Abstract
The extracellular phosphate concentration permissive for the expression of different amounts of the active high-affinity Pho84 phosphate transporter in the plasma membrane as well as the PHO84 messenger RNA levels in low-phosphate-grown Saccharomyces cerevisiae cells is very narrow and essential for a tight regulation of the transporter. The Pho84 transporter undergoes a rapid degradation once the supply of phosphate and/or carbon source is exhausted.
Orthophosphate plays a pivotal role in cell functioning, being involved in most metabolic energy transductions, serving as an intermediate in the biosynthesis of numerous metabolites. Regulation of the phosphate uptake process represents a common biological strategy for modulation of and response to phosphate metabolism and cellular activities (18). The phosphate transport process in Saccharomyces cerevisiae is characterized by a high-affinity transport system operative at low (μM) concentrations of phosphate and a low-affinity transport system operative at high concentrations (mM) of phosphate. The low-affinity system, with a Km for phosphate of approximately 1 mM at its proposed optimum of pH 4.5, is considered to be a constitutively expressed Pi/H+ cotransporter (16, 25). In contrast, the high-affinity system (Km, 1 to 15 μM) is derepressible by phosphate starvation during aerobic and anaerobic cell growth. Of the proteins responsible for the high-affinity transport of phosphate into the cell, one consists of a Pi/H+ cotransporter (Pho84p) with a pH optimum for phosphate uptake similar to that of the constitutive low-affinity system (1, 2, 4), and the other is a Pi/Na+ cotransporter with an alkaline pH optimum, being largely inactive at pH 4.5 (21). The identities of the genes encoding the proposed constitutively expressed low-affinity Pi/H+ and the high-affinity Pi/Na+ transporters have not yet been published.
The signal on the level of extracellular phosphate is known to be conveyed through the so-called PHO regulon (12, 18, 23). Although significant insight into the genetic regulation of phosphate signalling has been gained, the complex nature of the phosphate transport processes and their control is so far poorly understood. The changes in intracellular concentrations of polyphosphates and Pi occurring during the cell cycle may play an important role in the regulation of the Pi transport systems (3). The metabolic signals that serve as corepressors in this system and their relation with the phosphate metabolism in S. cerevisiae cells are presently unknown.
The aim of this investigation was to examine the factors, in addition to extracellular phosphate concentration, involved in the mechanisms underlying the physiological regulation of derepressible H+-coupled high-affinity Pi transport.
The S. cerevisiae CW04 strain (Mata ade2 his3 leu2 trp1 ura3 canr) was used. Cells were routinely grown in shaking Erlenmeyer flasks at 30°C in low-phosphate (LPi) medium prepared according to the method of Kaneko et al. (13). One liter of YEP (1% yeast extract, 2% Bacto Peptone) medium was supplemented with 10 ml of 1 M MgSO4 and 10 ml of 25% NH3 solution with stirring, allowed to stand at 25°C for 1 h to precipitate phosphate, and filtered through a Munktell no. 3 filter. The pH of the clear filtrate was adjusted to pH 4.5 with HCl, and 2% glucose was added. In some control experiments 0.2% KH2PO4 was used in high-phosphate (HPi) medium. Growth was monitored by the change in optical density at 590 nm (OD590). At specified time points, samples of the culture were aseptically withdrawn, centrifuged at 2,300 × g for 10 min, and washed either once with ice-cold 25 mM Tris-succinate buffer (pH 4.5) (for Pi uptake assays) or twice with ice-cold bidistilled water (for 31P nuclear magnetic resonance [NMR] and respiratory rate analysis). The supernatants were subjected to glucose and phosphate concentration measurements.
In the phosphate uptake studies 1 μl of [32P]orthophosphate (0.18 Ci/μmol; 1 mCi = 37 MBq; Amersham) was added to aliquots (30 μl, 0.546 mg of dry weight) of cell suspension in 25 mM Tris-succinate buffer, pH 4.5, supplemented with 3% glucose, to a final concentration of 0.11 mM. The suspension was blended and incubated for 1 min at 25°C. Phosphate uptake was terminated by adding 3 ml of ice-cold Tris-succinate dilution buffer. The sample was filtered immediately, the filter (Whatman GF/F) was washed once with the same cold solution, and the radioactivity retained on the filters was determined by liquid scintillation spectrometry. The maximum rate of phosphate transport catalyzed by the cells, estimated as the initial activity during the first minute of uptake per mg of cells (dry weight), is shown.
For sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting, the membrane fraction of S. cerevisiae cells was prepared as described by Ljungdahl et al. (15). Samples containing 20 mg of plasma membrane protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis by using a 12% polyacrylamide and bispolyacrylamide gel system (14). The electrophoresed proteins were transferred onto polyvinylidene difluoride membranes (Immobilon polyvinylidene difluoride; Millipore) according to the Amersham Western blotting protocol. Immunological detection was accomplished by using affinity-purified Pho84 anti-C terminal antibody (6) and anti-rabbit immunoglobulin donkey antibody-conjugated horseradish peroxidase (Amersham). After a short incubation with enhanced chemiluminescent substrate the blot was exposed to film for 2 min.
In the Northern analysis total RNA (15 μg) was isolated from CW04 cells grown in HPi and LPi media as described elsewhere (24), separated by electrophoresis on 1.5% agarose gels containing 2.2 M formaldehyde, blotted onto Hybond-N membranes (Amersham) according to the manufacturer’s instructions, and hybridized under high-stringency conditions in accordance with standard procedures (22). The probes used were a 32P-labeled 0.7-kbp NdeI-KpnI PHO84 gene fragment contained in pUC19 (1) and the 1.65-kbp BamHI-HindIII ACTI gene (7) as a loading control. The probes were labeled by the random primer technique by using an oligolabeling kit (Pharmacia) according to the instructions of the manufacturer. Filters were exposed to film at −80°C.
All NMR experiments were conducted on a Varian Unity Plus 400 instrument. Aliquots (3.5 ml) of CW04 cell suspensions of 0.5 g (wet weight)/ml in 25 mM Tris-succinate buffer, pH 4.5, were subjected to 31P NMR analyses. A broad-band probe designed for 10-mm sample tubes was used. The spectral width was 10,000 Hz, centered on the 85% phosphoric acid peak at 0 ppm in a separate experiment. The pulse delay was 2 s, and 512 scans of 2,048 complex data points were collected during an experimental time range of approximately 20 min. The 90°C excitation pulse length was determined to be 21 μs. No deuterium frequency lock or proton decoupling was used during the experiments. Experiments performed on one sample with pulse delays of 1, 2, and 4 s revealed no systematic changes of intensities, indicating that the 31P longitudinal relaxation rates are rapid. The relative contributions of different 31P-containing molecules could thus be calculated from the corresponding integrated intensities in the 31P NMR spectra. The NMR data were evaluated using the built-in VNMR software version 5.1 (Varian). The free induction decays were multiplied with an exponential window of 10 Hz, zero filled to 8,192 complex points, and Fourier transformed. The frequency domain spectra were baseline corrected, and the intensities and integrals were obtained using standard techniques. The assignment of the 31P NMR peaks of intra- and extracellular orthophosphate, ATP, and nonterminal Pi of polyphosphate were obtained as previously described (11). The total amount of ATP was calculated from the β-Pi of the ATP peak since other phosphorous compounds concur with the α-Pi and γ-Pi of the ATP peaks.
Phosphate and glucose concentrations in the growth media were assayed spectrophotometrically at 850 nm essentially as described by Nyrén et al. (17) and determined polarographically with glucose peroxidase according to the protocol of Okuda and Miwa (19), respectively.
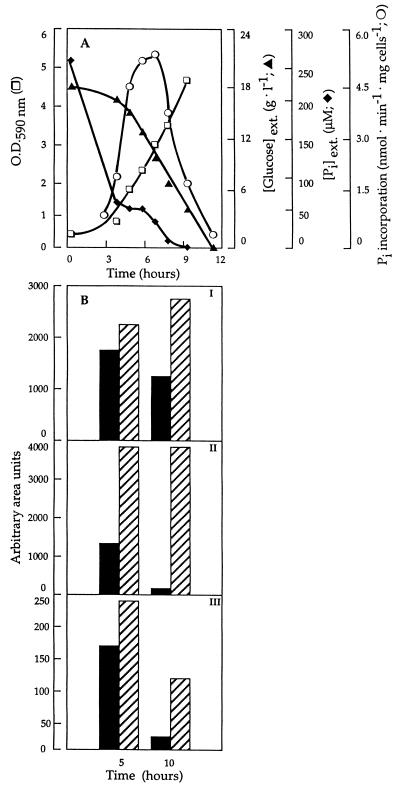
Under HPi growth conditions, the Pi transport activity of the S. cerevisiae cells withdrawn at different growth phases was very low, 0.5 nmol of Pi transported per min and mg of cells (dry mass), and unaffected by the prevailing growth phase (data not shown). In contrast, in cells grown in LPi medium (Fig. 1A), containing approximately 200 to 300 μM phosphate, phosphate transport changes with cell growth. The uptake rate increases along the exponential phase to reach its maximum rate (5.3 nmol of Pi transported per min and mg of dry mass) in mid- to late-exponential-growth phase (an OD590 of approximately 3) before rapidly declining. The cell growth was accompanied by an initial rapid rate of extracellular phosphate consumption, from approximately 275 to 70 μM in the first 4 h of growth, followed by a slower rate of utilization during the early-exponential-growth phase (an OD590 of approximately 1) (Fig. 1A). The highest transport activity was achieved when the extracellular Pi concentration was in the range of 50 to 70 μM. Interestingly, the onset of the decline in transport activity coincided with a situation where the extracellular phosphate concentration was very low, close to the Km of 10 μM for the transporter, while glucose was still abundant (approximately 10 g/liter). This observation suggests not only, in agreement with earlier proposals, that the derepression of the PHO84 is under control of the extracellular phosphate level (4) but also that its inactivation is subjected to the same control.
FIG. 1.
(A) Phosphate uptake by CW04 cells during growth (□) in LPi medium. At specified time intervals, samples were withdrawn and assayed for inorganic phosphate uptake (○), and the supernatants were used for glucose (▴) and phosphate (⧫) determination. (B) Intracellular levels of inorganic phosphate (I), polyphosphate (II), and ATP (III) in CW04 cells grown in HPi medium (hatched bars) and LPi medium (solid bars) and harvested after 5 and 10 h of growth determined by 31P NMR spectroscopy. The standard deviations of the values shown are 1 to 3%.
The results obtained (Fig. 1A) clearly indicate that cell growth during the first 3 to 4 h is supported by a rapid uptake of external phosphate after which the remaining low extracellular phosphate concentration is not sufficiently high to maintain further exponential cell growth. This implies that in the latter case internal phosphate pools are being utilized. In order to study the intracellular changes of phosphorous compounds and their putative role in the Pi-sensitive regulation of the Pi transporters, the intracellular amounts of Pi, polyphosphate, and ATP in cells grown in LPi and HPi media were measured by the 31P NMR technique. The samples analyzed were cells grown for 5 and 10 h, corresponding to the situations when there was a dramatic increase in the rate of phosphate consumption and when the extracellular phosphate concentration was close to zero, respectively. In a composite of a series of 31P NMR analyses (Fig. 1B), the changes of intracellular phosphorous compounds, such as free orthophosphate (panel I), polyphosphates (panel II), and ATP (panel III) are depicted. It can be seen that cells grown in LPi medium maintained much lower levels of free phosphate, polyphosphates, and ATP than HPi-grown cells. Remarkably, in cells grown in LPi medium under conditions of extracellular phosphate deprivation (10 h), the polyphosphate pool was diminished to almost zero, whereas it was unaffected in cells grown in HPi. In contrast, the amount of intracellular free Pi was maintained at a significant level during growth in LPi. Thus, it appears that under conditions when the cell meets no Pi limitations, free Pi is predominantly stored in the form of polyphosphates, whereas only low amounts of Pi reserves are maintained during Pi starvation, indicating that Pi taken up by the high-affinity system must be used immediately by the cell in essential cellular functions. It is conceivable that the intracellular polyphosphate pool might be responsible for sustaining cell growth when the extracellular phosphate is exhausted.
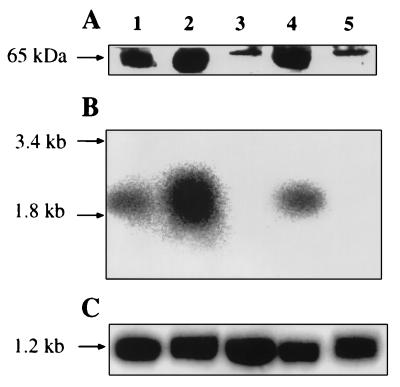
To investigate whether the transcription level of PHO84 as well as the amount of Pho84p in the plasma membrane correlated with the phosphate transport activity, Northern and Western blot analyses were performed on cells grown in LPi medium and harvested at different growth phases (Fig. 2). For comparison purposes, blot analyses were also performed for cells grown for 10 h in HPi medium. Figure 2A illustrates the result of a Western blot analysis of the presence of the Pho84 transporter in LPi-grown cells harvested at OD590s of 0.9, 3, and 7 (cf. Fig. 1A) as well as in cells harvested at an OD590 of 7 and incubated for 45 min in medium containing 17 g of glucose/liter and 50 μM Pi. The samples analyzed revealed significant variations in the intensity of the immunolabeled major band corresponding to Pho84p (65.4 kDa). The variations in intensity of the bands correlate well with the changes observed in the rate of Pi uptake (cf. Fig. 1A). The immunoreactive band corresponding to the 65.4-kDa Pho84 transporter was absent in cells grown for 10 h under phosphate starvation and in HPi medium. The Pi transport activities for the two conditions were similar (less than 1 nmol of Pi transported per min and mg of cells, dry mass), suggesting that the low-affinity system active under repressive growth conditions in HPi medium is also responsible for the transport determined in 10-h-old cells. The sharp immunoreactive band of approximately the same molecular size as the Pho84p band present in these samples probably reflects the immunoreactivity of another protein which comigrates with the Pho84p. These results clearly indicate a derepressive synthesis of the high-affinity carrier proportional with the initial decrease in the extracellular phosphate concentration and its rapid degradation upon extracellular phosphate and intracellular polyphosphate depletion. Proteolysis of a plasma membrane protein could be accomplished by a direct breakdown of a selected protein at the level of the plasma membrane or by selective internalization and transport to the vacuole for nonspecific proteolysis (10). The proteolytic pathway involved in the degradation of Pho84p still remains to be clarified. Transfer of the cells devoid of Pho84p to medium containing glucose and phosphate results in the reappearance of the immunoreactive Pho84p band and is paralleled by reactivation of the phosphate transport activity up to 2.9 nmol of Pi per min and mg of cells, dry mass (data not shown). When the PHO84 mRNA levels were studied in the same samples (Fig. 2B) it could be seen that the amount of the PHO84 transcripts increases during the exponential-growth phase in LPi medium (samples at OD590s of 0.9 and 3). After 10 h of growth, transcription was completely repressed, and the expressed Pho84p carrier was being degraded. The transcription of the PHO84 gene was rapidly turned on when starved cells were transferred to fresh medium, explaining the reappearance of the Pho84p immunoreactive band in the Western blot analysis. Furthermore, the PHO84 transcription was repressed in HPi-grown cells.
FIG. 2.
(A) Western blot analysis of the Pho84 transporter in isolated plasma membrane fractions of CW04 cells harvested at different phases of growth in LPi medium. The estimated molecular mass of the immunodecorated band is shown on the left. (B) Detection of PHO84 transcripts by Northern hybridization. (C) Detection of ACT1 transcript as a loading control. 25S and 18S rRNAs indicated on the left, visualized by staining with ethidium bromide, were used as size markers as described by Philipsen et al. (20). In both analyses, cells were collected at different OD590s during growth in LPi medium: 0.9 (lane 1), 3 (lane 2), and 7 (lane 3). For lanes 4 and 5, cells were collected at an OD590 of 7 followed by transfer to fresh medium (lane 4) or were grown in HPi medium to an OD590 of 4 (lane 5).
The results obtained strongly suggest that derepression of the transporter is maintained by the availability of extracellular phosphate rather than the level of intracellular phosphate, which is affected only to a minor extent by cell growth for 10 h. Even under conditions when external phosphate is fully depleted after 10 h of growth the cells still contain a considerable amount of intracellular phosphate and a significant level of ATP (Fig. 1B). Part of the intracellular phosphate reserve is contained in the vacuoles, where it can be mobilized when phosphate in the medium is limiting (5, 8, 9). The fact that essentially no polyphosphate was found in the cells showing the highest PHO84 expression level, i.e., after 10 h of growth, suggests that the Pi-sensitive regulation possibly is mediated by the concentration of these phosphate polymers. Bostian and coworkers (3) also found in their studies of the expression of the repressible acid phosphatase (rAPase) that changes in intracellular Pi levels did not correlate with rAPase derepression and concluded that Pi therefore may not serve as a corepressor. The same authors suggested that Pi or low-molecular-weight polyphosphates may serve as a metabolic regulator controlling the rAPase expression (3).
In summary, the results presented in this work clearly reflect a derepressive synthesis of the Pho84p carrier proportional with the initial decrease in the extracellular phosphate concentration and its rapid degradation upon glucose, extracellular phosphate, and intracellular polyphosphate depletion. The activation of Pi uptake under Pi starvation is due to a derepression of the transcription of the PHO84 gene. The inactivation of this transporter by nutrient (Pi, glucose) depletion is due to a negative regulation by which the carrier is degraded and the PHO84 transcription turned off.
Acknowledgments
During this work P.M. was a postdoctoral fellow of The Swedish Institute and of Programa Nacional de Formacion de Personal Investigador, Ministerio de Educación y Ciencia, Spain. This work was supported by grants from the Royal Swedish Academy of Sciences and the Swedish Natural Science Research Council.
REFERENCES
- 1.Berhe A, Fristedt U, Persson B L. Expression and purification of the high-affinity phosphate transporter of Saccharomyces cerevisiae. Eur J Biochem. 1995;227:566–572. doi: 10.1111/j.1432-1033.1995.tb20426.x. [DOI] [PubMed] [Google Scholar]
- 2.Borst-Pauwels G W F H. Kinetical parameters of monovalent cation uptake in yeast calculated on accounting for the mutual interaction of cation uptake and membrane potential. Biochim Biophys Acta. 1993;1152:201–206. doi: 10.1016/0005-2736(93)90250-4. [DOI] [PubMed] [Google Scholar]
- 3.Bostian K A, Lemire J M, Halvorson H O. Physiological control of repressible acid phosphatase gene transcripts in Saccharomyces cerevisiae. Mol Cell Biol. 1983;3:839–853. doi: 10.1128/mcb.3.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bun-ya M, Nishimura M, Harashima S, Oshima Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol. 1991;11:3229–3238. doi: 10.1128/mcb.11.6.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell-Burk S L, Schulman R G. High-resolution NMR studies of Saccharomyces cerevisiae. Annu Rev Microbiol. 1987;41:595–616. doi: 10.1146/annurev.mi.41.100187.003115. [DOI] [PubMed] [Google Scholar]
- 6.Fristedt U, Berhe A, Ensler K, Norling B, Persson B L. Isolation and characterization of membrane vesicles of Saccharomyces cerevisiae harboring the high-affinity phosphate transporter. Arch Biochem Biophys. 1996;330:133–141. doi: 10.1006/abbi.1996.0235. [DOI] [PubMed] [Google Scholar]
- 7.Gallwitz D, Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Biochemistry. 1980;5:2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillies R J, Ugurbil K, den Hollander J A, Schulman R G. 31P NMR studies of intracellular pH and phosphate metabolism during cell division cycle of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1981;78:2125–2129. doi: 10.1073/pnas.78.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenfield N J, Hussain M, Lenard J. Effects of growth state and amines on cytoplasmic and vacuolar pH, phosphate and polyphosphate levels in Saccharomyces cerevisiae: a 31P-nuclear magnetic resonance study. Biochim Biophys Acta. 1987;926:205–214. doi: 10.1016/0304-4165(87)90205-4. [DOI] [PubMed] [Google Scholar]
- 10.Hare J F. Mechanisms of membrane protein turnover. Biochim Biophys Acta. 1990;1031:71–90. doi: 10.1016/0304-4157(90)90003-u. [DOI] [PubMed] [Google Scholar]
- 11.Höfeler H, Jensen D, Pike M M, Delayre J L, Cirillo V P, Springer C S, Jr, Fossel E T, Balschi J A. Sodium transport and phosphorous metabolism in sodium-loaded yeast: simultaneous observation with sodium-23 and phosphorous-31 NMR spectroscopy in vivo. Biochemistry. 1987;26:4953–4962. doi: 10.1021/bi00390a011. [DOI] [PubMed] [Google Scholar]
- 12.Kaffman A, Herskowitz I, Tjian R, O’Shea E K. Phosphorylation of the transcription factor PHO4 by a cyklin-CDK complex, PHO80-PHO85. Science. 1994;263:1153–1156. doi: 10.1126/science.8108735. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko Y, Toh-e A, Oshima Y. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol Cell Biol. 1982;2:127–137. doi: 10.1128/mcb.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Ljungdahl P O, Gimeno C J, Styles C A, Fink G R. SHR3: a novel component of the secretory pathway specifically required for localization of amino acid permeases in yeast. Cell. 1992;71:463–478. doi: 10.1016/0092-8674(92)90515-e. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwenhuis B J W M, Borst-Pauwels G W F H. Derepression of the high-affinity phosphate uptake in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984;770:40–46. doi: 10.1016/0005-2736(84)90071-3. [DOI] [PubMed] [Google Scholar]
- 17.Nyrén P, Nore B F, Baltscheffsky M. Studies of photosynthetic inorganic pyrophosphate formation in Rhodospirillum rubrum chromatophores. Biochim Biophys Acta. 1986;851:276–282. doi: 10.1016/0005-2728(86)90135-0. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa N, Hayashi N, Saito H, Noguchi K-I, Yamashita Y, Oshima Y. Regulatory circuit for phosphatase genes in Saccharomyces cerevisiae: specific cis-acting sites in PHO promoters for binding the positive regulator Pho4p. In: Torriani-Gorini A, Yagil E, Silver S, editors. Phosphate in microorganisms: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1994. pp. 56–62. [Google Scholar]
- 19.Okuda J, Miwa I. Newer developments in enzynic determination of D-glucose and its anomers. Methods Biochem Anal. 1973;21:155–189. doi: 10.1002/9780470110416.ch4. [DOI] [PubMed] [Google Scholar]
- 20.Philipsen P, Thomas M, Kramer R A, Davis R W. Unique arrangement of coding sequences for 5S, 5.8S, 18S and 25S ribosomal RNA in Saccharomyces cerevisiae as determined by R-loop and hybridization analysis. J Mol Biol. 1978;123:387–404. doi: 10.1016/0022-2836(78)90086-4. [DOI] [PubMed] [Google Scholar]
- 21.Roomans G M, Blasco F, Borst-Pauwels G W F H. Cotransport of phosphate and sodium by yeast. Biochim Biophys Acta. 1977;467:65–71. doi: 10.1016/0005-2736(77)90242-5. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Schneider K R, Smith R L, O’Shea K. Phosphate regulated inactivation of the kinase PHO80-PHO85 by the CDK inhibitor PHO81. Science. 1994;266:122–126. doi: 10.1126/science.7939631. [DOI] [PubMed] [Google Scholar]
- 24.Sherman F, Fink G R, Hicks J B. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- 25.Tamai Y, Toh-e A, Oshima Y. Regulation of inorganic phosphate transport systems in Saccharomyces cerevisiae. J Bacteriol. 1985;164:964–968. doi: 10.1128/jb.164.2.964-968.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]