Abstract
Objective
Gut microbiome is an intricate micro-ecosystem mediating the human health and drug efficacy. Physalis alkekengi (PAL) is an edible and time-honored traditional Chinese medicine. Several pharmacological effects of PAL have been verified and gut bacteria are implied in its therapeutic actions. However, the detailed modulation of PAL on gut bacterial species and on gut fungi remains largely unknown. We, therefore, designed a preliminary experiment in normal mice to reveal the modulation effect of PAL on both gut bacteria and fungi, and explore the interaction between them.
Methods
Herein, the aqueous extract of PAL was orally administrated to normal C57BL/6 mice for four weeks. The full-length 16S rRNA and ITS1/2 gene sequencing were explored to detect the taxa of gut bacteria and gut fungi after PAL treatment, respectively.
Results
Oral administration of PAL notably enriched anti-inflammatory bacterial species such as Duncaniella spp. and Kineothrix alysoides, whereas decreased pro-inflammatory species such as Mucispirillum schaedleri. Simultaneously, PAL increased the abundance of gut fungi Aspergillus ochraceus, Cladosporium sp. and Alternaria sp., and decreased Penicillium janthinellum. Correlation network analysis identified two co-existing microbial groups (groups 1 and 2) that were negatively associated with each other. The group 1 comprised PAL-enriched bacteria and fungi, while group 2 was mainly normal chow-enriched bacteria and fungi. In group 1, Antrodia monomitica, Aspergillus clavatus, Mortierella kuhlmanii and Sarcinomyces sp. MA 4787 were positively correlated with Bifidobacterium globosum, Romboutsia ilealis and so on. In group 2, Chaetomium subspirilliferum, Septoria orchidearum and Cephaliophora tropica were positively related to Lactobacillus spp.
Conclusion
Altogether, this preliminary study first demonstrated the modulation effect of PAL on both gut bacteria and gut fungi, which may shed light on the elucidation of PAL’s pharmacological mechanism.
Keywords: gut bacteria, gut fungi, Physalis alkekengi L., traditional Chinese medicine, micro-ecosystem
1. Introduction
Physalis alkekengi L. (PAL) is a widely used medicinal plant in China with multiple pharmacological activities such as anti-inflammation (Kang, Kwon, & Choi, 2011), anti-bacteria (Helvaci et al., 2010), anti-diabetes (Zhao, Chen, Yin, Y. & Li, & X., 2017), anti-ulcer (Wang et al., 2018), liver and lung protection (Wu et al., 2022b, Zhao et al., 2017). Several mechanisms have been proposed for the pharmacological effects of PAL in recent years. For example, Yang et al. (2019) found the polysaccharide fraction of PAL fruits could activate mitogen-activated protein kinases (MAPKs) and nuclear factor-kappaB (NF-κB) signaling pathway in RAW264.7 cells in a Toll-like receptor 2 (TLR2) and TLR4-dependent manner. Physalin B, an active component in PAL, could inhibit dextran sulfate sodium (DSS)-induced activation of NF-κB, signal transducer and activator of transcription 3 (STAT3), β-arrestin1 and NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, which contributed to its anti-colitis effects (Zhang et al., 2020b). Although some progress has been achieved, the potential targets and exact mechanisms of PAL remain largely mystery and need extensive exploration.
Gut microbiota is a collective name of tremendous amounts of microbes residing in our intestine, playing a pivotal role in maintenance of human health. Growing evidences have proved that gut microbiota is a promising target mediated the efficacy of various drugs, such as Momordica charantia L. (Zhang et al., 2020a), mulberry leaf (Li et al., 2020b) and berberine (Zhang et al., 2020c). The modulation of gut microbiota is closely related to the nature and properties of traditional Chinese medicines (TCMs) (Yang et al., 2022b, Yang et al., 2022c, Yin et al., 2022, Zhang et al., 2021a). Our previous study also revealed that specific bacterium such as Blautia producta was a key mediator for anti-hyperlipidemia effect of berberine (Wu et al., 2022a, Yang et al., 2022d). It was also reported that the beneficial effect of PAL polysaccharides on diabetes and inflammation was related with the decrease of lipopolysaccharide-producing and inflammation-related bacteria, such as Desulfovibrio and Acetatifactor, and the increase of short-chain fatty acid-producing bacteria in advanced glycation end products-induced mice (Wu et al., 2022c). However, the specific bacterial species that may mediate the pharmacological effect of PAL have not been identified. More precise method is needed to explore the modulation of PAL on gut bacterial in detail.
The gut fungi are another important component of gut microbiota in addition to bacteria. Recent investigations have revealed that gut fungi are also link to the maintenance of human healthy homeostasis. They are crucial for our immune system development and inflammatory reaction (Underhill and Iliev, 2014, Wheeler et al., 2016). Sun et al. (2020) found that the commensal fungus Meyerozyma guilliermondii could induce the production of PGE2 in the liver, which was regarded as one of the mechanisms of alcoholic hepatic steatosis development. Manipulation of the gut fungi and bacteria by the water-insoluble polysaccharides from Wolfporia cocos could effectively alleviate alcoholic liver diseases. We also revealed that the modulation of gut fungi plays an important role in the anti-hyperlipidemic effect of Coptis chinensis (Yang et al., 2022a). In light of the significant role of gut fungi in disease development and drugs’ action, simultaneously analyzing the impact on both gut bacteria and fungi will greatly stimulate the clarification of the intestinal mechanisms of various TCMs to ameliorate disease in vivo.
To systemically elucidate the modulation effects of TCMs on both gut bacterial and fungal communities, we gavaged mice with various TCMs for four weeks and analyzed the fecal bacteria and fungi using full-length 16S rRNA sequencing and ITS1/2 gene sequencing, respectively. This is a part of the systematic study, which focused on the regulating effect of PAL, an edible and medical fruit, on both gut bacteria and fungi. In folk medicine, PAL is often taken by healthy residents to prevent inflammation and metabolic disorders. Therefore, our investigation on PAL-treated normal mice may not only elucidate the potential intestinal mechanism of PAL to deal with various diseases but also provides an evidence for the health-care use of this edible herb.
2. Material and methods
2.1. Preparation of P. alkekengi aqueous extract
PAL was purchased from Bohaotang Chinese Traditional Medicinal Co., Ltd. (Bozhou, China) and it was authentic identified by professor Niankai Zeng and deposited in Hainan Medical University with the numbers of FHMU6613. The aqueous extract of PAL was prepared as previous study. Briefly, 200 g of unprocessed raw medicinal materials was extracted with 2000 mL distilled water for two times by decoction. After extraction, the extracts were merged, filtered, and concentrated to obtain the extract of PAL (200 mL).
2.2. Animals and treatment
A total of 17 8-week-old male C57BL/6 mice (22–25 g) were purchased from GemPharmatech Co., Ltd. (Haikou, China). The animals were maintained in an SPF environment with a controlled humidity (40 ± 5) % and temperature (23 ± 2) ℃, and 12 h light/dark cycle during experiment. All experimental procedures in this study were designed according to the guidelines for the care and use of laboratory animals of the National Institutes of Health and were approved by the Medical Ethics Committee of Hainan Medical University (No. SYXK-2017-0013).
After one week adapting feeding, the mice were randomly distributed into two groups. The control group (CK, n = 8) was daily administration with equal volume of distilled water and the PAL group (n = 9) was gavaged with PAL aqueous extract (10 g/kg) for four weeks. The dosage of PAL was equivalent to their recommended clinical dosage in Pharmacopoeia of the People’s Republic of China (2020 edition). Food/water intake and body weight were monitored once a week. At the end of the experiment, fresh fecal samples were collected from each mouse, snap-frozen in liquid nitrogen, and stored at −80 ℃ for further gut bacteria and gut fungi analysis.
2.3. DNA extraction and gene sequencing
The mouse fecal bacteria DNA was extracted by FastDNA Spin Kit (MP Biomedicals, Santa Ana, US) according to the manufacturer's instruction. The V1–V9 region of bacterial 16S rRNA gene and ITS1/2 region of fungi gene were amplified by TransGen AP221-02 and TransStart Fastpfu DNA Polymerase. The PacBio libraries were built and sequenced using a SEQUEL IIe system by Shanghai Biozeron Biothchnology Co., Ltd. (Shanghai, China).
2.4. Bioinformatics analysis
The full-length 16S rRNA and ITS1/2 sequencing data were subjected to the bioinformatics data analysis including quality control, assembly, and abundance quantification. Alpha- and beta-diversity of gut bacteria or gut fungi were calculated by vegan package (v2.7) of R version 4.0.2. The difference of Bray-Curtis distance of principal coordinates analysis (PCoA) and clustering analysis at operational taxonomic units (OTU) level was assessed by Adonis analysis. Correlation analysis was calculated by spearman algorithm. The interaction network of gut fungi-bacteria was visualized using Cytoscape 3.8.2 software. The differences among groups were analyzed by Student's t-test and P < 0.05 was considered as statistical significance.
3. Results
3.1. Alteration of gut bacterial diversity and structure in PAL feeding mice
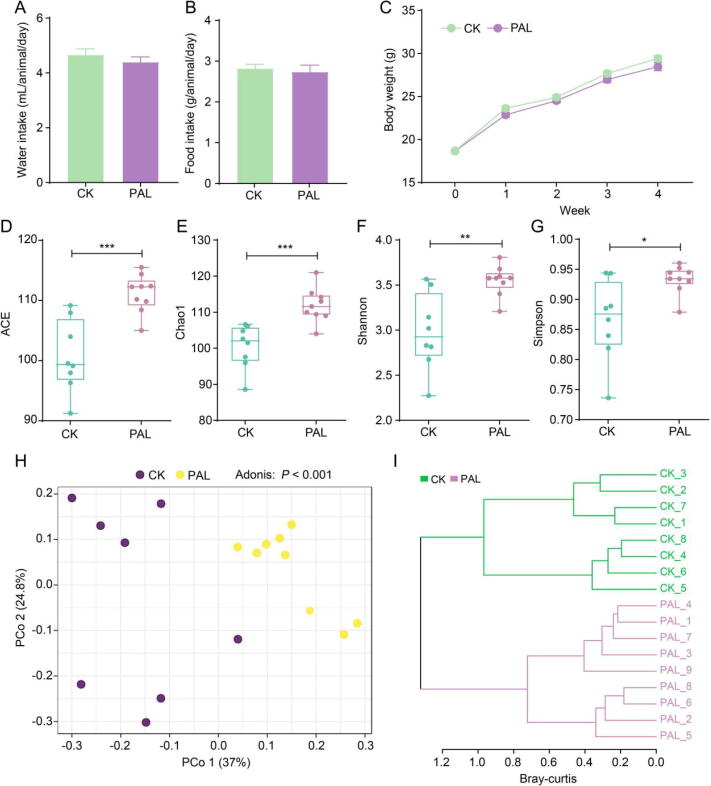
Oral administration of PAL did not influence the food intake of mice (Fig. 1A and B). The body weight of PAL-treated mice was slightly lower than normal control but the difference did not reach significance (Fig. 1C). These results indicated that four weeks administration of PAL showed no adverse effect on animals. In folk medicine, it is believed that PAL is also beneficial for healthy people to prevent diseases such as inflammation and diabetes. We therefore evaluated the impact of PAL on gut microbiota in normal animals to provide potential evidence for the beneficial effect of PAL on healthy persons.
Fig. 1.
PAL altered gut bacterial diversity and structure in mice. Average daily (A) water intake and (B) food intake of each mouse. (C) Change of body weight during four weeks treatment. The alpha diversity of gut bacteria after PAL treatment was assessed by (D) ACE, (E) Chao1, (F) Shannon and (G) Simpson indices. The statistical difference was evaluated by Student's t test. The structure of gut bacteria was assessed by (H) principal coordinate analysis (PCoA) and (I) hierarchical cluster analysis. The statistical difference was evaluated by Adonis analysis. *P < 0.05, **P < 0.01, ***P < 0.001.
Alpha-diversity is a critical indicator to manifest the health state of the gut microbiota, which is mainly indicated by ACE, Chao1, Shannon and Simpson indices. ACE and Chao1 usually reflect the richness of gut microbiota, while Shannon and Simpson generally reflect both the richness and evenness of gut microbiota. Fig. 1D–G showed that the ACE (P < 0.001), Chao1 (P < 0.001), Shannon (P < 0.01) and Simpson (P < 0.05) indices were all increased after PAL treatment compared with CK group, indicating that PAL could elevate the richness and evenness of gut bacteria. Beta-diversity is another pivotal indicator to embody the overall structure of gut microbiota, whose can be assessed by principal coordinate analysis (PCoA). PCoA diagram based on bray-curtis distance showed that the structure of gut bacteria was separated between CK and PAL group (P < 0.001) (Fig. 1H). The hierarchical cluster analysis also displayed the similar result, which CK group was obviously separated from PAL group (Fig. 1I). These results implied that administration of PAL significantly increased the evenness and richness of gut bacteria, along with notably changed its overall structure.
3.2. Modulation of gut bacterial composition by PAL
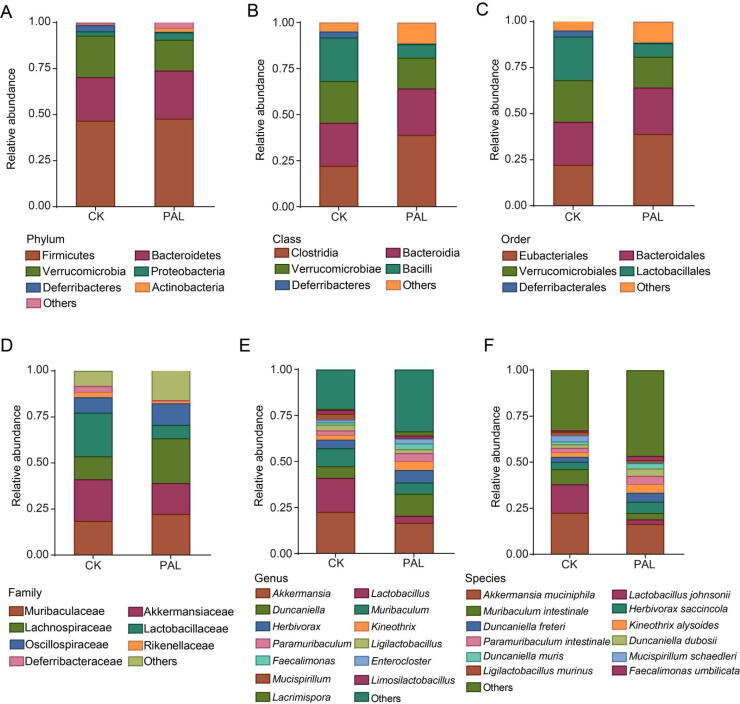
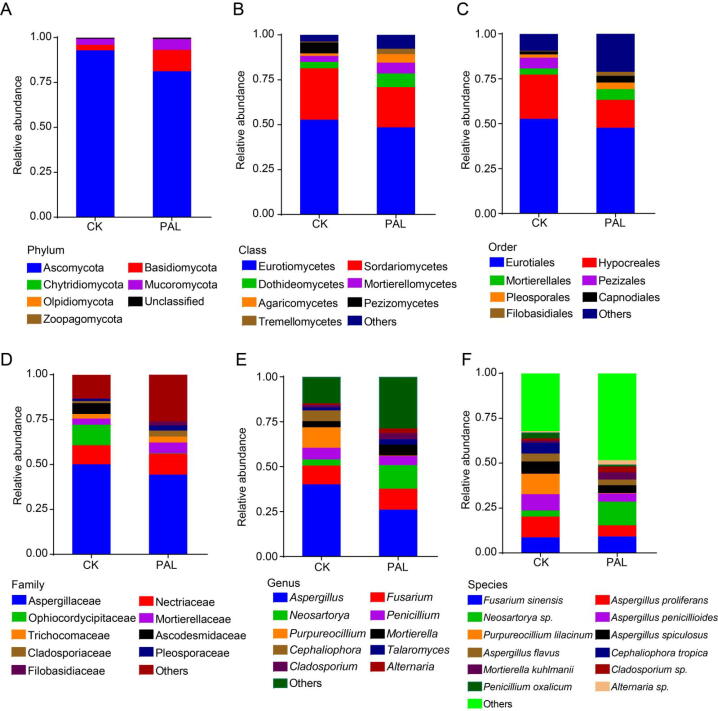
We then depicted the gut bacterial composition profile from phylum to species levels to identify the modulation effect of PAL on gut bacteria. At the phylum level, the dominant bacteria were Firmicutes, Bacteroidetes and Verrucomicrobia (Fig. 2A). The class of Bacilli and Deferribacteres, as well as the order of Lactobacillales and Deferribacterales were decreased after PAL treatment, while Eubacteriales order was increased (Fig. 2B and C). At family level, Lachnospiraceae and Oscillospiraceae were enriched by PAL, while Lactobacillaceae and Deferribacteraceae were reduced (Fig. 2D). Genera Duncaniella and Paramuribaculum of Muribaculaceae family, as well as genera Kineothrix, Faecalimonas and Lacrimispora of Lachnospiraceae were heightened, whereas genus Lactobacillus of Lactobacillaceae, Muribaculum of Muribaculaceae and Mucispirillum of Deferribacteraceae were lowered in PAL group (Fig. 2E). At species level, PAL elevated the relative abundance of Kineothrix alysoides, Duncaniella dubosii, Duncaniella muris, Faecalimonas umbilicata, but reduced the levels of Lactobacillus johnsonii, Muribaculum intestinale and Mucispirillum schaedleri (Fig. 2F).
Fig. 2.
PAL changed gut bacterial composition in mice. The gut bacterial composition profile diagram at phylum (A), class (B), order (C), family (D), genus (E) and species (F) levels. All of data were presented as mean.
3.3. Differential gut bacteria altered by PAL
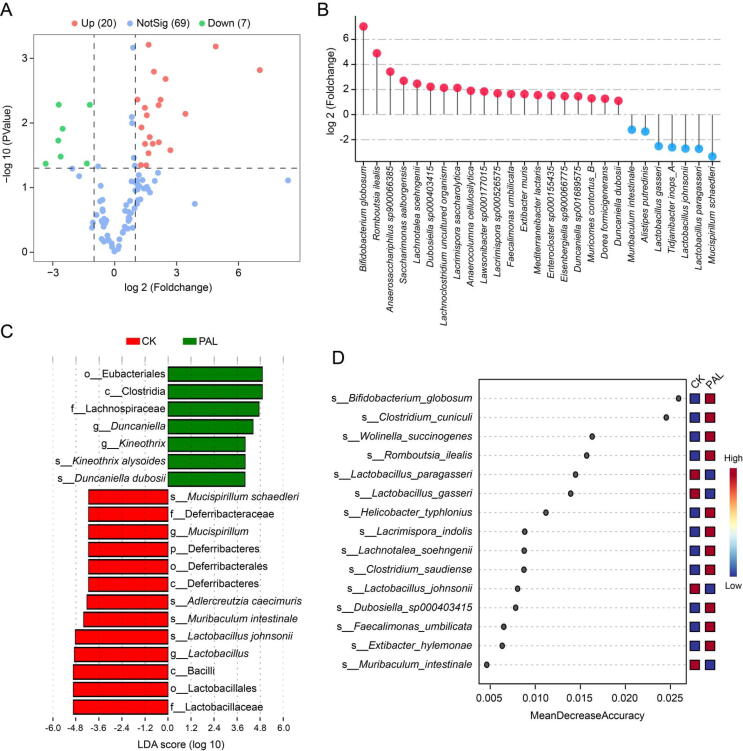
To clarify PAL-altered gut bacteria, we performed Student's t-test, linear discriminant analysis effect size (LEfSe) analysis and random forest analysis at the species level. Volcano plot displayed that there were 27 differential gut bacteria between CK and PAL groups (relative abundance >0.1%; P < 0.05), among which 20 bacteria were up-regulated while seven bacteria were down-regulated (Fig. 3A). The up-regulated species were Bifidobacterium globosum, Romboutsia ilealis, Anaerosacchariphilus sp900066385, Saccharimonas aalborgensis, Lachnotalea soehngenii, etc. and the down-regulated species were M. schaedleri, Lactobacillus paragasseri, L. johnsonii, Tidjanibacter inops A, Lactobacillus gasseri, Alistipes putredinis and M. intestinale (Fig. 3B). The distribution of differential bacteria was also detected by LEfSe analysis. As shown in Fig. 3C, the levels of K. alysoides and Duncaniella dubosii were increased in PAL group; otherwise, M. intestinale, M. schaedleri, Adlercreutzia caecimuris and L. johnsonii were increased in CK group. Random forest diagram showed that B. globosum, Clostridium cuniculi, Wolinella succinogenes, R. ilealis, L. paragasseri and L. gasseri were contributed greatly in the distinction of the two groups (Fig. 3D). Altogether, B. globosum, R. ilealis, L. johnsonii, Lactobacillus gasseri, M. intestinale and M. schaedleri may be differential bacterial species in PAL feeding mice.
Fig. 3.
Differential gut bacteria altered by PAL. (A) A volcano plot displayed the differential gut bacteria between CK and PAL groups (relative abundance >0.1%; P < 0.05). (B) Bar chart presented 20 up-regulated bacteria and seven down-regulated bacteria. (C) Distinct bacteria identified in PAL and CK group via LEfSe analysis. (D) The contribution of gut bacteria was predicted by random forest analysis.
3.4. Alteration of gut fungal diversity and structure by PAL
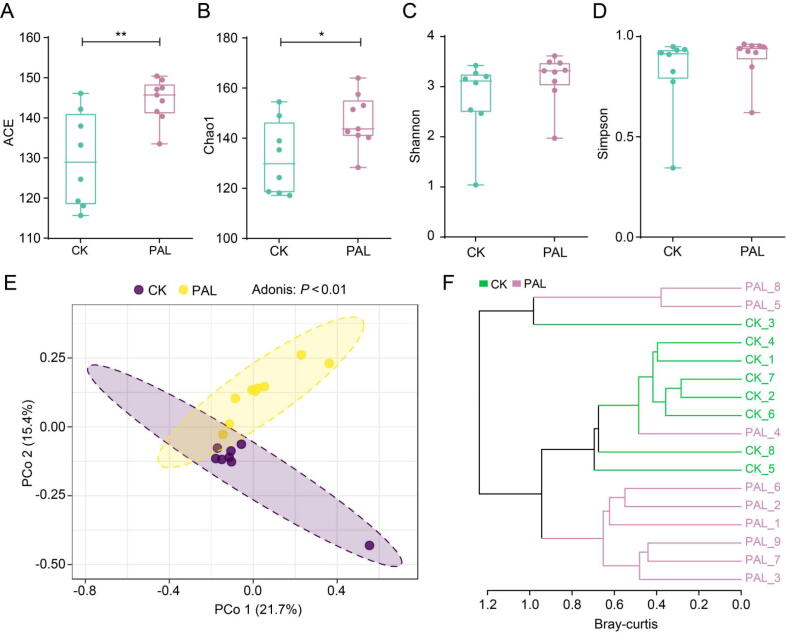
The intestinal micro-ecosystem is consisted of bacteria, fungi, virus and so on. Besides bacteria, gut fungi also participate in the regulation of human health. We therefore conducted ITS1/2 gene sequencing to investigate the change of gut fungi after PAL administration. The alpha-diversity analysis based on ACE (P < 0.01), Chao1 (P < 0.05), Shannon (P > 0.05) and Simpson (P > 0.05) indices demonstrated that PAL markedly increased the richness of gut fungi community, but had no significant effect on evenness (Fig. 4A–D). PCoA analysis and hierarchical cluster analysis based on bray-curtis distance showed that the structure of gut fungi in PAL group was separated from PAL group (P < 0.01) (Fig. 4E and F). These results suggested that PAL significantly increased the richness of gut fungi and markedly changed its overall structure.
Fig. 4.
PAL altered the gut fungal diversity and structure in mice. The alpha diversity of gut fungi after PAL treatment was assessed by (A) ACE, (B) chao1, (C) shannon and (D) simpson indices. The statistical difference was evaluated by Student's t-test. The structure of gut fungi was assessed by (E) principal coordinate analysis (PCoA) and (F) hierarchical cluster analysis. The statistical difference was evaluated by Adonis analysis. *P < 0.05, **P < 0.01.
3.5. Modulation of gut fungal composition by PAL
We further detected the composition of gut fungi from the phylum to species levels to assess the modulation effect of PAL on gut fungal composition. At phylum level, Ascomycota and Basidiomycota were dominant fungi, among which Ascomycota was reduced in PAL group, while Basidiomycota was elevated (Fig. 5A). As for class and order levels, Pezizomycetes class and Pezizales order were decreased, whereas Dothideomycetes, Mortierellomycetes, Agaricomycetes, Tremellomycetes class and Pleosporales, Capnodiales, Filobasidiales order were increased in PAL group as compared to PAL group (Fig. 5B and C). At family level, Ophiocordycipitaceae and Ascodesmidaceae levels were lower in PAL group than those in CK group, otherwise, Cladosporiaceae, Pleosporaceae and Filobasidiaceae were higher in PAL group than those in CK group (Fig. 5D). Additionally, PAL enriched Neosartorya, Cladosporium and Alternaria, but reduced Purpureocillium and Cephaliophora at the genus level (Fig. 5E). Fig. 5F also showed that the relative abundance of Purpureocillium lilacinum, Penicillium oxalicum, Aspergillus proliferans and A. penicillioides were obviously declined, and Neosartorya sp., Mortierella kuhlmanii, Cladosporium sp. and Alternaria sp. were increased after PAL treatment.
Fig. 5.
PAL changed the gut fungal composition in mice. The gut fungal composition profile diagram at phylum (A), class (B), order (C), family (D), genus (E) and species (F) levels. All of data were presented as mean.
3.6. Differential gut fungi altered by PAL
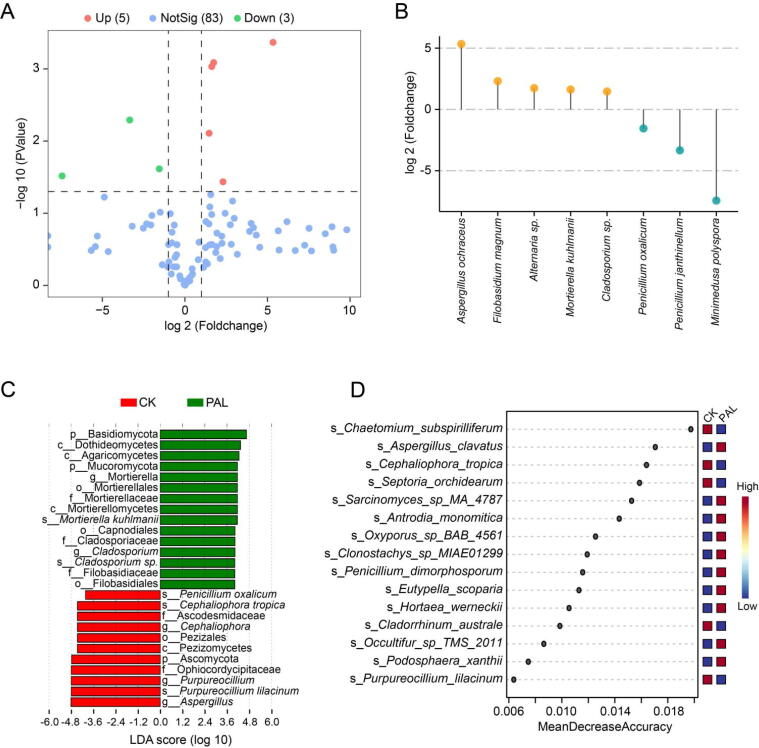
We also conducted Student's t-test, LEfSe analysis and random forest analysis to identify the differential fungal species in PAL feeding mice. As compared with CK group, PAL notably up-regulated five species, but down-regulated three species (Fig. 6A), that was, A. ochraceus, Filobasidium magnum, Alternaria sp., M. kuhlmanii and Cladosporium sp. were increased, P. oxalicum, P. janthinellum and Minimedusa polyspora were decreased (Fig. 6B). LEfSe diagram showed that P. oxalicum, Cephaliophora tropica and P. lilacinum were enriched in CK group, while M. kuhlmanii and Cladosporium sp. were enriched in PAL group (Fig. 6C). Random forest analysis revealed that Chaetomium subspirilliferum, A. clavatus, C. tropica, Septoria orchidearum, Sarcinomyces sp. MA 4787 and Antrodia monomitica were contributed greatly in the distinction of the two groups (Fig. 6D). These results demonstrated that PAL markedly altered the composition of gut fungal community, among which Cladosporium sp., M. kuhlmanii, A. ochraceus, F. magnum and Alternaria sp. may be the differential gut fungi that characterizing PAL.
Fig. 6.
Differential gut fungi altered by PAL. (A) A volcano plot displayed the differential gut fungi between CK and PAL groups (relative abundance >0.1%; P < 0.05). (B) Bar chart presented five up-regulated fungi and three down-regulated fungi. (C) Distinct fungi identified in PAL and CK group via LEfSe analysis. (D) The contribution of gut fungi was predicted by random forest analysis.
3.7. Interaction of gut bacteria and gut fungi in PAL feeding mice
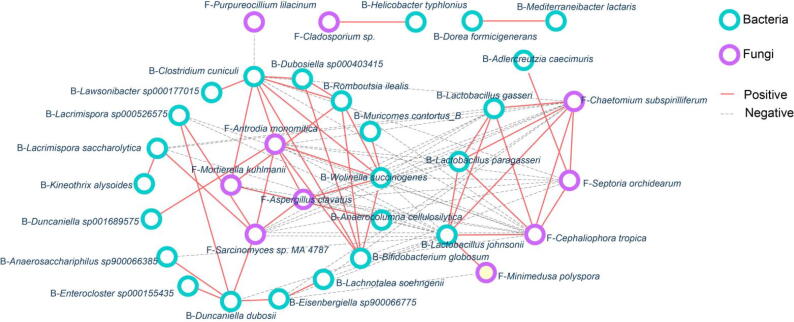
To explore the intrinsic association of gut bacteria and gut fungi in PAL feeding mice, we built an interaction network between differential bacteria and fungi based on Spearman algorithm. The results showed that there were mainly two interaction groups (Fig. 7). In group 1, four fungi, including A. monomitica, A. clavatus, M. kuhlmanii and Sarcinomyces sp. MA 4787, were positively related to each other; Simultaneously, they were positively associated with 17 bacteria such as R. ilealis, Duncaniella dubosii, Wolinella succinogenes, B. Globo sum and so on. In group 2, three fungi including C. subspirilliferum, S. orchidearum and C. tropica were positively related with each other, along with showed a positive interaction with Lactobacillus (L. paragasseri, L. johnsonii, L. gasseri). Moreover, the negative correlation was presented between group 1 and group 2. Based on above results, we found that there were complex interactions between gut bacteria and gut fungi in PAL feeding mice, which may mediate the pharmacological effect of PAL.
Fig. 7.
Interaction of gut bacteria and gut fungi in PAL feeding mice. Fungi–bacteria network in mice with or without PAL treatment. The correlation was calculated by Spearman coefficient. Green dots: bacteria; purple triangle: fungi; red solid line: positive correlation; gray dotted line: negative correlation.
4. Discussion
An increasing number of data have unveiled that TCMs usually interplay with gut microbiota after oral administration to exert their efficacy. However, due to the complex of intestinal micro-environment that composed of bacteria, fungi and so on, there is still a lacking of study simultaneously explored the modulation of TCMs on gut bacteria and gut fungi. This systematic study, to our knowledge, is the first to elucidate bacteria and mycobiota alterations after TCM treatment. In this paper, we found that PAL treatment enriched bacteria of B. globosum, R. ilealis, and fungi of Cladosporium sp., M. kuhlmanii, A. ochraceus, Filobasidium magnum, Alternaria sp. While bacteria of Lactobacillus (L. johnsonii, L. gasseri) and fungi of Penicillium (P. oxalicum, P. janthinellum) were decreased in PAL group. Furthermore, correlation network revealed that normal chow-enriched fungi including C. subspirilliferum, S. orchidearum and C. tropica were positively related to Lactobacillus; whereas PAL-enriched fungi including A. monomitica, A. clavatus, M. kuhlmanii and Sarcinomyces sp. MA 4787 were positively associated with PAL-enriched bacteria such as B. globosum, R. ilealis and so on. This exploratory study will provide a new direction for mechanistic research of TCM.
Currently, the hypothesis of the crucial role of some gut microbial species or strains in maintenance of human health and disease course has been supported by several basic researches and clinical researches (Dong et al., 2022). The experiment based on culturomics and mono-colonialization revealed that the genus of Duncaniella could protect host from dextran sulfate sodium (DSS)-induced colitis injury (Chang et al., 2021). Unsurprisingly, Duncaniella genus (D. dubosii and D. muris) were enriched by PAL, suggesting that the enrichment of Duncaniella possibly involved in the anti-colitic effects of PAL on DSS-induced mice (Zhang et al., 2020b). As a short-chain fatty acid (SCFA)-producing bacteria, K. alysoides could promote the production of SCFA, which then enhance the expression of TLR4 and further activate mitogen-activated protein kinase (MAPK) and NF-κB signaling pathway to initiate innate immunity against tumor cells (Xiao et al., 2018, Xie et al., 2021). We also observed the increasing of K. alysoides after PAL treatment, which may contribute to the activation of MAPK/NK‑κB signaling pathway by PAL. In contrast, the species of M. schaedleri was decreased in present study due to it was a pro-inflammatory species which was enriched in genetic and chemical colitis models and infection (Herp, Durai Raj, Salvado Silva, Woelfel, & Stecher, 2021). In addition, it is reported that supplementation with L. johnsonii and L. gasseri is an effectively strategy for preventing Citrobacter rodentium-induced colitis and allergic airway inflammation, respectively. (Hsieh et al., 2018, Zhang et al., 2021b). The present results, however, showed that L. johnsonii and L. gasseri were reduced after PAL treatment, which might be caused by the normal mice were applied in this study, not corresponding animal model.
Gut fungi has evolved along with bacteria and host, and is an indispensable part of the human body. There is a strong connection between human health and the gut fungi and its metabolites. Tong et al. (2021) found that an ergostane-type sterol derivative extracted from A. ochraceus notably enhanced the GSH level and scavenged ROS, thereby protected SH-SY5Y cells from H2O2 damage. Similarly, flavone isolated from P. alkekengi could significantly inhibit the production of MDA, and oxidative hemolysis of erythrocytes induced by H2O2 in mice (Yang, Li, & Yang, 2014), indicating that the enrichment of A. ochraceus by PAL may participate in its anti-oxidation effect. Furthermore, a new indole diterpenoid alkaloid, cladosporine A, derived from fungal strain Cladosporium sp. JNU17DTH12-9-01 possessed antimicrobial activity (Han et al., 2021), and resveratrol produced by strain Alternaria sp. MG1 showed protective effect for many diseases (Shi et al., 2012). In current study, we found the relative abundance of Cladosporium sp. and Alternaria sp. were all elevated by PAL. A case study reported that P. janthinellum could cause infection in immunosuppressed patients, such as a systemic lupus erythematosus patient (Li et al., 2020a). We found that PAL could decrease the level of P. janthinellum, which may enhance the immunomodulatory effect of PAL. Even though the human gut microbiome has received extensive attention, a lot is not known about gut fungi, especially the composition of the TCM-associated mycobiota and dynamic changes. Further research should concentrate more on the modulation effect of TCM on gut fungi and explore the association between gut fungal species and disease development.
Several gut bacteria and fungi live together and influence one another, so it is likely that an alteration in fungal abundance could affect a composition of gut bacteria and vice versa. In our previous study, a complex relationship between the gut bacteria and fungi was identified in the Coptidis Rhizoma treated mice (Yang et al., 2022a). In this study, we found that both gut bacterial and gut fungal diversity was increased and the composition was altered after PAL treatment. Correlation network depicted that PAL-enriched group 1 was negatively related to CK-enriched group 2. In group 1, fungi of A. monomitica, A. clavatus, M. kuhlmanii and Sarcinomyces sp. MA 4787 were positively associated with bacteria of B. globosum, R. ilealis and so on. While in group 2, fungi including C. subspirilliferum, S. orchidearum and C. tropica were positively related with Lactobacillus. In general, less fungi-bacteria interactions were identified so that the consequences of the interaction between bacteria and fungi remain a mystery. Future studies are also required to identify the fungal and bacteria composition in detail after TCM treatment, and define the mechanism underlying the interaction between gut fungi and gut bacteria.
Although the present study, for the first time, demonstrated the modulation effect of PAL on specific gut bacteria and gut fungi, along with elucidated the interaction between differential bacteria and fungi, we have to point out that this is only a preliminary study and it needs more delicate and rigorous design to further clarify the intrinsic mechanisms of TCMs such as PAL from the perspective of gut microbiome. In the future study, we will establish the corresponding pathological animal models to investigate the modulation effect of PAL on gut microbiome. Further, we will obtain the corresponding characteristic gut bacterial and fungal strains by culturomics approach, verify the efficacy of the strains and explore the potential mechanism. Such systematic study will promote the interpretation of TCM’s mechanism to some extent.
5. Conclusion
In sum, this preliminary study first reported that PAL could alter the structure and composition of both gut bacteria and gut fungi, as well as regulate their interaction, indicating that modulation of gut microbiome may be a new mechanism for pharmacological effect of PAL.
CRediT authorship contribution statement
Yanan Yang: Data curation, Formal analysis, Visualization, Writing – original draft. Xiaohui Zhao: Writing – review & editing. Yong Xie: Supervision, Writing – review & editing. Chongming Wu: Conceptualization, Data curation, Project administration, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (No. 81973217).
Contributor Information
Yong Xie, Email: yxie@implad.ac.cn.
Chongming Wu, Email: chomingwu@163.com.
References
- Chang C.S., Liao Y.C., Huang C.T., Lin C.M., Cheung C.H.Y., Ruan J.W.…Kao C.Y. Identification of a gut microbiota member that ameliorates DSS-induced colitis in intestinal barrier enhanced Dusp6-deficient mice. Cell Reports. 2021;37 doi: 10.1016/j.celrep.2021.110016. [DOI] [PubMed] [Google Scholar]
- Dong C., Yang Y., Wang Y., Hu X., Wang Q., Gao F.…Zhu H. Gut microbiota combined with metabolites reveals unique features of acute myocardial infarction patients different from stable coronary artery disease. Journal of Advanced Research. 2022;S2090–1232 doi: 10.1016/j.jare.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Bao X.F., Wang C.X., Xie J., Song X.J., Dai P.…Gao H. Cladosporine A, a new indole diterpenoid alkaloid with antimicrobial activities from Cladosporium sp. Natural Product Research. 2021;35:1115–1121. doi: 10.1080/14786419.2019.1641807. [DOI] [PubMed] [Google Scholar]
- Helvaci S., Kokdil G., Kawai M., Duran N., Duran G., Guvenc A. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D. Pharmaceutical Biology. 2010;48:142–150. doi: 10.3109/13880200903062606. [DOI] [PubMed] [Google Scholar]
- Herp S., Durai Raj A.C., Salvado Silva M., Woelfel S., Stecher B. The human symbiont Mucispirillum schaedleri: Causality in health and disease. Medical Microbiology Immunology. 2021;210:173–179. doi: 10.1007/s00430-021-00702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M.H., Jan R.L., Wu L.S., Chen P.C., Kao H.F., Kuo W.S., Wang J.Y. Lactobacillus gasseri attenuates allergic airway inflammation through PPARgamma activation in dendritic cells. Journal of Molecular Medicine (Berl) 2018;96:39–51. doi: 10.1007/s00109-017-1598-1. [DOI] [PubMed] [Google Scholar]
- Kang H., Kwon S.R., Choi H.Y. Inhibitory effect of Physalis alkekengi L. var. franchetii extract and its chloroform fraction on LPS or LPS/IFN-gamma-stimulated inflammatory response in peritoneal macrophages. Journal of Ethnopharmacology. 2011;135:95–101. doi: 10.1016/j.jep.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Li X., Zong L., Zhu Y., Li Y., Zhou Y., Zhou H. Penicillium janthinellum pneumonia in an SLE patient: A case study. Infection and Drug Resistance. 2020;13:2745–2749. doi: 10.2147/IDR.S255968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Xu, W., Zhang, F., Zhong, S., Sun, Y., Huo, J., Zhu, J. & Wu, C. (2020b). The gut microbiota-produced indole-3-propionic acid confers the antihyperlipidemic effect of mulberry-derived 1-deoxynojirimycin. mSystems, 5, e00313-00320. [DOI] [PMC free article] [PubMed]
- Shi J., Zeng Q., Liu Y., Pan Z. Alternaria sp. MG1, a resveratrol-producing fungus: Isolation, identification, and optimal cultivation conditions for resveratrol production. Applied Microbiology Biotechnology. 2012;95:369–379. doi: 10.1007/s00253-012-4045-9. [DOI] [PubMed] [Google Scholar]
- Sun S., Wang K., Sun L., Cheng B., Qiao S., Dai H.…Liu H. Therapeutic manipulation of gut microbiota by polysaccharides of Wolfiporia cocos reveals the contribution of the gut fungi-induced PGE2 to alcoholic hepatic steatosis. Gut Microbes. 2020;12 doi: 10.1080/19490976.2020.1830693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z., Xiao X., Lu Y., Zhang Y., Hu P., Jiang W.…Hu L. New metabolites from Aspergillus ochraceus with antioxidative activity and neuroprotective potential on H2O2 insult SH-SY5Y cells. Molecules. 2021;27:52. doi: 10.3390/molecules27010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill D.M., Iliev I.D. The mycobiota: Interactions between commensal fungi and the host immune system. Nature Reviews Immunology. 2014;14:405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang S.L., Zhang J.Y., Song X.N., Zhang Z.Y., Li J.F., Li S. Anti-ulcer and anti-helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. Journal of Ethnopharmacology. 2018;211:197–206. doi: 10.1016/j.jep.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Wheeler M.L., Limon J.J., Bar A.S., Leal C.A., Gargus M., Tang J.…Iliev I.D. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe. 2016;19:865–873. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.M., Zhao Y., Zhang Y.Y., Yang Y.N., Su W.Q., Yang Y.Y.…Wu S.X. Gut microbiota specifically mediates the anti-hypercholesterolemic effect of berberine (BBR) and facilitates to predict BBR's cholesterol-decreasing efficacy in patients. Journal of Advanced Research. 2022;37:197–208. doi: 10.1016/j.jare.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.Y., Wang T., Hu H.X., Zhang K., Zhao Y., Zhao B.B.…Shen T. The alleviative effect of flavonol-type Nrf2 activator rhamnazin from Physalis alkekengi L. var. franchetii (Mast.) Makino on pulmonary disorders. Phytotherapy Research. 2022;36:1692–1707. doi: 10.1002/ptr.7403. [DOI] [PubMed] [Google Scholar]
- Wu Y., Dong L., Song Y., Wu Y., Zhang Y., Wang S. Preventive effects of polysaccharides from Physalis alkekengi L. on dietary advanced glycation end product-induced insulin resistance in mice associated with the modulation of gut microbiota. International Journal of Biological Macromolecules. 2022;204:204–214. doi: 10.1016/j.ijbiomac.2022.01.152. [DOI] [PubMed] [Google Scholar]
- Xiao T., Wu S., Yan C., Zhao C., Jin H., Yan N.…Xia S. Butyrate upregulates the TLR4 expression and the phosphorylation of MAPKs and NK-kappaB in colon cancer cell in vitro. Oncology Letters. 2018;16:4439–4447. doi: 10.3892/ol.2018.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X.Q., Geng Y., Guan Q., Ren Y., Guo L., Lv Q.…Xu Z.H. Influence of short-term consumption of Hericium erinaceus on serum biochemical markers and the changes of the gut microbiota: A pilot study. Nutrients. 2021;13:1008. doi: 10.3390/nu13031008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Li X., Yang Y., Ayivi-Tosuh S.M., Wang F., Li H., Wang G. A polysaccharide isolated from the fruits of Physalis alkekengi L. induces RAW264.7 macrophages activation via TLR2 and TLR4-mediated MAPK and NF-kappaB signaling pathways. International Journal of Biological Macromolecules. 2019;140:895–906. doi: 10.1016/j.ijbiomac.2019.08.174. [DOI] [PubMed] [Google Scholar]
- Yang J.Y., Li J.J., Yang D.C. Advances in studies on pharmacological functions of Physalis alkekengi L. var. franchetii. Inner Mongolia Journal of Traditional Chinese Medicine. 2014;33:116–117. [Google Scholar]
- Yang Y., Cao S., Xu W., Zang C., Zhang F., Xie Y., Wu C. Dual modulation of gut bacteria and fungi manifests the gut-based anti-hyperlipidemic effect of Coptidis Rhizoma. Biomedicine & Pharmacotherapy. 2022;153 doi: 10.1016/j.biopha.2022.113542. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lu W., Zhang X., Wu C. Gut fungi differentially response to the antipyretic (heat-clearing) and diaphoretic (exterior-releasing) traditional Chinese medicines in Coptis chinensis-conditioned gut microbiota. Frontiers in Pharmacology. 2022;13:1032919. doi: 10.3389/fphar.2022.1032919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.N., Deng Y.T., Zang C.C., Zhang F., Huang Z.B., Dong L.…Wu C.M. The gut microbial co-abundance gene groups (CAGs) differentially respond to the flavor (Yao-Wei) of Chinese materia medica. American Journal of Chinese Medicine. 2022;50:2223–2244. doi: 10.1142/S0192415X22500963. [DOI] [PubMed] [Google Scholar]
- Yang Y.N., Wang Q.C., Xu W., Yu J., Zhang H., Wu C. The berberine-enriched gut commensal Blautia producta ameliorates high-fat diet (HFD)-induced hyperlipidemia and stimulates liver LDLR expression. Biomedicine & Pharmacotherapy. 2022;155 doi: 10.1016/j.biopha.2022.113749. [DOI] [PubMed] [Google Scholar]
- Yin L., Xia W.R., Huang G.X., Xiao W.L. Research progress on correlation between traditional Chinese medicine-gut microbiota and host’s own metabolic immune homeostasis. Chinese Traditional and Herbal Drugs. 2022;53(8):2526–2538. [Google Scholar]
- Zhang F., Zhang X.P., Yu J.Q., Tan Y.F., Guo P., Wu C.M. The gut microbiota confers the lipid-lowering effect of bitter melon (Momordica charantia L.) in high-fat diet (HFD)-induced hyperlipidemic mice. Biomedicine & Pharmacotherapy. 2020;131 doi: 10.1016/j.biopha.2020.110667. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Xu N., Hu X., Zheng Y. Anti-colitic effects of Physalin B on dextran sodium sulfate-induced BALB/c mice by suppressing multiple inflammatory signaling pathways. Journal of Ethnopharmacology. 2020;259 doi: 10.1016/j.jep.2020.112956. [DOI] [PubMed] [Google Scholar]
- Zhang X.P., Yang Y.N., Zhang F., Yu J.Q., Sun W.Y., Wang R.Q., Wu C.M. Traditional Chinese medicines differentially modulate the gut microbiota based on their nature (Yao-Xing) Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2021.153496. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gu Y., Ren H., Wang S., Zhong H., Zhao X.…Wang W. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study) Nature Communications. 2020;11:5015. doi: 10.1038/s41467-020-18414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mu T., Yang Y., Zhang J., Ren F., Wu Z. Lactobacillus johnsonii attenuates Citrobacter rodentium-induced colitis by regulating inflammatory responses and endoplasmic reticulum stress in mice. Journal of Nutrition. 2021;151:3391–3399. doi: 10.1093/jn/nxab250. [DOI] [PubMed] [Google Scholar]
- Zhao X., Chen Z., Yin, Y. & Li, X. Effects of polysaccharide from Physalis alkekengi var. francheti on liver injury and intestinal microflora in type-2 diabetic mice. Pharmaceutical Biology. 2017;55:2020–2025. doi: 10.1080/13880209.2017.1345953. [DOI] [PMC free article] [PubMed] [Google Scholar]