The discovery of cyclooxygenase 2 (COX-2) a decade ago heralded one of the most rapid and expensive development and marketing of a new class of drug that we have witnessed. This has resulted in the publication of an interesting mixture of exceptionally high-quality basic and clinical research. Based on the COX dogma, that COX-1 is good and COX-2 is bad, we were promised equal therapeutic efficacy to conventional nonsteroidal anti-inflammatory drugs (NSAIDs) with the COX-2 selective inhibitors and the absence of gastrointestinal side effects that otherwise represent a significant public health problem. Have these promises come to fruition?
The semantic problem about the term COX-2 selective agents is acknowledged,1,2,3 but for our purpose (accepting that nimesulide and etodolac, at least, have a case for using this label) our discussion relates to celecoxib and rofecoxib because data on these agents are now abundant.
Gastroenterologists are usually unconcerned about therapeutic efficacy of anti-inflammatory analgesic drugs, but the COX-2 selective agents have equivalent efficacy to conventional NSAIDs, and no new unexpected side effects have been encountered. Curiously, the prevalence of dyspeptic symptoms is similar to that with the use of conventional NSAIDs.4 The COX-2 selective agents have otherwise come through the conventional gastroduodenal safety assessments with flying colors.
Equivalent short-term endoscopy damage to placebo in volunteers, even at high doses.5
Equivalent long-term endoscopy damage to placebo in patients.6
Significant reduction (about 60%) in serious outcomes (perforation, hemorrhages) in patients taking the drugs long term (reported at Digestive Disease Week, San Diego, California).
Also, nimesulide and rofecoxib cause no short-term small bowel damage in healthy volunteers, which is a good predictor of long-term tolerability.7,8 Is this the proof to the COX dogma, or are there still some concerns?
Of special note is the high (3%-11%) prevalence of gastric damage in the placebo arms of the long-term endoscopy studies. Some of this damage may be due to concomitant ingestion of aspirin, used for cardiovascular prophylaxis.
Interestingly, the normal intestinal appearances in COX-1 knockout (genetically engineered) animals rang warning bells for the COX dogma for some of us. It is, therefore, particularly interesting that in the absence of a “topical” effect (Peter Isakson, oral communication, 1999, and widely confirmed at the Digestive Disease Week, 2000), selective COX-1 inhibition (SC-560) is not associated with gastrointestinal damage. Rather, it is the dual inhibition of COX-1 and COX-2 that is important. These deviations from the COX dogma pose potential problems because even minidoses of aspirin inhibit gastric COX-1 almost completely. The precise importance of concomitant aspirin ingestion and COX-2 inhibitory agents demands further study, but these findings should also be a stimulus to develop selective COX-1 inhibitors that may be devoid of the gastric toxicity of aspirin.
Selective COX-2 inhibition does not, therefore, appear to cause significant new gastrointestinal damage in humans. Their possible detrimental effect on preexisting intestinal disease,9 however, requires further clarification. COX-2 selective agents delay healing of experimental gastric ulcers in animals and, if substantiated in humans, may have implications for patients with Helicobacter pylori-driven gastroduodenal ulcer diathesis. Second, COX-2 inhibitors may exacerbate10 or ameliorate11 the severity of experimental colitis in rodents. This needs to be studied in humans because NSAIDs may cause relapse of inflammatory bowel disease, and many of these patients require anti-inflammatory analgesics for arthritis, metabolic bone disease, and the like. NSAIDs play a detrimental role in some other intestinal diseases, and it is also possible that selective COX-2 inhibitors may perpetuate NSAID-induced damage. This should not, however, deter from the fact that on current evidence, it is likely that selective COX-2 inhibitors will transform the care of arthritic patients. For many, this development has not come too soon.
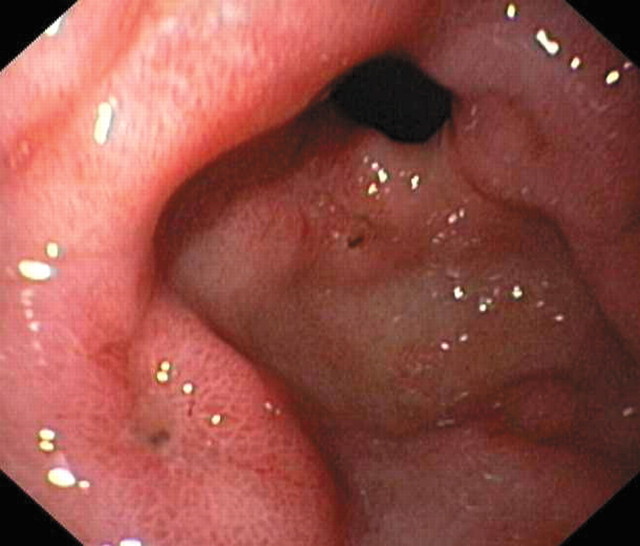
Figure 1.
COX-2 inhibitors do not cause new gastrointestinal damage but may exacerbate nonsteroidal anti-inflammatory drug-induced gastric ulcers
Gastrolab
Competing interests: None declared Adapted from Gut 2001;48:451
References
- 1.Jacob M, Simpson R, Bjarnason I. Nonsteroidal anti-inflammatory drugs, cyclooxygenase selectivity and gastrointestinal toxicity. Ital J Gastroenterol Hepatol 1998;30: 12-18. [PubMed] [Google Scholar]
- 2.Scarpignatio C, Bjarnason I, Bretagne JF, et al. Towards a GI safer antiinflammatory therapy. Gastroenterol Int 1999;12: 180-215. [Google Scholar]
- 3.Brooks P, Emery P, Evans JF, et al. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology (Oxford) 1999;38: 779-788. [DOI] [PubMed] [Google Scholar]
- 4.Cannon GW, Caldwell JR, Holt P, et al. Rofecoxib, a specific inhibitor of cyclooxygenase 2, with clinical efficacy comparable with that of diclofenac sodium: results of a one-year, randomized, clinical trial in patients with osteoarthritis of the knee and hip. Rofecoxib Phase III Protocol 035 Study Group. Arthritis Rheum 2000;43: 978-987. [DOI] [PubMed] [Google Scholar]
- 5.Lanza FL, Rack MF, Simon TJ, et al. Specific inhibition of cyclooxygenase-2 with MK-0966 is associated with less gastroduodenal damage than either aspirin or ibuprofen. Aliment Pharmacol Ther 1999;13: 761-767. [DOI] [PubMed] [Google Scholar]
- 6.Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA 1999;282: 1921-1928. [DOI] [PubMed] [Google Scholar]
- 7.Sigthorsson G, Crane R, Simon T, et al. COX-2 inhibition with rofecoxib does not increase intestinal permeability in healthy subjects: a double blind crossover study comparing rofecoxib with placebo and indomethacin. Gut 2000;47: 527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah AA, Thjodleifsson B, Murray FE, et al. Selective inhibition in humans of COX-2 is associated with less gastrointestinal injury: a comparison of nimesulide and naproxen. Gut 2001;48: 339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993;104: 1832-1847. [DOI] [PubMed] [Google Scholar]
- 10.Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J Clin Invest 1996;98: 2076-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karmeli F, Cohen P, Rachmilewitz D. Cyclo-oxygenase-2 inhibitors ameliorate the severity of experimental colitis in rats. Eur J Gastroenterol Hepatol 2000;12: 223-231. [DOI] [PubMed] [Google Scholar]