation discharged on oral anticoagulants
Proportion of patients with stroke discharged on antihypertensives
Proportion of patients with ischaemic stroke discharged on lipid-lowering therapy
Proportion of patients with modified Rankin scores of 0–2 at 3 months after index stroke
Proportion of patients with ischaemic stroke or intracerebral haemorrhage who die within 3 months of index stroke
Median door-to-puncture time (ie, time from arrival at hospital to the start of endovascular thrombectomy by groin [femoral artery] or elbow [radial artery] puncture) at advanced stroke centres, and proportion of patients with door-to-puncture time <120 min
Median time from puncture to recanalisation, and proportion of patients with complete or almost complete reperfusion after thrombectomy
Stroke registry.
A systematic approach to identification, diagnostic assessment, and registration of stroke cases in a community
A major gap in acute stroke care worldwide is the implementation of reperfusion therapy, even though alteplase and tenecteplase are included on the WHO list of essential medicines (2021)187 and devices for thrombectomy are included on the WHO list of priority devices (2021).188 In well resourced and organised health-care systems, only around 20% of patients with ischaemic stroke are treated with thrombolysis. In LMICs, less than 1% of patients are treated with thrombolysis,177,189–191 and some countries such as Ethiopia and Cameroon do not have any hospitals able to deliver thrombolysis, because the drugs are not available there.192–194 The use of intravenous thrombolysis for stroke increases according to World Bank income level. In our survey, less than 50% of participant hospitals could deliver intravenous thrombolysis available to treat stroke patients, but access was higher in HICs. In the Registry of Stroke Care Quality, in which participating hospitals are mostly stroke centres, have CT facilities, and can offer thrombolysis, the proportion of patients undergoing reperfusion treatment increased according to income level, showing gaps in access to the best treatments and disparities among and within countries. For example, within Nigeria, there are disparities in access to CT and stroke units, with available facilities located mainly in major cities with tertiary hospitals.13
Although mechanical thrombectomy is cost-effective, the needs for the treatment far outstrip supply in LMICs. A survey195 in India, for example, showed that only 1000–1500 mechanical thrombectomies are done per year, even though more than 270 000 are needed. This lack of treatment in LMICs is mostly due to its cost, which is not affordable to patients, and which is not covered by government or insurance reimbursement in several countries, despite its cost-effectiveness. A paucity of trained interventionalists and angiography-equipped centres, and a lack of awareness of mechanical thrombectomy among physicians, also contribute to its low use in LMICs.
A review192 of stroke care in Africa showed that mortality at 3 years after stroke was greater than 80%. In 2015, a geospatial analytic study196 showed that over 70% of people with stroke in Africa took more than 2 h to arrive at a hospital. Only ten countries have stroke units: Algeria, Central African Republic, Egypt, Ghana, Guinea, Mauritania, Morocco, Nigeria, Tunisia, and South Africa.197 The availability of neuroimaging services (CT or MRI) is very low in many African settings and access to reperfusion therapies is limited but growing. Reperfusion treatments are now available in Algeria, Central African Republic, Egypt, Morocco, Tunisia, and South Africa. Several countries do not have access to thrombolytic agents. A global survey194 of disparities in the cost of alteplase and its effect on health-care expenditure showed that alteplase costs account for over 200% of per-person health expenditure adjusted for purchasing power parity in LMICs, compared with 18% in HICs.
In Latin American and Caribbean countries, the organisation of acute stroke care has improved substantially in recent years.177,198 The number of stroke centres, all of which provide thrombolysis, increased from 322 in 2018, to 448 in 2020. The number of countries with dedicated stroke units also increased. All countries in Latin American and the Caribbean deliver thrombectomy in some centres, but the intervention is mostly restricted to a few private hospitals. Ambulance services remain limited in several countries. Use of telemedicine for stroke care has increased, but is restricted to a few hospitals and is not widely available throughout each country. Irrespective of the increasing number of stroke centres, in some countries, patients have to pay for reperfusion therapies, at least in part. For example, in public hospitals, the cost of thrombolysis is covered in full by patients in Bolivia, whereas in three (25%) of 12 countries patients cover half the cost.177,197 Mechanical thrombectomy is even less available, and the cost is covered by the government in public hospitals in only four (33%) countries, and by health-care insurance in private hospitals in five (42%) countries. The lack of reimbursement for reperfusion therapy limits its access for many patients. Despite this situation, there is evidence supporting the feasibility and cost-effectiveness of mechanical thrombectomy in Latin America, with the same efficacy as in HICs shown in a clinical trial199 done in public hospitals in Brazil.200,201 Before these studies, clinical trials of mechanical thrombectomy had been done only in HICs and the Brazilian health-care authorities had thus claimed that efficacy and cost-effectiveness were unproven in a health system with limited resources.
Barriers and facilitators of acute stroke care
In LMICs, the reasons for governments not to implement acute care are several and include lack of knowledge about its cost-effectiveness, insufficiently trained staff, and concerns about the efficacy of the treatment in these settings because of the complexity of the procedure and the logistic requirements (angiography, interventionalists, anaesthetists, specialist equipment, etc).36 The good results of the Brazilian trial,201 along with observational studies of service assessment in other countries, show that improved outcomes from implementation of reperfusion therapies are helping to drive change.199,202
Interviews with experts from nine countries (appendix pp 7–14, 106–07) allowed us to identify barriers and facilitators for acute stroke services. The major barriers that we identified were related to awareness, investment, and strategy. Awareness refers to lack of understanding of acute stroke care, which affected availability and access. There was agreement among the experts interviewed that low community awareness of stroke in the general population decreased access to evidence-based care. Lack of awareness among policy makers about stroke care resulted in low prioritisation and funding of stroke services. Furthermore, health professionals, particularly generalists, receive little stroke-specific training, which possibly precludes access to the best care. In terms of investment, all respondents agreed that funding for stroke care was inadequate and that lack of investment resulted in inequalities in access according to location and socioeconomic status. The best care was almost always available only in major large towns with populations of more than 100 000 people. In many countries, people outside major cities, and those without the means to pay out of pocket, have little access to stroke units, thrombolysis, or thrombectomy. Finally, strategy refers to the fact that many countries did not have a national strategy or guidelines for stroke care. Respondents said that countries often did not have professional organisations for health-care professionals interested in stroke, which meant that no national approach to training, accreditation, and advocacy on stroke is available.
The major facilitators for the delivery of high-quality acute care services were related to training, innovation, and networks. The provision of regular, structured training was viewed by respondents as essential for building a stroke care workforce. The best training services, according to respondents, were interdisciplinary, targeted health-care professionals who did not specialise in stroke care (who provide most in-hospital care), and were supported by various public and private funding sources. The increasing demand for thrombectomy has created an urgent need for more interventionalists. Some countries have addressed the growing demand for specialists that can perform thrombectomy by training people from different specialties—eg, cardiology—to perform endovascular clot retrieval. In other countries, tight restrictions on who can perform this intervention means that access to thrombectomy is scarce. In the context of facilitators of acute stroke care, innovation refers to highly motivated and invested workforces driving investments to improve stroke care. Innovation can occur via both formal (eg, government-funded) and informal (eg, WhatsApp groups, telehealth networks providing connected, regionalised stroke care) channels. Governments in some regions are investing in mobile stroke units to increase coverage of care. Innovations in data collection for stroke care are driving quality improvement, with public recognition of high-performing centres viewed as important. Finally, networks are facilitators of acute stroke care because well organised national bodies for stroke care, with strong leadership, increase access to high-quality care, according to respondents. The most effective networks were connected to governments through clear reporting structures (eg, to a minister of health), resulting in endorsed guidelines and funding. Living guidelines provide the potential for harmonised, internationally recognised stroke care guidelines that could be locally adapted.
We describe models of acute stroke care in LMICs, such as Brazil, Chile, China, Colombia, Egypt, Ethiopia, India, and Uruguay in the appendix (appendix pp 16–21). Various successful global initiatives for acute stroke care (the Angel’s Initiative, Mission Thrombectomy 2020+, the Latin American Stroke Ministerial Meeting, Global Stroke Alliance, the World Stroke Organization’s certification of stroke centres programme, the Registry of Stroke Care Quality, Safe Implementations in Treatments in Stroke, the World Stroke Organization implementation task force, and various American Stroke Association programmes) are also described in the appendix (pp 20–24).
Pragmatic solutions to improve acute stroke care
Effective planning of stroke care at the national or regional level, and adequate resource allocation are key to improving stroke outcomes, and thus reducing the burden of stroke. To effectively organise the delivery of acute stroke care and ensure implementation of guidelines, CT needs to be available to all patients with stroke to, at a minimum, enable health-care professionals to distinguish between haemorrhagic and ischaemic stroke. In the WHO–World Stroke Organization survey,13 only 179 (57%) of 314 hospitals had access to CT. Even where no CT scanners can be made available, care improvement is still feasible. In 2014, the World Stroke Organization launched the global stroke services guidelines and action plan,107 and created a roadmap with recommendations for regions with low resources, few physicians, and no CT scanners. The guidelines propose general basic stroke care that can be implemented anywhere (including swallowing assessment, fever management, and early mobilisation). These strategies depend only on the availability of trained health-care professionals. Several international initiatives provide free virtual training for basic organisation of stroke services and stroke unit protocols, such as the World Stroke Academy (delivered in English and Spanish),203 the Angels Initiative (delivered in several languages), and the Global Stroke Alliance (delivered in Portuguese and Spanish).
The World Stroke Organization’s action plan43 suggests that centres should gradually work to improve their status to achieve all the components required for the essential services level, and for some hospitals to reach the advanced care level. Monitoring is key to achieve improvement over time. To enable the assessment of services, the World Stroke Organization’s roadmap is freely available online in English, Portuguese, and Spanish, as a self-assessment tool. A centre can easily assess its status by comparing the proportion of elements implemented with those in the full list of recommendations. The tool can be used by a hospital, by stroke or neurological societies, and by health-care managers to assess local, regional, or national progress towards meeting recommendations.
Dialogue between stroke physicians and policy makers is strategically relevant to raise awareness of the importance of implementing evidence-based strategies. A key step is convincing hospital directors, administrators, and other health-care managers about the cost-effectiveness of stroke units (by citing evidence and showcasing examples from other hospitals or countries). The implementation of stroke units usually depends on the organisation of a team of professionals already available in the hospital (and how they are trained), and the positioning of all stroke beds in the same part of the hospital.
Key evidence-based recommendations for improving acute care by type of stroke are presented in panel 7, table 6, and the appendix (pp 108–12). Although reperfusion is cost-effective, access to interventions is poor in resource-constrained settings. To implement intravenous thrombolysis and mechanical thrombectomy, the drug and devices should be available affordably. Governments should negotiate with pharmaceutical companies to achieve lower costs for LMICs. The World Stroke Organization has previously helped negotiate in discussions between stroke specialists and ministries of health worldwide. The use of conceptual frameworks, such as the behaviour-change wheel, can help countries and regions in the identification, design, and implementation of evidence-based complex interventions like reperfusion treatments.17
Panel 7: Recommendations for improving acute stroke care.
Organisation of acute stroke care starts by recognising local gaps in structures and care. Many countries, particularly low-income and lower-middle-income countries, have huge intra-country disparities in access to care, and interventions should be tailored to address local needs. The World Stroke Organization roadmap can help in the assessment of available services.
The next step is to discuss these gaps and recommendations on how to close them with the directors of hospitals and local or national health authorities, and to elaborate action plans. It is crucial to surmount the barriers to availability and affordability of reperfusion treatments.
Efforts should be made to provide sufficient supply of alteplase worldwide and to reduce the cost of alteplase and tenecteplase in resource-limited settings.146 Stroke care should be included in universal health coverage packages in WHO member countries. Expensive treatments like intravenous thrombolysis and mechanical thrombectomy should be made affordable. Governments can negotiate with pharmaceutical companies and medical device companies to reduce these costs, as occurred in Brazil.204
Initiatives such as the Latin American Stroke Ministerial Meeting and Global Stroke Alliance can be organised in all regions of the world to bring together health-care managers, including ministers of health, to facilitate discussions and formulation of action plans. Identification of recognised regional and national stroke champions among the World Stroke Organization task force could help in discussions with local health-care authorities and the implementation of evidence-based recommendations.
The number of stroke units with a multidisciplinary approach needs to increase (the World Stroke Organization suggests at least 50 beds per 1 million people),43,183 and implementation of evidence-based acute treatments is essential (thrombolysis as a first step, followed by thrombectomy in advanced centres).177 We recommend the establishment of advanced stroke centres, with at least one centre per 2 million people.177 The multidisciplinary team should include at least a physician, a nurse, a nurse assistant, a physiotherapist, and a speech therapist.43
Telemedicine and training
In areas without trained stroke specialists, telemedicine can be used to increase access to acute care. Telemedicine has been successfully used in in Ethiopia (appendix pp 21, 25), India,205 Chile,177 and Brazil.177,206
Acute stroke care requires well trained staff in ambulances and emergency services for early recognition of stroke signs and rapid transfer of patients to a stroke centre, rapid assessment in emergency departments, initiation of acute stroke treatments in a timely manner, admission to a stroke unit, management by a multidisciplinary team, and early initiation of the rehabilitation interventions.43,163,186
Training of health professionals is fundamental for effective implementation of stroke care guidelines. There are large disparities in the availability of neurology training between high-income countries and low-income and middle-income countries (LMICs).54,207
Digital-based training can be used to build capacity among physicians and nurses to deliver evidence-based stroke care, especially in remote areas without access to trained professionals. Examples of successful training programmes are the Chilean ministry of health programme,208 which offers free virtual courses for health professionals, the World Stroke Academy, the Angels Initiative, and the Global Stroke Alliance virtual platforms, which provide free, high-quality training for several countries.
Practical hands-on and simulation-based training for neurointerventionalists are fundamental to develop mechanical thrombectomy skills and to improve the quality of the procedure and patient outcomes. A good example of a peer network to advocate for, and facilitate the global implementation of, mechanical thrombectomy is Mission Thrombectomy 2020+ in the USA.209
A paucity of speech therapists is quite common in LMICs, but nurses or physiotherapists can be trained to do swallowing assessments in the acute phase of stroke care and during rehabilitation.
Monitoring and certification
For quality monitoring, a nationwide registry with data from all patients with stroke admitted to hospitals should be implemented with a minimum dataset (such as the Registry of Stroke Care Quality, Safety Implementation of Treatments in Stroke Quality Registry, or other platforms). We recommend that hospitals publicly report stroke outcomes. There is some evidence of better outcomes among institutions that publicly report mortality for stroke and myocardial infarction.210
Certification of stroke centres based on an external audit by a national or international agency can help to improve the quality of care delivered and to ensure the implementation of stroke centres, as occurred in Latin America211 and China.212
Research priorities
We recommend that funding agencies, professional societies, and health-care authorities prioritise and fund research in stroke, especially in LMICs. The research priorities are:
Assessment of disparities in access to stroke care and prevention within and among countries and regions.
Further investigation of the use of telemedicine to increase access to specialised stroke treatment in LMICs.
Development of optimal strategies to increase early recognition, early hospital admission, and access to acute stroke care in LMICs (including reperfusion treatments).
Assessment of triage strategies in pre-hospital settings and strategies to distribute patients to stroke centres in different health-care systems.
Assessment of the best approach to manage hypertension in hyperacute (ie, pre-hospital) and acute patients in resource-constrained settings.
Development and assessment of treatments for dysphagia.
Clarification of the underlying causes of stroke and risk factors, to unravel new targets for diagnostic and prognostic tools and new treatments.
Development and assessment of neuroprotective drugs.
Table 6:
Pragmatic recommendations to improve acute stroke care by 2030
| Goals | Targets | Recommendations | Measures of progress | |
|---|---|---|---|---|
| Some countries do not have acute stroke care services | To establish acute stroke services in all countries | To ensure the availability of acute stroke services in all countries by 2030, so that acute stroke care is accessible to all patients worldwide | All countries without acute stroke services should initiate a government programme to establish services and train personnel to deliver interdisciplinary care to patients with stroke in pre-hospital and hospital settings Funding programmes should be developed to facilitate access to acute stroke services in low-income and middle-income countries (government or public–private partnerships) Telemedicine approaches should be used to facilitate access to stroke specialists | The number of countries without acute stroke services in 2022 that have established services by 2030 |
| Some countries do not have sufficient acute stroke care services (in terms of both number and quality) | To increase the number and quality of hospitals with acute stroke care | To increase the number of acute stroke centres, so that every patient with stroke has access to services by 2030 (at least 50 beds per 1 million inhabitants, with a multidisciplinary approach and implementation of evidence-based treatments: thrombolysis as a first step and thrombectomy in advanced centres as the next step) To create more advanced stroke centres providing all necessary treatment options, including thrombectomy, with at least one centre per 2 million inhabitants |
Countries with acute stroke hospitals should improve the quality of services provided according to evidence-based guidelines Training programmes should be enhanced to increase the number of personnel available to offer multidisciplinary care Telemedicine can be used to increase access to stroke specialists Certification programmes can be used to improve and maintain the quality of services |
The number of new acute stroke services established between 2022 and 2030 in each country National or international quality-indicator registries in hospitals to monitor and improve quality Number of certified stroke services in each country |
Behaviour-change wheel.
A conceptual model that combines 19 frameworks for behaviour change. It is a guide for policy makers, practitioners, intervention designers, and researchers and incorporates systematic, theory-based methods, key concepts, and practical tasks
It is imperative that regional stroke centres are fully equipped and able to deliver and provide training in mechanical thrombectomy. The training of providers in these techniques can follow the model of regional surgical training centres established in response to a study19 on the provision of essential surgical care in LMICs.213 Training should be provided by certified neurointerventionalists in large regional comprehensive certified stroke centres that do at least 50 thrombectomies yearly (as recommended by an international guideline),214 and should be accredited by professional societies or universities. The lack of trained specialists for acute stroke care is an important gap. Telemedicine can be used to increase access to acute care treatments.201,215–219 Mobile telemedicine could be a cheaper alternative to conventional telemedicine to increase access to reperfusion therapies in areas without neurologists or stroke specialists.207
Part 4: Tackling the global burden of disability with rehabilitation
Stroke is the third leading cause of disability worldwide.1,220,221 Any genuine effort to reduce the global burden of disability needs therefore to identify solutions that can be implemented worldwide to address functional limitations in people after a stroke. The proportion of people with disability (measured as a modified Rankin score 3–5) 5 years after stroke ranges from 25% among those who had minor strokes to about 50% among those who had moderate strokes and 80% among those who had severe strokes.222 10 years after a stroke, roughly half of survivors are disabled.223 Up to two-thirds (or more) of stroke survivors experience motor, sensory, visual, swallowing, language, cognitive, and psychological impairments (ie, functional deficits) that can limit daily activities (such as bathing, or dressing and grooming) and restrict participation in family, work, and social life.1,222–224 The extent of functional recovery after stroke is variable and depends on several factors, including health and socioeconomic status before the stroke, age, the severity of the stroke, its location and size, comorbidities, and the quality and quantity of rehabilitation received after the event.222,225
The aim of stroke rehabilitation is to improve functioning and prolong life without disability. Rehabilitation endeavours to facilitate neuroplasticity via function-specific or task-specific and goal-directed training, which is repetitive and challenging enough to maintain attention and to promote gains in functions and capacities. Rehabilitation also involves adaptative and compensatory interventions based on behavioural strategies (such as coping mechanisms) and assistive devices to enable completion of specific tasks,173 participation in domestic, social, and work life, and optimisation of overall quality of life and wellbeing.226,227 Research is underway to augment neural repair, regeneration, and connectivity by means of stem cells, growth factors, and other therapies228,229
Neuroplasticity.
The ability of the nervous system to change its activity in response to intrinsic or extrinsic stimuli by reorganising its structure, functions, or connections after injuries, such as a stroke
Differences in stroke rehabilitation worldwide
There are substantial gaps in the availability of inpatient, outpatient, and community rehabilitation services for stroke across and within countries and in the quality of services provided. These gaps are caused by the scarcity of evidence-based protocols for stroke rehabilitation, a lack of evidence for some interventions, and a lack of trained personnel and equipment (appendix pp 26–33).230–232 According to various comprehensive datasets,13,233–235 most countries offer less than half the stroke rehabilitation services recommended by the American Heart Association. Compared with the other three pillars of the stroke-tackling quadrangle (ie, surveillance, prevention, and acute care), availability of rehabilitation services was the lowest in terms of proportions of LMICs with such services available in every global region.12 Compared with HICs, LMICs have a substantially lower availability and provision of (and substantially lower quality) inpatient rehabilitation, home assessments, community rehabilitation services, education of carers and patients, early hospital discharge programmes, and rehabilitation protocols.13 The 2017 WHO Atlas for neurological disorders236 showed that only 17 (16%) of the 105 countries investigated had specialised neurorehabilitation services and only 18 (17%) had general rehabilitation services. This lack of rehabilitation services affects economic productivity in LMICs, especially in places where stroke occurs at a younger age, such as in Africa and India, thus affecting the workforce.192,237–239 The availability of rehabilitation services increases with increasing income strata.13,240
The lack of rehabilitation for stroke survivors is associated with depression, poor quality of life, and low frequency of return to work.240 Older people, women, people with impaired consciousness at admission, people with a previous history of stroke, people in rural and remote regions, and people not referred to, or counselled on, the need for rehabilitation are less likely to undergo rehabilitation.241,242
Effective rehabilitation services should include several components: an experienced multidisciplinary team (including physicians, speech therapists, physiotherapists, occupational therapists, nurses, prosthetists, orthotists, and other health-care professionals trained in stroke rehabilitation; appendix p 62), an individualised goal-oriented approach, and equipment and facilities to provide rehabilitation interventions (a lack of which substantially affects community reintegration of stroke survivors). Low availability of effective services, alongside low intensity of services (ie, delivering interventions to patients at too low a frequency or too short a duration, or not offering therapies that are engaging, challenging, and of sufficiently high quality), could explain the differing recovery patterns after stroke in patients in LMICs compared with those in HICs.232,243–245 The provision of simple interventions, including education of patients about their self-management, was recorded in 29 (35%) of the 84 countries included in the World Stroke Organization survey.13
Telemedicine and telerehabilitation are potential solutions to poor availability and low uptake of rehabilitation. Although telerehabilitation is viewed positively by both health-care staff and patients with stroke when offered in a research setting, there can be challenges with equipment setup, the scope of exercises that can be counselled on is restricted,219,246 and there are difficulties with patient assessment, interface problems, and time constraints (ie, health-care professionals do not always have sufficient time to offer telerehabilitation assistance in addition to their other duties) in a clinical setting.247 Further investigations into the feasibility, safety, efficacy, and cost-effectiveness of telerehabilitation in different regions are needed.
Meanwhile, poor service organisation and a lack of protocols for rehabilitation in LMICs and some HICs has led to prolonged waiting times for the assessment of individual needs and intervention, to hospital discharge without a rehabilitation plan in place (a major requirement in discharge schemes), inadequate long-term support, inadequate screening and care for depression, and little psychological and social support.245,248
Barriers to, and facilitators of, rehabilitation
On the basis of in-depth interviews with experts, we have identified major barriers to, and facilitators of, the delivery of high-quality stroke rehabilitation services (appendix p 89). Barriers were related to the complexity and scope of rehabilitation services and lack of awareness. The complexity of rehabilitation services, due to the interdisciplinary (rehabilitation specialists, physiotherapists, speech therapists, psychologists, prosthetists and orthotists, occupational therapists, nutritionists, and nurses) and inter-setting (ie, acute care, inpatient care, outpatient care, community care, and home care via public and private providers) nature of rehabilitation, creates barriers in terms of prioritising and securing funding, governance of workforce and services, data capture and reporting, and quality improvement and care coordination. According to our interviewees, most stroke rehabilitation efforts were focused on a small range of services, for a limited time, and in only some settings. Respondents stated that often only physiotherapy was available, with very little access to other specialties (eg, speech therapy, occupational therapy, and neuropsychology). Publicly funded rehabilitation services were mostly provided with acute care, and not in the community or patients’ homes, or via telemedicine approaches. Generally, the broadest scope of services was available only in large cities to patients who could afford to pay privately. Respondents reported low awareness of the role of rehabilitation after stroke across a broad range of stakeholders (eg, in the community and among health-care professionals and policy makers). This lack of awareness resulted in low political will to fund services and little advocacy to increase access to, or the scope of, services. However, some shifts in awareness, particularly among health-care professionals, linked to the growing evidence base and professionalisation of the allied health workforce, were reported in countries with trained therapists across several regions.
The major facilitators of the delivery of high-quality rehabilitation services were related to implementation of evidence-based care, universal health care, and capacity building. Evidence-based guidelines, frameworks, and protocols for stroke rehabilitation can assist with service planning and ensure quality of care. Respondents expressed regret that data were not used to plan services nationally and that data were recorded only for people with strokes treated in hospital settings. In countries with universal health coverage, most people get access to some rehabilitation after stroke. Although most respondents agreed that public services were underfunded, they also thought that allied health professionals were doing their best with available resources. The importance of the private sector for providing access to a wide range of services for stroke rehabilitation in diverse settings, including long-term services for discharged patients in the community, cannot be understated.
Capacity building facilitates stroke rehabilitation through training (undergraduate and postgraduate education) and certification by professional organisations of allied health professionals. There is little capacity for allied health training outside nursing and physiotherapy in many LMICs. In several regions, stroke rehabilitation was the responsibility of a few individuals who propagate services and run training programmes. In this context, mentoring and communities of practitioners can contribute to capacity building. Online, internationally led mentoring programmes for people in LMICs are important to prevent the so-called brain drain of health professionals who have to emigrate to get training, but do not subsequently return to apply the skills gained in their country of origin.
A survey from the World Federation for Neurorehabilitation
The World Federation for Neurorehabilitation issued more than 150 specific evidence-based recommendations for stroke rehabilitation practice in 2021.177 In 2022, this organisation did an international survey of health-care professionals (clinicians) involved in neurorehabilitation. The survey asked them about the extent to which these practice recommendations could be implemented in their region (not necessarily in their institution or by themselves). If the recommendations could not be implemented as indicated, respondents were asked why not (appendix pp 120–23). A fundamental lack of implementation of basic approaches to stroke rehabilitation—such as a multidisciplinary team approach, early initiation of stroke rehabilitation on stroke units (and continuation after discharge), integration and coordination of health-care professionals involved in care (and team work), use of WHO’s International classification of functioning, and identification of patient-centred individualised rehabilitation goals—was reported in 20–40% of health-care settings worldwide. Thus, a global perspective is required to address such service deficiencies. Additionally, disparities were noted between HICs and LMICs in terms of availability of and access to rehabilitation services and of the needs to be addressed regionally, nationally, and internationally. Whereas a multidisciplinary approach to stroke rehabilitation seemed to be standard in HICs, it was less common in LMICs, where more than a third of services did not have multidisciplinary input. A similar disparity between HICs and LMICs was noted in terms of continuation of stroke rehabilitation after discharge (26% of LMIC respondents reported much less post-discharge continuation of rehabilitation than stipulated in recommendations vs 6% of respondents in HICs). Accordingly, there is a substantial need for capacity building in LMICs. Conversely, in HICs where continuation of rehabilitation is implemented, the lack of specific knowledge of stroke rehabilitation among health-care workers in community settings seemed to be an issue. Thus, training of clinicians in a multidisciplinary rehabilitation approach in the community needs to be addressed.
Guidelines and priorities
There is a paucity of evidence-based guidelines, especially in LMICs, for stroke rehabilitation.14 Some guidelines in LMICs recommend treatments that are not recommended in guidelines in HICs and for which there is little evidence of effect (eg, cryotherapy, range-of-motion exercises for paresis, magnetotherapy for spasticity). Also, some guidelines in LMICs include recommendations that might be taken for granted in HICs (eg, to train nursing staff in early stroke rehabilitation).249
Equally apparent are gaps when practice recommendations are not supported by evidence of at least low quality (according to GRADE).249 Examples include recommendations for complex interdisciplinary rehabilitation to be provided for patients with prolonged disorders of consciousness after stroke, and recommendations about the optimal approach to speech therapy in patients with aphasia.250 Evidence for how best to treat post-stroke neuro-visual disorders, including unilateral spatial neglect, is of low quality, except for prism adaptation,251 and is hardly sufficient to guide clinical decision making, even though these sequelae are frequent and have substantial effects on activities of daily living and recovery. Similarly, there are gaps in the evidence base for specific interventions for post-stroke fatigue and cognitive, emotional, and behavioural disorders. These disorders greatly affect activities of daily living and social participation and yet there is little evidence for rehabilitation protocols. These evidence gaps highlight an urgent need to engage in collaborative rehabilitation research, ideally globally. Brain recovery research (by basic science) could help to generate new therapeutic notions that can be tested with translational research. Translational findings could then lead to clinical trials and consequently systematic reviews and meta-analyses to guide clinical decision making.252
Grading of Recommendations, Assessment, Development, and Evaluations (GRADE).
A transparent framework for developing and presenting summaries of evidence that provides a systematic approach for making clinical practice recommendations
Prism adaptation.
Adaptation of the motor and sensory systems that occurs after the visual field has been artificially shifted by using prisms
The WHO Package of interventions for rehabilitation253 provides an overview on interventions for rehabilitation relevant to people with stroke. To implement these interventions, there is a great need to establish interdisciplinary rehabilitation facilities and training programmes in tertiary institutions, especially in LMICs. In the face of accumulating evidence for stroke rehabilitation interventions that promote functional recovery, health-care staff need training in clinical decision-making (ie, knowledge about the best available evidence) and the skills to provide newly developed rehabilitation interventions.252 In the interim, implementation of the forthcoming WHO Basic rehabilitation package253 and supervised task sharing among available personnel and caregivers (to avoid stress and burnout, caregivers should be provided with adequate support) can bridge the gap and deliver sufficient task-oriented practice to facilitate recovery.9,254–256 In a family-led stroke rehabilitation trial257 in India, 1250 patients were randomly assigned to trained rehabilitation by caregivers at home or usual care after stroke. The functional outcomes at 6 months did not differ between the two groups. However, semi-structured interviews with participants showed that family-led rehabilitation (with education as an important component for raising stroke awareness) was an acceptable community-based package for patients and their relatives, and was considered a necessary model of care for poor and rural populations who could not access rehabilitation in a clinical setting.257
Telerehabilitation could be offered in various settings, including intensive care, inpatient care, outpatient care, and community-based and family-based care.258–261 Therefore, training gaps could be targeted by developing educational tools (including videos and apps) that can be disseminated globally. The results of a trial262 suggest that use of video conferencing to connect rehabilitation specialists in urban areas to specialists in rural areas is feasible in LMICs.
Local manufacturing of drugs, assistive devices, and rehabilitation equipment could improve access by reducing out-of-pocket costs in LMICs. Health insurance systems and subsidies could also improve access. Meanwhile, better use of available resources through protocols is essential14 globally, including in some HICs263 that do not have standardised approaches to follow-up.264 Most guidelines originate in HICs. In the absence of alternatives, there is a need to adapt these guidelines to LMICs until local evidence becomes available. A major facilitator of high-quality rehabilitation is the regional adaptation of practice recommendations by making use of the evidence265 to actively transform recommendations into clinical pathways that reflect local health-care priorities, settings, and capacities.266
Research priorities
Future research in stroke rehabilitation should investigate the determinants of functional dependence in different populations, the effectiveness of supervised task sharing of rehabilitation with available personnel and caregivers to overcome the shortage of health-care professionals (as compared with traditional care models), the effectiveness of community-delivered and home-delivered (including self-management) rehabilitation versus facility-based rehabilitation models, regenerative interventions, and low-cost and accessible robotics, neuromodulation devices, and brain–computer interfaces.267 Validation studies to clarify the effectiveness of rehabilitation-based educational tools, including telerehabilitation, training videos (eg, demonstrations of therapeutic procedures and sessions), and mobile health are also needed. Validation studies are also needed to assess the effectiveness and cost-effectiveness of locally manufactured rehabilitation and assistive devices. Assessment of health-care services and workforces for stroke rehabilitation in terms of training and availability of tools, and research into tailored rehabilitation protocols for LMICs, are also urgently needed.
Traditional care models.
Models that are focused on medical diagnoses, disability, and deficits, based on standardised assessments and treatments
Pragmatic solutions to improve stroke rehabilitation
We present some pragmatic solutions to improve stroke rehabilitation services worldwide in table 7 and the appendix (pp 124–70).
Table 7:
Pragmatic recommendations to improve stroke rehabilitation by 2030
| Goals | Targets | Recommendations | Measures of progress | |
|---|---|---|---|---|
| Some countries do not have stroke rehabilitation services | To establish and strengthen neurorehabilitation services in various settings in all countries | To ensure the availability of multidisciplinary neurorehabilitation facilities and personnel in all countries by 2030, so that rehabilitation is accessible to all patients worldwide | All countries without multidisciplinary neurorehabilitation services should initiate a programme to establish these services and train personnel to deliver multidisciplinary care to patients after a stroke in hospital and in community settings Funding solutions are needed to facilitate access to neurorehabilitation services for patients with stroke in low-income and middle-income countries |
Number of countries without multidisciplinary neurorehabilitation services in 2019 for stroke patients who have established such services by 2030 |
| Many countries do not have sufficient stroke rehabilitation services (in terms of both number and quality) | To increase the number of facilities offering high-quality multidisciplinary care for patients with stroke and to increase adherence to best practice guidelines along the continuum of care, including inpatient, outpatient, community, and home-based rehabilitation | To increase the number of facilities offering high-quality multidisciplinary care for patients with stroke with a continuum-of-care approach and inter-setting organisation, such that every patient with stroke has access to these services by 2030 The number and type of services required will depend on the burden of stroke in each region and country |
Multidisciplinary care and inter-setting organisation along the continuum of care should be available for patients with stroke Countries with stroke rehabilitation services should improve the quality of these services according to evidence-based guidelines Training programs should be enhanced to increase the number of personnel available to offer services Performance indicators for rehabilitation that address major impairments and patient and carer needs should be developed A repository for best-practice rehabilitation protocols for sharing and adaptation to different countries and settings should be created |
Number of new rehabilitation services established between 2019 and 2030 in each country Improved adherence to clinical practice guidelines |
A novel approach is the concept of so-called living guidelines, whereby experts from across the world participate in the continuous development and updating of evidence-based guidelines that can be adapted in the local context. For example, an online living guideline268 with a dynamically updating summary of evidence to guide clinical practice and policy development for stroke rehabilitation services has been adopted in Australia. The success of living guidelines is dependent on strong leadership and coordination, and would need to be adequately financed.
The development of international, evidence-based stroke rehabilitation guidelines that focus on therapeutic approaches rather than on organisational issues could also be useful to set up regional or local stroke rehabilitation pathways.266 Such international practice recommendations for stroke rehabilitation were developed by the World Federation for Neurorehabilitation.266,269 These recommendations followed a dual process: the context-independent identification of evidence-based practice recommendations for interventions in stroke rehabilitation, and then integration into contextualised regional clinical pathways that take into account regional priorities, resources, and organisational backgrounds (including health-care structures).269 These recommendations have been integrated into our recommendations (table 7; appendix pp 124–70).
In view of the increasing number of stroke survivors and the limited resources for community rehabilitation, effective and accessible self-management programmes or tools for stroke survivors and caregivers are crucial. Evidence suggests that self-management programmes are feasible and can improve survivors’ outcome expectations.270,271 Good educational tools include videos about stroke self-management endorsed by both the World Stroke Organization and World Federation for Neurorehabilitation.271 Our key messages on stroke rehabilitation are presented in panel 8.
Panel 8: Key messages for stroke rehabilitation.
There is an urgent need to invest in the creation of multidisciplinary rehabilitation services, and in research to generate innovative low-cost interventions (especially in low-income and middle-income countries), and in training of stroke rehabilitation professionals.
Assessment tools such as the modified Rankin Scale, the US National Institutes of Health Stroke Scale, and quality-of-life scales should be used to document the type and severity of disability and impairments.
Dissemination of multidimensional assessment tools, solutions, training videos (including self-management), and advocacy targeting all stakeholders should be implemented for stroke rehabilitation in all regions. Telemedicine and digital channels could be harnessed.
Research priorities
Multidimensional characterisation of the life course after a stroke.
Investigation of the prevalence and management of risk factors for functional dependence and mortality after stroke at the population level.
Establishment of the capacity and needs of the health services and workforce for stroke rehabilitation in terms of education, skill and competencies, and availability of required tools and equipment—eg, by using the WHO rehabilitation competency framework.272
Development of performance indicators to monitor rehabilitation quality.
Development of tailored rehabilitation protocols for low-income and middle-income countries.
Assessment and monitoring of country coverage and outcomes of stroke rehabilitation with routine data collection from facilities—eg, by using WHO’s Routine health information systems—rehabilitation toolkit.273
Validation of the effectiveness of educational tools for stroke rehabilitation, including telerehabilitation, training videos (including self-management tools and programmes), and mobile health (including the role for delivering remote care).
Investigation of the feasibility, safety, effectiveness, and coverage of home-based rehabilitation (including self-management), and community-based rehabilitation.
Investments in regenerative medicine, novel medications to modify neuroplasticity, low-cost and accessible robotics, neuromodulation tools, and brain–computer interface approaches.
Discovery of novel biomarkers for prognostication and quantification of neural repair and recovery.
Conclusions
Despite available evidence-based interventions, there are huge intra-country and inter-country variations in stroke surveillance, prevention, acute care, and rehabilitation worldwide, with fewer services in LMICs. Effective planning of stroke surveillance, prevention, acute care, and rehabilitation is needed to tackle the global burden of stroke. To maximise the effect of the limited resources available, cost-effective and evidence-based pragmatic solutions need to be deployed, with active engagement of all stakeholders, including policy makers and local communities. Region-specific adaptations of stroke prevention and care guidelines and incorporation of these guidelines into clinical practice are essential to bridge the gaps in stroke care between HICs and LMICs. Population-wide detection and control of modifiable risk factors through task sharing and digital tools are needed to reduce the incidence of stroke across the life course. Establishment of stroke units, stroke centres, and rehabilitation services should be prioritised worldwide, particularly in resource-limited settings. Promotion of universal health coverage will enable wider usage of thrombolysis and mechanical thrombectomy. Simple interventions focusing on managing fever, swallowing assessments, and control of blood glucose are low-cost strategies that improve stroke outcomes.
The ecosystem proposed by the World Stroke Organization (figure 8) could play a pivotal role in supporting the implementation of recommended cost-effective strategies to reduce stroke burden worldwide. Implementation will also require training of health-care professionals, research to generate innovative, low-cost interventions, empowerment of both patients and health-care providers with updated information on evidence to improve outcomes, and advocacy to mobilise resources and financial solutions. A biennial ranking of countries in terms of their provision of stroke surveillance, prevention, acute care, and rehabilitation will stimulate healthy competition and improvement. Efforts to mobilise funding via the international stakeholders and implementation partners in the ecosystem are crucial, because stroke, a leading cause of disability, is also a leading cause of catastrophic spending, especially in LMICs. Most LMICs rely on out-of-pocket payments for health care, which is grossly deficient and inadequate for the life-long care required for prevention and functional recovery after a stroke.171,172
Figure 8: Global ecosystem for the management, implementation, and dissemination of tangible actions to reduce stroke burden devised by the World Stroke Organization—Lancet Neurology Commission.
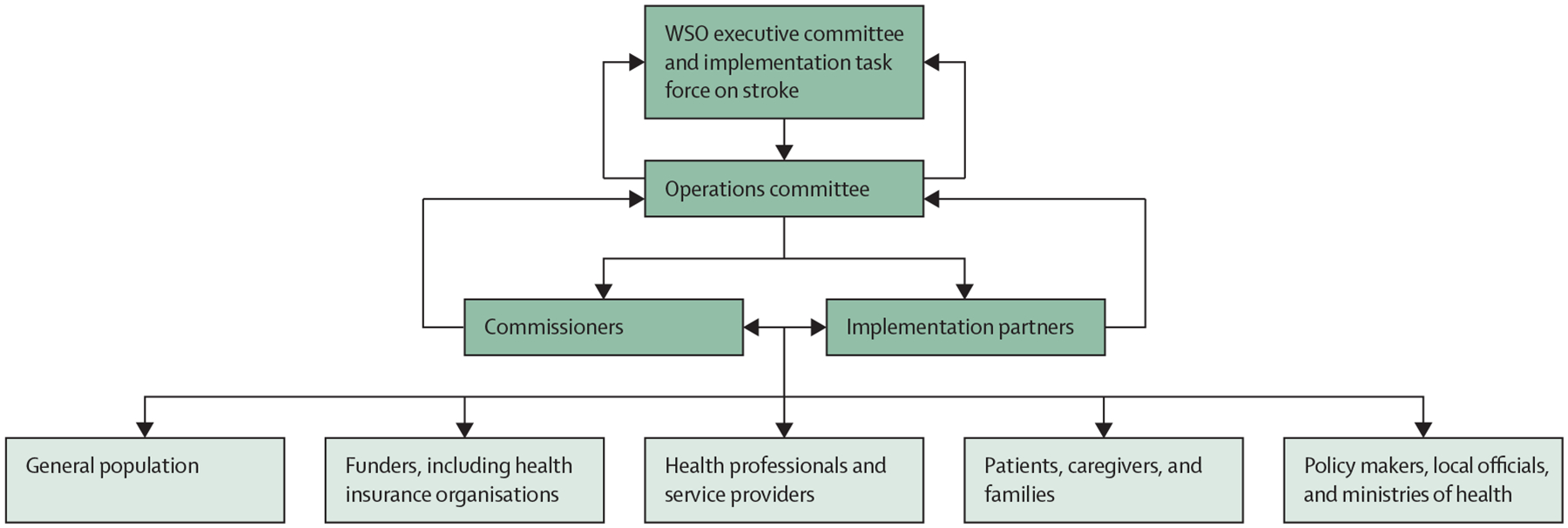
Monitoring and reducing the global burden of stroke necessitates equitable and inclusive pragmatic solutions for maximum effect. Therefore, we adopted a synergistic approach to motivate all relevant stakeholders. Bidirectional arrows show a symbiotic bottom-to-top and top-to-bottom approach and intend to convey a sense of joint ownership, which should help to attract global resources. The WSO executive committee implementation task force and the operations committee will communicate with global implementation partners. The operations committee, comprising stroke experts from the six WHO regions, can also advocate for the necessary implementation work through the commissioners. Coordination of the ecosystem by the implementation task force and executive committee will include contextualisation of the key performance indicators and targets, and implementation of the pragmatic solutions across the six WHO regions in collaboration with regional stroke organisations. In addition to WHO, which co-initiated our Commission, our international partners will include the World Federation of Neurology, the World Federation for Neurorehabilitation, the World Hypertension League, the World Heart Federation, the Global Coalition for Circulatory Health, and the Non-Communicable Disease Alliance. Implementation partners will include national stroke support organisations and national ministries of health. The World Bank and other agencies and philanthropists will be approached for funding and support by the executive committee or by the implementation task force. Adapted versions of the global ecosystem will be adopted as regional and national ecosystems to suit local environments. Commissioners will become country ambassadors. WSO=World Stroke Organization. Adapted with permission from Owolabi et al, 2023.11
Synergy with existing international initiatives
A neurological revolution is taking place.11,274 The new WHO Intersectoral Global Action Plan on epilepsy and other neurological disorders 2022–2031 is an integrated approach to brain health throughout the life course that is propelling neurology to the forefront of the global health and development agenda.47,274–280 The Global Action Plan leverages the recommendations and actions from other WHO initiatives, including WHO Rehabilitation 2030,47,281 WHO global action plan for the prevention and control of NCDs,282 the UN Joint action plan on One Health,283–285 and other international brain health programmes.286 These WHO programmes are important for stroke, which is a leading cause of death among NCDs.
Overall, NCDs cause about 74% of deaths globally.282 The determinants of NCD deaths are not only biological, but also social, environmental, and commercial. Addressing the major risk factors that can lead to NCDs—hypertension, diabetes, dyslipidaemia, smoking, unhealthy diet, misuse of alcohol, physical inactivity, and air pollution—could simultaneously prevent and delay substantial disability and a large number of deaths from stroke and other NCDs. Every member state of the UN has committed to the SDGs, which include a target to reduce premature death from NCDs by a third by 2030.282 Achieving this goal could save millions of lives, but few countries are on track to meet it. Small additional investments in NCD prevention and treatment could make a big difference: spending an additional $18 billion per year across all LMICs could generate net economic benefits of $2·7 trillion over the next 7 years.282 This investment would have benefits far beyond health. Poverty and inequity can be reduced through the introduction of universal health coverage, which would prevent catastrophic expenditure on treatment for many people with NCDs who are uninsured. Furthermore, introduction of healthy food systems as part of stroke prevention programmes would also have wider health and socioeconomic benefits.
The WHO Rehabilitation 2030 Initiative281 draws attention to the profound unmet need for rehabilitation worldwide for stroke and other conditions. The initiative emphasises that rehabilitation care should be available for everyone, throughout the life course, that efforts to strengthen rehabilitation should be directed towards supporting the health system as a whole and integrating rehabilitation into all levels of health care, and that rehabilitation is an essential health service that should be included in universal health coverage. WHO’s call for action articulates strategic steps for improving access to rehabilitation worldwide.47,281
A global partnership
Overall, partnering with global and regional professional organisations, WHO, and policy makers is essential in the dissemination and implementation of evidence-based interventions for stroke.
The World Stroke Organization implementation ecosystem will work with partners, including non-governmental organisations and national and regional stroke societies to create, implement, and monitor pragmatic solutions to reduce the global burden of stroke. Global, regional, and national key performance indicators and targets will be devised to improve stroke surveillance, prevention, acute care, and rehabilitation. Our implementation partners include WHO, the NCD Alliance, the Global Coalition for Circulatory Health, the Global Rehabilitation Alliance, the World Federation of Neurology, the World Heart Federation, One Neurology, the Angels Initiative, Resolve to Save Lives, and Mission Thrombectomy 2020. Regional and national stroke societies are crucial drivers in the ecosystem for the adoption of pragmatic solutions across regions—eg, the European Stroke Organisation incorporating the Stroke Action Plan Europe, in collaboration with the Stroke Alliance for Europe, the African Stroke Organization,287,288 the Middle East and North Africa Stroke Organization, the Asia Pacific Stroke Organization, the Stroke Society of Australasia, the Indian Stroke Association, the Ibero-American Stroke Organization, the International Stroke Recovery and Rehabilitation Alliance, and the American Heart Association and American Stroke Association.
Because of the wide availability of smartphones, including in LMICs, mobile health and telerehabilitation solutions, including training videos and advocacy videos, can be widely disseminated in various languages to enhance stroke prevention, care, and rehabilitation. Multiple interactive channels to communicate and deploy solutions should be targeted to all stakeholders (including patients, the wider population, and policy makers). The implementation cycle18 and stroke control measures based on the behaviour-change wheel approach should be implemented to inform, involve, and empower all stakeholders to collaborate. Interventions should be developed to improve awareness and increase demand for, and use of, stroke prevention, diagnosis, and rehabilitation services.289 The importance of awareness in the general population about risk factors, stroke warning signs, adequate acute care, and rehabilitation is illustrated by a case study (panel 9).
Panel 9: A case study in Nigeria.
P, a 39-year-old man, was going about his duties in a biochemistry laboratory at the University College Hospital, Ibadan (Ibadan, Nigeria), when a colleague suddenly heard him repeatedly mutter to himself “It is well!”, after which he staggered and tried to grab the wall to avoid falling. He was rushed to the emergency department of the hospital, where he received a diagnosis of ischaemic stroke. P also had raised blood pressure and serum lipid concentrations, which he had not been previously aware of. His stroke was managed conservatively, because reperfusion treatment was unavailable. He was discharged to his home after 10 days in hospital. At discharge, he was in a wheelchair and had severe functional disability: he could not handle basic activities of daily living without assistance and could neither talk nor walk unassisted.
P was the main income provider for his family before his stroke. However, he did not have health insurance. As the family income dwindled, he could not pay for physiotherapy or nursing services, which led to the development of joint contractures and decubitus ulcers, complicating his functional impairment. He did not have access to speech therapy either. He was unable to keep his outpatient clinic appointments, and his family considered traditional medicine. He developed post-stroke depression. Eventually, about 3 months after discharge, he also developed a fever, difficulty breathing, and unilateral limb swelling, so he was rushed to the emergency department again. Investigations revealed that he had a deep vein thrombosis, probably because he was sedentary and not moving the affected limb actively enough.
Although P clearly had an inadequate support system as a result of major gaps in the availability of acute care and organisation of post-stroke rehabilitation services in his country, provision of educational materials to him or his caregivers about self-management and some rehabilitation tips could have gone a long way towards avoiding some of the complications he experienced after discharge. Furthermore, community-based rehabilitation, which is generally less expensive than hospital care, could have been useful, or perhaps telemedicine-based care.
The practical goal of these efforts would have been to help P with basic activities of daily living and to reintegrate him into family and social life (and, if possible, into his work), which would have reduced the burden of care on his family, and would have given his child a better opportunity to survive. His stroke could have been prevented if P had been aware of his risk factors and encouraged to adopt lifestyle modifications, and attend regular annual check-ups, to get his blood pressure and lipid profile under control.
We recommend the collection and monitoring of key quality indicators for stroke services worldwide. Such monitoring of acute stroke care, for example, would facilitate planning actions for improvement of stroke centres and enable comparison between different centres and countries. Adherence to key indicators of service availability and quality is consistently associated with reduced risk of death and disability after stroke.290 Quality indicators can be used to monitor adherence to guidelines and to support the transfer of evidence into everyday clinical practice.291,292 Standardised core outcome measures for stroke rehabilitation, which are broadly not available in LMICs,293 are also needed for comparisons of the effectiveness of interventions across settings.
Overall, if the recommendations of this Commission are implemented, the burden of stroke will be reduced substantially worldwide by 2031 and beyond, which will accelerate the attainment of SDG 3.4 and other SDGs, and improve brain health and overall wellbeing worldwide.
Supplementary Material
Key messages
Stroke is the second leading cause of death, the third leading cause of disability, and a leading cause of dementia worldwide. The age-standardised incidence of stroke in younger individuals (ie, <55 years) is increasing in both high-income countries and low-income and middle-income countries. The absolute number of people affected by stroke (ie, who die from or remain disabled by stroke) has almost doubled during the past three decades, with more than 86% of the stroke burden in low-income and middle-income countries. Our projections show that the global burden of stroke (ie, deaths and disability-adjusted life-years) will continue to grow, with widening gaps between high-income countries and poorer countries.
Multiple factors contribute to the high burden of stroke in low-income and middle-income countries, including undetected and uncontrolled hypertension, lack of easily accessible, high-quality health services, insufficient attention to and investment in prevention, air pollution, population growth, unhealthy lifestyles (eg, poor diet, smoking, sedentary lifestyle, obesity), an earlier age of stroke onset and greater proportion of haemorrhagic strokes than in high-income countries, and the burden of infectious diseases resulting in competition for limited healthcare resources.
Major barriers to high-quality stroke surveillance, prevention, acute care, and rehabilitation are: low awareness of stroke and its evidence-based management among communities, health-care professionals, and policy makers, and scarce surveillance data for stroke risk factors, events, management, and outcomes to enable quality improvement and priority setting. Major facilitators include professional stroke organisations and networks that could advocate and build capacity for stroke care and research, and universal health coverage that can facilitate population-wide access to evidence-based care (pre-hospital care, acute care, rehabilitation, and prevention).
The total cost of stroke (both direct treatment and rehabilitation costs and indirect costs due to loss of income) will rise from US$891 billion per year in 2017 to as much as $2·31 trillion by 2050. However, this increase can be avoided because stroke is highly preventable and treatable. To mitigate this massive expense and reduce the burden of stroke globally, governments, health ministries, and other stakeholders need to apply the pragmatic approaches that we suggest.
Global investment in stroke surveillance, prevention, treatment, and rehabilitation will accelerate the achievement of Sustainable Development Goal 3.4, which aims to reduce premature mortality from non-communicable diseases by a third by 2030. Reducing the global burden of stroke is essential for promoting brain health and for overall health and wellbeing.
Key priorities to reduce the burden of stroke
Surveillance
Incorporate stroke event and risk factor surveillance into national stroke action plans.
Establish low-cost surveillance systems, ideally within existing systems for non-communicable diseases, to adequately guide prevention and treatment.
Embed regular national risk factor surveillance in national censuses.
Prevention
Establish an intersectoral system for population-wide primordial, primary, and secondary stroke prevention. Preventive strategies, with emphasis on lifestyle modification, should be implemented for people at any level of risk of stroke and cardiovascular disease. Primary and secondary stroke prevention services should be freely accessible and supported by universal health coverage, with access to affordable drugs for management of hypertension, dyslipidaemia, diabetes, and clotting disorders. Governments must allocate a fixed proportion of their annual health-care funding for prevention of stroke and related non-communicable diseases. This funding could come from taxation of tobacco, salt, alcohol, and sugar.
Raise public awareness and take action to encourage a healthy lifestyle and prevent stroke via population-wide deployment of digital technologies (a so-called motivational mass individual strategy for stroke prevention) with simple, inexpensive screening for cardiovascular disease and modifiable risk factors. This strategy should be reinforced by health-care professionals through digital technologies for person-centred primary and secondary prevention of stroke and cardiovascular disease, linked to national electronic health databases.
Establish protocol-based shifting (or sharing) of tasks from highly-trained health-care professionals to incentivised, supervised, and certified paramedical health-care workers, particularly community-based health-care workers, to facilitate population-wide primary stroke prevention interventions across rural and urban settings.
Acute care
Prioritise effective planning of acute stroke care services; capacity building, training, and certification of a multidisciplinary workforce; provision of evidence-based equipment and affordable medicines; and adequate resource allocation at national and regional levels.
Establish regional networks and protocol-driven services, including community-wide awareness campaigns for early recognition of a stroke, regionally coordinated pre-hospital services, telemedicine networks, and stroke centres that can triage and treat all cases of acute stroke, and facilitate timely access to reperfusion therapy, including intravenous thrombolysis or mechanical thrombectomy for ischaemic stroke.
Integrate acute care networks across the pillars of the quadrangle of resources, including surveillance, prevention, and rehabilitation services, by involving all relevant stakeholders (ie, communities, policy makers, non-governmental organisations, national and regional stroke organisations, and public and private health-care providers) in the stroke care continuum.
Rehabilitation
Establish multidisciplinary rehabilitation services and adapt evidence-based recommendations to the local context, including the training, support, and supervision of community health-care workers and caregivers to assist in long-term care.
Invest in research to generate innovative low-cost interventions, in public awareness to improve demand for rehabilitation services, and in advocacy to mobilise resources and financial solutions for multidisciplinary rehabilitation, especially in low-income and middle-income countries.
Promote the training of stroke rehabilitation professionals. Use digital portals to improve training and to extend the use of assessment tools—such as the modified Rankin Scale and the US National Institutes of Health Stroke Scale—and quality-of-life measures to assess functional impairment and monitor recovery.
Acknowledgments
The survey part of the Commission was partly funded by the World Stroke Organization. Stroke burden projections 2020-50 were funded by the Bill & Melinda Gates Foundation. The thematic analysis was partly funded by the Australian National Health and Medical Research Council Synergy STOPstroke grant (GNT1182071) and the World Stroke Organization. MOO is supported by Stroke Investigative Research and Educational Network (U54HG007479), Systematic Investigation of Blacks with Stroke using genomics (R01NS107900), African Rigorous Innovative Stroke Epidemiological Surveillance (R01NS115944-01), Copy number variation and stroke risk (1R01NS114045-01), the Sub-Saharan Africa Conference on Stroke (1R13NS115395-01A1), and Training Africans to Lead and Execute Neurological Trials & Studies (D43TW012030). VLF declares grants from the Brain Research New Zealand Centre of Research Excellence (16/STH/36), the Australian National Health and Medical Research Council (APP1182071), and the World Stroke Organization. VLF is an executive committee member of the World Stroke Organization, honorary medical director of Stroke Central New Zealand, and chief executive of the New Zealand Stroke Education Trust. AGT has received funding from the Australian National Health and Medical Research Council (1143155, 1171966, 1182071) and the Medical Research Future Fund (2015976), and is a board member of the Australian Stroke Foundation. SG is funded by the World Stroke Organization and the National Heart Foundation of Australia Future Leader Fellowship (GNT102061), and has received grants from the Australian National Health and Medical Research Council (GNT1182071), the Medical Research Future Fund Cardiovascular Health Mission, and the Royal Hobart Hospital Research Foundation. AM has received support from the Nossal Institute for Global Health. TPN has received support from the World Stroke Organization and an Australian National Health and Medical Research Council STOPstroke Synergy Grant (GNT1182071). AR has received grants from the Health Research Council of New Zealand, and is an executive board member of the World Stroke Organization and Neurological Association of New Zealand, executive secretary of the Stroke Society of Australasia, and a board member of the New Zealand Stroke Foundation. SR is funded by STOPStroke. ROA has received grants from the National Institutes of Health (U01HG010273), the UK Royal Society and African Academy of Sciences (FLR/R1/191813, FCG/R1/201034), the US National Institute on Aging and National Institutes of Health (U19AG074865, R01AG072547), and Global Brain Health Institute and Alzheimer’s Association (GBHI ALZ UK-21-24204). The World Federation for Neurorehabilitation Specialist Interest Group Clinical Pathways survey was supported by the BDH Bundesverband Rehabilitation.
We acknowledge the contributions of of Prebo Barango, Alarcos Cieza, Tarun Dua, Wouter De Groote, Taskeen Khan, Pauline Kleinitz, Jody-Anne Mills, Alexandra Rauch, Nicoline Schiess, Slim Slama, and Cherian Varghese of WHO in reviewing and editing this Commission.
Declaration of interests
VLF states that the free Stroke Riskometer app and PreventS-MD webapp are owned and copyrighted by AUT Ventures, which is owned by his employer, Auckland University of Technology (Auckland, New Zealand). VLF has no financial relationship with the apps, but AUT Ventures intends to commercialise PreventS-MD via its social enterprise business, for which VLF serves as chief scientific advisor. PreventS-MD was not involved in any aspect of the design, conduct, analysis, or content of this Commission. GAF has received grants from Novartis, consulting fees from CSL Behring, and honoraria from Astellas and Bayer; has served on data safety monitoring boards, data-monitoring committees, and steering committees for Pharmagenesis and Boehringer Ingelheim; and is a non-executive director of the UK National Institute for Health and Care Excellence and a trustee of Health Services Research UK. DCG has received travel support from the World Federation for Neurorehabilitation and Conventus Travel, and is a past president of the World Federation for Neurorehabilitation. WH has participated on data safety monitoring boards for IFS Institut für anwendungsorientierte Forschung und klinische Studien and Imperical College, London, and is a past president of the World Stroke Organization. GJH has received consultation fees from Janssen Research and Development, Bristol Myers Squibb, and Bayer, and has participated on data safety monitoring boards for Janssen Research and Development. PL has received honoraria from Pfizer, Boehringer Ingelheim, and RAPID-AI iSchemaView; has received travel support from Angels–Boehringer Ingelheim; has participated on data safety monitoring boards and steering committees for Pfizer and Janssen; and is the president of the Chilean Stroke Association and vice-president of the Iberoamerican Stroke Society. BN has received honoraria for serving on data safety monitoring boards work for Simbec-Orion. TP is a member of the Data Safety Monitoring Board for a publicly funded trial in Germany, with public grant money paid to his institution. AR has received honoraria from the University of Texas at Austin (Austin, TX, USA) and Medical College of Virginia (Richmond, VA, USA), and travel support from Fiji National University. PNS is a leader or member of advisory boards for Medtronic and the Angels Initiative (funded by Boehringer-Ingelheim). DY has served as consultant for the Athersys, Johnson & Johnson, Medtronic, Rapid Medical, Gravity Medical Technology, Guidepoint Global, Poseydon, Vascular Dynamics, Royal Caribbean Cruises, and Stryker; holds stocks or stock options in Athersys, Poseydon, and Rapid Medical; has received honoraria payments from Emory University (Atlanta, GA, USA); and is founder and global co-chair of the Society for Vascular and Interventional Neurology’s Mission Thrombectomy. All other authors declare no competing interests.
World Stroke Organization Implementation Task Force on Stroke
Bo Norrving (co-chair), Valery L Feigin (co-chair), Michael Brainin, Sheila Martins, Jeyaraj Pandian, Graeme J Hankey, Seana Gall, Mayowa O Owolabi, Taskeen Khan.
World Stroke Organization–Lancet Neurology Commission Steering Committee
Mayowa O Owolabi (co-chair), Valery L Feigin (co-chair), Foad Abd-Allah, Semaw Ferede Abera, Rufus O Akinyemi, Michael Brainin, Valeria Caso, Robert J Dempsey, Gary A Ford, Seana Gall, Dorcas Gandhi, Vladimir Hachinski, Werner Hacke, Graeme J Hankey, Norlinah Mohamed Ibrahim, Walter Johnson, Pablo Lavados, Liping Liu, Patrice Lindsay, Sheila Martins, Bo Norrving, Muideen Olaiya, Bruce Ovbiagele, Jeyaraj Pandian, Hoang Phan, Michael Piradov, Thomas Platz, Anna Ranta, Greg Roth, Ivy Anne Sebastian, Nijasri Suwanwela, P N Sylaja, Amanda G Thrift, Ezinne Uvere, Joseph Yaria.
World Stroke Organization–Lancet Neurology Commission writing group
Valery L Feigin, Foad Abd-Allah, Rufus O Akinyemi, Natalia V Bhattacharjee, Michael Brainin, Jackie Cao, Valeria Caso, Bronte Dalton, Alan Davis, Robert Dempsey, Joseph Duprey, Wuwei Feng, Gary A Ford, Seana Gall, Dorcas Gandhi, David C Good, Vladimir Hachinski, Werner Hacke, Graeme J Hankey, Marie Ishida, Walter Johnson, Julie Kim, Pablo Lavados, Patrice Lindsay, Ajay Mahal, Sheila Martins, Christopher Murray, Thuy Phuong Nguyen, Bo Norrving, Muideen T Olaiya, Oladotun V Olalusi, Jeyaraj Pandian, Hoang Phan, Thomas Platz, Anna Ranta, Sabah Rehman, Greg Roth, Ivy Anne Sebastian, Amanda E Smith, Nijasri C Suwanwela, P N Sylaja, Rajshree Thapa, Amanda G Thrift, Ezinne Uvere, Stein Emil Vollset, Dileep Yavagal, Joseph Yaria, Mayowa O Owolabi, on behalf of the World Stroke Organization—Lancet Neurology Commission Stroke Collaboration Group.
Affiliations
Faculty of Medicine, Cairo University, Cairo, Egypt (Prof F Abd-Allah MD); School of Public Health, College of Health Sciences, Mekelle University, Mekelle, Ethiopia (S F Abera PhD); Centre for Genomics and Precision Medicine, College of Medicine (Prof R O Akinyemi PhD, Prof M O Owolabi MD), Faculty of Clinical Sciences (Prof M O Owolabi), and Department of Medicine (Prof M O Owolabi, E Uvere MPH), University of Ibadan, Ibadan, Nigeria; Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA (N V Bhattacharjee PhD, J Cao MS, B Dalton BA, J Duprey MS, Prof V L Feigin MD, J Kim MS, Prof C Murray MD, G Roth MD, A E Smith MPA, Prof S E Vollset MD); Danube University Krems, Krems, Austria (Prof M Brainin MD); University of Perugia Stroke Unit, Perugia, Italy (Prof V Caso MD); Te Whatu Ora/Health New Zealand, Whangārei, New Zealand (A Davis MD); University of Wisconsin School of Medicine and Public Health, Madison, WI, USA (R Dempsey MD); National Institute for Stroke and Applied Neurosciences, Auckland University of Technology, Auckland, New Zealand (Prof V L Feigin); Research Centre of Neurology, Moscow, Russia (Prof V L Feigin); Department of Neurology, Duke University School of Medicine, Durham, NC, USA (W Feng MD); Radcliffe Department of Medicine, University of Oxford, Oxford, UK (Prof G A Ford PhD); Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia (S Gall PhD, T P Nguyen PhD, H Phan PhD, S Rehman PhD); Christian Medical College & Hospital, Ludhiana, India (Prof D Gandhi MPT, Prof J Pandian MD); Penn State Health, Hershey, PA, USA (D C Good MD); Department of Clinical Neurological Sciences, London’s Robarts Research Institute, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada (Prof V Hachinski MD); University of Heidelberg, Heidelberg, Germany (Prof W Hacke MD); University of Western Australia Medical School, The University of Western Australia, Perth, WA, Australia (Prof G J Hankey MD); Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia (M Ishida MPH, Prof A Mahal PhD); Perron Institute for Neurological and Translational Science, Perth, WA, Australia (Prof G J Hankey); Universiti Kebangsaan Malaysia Medical Center, Kuala Lumpur, Malaysia (Prof N M Ibrahim MB); School of Medicine and School of Public Health, Loma Linda University, Loma Linda, CA, USA (Prof W Johnson MD); World Health Organization, Geneva, Switzerland (T Khan MD); Clinica Alemana, Universidad del Desarrollo, Santiago, Chile (Prof P Lavados PhD); Universidad de Chile, Santiago, Chile (Prof P Lavados); Heart and Stroke Foundation of Canada, Toronto, ON, Canada (P Lindsay PhD); Beijing Tiantan Hospital, Capital Medical University, Beijing, China (L Liu MD); World Stroke Organization, Geneva, Switzerland (Prof S Martins MD); Medical School of Universidade Federal do Rio Grande do Sul, Port Alegre, Brazil (Prof S Martins); Hospital de Clínicas de Porto Alegre, Port Alegre, Brazil (Prof S Martins); Hospital Moinhos de Vento, Port Alegre, Brazil (Prof S Martins); Department of Clinical Sciences, Lund University, Lund, Sweden (Prof B Norrving PhD); Department of Clinical Sciences, Lund University, and Skåne University Hospital, Lund, Sweden (Prof B Norrving); Weill Institute for Neurosciences, University of California, San Francisco, San Francisco, CA, USA (Prof B Ovbiagele MD); Department of Medicine, School of Clinical Sciences at Monash Health, Monash University, Melbourne, VIC, Australia (M T Olaiya PhD, Prof A G Thrift PhD); Faculty of Clinical Sciences, National Research Medical University, Moscow, Russia (O V Olalusi MD); BDH Klinik Greifswald, Greifswald, Germany (Prof T Platz DrMed Habil); Institute for Neurorehabilitation and Evidence-based Practice, University of Greifswald, Greifswald, Germany (Prof T Platz); University Medical Centre, Greifswald, Germany (Prof T Platz); Research Center of Neurology, Moscow, Russia (E V Gnedovskaya MD, M Kravchenko MD, Prof M Piradov MD); University of Otago, Wellington, New Zealand (Prof A Ranta MD); St Stephen’s Hospital, Delhi, India (Prof I A Sebastian MD); Sree Chitra Tirunal Institute for Medical Sciences and Technology, Kerala, India (P N Sylaja MD); Department of Neurology and Department of Neurosurgery, University of Miami Miller School of Medicine, Miami, FL, USA (Prof D Yavagal MD); University College Hospital, Ibadan, Nigeria (O V Olalusi, J Yaria MBBS); Lebanese American University of Beirut, Beirut, Lebanon (Prof M O Owolabi); Chulalongkorn Comprehensive Stroke Center, King Chulalongkorn Memorial Hospital, Bangkok, Thailand (N C Suwanwela MD); Faculty of Medicine, Chulalongkorn University, Bangkok, Bangkok, Thailand (N C Suwanwela); Eastern Health Clinical School, Monash University, Melbourne, VIC, Australia (R Thapa PhD).
Other members of the World Stroke Organization—Lancet Neurology Commission Stroke Collaboration Group
Carlos Abanto, Adamu Addissie, Amos O Adeleye, Yerzhan Adilbekova, Bibigul Adilbekova, Thierry A Adoukonou, Diana Aguiar de Sousa, Zauresh Akhmetzhanova, Albert Akpalu, Mustapha El Alaoui-Faris, Sebastian F Ameriso, Silva Andonova, Anita Arsovska, Folorunso Emmanuel Awoniyi, Moiz Bakhiet, Miguel A Barboza, Hamidon Basri, Philip M Bath, Daniel Bereczki, Simone Beretta, Aaron L Berkowitz, Julie Bernhardt, Guna Berzina, Bhavan Bhavsar, Mher S Bisharyan, Manoj Bohara, Pacal Bovet, Hrvoje Budincevic, Dominique A Cadilhac, Denis Čerimagić, Augustina Charway-Felli, Christopher Chen, Jerome H Chin, Hanne Christensen, Kamil Chwojnicki, Adriana B Conforto, Manuel Correia, Daissy Liliana Mora Cuervo, Anna Członkowska, Marco D’Amelio, Kristine Edgar Danielyan, Stephen Davis, Vida Demarin, Andrew M Demchuk, Martin Dichgans, Klara Dokova, Geoffrey Donnan, Juan Carlos Duran, Gloria Ekeng, Mitchell S Elkind, Matthias Endres, Urs Fischer, Yuriy Flomin, Fortuné Gankpe, Martin Gavidia, Andrés Gaye-Saavedra, Mehari Gebreyohanns, Mary George, Marek Gierlotka, Maurice Giroud, Elena V Gnedovskaya, Ivete Pillo Gonçalves, Fernando Gongora-Rivera, Padma S Gunaratne, Randah R Hamadeh, Tal-hatu K Hamzat, Mirjam R Heldner, Etedal Ibrahim, Hanne Ihle-Hansen, Sungju Jee, Jeng Jiann-Shing, S Clay Johnston, Dejana Jovanovic, Kristaps Jurjāns, Rizwan Kalani, Yogeshwar Kalkonde, Saltanat Kamenova, Bartosz Karaszewski, Peter Kelly, Stefan Kiechl, Aida Kondybayeva, Janika Kõrv, Grzegorz Kozera, Michael Kravchenko, Yakup Krespi, Rita Krishnamurthi, Jera Kruja, Kursad Kutluk, Peter Langhorne, Zhe K Law, Dmytro Lebedynets, Tsong-Hai Lee, Thomas W Leung, David S Liebeskind, Patricio López-Jaramillo, Paulo A Lotufo, M Julia Machline-Carrion, Luis F Maia, Branko Malojcic, Hugh S Markus, Juan Manuel Marquez-Romero, Marco T Medina, Sabina Medukhanova, Man Mohan Mehndiratta, Evija Miglāne, Illa Mihejeva, Robert Mikulik, Erkin Mirrakhimov, Stephanie Mohl, Sunil Munakomi, Sean Murphy, Kamarul Imran Musa, Ahmed Nasreldein, Raul Gomes Nogueira, Christian H Nolte, Jean Jacques Noubiap, Nelson Novarro-Escudero, Cassandra Ocampo, Martin O’Donnell, Yomi Ogun, Adesola Ogunniyi, Mohammed I Oraby, Dilek N Ōrken, Atilla Ozcan Ōzdemir, Serefnur Ozturk, Mélanie Paccot, Telmo Pereira, A André Peeters, Tatjana Potpara, Farooq A Rathore, Ralph L Sacco, Ramesh Sahathevan, Else S Sandset, Irving Renato Santos, Gustavo Saposnik, Fred S Sarfo, João Sargento-Freitas, Mukul Sharma, Louise Shaw, Kevin N Sheth, Yong-Il Shin, A Shobhana, S Nishan Silva, V Tedim Cruz, Kiran Thakur, Lekh Jung Thapa, Danilo Toni, Mehmetakif A Topcuoglu, Julio Torales, Amytis Towfighi, Thomas Truelsen, Alexander Tsiskaridze, Marshall Tulloch-Reid, Juan Nicolas Useche, Peter Vanacker, Sophia Vassilopoulou, Gorana Vukorepa, Vladimira Vuletic, Kolawole W Wahab, Wenzhi Wang, Tissa Wijeratne, Bogdan Wojtyniak, Charles Wolfe, Mapoure N Yacouba, Jie Yang, Yared M Yifru, Adriana Yock-Corrales, Naohiro Yonemoto, Laetitia Yperzeele, Pawel Zagożdżon.
Affiliations
Departamento de Enfermedades Neurovasculares, Instituto Nacional de Ciencias Neurológicas, Lima, Peru (C Abanto MD); School of Public Health (A Addissie MD), and Department of Neurology, College of Health Science (Y M Yifru MD) Addis Ababa University, Addis Ababa, Ethiopia; College of Medicine, University of Ibadan, Ibadan, Nigeria (A Adeleye MD, T K Hamzat PhD); Department of Neurological Surgery, University College Hospital, Ibadan, Nigeria (A Adeleye, Prof A Ogunniyi MBChB); Astana Medical University, Astana, Kazakhstan (B Adilbekov MD, Prof Z Akhmetzhanova MD); National Coordination Center for Emergency Medicine, Astana, Kazakhstan (Y Adilbekov MD, S Medukhanova MD); Department of Neurology, Faculty of Medicine, Université de Parakou, Parakou, Benin (Prof T A Adoukonou MD); Stroke Center, Lisbon Central University Hospital, Lisbon, Portugal (D Aguiar de Sousa MD); Institute of Anatomy, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal (D Aguiar de Sousa); University of Ghana Medical School, Accra, Ghana (A Akpalu MB); Mohamed V University, Rabat, Morocco (M El Alaoui-Faris MD); Department of Neurology, Institute for Neurological Research, Fundación para la Lucha contra las Enfermedades Neurológicas de la Infancia, Montañeses, Buenos Aires, Argentina (S F Ameriso PhD); Medical University and University Hospital St Marina, Varna, Bulgaria (S Andonova MD, Prof K Dokova MD); University Clinic of Neurology, University SS Cyril and Methodius, Skopje, North Macedonia (A Arsovska MD); Department of Linguistics and Communication Studies, Osun State University of Technology, Osogbo, Nigeria (F E Awoniyi MSc); Department of Molecular Medicine, College of Medicine and Medical Sciences, and Princess Al-Jawhara Center for Genetics and Inherited Diseases, Arabian Gulf University, Manama, Bahrain (M Bakhiet MD); Escuela de Medicina, San José Universidad de Costa Rica, San José, Costa Rica (M A Barboza MD); Department of Medicine, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Selangor, Malaysia (H Basri MD); Stroke Trials Unit, University of Nottingham, Nottingham, UK (P M Bath DSc); Semmelweis University, Budapest, Hungary (D Bereczki MD); San Gerardo Hospital ASST Monza, Monza, Italy; University of Milano Bicocca, Milan, Italy (S Beretta MD); University of California, San Francisco, San Francisco, CA, USA (A L Berkowitz MD); The Florey Institute of Neuroscience & Mental Health (Prof J Bernhardt MD), University of Melbourne, Melbourne, VIC, Australia (Prof G Donnan MD); Riga East University Hospital, Riga, Latvia (G Berzina MD, I Mihejeva MD); Shah Hospital, Nairobi, Kenya (B Bhavsar MSc); Yerevan State Medical University after Mkhitar Heratsi, Yerevan, Armenia (M S Bisharyan MD); Department of Neurosurgery, Hospital for Advanced Medicine & Surgery, Kathmandu, Nepal (M Bohara MD); University Center of Primary Care and Public Health (Unisanté), Lausanne, Switzerland (P Bovet MD); Sveti Duh University Hospital, Zagreb, Croatia (H Budincevic MD); Department of Medicine, School of Clinical Sciences at Monash Health, Monash University, Melbourne, VIC, Australia (Prof D A Cadilhac PhD); Poliklinika Glavić, Dubrovnik, Croatia (D Čerimagić MD); Department of Neurology, Dubrovnik General Hospital, Croatia (D Čerimagić); 37 Military Hospital, Accra, Ghana (A Charway-Felli MD); Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore (C Chen MD); New York University School of Medicine, New York, NY, USA (J H Chin MD); Copenhagen University Hospital, Copenhagen, Denmark (H Christensen MD); Bispebjerg og Frederiksberg Hospital, Frederiksberg, Denmark (H Christensen); Department of Adult Neurology and Brain Disease Centre (B Karaszewski MD), Medical Simulation Center (Prof G Kozera MD), Department of Hygiene and Epidemiology (P Zagożdżon MD), and Department of Anesthesiology and Intensive Care (K Chwojnicki MD) Medical University of Gdansk, Gdansk, Poland; Hospital das Clinicas, Faculty of Medicine, University of São Paulo, São Paulo, Brazil (A B Conforto MD); Universidade do Porto Instituto de Ciências Biomédicas Abel Salazar, Porto, Portugal (M Correia MD); Department of Neurology, Hospital Moinhos de Vento, Porto Alegre Brazil (D L M Cuervo MD); Department of Neurology, Institute of Psychiatry and Neurology, Warsaw, Poland (A Członkowska MD); Department of Biomedicine, Neuroscience and Advanced Diagnostics, University of Palermo, Palermo, Italy (M D’Amelio MD); Institute of Biochemistry named after H Buniatyan, Yerevan, Armenia (K E Danielyan PhD); Royal Melbourne Hospital, Melbourne, VIC, Australia (Prof S Davis MD); Department of Medical Sciences, Croatian Academy of Sciences and Arts, Zagreb, Croatia (Prof V Demarin MD); Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada (A M Demchuk MD); Institute for Stroke and Dementia Research, Ludwig-Maximilians University, Munich Medical Center, Munich, Germany (Prof M Dichgans MD); Munich Cluster for Systems Neurology, Munich, Germany (Prof M Dichgans); Neurocentro, La Paz, Bolivia (J Duran MD); Stroke Care International, London, UK (G Ekeng MSc); Department of Neurology, Vagelos College of Physicians and Surgeons, New York, NY, USA (M S Elkind MD); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, USA (M S Elkind); Department of Neurology with Experimental Neurology, Charité—Universitätsmedizin, Berlin, Germany (M Endres MD); University Hospital Basel, Basel Switzerland (U Fischer MD); University Hospital Bern, Bern, Switzerland (U Fischer); Stroke and Neurorehabilitation Unit, MC Universal Clinic Oberig, Kyiv, Ukraine (Y Flomin MD); CNHU Hassan Hubert Koutoukou Maga and Leras, Cotonou, Benin (F Gankpe MD); Medico Neurologo, Clinica Angloamericana, Lima, Peru (M Gavidia MD); Unidad de de Ataque Cerebro Vascular, Instituto de Neurología, Hospital de Clínicas, Montovideo, Uruguay (A Gaye-Saavedra MD); Department of Neurology, University of Texas Southwestern Medical Centre, Dallas, TX, USA (M Gebreyohanns MD); US Centres for Disease Control and Prevention, Atlanta, GA, USA (M George MD); Department of Cardiology, University Hospital in Opole, and Institute of Medical Sciences, University of Opole, Opole, Poland (M Gierlotka MD); Dijon Stroke Registry, University Hospital of Dijon, University of Burgundy, Dijon, France (Prof M Giroud MD); Research Center of Neurology, Moscow, Russia (E V Gnedovskaya MD, M Kravchenko MD); Assoc Hospitalar Moinhos De Vento, Porto Alegre, Brazil (I P Gonçalves MD); Department of Neurology and Stroke Unit, University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, San Nicolas de los Garza, Mexico (F Gongora-Rivera PhD); National Hospital of Sri Lanka, Colombo, Sri Lanka (P S Gunaratne MD); Department of Family and Community Medicine, College of Medicine and Medical Sciences, Arabian Gulf University, Bahrain (R R Hamadeh Dphil); Department of Neurology, Inselspital, University Hospital Bern, Bern, Swizerland (M R Heldner MD); Department of Neurology, University of Bern, Bern, Switzerland (M R Heldner); Alneelain University, Khartoum, Sudan (E Ibrahim MD); Department of Neurology (E S Sandset MD), Oslo University Hospital, Oslo, Norway (H Ihle-Hansen MD); Department of Rehabilitation Medicine, College of Medicine, Chungnam National University Hospital, Daejeon, South Korea (S Jee MD); Department of Neurology, National Taiwan University Hospital, Taipei, Taiwan (J Jiann-Shing MD); School of Medicine, University of Texas, Austin, TX, USA (S C Johnston MD); Department of Emergency Neurology, Medical Faculty (Prof D Jovanovic MD), and School of Medicine (T Potpara MD), University of Belgrade, Belgrade, Serbia; Department of Neurology and Neurosurgery (K Jurjāns PhD, E Miglāne MD) and Department of Rehabilitation (G Berzina), Riga Stradins University, Riga, Latvia; University of Washington, Seattle, WA, USA (R Kalani MD); Sangwari, Surguja, Chhattisgar, India (Y Kalkonde MD); Higher School of Medicine, Al-Farabi Kazakh National University, Almaty, Kazakhstan (S Kamenova MD, A Kondybayeva MD); Mater Misericordiae University Hospital, Dublin, Ireland (P Kelly MD, S Murphy MD); Stroke Clinical Trials Network Ireland (P Kelly) and School of Medicine (P Kelly, S Murphy), University College Dublin, Dublin, Ireland; Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria (S Kiechl MD); Research Centre on Vascular Ageing and Stroke, Innsbruck, Austria (S Kiechl); Department of Neurology and Neurosurgery, University of Tartu, Tartu, Estonia (Prof J Kõrv MD); Department of Neurology, University of Istanbul, Istanbul, Türkiye (Y Krespi MD); National Institute for Stroke and Applied Neurosciences, School of Clinical Sciences, Auckland University of Technology, Auckland, New Zealand (Prof R Krishnamurthi PhD); Faculty of Medicine, University of Medicine, Tirana, Albania (J Kruja MD); Department of Neurology, Dokuz Eylül University, Izmir, Türkiye (K Kutluk MD); University of Glasgow, Glasgow, UK (Prof P Langhorne MD); Universiti Kebangsaan Malaysia Medical Center, Kuala Lumpur, Malaysia (Z K Law MD); Stroke Centre and Department of Neurology, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan (T H Lee MD); College of Medicine, Chang Gung University Taoyuan, Taiwan (T H Lee); Department of Neurology, University of California, Los Angeles, Los Angeles, CA, USA (D S Liebeskind MD); The Chinese University of Hong Kong, Hong Kong Special Administrative Regon, China (T W Leung MBChB); VN Karazin Kharkiv National University, Kharkiv, Ukraine (D Lebedynets MD); Universidad de Santander, Bucaramanga, Colombia P López-Jaramillo MD); Universidad Autonoma de Bucaramanga, Bucaramanga, Colombia (P López-Jaramillo); Department of Medicine, University of São Paulo, São Paulo, Brazil (P A Lotufo MD); epHealth Primary Care Solutions, Florianópolis, Brazil (M J Machline-Carrion MD); Department of Neurology, Centro Hospitalar Universitário de Porto, Porto, Portugal (L F Maia MD); University Hospital Centre Zagreb, Zagreb, Croatia (B Malojcic MD); Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (Prof H S Markus MD); Instituto Mexicano del Seguro Social HGZ 2, Aguascalientes, Mexico (J M Marquez-Romero MD); Faculty of Medical Sciences, National Autonomous University of Honduras, Tegucigalpa, Honduras (M T Medina PhD); Department of Neurosciences, Dr BL Kapur Hospital, Max Healthcare, New Delhi, India (Prof M M Mehndiratta PhD); St Anne’s University Hospital, Brno, Czech Republic (Prof R Mikulik MD); Masaryk University, Brno, Czech Republic (Prof R Mikulik); Department of Internal Medicine, Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan (Prof E Mirrakhimov MD); American Heart Association and American Stroke Association, Dallas, TX, USA (S Mohl MD); Kathmandu University, Kathmandu, Nepal (S Munakomi MCh); Royal College of Surgeons in Ireland Medical School, Dublin, Ireland (S Murphy); Department of Community Medicine, School of Medical Sciences, Universiti Sains, Kelantan, Malaysia (K I Musa PhD); Department of Neurology and Psychiatry, Assiut University, Assiut, Egypt (A Nasreldein MD); University of Pittsburgh, Pittsburgh, PA, USA (R Nogueira MD); Department of Neurology, Center for Stroke Research, Berlin, Germany (C H Nolte MD); Berlin Institute of Health, Charité—Universitätsmedizin, Berlin, Germany (C H Nolte); Division of Cardiology, Department of Medicine, University of California San Fransisco, San Fransisco, CA, USA (J J Noubiap MD); Hospital Punta Pacifica, En Punta Pacífica, Ciudad de Panamá, Panama (N Novarro-Escudero MD); University of Botswana, Gaborone, Botswana (C Ocampo MD); National University of Ireland Galway Health Research Board, Galway, Ireland (Prof M O’Donnell PhD); Lagos State University College of Medicine and Lagos State University Teaching Hospital, Lagos, Nigeria (Prof Y Ogun MPH);Beni Suef University Faculty of Medicine, Beni Suef, Egypt (M I Oraby PhD); İstanbul Arel University, Istanbul, Türkiye (Prof D N Ōrken MD); Eskişehir Osmangazi University, Eskişehir, Türkiye (A O Ōzdemir MD); Selcuk University Faculty of Medicine, Selcuklu-Konyu, Türkiye (S Ozturk MD); Non-Communicable Diseases Department, Ministry of Health, Santiago, Chile (M Paccot MD I R Santos MD); Polytechnic Institute of Coimbra, Coimbra Health School, Coimbra, Portugal (T Pereira PhD); UCL St Luc Unité neuro-vasculaire, Brussels, Belgium (A Peeters MD); PNS Shifa Hospital, Karachi, Pakistan (F A Rathore MBBS); Miller School of Medicine, University of Miami, Miami, FL, USA (Prof R L Sacco MD); Grampians Health and Deakin University, Ballarat, VIC, Australia (R Sahathevan MD); Department of Neurology and Neurosurgery, Faculty of Medicine, University of Chile, Santiago, Chile (I R Santos); Stroke Outcomes and Decision Neuroscience Research Unit, University of Toronto, Toronto, ON, Canada (G Saposnik MD); Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (F S Sarfo MD); Department of Neurology, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal (J Sargento-Freitas MD); Population Health Research Institute at Hamilton Health Sciences, Hamilton, ON, Canada (M Sharma PhD); Integrated Stroke Delivery Network, NHS England, Bath, UK (L Shaw MBChB); Department of Neurology and Neurosurgery, Yale School of Medicine, New Haven, CT, USA (K N Sheth MD); Department of Rehabilitation Medicine, Rehabilitation Hospital, Yangsan, South Korea (Y Shin MD); Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, South Korea (Y Shin); Institute of Neuroscience, Kolkata, India (A Shobhana MD); Ochre Medical Centre Deniliquin, Deniliquin, NSW, Australia (S N Silva MD); Australasian Institute of Digital Health, Melbourne, VIC, Australia (S N Silva); School of Medicine, Johns Hopkins University, Baltimore, MD, USA (V Tedim Cruz MD); Columbia University Irving Medical Center, New York, NY, USA (K Thakur MD); National Neuro Center, Maharajgupi, Kathmandu, Nepal (L Thapa MD); Department of Human Neurosciences, Sapienza University, Rome, Italy (D Toni MD); Department of Neurology, Hacettepe University Hospitals, Ankara, Türkiye (M A Topcuoglu MD); Department of Psychiatry, School of Medical Sciences, National University of Asunción, Asunción, Paraguay (J Torales MD); University of Southern California, Los Angeles, CA, USA (A Towfighi MD); Department of Neurology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (T Truelsen MD); Department of Neurology, Ivane Javakhishvili Tbilisi State University, Tbilisi, Georgia (A Tsiskaridze MD); University of the West Indies, Mona, Jamaica (M Tulloch-Reid MD); Department of Neuroradiology and Radiology, Universidad El Bosque, Bogota, Colombia (J N Useche MD); University Hospital Fundación Santa Fe de Bogotá, Bogota, Colombia (J N Useche); Stroke Unit and Neurovascular Reference Center (L Yperzeele MD), Antwerp University Hospital, Edegem, Belgium (P Vanacker MD); Department of Translational Neurosciences, Antwerp University, Antwerp, Belgium (P Vanacker); First Department of Neurology, National and Kapodistrian University of Athens, Athens, Greece (S Vassilopoulou MD); University Hospital Dubrava, Zagreb, Croatia (G Vukorepa MD); Medical Faculty, University of Rijeka, Rijeka, Croatia (V Vuletic MD); Department of Medicine, University of Ilorin, Ilorin, Nigeria (K W Wahab MD); Beijing Neurosurgical Institute, Beijing, China (W Wang PhD); Department of Neurology, The Sunshine Hospital, St Albans, VIC, Australia (Prof T Wijeratne MD); Polish Institute of Public Health, Warsaw, Poland (B Wojtyniak PhD MD); School of Lifecourse and Population Health Sciences, King’s College London, London, UK (Prof C Wolfe MD); Faculty of Medicine and Pharmaceutical Sciences, University of Douala, Douala, Cameroon (M N Yacouba MD); Department of Internal Medicine, Douala General Hospital, Douala, Cameroon (M N Yacouba); Department of Neurology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology, Chengdu, China (J Yang MD); Department of Emergency Medicine, National Children’s Hospital Dr Carlos Saenz Herrera, San José, Costa Rica (A Yock-Corrales MD); Department of Public Health, Juntendo University School of Medicine, Tokyo, Japan (N Yonemoto PhD).
Footnotes
See Online for appendix
For World Bank income groups see https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2022-2023
For more on the Brain Health Initiative see https://brainhealthinitiative.org/
For more on the NCD Alliance see https://ncdalliance.org
For more on the World Stroke Organization’s roadmap see https://www.wsoroadmap.com/panel/EN/
For more on the WHO global action plan for NCDs see https://www.who.int/teams/noncommunicable-diseases/governance/roadmap
For the Global Burden of Disease study see https://www.healthdata.org/research-analysis/gbd
For more on the Registry of Stroke Care Quality see https://www.qualityregistry.eu/
For more on Safe Implementations in Treatments in Stroke see https://www.sitsinternational.org
For more on the World Stroke Academy see https://www.world-stroke-academy.org/
For more on the Angels Initiative see https://www.angels-initiative.com/
For more on the Global Stroke Alliance see https://www.globalstrokealliance.com/
The Video is available here: https://youtu.be/KWHpEtfbHL0
The Video is also embedded on the Lancet website along with other materials including infographics at www.thelancet.com/commissions/global-burden-stroke
References
- 1.Feigin VL, Stark BA, Johnson CO, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurology 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott CA, Li L, Rothwell PM. Diverging temporal trends in stroke incidence in younger vs older people: a systematic review and meta-analysis. JAMA Neurol 2022; 79: 1036–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mijajlović MD, Pavlović A, Brainin M, et al. Post-stroke dementia—a comprehensive review. BMC Med 2017; 15: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: a 2020 updated review. Gen Hosp Psychiatry 2020; 66: 70–80. [DOI] [PubMed] [Google Scholar]
- 6.Watkins DA, Msemburi WT, Pickersgill SJ, et al. NCD Countdown 2030: efficient pathways and strategic investments to accelerate progress towards the Sustainable Development Goal target 3.4 in low-income and middle-income countries. Lancet 2022; 399: 1266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nugent R, Bertram MY, Jan S, et al. Investing in non-communicable disease prevention and management to advance the Sustainable Development Goals. Lancet 2018; 391: 2029–35. [DOI] [PubMed] [Google Scholar]
- 8.Owolabi MO, Thrift AG, Mahal A, et al. Primary stroke prevention worldwide: translating evidence into action. Lancet Public Health 2022; 7: e74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owolabi MO, Suwanwela NC, Yaria J. Barriers to implementation of evidence into clinical practice in low-resource settings. Nat Rev Neurol 2022; 18: 451–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owolabi MO, Leonardi M, Bassetti C, et al. The neurology revolution. Lancet Neurol 2022; 21: 960–61. [DOI] [PubMed] [Google Scholar]
- 11.Owolabi MO, Leonardi M, Bassetti C, et al. Global synergistic actions to improve brain health for human development. Nat Rev Neurol 2023; 19: 371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ 2016; 94: 634–634A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owolabi MO, Thrift AG, Martins S, et al. The state of stroke services across the globe: report of World Stroke Organization—World Health Organization surveys. Int J Stroke 2021; 16: 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaria J, Gil A, Makanjuola A, et al. Quality of stroke guidelines in low- and middle-income countries: a systematic review. Bull World Health Organ 2021; 99: 640–52E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owolabi MO. Taming the burgeoning stroke epidemic in Africa: stroke quadrangle to the rescue. West Indian Med J 2011; 60: 412–21. [PubMed] [Google Scholar]
- 16.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011; 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. 2013. https://www.who.int/publications/i/item/9789241506236 (accessed Aug 23, 2023).
- 18.Noncommunicable Disease Alliance. NCD Alliance’s submission to the first WHO consultation on the updated Appendix 3 of the Global action plan for the prevention and control of NCDs 2013–2030. https://ncdalliance.org/resources/new-submission-who-global-action-plan-for-the-prevention-and-control-of-noncommunicable-diseases-ncds-2013%E2%80%932030-appendix-3 (accessed Oct 21, 2022).
- 19.Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018; 392: 2052–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 2020; 10: 14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Med 2020; 17: e1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schutte AE, Srinivasapura Venkateshmurthy N, Mohan S, Prabhakaran D. Hypertension in low- and middle-income countries. Circ Res 2021; 128: 808–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UN Department of Economic and Social Affairs. Transforming our world: the 2030 agenda for sustainable development. 2015. https://sdgs.un.org/publications/transforming-our-world-2030-agenda-sustainable-development-17981 (accessed Feb 3, 2023).
- 24.World Economic Forum, WHO. From burden to “best buys”: reducing the economic impact of non-communicable disease in low and middle-income countries. 2011. https://ncdalliance.org/sites/default/files/resource_files/WHO%20From%20Burden%20to%20Best%20Buys.pdf (accessed Feb 3, 2023).
- 25.Smith SC Jr, Collins A, Ferrari R, et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). J Am Coll Cardiol 2012; 60: 2343–48. [DOI] [PubMed] [Google Scholar]
- 26.Bertram MY, Sweeny K, Lauer JA, et al. Investing in non-communicable diseases: an estimation of the return on investment for prevention and treatment services. Lancet 2018; 391: 2071–78. [DOI] [PubMed] [Google Scholar]
- 27.Economist Impact. The value of action. Mitigating the global impact of neurological disorders. 2022. https://impact.economist.com/perspectives/sites/default/files/ei_roche_executive_summary.pdf (acccessed Nov 29, 2022).
- 28.O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016; 388: 761–75. [DOI] [PubMed] [Google Scholar]
- 29.Zhou B, Carrillo-Larco RM, Danaei G, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021; 398: 957–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.New Zealand Ministry of Health. Cardiovascular disease risk assessment and management for primary care. 2018. https://www.health.govt.nz/publication/cardiovascular-disease-risk-assessment-and-management-primary-care (accessed April 26, 2019).
- 31.UK National Institute for Health and Care Excellence. Guideline: hypertension in adults: diagnosis and management. Draft for consultation, March 2019. 2019. https://www.nice.org.uk/guidance/GID-NG10054/documents/draft-guideline (accessed Oct 10, 2022).
- 32.Williams B, Mancia G, Spiering W, et al. 2018. practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension ESC/ESH task force for the management of arterial hypertension. J Hypertens 2018; 36: 2284–309. [DOI] [PubMed] [Google Scholar]
- 33.Whelton PK, Carey RM, Aronow WS, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/AphA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138: e426–83. [DOI] [PubMed] [Google Scholar]
- 34.Herrett E, Gadd S, Jackson R, et al. Eligibility and subsequent burden of cardiovascular disease of four strategies for blood pressure-lowering treatment: a retrospective cohort study. Lancet 2019; 394: 663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brindle P, Emberson J, Lampe F, et al. Predictive accuracy of the Framingham coronary risk score in British men: prospective cohort study. BMJ 2003; 327: 1267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasan RS, Sullivan LM, Wilson PWF, et al. Relative importance of borderline and elevated levels of coronary heart disease risk factors. Ann Intern Med 2005; 142: 393–402. [DOI] [PubMed] [Google Scholar]
- 37.Feigin VL, Brainin M, Norrving B, et al. What is the best mix of population-wide and high-risk targeted strategies of primary stroke and cardiovascular disease prevention? J Am Heart Assoc 2020; 9: e014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feigin VL. Primary stroke prevention: useful thresholds? Lancet Neurol 2022; 21: 116. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf S, Pinto FJ. The polypill: from concept and evidence to implementation. Lancet 2022; 400: 1661–63. [DOI] [PubMed] [Google Scholar]
- 40.WHO. Guideline for the pharmacological treatment of hypertension in adults. Geneva: World Health Organization, 2021. [PubMed] [Google Scholar]
- 41.Rahimi K, Bidel Z, Nazarzadeh M, et al. Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet 2021; 398: 1053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brainin M, Grisold W, Hankey GJ, Norrving B, Feigin VL. Time to revise primary prevention guidelines for stroke and cardiovascular disease. Lancet Neurol 2022; 21: 686–87. [DOI] [PubMed] [Google Scholar]
- 43.Lindsay P, Furie KL, Davis SM, Donnan GA, Norrving B. World Stroke Organization global stroke services guidelines and action plan. Int J Stroke 2014; 9 (suppl A100): 4–13. [DOI] [PubMed] [Google Scholar]
- 44.Bloom DE, Canning D, Hu L, Liu Y, Mahal A, Yip W. The contribution of population health and demographic change to economic growth in China and India. J Comp Econ 2010; 38: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nsubuga P, White ME, Thacker SB, et al. Public health surveillance: a tool for targeting and monitoring interventions. In: Jamison DT, Breman JG, Measham AR, et al. , eds. Disease control priorities in developing countries. Washington, DC: World Bank, 2006: 997–1015. [Google Scholar]
- 46.Truelsen T, Bonita R, Jamrozik K. Surveillance of stroke: a global perspective. Int J Epidemiol 2001; 30 (suppl 1): S11–16. [DOI] [PubMed] [Google Scholar]
- 47.WHO. Intersectoral global action plan on epilepsy and other neurological disorders 2022–2031. 2021. https://www.who.int/publications/m/item/intersectoral-global-action-plan-on-eepilepsy-and-other-neurological-disorders-2022-2031 (accessed Nov 14, 2022). [DOI] [PubMed]
- 48.WHO. Draft intersectoral global action plan on epilepsy and other neurological disorders 2022–2031. 2021. https://cdn.who.int/media/docs/default-source/mental-health/dementia/global-targets-and-indicators_summary.pdf?sfvrsn=33602d19_8 (accessed June 24, 2023).
- 49.Olaiya MT, Sodhi-Berry N, Dalli LL, et al. The allure of big data to improve stroke outcomes: review of current literature. Curr Neurol Neurosci Rep 2022; 22: 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thayabaranathan T, Kim J, Cadilhac DA, et al. Global stroke statistics 2022. Int J Stroke 2022; 17: 946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feigin V, Norrving B, Sudlow CLM, Sacco RL. Updated criteria for population-based stroke and transient ischemic attack incidence studies for the 21st century. Stroke 2018; 49: 2248–55. [DOI] [PubMed] [Google Scholar]
- 52.Aboa-Eboulé C, Mengue D, Benzenine E, et al. How accurate is the reporting of stroke in hospital discharge data? A pilot validation study using a population-based stroke registry as control. J Neurol 2013; 260: 605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Binney LE, Luengo-Fernandez R, Silver LE, Rothwell PM. Temporal trends in the accuracy of hospital diagnostic coding for identifying acute stroke: a population-based study. Eur Stroke J 2020; 5: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.GBD 2019 Ageing Collaborators. Global, regional, and national burden of diseases and injuries for adults 70 years and older: systematic analysis for the Global Burden of Disease 2019 Study. BMJ 2022; 376: e068208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feigin VL, Stark BA, Johnson CO, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray CJL, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1223–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bam K, Olaiya MT, Cadilhac DA, Donnan GA, Murphy L, Kilkenny MF. Enhancing primary stroke prevention: a combination approach. Lancet Public Health 2022; 7: e721–24. [DOI] [PubMed] [Google Scholar]
- 58.US National Center for Health Statistics. About the National Health Interview Survey. 2022. https://www.cdc.gov/nchs/nhis/about_nhis.htm (accessed June 24, 2023).
- 59.Farooq MU, Chaudhry AH, Amin K, Majid A. The WHO STEPwise approach to stroke surveillance. J Coll Physicians Surg Pak 2008; 18: 665. [DOI] [PubMed] [Google Scholar]
- 60.Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018–2030. Eur Stroke J 2018; 3: 309–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.WHO. WHO steps surveillance manual: the WHO stepwise approach to chronic disease risk factor surveillance. Geneva: World Health Organization, 2005. [Google Scholar]
- 62.Sajjad A, Chowdhury R, Felix JF, et al. A systematic evaluation of stroke surveillance studies in low-and middle-income countries. Neurology 2013; 80: 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feigin VL. Stroke in developing countries: can the epidemic be stopped and outcomes improved? Lancet Neurol 2007; 6: 94–97. [DOI] [PubMed] [Google Scholar]
- 64.Rocca WA, Reggio A, Savettieri G, et al. Stroke incidence and survival in three Sicilian municipalities. Ital J Neurol Sci 1998; 19: 351–56. [DOI] [PubMed] [Google Scholar]
- 65.Li SC, Schoenberg BS, Wang CC, Cheng XM, Bolis CL, Wang KJ. Cerebrovascular disease in the People’s Republic of China: epidemiologic and clinical features. Neurology 1985; 35: 1708–13. [DOI] [PubMed] [Google Scholar]
- 66.Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 2017; 135: 759–71. [DOI] [PubMed] [Google Scholar]
- 67.Rothwell PM, Coull AJ, Giles MF, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004; 363: 1925–33. [DOI] [PubMed] [Google Scholar]
- 68.Cabral NL, Cougo-Pinto PT, Magalhaes PSC, et al. Trends of stroke incidence from 1995 to 2013 in Joinville, Brazil. Neuroepidemiology 2016; 46: 273–81. [DOI] [PubMed] [Google Scholar]
- 69.Cadilhac DA, Kim J, Lannin NA, et al. National stroke registries for monitoring and improving the quality of hospital care: a systematic review. Int J Stroke 2016; 11: 28–40. [DOI] [PubMed] [Google Scholar]
- 70.Asplund K, Hulter Åsberg K, Appelros P, et al. The Riks-Stroke story: building a sustainable national register for quality assessment of stroke care. Int J Stroke 2011; 6: 99–108. [DOI] [PubMed] [Google Scholar]
- 71.Nichols EK, Byass P, Chandramohan D, et al. The WHO 2016 verbal autopsy instrument: an international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0. PLoS Med 2018; 15: e1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tran HT, Nguyen HP, Walker SM, Hill PS, Rao C. Validation of verbal autopsy methods using hospital medical records: a case study in Vietnam. BMC Med Res Methodol 2018; 18: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Varkila MRJ, Cremer OL. Is research from databases reliable? Not sure. Intensive Care Med 2019; 45: 122–24. [DOI] [PubMed] [Google Scholar]
- 74.Ozair FF, Jamshed N, Sharma A, Aggarwal P. Ethical issues in electronic health records: a general overview. Perspect Clin Res 2015; 6: 73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Porsdam Mann S, Savulescu J, Sahakian BJ. Facilitating the ethical use of health data for the benefit of society: electronic health records, consent and the duty of easy rescue. Philos Trans Royal Soc Math Phys Eng Sci 2016; 374: 20160130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu K The patient empowerment in the context of electronic health records: a comparison of the legal systems of the enectep union and the United States. Med Law 2018; 37: 595–608. [Google Scholar]
- 77.Mamun Q A conceptual framework of personally controlled electronic health record (PCEHR) system to enhance security and privacy. In: Abawajy J, Choo K-KR, Islam E, Xu Z, Atiquzzaman M, eds. International Conference on Applications and Techniques in Cyber Security and Intelligence: applications and techniques in cyber security and intelligence. London: Springer, 2018: 304–14. [Google Scholar]
- 78.Wilson K, Khansa L. Migrating to electronic health record systems: a comparative study between the United States and the United Kingdom. Health Policy 2018; 122: 1232–39. [DOI] [PubMed] [Google Scholar]
- 79.Owolabi M, Miranda JJ, Yaria J, Ovbiagele B. Controlling cardiovascular diseases in low and middle income countries by placing proof in pragmatism. BMJ Glob Health 2016; 1: e000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathur P, Kulothungan V, Leburu S, et al. National noncommunicable disease monitoring survey (NNMS) in India: estimating risk factor prevalence in adult population. PLoS One 2021; 16: e0246712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mishra A, Malik R, Hachiya T, et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature 2022; 611: 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feigin VL, Nguyen G, Cercy K, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 2018; 379: 2429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021; 52: e364–467. [DOI] [PubMed] [Google Scholar]
- 84.Hankey GJ. Secondary stroke prevention. Lancet Neurol 2014; 13: 178–94. [DOI] [PubMed] [Google Scholar]
- 85.cker V, Heuschmann PU, O’Flaherty M, et al. Twenty-year time trends in long-term case-fatality and recurrence rates after ischemic stroke stratified by etiology. Stroke 2020; 51: 2778–85. [DOI] [PubMed] [Google Scholar]
- 86.Diener H-C, Hankey GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol 2020; 75: 1804–18. [DOI] [PubMed] [Google Scholar]
- 87.Hankey GJ, Spiesser J, Hakimi Z, Carita P, Gabriel S. Time frame and predictors of recovery from disability following recurrent ischemic stroke. Neurology 2007; 68: 202–05. [DOI] [PubMed] [Google Scholar]
- 88.Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative enectepl study. Stroke 2007; 38: 1881–85. [DOI] [PubMed] [Google Scholar]
- 89.Richards A, Jackson NJ, Cheng EM, et al. Derivation and application of a tool to estimate benefits from multiple therapies that reduce recurrent stroke risk. Stroke 2020; 51: 1563–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370: 1432–42. [DOI] [PubMed] [Google Scholar]
- 91.Hobeanu C, Lavallée PC, Charles H, et al. Risk of subsequent disabling or fatal stroke in patients with transient ischaemic attack or minor ischaemic stroke: an international, prospective cohort study. Lancet Neurol 2022; 21: 889–98. [DOI] [PubMed] [Google Scholar]
- 92.Kolmos M, Christoffersen L, Kruuse C. Recurrent ischemic stroke—a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2021; 30: 105935. [DOI] [PubMed] [Google Scholar]
- 93.Drescher C, Buchwald F, Ullberg T, Pihlsgård M, Norrving B, Petersson J. Epidemiology of first and recurrent ischemic stroke in Sweden 2010–2019—a Riksstroke study. Neuroepidemiology 2022; 56: 433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke 2020; 51: 2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Modig K, Talbäck M, Ziegler L, Ahlbom A. Temporal trends in incidence, recurrence and prevalence of stroke in an era of ageing populations, a longitudinal study of the total Swedish population. BMC Geriatr 2019; 19: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim J, Andrew NE, Thrift AG, Bernhardt J, Lindley RI, Cadilhac DA. The potential health and economic impact of improving stroke care standards for Australia. Int J Stroke 2017; 12: 875–85. [DOI] [PubMed] [Google Scholar]
- 97.Hardy ST, Loehr LR, Butler KR, et al. Reducing the blood pressure-related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc 2015; 4: e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brainin M, Feigin VL, Norrving B, Martins SCO, Hankey GJ, Hachinski V. Global prevention of stroke and dementia: the WSO Declaration. Lancet Neurol 2020; 19: 487–88. [DOI] [PubMed] [Google Scholar]
- 99.Gamage DG, Riddell MA, Joshi R, et al. Effectiveness of a scalable group-based education and monitoring program, delivered by health workers, to improve control of hypertension in rural India: a cluster randomised controlled trial. PLoS Med 2020; 17: e1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joseph P, Roshandel G, Gao P, et al. Fixed-dose combination therapies with and without aspirin for primary prevention of cardiovascular disease: an individual participant data meta-analysis. Lancet 2021; 398: 1133–46. [DOI] [PubMed] [Google Scholar]
- 101.Brainin M, Feigin V, Martins S, et al. Cut stroke in half: polypill for primary prevention in stroke. Int J Stroke 2018; 13: 633–47. [DOI] [PubMed] [Google Scholar]
- 102.Feigin VL, Brainin M, Martins SCO. The polypill from a public-health perspective. Lancet Neurol 2023; 22: 294–95. [DOI] [PubMed] [Google Scholar]
- 103.Castellano JM, Pocock SJ, Bhatt DL, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med 2022; 387: 967–77. [DOI] [PubMed] [Google Scholar]
- 104.Feigin VL, Krishnamurthi R, Merkin A, Nair B, Kravchenko M, Jalili-Moghaddam S. Digital solutions for primary stroke and cardiovascular disease prevention: a mass individual and public health approach. Lancet Reg Health West Pac 2022; 29: 100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feigin VL, Owolabi M, Hankey GJ, Pandian J, Martins SC. Digital health in primordial and primary stroke prevention: a systematic review. Stroke 2022; 53: 1008–19. [DOI] [PubMed] [Google Scholar]
- 106.Krishnamurthi R, Barker-Colllo SAT, Bhattacharjee R, et al. Mobile technology for primary stroke prevention: a proof-of-concept pilot randomised controlled trial—a brief report. Stroke 2018; 50: 196–98. [DOI] [PubMed] [Google Scholar]
- 107.Parmar P, Krishnamurthi R, Ikram MA, et al. The Stroke Riskometer app: validation of a data collection tool and stroke risk predictor. Int J Stroke 2015; 10: 231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feigin VL, Krishnamurthi R, Bhattacharjee R, et al. New strategy to reduce the global burden of stroke. Stroke 2015; 46: 1740–47.25882050 [Google Scholar]
- 109.Medvedev O, Truong Q, Merkin A, Borotkanics R, Krishnamurthi R, Feigin V. Cross-cultural validation of the Stroke Riskometer using generalizability theory. Sci Rep 2021; 11: 19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feigin VL, Krishnamurthi R, Medvedev O, et al. Usability and feasibility of PreventS-MD webapp for stroke and cardiovascular disease prevention. Int J Stroke 2023; published online Aug 19. 10.1177/17474930231190745. [DOI] [Google Scholar]
- 111.Adcock AK, Haggerty T, Crawford A, Espinosa C. mHealth impact on secondary stroke prevention: a scoping review of randomized controlled trials among stroke survivors between 2010–2020. mHealth 2022; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamran S, Bener AB, Deleu D, et al. The level of awareness of stroke risk factors and symptoms in the Gulf Cooperation Council countries: Gulf Cooperation Council stroke awareness study. Neuroepidemiology 2007; 29: 235–42. [DOI] [PubMed] [Google Scholar]
- 113.Tibebu NS, Emiru TD, Tiruneh CM, Nigat AB, Abate MW, Demelash AT. Knowledge on prevention of stroke and its associated factors among hypertensive patients at Debre Tabor General Hospital: an institution-based cross-sectional study. Risk Manag Healthc Policy 2021; 14: 1681–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Worthmann H, Schwartz A, Heidenreich F, et al. Educational campaign on stroke in an urban population in northern Germany: influence on public stroke awareness and knowledge. Int J Stroke 2013; 8: 286–92. [DOI] [PubMed] [Google Scholar]
- 115.Brainin M, Teuschl Y, Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol 2007; 6: 553–61. [DOI] [PubMed] [Google Scholar]
- 116.Webb A, Heldner MR, Aguiar de Sousa D, et al. Availability of secondary prevention services after stroke in Europe: an ESO/SAFE survey of national scientific societies and stroke experts. Eur Stroke J 2019; 4: 110–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X, Lu J, Yang Y, et al. Cardiovascular disease prevention and mortality across 1 million urban populations in China: data from a nationwide population-based study. Lancet Public Health 2022; 7: e1041–50. [DOI] [PubMed] [Google Scholar]
- 118.Bayona H, Owolabi M, Feng W, et al. A systematic comparison of key features of ischemic stroke prevention guidelines in low-and middle-income vs high-income countries. J Neurol Sci 2017; 375: 360–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.World Stroke Organization. Post-stroke checklist (PSC): improving life after stroke. 2013. https://www.world-stroke.org/assets/downloads/psc-us-version-wso-livery-03-19-13.pdf (accessed June 24, 2023).
- 120.Willeit P, Toell T, Boehme C, et al. STROKE-CARD care to prevent cardiovascular events and improve quality of life after acute ischaemic stroke or TIA: a randomised clinical trial. EclinicalMedicine 2020; 25: 100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.The Lancet Neurology. WHO launches its Global Action Plan for brain health. Lancet Neurol 2022; 21: 671. [DOI] [PubMed] [Google Scholar]
- 122.OneNeurology. What is OneNeurology? An initiative to address neurology as one. 2022. http://oneneurology.net/about/ (accessed Aug 28, 2021).
- 123.Healthy Brain Initiative, Alzheimer’s Association. Protecting the heart and the brain: a call to action for public health professionals. 2020. https://www.alz.org/media/Documents/executive-summary-healthy-brain-initiative-protecting-heart-and-brain.pdf (accessed June 24, 2023).
- 124.World Heart Federation. The road to UHC: why integration of circulatory health interventions in primary care is essential. 2023. https://world-heart-federation.org/resource/the-road-to-uhc-why-integration-of-circulatory-health-interventions-in-primary-care-is-essential/ (accessed June 24, 2023).
- 125.WHO. WHO Technical Advisory Group of Experts on NCD-related Research and Innovation (TAG-NCD-R&I). 2021. https://www.who.int/groups/who-technical-advisory-group-of-experts-on-ncd-research-and-innovation (accessed June 24, 2024).
- 126.Balsari S, Phadke M, Simon G, Goyal R, Mulholland I. Task shifting in Indian healthcare: reframing the AYUSH debate. Cambridge, MA: Harvard University South Asia Institute, 2018. [Google Scholar]
- 127.Sylaja PN, Singh G, Sivasambath S, et al. Secondary prevention of stroke by a primary health care approach: an open-label cluster randomised trial. J Clin Neurosci 2021; 84: 53–59. [DOI] [PubMed] [Google Scholar]
- 128.Kaur P, Kunwar A, Sharma M, et al. India Hypertension Control Initiative—Hypertension treatment and blood pressure control in a cohort in 24 sentinel site clinics. J Clin Hypertens (Greenwich) 2021; 23: 720–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pandian JD, Kate MP, Sylaja PN, et al. Secondary prevention with a structured semi-interactive stroke prevention package in INDIA (SPRINT INDIA): a multicentre, randomised controlled trial. Lancet Glob Health 2023; 11: e425–35. [DOI] [PubMed] [Google Scholar]
- 130.Feigin VL, Norrving B, Mensah GA. Primary prevention of cardiovascular disease through population-wide motivational strategies: insights from using smartphones in stroke prevention. BMJ Glob Health 2017; 2: e000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsakpounidou K, Tsitiroki A, Keramydas C, Proios H. Digital implementation of originally school-based stroke educational programme in Greece due to the COVID-19 pandemic. Int J Health Promot Educ 2022; published online May 17. 10.1080/14635240.2022.2073552. [DOI] [Google Scholar]
- 132.Pandian JD, Gall SL, Kate MP, et al. Prevention of stroke: a global perspective. Lancet 2018; 392: 1269–78. [DOI] [PubMed] [Google Scholar]
- 133.WHO. Seventy-fifth World Health Assembly. Provisional agenda item 14.1. Follow-up to the political declaration of the third high-level meeting of the General Assembly on the prevention and control of non-communicable diseases. 2022. https://apps.who.int/gb/ebwha/pdf_files/WHA75/A75_10Add8-en.pdf (accessed May 14, 2022).
- 134.Esenwa C, Gutierrez J. Secondary stroke prevention: challenges and solutions. Vasc Health Risk Manag 2015; 11: 437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Graham GD. Secondary stroke prevention: from guidelines to clinical practice. J Natl Med Assoc 2008; 100: 1125–37. [DOI] [PubMed] [Google Scholar]
- 136.Spence JD. Secondary stroke prevention. Nat Rev Neurol 2010; 6: 477–86. [DOI] [PubMed] [Google Scholar]
- 137.Arnett DK, Blumenthal RS, Albert MA, et al. 2019. ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 140: e563–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Visseren FLJ, Mach F, Smulders YM, et al. 2021. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227–337. [DOI] [PubMed] [Google Scholar]
- 139.Diener H-C, Hankey GJ, Easton JD, Lip GYH, Hart RG, Caso V. Non-vitamin K oral anticoagulants for secondary stroke prevention in patients with atrial fibrillation. Eur Heart J Suppl 2020; 22 (suppl I): I13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dawson J, Béjot Y, Christensen LM, et al. European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur Stroke J 2022; 7: I–II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fonseca AC, Merwick Á, Dennis M, et al. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur Stroke J 2021; 6: V. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Emberson J, Whincup P, Morris R, Walker M, Ebrahim S. Evaluating the impact of population and high-risk strategies for the primary prevention of cardiovascular disease. Eur Heart J 2004; 25: 484–91. [DOI] [PubMed] [Google Scholar]
- 143.Eriksen CU, Rotar O, Toft U, Jørgensen T. What is the effectiveness of systematic population-level screening programmes for reducing the burden of cardiovascular diseases? Copenhagen: WHO Regional Office for Europe, 2021. [PubMed] [Google Scholar]
- 144.Navar AM, Fonarow GC, Pencina MJ. Time to revisit using 10-year risk to guide statin therapy. JAMA Cardiol 2022; 7: 785–86. [DOI] [PubMed] [Google Scholar]
- 145.Al-Makki A, DiPette D, Whelton PK, et al. Hypertension pharmacological treatment recommendations in adults. A World Health Organization guidelines executive summary. Hypertension 2022; 79: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaptoge S, Pennells L, De Bacquer D, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019; 7: e1332–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.WHO. Technical package for cardiovascular disease management in primary health care: risk-based CVD management. 2020. https://apps.who.int/iris/bitstream/handle/10665/333221/9789240001367-eng.pdf (accessed Dec 20, 2021).
- 148.Rose G Sick individuals and sick populations. Int J Epidemiol 1985; 14: 32–38. [DOI] [PubMed] [Google Scholar]
- 149.WHO Noncommunicable Diseases and Mental Health Cluster. WHO STEPS stroke manual: the WHO STEPwise approach to stroke surveillance. Geneva, World Health Organization: 2005. [Google Scholar]
- 150.Pandian JD, William AG, Kate MP, et al. Strategies to improve stroke care services in low- and middle-income countries: a systematic review. Neuroepidemiology 2017; 49: 45–61. [DOI] [PubMed] [Google Scholar]
- 151.WHO. WHO package of essential noncommunicable (PEN)disease interventions for primary health care. 2020. https://apps.who.int/iris/handle/10665/334186 (accessed Aug 23, 2023).
- 152.Feigin VL, Owolabi M, Hankey GJ, Pandian J, Martins SC. Digital health in primordial and primary stroke prevention: a systematic review. Stroke 2022; 53: 1008–19. [DOI] [PubMed] [Google Scholar]
- 153.Brainin M, Sliwa K. WSO and WHF joint position statement on population-wide prevention strategies. Lancet 2020; 396: 533–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.UN Development Group. Theory of change: UNDAF campanion guidance. 2017. https://unsdg.un.org/sites/default/files/UNDG-UNDAF-Companion-Pieces-7-Theory-of-Change.pdf (accessed June 13, 2022).
- 155.Mishra A, Malik R, Hachiya T, et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature 2022; 611: 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013; 2013: CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Langhorne P, Ramachandra S. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev 2020; 4: CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Waje-Andreassen U, Nabavi DG, Engelter ST, et al. European Stroke Organisation certification of stroke units and stroke centres. Eur Stroke J 2018; 3: 220–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Te Ao BJ, Brown PM, Feigin VL, Anderson CS. Are stroke units cost effective? Evidence from a New Zealand stroke incidence and population-based study. Int J Stroke 2012; 7: 623–30. [DOI] [PubMed] [Google Scholar]
- 160.Canavan M, Ni Mhaille G, Mulkerrin EC. Development of acute stroke units—a cost effective reconfiguration which benefits patients. QJM 2012; 105: 99–102. [DOI] [PubMed] [Google Scholar]
- 161.Zhai S, Gardiner F, Neeman T, Jones B, Gawarikar Y. The cost-effectiveness of a stroke unit in providing enhanced patient outcomes in an Australian Teaching Hospital. J Stroke Cerebrovasc Dis 2017; 26: 2362–68. [DOI] [PubMed] [Google Scholar]
- 162.Langhorne P, Williams BO, Gilchrist W, Howie K. Do stroke units save lives? Lancet 1993; 342: 395–98. [DOI] [PubMed] [Google Scholar]
- 163.Boulanger JM, Lindsay MP, Gubitz G, et al. Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6 th edition, update 2018. Int J Stroke 2018; 13: 949–84. [DOI] [PubMed] [Google Scholar]
- 164.Stroke Alliance for Europe, King’s College London. The burden of stroke in Europe. 2017. http://www.strokeeurope.eu/downloads/The_Burden_of_Stroke_in_Europe_Report_-_Appendix.pdf (accessed Aug 24, 2023).
- 165.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Campbell BCV, Ma H, Ringleb PA, et al. Extending thrombolysis to 4·5–9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet 2019; 394: 139–47. [DOI] [PubMed] [Google Scholar]
- 167.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010; 41: 2254–58. [DOI] [PubMed] [Google Scholar]
- 168.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–31. [DOI] [PubMed] [Google Scholar]
- 169.Roaldsen MB, Jusufovic M, Berge E, Lindekleiv H. Endovascular thrombectomy and intra-arterial interventions for acute ischaemic stroke. Cochrane Database Syst Rev 2021; 6: CD007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 171.Albers GW, Lansberg MG, Kemp S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke 2017; 12: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–31. [DOI] [PubMed] [Google Scholar]
- 173.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Adeoye O, Nyström KV, Yavagal DR, et al. Recommendations for the establishment of stroke systems of care: a 2019 update. Stroke 2019; 50: e187–210. [DOI] [PubMed] [Google Scholar]
- 175.Zachrison KS, Dhand A, Schwamm LH, Onnela JP. A network approach to stroke systems of care. Circ Cardiovasc Qual Outcomes 2019; 12: e005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schoenfelder J, Zarrin M, Griesbaum R, Berlis A. Stroke care networks and the impact on quality of care. Health Care Manage Sci 2022; 25: 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ouriques Martins SC, Sacks C, Hacke W, et al. Priorities to reduce the burden of stroke in Latin American countries. Lancet Neurol 2019; 18: 674–83. [DOI] [PubMed] [Google Scholar]
- 178.Aref H, Zakaria M, Shokri H, Roushdy T, El Basiouny A, El Nahas N. Changing the Landscape of Stroke in Egypt. Cerebrovasc Dis Extra 2021; 11: 155–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cisse FA, Damien C, Bah AK, et al. Minimal setting stroke unit in a sub-Saharan African public hospital. Front Neurol 2019; 10: 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dos Santos E, Wollmann GM, Nagel V, et al. Incidence, lethality, and post-stroke functional status in different Brazilian macro-regions: the SAMBA study (analysis of stroke in multiple Brazilian areas). Front Neurol 2022; 13: 966785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J 2019; 4: 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.WHO. Assessing national capacity for the prevention and control of NCDs. 2018. http://www.who.int/ncds/surveillance/ncd-capacity/en/ (accessed Oct 16, 2020).
- 183.Mead GE, Sposato LA, Sampaio Silva G, et al. A systematic review and synthesis of global stroke guidelines on behalf of the World Stroke Organization. Int J Stroke 2023; 18: 499–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shahjouei S, Bavarsad-Shahripour R, Assarzadegan F, et al. Acute management of stroke in Iran: obstacles and solutions. Iran J Neurol 2017; 16: 62–71. [PMC free article] [PubMed] [Google Scholar]
- 185.Ince B, Necioglu D. Organization of stroke care in Turkey. Int J Stroke 2017; 12: 105–07. [DOI] [PubMed] [Google Scholar]
- 186.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–418. [DOI] [PubMed] [Google Scholar]
- 187.WHO. WHO model list of essential medicines—22nd list, 2021. 2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed Jan 13, 2023).
- 188.WHO. WHO list of priority medical devices for management of cardiovascular diseases and diabetes. 2021. https://www.who.int/publications/i/item/9789240027978 (accessed March 21, 2023).
- 189.Chimatiro GL, Rhoda AJ. Scoping review of acute stroke care management and rehabilitation in low and middle-income countries. BMC Health Serv Res 2019; 19: 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li Z, Pandian J, Sylaja PN, et al. Quality of care for ischemic stroke in China vs India: findings from national clinical registries. Neurology 2018; 91: e1348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khatib R, Arevalo YA, Berendsen MA, Prabhakaran S, Huffman MD. Presentation, evaluation, management, and outcomes of acute stroke in low- and middle-income countries: a systematic review and meta-analysis. Neuroepidemiology 2018; 51: 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Akinyemi RO, Ovbiagele B, Adeniji OA, et al. Stroke in Africa: profile, progress, prospects and priorities. Nat Rev Neurol 2021; 17: 634–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baatiema L, Otim M, Mnatzaganian G, Aikins AD-G, Coombes J, Somerset S. Towards best practice in acute stroke care in Ghana: a survey of hospital services. BMC Health Serv Res 2017; 17: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.de Souza AC, Sebastian IA, Zaidi WAW, et al. Regional and national differences in stroke thrombolysis use and disparities in pricing, treatment availability, and coverage. Int J Stroke 2022; 17: 990–96. [DOI] [PubMed] [Google Scholar]
- 195.Sundar K, Panwar A, Yagaval DR, Huded V, Sylaja PN. Mission Thrombectomy 2020 (MT2020)—India’s biggest healthcare challenge yet. J Stroke Med 2020; 3: 62–71. [Google Scholar]
- 196.Ouma PO, Maina J, Thuranira PN, et al. Access to emergency hospital care provided by the public sector in sub-Saharan Africa in 2015: a geocoded inventory and spatial analysis. Lancet Glob Health 2018; 6: e342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Al-Rukn S, Mazya M, Akhtar N, et al. Stroke in the Middle-East and North Africa: a 2-year prospective observational study of intravenous thrombolysis treatment in the region. Results from the SITS-MENA Registry. Int J Stroke 2020; 15: 980–87. [DOI] [PubMed] [Google Scholar]
- 198.Martins SCO, Lavados P, Secchi TL, et al. Fighting against stroke in Latin America: a joint effort of medical professional societies and governments. Front Neurol 2021; 12: 743732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Marquez-Romero JM, Góngora-Rivera F, Hernández-Curiel BC, et al. Endovascular treatment of ischemic stroke in a developing country. Vasc Endovascular Surg 2020; 54: 305–12. [DOI] [PubMed] [Google Scholar]
- 200.de Souza AC, Martins SO, Polanczyk CA, et al. Cost-effectiveness of mechanical thrombectomy for acute ischemic stroke in Brazil: results from the RESILIENT trial. Int J Stroke 2021; 17: 17474930211055932. [DOI] [PubMed] [Google Scholar]
- 201.Martins SO, Mont’Alverne F, Rebello LC, et al. Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med 2020; 382: 2316–26. [DOI] [PubMed] [Google Scholar]
- 202.Gongora-Rivera F, Gonzalez-Aquines A, Marquez-Romero JM. Identification of barriers to access endovascular treatment for acute ischemic stroke in the health care system of Mexico: results from a national survey among endovascular neurologists. Front Neurol 2021; 12: 601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Saposnik G, Galanos LC, Guerrero R, et al. The World Stroke Academy: a World Stroke Organization global pathway to improve knowledge in stroke care. Int J Stroke 2022; 17: 17474930221085895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Martins SC, Pontes-Neto OM, Alves CV, et al. Past, present, and future of stroke in middle-income countries: the Brazilian experience. Int J Stroke 2013; 8 (suppl A100): 106–11. [DOI] [PubMed] [Google Scholar]
- 205.Sharma S, Padma MV, Bhardwaj A, Sharma A, Sawal N, Thakur S. Telestroke in resource-poor developing country model. Neurol India 2016; 64: 934–40 [DOI] [PubMed] [Google Scholar]
- 206.Martins SCO, Weiss G, Almeida AG, et al. Validation of a smartphone application in the evaluation and treatment of acute stroke in a comprehensive stroke center. Stroke 2020; 51: 240–46. [DOI] [PubMed] [Google Scholar]
- 207.Hillis JM, Berkowitz AL. Neurology training worldwide. Semin Neurol 2018; 38: 135–44. [DOI] [PubMed] [Google Scholar]
- 208.Chilean Ministry of Health. Ataque cerebrovascular. 2019. https://redcronicas.minsal.cl/ataque-cerebrovascular/ (accessed June 26, 2023).
- 209.Society of Vascular and Interventional Neurology. SVIN2023 annual meeting. Mission Thrombectomy. https://www.svin.org/i4a/pages/index.cfm?pageid=1 (accessed June 26, 2023).
- 210.Hollenbeak CS, Gorton CP, Tabak YP, Jones JL, Milstein A, Johannes RS. Reductions in mortality associated with intensive public reporting of hospital outcomes. Am J Med Qual 2008; 23: 279–86. [DOI] [PubMed] [Google Scholar]
- 211.Global Stroke Alliance. Cerificate your hospital. https://globalstrokealliance.com/en/certification/ (accessed June 26, 2023).
- 212.Liu L, Liu J, Wang Y, Wang D, Wang Y. Substantial improvement of stroke care in China. Stroke 2018; 49: 3085–91. [DOI] [PubMed] [Google Scholar]
- 213.Rose J, Weiser TG, Hider P, Wilson L, Gruen RL, Bickler SW. Estimated need for surgery worldwide based on prevalence of diseases: a modelling strategy for the WHO Global Health Estimate. Lancet Glob Health 2015; 3 (suppl 2): S13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pierot L, Jayaraman MV, Szikora I, et al. Standards of practice in acute ischemic stroke intervention: international recommendations. J Neurointerv Surg 2018; 10: 1121–26. [DOI] [PubMed] [Google Scholar]
- 215.Wechsler LR, Demaerschalk BM, Schwamm LH, et al. Telemedicine quality and outcomes in stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e3–25. [DOI] [PubMed] [Google Scholar]
- 216.Demaerschalk BM, Switzer JA, Xie J, Fan L, Villa KF, Wu EQ. Cost utility of hub-and-spoke telestroke networks from societal perspective. Am J Manag Care 2013; 19: 976–85. [PubMed] [Google Scholar]
- 217.Switzer JA, Demaerschalk BM, Xie J, Fan L, Villa KF, Wu EQ. Cost-effectiveness of hub-and-spoke telestroke networks for the management of acute ischemic stroke from the hospitals’ perspectives. Circ Cardiovasc Qual Outcomes 2013; 6: 18–26. [DOI] [PubMed] [Google Scholar]
- 218.Nelson RE, Okon N, Lesko AC, Majersik JJ, Bhatt A, Baraban E. The cost-effectiveness of telestroke in the Pacific Northwest region of the USA. J Telemed Telecare 2016; 22: 413–21. [DOI] [PubMed] [Google Scholar]
- 219.Sarfo FS, Adamu S, Awuah D, Sarfo-Kantanka O, Ovbiagele B. Potential role of tele-rehabilitation to address barriers to implementation of physical therapy among West African stroke. survivors: a cross-sectional survey. J Neurol Sci 2017; 381: 203–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Institute for Health Metrics and Evaluation. GBD results. 2023. https://vizhub.healthdata.org/gbd-results/ (accessed May 4, 2023).
- 221.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017; 120: 439–48. [DOI] [PubMed] [Google Scholar]
- 222.Luengo-Fernandez R, Paul NL, Gray AM, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke 2013; 44: 2854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke 2004; 35: 731–35. [DOI] [PubMed] [Google Scholar]
- 224.Divani AA, Majidi S, Barrett AM, Noorbaloochi S, Luft AR. Consequences of stroke in community-dwelling elderly: the health and retirement study, 1998 to 2008. Stroke 2011; 42: 1821–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011; 377: 1693–702. [DOI] [PubMed] [Google Scholar]
- 226.Bernhardt J, Borschmann K, Boyd L, et al. Moving rehabilitation research forward: developing consensus statements for rehabilitation and recovery research. Int J Stroke 2016; 11: 454–58. [DOI] [PubMed] [Google Scholar]
- 227.Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017; 12: 451–61. [DOI] [PubMed] [Google Scholar]
- 228.Gupta A, Singh S. Potential role of growth factors controlled release in achieving enhanced neuronal trans-differentiation from mesenchymal stem cells for neural tissue repair and regeneration. Mol Neurobiol 2022; 59: 983–1001. [DOI] [PubMed] [Google Scholar]
- 229.Hu X, Zhou X, Li Y, et al. Application of stem cells and chitosan in the repair of spinal cord injury. Int J Dev Neurosci 2019; 76: 80–85. [DOI] [PubMed] [Google Scholar]
- 230.Dieleman JL, Campbell M, Chapin A, et al. Future and potential spending on health 2015–40: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet 2017; 389: 2005–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dieleman J, Campbell M, Chapin A, et al. Evolution and patterns of global health financing 1995–2014: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet 2017; 389: 1981–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tawa N, Rhoda A, Brink Y, et al. Human functioning, technology and health. Stroke rehabilitation services in Africa—challenges and opportunities: a scoping review of the literature. In: Louw Q, ed. Collaborative capacity development to complement stroke rehabilitation in Africa. Cape Town: Aosis, 2020: 3–54. [Google Scholar]
- 233.WHO. Assessing national capacity for the prevention and control of noncommunicable diseases: report of the 2019 global survey. Geneva: World Health Organization, 2020. [Google Scholar]
- 234.Grecu A, Simsic S, Svobodová V, Mikulík R. Registry for stroke care quality (res-q)—a registry tool for evaluating stroke care quality indices in eastern Europe and western Asia. Prague: European Stroke Organisation Conference, May 16–18, 2023. [Google Scholar]
- 235.Mikulik R, Bar M, Grecu A, et al. The registry of stroke care quality (RES-Q): the first nation-wide data on stroke care quality. J Neurol Sci 2017; 381: 91. [Google Scholar]
- 236.WHO. ATLAS country resources for neurological disorders. 2017. https://www.who.int/publications/i/item/atlas-country-resources-for-neurological-disorders (accessed June 24, 2023).
- 237.Gorthi SP, Garg D. Stroke Epidemiology among young persons in India: every step counts. Ann Indian Acad Neurol 2022; 25: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Owolabi MO, Sarfo F, Akinyemi R, et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Health 2018; 6: e436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Owolabi MO, Akarolo-Anthony S, Akinyemi R, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J S Afr 2015; 26 (suppl 1): S27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Walters R, Collier JM, Braighi Carvalho L, et al. Exploring post acute rehabilitation service use and outcomes for working age stroke survivors (≤65 years) in Australia, UK and South East Asia: data from the international AVERT trial. BMJ Open 2020; 10: e035850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Labberton AS, Barra M, Rønning OM, et al. Patient and service factors associated with referral and admission to inpatient rehabilitation after the acute phase of stroke in Australia and Norway. BMC Health Serv Res 2019; 19: 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Unrath M, Kalic M, Berger K. Who receives rehabilitation after stroke?: Data from the quality assurance project “Stroke Register Northwest Germany”. Dtsch Arztebl Int 2013; 110: 101–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rhoda A, Smith M, Putman K, Mpofu R, DeWeerdt W, DeWit L. Motor and functional recovery after stroke: a comparison between rehabilitation settings in a developed versus a developing country. BMC Health Serv Res 2014; 14: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Roushdy T, Aref H, Kesraoui S, et al. Stroke services in Africa: what is there and what is needed. Int J Stroke 2022; 17: 972–82. [DOI] [PubMed] [Google Scholar]
- 245.Murie-Fernández M, Laxe S. Comparison of stroke neurorehabilitation in Spain and in Europe. Neurología (Engl Ed) 2020; 35: 354–55. [DOI] [PubMed] [Google Scholar]
- 246.Tyagi S, Lim DSY, Ho WHH, et al. Acceptance of tele-rehabilitation by stroke patients: perceived barriers and facilitators. Arch Phys Med Rehabil 2018; 99: 2472–77.e2. [DOI] [PubMed] [Google Scholar]
- 247.Davoody N, Hägglund M. Care professionals’ perceived usefulness of a rehabilitation ehealth service in stroke care. Stud Health Technol Inform 2015; 216: 992. [PubMed] [Google Scholar]
- 248.Wafa HA, Wolfe CDA, Emmett E, Roth GA, Johnson CO, Wang Y. Burden of stroke in enect: thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke 2020; 51: 2418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bernhardt J, Urimubenshi G, Gandhi DBC, Eng JJ. Stroke rehabilitation in low-income and middle-income countries: a call to action. Lancet 2020; 396: 1452–62. [DOI] [PubMed] [Google Scholar]
- 250.Brady MC, Ali M, VandenBerg K, et al. Dosage, intensity, and frequency of language therapy for aphasia: a systematic review-based, individual participant data network meta-analysis. Stroke 2022; 53: 956–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Longley V, Hazelton C, Heal C, et al. Non-pharmacological interventions for spatial neglect or inattention following stroke and other non-progressive brain injury. Cochrane Database Syst Rev 2021; 7: CD003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Platz T, Sandrini G. Specialty grand challenge for neurorehabilitation research. Front Neurol 2020; 11: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.WHO. Package of interventions for rehabilitation. 2023. https://www.who.int/activities/integrating-rehabilitation-into-health-systems/service-delivery/package-of-interventions-for-rehabilitation (accessed June 24, 2023).
- 254.He M, Wang J, Dong Q, et al. Community-based stroke system of care improves patient outcomes in Chinese rural areas. J Epidemiol Community Health 2018; 72: 630–35. [DOI] [PubMed] [Google Scholar]
- 255.Niama Natta DD, Lejeune T, Detrembleur C, et al. Effectiveness of a self-rehabilitation program to improve upper-extremity function after stroke in developing countries: a randomized controlled trial. Ann Phys Rehabil Med 2021; 64: 101413. [DOI] [PubMed] [Google Scholar]
- 256.Zhou B, Zhang J, Zhao Y, et al. Caregiver-delivered stroke rehabilitation in rural China. Stroke 2019; 50: 1825–30. [DOI] [PubMed] [Google Scholar]
- 257.Liu H, Lindley R, Alim M, et al. Family-led rehabilitation in India (ATTEND)—findings from the process evaluation of a randomized controlled trial. Int J Stroke 2019; 14: 53–60. [DOI] [PubMed] [Google Scholar]
- 258.Olaleye OA, Hamzat TK, Owolabi MO. Development and evaluation of the primary healthcare-based physiotherapy intervention and its effects on selected indices of stroke recovery. Int J Ther Rehabil 2013; 20: 443–49. [Google Scholar]
- 259.Olaleye OA, Hamzat TK, Owolabi MO. Stroke rehabilitation: should physiotherapy intervention be provided at a primary health care centre or the patients’ place of domicile? Disabil Rehabil 2014; 36: 49–54. [DOI] [PubMed] [Google Scholar]
- 260.Kuo LM, Huang HL, Huang HL, et al. A home-based training program improves Taiwanese family caregivers’ quality of life and decreases their risk for depression: a randomized controlled trial. Int J Geriatr Psychiatry 2013; 28: 504–13. [DOI] [PubMed] [Google Scholar]
- 261.Vloothuis JD, Mulder M, Veerbeek JM, et al. Caregiver-mediated exercises for improving outcomes after stroke. Cochrane Database Syst Rev 2016; 12: CD011058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cramer SC, Dodakian L, Le V, et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: a randomized clinical trial. JAMA Neurol 2019; 76: 1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hempler I, Maun A, Kampling H, Thielhorn U, Farin E. Poststroke care in Germany: results of an online survey of inpatient and outpatient experts in southern Germany. Nervenarzt 2019; 90: 824–31 (in German). [DOI] [PubMed] [Google Scholar]
- 264.Hempler I, Woitha K, Thielhorn U, Farin E. Post-stroke care after medical rehabilitation in Germany: a systematic literature review of the current provision of stroke patients. BMC Health Serv Res 2018; 18: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ekechukwu END, Olowoyo P, Nwankwo KO, et al. Pragmatic solutions for stroke recovery and improved quality of life in low- and middle-income countries—a systematic review. Front Neurol 2020; 11: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Platz T Evidence-based guidelines and clinical pathways in stroke rehabilitation—an international perspective. Front Neurol 2019; 10: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hill G, Regan S, Francis R, et al. Research priorities to improve stroke outcomes. Lancet Neurol 2022; 21: 312–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Stroke Foundation. Living stroke guidelines. https://strokefoundation.org.au/what-we-do/for-health-professionals/living-stroke-guidelines (accessed June 26, 2023).
- 269.Platz T Clinical pathways in stroke rehabilitation: evidence-based clinical practice recommendations. Cham: Springer: 2021. [PubMed] [Google Scholar]
- 270.Lo SHS, Chang AM, Chau JPC. Stroke self-management support improves survivors’ self-efficacy and outcome expectation of self-management behaviors. Stroke 2018; 49: 758–60. [DOI] [PubMed] [Google Scholar]
- 271.Jones KM, Bhattacharjee R, Krishnamurthi R, et al. Determining the feasibility and preliminary efficacy of a stroke instructional and educational DVD in a multinational context: a randomized controlled pilot study. Clin Rehabil 2018; 32: 1086–97. [DOI] [PubMed] [Google Scholar]
- 272.WHO. Rehabilitation competency framework. 2021. https://www.who.int/teams/noncommunicable-diseases/sensory-functions-disability-and-rehabilitation/rehabilitation-competency-framework (accessed June 24, 2023).
- 273.WHO. Routine health information systems—rehabilitation toolkit. 2022. https://www.who.int/activities/integrating-rehabilitation-into-health-systems/routine-health-information-systems---rehabilitation-toolkit (accessed June 24, 2023).
- 274.Owolabi MO, Leonardi M, Bassetti C, et al. The neurology revolution. Lancet Neurol 2022; 21: 960–61. [DOI] [PubMed] [Google Scholar]
- 275.Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 459–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Feigin VL, Vos T, Nichols E, et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 2020; 19: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.The Lancet Neurology. A decisive year for the neurological community. Lancet Neurol 2022; 21: 103. [DOI] [PubMed] [Google Scholar]
- 278.Guekht A, Brodie M, Secco M, Li S, Volkers N, Wiebe S. The road to a World Health Organization global action plan on epilepsy and other neurological disorders. Epilepsia 2021; 62: 1057–63. [DOI] [PubMed] [Google Scholar]
- 279.Wiebers DO, Feigin VL, Winkler AS. Brain health, One Health, and COVID-19. Neuroepidemiology 2021; 55: 425–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wiebers DO, Feigin VL, Winkler AS. All life protection and our collective future. Neuroepidemiology 2022; 56: 147–50. [DOI] [PubMed] [Google Scholar]
- 281.WHO. Rehabilitation 2030 Initiative. https://www.who.int/initiatives/rehabilitation-2030 (accessed March 24, 2023).
- 282.WHO. Invisible numbers: the true extent of noncommunicable diseases and what to do about them. 2022. https://apps.who.int/iris/handle/10665/362800 (accessed Nov 14, 2022).
- 283.WHO. One Health. 2017. https://www.who.int/news-room/questions-and-answers/item/one-health (accessed Jan 13, 2023).
- 284.UN Environment Programme. One Health joint plan of action to address health threats to humans, animals, plants and environment. 2022. https://www.unep.org/news-and-stories/press-release/one-health-joint-plan-action-address-health-threats-humans-animals (accessed Jan 13, 2023).
- 285.UN Environment Programme. One Health Joint Plan of Action (2022–2026). 2022. https://www.unep.org/resources/publication/one-health-joint-plan-action-2022-2026 (accessed Jan 13, 2023).
- 286.Amorín I, Alessi C, Angellsen SN, et al. Optimizing brain health across the life course: WHO position paper. Geneva: World Health Organization, 2022. [Google Scholar]
- 287.Akinyemi R, Sarfo F, Abd-Allah F, et al. Conceptual framework for establishing the African Stroke Organization. Int J Stroke 2021; 16: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Owolabi M, Sarfo F, Akinyemi R, Gebreyohanns M, Ovbiagele B. The Sub-Saharan Africa Conference on Stroke (SSACS): an idea whose time has come. J Neurol Sci 2019; 400: 194–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Stuifbergen AK, Gordon D, Clark AP. Health promotion: a complementary strategy for stroke rehabilitation. Top Stroke Rehabil 1998; 5: 11–18.26368582 [Google Scholar]
- 290.Urimubenshi G, Langhorne P, Cadilhac DA, Kagwiza JN, Wu O. Association between patient outcomes and key performance indicators of stroke care quality: a systematic review and meta-analysis. Eur Stroke J 2017; 2: 287–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Grube MM, Dohle C, Djouchadar D, et al. Evidence-based quality indicators for stroke rehabilitation. Stroke 2012; 43: 142–46. [DOI] [PubMed] [Google Scholar]
- 292.Hubbard IJ, Harris D, Kilkenny MF, Faux SG, Pollack MR, Cadilhac DA. Adherence to clinical guidelines improves patient outcomes in Australian audit of stroke rehabilitation practice. Arch Phys Med Rehabil 2012; 93: 965–71. [DOI] [PubMed] [Google Scholar]
- 293.Ntsiea MV. Current stroke rehabilitation services and physiotherapy research in South Africa. S Afr J Physiother 2019; 75: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


