Abstract
Cardiogenic shock (CS) is a life-threatening condition characterized by a state of inadequate systemic tissue perfusion caused by cardiac dysfunction. When to implement, change, or remove the use of a temporary mechanical circulatory support (tMCS) in patients with CS is dependent on the aetiology and severity. Here, patient scenarios underlying the need to escalate, de-escalate, wean, or bridge from tMCS devices are taken into consideration by interdisciplinary heart failure and CS teams. This includes a comprehensive review of and focus on the rationale for specific device escalation and de-escalation strategies, device selection, and general management.
Keywords: Cardiogenic shock, Short-term device, Mechanical circulatory support, Ventricular-assisted device, Heart transplantation, Recovery
Introduction
While cardiogenic shock (CS) is often related to acute myocardial infarction (AMI), it can also be caused by other underlying pathologies such as acute decompensated chronic heart failure. Whether a patient needs to be escalated, de-escalated, or weaned from a device depends on the underlying disease, comorbidities, native heart function, right heart function, and the circulatory demand of the patient. Here, these concepts will be considered in the context of different patient- and disease-specific aspects by interdisciplinary heart failure and CS teams.
Escalation
Cardiogenic shock is a severe and life-threatening condition characterized by a state of inadequate systemic tissue perfusion caused by cardiac dysfunction. Despite advancements in medical therapies, the mortality rate associated with CS remains high (up to 50%).1 Device escalation, involving the implantation of temporary mechanical circulatory support (tMCS), has emerged as a promising approach to improve outcomes in patients with refractory CS. It is important to identify patients who do not respond effectively to the initial treatment in order to reverse the rapid downward spiral in fulminant CS. If escalation occurs too late, it is often extremely challenging to reverse multi-organ failure.2 In this section, we provide an overview of current device escalation strategies, highlighting the rationale, clinical evidence, and considerations for device selection and management.
Rationale for device escalation
The rationale for device escalation lies in the ability to implement a tMCS device, augment cardiac output, and improve end-organ perfusion. Several devices, including intra-aortic balloon pumps (IABPs), microaxial flow pumps (mAFPs), and extracorporeal membrane oxygenation (ECMO) have been utilized to escalate support in patients with CS.3
Intra-aortic balloon pump
The intra-aortic balloon pump is a widely used temporary short-term device in CS. However, recent studies, including the IABP-SHOCK II trial, have shown conflicting results regarding its efficacy in improving survival.4 Therefore, the use of IABP as a standalone therapy is not recommended and is not typically considered to play a role in device escalation strategies.3
Microaxial flow pump devices
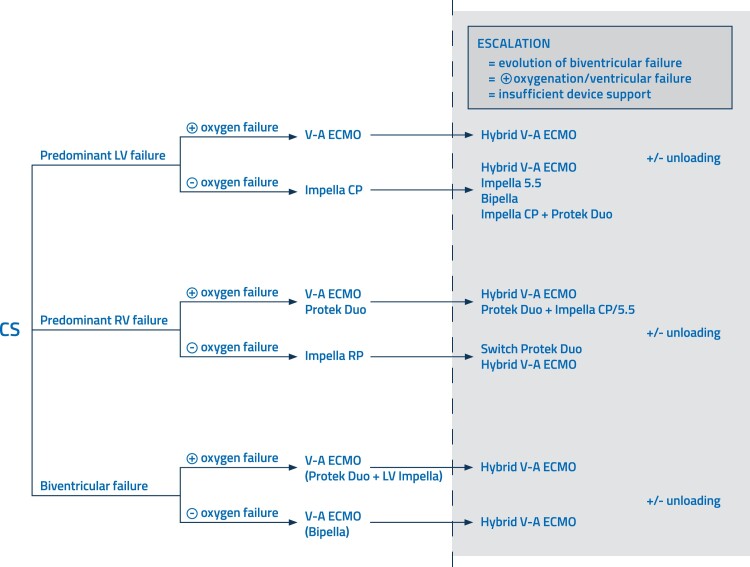
Impella devices are a type of mAFP (up to 5.8 L/min) that directly unload the left ventricle (LV), without the possibility of providing respiratory support.1 The Impella devices have shown promise in improving haemodynamic parameters and end-organ function in patients with CS.5 The axillary-implanted Impella 5.5 devices are more powerful and offer numerous benefits. However, the femoral-inserted devices (Impella CP) are established as primary therapy and provide powerful support and unloading. The axillary position of mAFPs is more stable, resulting in fewer dislocations and less haemolysis. Patient mobilization is enabled due to the axillary position, and the device (Impella 5.5) can remain in place for a longer duration as a bridge to an LV-assisted device (LVAD) or heart transplantation (HTx).6 In the case of isolated right ventricular (RV) failure with the absence of oxygenation/ventilation issues, Impella RP (flex) might be an option as it is specifically designed for right heart use (Figure 1).
Figure 1.
Proposed treatment and escalation strategies in cardiogenic shock with predominant left ventricular failure, predominant right ventricular failure, and biventricular failure.
Veno-arterial extracorporeal membrane oxygenation
Veno-arterial ECMO (V-A ECMO) provides both circulatory (up to 7 L/min) and respiratory support, making it a versatile option for patients with severe CS and those in cardiac arrest. Peripheral V-A ECMO can rapidly restore cardiac output and oxygenation, allowing time for myocardial recovery or facilitating a bridge to further therapies such as LVAD or HTx. However, V-A ECMO is associated with high rates of complications.1
ECMELLA
ECMELLA is the combined use of peripheral V-A ECMO and Impella (CP or 5.5 devices), which is increasingly employed as an elegant method of LV unloading to provide the LV with optimal chances of recovery and reduced infarct size. More recent literature suggests that unloading may potentially improve outcomes in this CS population, but more importantly, unloading should ideally be initiated early to have a significant effect on mortality.7
Other escalation strategies
In complex situations, implantation of additional cannulas into the existing ECMO cannulas to drain blood or add oxygenated blood can be utilized. The addition of cannulas and devices for severe RV failure during left-sided support or LV unloading is also considered an escalation. These configurations require expertise and a carefully considered approach after consultation with the heart team.8 A possible solution to treat isolated RV failure (with or without the need for oxygenation/ventilation) or escalation from LV to biventricular support is the Protek Duo cannula used with an V-A ECMO circuit or an extracorporeal pump (Figure 1). This cannula is inserted via the right internal jugular vein and ejects blood directly into the pulmonary artery, bypassing the RV.9 Lastly, microaxial pumps can be combined on the right and left sides (BiPella approach), with the flow of the RV pump set lower than that of the left to prevent pulmonary oedema.10
Device selection and management considerations
The selection of the appropriate device for device escalation should be determined based on the patient’s clinical presentation, haemodynamic profile, and institutional expertise. First and foremost, the cause of CS must be identified using appropriate diagnostics (echocardiography, pulmonary artery catheter, clinical assessment, fluid and positive end-expiratory pressure challenges, etc.). This is helpful in determining next steps in patients who do not respond, or in cases where the shock condition deteriorates during tMCS use (insufficient lactate clearance, persistent inadequate organ perfusion, and failure to achieve set clinical and haemodynamic targets). A direct approach targeting the cause of CS with the appropriate device increases the chances of success of the therapy while minimizing the occurrence of major complications. Factors such as device availability, patient comorbidities, and resource allocation should also be considered. Optimal management of these devices requires a multidisciplinary approach involving an experienced heart team available 24/7.
De-escalation
A recently published scientific statement from the American Heart Association aims to provide a standardized approach in device management, defining de-escalation as the weaning or subsequent explantation of one or multiple tMCS devices in CS.11 In our opinion, ‘de-escalation’ and ‘weaning’ are different processes, sometimes going in parallel, sometimes not. De-escalation is a strategic process in the care of patients with CS with the intent of quickly removing V-A ECMO in order to reduce ECMO-associated complications that significantly increase over time,12,13 while the addition of a LV unloading/venting (mAFP) improves outcomes.14
The term de-escalation covers multiple aspects of evaluation of these patients.
Severity
The de-escalation strategy should be reserved for Society for Cardiovascular Angiography and Interventions (SCAI) Classes D and E patients (deteriorating and extreme shock or extracorporeal cardiopulmonary resuscitation) who have transitioned to SCAI C with tMCS and remain stable.
Pump support
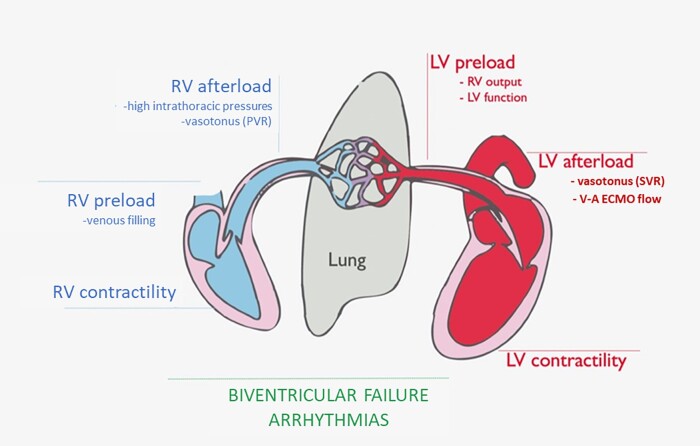
De-escalation does not necessarily mean less systemic support by pump flow, but rather a shift to a less-invasive pump in univentricular configuration and upper-body (axillary) cannulation to promote patient mobilization and ambulation. In LV failure, de-escalation devices could be axillary Impella 5.0/5.5 or temporary apical LVAD with axillary return (if an LV apical venting is already in place) or IABP via axillary access in case of contraindications for the use of mAFP. In the days after device implantation, V-A ECMO flow must be progressively decreased until removal. Given that the evaluation of the RV is not straightforward during full-flow V-A ECMO, the latter should be weaned slowly over the following hours (Figure 2).
Figure 2.
Biventricular failure arrhythmias. RV, right ventricle; LV, left ventricle; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance; V-A ECMO, veno-arterial extracorporeal membrane oxygenation.
Why
The de-escalation strategy allows for an extended period for native heart recovery, especially if it provides full unloading and implementation of heart failure medications, and for resolution of inter-current clinical conditions such as infections and end-organ damage. Furthermore, this type of patient often experiences cardiac arrest with anoxic brain damage and would benefit from more time for neurological evaluation and recovery. Concomitantly, axillary support allows patient extubation, oral feeding, and mobilization while receiving maximal haemodynamic support, which has been demonstrated to be associated with a better prognosis at discharge.15 The scenario is also optimal for a thorough evaluation of RV function in the perspective of a durable LVAD or heart transplant, given the reduced perioperative complications associated with these procedures compared with the direct transition from V-A ECMO to any heart replacement therapy.16
When to consider de-escalation
This strategy should be considered in the absence of consistent signs of native heart recovery to foresee weaning in a short time frame in a patient on V-A ECMO with or without LV unloading. De-escalation should be executed as soon as shock parameters have recovered, inotropes are weaned, and euvolemic status is achieved.
Timing
De-escalation as intended, by shifting to an upper-body device cannulation, is an elective and strategic procedure. As such, pre-procedural optimization and vascular accesses must be assessed before the next implantation.16
Limitations of de-escalation in the current clinical setting
Patients need to stay in intensive care units for the duration of mAFP support, as logistics outside these units are still underdeveloped. The use of the pulmonary artery catheter is advised during this process, even when the patient is ambulatory, as it allows heart failure medications to be titrated and avoids weaning patients who would, therefore, suffer from refractory heart failure and low quality of life (New York Heart Association III). The availability of skilled intensivists, cardiologists, cardiac surgeons, and nurses is crucial for the management of patients with CS to maintain a low risk of mortality and morbidity.
Weaning
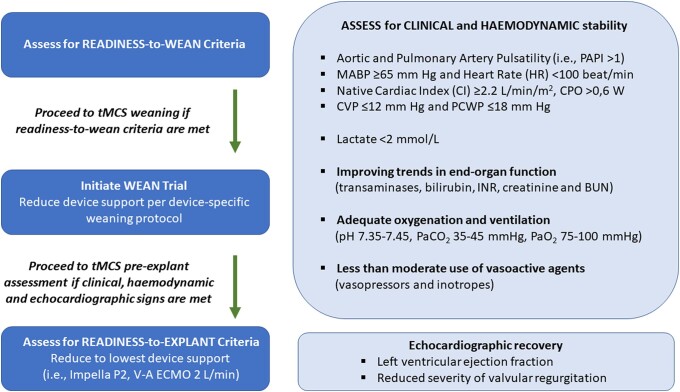
While indications, contraindications, and complications of use guide device selection, optimal standardized strategies for device-specific weaning and explantation remain poorly defined.17 However, general clinical, haemodynamic, metabolic, and echocardiographic parameters can help guide a patient-tailored approach to weaning and explant. Weaning should begin whenever feasible, with careful consideration to maintain a balance between the risks (limb ischaemia, stroke, haemolysis, bleeding, and deconditioning) and the benefits of continued therapy. As such, patients should be evaluated daily for readiness to wean characterized by improved clinical status. This does not necessarily constitute full restoration of underlying cardiac function, but reflects an improvement in clinical, haemodynamic, metabolic, and imaging parameters. Readiness to explant is marked by successful device wean trials and guides the timing of the explantation (Figure 2). Still, more research is needed to better understand optimal timing and implementation of standardized protocols to achieve successful tMCS weaning and explant.
Impella
The overall weaning strategy for Impella is to achieve adequate organ perfusion at the lowest support level to minimize device-related complications and determine candidature for device removal. Complex cases using Impella support utilize a pulmonary artery catheter in order to define patient-specific haemodynamics.18,19 The patient should be free from inotropic support and vasopressors. Serial echocardiography studies should be performed during full support and during flow reduction. Further, sufficient end-organ perfusion should be monitored during the weaning period. Impella weaning should take 2–4 days, while the lowest level should be maintained for at least 24 h before explantation. While several studies have established the cardioprotective physiological basis of left-ventricular unloading,20,21 a more recent investigation indicates the detrimental effects of rapid reloading by abruptly increased LV end-diastolic wall stress resulting in cardiomyocyte stretch-induced injury and/or microvascular flow impairment.22
Left-sided Impella devices with SmartAssist technology are equipped with optical sensors that sense the pressure at the outlet of the device (i.e. aortic pressure) and provide the automated Impella controller with exact information on device positioning. In addition, a sensor close to the microaxial motor identifies the pressure difference between the inlet and the outlet of the Impella device (i.e. the pressure difference between the aorta and the LV), which aids in managing and positioning the device. Left-sided SmartAssist technology provides continuous calculation of data on LV pressure, end-diastolic pressure, continuous cardiac output, and cardiac power output [CPO: (cardiac output × mean arterial pressure)/451.8] that can provide useful information for the characterization of the patient’s haemodynamic state.
In general, weaning from Impella support can be advised if CPO and other haemodynamic parameters are adequate with no or very low-level use of vasopressors and without inotropes (Figure 3). The Impella performance level (P-level) should be reduced in a stepwise fashion every hour and the mixed venous oxygen saturation (target SvO2 > 60%), lactic acid (<1.5 mmol/L) and urine output measured to ensure that the patient continues to maintain adequate CPO (>0.6 W) and a targeted mean arterial pressure of >65 mmHg.23
Figure 3.
Proposed algorithm for weaning and explanting percutaneous ventricular assist device (pVAD). Figure modified from Randhawa et al.17 HTx, heart transplantation; IABP, intra-aortic balloon pump; LV, left ventricle; LVAD, left ventricular-assisted device; RV, right ventricle; tMCS, temporary mechanical circulatory support; V-A ECMO, veno-arterial extracorporeal membrane oxygenation.
Impella RP
Monitoring and managing RV function relies on clinical situation, invasive haemodynamics, and ultrasound imaging. Fluid balance should be titrated to target a right atrial pressure of 8–12 mmHg and a pulmonary artery pulsatility index >1 [(PAPI, systolic PAP − diastolic PAP)/right atrial pressure]. RECOVER RIGHT24 and post-marketing Impella RP studies used stepwise flow reduction (0.5–1 L/min every 2–3 h) for Impella RP weaning. Overall, a CPO > 0.6 W and PAPI > 1.0 are the target measures that guide the weaning of Impella RP support.23
Bridging to recovery
In some indications, such as AMI, vasoactive drug poisoning or acute myocarditis, recovery of the native heart is the main goal of mAFP use. This concept of bridge to recovery can be performed by a femoral percutaneous implantation of an Impella CP that does not require surgical cut-down. However, if higher circulatory support is needed, such as more unloading of the LV and longer duration of support, escalation to an axillary implanted Impella 5.0/5.5 device is recommended. After the myocardial recovery occurs, weaning should be performed as described above.
Bridging to heart transplantation
The Impella device is indicated to provide temporary circulatory support in patients with severe acute de novo heart failure and acute decompensated chronic heart failure.25 For patients with advanced heart failure or those who cannot be weaned from the Impella, durable ventricular-assisted devices (VADs) or HTx are considered viable treatment options.3 However, in patients with CS (IM I and II), durable VADs have inferior outcomes compared with stable patients with heart failure.26 Often, these patients have temporary contraindications for these heart replacement therapies and should therefore be stabilized and optimized.27 Impella support can restore haemodynamic stability in critically ill patients, allowing for optimization of end-organ function and assessment of candidature for long-term LVADs or transplantation. By improving cardiac output and end-organ perfusion, Impella can mitigate multiorgan dysfunction, reduce the risk of complications, and improve patient outcomes after durable LVAD implantation and HTx.
Surgical axillary Impella implantation is feasible and safe to improve the patient’s overall condition and to evaluate RV function under ‘LVAD-like’ conditions.28 Although clinical data comparing preoperative extracorporeal life support vs. Impella 5.0/5.5 therapy are lacking, the potential advantages of axillary Impella bridging are a feasible solution to mobilize patients and evaluate the right heart.29 Right heart failure is one of the risk factors for early mortality after LVAD implantation. Thus, an evaluation of potential LVAD recipients is crucial for achieving optimal outcomes. The goal of Impella 5.5 therapy is to optimize the overall condition of the patient preoperatively and to overcome potential contraindications for LVAD implantation.
Early series of Impella-bridged patients to durable devices have demonstrated close to 90% 1-year survival.28,29 These findings will be further supported by an upcoming multicentre retrospective study with a 1-year survival of over 70%. These positive outcomes are partially attributed to pre-LVAD optimization and careful patient selection, with particular focus on adequate RV function. As such, many patients in CS require the use of both mAFP and ECMO.30 While unloading the LV with an mAFP appears beneficial compared with ECMO alone, it is known that ECMO-bridged LVAD recipients have a 1-year survival rate of ∼50%. Therefore, switching patients from ECMO to mAFP first and then implantation of a durable VAD may help improve a patient’s clinical status and outcomes following LVAD implantation.28 The rationale is to overcome contraindications such as unclear neurologic status, optimize end-organ function, especially right heart and renal function, and treat infections before LVAD implantation.28,31 The axillary approach, in particular, has advantages over femoral approaches in weaning from mechanical ventilation and ambulation, which improves functional status before durable LVAD implantation.
With long wait times for HTx in many European countries, bridging to HTx is a rare occurrence. However, in countries with higher organ donation rates such as the USA, bridging to HTx is an established treatment strategy. In 2018, the heart allocation policy in the USA has changed in favour of short-term devices. The goal of the allocation guideline change was to reduce waitlist mortality and shorten wait times without negatively affecting post-transplant outcomes. The first results from evaluations of before and after the allocation change indicate the success of this modification. The 1-year survival in patients bridged by a short-term device is around 90% and not significantly different from patients who did not need a short-term device.32
Impella support plays a crucial role in bridging patients with severe heart failure to durable VADs or HTx. Its ability to provide temporary circulatory support, stabilize haemodynamics, and improve end-organ function has shown promising outcomes. However, careful patient selection, close monitoring, and ongoing research are essential for optimizing outcomes and expanding the application of Impella bridging therapy in the future.
Conclusions
The decision to escalate, de-escalate, or wean is highly dependent on patient presentation, capabilities of the institution, and the expertise of the heart team in place. As such, standardized strategies and subsequent training are likely to aid in achieving consistent optimal outcomes for patients with CS.
Acknowledgements
This manuscript is one of eight manuscripts published as a Supplement to address best practices in the Management of Cardiogenic Shock. JetPub Scientific Communications, LLC, supported by funding from Abiomed Europe GmbH, provided editorial assistance to the authors during the preparation of this manuscript.
Contributor Information
Alexander M Bernhardt, Department of Cardiovascular Surgery, University Heart and Vascular Center, Martinistrasse 52, Hamburg 20246, Germany.
Evgenij Potapov, Department of Cardiothoracic and Vascular Surgery, Deutsches Herzzentrum der Charite Campus Virchow-Klinikum, Augustenburger 1, Berlin 13353, Germany.
Christophe Vandenbriele, Department of Cardiolovascular Sciences, KU Leuven, Herestraat 49, Leuven 3000, Belgium.
Carsten Skurk, Klinik fur Kardiologie, Angiologie and Intensivmedizin, Deutsches Herzzentrum der Charite, Campus Benjamin Franklin, Hindenburgdamn 39, Berlin 12203, Germany.
Letizia F Bertoldi, Cardio Center, IRCCS Humanitas Clinical and Research Center, Via Alessandro Manzoni 56, Rozzano (MI) 20089, Italy.
Federico Pappalardo, Department of Cardiothoracic and Vascular Anesthesia, AO SS Antonio e Biagio e Cesare Arrigo, Via Venezia 16, Alessandria 15121, Italy.
Funding
This work has been supported by Abiomed Europe GmbH to cover publication costs as well as professional language editing of each manuscript. No individual fees were paid to the authors during the generation of this publication. This paper was published as part of a supplement financially supported by Abiomed GmbH.
Data availability
All research data is available through the corresponding author and can be used for future research.
References
- 1. Chieffo A, Dudek D, Hassager C, Combes A, Gramegna M, Halvorsen S et al. Joint EAPCI/ACVC expert consensus document on percutaneous ventricular assist devices. Eur Heart J Acute Cardiovasc Care 2021;10:570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balthazar T, Vandenbriele C, Verbrugge FH, Den Uil C, Engström A, Janssens S et al. Managing patients with short-term mechanical circulatory support: JACC review topic of the week. J Am Coll Cardiol 2021;77:1243–1256. [DOI] [PubMed] [Google Scholar]
- 3. McDonagh TA, Metra M, Adamo M, Baumbach A, Böhm M, Burri H et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC), with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599–3726.34447992 [Google Scholar]
- 4. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–1296. [DOI] [PubMed] [Google Scholar]
- 5. Tan SR, Low CJW, Ng WL, Ling RR, Tan CS, Lim SL et al. Microaxial left ventricular assist device in cardiogenic shock: a systematic review and meta-analysis. Life (Basel, Switzerland) 2022;12:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salas De Armas I, Bergeron A, Bhardwaj A, Patarroyo M, Akay MH, Al Rameni D et al. Surgically implanted Impella device for patients on Impella CP support experiencing refractory hemolysis. ASAIO J 2022;68:e251–e255. [DOI] [PubMed] [Google Scholar]
- 7. Uriel N, Sayer G, Annamalai S, Kapur NK, Burkhoff D. Mechanical unloading in heart failure. J Am Coll Cardiol 2018;72:569–580. [DOI] [PubMed] [Google Scholar]
- 8. Wilson J, Fisher R, Caetano F, Soliman-Aboumarie H, Patel B, Ledot S et al. Managing Harlequin syndrome in VA-ECMO—do not forget the right ventricle. Perfusion 2022;37:526–529. [DOI] [PubMed] [Google Scholar]
- 9. Alam A, Baran DA, Doshi H, Van Zyl J, Patlolla S, Salem M et al. Safety and efficacy of ProtekDuo right ventricular assist device: a systemic review. Artif Organs 2023;47:1094–1103. [DOI] [PubMed] [Google Scholar]
- 10. Yalcin YC, Muslem R, Veen KM, Soliman OI, Hesselink DA, Constantinescu AA et al. Impact of continuous flow left ventricular assist device therapy on chronic kidney disease: a longitudinal multicenter study. J Card Fail 2020;26:333–341. [DOI] [PubMed] [Google Scholar]
- 11. Geller BJ, Sinha SS, Kapur NK, Bakitas M, Balsam LB, Chikwe J et al. Escalating and de-escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2022;146:E50–E68. [DOI] [PubMed] [Google Scholar]
- 12. Bertoldi LF, Delmas C, Hunziker P, Pappalardo F. Escalation and de-escalation of mechanical circulatory support in cardiogenic shock. Eur Heart J Suppl 2021;23:A35–A40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tarzia V, Bortolussi G, Bianco R, Buratto E, Bejko J, Carrozzini M et al. Extracorporeal life support in cardiogenic shock: impact of acute versus chronic etiology on outcome. J Thorac Cardiovasc Surg 2015;150:333–340. [DOI] [PubMed] [Google Scholar]
- 14. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G et al. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404–412. [DOI] [PubMed] [Google Scholar]
- 15. Esposito ML, Jablonski J, Kras A, Krasney S, Kapur NK. Maximum level of mobility with axillary deployment of the Impella 5.0 is associated with improved survival. Int J Artif Organs 2018;41:236–239. [DOI] [PubMed] [Google Scholar]
- 16. Saeed D, Potapov E, Loforte A, Morshuis M, Schibilsky D, Zimpfer D et al. Transition from temporary to durable circulatory support systems. J Am Coll Cardiol 2020;76:2956–2964. [DOI] [PubMed] [Google Scholar]
- 17. Randhawa VK, Al-Fares A, Tong MZY, Soltesz EG, Hernandez-Montfort J, Taimeh Z et al. A pragmatic approach to weaning temporary mechanical circulatory support: a state-of-the-art review. JACC Heart Fail 2021;9:664–673. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW et al. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Card Fail 2019;25:364–371. [DOI] [PubMed] [Google Scholar]
- 19. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 20. Esposito ML, Zhang Y, Qiao X, Reyelt L, Paruchuri V, Schnitzler GR et al. Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J Am Coll Cardiol 2018;72:501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swain L, Reyelt L, Bhave S, Qiao X, Thomas CJ, Zweck E et al. Transvalvular ventricular unloading before reperfusion in acute myocardial infarction. J Am Coll Cardiol 2020;76:684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazurek R, Kariya T, Sakata T, Mavropoulos SA, Ravichandran AJ, Romeo FJ et al. Negative impact of acute reloading after mechanical left ventricular unloading. J Cardiovasc Transl Res. doi: 10.1007/s12265-023-10371-z. Published online ahead of print 6 April 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basir MB, Kapur NK, Patel K, Salam MA, Schreiber T, Kaki A et al. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv 2019;93:1173–1183. [DOI] [PubMed] [Google Scholar]
- 24. Anderson MB, Goldstein J, Milano C, Morris LD, Kormos RL, Bhama J et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: the prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant 2015;34:1549–1560. [DOI] [PubMed] [Google Scholar]
- 25. Bernhardt AM, Copeland H, Deswal A, Gluck J, Givertz MM. The International Society for Heart and Lung Transplantation/Heart Failure Society of America Guideline on Acute Mechanical Circulatory Support. J Heart Lung Transplant 2023;42:e1–e64. [DOI] [PubMed] [Google Scholar]
- 26. De By TMMH, Schoenrath F, Veen KM, Mohacsi P, Stein J, Alkhamees KMM et al. The European Registry for Patients with Mechanical Circulatory Support of the European Association for Cardio-Thoracic Surgery: third report. Eur J Cardiothorac Surg 2022;62:ezac032. [DOI] [PubMed] [Google Scholar]
- 27. Potapov EV, Antonides C, Crespo-Leiro MG, Combes A, Färber G, Hannan MM et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg 2019;56:230–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertoldi LF, Pappalardo F, Lubos E, Grahn H, Rybczinski M, Barten MJ et al. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: de-escalate and ambulate. J Crit Care 2020;57:259–263. [DOI] [PubMed] [Google Scholar]
- 29. Bernhardt AM, Potapov E, Schibilsky D, Ruhparwar A, Tschöpe C, Spillmann F et al. First in man evaluation of a novel circulatory support device: early experience with the Impella 5.5 after CE mark approval in Germany. J Heart Lung Transplant 2021;40:850–855. [DOI] [PubMed] [Google Scholar]
- 30. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation 2020;142:2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernhardt AM, Zipfel S, Reiter B, Hakmi S, Castro L, Söffker G et al. Impella 5.0 therapy as a bridge-to-decision option for patients on extracorporeal life support with unclear neurological outcomes. Eur J Cardiothorac Surg 2019;56:1031–1036. [DOI] [PubMed] [Google Scholar]
- 32. Gill G, Rowe G, Chen Q, Malas J, Thomas J, Peiris A et al. Bridging with surgically placed microaxial left ventricular assist devices: a high-volume centre experience. Eur J Cardiothorac Surg 2023;63:ezad116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All research data is available through the corresponding author and can be used for future research.