Abstract
PURPOSE
To review the literature exploring endometrial cancer (EC) risk among surgical candidates with germline BRCA1/2 pathogenic variants (PVs) to guide decisions around risk-reducing (rr) hysterectomy in this population.
DESIGN
A comprehensive review was conducted of the current literature that influences clinical practice and informs expert consensus. We present our understanding of EC risk among BRCA1/2 PV carriers, the risk-modifying factors specific to this patient population, and the available research technology that may guide clinical practice in the future. Limitations of the existing literature are outlined.
RESULTS
Patients with BRCA1/2 PVs, those with a personal history of tamoxifen use, those who desire long-term hormone replacement therapy, and/or have an elevated BMI are at higher risk of EC, primarily endometrioid EC and/or uterine papillary serous carcinoma, and may benefit from rr-hysterectomy. Although prescriptive clinical guidelines specific to BRCA1/2 PV carriers could inform decisions around rr-hysterectomy, limitations of the current literature prevent more definitive guidance at this time. A large population-based study of a contemporary cohort of BRCA1/2 PV carriers with lifetime follow-up compared with cancer-gene negative controls would advance this topic and facilitate care decisions.
CONCLUSION
This review validates a potential role for rr-hysterectomy to address EC risk among surgical candidates with BRCA1/2 PVs. Evidence-based clinical guidelines for rr-hysterectomy in BRCA1/2 PV carriers are essential to ensure equitable access to this preventive measure, supporting insurance coverage for patients with either BRCA1 or BRCA2 PVs to pursue rr-hysterectomy. Overall, this review highlights the complexity of EC risk in BRCA1/2 PV carriers and offers a comprehensive framework to shared decision making to inform rr-hysterectomy for BRCA1/2 PV carriers.
BRCA1/2 carriers face ↑ EC risk; ↑ research needed to inform rr-hysterectomy in this population.
INTRODUCTION
Among women with BRCA1/2 germline pathogenic variants (PVs), the lifetime risk of endometrial cancer (EC) is low but higher than that of the general population. Our current understanding of BRCA1/2-associated EC, including its pathogenesis, tumor biology, stage and age at presentation, and associated risk factors, remains limited. There are no discrete recommendations for risk-reducing hysterectomy (rr-hysterectomy) for females with BRCA1/2 PVs. By contrast, the risk of EC is well established in women with Lynch syndrome and PTEN hamartoma tumor syndrome/Cowden syndrome for whom rr-hysterectomy is recommended when childbearing is complete.1,11,12 In this article, we review the existing literature and its limitations, which preclude more definitive clinical guidelines for rr-hysterectomy in BRCA1/2 PV carriers and outline the research necessary to fill this knowledge gap.
EC IN THE GENERAL POPULATION
Uterine cancer consists of uterine sarcoma and EC, which is further subdivided into endometrioid, serous, clear cell, carcinosarcoma (also known as malignant mixed Müllerian tumor), and undifferentiated/dedifferentiated carcinoma. Few studies among BRCA1/2 PV carriers include uterine sarcoma and most work is focused on EC.2,3 In addition to these histologic subtypes, molecular subtypes of EC (ie, polymerase epsilon ultramutated, microsatellite instability hypermutated, copy-number low, copy-number high, and no specific molecular profile) are gaining greater recognition as specific features play increasing roles in treatment decisions.4
The median age for EC in the general population is 63 years, with >75% of cases diagnosed after age 55 years. Most patients are diagnosed with stage I endometrioid EC, which is localized in the uterus.5 As endometrioid EC is an estrogen-sensitive cancer, risk factors include sources of elevated unopposed estrogen (eg, nulliparity, early menarche, late menopause, polycystic ovarian syndrome, and tamoxifen use).6 A particularly aggressive subtype of EC, serous EC, also known as uterine papillary serous carcinoma (UPSC), accounts for <10% of all uterine cancer cases but over 40% of deaths.7 UPSC and clear cell are estrogen-insensitive, more common among Black Americans, and associated with aggressive biology.8 Although survival outcomes among Black Americans with UPSC are poor, tumor biology when characterized by microRNA profiling does not differ by race.9 In recent years, the incidence and mortality of EC has risen in parallel to increasing rates of obesity in the United States.10
Noninvasive screening modalities currently do not exist for EC. Individuals with Lynch syndrome are recommended to receive annual endometrial biopsy and ultrasound starting at age 30-35 years.6 Similarly, there is growing support to screen for EC starting at 35 years in patients with Cowden syndrome.11 However, there are no screening recommendations for EC in the general population or among additional high-risk groups, such as women with elevated BMI or a BRCA1/2 PV.6,12 Moreover, irrespective of personal or familial gene mutations, family history plays a key role in modifying EC risk and warrants consideration if a screening protocol was established for those at risk.13
Among patients who are starting on tamoxifen for breast cancer (BC) treatment or prevention, there were efforts to explore EC screening with serial transvaginal ultrasounds.14-16 Most studies found that patients with abnormal endometrium had abnormal vaginal bleeding, and therefore, the value of screening with transvaginal ultrasounds was low.17 However, because of the higher rate of benign endometrial polyps among patients with postmenopausal BC (16%), the American College of Obstetricians and Gynecologists (ACOG) does recommend consideration of a baseline transvaginal ultrasound before initiating tamoxifen therapy among postmenopausal women.17 Per ACOG guidelines, premenopausal women with no known increased risk of uterine cancer who are starting tamoxifen do not require additional monitoring beyond routine care.17 By contrast, this screening practice is not supported by the National Comprehensive Cancer Network (NCCN) guidelines, which state that endometrial ultrasound and biopsy are not recommended in the absence of symptoms.18
If a patient does develop symptoms of EC, such as abnormal uterine bleeding, appropriate gynecologic care should be pursued. Diagnosis of EC typically consists of a transvaginal ultrasound, followed by hysteroscopy and endometrial biopsy.
LIMITATIONS OF THE CURRENT LITERATURE INFORMING RR-HYSTERECTOMY FOR BRCA1/2 PV CARRIERS
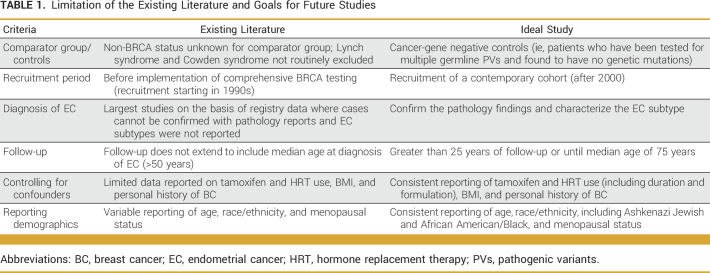
In recent years, systematic reviews and meta-analyses exploring EC risk in BRCA1/2 PV carriers have highlighted the shortcomings of previous work.19-21 Most early studies focused on patients with EC who were subsequently identified as BRCA1/2 PV carriers. Moreover, much of the data are from the 1990s, an era when this patient population was not offered high-risk screening and rr-interventions to manage their breast and ovarian cancer risk. Since many of these reports were not intended as genotype-phenotype correlation studies, histopathologic characteristics of the tumors, including EC subtype, were often unavailable. Therefore, these historic data sets may underrepresent the number of BRCA1/2 PV carriers at risk of EC, particularly UPSC. Now that patients with BRCA1/2 PVs have more effective management of their breast and ovarian cancer risks and improved life expectancy, their lifetime risk of EC may be higher than what historic data indicate.22-24
The longest prospective cohort study was performed by de Jonge et al.25 This study followed 5,980 BRCA1/2 PV carriers for a median of 22.5 years, from a median age ranging from 27.4-51.9 years,25 which is younger than the average age of EC in the general population. Fifty-eight cases of EC were reported.25 As expected in this cohort, the majority of EC occurred after age 40 years. To estimate the risk of developing EC by age 75 years, data were extrapolated using a competing risk analysis. The risk of overall EC was increased among all BRCA1/2 PV carriers (hazard ratio [HR], 2.37; 95% CI, 1.53 to 3.69); in particular, overall EC risk was noted to be higher for BRCA1 versus BRCA2 PV carriers at 3.4% (95% CI, 2.46 to 4.8) versus 2.0% (95% CI, 1.09 to 3.30), respectively.25 Using a competing risk analysis, the risk of developing UPSC by age 75 years was estimated to be 1.1% among all BRCA1/2 PV carriers, with a higher risk for BRCA1 (HR, 10.48; 95% CI, 2.95 to 37.20).25
Shu et al2 performed a multicenter prospective cohort study to estimate risk of EC for 1,083 BRCA1/2 PV carriers who received rr-bilateral salpingo-oophorectomy (rr-BSO) but did not pursue hysterectomy with a median follow-up of 5.1 years. A comparator group was not included. Eight cases of EC were reported.2 In contrast to the study by de Jonge et al,25 overall EC risk was not increased for BRCA1/2 PV carriers (observed:expected [O:E] ratio, 1.9; 95% CI, 0.8 to 3.7).2 However, similar to the study by de Jonge et al,25 risk of UPSC was elevated for BRCA1/2 PV carriers (O:E ratio, 14.8; 95% CI, 4.8 to 34.6), with increased risk for BRCA1 PV carriers (O:E ratio, 22.2; 95% CI, 6.1 to 56.9).2 Assuming a constant annual risk, the risk of developing UPSC by age 70 years was estimated to be 2.6% or 4.7% assuming a relative risk compared with Surveillance, Epidemiology, and End Results data.2
A meta-analysis by Matanes et al20 including 11 studies reported even lower rates of EC for BRCA1/2 PV carriers, 0.58% (82/13,827), which likely reflects limitations of the included studies and not the actual prevalence. Although these estimates are informative, they reveal a need to better characterize the lifetime risk of EC in a prospective cohort of BRCA1/2 PV carriers with long-term follow-up.
As broad population-based cancer genetic testing is not a routine practice, there is no comprehensive data set to compare EC rates in BRCA1/2 PV carriers with cancer-gene negative controls. At a minimum, controls would ideally exclude other high-risk patients with Lynch or Cowden syndrome. In the study by de Jonge et al,25 for example, standardized incidence ratios were calculated comparing cancer rates in BRCA1/2 PV carriers with controls defined as noncarriers, but one third of controls had a previous history of BC, which is almost three-folds higher than expected for the general population (12%-13%). Consequently, 13.7% of controls had received tamoxifen.25 We can glean information from the study by Heald et al,26 where the incidence rates of hereditary EC susceptibility mutations were reported in women with uterine cancer, but this does not inform risk with BRCA1/2 PVs. Previous work has also focused on somatic tumor testing to infer germline BRCA1/2 status, and albeit informative to assess eligibility for therapies such as poly (ADP-ribose) polymerase inhibitors that target tumor cells with BRCA1/2 PVs, these data do not provide an accurate estimate of EC risk in BRCA1/2 PV carriers.2,19
Despite limitations of the existing literature (summarized in Table 1), insurance coverage for prophylactic hysterectomies has been restricted generally to BRCA1 PV carriers in the United States. Historically, NCCN guidelines have emphasized the potential benefit of hysterectomy among BRCA1 PV carriers because of increased risk of UPSC.2,11 Further research is critical to inform evidence-based guidelines for prophylactic hysterectomy among BRCA1 and BRCA2 PV carriers to ensure equitable access to this potentially life-saving intervention.
TABLE 1.
Limitation of the Existing Literature and Goals for Future Studies
BRCA1/2 PV carriers undergo several interventions that may influence the risk of EC. Many of the previous studies do not account for potential confounding factors, such as rr-BSO, type and formulary of hormone replacement therapy (HRT), obesity, tamoxifen, parity, and oral contraceptive use, each of which can affect EC risk. We will discuss four of these factors pertaining to BRCA1/2 PV carriers.
rr-BSO and HRT
Women with germline BRCA1/2 PV are recommended to undergo a rr-BSO after they complete childbearing and before menopause, typically at age 35-40 years for BRCA1 PV carriers and 40-45 years for BRCA2 PV carriers,11 resulting in surgical menopause. HRT is recommended until age 50-51 years, the approximate age of natural menopause, for women in the general population who undergo surgical menopause and have no personal history of BC.27 HRT raises estrogen levels with the goal of reducing risk of cardiovascular disease and osteoporosis, among other mordibidites.28 In addition, vaginal estrogen therapy plays a critical role in the management of pelvic floor dysfunction and sexual health.29,30 Although the data are limited, a small US-based study suggests that hysterectomy adversely affects pelvic floor function, irrespective of menopausal status.31 While hysterectomy alone has not been shown to worsen sexual function, rr-BSO independently and when combined with hysterectomy adversely affects sexual health because of the induced hypoestrogenic state.32,33
Consequently, women with BRCA1/2 PVs who undergo rr-BSO are more likely to start HRT at a younger age and for a longer duration than the general population, particularly BRCA1 PV carriers. The impact of altered estrogen levels after these interventions on EC risk is unknown. Four single-arm studies in patients who have undergone rr-BSO, two of which are prospective studies, reported a low prevalence of EC (0.28%-0.74%) among women with a mean age of 45-50 years with 2-6 years of follow-up.2,34-36 However, as previously noted, the follow-up remains insufficient relative to the age of onset of EC. None of the four studies report on HRT use.2,34-36
To our knowledge, only two studies that explored EC rates among women with BRCA1/2 PV report HRT use for all study participants. Segev et al37 conducted a prospective cohort study of 4,893 women with a germline BRCA1/2 PV across 11 countries wherein participants completed questionnaires at baseline and every 2 years for a mean follow-up of 5.7 years. A subgroup analysis demonstrated no increased risk of EC among women with a history of HRT use. A subsequent case-control study that matched 83 EC cases to 1,027 controls confirmed no association between HRT use and EC.38 Although the type of HRT was reported and stratified by estrogen plus progestin, estrogen-only, and progestin-only, the limited number of observed outcomes for each formulation precludes any substantive inference. An additional four studies describe HRT use in BRCA1/2 PV carriers diagnosed with EC, but no comparator group was included in these reports.39-42
It is also important to consider the impact of HRT duration and formulation not only for EC risk, but also on BC risk in BRCA1/2 PV carriers. Distinct from the general population, HRT after natural menopause and after rr-BSO for BRCA1 PV carriers is not associated with increased BC risk.43,44 In the general population, a meta-analysis by the Collaborative Group on Hormonal Factors in Breast Cancer showed that a longer duration of any HRT and estrogen-progestin HRT (v estrogen-only) is associated with an increased risk of BC.45 Among BRCA1/2 PV carriers with increased BC risk, these findings suggest that estrogen-only HRT is preferred in this setting, and, in fact, among BRCA1 PV carriers who underwent surgical menopause after rr-BSO, the cumulative incidence of BC at 10 years of follow-up was 12% for those who used estrogen-only versus 22% for those who received estrogen plus progestin HRT (P = .04).46 Most likely similar effects occur among women with BRCA2 PVs as these tumors tend to be more hormonally driven and a shorter duration of HRT is required because of later onset of surgical menopause, but this has yet to be reported.
For patients with an intact uterus without the protective effects of progestin, the risks of endometrial hyperplasia and EC are significant, particularly with estrogen-only HRT administered >10 years.47,48 Hence, estrogen-only HRT is contraindicated for patients with an intact uterus.6 Although BRCA1/2 PV carriers are excluded, a phase IIB trial is underway evaluating the use of bazedoxifene and conjugated estrogen among women with a high risk of BC. Bazedoxifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen-receptor agonist in bones but as an antagonist in the uterus and breast tissue. Therefore, this approach may offer an alternative form of HRT that better modulates BC risk in an individual with an intact uterus (ClinicalTrials.gov identifier: NCT04821141).49 Future clinical studies of bazedoxifene and conjugated estrogen in the BRCA1/2 PV carrier population are necessary to minimize the risk of breast and gynecologic cancers.
Obesity
The risk of EC from HRT is influenced by body weight; women who are overweight or obese and receive HRT are at a greater risk of developing EC, specifically the endometrioid histology.50 Independent of HRT, elevated BMI is a well-accepted risk factor for overall EC, including non–estrogen-dependent subtypes.51-53 Only few studies have reported the BMI of patients with BRCA1/2 PVs diagnosed with EC, and these lack a comparator/control group.38,40,54,55 Most studies did not discern a pattern in regard to BMI and EC risk.38,40,55 One study by Lee et al56 noted an elevated BMI in four of five cases of endometrioid EC.
As higher BMI is associated with higher perioperative risk, it bears consideration when recommending prophylactic hysterectomy.57 Although vaginal and laparoscopic hysterectomies are preferred to an abdominal approach,58 complication rates increase with higher BMI for any surgical technique.59 An elevated BMI is also associated with pelvic floor dysfunction after hysterectomy in the general population.60 Therefore, balancing the risk of future EC with operative risks and future quality of life becomes an important factor when considering the benefits of rr-hysterectomy among BRCA1/2 PV carriers with an elevated BMI.
Tamoxifen Use
As a SERM, tamoxifen can act as an antagonist or agonist depending on the tissue. Although tamoxifen blocks estrogen receptors (ER) in the breast tissue, it has an agonistic effect on the endometrium, increasing the risk of endometrial hyperplasia and EC. Consequently, tamoxifen use has been demonstrated to increase the risk of endometrioid EC, uterine sarcomas, and uterine carcinosarcomas.61-63 There is growing evidence suggesting tamoxifen may increase EC risk through the phosphoinositol-3-kinase signaling pathway.64
EC risk bears consideration when prescribing tamoxifen.65 Among BRCA1/2 PV carriers, tamoxifen is used for both BC treatment and prevention. In patients with a perceived ER-positive BC risk, there are now data to support a role for low-dose tamoxifen for prevention.66 Beiner et al40 conducted a prospective cohort study of 857 women with a BRCA1/2 PV, identifying six incident cases of EC after a mean follow-up of 3.3 years, and in four of the cases, tamoxifen use was ≥5 years. A subgroup analysis revealed an increased risk of EC with tamoxifen use. The authors postulated that the increased EC risk among all BRCA1/2 PV carriers can be primarily attributed to tamoxifen treatment for BC.40 Similarly, Segev et al37 noted an increased EC risk with tamoxifen use among all BRCA1/2 PV carriers and specifically among BRCA1/2 PV carriers with a history of BC, concluding that tamoxifen use is the most relevant risk factor for developing EC. Further work confirmed an increased risk of EC with the use of tamoxifen for treatment and prevention of BC among BRCA1 carriers; the odds ratio for EC was 3.66 for any use of tamoxifen and 7.19 for patients on tamoxifen with previous BC.38 Other studies have reported tamoxifen use among BRCA1/2 PV carriers diagnosed with EC, but these are descriptive studies, often with missing information on the dose and duration of use.3,34,36,39,41,67
Studies conducted among patients with BC irrespective of BRCA1/2 PV status have not associated tamoxifen with a specific EC subtype.61,68,69 Although endometrioid EC has historically been considered the most estrogen-sensitive,70 in studies limited to BRCA1/2 PV carriers, the association between endometrioid EC and tamoxifen has not been consistently reported. Shu et al2 reported a higher risk of UPSC among tamoxifen-exposed women (3/273; O:E ratio, 24.4; 95% CI, 5.0 to 71.3). By contrast, other investigators noted that all cases of EC in the BRCA1/2 PV population were of endometrioid subtype and associated with tamoxifen use.40,54
In BRCA1/2 PV carriers diagnosed with an ER-positive BC who do not undergo hysterectomy, the use of an aromatase inhibitor (AI) may be preferable to tamoxifen, particularly in light of the recent SOFT-EST substudy demonstrating greater estrogen suppression with the AI exemestane compared with tamoxifen when used with triptorelin.71,72 However, this is not always possible, given that patients with a higher BMI have higher levels of endogenous estrogen, such that achieving estrogen suppression with AI is more challenging, and therefore, these patients will often receive tamoxifen with ovarian suppression.71 For this subpopulation, the benefits of ovarian function suppression will outweigh the risk of future EC, and therefore, tamoxifen should be continued but in light of existing evidence, and furthermore, obese BRCA1/2 PV carriers diagnosed with ER-positive BC may warrant greater consideration for rr-hysterectomy. It is important to note that future studies would ideally stratify patients on the basis of menopausal status and include duration of tamoxifen use. Consistent reporting of duration and dose of tamoxifen is particularly relevant, given changing recommendations during the past decade for BC treatment and chemoprevention.73
Role of Hereditary Cancer Genetic Testing
Comprehensive hereditary cancer genetic testing, including rearrangement and deletion testing, is recommended for all individuals with a strong family history of BC as of 2005.74 However, in the context of more advanced genetic technology in recent years, the genotype-phenotype correlation of BRCA1/2 PV carriers requires improved calibration to better understand variations in tumor characteristics that are distinct to germline BRCA1 and BRCA2 PVs. Consequently, without greater understanding of EC tumor biology in BRCA1/2 PV carriers, it is unclear how much the EC risk is attributable to other factors, namely those aforementioned, versus driven by homologous recombination deficiency.
There may be an emerging role for single-nucleotide polymorphism (SNP) and polygenic risk score (PRS) testing to predict risk and outcomes of nonendometrioid EC among BRCA1/2 PV carriers.75 However, the low incidence of EC compared with other cancer types with established PRS, such as BC, suggests that it will be challenging to stratify risk and calibrate these diagnostics, and therefore, lowers their utility in clinical practice.
The existing literature on SNPs for EC consists primarily of candidate-gene studies. The largest genome-wide association study (GWAS) consisted of 12,096 cases and 108,979 controls.76 The authors noted that 350,000-400,000 cases would be needed for a GWAS to account for 80% of genetic variance in EC.77 Even if a larger EC GWAS was performed, the findings would not be generalizable to the small subset of patients with a germline BRCA1/2 PV.
Noninvasive biomarkers to help identify patients at risk of EC offer a more promising and feasible solution for clinical practice. This could include methylated DNA, circulating tumor DNA, circulating cell-free DNA, or serum protein biomarkers.78,79 Serum human epididymis protein 4 (HE4) appears to be a promising biomarker, although current data are insufficient to introduce its use in clinical practice.79,80 Circulating tumor markers, such as L1 cell adhesion molecule (L1CAM) and DJ-1, have been associated with nonendometrioid EC and may be of value in this setting as there is a higher prevalence of UPSC in BRCA1 PV carriers.81 Large validation studies are needed in this realm to determine which biomarker adds value to care.79
Tissue biomarkers and tumor genomic testing can inform risk stratification and prognostication of patients diagnosed with EC to allow for a more personalized treatment.79,82 Future work is needed to better characterize the EC tumors that develop in BRCA1/2 PV carriers. These findings can be compared with EC tumor characteristics of patients with Cowden or Lynch syndrome.
Organoid models offer an additional means of understanding tumor biology and cancer progression among BRCA1/2 PV carriers. Similar to studies underway for Lynch syndrome,83 organoid models derived from hyperplastic endometrium from BRCA1/2 PV carriers at the time of hysterectomy can improve our understanding of precancerous changes, including potential biomarkers and genetic alterations in this select population, as well as identifying susceptibility to specific systemic therapies.84
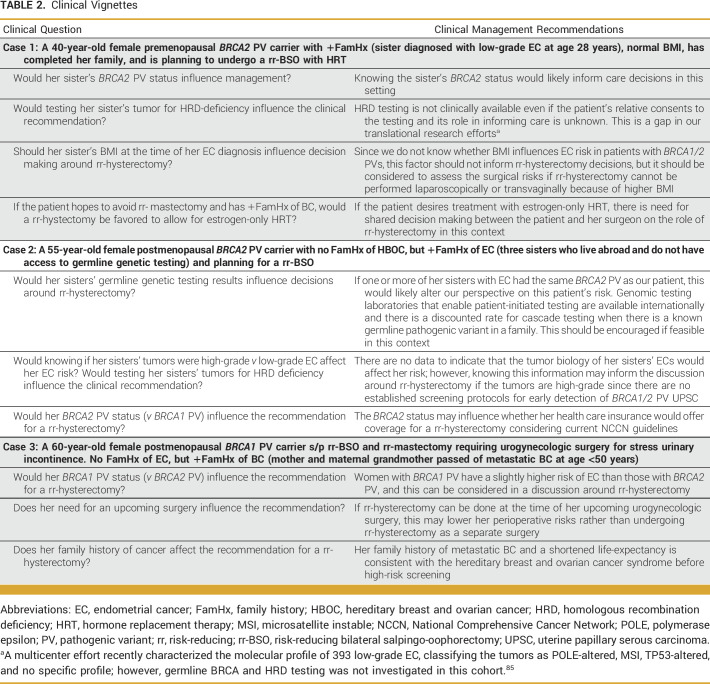
In summary, despite mounting evidence indicating the EC risk in germline BRCA1/2 PV carriers, there are insufficient data to indicate hysterectomy for all women with BRCA1/2 PVs, given the low EC risk and the perioperative risks with surgical intervention. There are special clinical considerations specific to BRCA1/2 PV carriers that must inform the decision about hysterectomy (Table 2), necessitating clinical guidelines for this patient population. Patients with a personal history of BC, tamoxifen use, interest in long-term HRT, and/or elevated BMI are at higher risk of EC, primarily endometrioid EC and/or UPSC as per available evidence, and may benefit from rr-hysterectomy if their life expectancy is favorable. However, limitations of the current literature prevent more definitive clinical guidelines. A large population-based study of a contemporary cohort with a lifetime follow-up compared with cancer-gene negative controls would advance this topic and facilitate care decisions. As data evolve, decisions regarding rr-hysterectomy should be shared between patients and clinicians with expertise in the field. Moreover, since germline PVs in both BRCA1 and BRCA2 increase EC risk, national guidelines and insurers should support coverage for patients with either BRCA1 or BRCA2 PVs who elect to pursue hysterectomy to reduce their risk of EC. The current understanding of EC risk in BRCA1/2 PV carriers is sufficient to suggest that rr-hysterectomy may have a potential life-saving impact in this patient population. Therefore, concerted efforts are required to facilitate development of clinical guidelines to establish a standard of care to manage EC risk among BRCA1/2 PV carriers.
TABLE 2.
Clinical Vignettes
Filipa Lynce
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo/Astra Zeneca, OncoSec, AmerisourceBergen, Pfizer, Prime Education
Research Funding: Bristol Myers Squibb, Immunomedics (Inst), Inivata (Inst), AstraZeneca/Daiichi Sankyo (Inst), CytomX Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1313721
Colleen M. Feltmate
Consulting or Advisory Role: Avania
Patents, Royalties, Other Intellectual Property: UpToDate
Michelle R. Davis
Consulting or Advisory Role: Vicarious Surgical
Panagiotis A. Konstantinopoulos
Consulting or Advisory Role: Merck, Vertex, AstraZeneca, Pfizer/EMD Serono, Tesaro, Bayer, Alkermes, Repare Therapeutics, Kadmon, Mersana, Novartis, AADi, Artios, Immunogen
Research Funding: Pfizer (Inst), Lilly (Inst), Tesaro (Inst), Merck Serono (Inst), AstraZeneca (Inst), Merck (Inst), Bayer (Inst), Bristol Myers Squibb/Sanofi (Inst), Novartis (Inst)
Elizabeth H. Stover
Patents, Royalties, Other Intellectual Property: Patent pending
Allison W. Kurian
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech, Myriad Genetics, Adela
Sarah J. Hill
Research Funding: Lilly, AstraZeneca, Merck
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for co-authoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, AstraZeneca, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Seagen, CytomX Therapeutics, Daiichi Sankyo, Immunomedics/Gilead, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Myovant Sciences, Umoja Biopharma, Menarini Group, AADi, Artios Biopharmaceuticals, Incyte, Zetagen, Bayer, Ellipses Pharma, OncoSec, Infinity Pharmaceuticals, Jazz Pharmaceuticals
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Seagen (Inst), OncoPep (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Lilly, Sanofi
Judy E. Garber
Consulting or Advisory Role: Novartis, Kronos Bio, GV20 Therapeutics, Belharra Therapeutics, Inc, Earli, Inc
Research Funding: Novartis, Ambry Genetics, InVitae, Amgen
Other Relationship: AACR, Diana Helis Henry Medical Foundation, James P. Wilmot Foundation, Adrienne Helis Malvin Medical Research Foundation, Breast Cancer Research Foundation, Facing our Risk of Cancer Empowered
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Kimia Sorouri, Brittany L. Bychkovsky
Collection and assembly of data: Kimia Sorouri, Brittany L. Bychkovsky
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Filipa Lynce
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo/Astra Zeneca, OncoSec, AmerisourceBergen, Pfizer, Prime Education
Research Funding: Bristol Myers Squibb, Immunomedics (Inst), Inivata (Inst), AstraZeneca/Daiichi Sankyo (Inst), CytomX Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1313721
Colleen M. Feltmate
Consulting or Advisory Role: Avania
Patents, Royalties, Other Intellectual Property: UpToDate
Michelle R. Davis
Consulting or Advisory Role: Vicarious Surgical
Panagiotis A. Konstantinopoulos
Consulting or Advisory Role: Merck, Vertex, AstraZeneca, Pfizer/EMD Serono, Tesaro, Bayer, Alkermes, Repare Therapeutics, Kadmon, Mersana, Novartis, AADi, Artios, Immunogen
Research Funding: Pfizer (Inst), Lilly (Inst), Tesaro (Inst), Merck Serono (Inst), AstraZeneca (Inst), Merck (Inst), Bayer (Inst), Bristol Myers Squibb/Sanofi (Inst), Novartis (Inst)
Elizabeth H. Stover
Patents, Royalties, Other Intellectual Property: Patent pending
Allison W. Kurian
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech, Myriad Genetics, Adela
Sarah J. Hill
Research Funding: Lilly, AstraZeneca, Merck
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for co-authoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, AstraZeneca, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Seagen, CytomX Therapeutics, Daiichi Sankyo, Immunomedics/Gilead, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Myovant Sciences, Umoja Biopharma, Menarini Group, AADi, Artios Biopharmaceuticals, Incyte, Zetagen, Bayer, Ellipses Pharma, OncoSec, Infinity Pharmaceuticals, Jazz Pharmaceuticals
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Seagen (Inst), OncoPep (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Lilly, Sanofi
Judy E. Garber
Consulting or Advisory Role: Novartis, Kronos Bio, GV20 Therapeutics, Belharra Therapeutics, Inc, Earli, Inc
Research Funding: Novartis, Ambry Genetics, InVitae, Amgen
Other Relationship: AACR, Diana Helis Henry Medical Foundation, James P. Wilmot Foundation, Adrienne Helis Malvin Medical Research Foundation, Breast Cancer Research Foundation, Facing our Risk of Cancer Empowered
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gupta S, Weiss JM, Axell L, et al. : NCCN Guidelines: Genetic/familial high-risk assessment: Colorectal, version 2.2022. http://NCCN.org
- 2.Shu CA, Pike MC, Jotwani AR, et al. : Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol 2:1434-1440, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laitman Y, Michaelson-Cohen R, Levi E, et al. : Uterine cancer in Jewish Israeli BRCA1/2 mutation carriers. Cancer 125:698-703, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Levine DA, Kandoth C, Schultz N, et al. : Integrated genomic characterization of endometrial carcinoma. Nature 497:67-73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute : Cancer of the endometrium: Cancer stat facts. Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/statfacts/html/corp.html
- 6.Chelmow D, Brooks R, Cavens A, et al. : Executive summary of the uterine cancer evidence review conference. Obstet Gynecol 139:626-643, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fader AN, Boruta D, Olawaiye AB, et al. : Uterine papillary serous carcinoma: Epidemiology, pathogenesis and management. Curr Opin Obstet Gynecol 22:21-29, 2010 [DOI] [PubMed] [Google Scholar]
- 8.McGunigal M, Liu J, Kalir T, et al. : Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: A national cancer database analysis. Int J Gynecol Cancer 27:85-92, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Lee L, Howitt B, Cheng T, et al. : MicroRNA profiling in a case-control study of African American women with uterine serous carcinoma. Gynecol Oncol 163:453-458, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Smrz SA, Calo C, Fisher JL, et al. : An ecological evaluation of the increasing incidence of endometrial cancer and the obesity epidemic. Am J Obstet Gynecol 224:506.e1-506.e8, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 1.2023. NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [DOI] [PubMed]
- 12.Tischkowitz M, Colas C, Pouwels S, et al. : Cancer Surveillance Guideline for individuals with PTEN hamartoma tumour syndrome. Eur J Hum Genet 28:1387-1393, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Win AK, Reece JC, Ryan S: Family history and risk of endometrial cancer: A systematic review and meta-analysis. Obstet Gynecol 125:89-98, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Barakat RR: Tamoxifen and the endometrium. Cancer Treat Res 94:195-207, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Kedar RP, Bourne TH, Collins W, et al. : Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trial. Lancet 343:1318-1321, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Seoud M, Shamseddine A, Khalil A, et al. : Tamoxifen and endometrial pathologies: A prospective study. Gynecol Oncol 75:15-19, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Committee on Gynecologic Practice : Committee Opinion No. 601: Tamoxifen and uterine cancer. Obstet Gynecol 123:1394-1397, 2014 [DOI] [PubMed] [Google Scholar]
- 18.NCCN Guidelines®: Breast cancer risk reduction, version 1.2023. NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
- 19.Gasparri ML, Bellaminutti S, Farooqi AA, et al. : Endometrial cancer and BRCA mutations: A systematic review. J Clin Med Res 11:3114, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matanes E, Volodarsky-Perel A, Eisenberg N, et al. : Endometrial cancer in germline BRCA mutation carriers: A systematic review and meta-analysis. J Minim Invasive Gynecol 28:947-956, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Huber D, Seitz S, Kast K, et al. : Hormone replacement therapy in BRCA mutation carriers and risk of ovarian, endometrial, and breast cancer: A systematic review. J Cancer Res Clin Oncol 147:2035-2045, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrag D, Kuntz KM, Garber JE, et al. : Decision analysis—Effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med 336:1465-1471, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Schrag D, Kuntz KM, Garber JE, et al. : Life expectancy gains from cancer prevention strategies for women with breast cancer and BRCA1 or BRCA2 mutations. JAMA 283:617-624, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Byrd LM, Shenton A, Maher ER, et al. : Better life expectancy in women with BRCA2 compared with BRCA1 mutations is attributable to lower frequency and later onset of ovarian cancer. Cancer Epidemiol Biomarkers Prev 17:1535-1542, 2008 [DOI] [PubMed] [Google Scholar]
- 25.de Jonge MM, de Kroon CD, Jenner DJ, et al. : Endometrial cancer risk in women with germline BRCA1 or BRCA2 mutations: Multicenter cohort study. J Natl Cancer Inst 113:1203-1211, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heald B, Mokhtary S, Nielsen SM, et al. : Unexpected actionable genetic variants revealed by multigene panel testing of patients with uterine cancer. Gynecol Oncol 166:344-350, 2022 [DOI] [PubMed] [Google Scholar]
- 27.ACOG Committee Opinion No: 698: Hormone therapy in primary ovarian insufficiency. Obstet Gynecol 129:e134-e141, 2017. https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2017/05/hormone-therapy-in-primary-ovarian-insufficiency.pdf [DOI] [PubMed] [Google Scholar]
- 28.Sullivan SD, Sarrel PM, Nelson LM: Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril 106:1588-1599, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzur T, Yohai D, Weintraub AY: The role of local estrogen therapy in the management of pelvic floor disorders. Climacteric 19:162-171, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Nappi RE, Tiranini L, Martini E, et al. : Medical treatment of female sexual dysfunction. Urol Clin North Am 49:299-307, 2022 [DOI] [PubMed] [Google Scholar]
- 31.Ji R, He B, Wu J: Application of transperineal ultrasound combined with shear wave elastography in pelvic floor function assessment after hysterectomy. Medicine 102:e32611, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doğanay M, Kokanalı D, Kokanalı MK, et al. : Comparison of female sexual function in women who underwent abdominal or vaginal hysterectomy with or without bilateral salpingo-oophorectomy. J Gynecol Obstet Hum Reprod 48:29-32, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Islam RM, Davis SR, Bell RJ, et al. : A prospective controlled study of sexual function and sexually related personal distress up to 12 months after premenopausal risk-reducing bilateral salpingo-oophorectomy. Menopause 28:748-755, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Reitsma W, Mourits MJE, de Bock GH, et al. : Endometrium is not the primary site of origin of pelvic high-grade serous carcinoma in BRCA1 or BRCA2 mutation carriers. Mod Pathol 26:572-578, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Minig L, Cabrera S, Oliver R, et al. : Pathology findings and clinical outcomes after risk reduction salpingo-oophorectomy in BRCA mutation carriers: A multicenter Spanish study. Clin Transl Oncol 20:1337-1344, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Saule C, Mouret-Fourme E, Briaux A, et al. : Risk of serous endometrial carcinoma in women with pathogenic BRCA1/2 variant after risk-reducing salpingo-oophorectomy. J Natl Cancer Inst 110:213-215, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Segev Y, Iqbal J, Lubinski J, et al. : The incidence of endometrial cancer in women with BRCA1 and BRCA2 mutations: An international prospective cohort study. Gynecol Oncol 130:127-131, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Segev Y, Rosen B, Lubinski J, et al. : Risk factors for endometrial cancer among women with a BRCA1 or BRCA2 mutation: A case control study. Fam Cancer 14:383-391, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casey MJ, Bewtra C, Lynch HT, et al. : Endometrial cancers in mutation carriers from hereditary breast ovarian cancer syndrome kindreds: Report from the Creighton University Hereditary Cancer Registry with review of the implications. Int J Gynecol Cancer 25:650-656, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Beiner ME, Finch A, Rosen B, et al. : The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations. A prospective study. Gynecol Oncol 104:7-10, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Bruchim I, Amichay K, Kidron D, et al. : BRCA1/2 germline mutations in Jewish patients with uterine serous carcinoma. Int J Gynecol Cancer 20:1148-1153, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Biron-Shental T, Drucker L, Altaras M, et al. : High incidence of BRCA1-2 germline mutations, previous breast cancer and familial cancer history in Jewish patients with uterine serous papillary carcinoma. Eur J Surg Oncol 32:1097-1100, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Eisen A, Lubinski J, Gronwald J, et al. : Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst 100:1361-1367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rebbeck TR, Friebel T, Wagner T, et al. : Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J Clin Oncol 23:7804-7810, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Collaborative Group on Hormonal Factors in Breast Cancer : Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 394:1159-1168, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotsopoulos J, Gronwald J, Karlan BY, et al. : Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol 4:1059-1065, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lethaby A, Suckling J, Barlow D, et al. : Hormone replacement therapy in postmenopausal women: Endometrial hyperplasia and irregular bleeding. Cochrane Database Syst Rev:CD000402, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Sjögren LL, Mørch LS, Løkkegaard E: Hormone replacement therapy and the risk of endometrial cancer: A systematic review. Maturitas 91:25-35, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Fabian CJ, Nye L, Powers KR, et al. : Effect of bazedoxifene and conjugated estrogen (Duavee) on breast cancer risk biomarkers in high-risk women: A pilot study. Cancer Prev Res 12:711-720, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Y, Jiao H, Qu L, et al. : Association between hormone replacement therapy and development of endometrial cancer: Results from a prospective US cohort study. Front Med 8:802959, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenabi E, Poorolajal J: The effect of body mass index on endometrial cancer: A meta-analysis. Public Health 129:872-880, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Liu H, Yang S, et al. : Overweight, obesity and endometrial cancer risk: Results from a systematic review and meta-analysis. Int J Biol Markers 29:e21-e29, 2014 [DOI] [PubMed] [Google Scholar]
- 53.McCullough ML, Patel AV, Patel R, et al. : Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev 17:73-79, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Lee YC, Milne RL, Lheureux S, et al. : Risk of uterine cancer for BRCA1 and BRCA2 mutation carriers. Eur J Cancer 84:114-120, 2017 [DOI] [PubMed] [Google Scholar]
- 55.Bogani G, Tagliabue E, Signorelli M, et al. : Assessing the risk of occult cancer and 30-day morbidity in women undergoing risk-reducing surgery: A prospective experience. J Minim Invasive Gynecol 24:837-842, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Lee SC, Chou YH, Tantoh DM, et al. : Risk of uterine leiomyoma based on BET1L rs2280543 single nucleotide polymorphism and vegetarian diet. BMC Womens Health 22:139, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Committee on Gynecologic Practice: Committee Opinion No. 619: Gynecologic surgery in the obese woman. Obstet Gynecol 125:274-278, 2015 [DOI] [PubMed] [Google Scholar]
- 58.ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol 114:1156-1158, 2009. [Retraction] [DOI] [PubMed] [Google Scholar]
- 59.Bogani G, Cromi A, Serati M, et al. : Laparoscopic and vaginal approaches to hysterectomy in the obese. Eur J Obstet Gynecol Reprod Biol 189:85-90, 2015 [DOI] [PubMed] [Google Scholar]
- 60.Wu JM, Vaughan CP, Goode PS, et al. : Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol 123:141-148, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bland AE, Calingaert B, Secord AA, et al. : Relationship between tamoxifen use and high risk endometrial cancer histologic types. Gynecol Oncol 112:150-154, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Wysowski DK, Honig SF, Beitz J: Uterine sarcoma associated with tamoxifen use. N Engl J Med 346:1832-1833, 2002 [DOI] [PubMed] [Google Scholar]
- 63.McCluggage WG, Abdulkader M, Price JH, et al. : Uterine carcinosarcomas in patients receiving tamoxifen. A report of 19 cases. Int J Gynecol Cancer 10:280-284, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Kubler K, Nardone A, Anand S, et al. : Abstract GS2-09: Tamoxifen instigates uterine cancer development by activating PI3K signaling and supersedes PIK3CA driver mutations. Cancer Res 82, 2022. (suppl 4; abstr GS2-09) [Google Scholar]
- 65.Ryu KJ, Kim MS, Lee JY, et al. : Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open 5:e2243951, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazzeroni M, Puntoni M, Guerrieri-Gonzaga A, et al. : Randomized placebo controlled trial of low-dose tamoxifen to prevent recurrence in breast noninvasive neoplasia: A 10-year follow-up of TAM-01 study. J Clin Oncol 41:3116-3121, 2023 [DOI] [PubMed] [Google Scholar]
- 67.Kwon JS, Lenehan J, Carey M, et al. : Prolonged survival among women with BRCA germline mutations and advanced endometrial cancer: A case series. Int J Gynecol Cancer 18:546-549, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Portela S, Cunningham A, Laios A, et al. : Breast cancer patients at increased risk of developing type II endometrial cancer: Relative and absolute risk estimation and implications for counseling. Cureus 13:e12981, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bergman L, Beelen ML, Gallee MP, et al. : Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Lancet 356:881-887, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Murali R, Soslow RA, Weigelt B: Classification of endometrial carcinoma: More than two types. Lancet Oncol 15:e268-e278, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Bellet M, Gray KP, Francis PA, et al. : Twelve-month estrogen levels in premenopausal women with hormone receptor-positive breast cancer receiving adjuvant triptorelin plus exemestane or tamoxifen in the suppression of ovarian function trial (SOFT): The SOFT-EST substudy. J Clin Oncol 34:1584-1593, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Francis PA, Regan MM, Fleming GF, et al. : Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 372:436-446, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Censi A: 10 year results of a phase 3 trial of low-dose tamoxifen in noninvasive breast cancer. Presented at San Antonio Breast Cancer Symposium, San Antonio, TX, December 9, 2022
- 74.US Preventive Services Task Force : Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: Recommendation statement. Ann Intern Med 143:355-361, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Bafligil C, Thompson DJ, Lophatananon A, et al. : Association between genetic polymorphisms and endometrial cancer risk: A systematic review. J Med Genet 57:591-600, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Mara TA, Glubb DM, Amant F, et al. : Identification of nine new susceptibility loci for endometrial cancer. Nat Commun 9:3166, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang YD, Hurson AN, Zhang H, et al. : Assessment of polygenic architecture and risk prediction based on common variants across fourteen cancers. Nat Commun 11:3353, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cicchillitti L, Corrado G, De Angeli M, et al. : Circulating cell-free DNA content as blood based biomarker in endometrial cancer. Oncotarget 8:115230-115243, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hutt S, Tailor A, Ellis P, et al. : The role of biomarkers in endometrial cancer and hyperplasia: A literature review. Acta Oncol 58:342-352, 2019 [DOI] [PubMed] [Google Scholar]
- 80.Bie Y, Zhang Z: Diagnostic value of serum HE4 in endometrial cancer: A meta-analysis. World J Surg Oncol 12:169, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bednarikova M, Vinklerova P, Gottwaldova J, et al. : The clinical significance of DJ1 and L1CAM serum level monitoring in patients with endometrial cancer. J Clin Med Res 10:2640, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou L, Wang W, Wang F, et al. : Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol Cancer 20:57, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boretto M, Maenhoudt N, Luo X, et al. : Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol 21:1041-1051, 2019 [DOI] [PubMed] [Google Scholar]
- 84.Cunnea P, Fotopoulou C, Ploski J, et al. : Changes in stem cell regulation and epithelial organisation during carcinogenesis and disease progression in gynaecological malignancies. Cancers 13:3349, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vrede SW, Kasius J, Bulten J, et al. : Relevance of molecular profiling in patients with low-grade endometrial cancer. JAMA Netw Open 5:e2247372, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]