Abstract
A novel engineered CCL20 locked dimer (CCL20LD) is nearly identical to the naturally occurring chemokine CCL20 but blocks CCR6-mediated chemotaxis and offers a new approach to treat the diseases of psoriasis and psoriatic arthritis. Methods for quantifying CCL20LD serum levels are needed to assess pharmacokinetics parameters and evaluate drug delivery, metabolism, and toxicity. Existing ELISA kits fail to discriminate between CCL20LD and the natural chemokine, CCL20WT (the wild type monomer). Herein, we tested several available CCL20 monoclonal antibodies to be able to identify one clone that can be used both as a capture and a detection antibody (with biotin-labeling) to specifically detect CCL20LD with high specificity. After validation using recombinant proteins, the CCL20LD-selective ELISA was used to analyze blood samples from CCL20LD treated mice, demonstrating the utility of this novel assay for preclinical development of a biopharmaceutical lead compound for psoriatic disease.
Keywords: ELISA, Psoriasis, Cytokine, Locked dimer
1. Introduction
Together with its receptor CCR6, the chemokine CCL20 plays a critical role in the development of psoriasiform dermatitis in mouse models (Cochez et al., 2017; Furue et al., 2020; Onuora, 2021). We previously screened a panel of CCL20 variants engineered to form dimers stabilized by intermolecular disulfide bonds. A single-atom substitution yielded a CCL20 variant (CCL20 S64C), later designated CCL20 locked dimer (CCL20LD), that could act as a partial, biased agonist for the chemokine receptor CCR6. Binding of CCL20LD to CCR6 resulted in G-protein receptor activation, evidenced by induction of intracellular calcium release, while exhibiting minimal chemotactic activity. Instead, CCL20LD was able to inhibit CCR6-mediated T cell trafficking with nominal impacts on other chemokine receptor signaling cascades (Getschman et al., 2017).
When administered in an IL23–dependent mouse model for psoriasis, CCL20LD prevented psoriatic inflammation and blocked upregulation of IL17A and IL22 signaling. Specific targeting of the CCR6/CCL20 axis with CCL20LD led to therapeutic reductions in entheseal inflammation as well as clinical and proinflammatory markers in the joints and skin of IL23 MC–treated mice, validating CCR6 as a potential therapeutic target for psoriasis and demonstrating the value of CCL20LD as a lead therapeutic compound (Shi et al., 2021).
To effectively prepare CCL20LD for therapeutic development, investigations into pharmaceutical kinetics (PK), drug delivery methods, and potential toxicities are needed (Tonge, 2018). Therefore, a specific ELISA assay for detecting CCL20LD levels in the serum would provide a much needed tool for drug development. Given the nearly identical composition of CCL20LD compared to the WT molecule, commercially available ELISA assays cannot differentiate the CCL20LD variant from the wild-type monomer, CCL20WT, which can be elevated in inflammatory conditions such as psoriasis (Furue et al., 2020). Herein, we develop an ELISA assay that is capable of differentiating CCL20LD from CCL20WT and, thus, quantifying the level of CCL20LD in serum of mice (and presumably humans) in order to perform PK studies (Tonge, 2018).
2. Methods
2.1. Reagents
Highly purified CCL20 wild type monomer (CCL20WT) and CCL20 locked dimer (CCL20LD) proteins were obtained from XLock Biosciences, LLC (XL), West Allis, WI. Purified anti-human CCL20 (MIP-3α) antibody was purchased from Biolegend (Clone L2412F11, San Diego, CA). Human MIP-3 α / CCL20 ELISA Kits were purchased from Sigma-Aldrich (RAB0059, Sigma-Aldrich, St. Louis, MO). Biotin Conjugation Kits (Fast, Type A) - Lightning-Link® (ab201795) were purchased from Abcam (Waltham, MA). Other CCL20 antibodies used in the experiments include products from SinoBiology US In. (Houston, TX), such as clone #21, Cat: 10485-MM21 and Clone #07, Cat: 10485-MM07.
2.2. Human plasma specimens
Stored human plasma specimens for broad research purposes, including six psoriasis patients and six healthy controls, were obtained from the Department of Dermatology, UCDAVIS. Our research using human specimens has been reviewed and approved by UCDAVIS Institutional Review Board (IRB).
2.3. Animals and CCL20LD in vivo delivery
Eight- to 10-week-old female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and utilized in line with institutionally approved animal protocols (University of California Davis, Sacramento, California). To detect serum levels of CCL20LD, each mouse was treated with 400 μg of CCL20LD dissolved in 200 μl of PBS via intraperitoneal, intravenous, or subcutaneous injection. Mice were euthanized after two hours of incubation, followed immediately by a blood draw from the heart. Whole blood was transferred to serum collection tubes (Micro tube 1.1 ml Z-Gel, SARSTEDT, Inc. Newton NC) and left undisturbed for 10 min, followed by centrifugation at 2500 rpm. The resulting supernatant was designated serum for either direct use in ELISA assay or stored as aliquots at −80 °C degree for later use.
2.4. A modified sandwich ELISA using a single unlabeled and biotin-label clone of a CCL20 specific mAb as capture and detection antibodies
Standard steps of a traditional sandwich ELISA were followed, with the key exception of use of identical clone antibodies for both capture and detection antibodies. A commercially available purified anti-human CCL20 (MIP-3α) antibody, Clone L2412F11 (described above), was coated in 96-well plates as a capture antibody, followed by incubation with CCL20LD test specimens at different concentrations or in unknown quantities from serum of mice that had been injected with CCL20LD. The same clonal anti-human CCL20 antibody was biotin-labeled and incubated (details below) and subsequently used as the detection antibody. The addition of streptavidin-HRP conjugate and substrate TMB (3,3′5,5’-Tetramethylbenzidine) yielded a yellow color, with a λ-max of 450 nm for evaluation with a plate reader (Bio-Rad, Hercules, California). Please be noted, the blocking buffer, diluent, Avidin-HRP, substrate TMB, and stop solution are all purchased from Invitrogen (Thermo-Fisher Scientific. Waltham, MA).
2.5. Biotin labeling
Following the product guidelines of the Biotinylation Kit / Biotin Conjugation Kit (Fast, Type A) - Lightning-Link® (ab201795, Waltham, MA), we conjugated biotin to the purified anti-human CCL20: Biolegend 534,502, 0.5 mg/ml, clone: L2412F11. A modifier mixture containing the biotin label was added to the antibody and incubated for 15 min, followed by addition of a quencher and further incubation for 5 min. No further purification was required and 100% of the antibody was recovered for immediate use.
2.6. Statistical analysis
Graph Pad Prism software was used to perform statistical analysis of the data.
3. Results
3.1. A modified ELISA assay to differentially detect CCL20 locked dimers but not CCL20 monomer
A sandwich ELISA measures antigen bound to two layers of antibodies (so-called capture and detection antibodies). The target antigen must contain at least two antigenic sites (epitopes) capable of binding to antibodies (Fig. 1A. a). Monoclonal or polyclonal antibodies can be used as the capture and detection antibodies in sandwich ELISA systems. Monoclonal antibodies recognize a single epitope that allows quantification of small differences in antigen. Sandwich ELISA assays can be difficult to optimize and are often validated after selecting match-paired antibodies. This ensures that the antibodies detect different epitopes on the target protein and do not interfere with the other antibody binding (Fig. 1A). We tested several commercially available ELISA kits for human CCL20. The one from Sigma-Aldrich, as shown (Fig. 1A. b), generated a standard curve of 0.8–600 pg/ml, and proved to be a sensitive and specific ELISA kit for CCL20. However, it cannot distinguish between WT CCL20 monomer and CCL20LD (Suppl. Fig. 1).
Fig. 1.
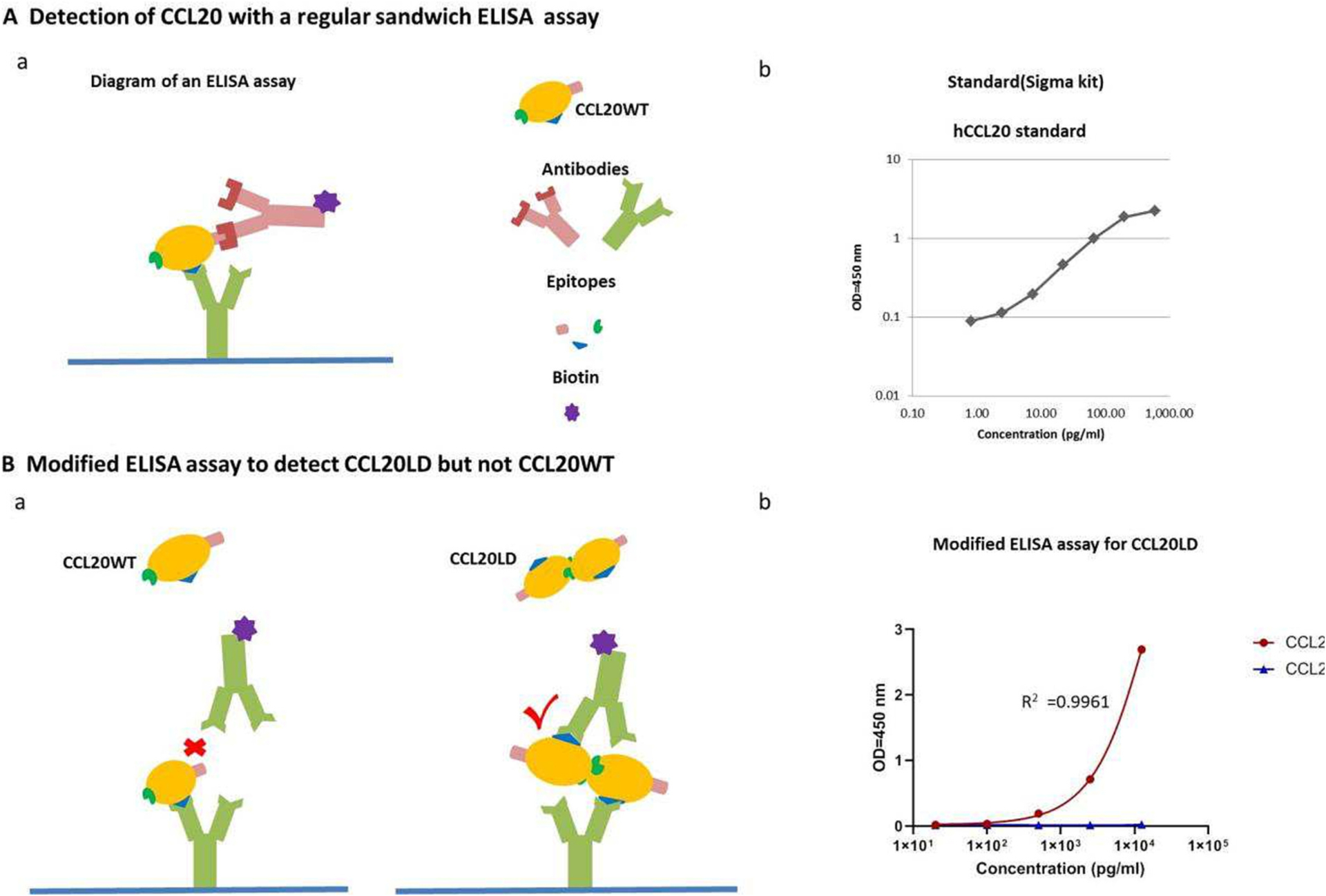
ELISA diagrams and modification for CCL20LD differentiation. A. Diagram (a) and an experimental example (b) for a traditional ELISA assay to detect cytokine human CCL20. B. (a) Diagram for how a modification of ELISA assay to detect CCL20LD but not CCL20WT. (b) Modified ELISA assay was performed for CCL20 LD and CCL20WT with concentrations ranging from 20 pg/ml to 62,500 pg/ml. The OD value versus concentration for both CCL20 WT and CCL20LD is shown in an interpolated standard curve generated by second order polynomial.
Based on the Sigma-Aldrich ELISA kit, we developed a modified assay for isolating CCL20LD by solely using the capture antibody, Clone L2412F11, and discarding the original polyclonal detection antibody. Instead, we labeled (using a commercial biotin-labeling kit) the same anti-CCL20 Clone L2412F11 with biotin for use as the detection antibody. As predicted (Fig. 1B, a), the combination of purified Clone L2412F11 and its biotin labeled partner was not able to detect CCL20 monomer, however, it readily detected CCL20LD (Fig. 1B, b). Of note, that other clones of CCL20 antibodies such as Clone #21(10485-MM21) and Clone #07 (10485-MM07) from Sino Biological, failed with this strategy, potentially indicating the importance of specific epitope and antibody binding in establishing this strategy.
3.2. Validation and establishment of the concentration range for CCL20LD ELISA
CCL20 and CCL20LD were prepared via serial dilution to achieve a concentration range of 20 pg/ml to 62.5 ng/ml respectively (Suppl. Fig. 2). The new assay was designed to show detectable elevation of ELISA signals for CCL20LD at concentrations above 100 pg/ml, while with clear saturations for concentrations above 12.5 ng/ml. In the range of 100 pg/ml to 12.5 ng/ml, the CCL20 from XLock was not detectable at all, validating the specificity of our assay for the locked dimer. We utilized a second order polynomial to interpolate a standard curve (GraphPad) after removing the concentration point at 62.5 ng/ml, generating a closely fit standard curve for CCL20LD (R2 = 0.9961) (Fig. 1B, b). Because CCL20 monomers generated a slight positive signal at a concentration of 62.5 ng/ml, it was removed from the standard curve analysis as well, which sets up the concentrations between 100 pg/ml and 12.5 ng/ml as a good dynamic range.
3.3. Interference assessments for CCL20LD ELISA assay applied with blood samples
To test if our modified ELISA assay could measure CCL20LD in blood specimens, we ran an additional ELISA for CCL20LD mixed with mouse serum. CCL20LD was again prepared via serial dilutions as described in 2.2; however, extra groups of CCL20LD serial dilutions were made, in parallel, containing mouse serum at 1/2, 1/5, 1/10, and 1/20 (v/v) (Fig. 2A, a) to assess for “Matrix Interference”, which indicates that the complex sample matrix, such as a human blood sample, contains a different mix of interfering factors that affect the accuracy and recovery (Waritani et al., 2017). Although the resulting standard curves generated from serum mixed assays were similar in shape to the initial assay without serum, the addition of larger volumes (1/2 and 1/5) of serum in the samples generally resulted in higher OD values than those with same amount of CCL20LD in serum-free diluent. The OD curve generated from the ½ serum group shifted to the left mostly, indicating serum existence results in high assay background. However, matrix interference seemed to diminish with higher dilution folds, i.e., 1/10 or 1/20, for serum samples (Fig. 2A, a).
Fig. 2.
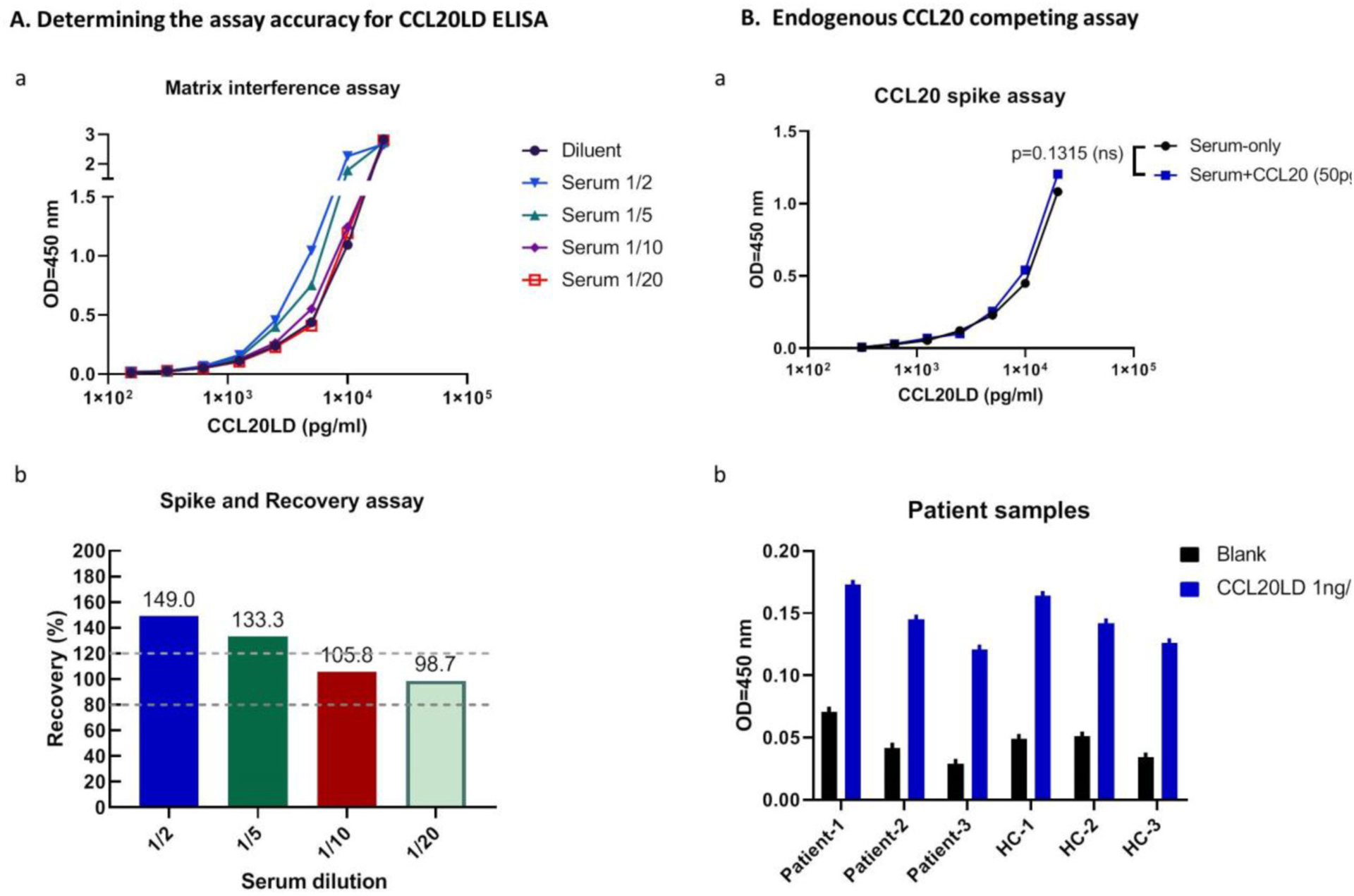
Assay accuracy and interference assessment for CCL20LD ELISA assay. A. (a) Comparison of OD values of CCL20LD in serum mixed at different ratios with standard diluent. Serial diluted CCL20LD in different matrix diluents, i.e., serum/diluent mixtures at the ratios of ½, 1/5, 1/10, or 1/20 were tested by CCL20LD ELISA to identify the Matrix interference. (b) Recovery of spiked CCL20LD in serum samples at different serum dilutions. Recovery rate is calculated for the percent of measured concentration in serum mixed samples to the expected concentration in diluent (percent values shown for the calculation with CCL20LD spiked at 5 ng/ml). Two dotted lines define the acceptable recovery rate between 80 and 120%. B. Probability evaluation of endogenous CCL20 interference from psoriatic specimen. (a) ELISA assay for CCL20LD in mouse serum spiked with and without CCL20 (50 pg/ml). OD values for serial diluted CCL20LD, ranging between 0 pg/ml and 12.5 ng/ml, showed no significant difference between the two groups (p = 0.1315). (b) CCL20LD ELISA assay was performed for plasma samples composed of both psoriasis patients and healthy controls (n = 3 in each group). OD values were shown for each sample spiked with, or without, CCL20LD protein.
Recoveries were then calculated for the above data to determine the accuracy of the modified assay on serum samples (Fig. 2A. b). At a known CCL20LD concentration, e.g., at 5 ng/ml level, the analyzed concentrations for serum added at ½, 1/5, 1/10 and 1/20 were calculated against the standard curve generated on using regular diluent. The percentage of the measured concentration to the expected concentration in diluent, defined as recovery, is considered acceptable when between 80 and 120% and indicates the matrix affect has been overcome. In the results (Fig. 2A. b), 1/2 and 1/5 dilution led to recoveries outside this range, indicating interference from serum matrix. Comparatively, 1/10 and 1/20 dilutions showed acceptable recovery rates (105.8% and 98.7% respectively), showing that serum samples can be accurately assayed with at least 1/10 dilution.
3.4. Evaluation of possible endogenous CCL20 interference from psoriatic specimen
Based on reports that patients with psoriasis have elevated CCL20 in serum, we considered the possibility that elevated endogenous CCL20 will compete with the therapeutic CCL20LD for the capture antibody, therefore leading to a falsely decreased CCL20LD serum concentration. In order to evaluate if this possibility will be problematic in the application of CCL20LD ELISA in psoriasis patients, we spiked mouse serum with CCL20 and compared the OD values with non-spiked serum for serially diluted CCL20LD. According to our quantification of plasma levels of CCL20 from patients with psoriasis (Suppl. Fig. 3) and a report from another research group (Ekman et al., 2013), 50 pg/ml represents the maximum level of CCL20 in psoriasis patients. By running the ELISA assay for CCL20LD in mouse serum spiked with and without CCL20, we found that the OD values for the serially diluted CCL20LD, ranging between 100 pg/ml and 12.5 ng/ml, showed no significant difference (p = 0.1315, Fig. 2B. a). To further confirm the ability to detect CCL20LD in patient samples, we spiked a known concentration, i.e., 1000 pg/ml, of CCL20LD to six human plasma samples composed of both psoriasis patients and healthy controls. By performing a modified CCL20LD ELISA assay, we observed equally elevated OD values in spiked versus non-spiked samples, regardless of patient or healthy control status (Fig. 2B. b). These data confirm that the actual concentration of CCL20 in psoriasis patients is insufficient to saturate the binding capacity of CCL20LD to capture antibodies and affect the detection of CCL20LD.
3.5. Application of the CCL20LD ELISA for measuring levels of CCL20LD in mouse serum and calculation of serum in vivo half-life
For preliminary assessments of detecting CCL20LD levels in mice, we injected 400 micrograms of CCL20LD intravenously, a known therapeutic dosage demonstrated in our previous studies (Shi et al., 2021). We then collected serum from whole blood via cardiac puncture two hours after CCL20LD administration. Compared to the control mice, CCL20LD-injected mice displayed high ELISA signals with serum levels between 50 and 100 ng/ml after calculation (Fig. 3B). In addition to intravenous (IV) administration, we also re-performed the assay to detect serum levels of CCL20LD in mice injected through subcutaneous (SC) and intraperitoneal (IP) routes. Both methods showed equivalent systemic delivery of CCL20LD to the IV route (Fig. 3C). Serum levels were also measured at multiple time points (0, 1, 8, and 24 h) after injection of CCL20LD via SC for pharmacokinetic analysis. Half-life was calculated by fitting the natural logarithm of concentration by time, which resulted in a half-life of 6 ± 2 h (fitted data are shown in Fig. 3D).
Fig. 3.
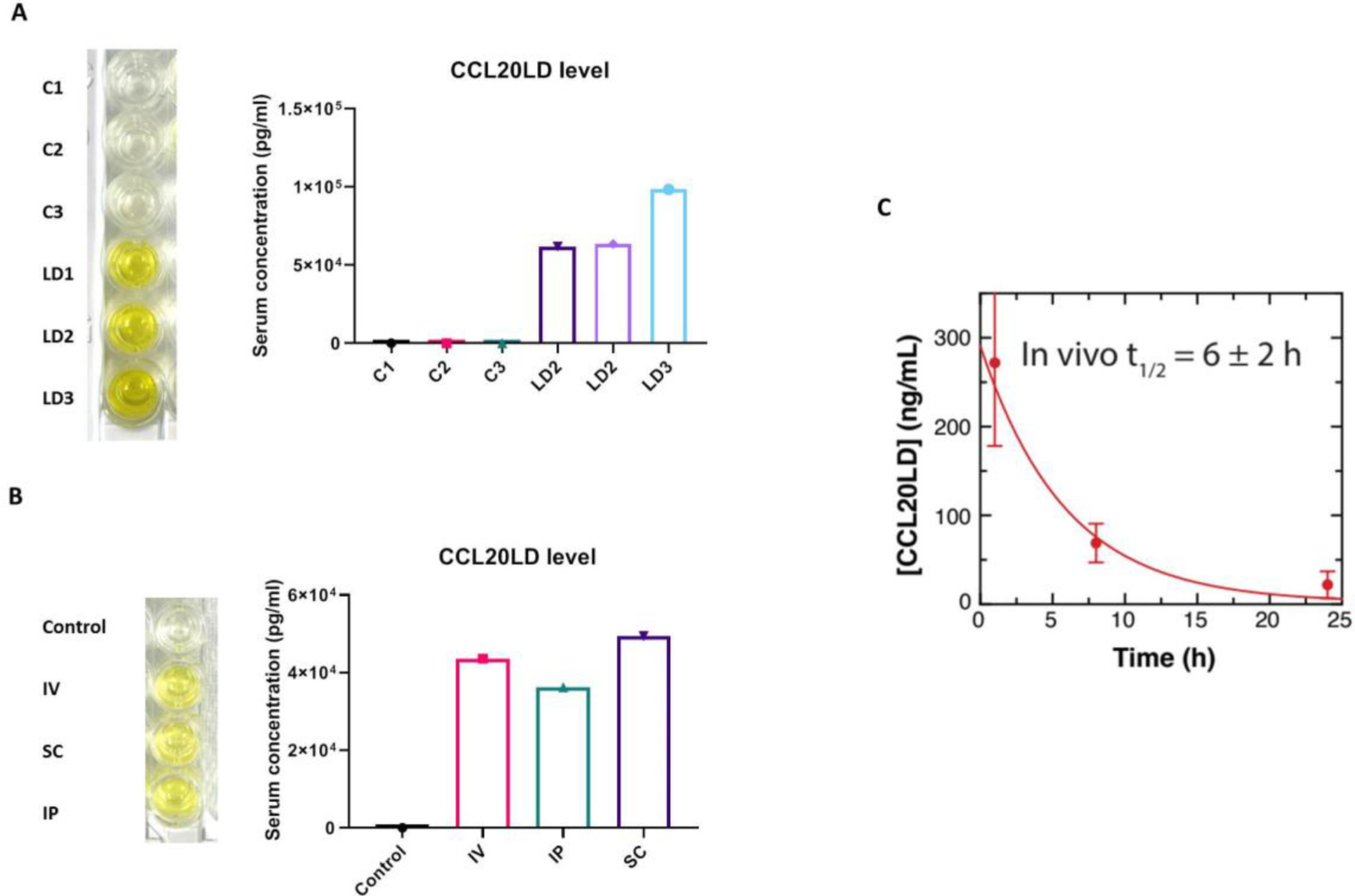
Application of CCL20LD ELISA assay for mouse in vivo study. A. CCL20LD was injected via IV with the dose of 400μg per mouse. Serum is collected at 2 h after administration followed by CCL20LD ELISA assay. Serum concentration is shown for both CCL20LD injected mice and un-injected controls (n = 3). B. CCL20LD ELISA data for serum samples from mice treated with CCL20LD through different routes, i.e., intravenous (IV), subcutaneous (SC), and intraperitoneal (IP). C. CCL20LD Half-life determination by collecting mouse serum at multiple time points (0, 1, 8, 24 h) after SC delivery (n = 3 mice per time point). Blood was collected through cardiac puncture at time indicated.
4. Discussion
CCL20LD is a prospective therapeutic drug for the treatment of psoriasis (Shi et al., 2021), but pharmacokinetic, dosing, and safety studies that require precisely measuring CCL20LD in serum (and possibly other tissues) in vivo are necessary for further preclinical development. The ELISA assay has been widely used for detecting cytokines, such as CCL20, in the serum. In the current study, since conventional CCL20 ELISA kits cannot distinguish between CCL20LD and WT CCL20 monomer, we modified the commercially available ELISA assay for CCL20 to enable selective detection of CCL20LD to enable PK analysis of drug half-life in mice or in humans.
The theoretical basis for this modified assay is the unique antigenic epitope on monomeric CCL20, which enables recognition and binding by a selected antibody. Once occupied, the WT CCL20 molecule no longer possesses any remaining binding sites for the same antibody (Abbott et al., 2014; Nilvebrant and Rockberg, 2018). The dimer-based CCL20LD, however, has another available epitope that is available to bind a labeled version of the capture antibody.
Epitope and solvent factors that affect the accessibility of a protein to an antibody are major considerations for establishing an accurate and valid ELISA assays. Different antigenicity of monomers, dimers, or higher order complex was reported on the impact of protein conformations for ELISA sensitivity and practicality. Khan WH, et al. demonstrated significant impacts of dimerization of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) N-protein on ELISA-based diagnostics (Khan et al., 2022). Dimerization stabilized proteins in solution from burying hydrophobic residues, which resulted in duplicated epitope numbers in the protein dimers and increased assay sensitivity (Khan et al., 2022). In our specialized strategy of focused on epitope features, we differentiate CCL20 dimers from monomers based on epitope binding specificities. To the best of our knowledge, this is the first demonstration of an ELISA based-assay approach to detect protein dimers, but not monomers (both of which have more on 99% similarity in amino acid sequence). Indeed, there are still unsatisfactory quality issues regarding the modified CCL20LD ELISA assay in its current status. The LOD (Limit of detection) in the modified assay was determined to be 312.5 pg/ml at under current conditions (Suppl. Fig. 4), and is not satisfactorily sensitive. Moreover, the required 1/10 dilution for assessing serum samples may further impact the evaluation of dynamic CCL20LD at lower amount. We acknowledge that this leaves a problem for us to work towards a solution.
A search of the literature helped us identify of possible approaches to further improve this assay, which include setting up extra plate-well controls to eliminate background noise caused by hydrophobic binding of immunoglobulin components in blood sample specimens to solid surfaces (Waritani et al., 2017). Also, further identifying superior blocking agents and optimizing the buffer/diluent components can be critical to eliminate a variety of false positive and negative reactions caused by non-specific binding of immunoglobulins to target-antigens.
In summary, our studies validate a modified ELISA with high specificity to detect the CCL20LD molecule with minimal reactivity to WT CCL20 which, in theory, may be present at the same time in vivo. This method will be readily used in preclinical studies to collect PK and pharmacodynamic (PD) data on CCL20LD in vivo and to establish therapeutic dose-exposure-response relationships.
Supplementary Material
Acknowledgment
This work was supported by NIH SBIR grant AR074363 and National Psoriasis Foundation Bridge grant A22-1304-001. We thank Dr. Zhenrui Shi (Dept. Dermatology, Sun Yat-sen University, China) for her critical comments and suggestions in every aspects of this study.
Abbreviation:
- ELISA
Enzyme-linked immunosorbent assay
- CCL20
C-C motif chemokine ligand 20
- CCL20LD
C-C motif chemokine ligand 20 locked dimer
- IL23MC
IL23 minicircle DNA
Footnotes
Declaration of Competing Interest
STH, BFV, MBD, WFC, FCP, and CAK have financial interest in and are officers of XLock Biosciences, which produces the CCL20LD.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jim.2023.113453.
Data availability
Data will be made available on request.
References
- Abbott WM, Damschroder MM, Lowe DC, 2014. Current approaches to fine mapping of antigen-antibody interactions. Immunology 142, 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochez PM, Michiels C, Hendrickx E, Dauguet N, Warnier G, Renauld JC, Dumoutier L, 2017. Ccr6 is dispensable for the development of skin lesions induced by Imiquimod despite its effect on epidermal homing of IL-22-producing cells. J Invest Dermatol 137, 1094–1103. [DOI] [PubMed] [Google Scholar]
- Ekman AK, Sigurdardottir G, Carlstrom M, Kartul N, Jenmalm MC, Enerback C, 2013. Systemically elevated Th1-, Th2- and Th17-associated chemokines in psoriasis vulgaris before and after ultraviolet B treatment. Acta Derm. Venereol 93, 527–531. [DOI] [PubMed] [Google Scholar]
- Furue K, Ito T, Tsuji G, Nakahara T, Furue M, 2020. The CCL20 and CCR6 axis in psoriasis. Scand. J. Immunol 91, e12846. [DOI] [PubMed] [Google Scholar]
- Getschman AE, Imai Y, Larsen O, Peterson FC, Wu X, Rosenkilde MM, Hwang ST, Volkman BF, 2017. Protein engineering of the chemokine CCL20 prevents psoriasiform dermatitis in an IL-23-dependent murine model. Proc. Natl. Acad. Sci. U. S. A 114, 12460–12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WH, Khan N, Mishra A, Gupta S, Bansode V, Mehta D, Bhambure R, Ansari MA, Das S, Rathore AS, 2022. Dimerization of SARS-CoV-2 nucleocapsid protein affects sensitivity of ELISA based diagnostics of COVID-19. Int. J. Biol. Macromol 200, 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilvebrant J, Rockberg J, 2018. An introduction to epitope mapping. Methods Mol. Biol 1785, 1–10. [DOI] [PubMed] [Google Scholar]
- Onuora S, 2021. Targeting the CCR6-CCL20 axis improves experimental PsA. Nat. Rev. Rheumatol 17, 441. [DOI] [PubMed] [Google Scholar]
- Shi Z, Garcia-Melchor E, Wu X, Getschman AE, Nguyen M, Rowland DJ, Wilson M, Sunzini F, Akbar M, Huynh M, Law T, Raychaudhuri SK, Raychaudhuri SP, Volkman BF, Millar NL, Hwang ST, 2021. Targeting the CCR6/CCL20 Axis in Entheseal and cutaneous inflammation. Arthritis Rheumatol 73, 2271–2281. [DOI] [PubMed] [Google Scholar]
- Tonge PJ, 2018. Drug-target kinetics in drug discovery. ACS Chem. Neurosci 9, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waritani T, Chang J, McKinney B, Terato K, 2017. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX 4, 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


