Abstract
Nasal irrigation (NI) for the local treatment of chronic rhinosinusitis (CRS) has some specificity due to the deep anatomical site of the sinuses. The purpose of this review is to help standardize the application of NI in healthcare practice, improve the prevention and treatment of CRS, and facilitate further research on the local treatment of CRS in the future. We searched the PubMed database for 342 articles in the last decade, using the keywords “saline nasal irrigation” and “chronic rhinosinusitis.” We summarize the studies on the mechanism of action, rinsing solution, rinsing apparatus, and rinsing method of NI for CRS. NI plays an important role in the treatment of CRS, and it is a beneficial low-risk treatment. Isotonic saline is the most accepted flushing solution, and large-volume low-pressure flushing bottles are the flushing devices with the best flushing effect and are generally tolerated by patients. Phage, colloidal silver, and hydrogen can be further studied as components of rinses. NI plays an important role in the treatment of CRS, and it is a beneficial low-risk treatment. Further high-quality and expanded sample size studies on other flushing solutions, flushing head position, flushing frequency, and treatment courses are still needed, and lessons learned in practice.
Keywords: Chronic rhinosinusitis, flushing solution, irrigating methods, nasal lavage, therapy
1. Introduction
Chronic rhinosinusitis (CRS) is a chronic inflammatory disease of the sinus mucosa, an inflammatory disease thought to be associated with allergies and immunity, mostly involving multiple sinuses, with symptoms such as nasal congestion, runny nose, loss of smell, and headache lasting more than 12 weeks, with or without nasal polyps [1]. It is a heterogeneous disease, usually refractory, with a prevalence of 6% to 27.1% [1] and an incidence of approximately 1,800 to 2,300 per 100,000 people [2], which negatively affects the quality of life of patients.
The origins of nasal irrigation (NI) can be traced back to the ancient Hindu art of Hatha yoga in the 1st millennium B.C. [3], which is an important component of the yogic system of bodily cleansing. NI is thought to act through a variety of mechanisms, generally based on the thinning of nasal secretions and the resulting improved mucociliary clearance, direct removal of mucus, antigens, bacterial membranes or inflammatory mediators, and possibly vasoconstriction, thus reducing congestion and nasal congestion to some extent. Simple and convenient to operate, NI is noted in many guidelines for the treatment of sinusitis and nasal polyps [1, 4, 5] as an important treatment for nasal and sinus disease as well as postoperatively, and occupies an important position in the recovery of nasal mucosal morphology and function. Simple and convenient to operate, even in children with CRS, NI may be beneficial [6] and is recommended as one of the initial medical management measures for CRS in children [7].
Although NI is recommended as effective for the treatment of CRS in various guidelines and consensus [1, 7–10], there are many corresponding studies showing that there is still a wide variation in flushing fluid composition, flushing volume, frequency duration, and flushing equipment. Moreover, patient compliance in clinical practice is uneven [8], with a recent study reporting [11] that only 24% of patients had daily compliance and only 19% used irrigation regularly, which does not reflect well on the true efficacy and makes it difficult to assess the results of clinical studies.
We used NI and chronic sinusitis as keywords and searched the PubMed database for 342 relevant literature over a 10-year period from 2012 to 2022, with the main eligibility criteria being articles in English, randomized and controlled trials, including retrospective studies, literature reviews, and CRS studies in children, excluding the acute sinusitis NI literature. Additional relevant literature was also identified by reviewing the reference lists of the selected articles. The authors independently assessed the full text of each literature, excluded those that were not considered relevant to the topic of this review, and summarized them according to the following aspects in the hope of informing clinical use and future related research.
2. Therapeutic mechanism of NI for sinusitis
The etiology and pathophysiological mechanisms of CRS are complex and are mainly related to a variety of factors, including anatomical structures, genetics, metabolic reactions, environment, and so on. Increasing evidence supports the predominance of inflammation in the pathogenesis of CRS [12–14], with intrinsic and extrinsic factors involved in a complex interplay, including bacterial biofilm formation. Given that CRS inflammation occurs at the interface between the mucosa and the external environment, it seems reasonable to target exogenous drugs or interventions that act at this interface. Treatment of CRS is a long-term process aimed at achieving local inflammatory control and alleviating patient symptoms, with a clinical preference for local treatment acting on the nasal cavity and sinuses, and NI is the main local treatment method. It is currently believed that the therapeutic mechanism of NI may have the following several aspects.
2.1. Physical or mechanical scouring effect
The therapeutic effect of NI may lie more in its physical flushing mechanism [15], which flushes out mucus, allergens, and air pollutants from the nasal cavity.
2.2. Improving mucosal cilia function
Normal ciliary function plays an important role in maintaining a clean nasal cavity and sinuses. Irrigation improves ciliary activity and allows cilia to transport mucus, particles, and microorganisms efficiently [16].
2.3. Alleviating mucosal inflammatory edema
NI increases local blood circulation in the nasal cavity and has a mild vasoconstrictive effect, so it can alleviate mucosal inflammatory edema and improve patients’ symptoms such as nasal congestion and headache [17].
2.4. Reduction of bacteria and biofilm formation
The biofilm-forming ability of bacteria plays an important role in the pathogenesis of sinusitis. NI reduces the diversity of the nasal flora structure and decreases the flora species and biofilm formation, allowing the timely removal of some of the more strongly adherent bacteria [18].
2.5. Immunomodulatory effects on the nasal mucosa
NI may also inhibit the development of chronic inflammation of the sinuses through immunomodulatory effects. NI was also found to reduce the level of IL-8 expression in CRS, possibly accompanied by a decrease in neutrophil chemotaxis, for local immunomodulation [19]. Relying on certain added ingredients of the flushing solution, such as hydrogen, can also have some immunomodulatory effects, as described in the later section on flushing solution ingredients.
2.6. Special mechanisms of NI after CRS surgery
Postoperative patients with CRS are routinely given biodegradable hemostatic sponges to stop bleeding, and when it degrades on its own, the residual hemostatic material and crusts will adhere to the operative cavity, thus delaying epithelialization of the operative cavity. Recent postsinus surgery flushing can effectively mechanically remove postoperative blood clots, degraded caulking material and crusts, and remove mucous snot, pathogens, and allergens from the nasal cavity to improve nasal mucosal cilia function, reduce inflammatory factor release, decrease nasal mucosal edema, unblock sinus drainage, promote sinus secretion drainage, facilitate surgical wound healing, and reduce the risk of postoperative adhesion [20, 21]. The distant irrigation after sinusitis surgery makes it easier to clean the sinus cavity locally during irrigation and to distribute the irrigating drugs into the sinus cavity because the surgery enlarges the sinus opening and opens the sinuses.
Endoscopic sinus surgery (ESS) is essential to provide effective local solution distribution, and flushing can be enhanced, especially in the frontal sinus and sphenoid sinus, by unblocking and clearing drainage through nasal endoscopy [22]. Barham et al. [23] using a cadaveric head, concluded that the Draf type III procedure (which involves removal of the entire frontal sinus floor, frontal sinus septum, and part of the superior nasal septum to create a common cavity) provides the best delivery pathway for local drug therapy in patients with frontal sinus disease. The clinical study by Li et al. [24]. resulted in good control of CRS after complete nasal endoscopic removal of the inter-sinus septum to form a new sinus cavity with NI.
3. Studies related to the composition of nasal rinse solution
3.1. Saline
Saline is most commonly used for NI, and the solution can be isotonic (saline), hypotonic, or hypertonic, and can be in buffered or nonbuffered form [1]. In several randomized trials, saline irrigation improved symptoms, quality of life, and endoscopic findings in CRS, and its good safety profile makes it an attractive long-term local treatment strategy [25, 26].
Although isotonic saline is the most commonly used NI solution, [18, 19] this search revealed more studies with higher acceptance of hypertonic saline in the last 5 years. A very early study found a statistically significant improvement in nasal mucociliary clearance when hypertonic saline was applied to patients with CRS. Kanjanawasee et al. [27] showed that hypertonic saline was superior to isotonic saline and that hypertonic solution promoted water drainage from cells, alleviated mucosal edema, and increased lysis layer hydration more significantly than isotonic solution, thereby reducing mucus adhesion and improving mucus cilia removal power. There was a meta-analysis on several different concentrations of saline for NI for CRS [28], which ultimately concluded that hypertonic saline was significantly more effective in improving nasal congestion, nasal secretion and headache symptoms, and nasal cilia movement with mild side effects compared with isotonic saline, but there were no significant differences in imaging performance and olfactory improvement. Wang et al. [17].searched the database until 2019 and found that the application of buffered hypertonic saline (1.25%, 2%, 2.7%, and 3%) compared with isotonic saline (0.9%) irrigation, local hypertonic saline alleviated nasal edema, improved the nasal cavity environment, and had a relatively lower rate of postoperative complications, and 3% hypertonic saline was more effective. However, it is worth noting that the concentration of hypertonic saline should not exceed 3% because higher concentrations (6% or higher) can have a deleterious effect on mucociliary clearance and may cause some severe mucosal irritation and other adverse effects [17], causing significant nasal pain when the hypertonic saline concentration is >2.7% [29] and nasal congestion secondary to vasodilation at concentrations >5.4%23. Low et al. [30] compared the effects of lactated Ringer’s solution, isotonic saline, and hypertonic saline for NI after sinus surgery, and they found that lactated Ringer’s solution improved the symptoms of sinusitis such as nasal congestion and headache with better results.
Although hypertonic saline rinses have their advantages based on the current literature review, after all, the sample sizes of these studies are small, and the 2020 EPOSS Steering Group still recommends that isotonic saline or lactated Ringer’s salts for nasal saline rinses are preferable [1].
3.2. Other components of the rinse solution
Local nasal administration directly contacts the mucous membranes of the nasal cavity and sinuses, theoretically allowing for higher local concentrations and fewer systemic effects. Based on this, there have been many studies on nasal rinses, but in general, not many have actually been promoted for clinical application. For example, the use of xylitol for CRS and local fluconazole for allergic fungal sinusitis seemed promising 10 years ago, but nasal rinses with xylitol are still not commonly promoted today, while nasal rinses with antifungal drugs are currently the majority of opposing views.
3.2.1. Corticosteroids from anti-inflammatory mechanism
Corticosteroids bind to specific cytoplasmic glucocorticoid receptors and have a multifactorial effect, activating anti-inflammatory gene transcription and inhibiting pro-inflammatory gene transcription, reducing inflammatory cell infiltration and cytokine production. Recent studies suggest that the utility of steroids in CRS treatment may not be limited to their anti-inflammatory effects but also exhibit in vitro antimicrobial effects [12, 31]. Delivery of local steroids by irrigation to improve CRS has also been recommended by many guidelines [1, 32]. The effectiveness and safety studies of steroids for CRS irrigation are the most numerous compared with other nasal rinse components, and some of our studies over the last 10 years are summarized in Table 1.
Table 1.
Clinical study of steroid rinsing of the nasal cavity for the treatment of CRS in recent 10 years
| Study | Methods | participants | Intervention | Duration | Outcomes | Better than the control group (yes/no) |
|---|---|---|---|---|---|---|
| Luz-Matsumoto GR [39], 2021 | Retrospective observational study | 205 CRSwNP 52 CRSsNP. |
CS spray group: CS spray CNI group: 1% compounded budesonide drops or betamethasone cream 0.5 mg in 250 mL alkaline homemade saline solution |
3–6months | SNOT-22 score, Lund---Kennedy endoscopic score | Yes |
| Thanneru M [38], 2020 | Prospective study | 60 postoperative CRSwNP | Experimental group (30 patients): 2 mg of budesonide was mixed in 250 mL normal saline control group (30 patients): isotonic saline |
1, 2, 6 and 10 weeks | SNOT-22 score, Lund-Kennedy endoscopy score | Yes |
| Jiramongkolchai P [45], 2020 | Double-blind, placebo-controlled, randomized clinical trial | 53 CRSsNP (43 were actually completed) | Experimental group (21 patients): mometasone nasal irrigation (2.4 mg in 240 mL) control group (22 patients): MFNS (nasal saline irrigation and mometasone furoate nasal spray, 50 µg in each spray, 2 sprays/nostril) |
8 weeks | SNOT-22 score, Lund-Kennedy endoscopy score | Yes |
| Huang ZZ [37], 2019 | Prospective cohort analysis of prospectively collected data | 60 CRS Post ESS | Experimental group (30 patients): budesonide nasal irrigation control group (30 patients): normal saline nasal irrigation no amount of irrigation, no dose of budesonide, no frequency of irrigation |
3 months | Lund-Kennedy endoscopy score, symptom VAS, SNOT-22 score, SF-36, SAS, SDS, side effects scale | Yes |
| Harvey RJ [44], 2018 | Randomized in a double-blind, randomized, placebo-controlled trial | 44 CRS, postoperative (35 were actually completed) |
Nasal irrigation followed by a nasal spray once a day (2 mg mometasone delivered by either spray or irrigation) | 12 months | VAS, SNOT-22 score, 13-point Likert score of overall (or global), sinonasal function, Lund-Mackay score, Modified Lund-Kennedy score | Yes |
| Tait S [41], 2018 | Double-blind, placebo-controlled, randomized clinical trial | 74 CRS (61 were actually completed) 29 budesonide 32 saline |
Both groups were washed with 8-ounce (approximately 240 mL) of normal saline once a day, and the budesonide group wash solution contained 0.5 mg of budesonide | 30 days | SNOT-22 score, Lund-Kennedy endoscopic score | Yes |
| Kang TW [35], 2017 | prospective study | 12 CRSwNP with asthma, postoperative | 0.5 mg budesonide in 250 mL normal saline | 6 months | SNOT-22 score, Lund-Kennedy endoscopy score | Yes |
| Dawson B [36], 2017 | Open-label study | 30 CRS, postoperative | betamethasone nasal irrigation 0.5 mg in 200 mL normal saline, once daily | 6 weeks | Endogenous cortisol levels, SNOT-22 score | Yes |
| Kosugi EM [40], 2016 | Prospective uncontrolled intervention trial | 16 difficult-to-treat CRS | 1 mg budesonide was mixed with 500 mL normal saline and the nasal cavity was irrigated twice a day | 3 months | SNOT-22 score, Lund-Kennedy endoscopy score | Yes |
| Rawal RB [42], 2015 | Prospective, single-blind, randomized controlled trial. | 50 CRSwNP post-FESS |
0.5 mg budesonide in 240 mL normal saline, 60 mL per nostril twice-daily irrigation for a total of 240 mL using a high -volume low -pressure irrigating device | 3–6 months | SNOT-22 score, RSOM-31, RSDI, UPSIT, PEA test | No |
| Jang DW [34], 2013 | Retrospective review of prospectively collected data | 60 CRS post ESS (30 eCRSwNP, 13 AFS, 13 ST, 4 eCRSsNP) |
0.5 mg budesonide mixed in 3 ounces of nasal saline. irrigate each nostril with 1.5 ounces using a syringe twice a day | 25 months | SNOT-20 score, Lund-Kennedy endoscopy score | Yes |
| Man LX [43], 2013 | Prospective, an open-label study |
23 CRS previously undergone bilateral ESS | 3 mg of fluticasone propionate powder in 240 mL of normal saline delivered via low-pressure, high-volume, twice-daily | 6 weeks | Adrenal function, IOP, cataracts | Yes |
| Snidvongs K [33], 2012 | Self control study | 111CRS, post ESS | Budesonide 1 mg or Betamethasone 1 mg delivered in a 240 mL squeeze bottle, once daily | 55.5 ± 33.9 weeks | Symptom score, SNOT-22 score, endoscopy score | Yes |
AFS, allergic fungal sinusitis; CNI, corticosteroid nasal irrigation; CS, corticosteroid; CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with polyposis; eCRSsNP, eosinophilic chronic rhinosinusitis without polyps; eCRSwNP, eosinophilic chronic rhinosinusitis with polyps; ESS, endoscopic sinus surgery; FESS, functional endoscopic sinus surgery; IOP, intraocular pressure; MFNS, mometasone furoate nasal spray; PEA, Phenyl Ethyl Alcohol; RSDI, Rhinosinusitis Disability Index; RSOM-31, Rhinosinusitis Outcomes Measurement Teste-31; SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; SF-36, Short Form36-Item Questionnaire; SNOT-22, Sino-Nasal Outcome Test22; UPSIT, University of Pennsylvania Smell Identification Test; ST, Samter’s triad; VAS, visual analog score.
One of the more studied corticosteroid flushing agents is budesonide and 9 of the 13 studies we summarized studied budesonide. Budesonide is an effective local corticosteroid with approximately 1,000 times the local anti-inflammatory potency of cortisol, and most of the relevant studies have used it for postoperative irrigation of CRS [33–39], all of which concluded that budesonide is safe and effective for postoperative irrigation of CRS and facilitates postoperative management of CRS. There is also a significant clinical benefit for patients with refractory CRS [40] or CRS who have not undergone sinus surgery [41]. However, Rawal et al. [42] found no significant difference between irrigation with saline and saline plus budesonide in patients with postoperative CRSwNP. The single flush dose of budesonide in these studies ranged from 0.5 to 2 mg, and the frequency of flushing varied from 1 to 3 times a day, but 0.5 mg/dose once a day was the most common.
Fluticasone propionate may have higher potency and lower systemic bioavailability compared with budesonide; therefore, Man et al. [43] used fluticasone propionate for postoperative rinsing in CRS and found no changes in intraocular pressure, blood, and urine cortisol and considered it a safe treatment option. Studies [38, 39] using mometasone NI in the treatment of CRS have found that the efficacy is better than that with mometasone topical spray.
There are also reviews on NI after sinus surgery for CRS [46, 47]. Some believe that NI after sinus surgery is not beneficial [46], while others suggest that using local corticosteroids in irrigation can be a first-line medical treatment for postoperative CRS patients [47], which is safe and effective [48]. Therefore, further clinical trials with strong evidence are needed to confirm this. There are also many inconsistent aspects, such as the dosage of irrigation ranging from 5 ml to 480 ml. It is recommended that randomized controlled trials in the future are necessary for CRS phenotypes, subtypes, and the dosage and duration of nasal corticosteroid irrigation [48]. Therefore, comparing the advantages and disadvantages of local corticosteroid irrigation treatment for CRS with other management schemes is still a field that needs further research [49].
3.2.2. From the antimicrobial perspective
3.2.2.1. Various antibiotics.
When antibacterial efficacy is mentioned, the first thing that comes to mind is a variety of antibiotics. Although there is growing evidence supporting the concept of CRS pathogenesis as an inflammation rather than an infection, it is widely accepted that bacteria play a major role in triggering or exacerbating chronic inflammation. Biofilms have been identified in the mucosa of patients with CRS and have been associated with the development of recalcitrant CRS, with the help of which bacteria can evade host defenses and the action of antibiotics. The aim of local antibiotic therapy is to inject higher concentrations of antibiotics directly into the sinuses and disrupt bacterial biofilms while minimizing potential systemic side effects, so the use of local antibiotics has been widely studied, including cephalosporin antibiotics, aminoglycoside antibiotics, macrolides, mupirocin, tobramycin, and so on. The most research has been done on the local use of mupirocin and aminoglycoside antibiotics.
In 2012, Jervis-Bardy et al. [50] conducted a randomized double-blind controlled study on mupirocin NI for postoperative CRS, in which the experimental group irrigated their nasal cavity with saline containing mupirocin at 240 ml (containing 125 mg of mupirocin) twice a day for 1 month, and then compared the bacterial culture results and other indicators with the control group (saline). It was concluded that mupirocin NI is a short-term effective treatment for recalcitrant CRS due to Staphylococcus aureus, but the efficacy may not be durable. Lee et al. [51] in a study published in 2020 compared the efficacy of povidone-iodine, mupirocin, and saline irrigation in the treatment of postoperative exacerbation of sinusitis in 3 groups irrigated twice daily for 30 days. In a study published in 2022, Hon et al. [52] found that mupirocin delivered after lowering the pH of the irrigation solution may help to eliminate the S. aureus biofilm present in the sinus mucosa of patients with CRS.
Snidvongs et al. [53] in a 2017 review concluded that there is no evidence yet to support the use of local antibiotics in the treatment of sinusitis. Carlton et al. [54] in 2019 summarized studies on the use of local antibiotics in chronic sinusitis since 2014 and showed that local antibiotic therapy may be useful in the short term in patients with CRS after sinusitis surgery and recalcitrant S. aureus infection. The EPOS2020 Steering Group concluded that it is uncertain whether the use of topical antimicrobial therapy has an impact on the prognosis of adult patients with CRS compared with placebo due to the very low quality of evidence [1].
On the whole, so far, NI with local antibiotics for CRS is still controversial, and better protocol design and more studies are needed to evaluate the efficacy and safety of local antibiotic irrigation, to study the problem of bacterial resistance caused by local administration, and the potential risk of local and systemic adverse effects.
3.2.2.2. Xylitol nasal irrigation.
Xylitol is an organic, insoluble, 5-carbon sugar alcohol that acts as an osmotic agent to reduce the salt concentration of airway surface fluids, thereby increasing bactericidal efficacy. Because Xylitol nasal irrigation (XNI) may disrupt S. aureus biofilms and inhibit the growth of Streptococcus pneumoniae [55], there was a study in 2011 where 12 mg of XNI mixed with 240 mL of water flush significantly improved CRS symptoms compared with the saline flush [56]. A later researcher [49] also used XNI as a rinse solution for CRS, and compared with the saline flush group, the XNI group showed significant improvement in various assessment indicators compared with the saline flush group, and the difference was also significant, but the sample size of the study was still too small (only 25 cases were actually completed in both groups in total), and further studies are needed to confirm this. Recently, Silva et al. [57] also did a study with a total of 52 patients with sinusitis, and after 30 days of irrigation, the “xylitol” group showed significant pain relief and reduction of nasal symptoms in the postoperative period compared with the saline group (P < 0.001). The drawback of this study is that there is no objective assessment index such as imaging assessment.
3.2.2.3. Manuka honey.
The advantages of Manuka honey (MH) over topical antibiotics are that it is natural, nontoxic, low side effects and hypoallergenic reactions, a broad antimicrobial spectrum, and reduces the emergence of resistant bacteria [58]. Lee et al. [59] conducted a randomized single-blind controlled trial of MH NI for CRS and completed the trial in a total of 42 individuals; both MH and saline irrigation improved prognosis, but there was no statistically significant difference between groups; however, in the subgroup that did not receive oral antibiotics/steroids, MH was statistically better in terms of negative bacterial cultures, so it is thought that MH may be effective for acute exacerbations of CRS. The combination of low pH, hypertonic blood pressure, hydrogen peroxide activity, and methylglyoxa (MGO) gives MH its unique antibiofilm properties [58]. Ooi et al. [60] conducted a randomized, single-blind, placebo-controlled phase 1 clinical trial of MH combined with MGO irrigation for the treatment of recalcitrant chronic sinusitis, and a total of 25 patients completed this study with twice-daily 16.5% MH and 1.3 mg/mL MGO combined NI and the final results concluded that it was safe to use for 14 days, and although the efficacy was not superior to oral antibiotics and twice-daily saline irrigation, it was concluded that MH irrigation had some potential benefit in the treatment of recalcitrant CRS. In future studies, they will look to optimize the duration of treatment or use MH as an adjunct to antibiotics and evaluate it in a larger sample of patients.
3.2.2.4. Povidone-iodine.
Povidone-iodine (PVP-I) has been shown to have an inactivating effect on a range of infection factors, including drug-resistant bacteria, viruses, fungi, protozoa, and biofilms [61]. PVP-I is an attractive candidate for the treatment of recalcitrant CRS because of its good tolerability, rare sensitization, and lack of drug resistance [61]. Panchmatia et al. [62] conducted a prospective cohort study on the efficacy and safety of diluted PVP-I nasal rinse as an adjunctive treatment for CRS, including analysis of 29 patients with recalcitrant CRS, requiring bilateral NI with 0.08% PVP-I every other day for 7 weeks, and based on the results concluded that diluted 0.08% PVP-I nasal rinse as an adjunctive treatment for recalcitrant CRS significantly reduced signs of infection while significantly improving symptoms without affecting thyroid function, mucociliary clearance, or olfaction. Lee et al. [51] concluded that 0.1% PVP-I solution was well tolerated as sinus irrigation and also suggested that it may be a viable method for temporary disinfection of sinus cavities for viruses and bacteria such as COVID-19.
3.2.2.5. Hypochlorous acid.
Hypochlorous acid (HOCl) at low concentrations has been shown to be effective against bacteria, fungi, and viruses and is not toxic to the nasal mucosa, so it may be an effective NI agent [63]. Cho et al. [64] evaluated the effectiveness of low-concentration HOCl NI compared with isotonic saline for pediatric CRS and concluded that HOCl NI for pediatric CRS is also an effective adjunctive therapy.
Other studies of nasal rinses with antibacterial effects include surfactants (eg, baby shampoo) and electrolyzed acid water, which, although found to be effective against CRS, are not currently recommended because they cause nasal irritation or discomfort [1].
3.2.3. In terms of promoting cilia and mucosal repair function: Hyaluronic acid
Hyaluronic acid, which has no allergenic properties, is considered a highly safe molecule and has a wide range of applications in many branches of medicine [65]. Due to the mucosal regenerative properties of sodium hyaluronate, there has been an increasing interest in its use for the treatment of patients after CRS surgery [66, 67], with beneficial effects. Mozzanica et al. [67] conducted a multicenter, prospective, randomized, double-blind, parallel-controlled comparison of isotonic saline and isotonic saline plus sodium hyaluronate rinsing of the nasal cavity for functional endoscopic sinus surgery after CRS. A total of 56 patients included in the analysis were randomly divided into 2 groups and the nasal cavity was irrigated with 250 mL of isotonic saline or 250 mL of isotonic saline plus 9 mg of sodium hyaluronate twice daily for a total of 6 weeks of follow-up. The results showed that the evaluation indexes of the hyaluronate group were significantly better than those of the saline group, so they hypothesized that the application of sodium hyaluronate facilitated the repair of nasal mucosal tissue, promoted wound healing, and reduced crust formation, and improved nasal congestion, hyposmia, and headache. While Savietto et al. [68] conducted a study on the use of hyaluronic acid for NI in CRS, 30 patients aged 18 to 65 years with chronic rhinosinusitis without nasal polyps were randomly administered hyaluronic acid or isotonic saline solution through a nasal spray twice a day, both 5 mL each time, twice a day for 30 days, and 26 cases were actually completed. Although both groups improved the symptoms of chronic rhinosinusitis without nasal polyps, especially nasal congestion and olfactory ability, hyaluronic acid did not show an advantage.
As mentioned earlier, there have been many studies on rinses in recent years, many of which are still controversial. Chen et al. [69] systematically evaluated and compared the results of 5 studies involving 331 post-CRS patients receiving nasal rinses with different solutions (hot water with iron in sulfur arsenic, lactated Ringer’s solution, electrolytic acidic water, amphotericin B, hyaluronic acid plus saline, or saline alone). It was found that, based on the limited evidence available, these different compositions of solutions were not superior to saline for irrigating. Therefore, the study of flushing solutions needs to be expanded and deepened.
3.3. Research progress on the composition of flushing solution
There are some studies of flushing solutions in the basic experimental phase or early clinical research phase.
3.3.1. Bacteriophage/phage
Bacteriophages/phages are viruses that can infect and lyse specific bacteria without harming the human host [70]. Zhang et al. [71] found that S. aureus isolated from CRS patients had a high incidence of multidrug resistance (20%) and that these resistant strains could still be killed by phages. Therefore, it is believed that phage could be a promising therapeutic alternative to antibiotics for the control of multidrug-resistant S. aureus infections and biofilms. Drilling et al. [72] have tested phages in animal models by saline irrigation and found that phages significantly reduced biofilm without causing host damage. Ooi et al. [73] first reported that a phase 1 clinical trial of phage for CRS caused by S. aureus has been completed in 9 patients studied who received twice-daily intranasal irrigations with phage for 1 to 2 weeks with good initial efficacy (but no statistical analysis due to the small sample size) and no serious adverse events or biochemical or physiological abnormalities, and further clinical trials are in development.
3.3.2. Silver
Recalcitrant CRS is associated with bacterial biofilms, and biofilm lesions are periodically shed, leading to recurrent disease. Some studies have confirmed that colloidal silver (CS) may be effective against bacterial biofilms [74]. Ooi et al. [75] conducted the first study of CS irrigation for recalcitrant sinusitis, enrolling 22 patients who completed a twice-daily saline (along with oral administration of a sensitive antibiotic chosen based on culture results) or CS irrigation for 10 days, and based on the results, they concluded that daily 2 CS (0.015 mg/mL) NIs per day for 10 days were considered safe but not superior to the oral antibiotic group. However, this study confirmed the safety of CS and found no serious adverse effects or olfactory changes. The study was limited by the sample size and further studies are needed. Feizi et al. [76] reported their results in 2021 on the in vitro antibacterial of Silver nanoparticles (AgNPs) (strains isolated from the nasal cavity of patients) and concluded that AgNPs appear to be a promising tool for the treatment of bacterial biofilms, especially in CRS.
3.3.3. Hydrogen
It is now clear that hydrogen is a therapeutic gas signaling molecule with selective antioxidant and anti-inflammatory effects and that oxidative stress is one of the pathogenic mechanisms of CRS [77, 78]. In recent years, there have been basic studies on the use of hydrogen-rich saline to improve chronic inflammation in the nasal mucosa, and it was found that hydrogen-rich saline achieved antioxidant effects by directly neutralizing free radicals and indirectly increasing superoxide dismutase levels [79], and also reduced the transcription and expression of inflammatory factors IL-4 and IL-13 in the nasal mucosa of experimental guinea pigs, reducing inflammation and promoting the recovery of nasal mucosa physiological functions [80]. On this basis, clinical studies have been conducted on the use of hydrogen-rich saline for chronic rhinitis [81] and CRS, with the hope that it will become another effective alternative to NI for CRS.
In short, future studies on flushing solutions need to be further developed to refine the study protocol, overcome current limitations, and provide more accurate clinical information.
4. Nasal irrigator
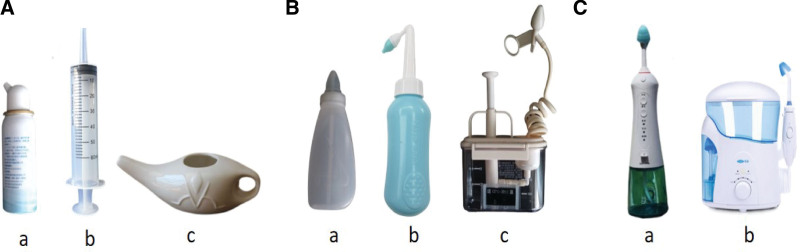
The efficacy of NI is related to its ability to reach the target tissue, that is, the distribution of the irrigation fluid. The complex nasal anatomy resulting in large regional differences, as well as pathological changes due to disease effects causing impaired ciliary motility and nasal mucosal edema, make it challenging to study the distribution of NI fluid [35]. The factor that affects the distribution of irrigation, besides the nasal cavity itself, is the rinsing apparatus. Nasal rinses can be delivered by a variety of instruments, including squeeze nebulizers, syringes, Neti pots (Fig. 1A), squeeze rinse bottles, pressurized rinses (Fig. 1B), and electric rinse devices (Fig. 1C).
Figure 1.
Commonly used flushing equipment. (A) Nebulizer (a, Shanghai Nasalcleaner Biotechnology Co., Ltd, China); Syringe (b); Neti jug (c, Jiangsu Kangshen Pharmaceutical Health Products Co., Ltd, China) (B) Squeeze flush bottle (a, Foshan Changming Medical Technology Co., Ltd, China; b, Shanghai Chunzi Biotechnology Co., Ltd, China); Push flusher (c, Yangzhou Qiangjian Medical Equipment Co., Ltd, China) (C) Electric flushing device (a, b, Shenzhen Miaomiaoce Medical Device Technology Co., Ltd, China).
The volume of appropriate nasal rinsers for adult patients ranges from approximately 30 to 500 mL and can be divided into small and large volumes. Small-volume irrigators are not as effective as high-volume delivery in terms of sinus distribution, so high-volume nasal irrigators (generally defined as >100 mL) are effective and commonly used [82]. The 2016 international consensus statement on allergy and sinology strongly recommends high-volume (>200 mL) NI as an adjunctive therapy for CRS [83].
Squeeze bottles are most commonly used because of their ease of handling, cleaning, and adding other medications. Chen et al. [84] in a 2017 cadaveric study comparing powered high-pressure irrigating devices with classic NI squeeze bottles showed that squeeze bottles were more effective at irrigating sinus cavities. Piromchai et al. [85] in their two investigative studies found that high-volume (>100 ml) NI devices were effective in treating sinusitis and more effective than other types of devices in removing nasal secretions and reducing postnasal drip.
Professor Zhang Luo’s team at China Capital Medical University also published a patent this year for a nasal sinus irrigator with a large capacity, adjustable, anti-reflux, and other advantages [86].
5. Irrigating methods
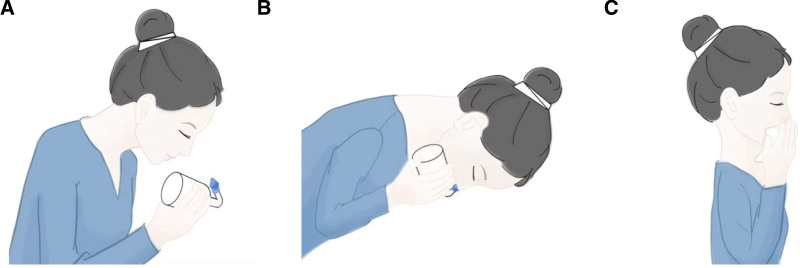
The common method of NI is nasal lavage, and the operation steps are: first, place the configured rinse solution in the rinse device, second, with the patient’s head in a low position, place the outlet of the nasal rinse device at one nostril, and finally, apply slight force so that there is no gap between the outlet and the nasal cavity (Fig. 2A), use the pressure difference to make the rinse solution flow into the nasal cavity from one nostril, pass through the nasopharynx, and then flow out from the other nostril or oropharynx Spit out (Fig. 2B), and when the irrigation is finished, the other side of the nasal cavity is changed to irrigate, and finally the residual rinsing fluid in the nasal cavity is drained by pressing both nostrils, respectively (Fig. 2C). There are also nasal spray method. Different NI methods can produce different adjuvant therapeutic effects. NI is characterized by high pressure and high flow rate, easy method, mild respiratory response, and higher comfort, but may produce damage to nasal mucosal tissues; nasal spray is characterized by low pressure and flow rate but is easy to operate.
Figure 2.
Commonly used flushing methods for squeeze bottles. (A) Put the water outlet of the nasal irrigator on one nostril. (B) Squeeze the rinse fluid from one nostril into the nasal cavity, pass through the nasopharynx, and then flow out from the other nostril or spit out from the oropharynx. (C) After rinsing, press two Lateral nostrils to drain the residual irrigation fluid from the nasal cavity.
NI is suitable for patients with CRS because their subjective symptoms are closely related to the accumulation of large amounts of nasal secretions, and the higher flow and pressure of NI can more thoroughly remove crusts and secretions from the nasal cavity and keep the nasal cavity clean and moist in real time [87].NI after nasal endoscopy in patients with CRS may be an ideal postoperative NI method because it can more effectively relieve patients’ discomfort and reduce the risk of postoperative nasal adhesions.
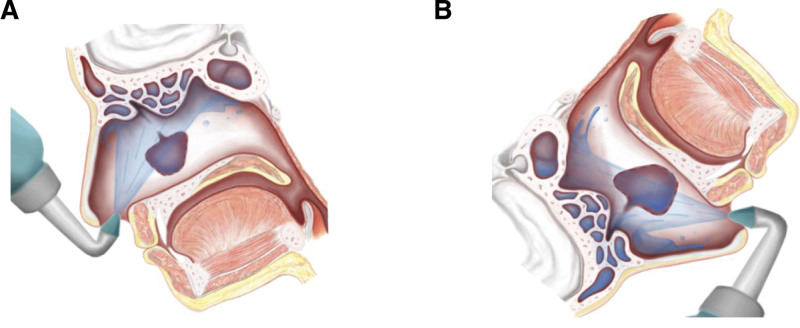
The optimal penetration of the solution into the sinuses also depends on the patient’s head position during irrigation. It is common to have a slightly anterior head position (Fig. 3A), but for the most efficient penetration, especially to access the frontal sinuses, the patient’s head should be in a head-down position as far as possible (Fig. 3B) [30]. In 2013, a systematic review by Thomas et al. [88] concluded that the evidence supports the use of high-volume devices in the anterior inferior head position (Fig. 3B) as the optimal sinus irrigation modality, and as for small-volume devices, the dorsal or lateral low head position is recommended for optimal low-volume irrigation penetration [88].
Figure 3.
Comparison of irrigating head positions. (A) Commonly used head position for irrigation (slightly tilted head): limited irrigation of the sinuses. (B) Head-down position with the head facing the floor: irrigation of the sinuses widely.
Griggs et al. [89] studied the head position and bottle angle used by 42 patients when irrigating according to the pictorial description of the irrigation device or the instructions of the rhinologist, and interestingly, there were no significant differences in head position and bottle angle between the different instruction groups of patients. It was also found that some patients completed irrigation within seconds, while others took several minutes, and some patients were unable to complete a 120 mL per side irrigation. This variability should be considered simultaneously in all future NI studies.
When flushing is performed with the vertex of the head in a downward position (Fig. 3B), all open sinuses are optimally distributed, but this head position is uncomfortable. In contrast, the entire nasal cavity and paranasal sinuses can also be filled if the flushing volume is large enough when the position of the head has less influence on the delivery of flushing fluid [88]. Therefore, if patients who cannot achieve the position of the head vertex toward the floor should use a squeeze bottle with 200 mL of flushing for better results, so a review by de Paiva Leite et al. [22] concluded that it is recommended that patients bend over a hand basin and do NI through a squeeze bottle using a high volume flushing apparatus with good results. Inthavong et al. [90] established a computational fluid dynamics model of the detailed physical mechanisms of sinus irrigation with fluid injected in a commonly used squeeze bottle and found that ipsilateral maxillary sinus and ethmoid sinus penetration was mainly due to overflow rather than direct jet entry, which confirmed the large volume irrigation to “flood” the sinus opening as suggested.
It has also been suggested that patients instructed to perform NI should be gentle [91], not apply strong pressure, and not insert the tip of the device too deeply or point the tip toward the nasal septum. The use of a bottle or natural fall rinse device is recommended over a pump type. In addition, check the nasal septum for any abnormalities during each postoperative examination. Therefore, more and more rigorous studies are needed to investigate the choice of the NI method.
6. Other aspects of NI
6.1. Rinse volume
In fact, the amount of fluid for NI has been mentioned earlier, and most recommend large volumes, supporting daily large volume (>100 mL) irrigation as one of the first-line treatments for CRS [92], and also recommending >200 mL [83], so it is recommended that at least >100 mL of irrigation volume is preferable.
6.2. Temperature
The ciliary function of the nasal mucosa is influenced by temperature. Since the rinse solution acts directly on the nasal mucosa during NI, an inappropriate temperature can directly damage the physiological function of the nasal mucosa. The most suitable temperature for NI is the one closest to the normal body temperature of the human body. Too high a temperature is not tolerated by the nasal mucosa, while too low a temperature will stimulate the vasoconstriction of the nasal mucosa.
In 2008, Liu et al. [93] studied the effect of nasal rinse temperature on nasal mucosal healing time, and they found that a 32°C to 34°C nasal rinse was sufficient. In 2012, Ottaviano et al. [94] studied a controlled study of iron-containing hot water (exact temperature unknown) with sulfite arsenic versus isotonic saline rinses, and the hot water treatment group had significantly lower nasal resistance and significantly improved cilia cell counts and nasal endoscopy findings. Nimsakul et al. [95] in a 2018 study compared heated saline (40°C) and room temperature saline nasal rinses on mucociliary clearance in patients with CRS and found that the effect of rinsing after heating the solution was not significantly increased, so rinsing after heating was considered unnecessary.
6.3. Frequency and duration
Pham et al. [96] found that NI once a day for 6 weeks was effective in relieving CRS symptoms through clinical observation of 144 pediatric CRS patients. In contrast, patients with refractory CRS can undergo long-term NI, that is, NI 1 to 3 times daily for several months or even more than 2 years. Maintaining the duration of NI for about 12 weeks after functional endoscopic sinus surgery for CRS maximizes the postoperative results of nasal endoscopy and reduces postoperative complications. Casale et al. [97] in their 2019 review on NI for CRS in adults analyzed the NI frequency ranged from 1 to 3 times and duration ranged from 3 to 6 months, all of which have not been standardized, making it difficult to conclude the optimal protocol. Therefore, the frequency and duration of individualized NI should be determined for patients with sinusitis based on their subtype, whether they are operated on or not, and the severity and tolerance of their disease.
7. Adverse effects of NI
NI is generally considered to be a highly safe treatment. It has rare and usually mild adverse effects, including a sensation of ear stuffiness, burning or stinging of the nasal mucosa, rhinorrhea, nausea, and headache, with no serious adverse effects [39]. High-volume devices cause a greater incidence of local irritation discomfort, burning, and eustachian tube dysfunction compared with small-volume devices [88]. However, some studies have found no statistically significant differences in the incidence of adverse events between different flushing device sets [85]. Sekine et al. [91] reported 3 cases of anterior septal perforation with NI after CRS (all without septal surgery), and because of the small number of cases, further epidemiological studies are pending to confirm these results.
In addition, the irrigation equipment and irrigation fluid may be contaminated with bacteria. Contamination of NI bottles is clinically important, most likely due to fluid reflux from the sinuses into the NI bottle, leading to bacterial colonization [98], and the presence of biofilm on the surface of NI bottles has been established [98]. Keen et al. [98] showed contamination of 97% of bottles in a study of regular replacement of irrigation bottles and nasal swabs. A review of sinus irrigation device contamination showed that the overall contamination rate ranged from 25% to 100%, with the most common bacteria isolated being S. aureus and Pseudomonas spp [99]. Although rare fatal primary amebic meningoencephalitis infections have been reported as a result of rinsing with contaminated tap water [100], according to a survey performed by Sowerby et al. [101], 48% of people still choose to use tap water for rinsing because of its ease of access, and more than a quarter of people never wash their bottles.
8. Hygiene recommendations for NI
One study found that the vast majority (70%) of patients with CRS did not follow the cleaning instructions for sinus irrigation bottles [101]. Although some studies have concluded [102] that contaminants from the irrigation water have little effect on the sinus microbiota regardless of the source of the water, patients are currently advised to observe the hygiene of the irrigation water and the irrigation apparatus.
The official guidelines of the Centers for Disease Control and Prevention recommend the use of boiled water that has been cooled (boiled for at least 1 min or 3 min at >6500 feet), microfiltered water (<1 µmpore), or bottled water (distilled or sterile). Hardy et al. [97], by studying 3 nasal rinses (cooled water after boiling for 5 min; bottled water, and distilled water) at room temperature and refrigerated conditions, recommended that the solutions should be refrigerated and should not be stored for more than 7 days. If the solutions are to be stored at ambient temperature, they should be isotonic or hypertonic and prepared from bottled or distilled water [103].
It is recommended that irrigation equipment be disinfected after each use and replaced every 3 months or according to instructions [99]. Morong et al. [104] concluded that irrigation bottle contamination may be time-dependent, so it is reasonable to microwave the irrigation apparatus before irrigation. Treatment with Milton’s solution (1% sodium hypochlorite + 19% sodium chloride) and microwaving was found to be an effective method for disinfection of bottles [98].
9. Conclusion
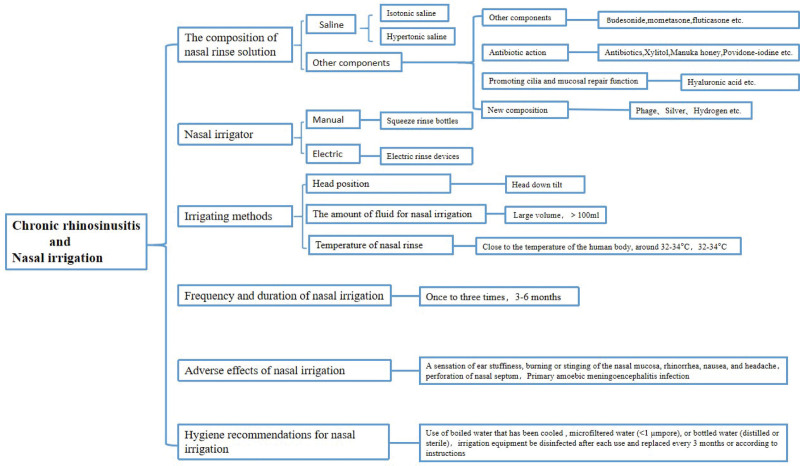
NI is a safer and more effective treatment for CRS, and its therapeutic effect has been confirmed by a large amount of clinical data and accepted and recognized by the majority of patients. According to the results of our review of the information contained in the literature (Fig. 4), low-pressure and high-volume flushing devices are mostly recommended, saline and hypertonic saline are currently more recognized, and other components are more studied and used with steroid hormones. Although there are more studies on antimicrobial mechanisms, they are still mostly controversial or defective and need to be further expanded or not yet generally promoted. There are fewer studies on irrigation solutions that accelerate epithelialization of the nasal mucosa and improve the efficiency of cilia transmission, etc. Phage, CS, and hydrogen are all directions that can be further studied in the future.
Figure 4.
The frame diagram of the main content of the article.
Most studies using NI in patients with CRS are characterized by small patient populations, short observation periods, different clinical and diagnostic parameters assessed, and adherence to the study assessed mainly by patient self-report, which is more subjective. Future studies should be designed as randomized controlled trials and include patients with and without nasal polyps (randomized by subgroup strata), uniform assessment metrics, and refined studies of irrigation frequency, duration of treatment, and so on. More studies are needed to develop individualized optimal delivery methods (rinse device, rinse volume, delivery method, and composition of the solution used), irrigation frequency and duration of treatment for patients with CRS, and to evaluate the safety of its long-term use, which is essential for the treatment of CRS.
Acknowledgements
Ju Lai and Shiwang Tan helped draw all the figures in the article. Chunyan Yao helped paper revision.
This work was supported by the National Science Foundation of China (No.81873689), Clinical Research Program of Shanghai Municipal Health Commission(No.202140293). Clinical Research Project of Tongji Hospital of Tongji University(No.ITJ(ZD)2207), Clinical Research Plan of SHDC (No. SHDC2020CR4090), National Key R&D Program of China (2022YFC2504100), National Science Foundation of Shanghai (No. 23ZR1458000), Shanghai General Hospital Integrated Traditional Chinese and Western Medicine (No.ZHYY-ZXYJHZX-202118).
Conflicts of interest
The authors have no financial conflicts of interest.
Authors contributions
Ling Jin and Kai Fan: conception of the study, literature retrieval, writing of article. Shaoqing Yu: review and revise the article. All authors approved the final version of the article.
Footnotes
Published online 11 December 2023
Ling Jin and Kai Fan contributed equally to this work and share the first authorship.
REFERENCES
- 1.Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, Toppila-Salmi S, Bernal-Sprekelsen M, Mullol J, Alobid I, Terezinha Anselmo-Lima W, Bachert C, Baroody F, von Buchwald C, Cervin A, Cohen N, Constantinidis J, De Gabory L, Desrosiers M, Diamant Z, Douglas RG, Gevaert PH, Hafner A, Harvey RJ, Joos GF, Kalogjera L, Knill A, Kocks JH, Landis BN, Limpens J, Lebeer S, Lourenco O, Meco C, Matricardi PM, O’Mahony L, Philpott CM, Ryan D, Schlosser R, Senior B, Smith TL, Teeling T, Tomazic PV, Wang DY, Wang D, Zhang L, Agius AM, Ahlstrom-Emanuelsson C, Alabri R, Albu S, Alhabash S, Aleksic A, Aloulah M, Al-Qudah M, Alsaleh S, Baban MA, Baudoin T, Balvers T, Battaglia P, Bedoya JD, Beule A, Bofares KM, Braverman I, Brozek-Madry E, Richard B, Callejas C, Carrie S, Caulley L, Chussi D, de Corso E, Coste A, El Hadi U, Elfarouk A, Eloy PH, Farrokhi S, Felisati G, Ferrari MD, Fishchuk R, Grayson W, Goncalves PM, Grdinic B, Grgic V, Hamizan AW, Heinichen JV, Husain S, Ping TI, Ivaska J, Jakimovska F, Jovancevic L, Kakande E, Kamel R, Karpischenko S, Kariyawasam HH, Kawauchi H, Kjeldsen A, Klimek L, Krzeski A, Kopacheva Barsova G, Kim SW, Lal D, Letort JJ, Lopatin A, Mahdjoubi A, Mesbahi A, Netkovski J, Nyenbue Tshipukane D, Obando-Valverde A, Okano M, Onerci M, Ong YK, Orlandi R, Otori N, Ouennoughy K, Ozkan M, Peric A, Plzak J, Prokopakis E, Prepageran N, Psaltis A, Pugin B, Raftopulos M, Rombaux P, Riechelmann H, Sahtout S, Sarafoleanu C-C, Searyoh K, Rhee C-S, Shi J, Shkoukani M, Shukuryan AK, Sicak M, Smyth D, Sindvongs K, Soklic Kosak T, Stjarne P, Sutikno B, Steinsvag S, Tantilipikorn P, Thanaviratananich S, Tran T, Urbancic J, Valiulius A, Vasquez de Aparicio C, Vicheva D, Virkkula PM, Vicente G, Voegels R, Wagenmann MM, Wardani RS, Welge-Lussen A, Witterick I, Wright E, Zabolotniy D, Zsolt B, Zwetsloot CP. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58(Suppl S29):1-464. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Quan H, Faris P, Garies S, Liu M, Bird C, Kukec E, Dean S, Rudmik L. Prevalence and incidence of diagnosed chronic rhinosinusitis in Alberta, Canada. JAMA Otolaryngol Head Neck Surg. 2016;142:1063-1069. [DOI] [PubMed] [Google Scholar]
- 3.Barham HP, Harvey RJ. Nasal saline irrigation: therapeutic or homeopathic. Braz J Otorhinolaryngol. 2015;81:457-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, Orlandi RR, Palmer JN, Patel ZM, Peters A, Walsh SA, Corrigan MD. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1-S39. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins C. Chronic rhinosinusitis with nasal polyps. N Engl J Med. 2019;381:55-63. [DOI] [PubMed] [Google Scholar]
- 6.Gallant JN, Basem JI, Turner JH, Shannon CN, Virgin FW. Nasal saline irrigation in pediatric rhinosinusitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;108:155-162. [DOI] [PubMed] [Google Scholar]
- 7.Chandy Z, Ference E, Lee JT. Clinical guidelines on chronic rhinosinusitis in children. Curr Allergy Asthma Rep. 2019;19:14. [DOI] [PubMed] [Google Scholar]
- 8.Yoo F, Ference EH, Kuan EC, Lee JT, Wang MB, Suh JD. Evaluation of patient nasal saline irrigation practices following endoscopic sinus surgery. Int Forum Allergy Rhinol. 2018;8:32-40. [DOI] [PubMed] [Google Scholar]
- 9.Borish L, Baroody FM, Kim MS, Lieberman JA, Peters A, Stevens WW, Bernstein JA. Yardstick for the medical management of chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2022;128:118-128. [DOI] [PubMed] [Google Scholar]
- 10.Shao S, Zheng M, Wang X, Latiff AH, Kim DY, Wang JY, Recto M, Lucas M, Sonomjamts M, Logi N, Lestari N, Irawati N, Tantilipikorn P, Bhargava S, Ms S, Shimizu T, Leung TF, Kamchaisatian W, Pawankar R, Zhang L. Asia-Pacific survey of physicians’ perceptions and managements of chronic rhinosinusitis. Asian Pac J Allergy Immunol. 2022. [DOI] [PubMed] [Google Scholar]
- 11.Phillips KM, Hoehle LP, Caradonna DS, Gray ST, Sedaghat AR. Intranasal corticosteroids and saline: usage and adherence in chronic rhinosinusitis patients. Laryngoscope. 2020;130:852-856. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH. Mechanisms of glucocorticoid action in chronic rhinosinusitis. Allergy Asthma Immunol Res. 2015;7:534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho SW, Kim DW, Kim JW, Lee CH, Rhee CS. Classification of chronic rhinosinusitis according to a nasal polyp and tissue eosinophilia: limitation of current classification system for Asian population. Asia Pac Allergy. 2017;7:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamad S, Hamid SSA, Azlina A, Md Shukri N. Association of IL-1 gene polymorphisms with chronic rhinosinusitis with and without nasal polyp. Asia Pac Allergy. 2019;9:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achilles N, Mösges R. Nasal saline irrigations for the symptoms of acute and chronic rhinosinusitis. Curr Allergy Asthma Rep. 2013;13:229-235. [DOI] [PubMed] [Google Scholar]
- 16.Fandino A, Douglas R. A historical review of the evolution of nasal lavage systems. J Laryngol Otol. 2021;135:110-116. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Jin L, Liu SX, Fan K, Qin ML, Yu SQ. Role of nasal saline irrigation in the treatment of allergic rhinitis in children and adults: a systematic analysis. Allergol Immunopathol (Madr). 2020;48:360-367. [DOI] [PubMed] [Google Scholar]
- 18.Cirkovic I, Pavlovic B, Bozic DD, Jotic A, Bakic L, Milovanovic J. Antibiofilm effects of topical corticosteroids and intranasal saline in patients with chronic rhinosinusitis with nasal polyps depend on bacterial species and their biofilm-forming capacity. Eur Arch Otorhinolaryngol. 2017;274:1897-1903. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Kang H, Hong S, Shen Y. Effect of postoperative specific immunotherapy combined with nasal irrigation on chronic rhinosinusitis with allergic rhinitis. Iran J Allergy Asthma Immunol. 2021;20:432-440. [PubMed] [Google Scholar]
- 20.Fandino A, Douglas R. A historical review of the evolution of nasal lavage systems. J Laryngol Otol. 2021;135:110-116. [DOI] [PubMed] [Google Scholar]
- 21.Michalik M, Samet A, Dmowska-Koroblewska A, Podbielska-Kubera A, Waszczuk-Jankowska M, Struck-Lewicka W, Markuszewski MJ. An overview of the application of systems biology in an understanding of Chronic Rhinosinusitis (CRS) development. J Pers Med. 2020;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Paiva Leite SH, Douglas RG. How does sinus surgery affect topical irrigation distribution? Curr Opin Otolaryngol Head Neck Surg. 2018;26:21-26. [DOI] [PubMed] [Google Scholar]
- 23.Barham HP, Hall CA, Hernandez SC, Zylicz HE, Stevenson MM, Zito BA, Harvey RJ. Impact of Draf III, Draf IIb, and Draf IIa frontal sinus surgery on nasal irrigation distribution. Int Forum Allergy Rhinol. 2020;10:49-52. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Ho J, Grayson JW, Alvarado R, Rimmer J, Sewell WA, Campbell R, Kalish L, Sacks R, Harvey RJ. Evaluation of diffuse type 2 dominant or eosinophilic chronic rhinosinusitis with corticosteroid irrigation after surgical neosinus cavity formation. JAMA Otolaryngol Head Neck Surg. 2021;147:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giotakis AI, Karow EM, Scheithauer MO, Weber R, Riechelmann H. Saline irrigations following sinus surgery - a controlled, single blinded, randomized trial. Rhinology. 2016;54:302-310. [DOI] [PubMed] [Google Scholar]
- 26.Nikakhlagh S, Abshirini H, Lotfi M, Mohammadi SM, Saki N. A comparison between the effects of nasal lavage with hypertonic, isotonic and hypotonic saline solutions for the treatment of chronic sinusitis. J Global Pharma Technol. 2016;8:68-73. [Google Scholar]
- 27.Kanjanawasee D, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Hypertonic Saline versus isotonic Saline Nasal irrigation: systematic review and meta-analysis. Am J Rhinol Allergy. 2018;32:269-279. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Pan M, Li Y, Tan G, Yang Y. Efficacy of nasal irrigation with hypertonic saline on chronic rhinosinusitis: systematic review and meta-analysis. Braz J Otorhinolaryngol. 2020;86:639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanan A, Rabinowitz M, Rosen M, Nyquist G. Topical therapies for refractory chronic rhinosinusitis. Otolaryngol Clin North Am. 2017;50:129-141. [DOI] [PubMed] [Google Scholar]
- 30.Low TH, Woods CM, Ullah S, Carney AS. A double-blind randomized controlled trial of normal saline, lactated Ringer’s, and hypertonic saline nasal irrigation solution after endoscopic sinus surgery. Am J Rhinol Allergy. 2014;28:225-231. [DOI] [PubMed] [Google Scholar]
- 31.Cherian LM, Cooksley C, Richter K, Ramezanpour M, Paramasivan S, Wormald P-J, Vreugde S, Psaltis AJ. Effect of commercial nasal steroid preparation on bacterial growth. Int Forum Allergy Rhinol. 2019;9:766-775. [DOI] [PubMed] [Google Scholar]
- 32.Orlandi RR, Kingdom TT, Smith TL, Bleier B, DeConde A, Luong AU, Poetker DM, Soler Z, Welch KC, Wise SK, Adappa N, Alt JA, Anselmo-Lima WT, Bachert C, Baroody FM, Batra PS, Bernal-Sprekelsen M, Beswick D, Bhattacharyya N, Chandra RK, Chang EH, Chiu A, Chowdhury N, Citardi MJ, Cohen NA, Conley DB, DelGaudio J, Desrosiers M, Douglas R, Eloy JA, Fokkens WJ, Gray ST, Gudis DA, Hamilos DL, Han JK, Harvey R, Hellings P, Holbrook EH, Hopkins C, Hwang P, Javer AR, Jiang RS, Kennedy D, Kern R, Laidlaw T, Lal D, Lane A, Lee HM, Lee JT, Levy JM, Lin SY, Lund V, McMains KC, Metson R, Mullol J, Naclerio R, Oakley G, Otori N, Palmer JN, Parikh SR, Passali D, Patel Z, Peters A, Philpott C, Psaltis AJ, Ramakrishnan VR, Ramanathan M, Jr, Roh HJ, Rudmik L, Sacks R, Schlosser RJ, Sedaghat AR, Senior BA, Sindwani R, Smith K, Snidvongs K, Stewart M, Suh JD, Tan BK, Turner JH, van Drunen CM, Voegels R, Wang Y, Woodworth BA, Wormald PJ, Wright ED, Yan C, Zhang L, Zhou B. International consensus statement on rhinology and allergy: rhinosinusitis. Int Forum Allergy Rhinol. 2021;11:213-739. [DOI] [PubMed] [Google Scholar]
- 33.Snidvongs K, Pratt E, Chin D, Sacks R, Earls P, Harvey RJ. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2012;2:415-421. [DOI] [PubMed] [Google Scholar]
- 34.Jang DW, Lachanas VA, Segel J, Kountakis SE. Budesonide nasal irrigations in the postoperative management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3:708-711. [DOI] [PubMed] [Google Scholar]
- 35.Kang TW, Chung JH, Cho SH, Lee SH, Kim KR, Jeong JH. The effectiveness of budesonide nasal irrigation after endoscopic sinus surgery in chronic rhinosinusitis with asthma. Clin Exp Otorhinolaryngol. 2017;10:91-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson B, Gutteridge I, Cervin A, Robinson D. The effects of nasal lavage with betamethasone cream post-endoscopic sinus surgery: clinical trial. J Laryngol Otol. 2018;132:143-149. [DOI] [PubMed] [Google Scholar]
- 37.Huang ZZ, Chen XZ, Huang JC, Wang Z-Y, Li X, Chen X-H, Lai X-P, Chang L-H, Zhang G-H. Budesonide nasal irrigation improved Lund-Kennedy endoscopic score of chronic rhinosinusitis patients after endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2019;276:1397-1403. [DOI] [PubMed] [Google Scholar]
- 38.Thanneru M, Lanke S, Kolavali S. The effectiveness of Budesonide nasal irrigation after endoscopic sinus surgery in chronic allergic rhinosinusitis with polyps. Indian J Otolaryngol Head Neck Surg. 2020;72:350-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luz-Matsumoto GR, Cabernite-Marchetti E, Sasaki LSK, Marquez GJ, de Lacerda LS, de Almeida TR, Kosugi EM. Nasal irrigation with corticosteroids in Brazil: the clinical response of 1% compounded budesonide drops and betamethasone cream. Braz J Otorhinolaryngol. 2021;88:S32-S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosugi EM, Moussalem GF, Simões JC, Souza Rafael de Paula e Silva Felici de, Chen VG, Saraceni Neto P, Mendes Neto JA. Topical therapy with high-volume budesonide nasal irrigations in difficult-to-treat chronic rhinosinusitis. Braz J Otorhinolaryngol. 2016;82:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tait S, Kallogjeri D, Suko J, Kukuljan S, Schneider J, Piccirillo JF. Effect of budesonide added to large-volume, low-pressure saline sinus irrigation for chronic rhinosinusitis: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2018;144:605-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawal RB, Deal AM, Ebert CS, Jr, Dhandha VH, Mitchell CA, Hang AX, Gore MR, Senior BA, Zanation AM. Post-operative budesonide irrigations for patients with polyposis: a blinded, randomized controlled trial. Rhinology. 2015;53:227-234. [DOI] [PubMed] [Google Scholar]
- 43.Man LX, Farhood Z, Luong A, Fakhri S, Feldman RM, Orlander PR, Citardi MJ. The effect of intranasal fluticasone propionate irrigations on salivary cortisol, intraocular pressure, and posterior subcapsular cataracts in postsurgical chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2013;3:953-957. [DOI] [PubMed] [Google Scholar]
- 44.Harvey RJ, Snidvongs K, Kalish LH, Oakley GM, Sacks R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double-blinded placebo-controlled trial for chronic rhinosinusitis after sinus surgery. Int Forum Allergy Rhinol. 2018;8:461-470. [DOI] [PubMed] [Google Scholar]
- 45.Jiramongkolchai P, Peterson A, Kallogjeri D, Lee JJ, Kukuljan S, Liebendorfer A, Schneider JS, Klatt-Cromwell CN, Drescher AJ, Piccirillo JF. Randomized clinical trial to evaluate mometasone lavage vs spray for patients with chronic rhinosinusitis without nasal polyps who have not undergone sinus surgery. Int Forum Allergy Rhinol. 2020;10:936-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon HY, Lee HS, Kim IH, Hwang SH. Post-operative corticosteroid irrigation for chronic rhinosinusitis after endoscopic sinus surgery: a meta-analysis. Clin Otolaryngol. 2018;43:525-532. [DOI] [PubMed] [Google Scholar]
- 47.Grayson JW, Harvey RJ. Topical corticosteroid irrigations in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9:S9-S15. [DOI] [PubMed] [Google Scholar]
- 48.Jiramongkolchai P, Patel S, Schneider JS. Use of off-label Nasal steroid irrigations in long-term management of chronic rhinosinusitis. Ear Nose Throat J. 2021;100:329-334. [DOI] [PubMed] [Google Scholar]
- 49.Freeman CM, Rank MA. Optimal use of intranasal corticosteroids and intranasal saline, is there a clear answer? Curr Opin Allergy Clin Immunol. 2021;21:394-400. [DOI] [PubMed] [Google Scholar]
- 50.Jervis-Bardy J, Boase S, Psaltis A, Foreman A, Wormald PJ. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope. 2012;122:2148-2153. [DOI] [PubMed] [Google Scholar]
- 51.Lee VS, Pottinger PS, Davis GE. Tolerability and effectiveness of povidone-iodine or mupirocin versus saline sinus irrigations for chronic rhinosinusitis. Am J Otolaryngol. 2020;41:102604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hon K, Liu S, Cooksley C, Vreugde S, Psaltis AJ. Low pH nasal rinse solution enhances mupirocin antimicrobial efficacy. Rhinology. 2022. [DOI] [PubMed] [Google Scholar]
- 53.Snidvongs K, Thanaviratananich S. Update on intranasal medications in rhinosinusitis. Curr Allergy Asthma Rep. 2017;17:47. [DOI] [PubMed] [Google Scholar]
- 54.Carlton DA, Beahm DD, Chiu AG. Topical antibiotic therapy in chronic rhinosinusitis: an update. Int Forum Allergy Rhinol. 2019;9:S27-S31. [DOI] [PubMed] [Google Scholar]
- 55.Lin L, Tang X, Wei J, Dai F, Sun G. Xylitol nasal irrigation in the treatment of chronic rhinosinusitis. Am J Otolaryngol. 2017;38:383-389. [DOI] [PubMed] [Google Scholar]
- 56.Weissman JD, Fernandez F, Hwang PH. Xylitol nasal irrigation in the management of chronic rhinosinusitis: a pilot study. Laryngoscope. 2011;121:2468-2472. [DOI] [PubMed] [Google Scholar]
- 57.Silva FERD, Pauna HF, Hurtado JGGM, Dos Santos MCJ. Symptom assessment after nasal irrigation with xylitol in the postoperative period of endonasal endoscopic surgery. Braz J Otorhinolaryngol. 2022;88:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayes G, Wright N, Gardner SL, Telzrow CL, Wommack AJ, Vigueira PA. Manuka honey and methylglyoxal increase the sensitivity of Staphylococcus aureus to linezolid. Lett Appl Microbiol. 2018;66:491-495. [DOI] [PubMed] [Google Scholar]
- 59.Lee VS, Humphreys IM, Purcell PL, Davis GE. Manuka honey sinus irrigation for the treatment of chronic rhinosinusitis: a randomized controlled trial. Int Forum Allergy Rhinol. 2017;7:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ooi ML, Jothin A, Bennett C, Ooi EH, Vreugde S, Psaltis AJ, Wormald P-J. Manuka honey sinus irrigations in recalcitrant chronic rhinosinusitis: phase 1 randomized, single-blinded, placebo-controlled trial. Int Forum Allergy Rhinol. 2019;9:1470-1477. [DOI] [PubMed] [Google Scholar]
- 61.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260-268. [DOI] [PubMed] [Google Scholar]
- 62.Panchmatia R, Payandeh J, Al-Salman R, Kakande E, Habib A-R, Mullings W, Javer AR. The efficacy of diluted topical povidone-iodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study. Eur Arch Otorhinolaryngol. 2019;276:3373-3381. [DOI] [PubMed] [Google Scholar]
- 63.Yu MS, Kim BH, Kang SH, Lim DJ. Low-concentration hypochlorous acid nasal irrigation for chronic sinonasal symptoms: a prospective randomized placebo-controlled study. Eur Arch Otorhinolaryngol. 2017;274:1527-1533. [DOI] [PubMed] [Google Scholar]
- 64.Cho HJ, Min HJ, Chung HJ, Park D-Y, Seong SY, Yoon J-H, Lee J-G, Kim C-H. Improved outcomes after low-concentration hypochlorous acid nasal irrigation in pediatric chronic sinusitis. Laryngoscope. 2016;126:791-795. [DOI] [PubMed] [Google Scholar]
- 65.Casale M, Moffa A, Sabatino L, Pace A, Oliveto G, Vitali M, Baptista P, Salvinelli F. Hyaluronic acid: perspectives in upper aero-digestive tract. A systematic review. PLoS One. 2015;10:e0130637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fong E, Garcia M, Woods CM, Ooi E. Hyaluronic acid for post sinus surgery care: systematic review and meta-analysis. J Laryngol Otol. 2017;131:S2-S11. [DOI] [PubMed] [Google Scholar]
- 67.Mozzanica F, Preti A, Gera R, Bulgheroni C, Cardella A, Albera A, Collurà F, Mevio N, Dragonetti A, Schindler A, Milanesi U, Ottaviani F. Double-blind, randomised controlled trial on the efficacy of saline nasal irrigation with sodium hyaluronate after endoscopic sinus surgery. J Laryngol Otol. 2019;133:300-308. [DOI] [PubMed] [Google Scholar]
- 68.Savietto E, Marioni G, Maculan P, Pettorelli A, Scarpa B, Simoni E, Astolfi L, Marchese-Ragona R, Ottaviano G. Effectiveness of micronized nasal irrigations with hyaluronic acid/isotonic saline solution in non-polipoid chronic rhinosinusitis: A prospective, randomized, double-blind, controlled study. Am J Otolaryngol. 2020;41:102502. [DOI] [PubMed] [Google Scholar]
- 69.Chen XZ, Feng SY, Chang LH, Lai XP, Chen XH, Li X, Zhang GH. The effects of nasal irrigation with various solutions after endoscopic sinus surgery: systematic review and meta-analysis. J Laryngol Otol. 2018;132:673-679. [DOI] [PubMed] [Google Scholar]
- 70.Cisek AA, Dabrowska I, Gregorczyk KP, Wyzewski Z. Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol. 2017;74:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang G, Zhao Y, Paramasivan S, Richter K, Morales S, Wormald P-J, Vreugde S. Bacteriophage effectively kills multidrug resistant Staphylococcus aureus clinical isolates from chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2018;8:406-414. [DOI] [PubMed] [Google Scholar]
- 72.Drilling AJ, Ooi ML, Miljkovic D, James C, Speck P, Vreugde S, Clark J, Wormald P-J. Long-term safety of topical 66. Bacteriophage application to the frontal sinus region. Front Cell Infect Microbiol. 2017;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ooi ML, Drilling AJ, Morales S, Fong S, Moraitis S, Macias-Valle L, Vreugde S, Psaltis AJ, Wormald P-J. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to staphylococcus aureus. JAMA Otolaryngol Head Neck Surg. 2019;145:723-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goggin R, Jardeleza C, Wormald PJ, Vreugde S. Colloidal silver: a novel treatment for Staphylococcus aureus biofilms? Int Forum Allergy Rhinol. 2014;4:171-175. [DOI] [PubMed] [Google Scholar]
- 75.Ooi ML, Richter K, Bennett C, Macias-Valle L, Vreugde S, Psaltis AJ, Wormald P-J. Topical colloidal silver for the treatment of recalcitrant chronic rhinosinusitis. Front Microbiol. 2018;9:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feizi S, Cooksley CM, Nepal R, Psaltis AJ, Wormald PJ, Vreugde S. Silver nanoparticles as a bioadjuvant of antibiotics against biofilm-mediated infections with methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in chronic rhinosinusitis patients. Pathology (Phila). 2021;54:453-459. [DOI] [PubMed] [Google Scholar]
- 77.Mrowicka M, Zielinska-Blizniewska H, Milonski J, Olszewski J, Majsterek I. Evaluation of oxidative DNA damage and antioxidant defense in patients with nasal polyps. Redox Rep. 2015;20:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramanathan M, Jr, Tharakan A, Sidhaye VK, Lane AP, Biswal S, London NR, Jr. Disruption of sinonasal epithelial Nrf2 enhances susceptibility to rhinosinusitis in a mouse model. Laryngoscope. 2021;131:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu S, Zhao C, Che N, Jin L, Ge R. Hydrogen-rich saline attenuates eosinophil activation in a guinea pig model of allergic rhinitis via reducing oxidative stress. J Inflamm (Lond). 2017;14:1.1-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao C, Yu S, Li J, Xu W, Ge R. Changes in IL-4 and IL-13 expression in allergic-rhinitis treated with hydrogen-rich saline in guinea-pig model. Allergol Immunopathol (Madr). 2017;45:350-355. [DOI] [PubMed] [Google Scholar]
- 81.Jin L, Fan K, Tan S, Liu S, Ge Q, Wang Y, Ai Z, Yu S. The beneficial effects of hydrogen-rich Saline irrigation on chronic rhinitis: a randomized, double-blind clinical trial. J Inflamm Res. 2022;15:3983-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang A, Govindaraj S. Topical therapy in the management of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2013;21:31-38. [DOI] [PubMed] [Google Scholar]
- 83.Orlandi RR, Kingdom TT, Hwang PH. International Consensus Statement on Allergy and Rhinology: rhinosinusitis executive summary. Int Forum Allergy Rhinol. 2016;6(suppl 1):S3-21. [DOI] [PubMed] [Google Scholar]
- 84.Chen PG, Murphy J, Alloju LM, Boase S, Wormald PJ. Sinus penetration of a pulsating device versus the classic squeeze bottle in cadavers undergoing sinus surgery. Ann Otol Rhinol Laryngol. 2017;126:9-13. [DOI] [PubMed] [Google Scholar]
- 85.Piromchai P, Puvatanond C, Kirtsreesakul V, Chaiyasate S, Suwanwech T. A multicenter survey on the effectiveness of nasal irrigation devices in rhinosinusitis patients. Laryngoscope Investig Otolaryngol. 2020;5:1003-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang L, Wang XD, Huang ZG, Wang XD, Wang CS, Kan C, Yang J, Zhou S. An adjustable irrigator for the nasal sinuses. C.N.Patent 218870810U, April 18, 2023. [Google Scholar]
- 87.Meera S, Vandana Rani M, Sreedhar C, Robin DT. A review on the therapeutic effects of NetiKriya with special reference to JalaNeti. J Ayurveda Integr Med. 2020;11:185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas WW, 3rd, Harvey RJ, Rudmik L, Hwang PH, Schlosser RJ. Distribution of topical agents to the paranasal sinuses: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013;3:691-703. [DOI] [PubMed] [Google Scholar]
- 89.Griggs ZH, Williams A M, Craig JR. Head and bottle angles achieved by patients during high-volume sinonasal irrigations. Am J Rhinol Allergy. 2019;33:302-309. [DOI] [PubMed] [Google Scholar]
- 90.Inthavong K, Shang Y, Wong E, Singh N. Characterization of nasal irrigation flow from a squeeze bottle using computational fluid dynamics. Int Forum Allergy Rhinol. 2020;10:29-40. [DOI] [PubMed] [Google Scholar]
- 91.Sekine M, Goto F, Saito K, Kaneda S, Yamamoto H, Murakami T, Okami K. Unusual complication of nasal irrigation: three case reports of nasal septal perforation. Tokai J Exp Clin Med. 2021;46:105-109. [PubMed] [Google Scholar]
- 92.Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: a systematic review. JAMA. 2015;314:926-939. [DOI] [PubMed] [Google Scholar]
- 93.Liu DQ, Liu JW, Liu JM. Effect of nasal endoscopic rinse fluid temperature on the healing time of nasal mucosa. Chin J Nur Tra. 2008;23:1782-1783. [Google Scholar]
- 94.Ottaviano G, Marioni G, Giacomelli L, La Torre FB, Staffieri C, Marchese-Ragona R, Staffieri A. Smoking and chronic rhinitis: effects of nasal irrigations with sulfurous-arsenical-ferruginous thermal water: a prospective, randomized, double-blind study. Am J Otolaryngol. 2012;33:657-662. [DOI] [PubMed] [Google Scholar]
- 95.Nimsakul S, Ruxrungtham S, Chusakul S, Kanjanaumporn J, Aeumjaturapat S, Snidvongs K. Does heating up saline for nasal irrigation improve mucociliary function in chronic rhinosinusitis? Am J Rhinol Allergy. 2018;32:106-111. [DOI] [PubMed] [Google Scholar]
- 96.Pham V, Sykes K, Wei J. Long-term outcome of once daily nasal irrigation for the treatment of pediatric chronic rhinosinusitis. Laryngoscope. 2014;124:1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Casale M, Moffa A, Cassano M, Carinci F, Lopez MA, Trecca EMC, Torretta S, Rinaldi V, Pignataro L. Saline nasal irrigations for chronic rhinosinusitis: from everyday practice to evidence-based medicine. An update. Int J Immunopathol Pharmacol. 2018;32:2058738418802676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keen M, Foreman A, Wormald PJ. The clinical signifi-cance of nasal irrigation bottle contamination. Laryngoscope. 2010;120:2110-2114. [DOI] [PubMed] [Google Scholar]
- 99.Psaltis AJ, Foreman A, Wormald JP, Schlosser RJ. Contamination of sinus irrigation devices: a review of the evidence and clinical relevance. Am J Rhinol Allergy. 2012;26:201-203. [DOI] [PubMed] [Google Scholar]
- 100.Piper KJ, Foster H, Susanto D, Maree CL, Thornton SD, Cobbs CS. Fatal Balamuthia mandrillaris brain infection associated with improper nasal lavage. Int J Infect Dis. 2018;77:18-22. [DOI] [PubMed] [Google Scholar]
- 101.Sowerby LJ, Wright ED. Tap water or “sterile” water for sinus irrigations: what are our patients using? Int Forum Allergy Rhinol. 2012;2:300-302. [DOI] [PubMed] [Google Scholar]
- 102.Hauser LJ, Ir D, Kingdom TT, Robertson CE, Frank DN, Ramakrishnan VR. Evaluation of bacterial transmission to the paranasal sinuses through sinus irrigation. Int Forum Allergy Rhinol. 2016;6:800-806. [DOI] [PubMed] [Google Scholar]
- 103.Hardy ET, Stringer SP, O’Callaghan R, Arana A, Bierdeman MA, May WL. Strategies for decreasing contamination of homemade nasal saline irrigation solutions. Int Forum Allergy Rhinol. 2016;6:140-142. [DOI] [PubMed] [Google Scholar]
- 104.Morong S, Lee JM. Microwave disinfection: assessing the risks of irrigation bottle and fluid contamination. Am J Rhinol Allergy. 2012;26:398-400. [DOI] [PubMed] [Google Scholar]