Abstract
A 31-year-old woman with a mechanical aortic valve for congenital aortic stenosis presented to the cardiology clinic for preconception counseling. After experiencing an acute stroke 4 weeks prior, she was subsequently discovered to have prosthetic valve thrombosis requiring replacement of the aortic valve. We discuss her clinical course and preconception considerations.
Key Words: aortic valve, cardiac risk, echocardiography, pregnancy, thrombosis, valve replacement
Central Illustration
Case Presentation
This is a 31-year-old woman with anomalous left coronary artery from the pulmonary artery and congenital aortic stenosis. At age 1 year, she underwent surgical transposition of her left main coronary to the aorta and aortic valve commissurotomy. She enjoyed good health until age 26 years, when she developed severe aortic regurgitation from endocarditis with Cardiobacterium, presumed to be related to a foodborne illness. She underwent aortic valve replacement (AVR) with a 23-mm On-X valve (Artivion). She subsequently presented to the cardiology-obstetrics clinic for preconception counseling. A transthoracic echocardiogram (TTE) demonstrated normal AVR function.
Learning Objectives
-
•
To explain pregnancy risks associated with mechanical heart valves.
-
•
To discuss maternal and fetal risks in pregnancies achieved after maternal stroke.
-
•
To explain diagnosis and management of mechanical prosthetic valve thrombosis.
-
•
To consider management of high-risk pregnancies after the Dobbs decision.
Question 1: What risks does this patient have during pregnancy? Can she remain on warfarin?
A mechanical AVR places the patient in the World Health Organization class III, with 19% to 23% predicted risk of mortality.1 Her CARPREG II (Cardiac Disease in Pregnancy study II) score is 3, and her ZAHARA (Zwangerschap bij Aangeboren Hartafwijkingen I) score is 4.25, conveying 15% and 70% predicted risk of maternal cardiac complications, respectively.2,3
Women with mechanical heart valves are at higher risk of maternal mortality, prosthetic valve thrombosis (PVT), and hemorrhagic events. High doses of Vitamin K antagonists (VKAs) increase risks of teratogenicity and fetotoxicity. Current guidelines recommend that women who require high doses of VKA (>5 mg warfarin daily) be transitioned to low-molecular-weight heparin.4,5 The teratogenicity and fetotoxicity risks from VKAs should be balanced with the risk of PVT and fetal loss. In the European Society of Cardiology Registry of Pregnancy and Cardiac Disease, PVT occurred in 10 of 212 (4.7%) patients with mechanical AVR; in 5 patients, PVT occurred during the first trimester in the setting of heparin use. VKA use during the first trimester was also associated with higher rates of miscarriage.6
These risks were discussed with the patient. As she attempted to get pregnant, her international normalized ratio (INR) goal was increased from 1.5 to 2 up to 2 to 3, given the hypercoagulable nature of pregnancy. This was achieved with warfarin 4 to 5 mg daily. Approximately 10 months later, she presented with acute onset of stuttering speech, word-finding difficulty, and right-sided weakness. On arrival to the hospital, her blood pressure was 126/72 mm Hg, heart rate was 67 beats/min, respiratory rate was 18/minute, oxygen saturation was 99%, and body mass index was 26.5 kg/m2. Imaging showed an acute left middle cerebral artery stroke. Urine human chorionic gonadotropin was negative.
Question 2: What is the most likely etiology of her stroke, and how should she be further evaluated?
In a young patient with a mechanical AVR and no other risk factors for stroke, the concern is highest for cardioembolic etiology. Evaluation includes INR measurement; cardiac imaging to evaluate her valve, cardiac function, and for the presence of an intracardiac thrombus; and telemetry monitoring to assess for atrial arrhythmias.
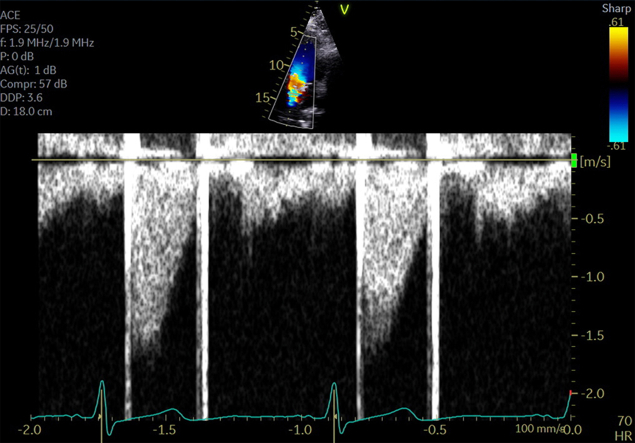
Her laboratory test results were notable for an INR of 1.0. The patient’s INR was therapeutic at 2.6 1 week prior. She reported compliance with warfarin. Her symptoms improved after tenecteplase administration. TTE demonstrated normal cardiac function, no intracardiac thrombus, and a normally functioning AVR with normal gradients (Figure 1). Telemetry monitoring did not reveal any arrhythmia. The patient was discharged with a therapeutic INR and returned to the clinic for additional preconception counseling.
Figure 1.
Poststroke Mechanical Aortic Valve Gradients on Transthoracic Echocardiogram
Echocardiogram during hospitalization for stroke revealed normal gradients across the mechanical aortic valve.
Question 3: Is it safe for her to attempt pregnancy again, and if so, when?
Risks of recurrent stroke during pregnancy are related to the etiology of the index stroke. Pregnant women are at risk because of the hypercoagulable state of pregnancy and can develop strokes de novo. In this patient, therapeutic anticoagulation should theoretically lessen risk for future cardioembolic stroke. The FUTURE (Follow-Up of Transient Ischemic Attack and Stroke Patients and Unelucidated Risk Factor Evaluation) study assessed the natural course of pregnancy and pregnancy-related complications in women with a history of ischemic stroke. In that study of 213 patients, miscarriages and fetal deaths were more common than in an age-matched cohort. In nulliparous women, like our patient, hypertensive disorders of pregnancy and early preterm delivery were also more common.7
There are no existing guidelines regarding optimal timing for pregnancy after a stroke. In the FUTURE study, the mean time after stroke to first pregnancy was 5.7 years, with a standard deviation of 2.7 years.7
These risks were discussed with the patient, who still desired pregnancy. Because of concerns regarding the hypercoagulable state of pregnancy and the mechanism of stroke, transesophageal echocardiogram (TEE) was recommended. One week following this discussion and approximately 30 days from her stroke, the patient notified our office of a positive pregnancy test. She again had a subtherapeutic INR level at 1.7, and thus, low-molecular-weight heparin was initiated for consistent systemic anticoagulation. The patient miscarried at 6 weeks gestation. She then presented for TEE.
Question 4: How is mechanical PVT diagnosed and managed?
Mechanical PVT should be suspected in any pregnant woman who experiences an embolic event, experiences heart failure symptoms, or has increasing/new valvular gradients on echocardiogram. PVT can be diagnosed on multiple imaging modalities, including TTE, TEE, cardiac computed tomography, cardiac magnetic resonance, and fluoroscopy. TTE is often initially performed to assess leaflet motion and for the presence of thrombus. Because of acoustic shadowing from the mechanical valve, subvalvular thrombus can be obscured. Current American Heart Association/American College of Cardiology guidelines recommend TEE in pregnant women with prosthetic valve obstruction or who experience an embolic event. Use of color and spectral Doppler in both TTE and TEE can assess turbulent flow and gradients across the valve. Prosthetic valve obstruction in a mechanical AVR is defined as a gradient 10 mm Hg higher than the postoperative gradient.8
Once prosthetic valve obstruction is identified, distinction between thrombus and pannus is necessary. Although pathologic confirmation is the gold standard, mass size, history of inadequate anticoagulation, and timing from initial surgery can elevate suspicion for thrombus. Additionally, left-sided arterial occlusion, including ischemic stroke, is highly suspicious for thrombus.8
The size of the thrombus guides management. For thrombi of <5 mm, therapeutic anticoagulation can often resolve the thrombus. Thrombi of >5 mm are at higher risk of embolization; surgery is recommended for thrombi of >10 mm and evidence of systemic embolization.8 Systemic fibrinolysis can also be considered instead of surgery in patients in whom surgical risk is high or prohibitive as well as those who have small thrombus burden, mild heart failure symptoms, and/or low bleeding risk.5
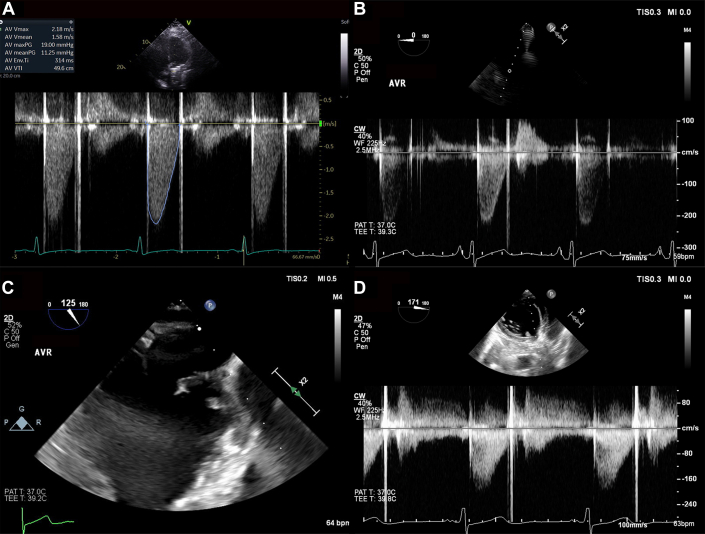
TEE demonstrated a 5- to 10-mm echodensity on the AVR with mobile elements in the left ventricular outflow tract concerning for thrombus. The peak gradient across the aortic valve was 18 mm Hg (Figure 2), compared to the postoperative gradient of 5 mm Hg.
Figure 2.
Mobile Echodensity on the Mechanical Aortic Valve
Normal gradients across the mechanical aortic valve on (A) transthoracic and (B) transesophageal echocardiograms. (C) A mobile, 5- to 10-mm echodensity prolapses from the mechanical valve into the left ventricular outflow tract. (D) The echodensity with normal gradients across the mechanical valve.
The patient was hospitalized, and a multidisciplinary consultation with cardiology, interventional cardiology, and cardiothoracic surgery was held. A repeat TEE performed after 8 days of therapeutic intravenous heparin demonstrated a highly mobile 10-mm echodensity on the mechanical AVR (Figure 2, Video 1). Given the size of the thrombus as well as her recent thromboembolic stroke, reoperation with a mechanical AVR rather than lytic therapy was pursued. During the operation, the thrombus was located below the valve discs, and no pannus was visualized (Video 2). A 23-mm CryoLife On-X aortic valve prosthesis was implanted. The patient had an uneventful postoperative course, resumed warfarin anticoagulation with a goal INR of 2 to 3, and started on aspirin 81 mg daily.
Question 5: If the patient had still been pregnant at the time of valve thrombosis diagnosis, how would you counsel her regarding her pregnancy risks and options in this post-Dobbs era?
This situation requires in-depth shared decision making with the patient and other members of the multidisciplinary cardio-obstetrics team, including maternal fetal medicine and cardiac anesthesia. This would be considered a high-risk pregnancy, with specific risks including recurrent stroke(s) and/or other embolic phenomenon in the setting of a known left-sided intracardiac thrombus. Cardiac surgery in the first trimester carries high risk to the fetus. Following the Dobbs decision, new challenges are present in reproductive choices, especially in the state of Missouri, where the patient resides.9 Here, it is a felony to perform an abortion except in the case of a medical emergency when a condition “complicates the medical condition of a pregnant woman as to necessitate the immediate abortion of her pregnancy to avert the death of the pregnant woman or for which a delay will create a serious risk of substantial and irreversible physical impairment of a major bodily function.”10 In our patient’s case, an abortion could be justified as risks could lead to irreversible physical impairment. Risk interpretation can vary, though, making it a challenge to access abortion care in such situations.
Question 6: The patient has asked about another pregnancy after recovery from the new AVR. How would you manage her anticoagulation?
If our patient desired pregnancy, we would recommend therapeutic low-molecular-weight heparin, verified by measurement of anti-Xa levels (goal: 0.8-1.2 U/mL, 4-6 hours after dose) given her history of subtherapeutic INR levels. If she preferred to continue warfarin, we would recommend a continued INR target of 2.0 to 3.0, as long as the daily dose was ≤5 mg. Regardless of anticoagulation strategy, stability of therapeutic anticoagulation is recommended for several months before pregnancy. Because the risks of developing PVT are highest in the weeks after valve implantation, we would recommend waiting at least 6 months after surgery before attempting pregnancy.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Echodensity on Mechanical Aortic Valve
An echodensity with attachment to the mechanical aortic valve is seen prolapsing into the left ventricular outflow tract.
Intraoperative Removal of Thrombus
A thrombus is removed from the mechanical aortic valve.
References
- 1.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 2.Silversides C.K., Grewal J., Mason J., et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. 2018;71:2419–2430. doi: 10.1016/j.jacc.2018.02.076. [DOI] [PubMed] [Google Scholar]
- 3.Drenthen W., Boersma E., Balci A., et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31:2124–2432. doi: 10.1093/eurheartj/ehq200. [DOI] [PubMed] [Google Scholar]
- 4.Economy K.E., Valente A.M. Mechanical heart valves in pregnancy: a sticky business. Circulation. 2015;132(2):79–81. doi: 10.1161/CIRCULATIONAHA.115.017349. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen I.M., Roos-Hesselink J.W., Ruys T.P., et al. Pregnancy in women with a mechanical heart valve: data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC) Circulation. 2015;132(2):132–142. doi: 10.1161/CIRCULATIONAHA.115.015242. [DOI] [PubMed] [Google Scholar]
- 7.van Alebeek M.E., de Vrijer M., Arntz R.M., et al. Increased risk of pregnancy complications after stroke: the FUTURE study (Follow-Up of Transient Ischemic Attack and Stroke Patients and Unelucidated Risk Factor Evaluation) Stroke. 2018;49(4):877–883. doi: 10.1161/STROKEAHA.117.019904. [DOI] [PubMed] [Google Scholar]
- 8.Soria Jiménez C., Papolos A., Kenigsberg B., et al. Management of mechanical prosthetic heart valve thrombosis. J Am Coll Cardiol. 2023;81(21):2115–2127. doi: 10.1016/j.jacc.2023.03.412. [DOI] [PubMed] [Google Scholar]
- 9.Gupta T., Cai A.W. Implications of restricting legal abortion access on cardio-obstetrics care. 2022. https://www.acc.org/Membership/Sections-and-Councils/Fellows-in-Training-Section/Section-Updates/2022/08/22/17/46/Implications-of-Restricting-Legal-Abortion-Access-on-Cardio-Obstetrics-Care
- 10.Right to Life of the Unborn Child Act, Title XII. §188.017. 2022. https://revisor.mo.gov/main/OneSection.aspx?section=188.017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echodensity on Mechanical Aortic Valve
An echodensity with attachment to the mechanical aortic valve is seen prolapsing into the left ventricular outflow tract.
Intraoperative Removal of Thrombus
A thrombus is removed from the mechanical aortic valve.