Abstract
Background
A lack of geographic and racial diversity in clinical trial populations may arise from a disproportionate focus on the United States and Europe for trial leadership and conduct. Inadequate diversity may compromise the external validity to the Asia-Pacific (APAC) region, where 60% of global cardiometabolic disease exists.
Objectives
This study aimed to assess the proportion and trends of Asian race participants and APAC authorship in cardiometabolic trials.
Methods
We performed a systematic review of all cardiovascular, diabetes and obesity-related randomized controlled trials (phase ≥2, n = ≥100) published in these major medical journals: the New England Journal of Medicine, the Lancet, and the Journal of the American Medical Association between January 1, 2011, and December 31, 2020. Trial leadership was defined by first authorship, and any listed author was considered a trial collaborator. Temporal trends were evaluated using the Jonckheere–Terpstra proportion test and correlations using Pearson’s correlation coefficient. Participant-to-prevalence ratios (PPR) were determined using Global Health Data Exchange registry data.
Results
A total of 8.3% (218,613 of 2,619,710) participants identified as being of Asian race and 7.7% of total enrollment occurred in APAC. APAC lead authorship occurred in 52 of 656 (7.9%) trials and collaboration in 10.1% (1312 of 13,000 of authors), which correlated with Asian enrollment (r = 0.63 and r = 0.76, respectively). A marginal increase in the proportion of Asian race (Δ1.40% ± 6.95%/year, P = 0.003) and APAC regional (Δ1.46% ± 8.67%/year, P = 0.003) enrollment was observed; however, severe regional underrepresentation persisted (PPR <0.30).
Conclusions
Despite a favorable trend over the past decade, Asian participants and authors from APAC remain significantly underrepresented in seminal cardiometabolic trials; barriers to trial conduct and leadership in this region must be addressed.
Key Words: cardiometabolic, clinical trials, diversity, race
Central Illustration
Clinical trial populations must be sufficiently diverse and representative of the clinical burden of disease their intervention ultimately intends to address. Insufficient diversity and inclusion threaten the external validity of a trial’s results. The peer-reviewed literature has shone light on inadequate representation of gender,1, 2, 3 race,4, 5, 6, 7 and age3,8, 9, 10, 11 in cardiovascular research, with regulators such as the U.S. Food and Drug Administration (FDA) providing voluntary guidance for industry in November 2020 aimed at improving trial diversity.12 This has gained further momentum with legislation changes currently underway in the United States that will require sponsors to submit a diversity action plan to the FDA when undertaking a phase 3 or pivotal study.13
However, from a global perspective, comparably less has been made of the need to address geographic and Asian race diversity. Cardiometabolic disease continues to be a leading cause of death globally, with at least 60% of the burden of disease experienced by people living in the Asia-Pacific (APAC) region,14, 15, 16 Despite this concerning statistic, a recently conducted bibliometric review of heart failure trials revealed that <15% of participants were recruited from Oceania and Asia.17 The same authors also showed that non-European, non-American investigators, although infrequent, were more likely to recruit participants from those regions. However, this analysis was restricted to heart failure trials and did not evaluate recruitment by race, nor did it report specific variables by APAC region.
Thus, the focus of our study was to evaluate trends in the proportion of Asian race and APAC regional enrollment as well as trends in lead and collaborative authorship within high-impact cardiometabolic trials over the past 10 years.
Methods
Our systematic review was registered in PROSPERO (CRD42023388798) and presented using STROBE guidance.
Search Strategy and Data Sources
We conducted a search of MEDLINE to identify cardiometabolic trials that were likely to have an impact on clinical care. We confined our search for studies published in these 3 high-impact journals: the New England Journal of Medicine, the Lancet, and the Journal of the American Medical Association. Trials published in the highest-impact journals generally have rigorous methods, larger sample sizes, and lower risk of bias, and by definition are more likely to publish practice-changing and guideline-informing studies.18 Our search strategy used key words and MESH terms to cover cardiovascular, cardiometabolic, diabetes, and obesity trials; our full strategy is included in the Supplemental Appendix. Our search period of interest was from January 1, 2011, until December 31, 2020. A 10-year period of evaluation was chosen because it was considered sufficiently long to evaluate trends yet recent enough to reflect contemporary practice. This study was considered exempt from ethical approval by the Monash Health Ethics Committee.
Study Selection
The authors independently screened titles and abstracts against predetermined inclusion/exclusion criteria (R.A., A.J.N.). Any discrepancies between reviewers' study selections were resolved by consensus. Studies were included if they satisfied the following criteria: 1) was a phase II, III, or IV trial; 2) included participants ≥18 years of age; 3) investigated patients with cardiovascular or cardiometabolic disease; and 4) enrolled ≥100 participants. Studies looking at post-hoc, intermediate, or secondary analyses were excluded.
Data Extraction
Extraction of data was completed using a standardized format. Full text publications and any supplemental material was evaluated for data pertaining to study variables. Extraction was performed by 1 author (R.A.), and a random sample of 10% was independently confirmed by another (A.N.).
Variables
Lead authorship was defined as the first or corresponding author of the publication, and any listed author was considered a trial collaborator. The authors’ countries were determined according to their listed primary affiliation details and segmented by region according to the Global Burden of Disease geographic convention. A study was considered multiregional when ≥2 regions were represented in the enrolled population. Trial interventions were categorized as follows: drug/pharmaceutical, procedure, counseling/nonpharmaceutical/health service, device, or biologic. Therapeutic areas were defined as follows: hypertension, arrhythmia, coronary artery disease (CAD), peripheral artery disease (PAD), cerebrovascular disease (CeVD), venous thromboembolism, critical care/resuscitation medicine, pericardial disease, hypertension, lipids, pulmonary hypertension, heart failure/cardiomyopathy, diabetes, obesity, valvular heart disease, aortic disease. Studies were categorized as “industry” if commercial organizations were acknowledged to be the primary source of funding in the publication or as “nonindustry” for all others.
Outcomes
The primary outcome was lead authorship in the APAC region. Secondary outcomes included trial collaboration in the APAC region, and participant enrollment by Asian race and by APAC region. Proportion of Asian race was determined as reported in the main manuscript or its supplemental appendix. In studies conducted within an APAC country with >95% Asian race population, Asian race enrollment was assumed to be 100% if not explicitly reported. Enrollment by APAC region was determined from the main manuscript (or its supplement) by calculating the number of participants enrolled from all APAC countries (as defined earlier by the Global Burden of Disease APAC geographic convention) divided by the number of all enrolled participants.
Statistical Analysis
Descriptive analysis was performed on trial characteristics and regions; categorical variables are presented as number (percentages) and continuous variables as median (Q1, Q3). Trends in lead authorship and collaboration over the study duration were analyzed with the Jonckheere-Terpstra proportion test of trend and presented as Δ mean ± SD % change/year (P value). Trial participant-to-prevalence ratio (PPR) was estimated for APAC in each of the therapeutic areas. We calculated PPRs from the APAC regional contribution of participants to the total randomized controlled trials (RCTs) divided by the APAC prevalence of that therapeutic area using the Global Health Data Exchange registry19 and other published sources.20 A PPR close to 1.0 indicates that the proportion of APAC participants in the clinical trials is comparable with that of the global prevalence, a PPR <0.80 indicates that the population is underrepresented, and >1.2 indicates that they are overrepresented.2 Given the breadth of the disease areas, prevalence data were not reportable for aortic disease, arrhythmia, critical care/resuscitation medicine, and pulmonary hypertension. Correlation between continuous variables was reported with Pearson’s correlation coefficient (r) and P value
Results
In the 10-year study period, 656 trials met the inclusion criteria; 306 were published in the New England Journal of Medicine, 181 in the Lancet, and 169 in the Journal of the American Medical Association (Figure 1). The median number of participants per trial was 1,203 (Q1, Q3: 426, 4,001) of median duration 12 (Q1, Q3: 3, 24) months. The majority of trials evaluated a pharmaceutical intervention (49.8%, 327/656) with approximately one third evaluating a procedure (28.8%, 189/656) and a smaller proportion testing nonpharmaceutical interventions (15.2%, 100/656). Patients with coronary artery disease constituted the most studied population (33.5%, 220/656), with a significant proportion of trials focused on patients with CeVD (12.8%, 84/656), diabetes (11.4%, 75/656), and heart failure (9.4%, 62/656).
Figure 1.
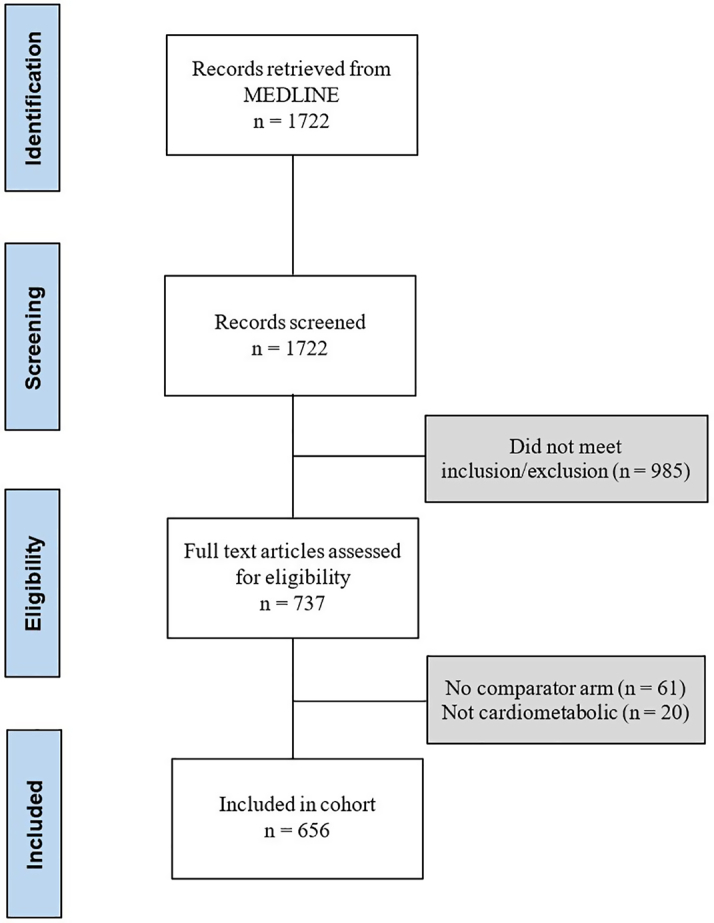
Study Flow Diagram
PRISMA flow diagram representing included studies based on screening and eligibility.
APAC Representation Among Trial Conduct
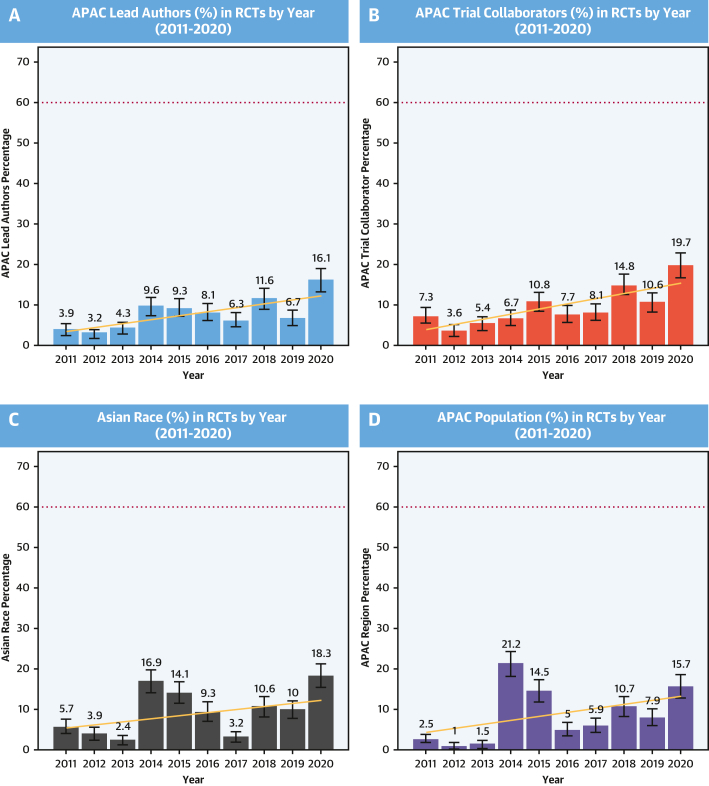
Lead authors were listed in 651 of the 656 eligible studies (Table 1). The majority of the 651 lead authors were from the United States (n = 275, 42.1%) and Europe (n = 242, 37.1%) with 52 (7.9%) from APAC countries. Of the 52 APAC lead authors, most were from Australia (n = 26), and the others were from China (n = 9), Korea (n = 9), Japan (n = 4), India (n = 2), and 1 each from New Zealand and Singapore. Relative to their overall contribution, APAC lead authorship was more common among CAD, CeVD, hypertension, and lipid trials, with minimal or no leadership of diabetes, heart failure, and obesity trials. Among APAC investigators, lead authorship was more common in nonindustry- than in industry-sponsored trials (10.6% vs 5.4%). The proportion of APAC lead authors increased over the study period (mean Δ1.36% ± 4.45%/year; P = 0.02) (Figure 2).
Table 1.
Characteristics of Trials by APAC Leadership and Collaborator Status
| Number of Trials (N = 656) | Lead Authors |
Trial Collaborators |
|||
|---|---|---|---|---|---|
| APAC (n = 52, 7.9%) | Non-APAC (n = 604, 92.1%) | APAC (n = 1,312, 10.1%) | Non-APAC (n = 11,688, 89.9%) | ||
| Disease type | |||||
| Aortic disease | 5 (0.8) | 0 (0) | 5 (100.0) | 0 (0) | 50 (100.0) |
| Arrhythmia | 48 (7.3) | 4 (8.3) | 44 (91.7) | 77 (8.9) | 790 (91.1) |
| CAD | 220 (33.5) | 28 (12.7) | 192 (87.3) | 563 (11.7) | 4,256 (88.3) |
| CeVD | 84 (12.8) | 12 (14.3) | 72 (85.7) | 444 (23.7) | 1,428 (76.3) |
| Critical care/resuscitation | 24 (3.7) | 0 (0) | 24 (100.0) | 21 (4.2) | 482 (95.8) |
| Diabetes | 75 (11.4) | 2 (2.7) | 73 (97.3) | 52 (4.7) | 1,048 (95.3) |
| DVT/PE | 21 (3.2) | 1 (4.8) | 20 (95.2) | 14 (3.8) | 354 (96.2) |
| HF/cardiomyopathy | 62 (9.5) | 0 (0) | 62 (100.0) | 36 (2.7) | 1,291 (97.3) |
| Hypertension | 19 (2.9) | 2 (10.5) | 17 (89.5) | 51 (13.6) | 325 (86.4) |
| Lipid disorders | 24 (3.7) | 2 (8.3) | 22 (91.7) | 17 (4.7) | 342 (95.3) |
| Obesity | 32 (4.9) | 0 (0) | 32 (100.0) | 15 (3.1) | 476 (97.0) |
| PAD | 14 (2.1) | 0 (0) | 14 (100.0) | 3 (1.4) | 211 (98.6) |
| PAH | 2 (0.3) | 0 (0) | 2 (100.0) | 3 (12.0) | 22 (88.0) |
| Pericardial disease | 3 (0.5) | 0 (0) | 3 (100.0) | 0 (0) | 67 (100.0) |
| Valvular heart disease | 23 (3.5) | 1 (4.3) | 22 (95.7) | 16 (2.9) | 546 (97.2) |
| Year of publication | |||||
| 2011 | 51 | 2 (3.9) | 49 (96.1) | 68 (7.3) | 859 (92.7) |
| 2012 | 62 | 2 (3.2) | 60 (96.8) | 35 (3.6) | 939 (96.4) |
| 2013 | 69 | 3 (4.3) | 66 (95.7) | 61 (5.4) | 1,077 (94.6) |
| 2014 | 52 | 5 (9.6) | 47 (90.4) | 70 (6.7) | 968 (93.3) |
| 2015 | 75 | 7 (9.3) | 68 (90.7) | 152 (10.8) | 1,259 (89.2) |
| 2016 | 62 | 5 (8.1) | 57 (91.9) | 92 (7.7) | 1,102 (92.3) |
| 2017 | 64 | 4 (6.3) | 60 (93.8) | 105 (8.1) | 1,195 (91.9) |
| 2018 | 69 | 8 (11.6) | 61 (88.4) | 238 (14.8) | 1,376 (85.3) |
| 2019 | 90 | 6 (6.7) | 84 (93.3) | 210 (10.6) | 1,770 (89.4) |
| 2020 | 62 | 10 (16.1) | 52 (83.9) | 281 (19.7) | 1,143 (80.3) |
| Site location | |||||
| Asia Pacific | 34 | 31 (91.2) | 3 (8.8) | 680 (94.7) | 38 (5.3) |
| Middle East/Africa | 2 | 0 (0) | 2 (100.0) | 0 (0) | 64 (100.0) |
| North America | 147 | 0 (0) | 147 (100.0) | 1 (0.04) | 2,633 (99.9) |
| South America | 5 | 0 (0) | 5 (100.0) | 0 (0) | 140 (100.0) |
| Western/Central Europe | 166 | 0 (0) | 166 (100.0) | 17 (0.5) | 3,225 (99.5) |
| Multiregional | 302 | 21 (7.0) | 281 (93.0) | 614 (9.9) | 5,588 (90.1) |
| Funding | |||||
| Industry | 335 | 18 (5.4) | 317 (94.6) | 430 (6.9) | 5,826 (93.1) |
| Nonindustry | 321 | 34 (10.6) | 287 (89.4) | 882 (13.1) | 5,862 (86.9) |
Values are n (%) unless otherwise indicated.
APAC = Asia-Pacific; CAD = coronary artery disease; CeVD = cerebrovascular disease; DVT = deep venous thrombosis; HF = heart failure; PAD = peripheral artery disease; PAH = pulmonary arterial hypertension; PE = pulmonary embolism.
Figure 2.
APAC Study Leadership/Collaboration and Asian Race Enrollment
Proportion of lead authors (A) and trial collaborators (ie, remaining non-lead authors) (B) from APAC region by year between 2011 and 2020. Proportion of enrolled participants of Asian race (C) and from APAC region (D) by year between 2011 and 2020. Dotted line represents the estimated APAC prevalence of CVD ∼60% as an aspirational benchmark for representativeness. APAC = Asia-Pacific; CVD = cardiovascular disease; RCT = randomized controlled trial.
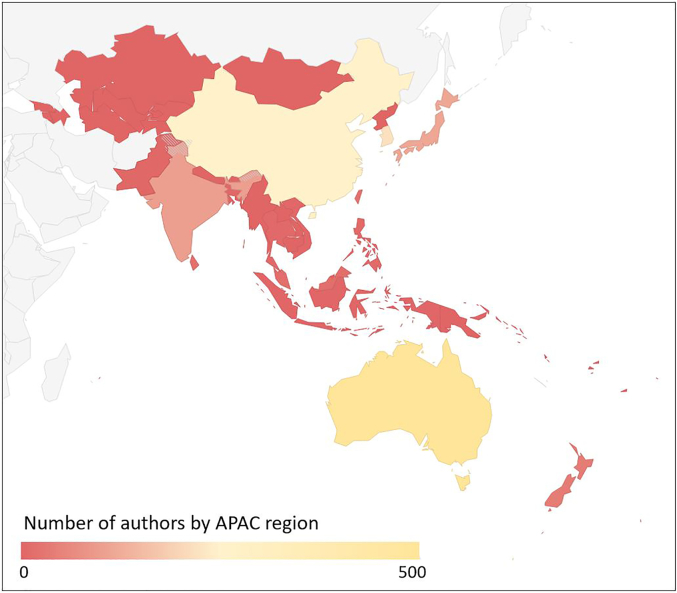
The characteristics of APAC collaboration were similar to those of lead authorship, with an overall 10.1% of all authors being from APAC with predominant contributions in CeVD, CAD, hypertension, and arrhythmia trials (Table 1). The majority of trial collaboration came from Oceania (40.1%) and East Asia (46.1%), with only modest contributions from South Asia (7.9%) and Southeast Asia (6.0%) and only a single author from Central Asia (0.1%) (Figure 3, Supplemental Table 1). As with lead authorship, APAC collaboration was more common among studies that were not industry funded (13.1% vs 6.9%). Despite a large number of multiregional trials, many of which involved the APAC region, APAC collaboration was uncommon (12.7%). Over the 10-year study period, APAC authorship increased (mean Δ1.38% ± 4.66%/year; P = 0.02) from 7.3% in 2011 to 19.7% in 2020 (Figure 2). However, when plotted by region, proportional increases were limited to the Oceanic (test of trend, P < 0.001) and East Asian regions (test of trend, P < 0.001), with essentially no appreciable change in South Asia, Central Asia, or Southeast Asia (test of trend, all P > 0.05) (Supplemental Figure 1).
Figure 3.
Heat Map of APAC Trial Collaborators
Heat map of 1311 trial collaborators from APAC region between 2011 and 2020. See Supplemental Table 2 for full data. APAC = Asia-Pacific.
Asian Race Representation
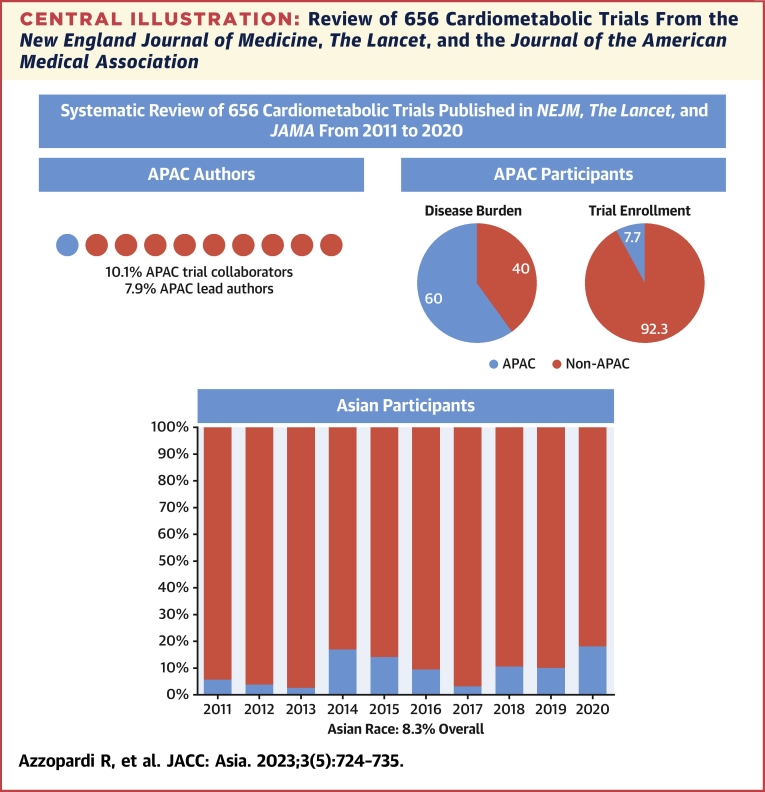
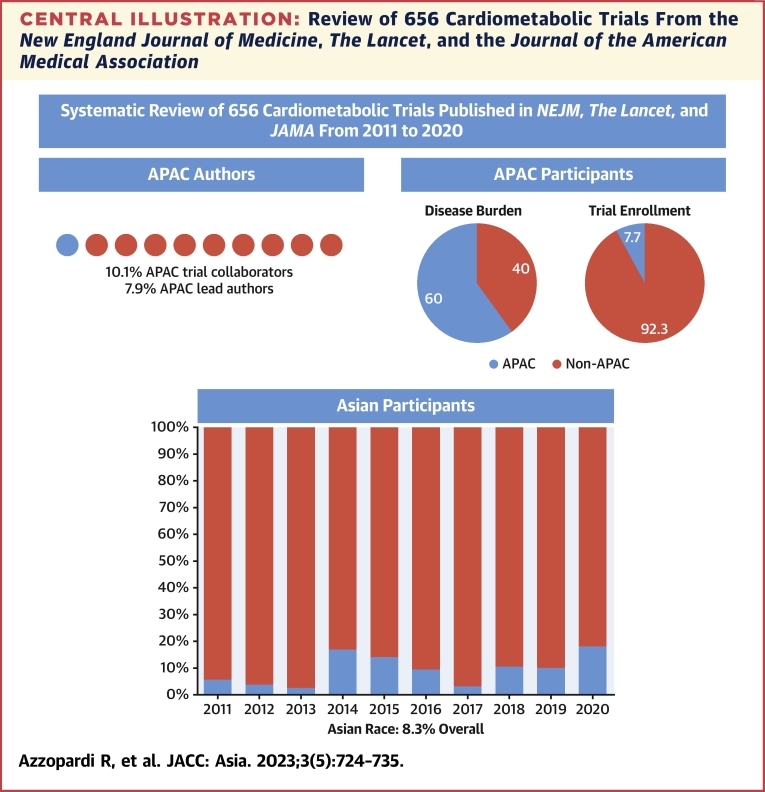
Of all 656 trials in the study period, 189 (28.8%) listed the proportion of Asian race participants (Table 2). Asian race enrollment was assumed in APAC trials with the exception of those performed in Oceania. Overall, the proportion of enrolled Asian race participants was low (8.3%) (Central Illustration), with higher proportions among participants with pulmonary arterial hypertension (PAH) (albeit only 2 in the sample, 27.8%) and CeVD (26.4%); lower proportions among hypertension (11.2%), heart failure (9.5%), and diabetes (9.5%) trials; and very low participation among lipid, obesity, and valvular heart disease trials. A total of 51 trials (51/656 = 7.7%) reported a proportion of Asian participants of at least 20% or more with 75 trials (75/656 = 11.4%), making an attempt to report Asian ethnicity (eg, East Asian vs South Asian). Asian race was more common in industry trials than in nonindustry trials (39.4 vs 26.2%). The overall proportion of Asian race participants increased over time (Figure 1C) (mean Δ1.40 ± 6.95%/year; P = 0.003) from 5.7% in 2011 to 18.3% in 2020. Asian race enrollment was more common among trials with APAC leaders (69.2%) than in those with non-APAC leaders (29.8%), with over one-half of the Asian population enrolled by trials led by APAC investigators (116,100/218,613 = 53.1%). Strong correlations were observed between proportion of Asian race enrollment and APAC leadership (r = 0.63; P < 0.001) and proportion of APAC collaborators (r = 0.76; P < 0.001).
Table 2.
Characteristics of Trial Population by Asian Race and APAC Region
| Asian Race |
APAC Region |
|||
|---|---|---|---|---|
| Number of Trials Reporting Asian Race >0 | Enrolled Asian Population | Number of Trials Reporting APAC Region >0 | Enrolled APAC Region | |
| Overall | 216/656 (32.9) | 218,613/2,619,710 (8.3) | 109/656 (16.6) | 202,295/2,619,710 (7.7) |
| Trial leaders | ||||
| APAC | 36/52 (69.2) | 116,100/229,275 (50.1) | 36/52 (69.2) | 115,117/229,275 (50.2) |
| Non-APAC | 180/604 (29.8) | 102,513/2,390,435 (4.3) | 73/604 (12.1) | 87,178/2,390,435 (3.6) |
| Disease type | ||||
| Aortic disease | 1/5 (20.0) | 8/3467 (0.2) | 0/5 (0) | 0/3,467 (0) |
| Arrhythmia | 6/48 (12.5) | 1,105/101,929 (1.1) | 8/48 (16.7) | 6,280/101,929 (6.2) |
| CAD | 70/220 (31.8) | 112,262/1,195,769 (9.4) | 42/220 (19.1) | 105,424/1,195,769 (8.8) |
| CeVD | 32/84 (38.1) | 58,506/221,897 (26.4) | 20/84 (23.8) | 54,284/221,897 (24.5) |
| Critical care/resuscitation | 1/24 (4.2) | 646/88,261 (0.7) | 0/24 (0) | 0/8,826 (0) |
| Diabetes | 49/75 (65.3) | 23,990/252,959 (9.5) | 16/75 (21.3) | 10,314/252,959 (4.1) |
| DVT/PE | 5/21 (23.8) | 1,976/39,238 (5.0) | 2/21 (9.5) | 1,509/39,238 (3.8) |
| HF/cardiomyopathy | 23/62 (37.1) | 9,213/97,192 (9.5) | 12/62 (19.4) | 6,448/97,192 (6.6) |
| Hypertension | 6/19 (31.6) | 6,804/61,004 (11.2) | 3/19 (15.8) | 4,239/61,004 (6.9) |
| Lipid disorders | 4/24 (16.7) | 2,226/391,241 (0.6) | 3/24 (12.5) | 11,840/391,241 (3.0) |
| Obesity | 11/32 (34.4) | 369/59,852 (0.6) | 1/32 (3.1) | 851/59,852 (1.4) |
| PAD | 5/14 (35.7) | 1,167/75,672 (1.5) | 1/14 (7.1) | 961/75,672 (1.3) |
| PAH | 2/2 (100.0) | 196/704 (27.8) | 0/2 (0) | 0/704 (0) |
| Pericardial disease | 0/3 (0) | 0/1,880 (0) | 0/3 (0) | 0/1,880 (0) |
| Valvular heart disease | 1/23 (4.4) | 145/28,645 (0.5) | 1/23 (4.3) | 145/28,645 (0.5) |
| Year of publication | ||||
| 2011 | 17/51 (33.3) | 9,353/164,177 (5.7) | 4/51 (7.8) | 4,165/164,177 (2.5) |
| 2012 | 18/62 (29.0) | 8,959/230,462 (3.9) | 3/62 (4.8) | 2,350/230,462 (1.0) |
| 2013 | 15/69 (21.7) | 11,149/467,237 (2.4) | 5/69 (7.2) | 6,806/467,237 (1.5) |
| 2014 | 19/52 (36.5) | 30,936/183,631 (16.8) | 11/52 (21.2) | 38,926/183,631 (21.2) |
| 2015 | 20/75 (26.7) | 34,251/243,081 (14.1) | 15/75 (20.0) | 35,223/243,081 (14.5) |
| 2016 | 17/62 (26.4) | 15,961/172,224 (9.3) | 5/62 (8.1) | 8,525/172,224 (4.9) |
| 2017 | 15/64 (23.4) | 10,485/330,485 (3.2) | 11/64 (17.2) | 19,473/330,485 (5.9) |
| 2018 | 22/69 (31.9) | 36,244/341,165 (10.6) | 18/69 (26.1) | 36,556/341,165 (10.7) |
| 2019 | 40/90 (44.4) | 33,726/336,317 (10.0) | 17/90 (18.9) | 26,606/336,317 (7.9) |
| 2020 | 33/62 (52.2) | 27,549/150,931 (18.3) | 20/62 (32.3) | 23,665/150,931 (15.7) |
| Funding | ||||
| Industry | 132/335 (39.4) | 111,205/1,397,491 (8.0) | 70/335 (20.9) | 98,563/1,397,491 (7.1) |
| Nonindustry | 84/321 (26.2) | 107,408/1,222,219 (8.8) | 39/335 (12.1) | 103,732/1,222,219 (8.5) |
Values are n/N (%).
Abbreviations as in Table 1.
Central Illustration.
Review of 656 Cardiometabolic Trials From the New England Journal of Medicine, The Lancet, and the Journal of the American Medical Association
Proportion of APAC trial collaborators and lead authors; proportion of APAC trial enrollment as a proportion of regional cardiovascular disease burden; proportion of Asian race enrollment by year.
APAC Regional Representation
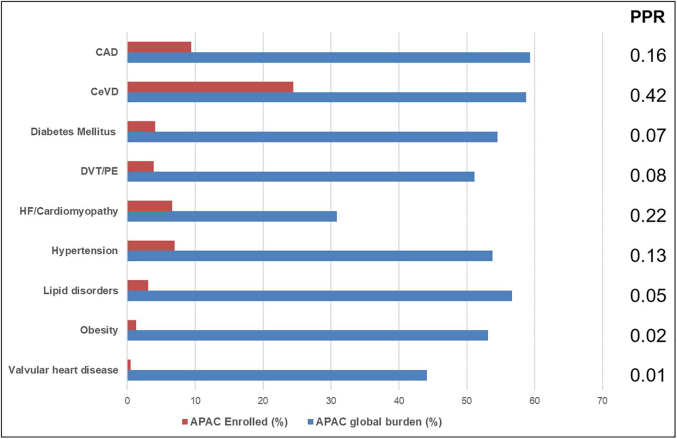
A total of 109 trials (16.6%) listed enrollment from any of the APAC countries. APAC participants constituted 7.7% of the total study population, with proportionally higher representation in CeVD (24.5%) and CAD (8.8%) trials and fewer APAC participants in diabetes (4.1%), obesity (1.4%), and lipid (3.0%) trials. There was an increase in the proportion of enrolled participants from the APAC over time (mean Δ1.46% ± 8.67%/year; P = 0.003) from 2.5% in 2011 to 15.7% in 2020. Proportion of APAC participant enrollment was strongly correlated with APAC trial collaboration (r = 0.85; P < 0.01) and APAC lead authorship (r = 0.71; P < 0.01). With the exception of CeVD, PPR was <0.30 for all cardiometabolic diseases and particularly low among diabetes (PPR = 0.07), lipid disorders (PPR = 0.05), obesity (PPR = 0.02), and valvular heart disease trials (PPR = 0.01) (Figure 4).
Figure 4.
Representativeness of APAC Trial Enrollment by Burden of Disease
Proportion of enrolled APAC participants (red) by therapeutic area. Proportion of global burden of disease arising from APAC region (blue) by therapeutic area using Global Health Data Exchange registry and other published sources. Participant-to-prevalence ratio (PPR) determined by dividing APAC enrollment by APAC prevalence. APAC = Asia-Pacific; CAD = coronary artery disease; CeVD = cerebral artery disease; DVT = deep venous thrombosis; HF = heart failure; PE = pulmonary embolism.
Discussion
Our study of 656 high-impact cardiometabolic publications in top-tier medical journals over the past decade has revealed the following key insights: 1) Asian race enrollment is low and has only marginally increased over the past decade; 2) regional APAC enrollment is low and significantly underrepresented based on the regional contribution to the global burden of cardiometabolic disease; and 3) lead authorship and trial collaboration is infrequent in APAC, and although there are optimistic trends, this has occurred disparately across the region. Importantly, greater representation of APAC in trial authorship was associated with higher proportion of Asian enrollment.
Seminal trials provide evidentiary support for regulatory approval of drugs and devices and provide the foundations for guideline recommendations. Accordingly, much has been written about the need for clinical trials to adequately represent population diversity from a gender and age perspective, but also with respect to race and ethnicity. Inability to achieve this diversity may compromise a study’s external validity, leaving extrapolation of results to be based on untested assumptions. This is of particular importance when one considers that as many as 1 in 5 approved therapies have meaningful differences in drug metabolism by race.21
Our finding that <10% of the seminal cardiometabolic trial population was of Asian race is concerning and raises questions about the confidence with which we can apply this evidence base to Asians and within countries whose racial base is predominantly Asian. These results are consistent with a recent analysis of the 2018 Cholesterol Guidelines, which demonstrated only 15% of all RCTs that were cited included Asian participants.22 Our analysis goes further and demonstrates that even when Asian participants are enrolled, their proportion is likely to be too low to extrapolate the evidence base. Notwithstanding the limitation of multiplicity common to most RCT subgroup testing approaches, our finding of small Asian subpopulation sample size is likely to result in low statistical power to demonstrate heterogeneity and also increase the chance of baseline imbalances between nonrandomized groups.23 Thus, trial-level interaction testing (in the absence of stratified randomization by region as a proxy for race) may not be fit for purpose among a large proportion of high-impact cardiometabolic trials evaluated in our study. Meta-analysis of individual patient-level data may overcome some of the sample size limitations; however, the lack of consistency in Asian race and ethnicity reporting that we observed, even among trials performed in APAC, will limit the validity of this approach. With evidence that cardiovascular risk is not experienced consistently across Asian race and ancestry,16,24, 25, 26 sensitive and inclusive reporting may permit disaggregated analysis to better understand these differences and tailor management appropriately.27 However, the degree to which the complex and dynamic construct of race and ethnicity can be practically disentangled from inextricable environmental, socioeconomic, genetic, and biological forces remains to be seen.28
Fewer than 1 in 12 patients in our study were enrolled from APAC, and only 1 in 6 trials listed recruitment from this region. With >50% of the global burden of cardiovascular disease residing in APAC, this represents a gross under-representation of patients from this region who have unmet treatment needs.14, 15, 16 APAC-led and -authored trials had a greater proportion of APAC recruitment, which suggests that a dedicated approach to investing in trial conduct in this region would improve regional enrollment. This is not straightforward to implement because trial conduct requires infrastructure and a skilled workforce that may be barriers to entry for many low- and middle-income countries that reside in APAC.29 At present, there is a fiscal reality that industry-sponsored trials must prioritize fulfilling the requirements of the FDA and the European Medicines Agency (EMA) because this will enable access to countries with the largest market share and thus prompt a return on investment. Accordingly, industry-driven trials focus on meeting FDA and EMA requirements by enrolling from their respective constituent countries in order to achieve these goals. Until there is greater globalization to a point where burden of disease more closely aligns with market share in these regions, quotas for enrollment by regulatory bodies outside the northern hemisphere are unlikely to be an effective mechanism to increase representation. Currently, approval from other large regulatory bodies (eg, the Pharmaceuticals and Medical Devices Agency of Japan or the National Medical Products Administration of China) requires either additional trials or dedicated pharmacokinetic studies. The conduct of these additional studies requires additional capital, and if there is insufficient market they may be abandoned altogether or, at the very least, likely delay access to an evolving standard of care for these nations. Thus, increasing geographic representation in trials may have the indirect outcome of achieving higher quality and more equitable care in these regions. An alternative approach would be to mandate that postmarketing studies are performed in a globally representative manner to ensure consistent safety and efficacy profiles in regions and groups that were under-represented in the pivotal studies. As investigators from APAC we acknowledge that ultimately our region needs to demonstrate more effective advocacy and unified leadership on behalf of our constituent patients; we can’t expect other continents to fix the lack of representation in our own region. Most of the APAC countries have cardiology societies and professional bodies; however, their mandates are inward facing, and intersociety and intercontinental relationships across the region are opportunistic and lacking in strategy.
Whereas there is an encouraging trend toward increased trial leadership and collaboration from APAC, the absolute numbers remain low, and temporal improvements are marginal and limited to higher-income regions such as East Asia and Oceania. Our finding that an overall 7.9% of trial leadership occurred from APAC is consistent with a recent analysis of 20-year trends of heart failure trials in which 7.1% were coordinated from Asia or Oceania.30 As mentioned earlier, trial leadership and authorship track with enrollment and could be considered an upstream determinant of achieving trial participant geographic diversity; thus, attempts at recruiting, upskilling, and incentivizing APAC investigators and sites are required. Given correlations between Asian race participant enrollment and trial leadership, a focus of funding bodies and industry sponsors should be on incentivizing and potentially stipulating non-US, non-European leadership. Mentoring and partnership programs with established institutions in larger and/or higher income countries may assist with upskilling individuals who can lead and grow the research enterprise in smaller and more remote APAC nations. If supported, these individuals are also likely to develop into key opinion leaders in the region, which can be further promoted and the cycle repeated. Other downstream levers are being applied by journals; one example being JACC HF which requires authors to explain the diversity of the study’s leadership and author list in the methodology section, and to defend its absence in the limitations.31
Study Strengths and Limitations
Our results need to be interpreted in light of the study’s strengths and weaknesses. We used first authorship to define trial leadership, as others have done;32 however, senior authorship can also be considered a leadership role. Prior studies, where proportions of both first and last authorship have been measured together, have revealed consistent results as when measured separately.33 Regional authorship was determined from primary affiliation status, although authors may have multiregional affiliations. Our search was limited to 3 top medical journals as a proxy for trial impact; however, other high-quality, well-conducted trials of clinical importance are published in specialty journals that were not included in our analysis and may have led to an underestimation of APAC trial activity. Estimates of the regional contribution to the global burden of disease are coarse, rely on population size estimates, and use multiple sources of data; these figures should be interpreted with caution.
Conclusions
Enrollment of participants of Asian race and from APAC regions remains low and disproportionate to the global burden of disease. Although there are encouraging trends in the proportion of APAC trial leaders and collaborators over the past decade, these improvements are occurring in a disparate manner and are limited to the Oceanic and East Asian countries. Strategies aimed at investing in the research enterprise and incentivizing trial conduct in APAC are likely to improve geographic and racial diversity in the cardiometabolic disease evidence base.
Perspectives.
COMPETENCY IN SYSTEMS-BASED PRACTICE: Less than 10% of enrolled participants in seminal cardiometabolic trials were of Asian race and thus extrapolation of these findings should be performed with caution.
TRANSLATIONAL OUTLOOK 1: Despite over 60% of the global cardiovascular disease residing in the Asia-Pacific, there has been minimal increase in the enrollment of participants or study leadership from this region
TRANSLATIONAL OUTLOOK 2: Sponsors and funding agencies need to identify and overcome barriers to trial conduct in the Asia-Pacific in order to achieve greater geographical and racial representativeness of their cohorts.
Funding Support and Author Disclosures
Dr Nicholls has received grants or contracts from AstraZeneca, New Amsterdam Pharma, Amgen, Anthera, Eli Lilly, Esperion, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience; and consulting fees from AstraZeneca, Amarin, Akcea, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. Dr Chandramouli has received grants from National Medical Research Council Singapore; philanthropic research grants from Lee Foundation Singapore; and consulting or speaker fees from Boehringer Ingelheim, Us2.ai, and Sanofi. Dr Lam has received research support from Bayer and Roche Diagnostics; has served as a consultant or on the advisory board/steering committee/executive committee for Actelion, Alleviant Medical, Allysta Pharma, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc, EchoNous Inc, Eli Lilly, Impulse Dynamics, Ionis Pharmaceutical, Janssen Research and Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics, and Us2.ai; and serves as cofounder and nonexecutive director of Us2.ai. Dr Wong has reported that the University of Adelaide has received on his behalf lecture, travel, and/or research funding from Abbott Medical, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, St. Jude Medical, and Vifor Pharma. Dr Zoungas has received payments to Monash University on her behalf from Eli Lilly Australia Ltd, Boehringer-Ingelheim, MSD Australia, AstraZeneca, Novo Nordisk, Sanofi, and Servier. Dr Yeo has received research funding from Medtronic and honoraria from Abbott Vascular, Boston Scientific, Amgen, and Menarini. Dr Nelson has received honoraria and speaking fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, and Sanofi. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Appendix
References
- 1.Jin X., Chandramouli C., Allocco B., Gong E., Lam C.S.P., Yan L.L. Women's participation in cardiovascular clinical trials from 2010 to 2017. Circulation. 2020;141:540–548. doi: 10.1161/CIRCULATIONAHA.119.043594. [DOI] [PubMed] [Google Scholar]
- 2.Scott P.E., Unger E.F., Jenkins M.R., et al. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol. 2018;71:1960–1969. doi: 10.1016/j.jacc.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 3.Tahhan A.S., Vaduganathan M., Greene S.J., et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3:1011–1019. doi: 10.1001/jamacardio.2018.2559. [DOI] [PubMed] [Google Scholar]
- 4.Michos E.D., Reddy T.K., Gulati M., et al. Improving the enrollment of women and racially/ethnically diverse populations in cardiovascular clinical trials: an ASPC practice statement. Am J Prev Cardiol. 2021;8 doi: 10.1016/j.ajpc.2021.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasanna A., Miller H.N., Wu Y., et al. Recruitment of black adults into cardiovascular disease trials. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tharakan S., Zhong X., Galsky M.D. The impact of the globalization of cancer clinical trials on the enrollment of black patients. Cancer. 2021;127:2294–2301. doi: 10.1002/cncr.33463. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T., Tsang W., Wijeysundera H.C., Ko D.T. Reporting and representation of ethnic minorities in cardiovascular trials: a systematic review. Am Heart J. 2013;166:52–57. doi: 10.1016/j.ahj.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Bourgeois F.T., Orenstein L., Ballakur S., Mandl K.D., Ioannidis J.P.A. Exclusion of elderly people from randomized clinical trials of drugs for ischemic heart disease. J Am Geriatr Soc. 2017;65:2354–2361. doi: 10.1111/jgs.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green P., Maurer M.S., Foody J.M., Forman D.E., Wenger N.K. Representation of older adults in the late-breaking clinical trials American Heart Association 2011 scientific sessions. J Am Coll Cardiol. 2012;60:869–871. doi: 10.1016/j.jacc.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanna M.G., Chen S.T., Nelson A.J., Navar A.M., Peterson E.D. Representation of older adults in cardiovascular disease trials since the inclusion across the lifespan policy. JAMA Intern Med. 2020;180:1531–1533. doi: 10.1001/jamainternmed.2020.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen Q.D., Peters E., Wassef A., et al. Evolution of age and female representation in the most-cited randomized controlled trials of cardiology of the last 20 years. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.004713. [DOI] [PubMed] [Google Scholar]
- 12.Roth L.K. Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs; Guidance for Industry; Availability. FDA, FR Doc. 2020;24881 [Google Scholar]
- 13.Hwang T.J., Brawley O.W. New federal incentives for diversity in clinical trials. N Engl J Med. 2022;387:1347–1349. doi: 10.1056/NEJMp2209043. [DOI] [PubMed] [Google Scholar]
- 14.Li J.J., Yeo K.K., Tan K., et al. Tackling cardiometabolic risk in the Asia Pacific region. Am J Prev Cardiol. 2020;4 doi: 10.1016/j.ajpc.2020.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaduganathan M., Mensah G.A., Turco J.V., Fuster V., Roth G.A. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80:2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC Asia. 2021;1:1–13. doi: 10.1016/j.jacasi.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J.W., Le N., Wei S., et al. Global representation of heart failure clinical trial leaders, collaborators, and enrolled participants: a bibliometric review 2000-20. Eur Heart J Qual Care Clin Outcomes. 2022;8:659–669. doi: 10.1093/ehjqcco/qcab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bala M.M., Akl E.A., Sun X., et al. Randomized trials published in higher vs. lower impact journals differ in design, conduct, and analysis. J Clin Epidemiol. 2013;66:286–295. doi: 10.1016/j.jclinepi.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 19.GBD results Institute for Health Metrics and Evaluation. Accessed August 1, 2022. https://vizhub.healthdata.org/gbd-results/
- 20.Lin C.-F., Chang Y.-H., Chien S.-C., Lin Y.-H., Yeh H.-Y. Epidemiology of dyslipidemia in the Asia Pacific region. Int JGerontol. 2018;12:2–6. [Google Scholar]
- 21.Ramamoorthy A., Pacanowski M.A., Bull J., Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97:263–273. doi: 10.1002/cpt.61. [DOI] [PubMed] [Google Scholar]
- 22.Sarraju A., Valencia A., Knowles J.W., Maron D.J., Rodriguez F. Diverse racial/ethnic group underreporting and underrepresentation in high-impact cholesterol treatment trials. Circulation. 2021;143:2409–2411. doi: 10.1161/CIRCULATIONAHA.120.050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui L., Hung H.M., Wang S.J., Tsong Y. Issues related to subgroup analysis in clinical trials. J Biopharm Stat. 2002;12:347–358. doi: 10.1081/bip-120014565. [DOI] [PubMed] [Google Scholar]
- 24.Huxley R.R., Hirakawa Y., Hussain M.A., et al. Age- and sex-specific burden of cardiovascular disease attributable to 5 major and modifiable risk factors in 10 Asian countries of the western Pacific region. Circ J. 2015;79:1662–1674. doi: 10.1253/circj.CJ-15-0661. [DOI] [PubMed] [Google Scholar]
- 25.Ohira T., Iso H. Cardiovascular disease epidemiology in Asia: an overview. Circ J. 2013;77:1646–1652. doi: 10.1253/circj.cj-13-0702. [DOI] [PubMed] [Google Scholar]
- 26.Roth G.A., Johnson C., Abajobir A., et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kader F., Chebli P. Disaggregation of race and ethnicity group data: research-to-practice issues in clinical environments. JAMA. 2022;328:1395–1396. doi: 10.1001/jama.2022.17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borrell L.N., Elhawary J.R., Fuentes-Afflick E., et al. Race and genetic ancestry in medicine: a time for reckoning with racism. N Engl J Med. 2021;384:474–480. doi: 10.1056/NEJMms2029562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray D.M., 2nd, Nolan T.S., Gregory J., Joseph J.J. Diversity in clinical trials: an opportunity and imperative for community engagement. Lancet Gastroenterol Hepatol. 2021;6:605–607. doi: 10.1016/S2468-1253(21)00228-4. [DOI] [PubMed] [Google Scholar]
- 30.Wei S., Le N., Zhu J.W., et al. Factors associated with racial and ethnic diversity among heart failure trial participants: a systematic bibliometric review. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.008685. [DOI] [PubMed] [Google Scholar]
- 31.Lindenfeld J., Fiuzat M., O'Connor C. Promoting diversity in clinical trial leadership: a call to action. J Am Coll Cardiol HF. 2021;9:401–402. doi: 10.1016/j.jchf.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Mehran R., Kumar A., Bansal A., Shariff M., Gulati M., Kalra A. Gender and disparity in first authorship in cardiology randomized clinical trials. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reza N., Tahhan A.S., Mahmud N., et al. Representation of women authors in international heart failure guidelines and contemporary clinical trials. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.