Abstract
We describe 2 pregnancies complicated by descending aortic dissections. Patient 1 suffered an acute dissection at 28 weeks. Patient 2 had residual dissection after ascending dissection repair and conceived after detailed preconception counseling. Both were complicated by hypertension, managed by a multidisciplinary team, and ended uneventfully with cesarean deliveries.
Key Words: Pregnancy, type B aortic dissection
Central Illustration
Case Presentation
Case 1
A 27-year-old patient (G3P2002) at 28 weeks presented to the emergency department with acute severe low back pain.
Learning Objectives
-
•
To identify high-risk periods for aortic dissection through pregnancy.
-
•
To manage chronic TBADs during pregnancy and delivery.
Her medical history consisted of genetically confirmed Marfan syndrome (FBN1 c.1090C>T pathogenic variant) and family history of mother and brother who had aortic dissections, the details of which were unknown. Her systemic signs included tall stature, arachnodactyly, and severe scoliosis requiring surgery. She did not have a history of ectopia lentis, and, notably, her aortic dimensions were consistently noted to be normal on echocardiogram and cross-sectional imaging studies. Although the nature of the preconception counseling that she previously received was not known at the time of her presentation, her 2 prior pregnancies were well tolerated without recorded elevated blood pressures or changes in the aortic dimensions. She was treated with metoprolol, which she continued during the current pregnancy. A first-trimester echocardiogram demonstrated effacement of the sinotubular junction with normal aortic root, ascending aortic arch, and transverse aortic arch diameters measuring 3.2 cm (z = 0.26), 2.8 cm, and 2.9 cm, respectively, all unchanged from prior measurements. Her most recent aortic computed tomography with angiography (CTA) showed a maximal descending aortic diameter of 2.1 × 2.0 cm.
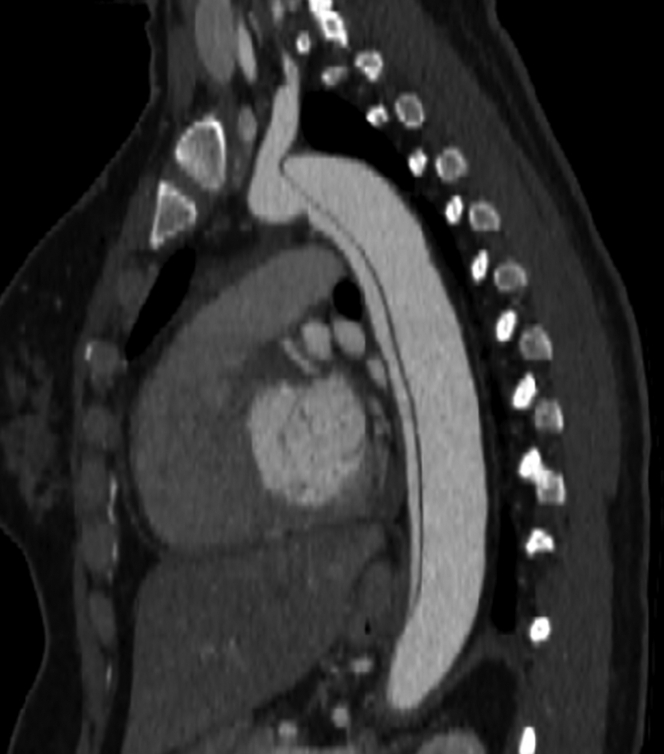
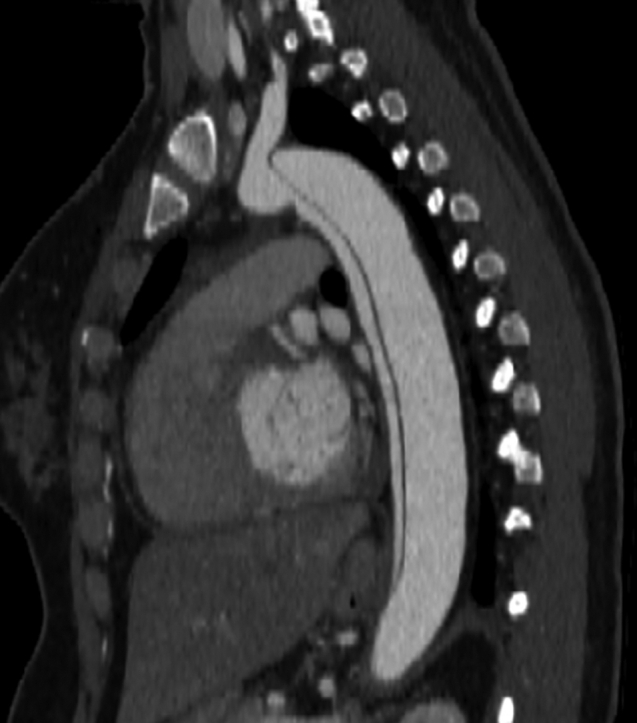
Her pregnancy was uncomplicated until 28 weeks when she was admitted to an outside hospital with acute severe back pain. A chest CTA was obtained, revealing a type B aortic dissection (TBAD), which was noted to extend into the left subclavian artery and both common iliac arteries (Figure 1). The measured descending thoracic aortic diameter was 2.6 × 2.5 cm. The left renal artery was noted to come off the true lumen, whereas the right renal artery was supplied by the false lumen. The aortic root and ascending aortic dimensions were again noted to be of normal diameters, which were unchanged from prior CTA. She was started on esmolol infusion and was transferred to our hospital.
Figure 1.
Acute Type B Aortic Dissection
Acute type B aortic dissection diagnosed by computed tomography angiogram after the patient presented at 28 weeks’ pregnancy with acute chest pain radiating to her back.
On arrival at our hospital, she was admitted to the intensive care unit. Her hemodynamic status was normal with normotension and no laboratory or clinical evidence of end-organ malperfusion. A multidisciplinary team consisting of experts in the fields of cardio-obstetrics, maternal fetal medicine, obstetrical anesthesia, vascular and cardiac surgery, and intensive care unit was assembled and continued to provide her care throughout the hospitalization. Although her initial blood pressure was 113/77 mm Hg, values increased to >140/80 mm Hg within several hours and intravenous nifedipine was added, with subsequent need to add hydralazine, which provided better blood pressure control. Laboratory studies revealed proteinuria, prompting the diagnosis of preeclampsia.
In the following 2 weeks, she continued to require high dose of triple antihypertensive therapy with systolic blood pressure values ranging between 130 and 140 mm Hg. It was ultimately decided that delivery at 30 + 2 weeks would best balance the risks of fetal prematurity and the unique maternal medical needs. Due to the history of prior cesarean deliveries, it was decided to proceed with a repeat cesarean delivery under general anesthesia, which was performed during daytime in the middle of the week and was uncomplicated. Neuraxial anesthesia was not an option given patient’s history of scoliosis and spinal fusion with Harrington rods from T10-S1. She also underwent elective bilateral salpingectomy because she did not desire any future pregnancies. Postpartum blood pressure was easier to control with down-titration of the blood pressure medications, and additional CTA of the aorta showed stable findings. She was observed for 7 days and was discharged home on losartan and metoprolol therapy.
Approximately 1 year after her discharge, an uncomplicated staged repair of the descending aortic dissection was performed, and she is currently followed regularly at the genetic aortopathy and vascular surgery clinics. Her blood pressure continues to be well controlled. Two of her 3 children were confirmed to have an identical FBN1 pathogenic mutation to hers.
Case 2
A 27-year-old patient (G1P1) with prior medical history consisting of postpartum types A and B aortic dissection presented for preconception counseling.
Prior to her presentation, she had completed an uncomplicated, well-tolerated pregnancy with vaginal delivery at 39 weeks. At that time her medical history was unremarkable, and she had no notable features or symptoms suggestive of connective tissue disorder and no family history of aortic dissection or surgeries. She had not had any prior imaging of her aorta.
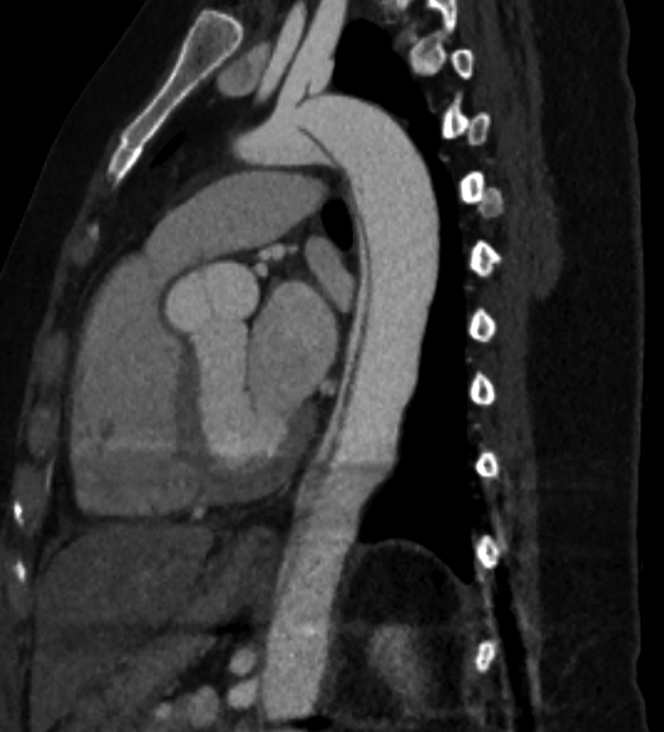
Five days postpartum, she presented to an outside emergency department with severe chest and upper back pain. Her blood pressure was elevated at 150/61 mm Hg, and a chest CTA demonstrated an acute type A aortic dissection, extending to the ostium of the left common iliac artery without extension into the head and neck, renal, or femoral vessels (Figure 2). She was transferred to our institution for further management and underwent an emergent David-V surgery during which her aortic valve was not replaced in setting of a morphologically normal trileaflet aortic valve. Her postoperative course was uneventful, and she was started on labetalol to achieve a goal systolic blood pressure of <120 mm Hg. A full aortopathy genetic panel was obtained, which revealed no evidence of pathogenic mutation to explain her aortic dissection. Follow-up imaging studies demonstrated stable aortic root dimensions, with the largest aortic diameter seen at the sinotubular junction measuring 4.2 × 3.7 cm. The residual TBAD was unchanged.
Figure 2.
Residual Unrepaired Aortic Dissection Following Type A Dissection Repair
Baseline computed tomography demonstrating residual unrepaired type B dissection following ascending aorta replacement in a woman desiring to become pregnant.
Three years after this event, she presented to the cardio-obstetrics clinic for preconception counseling. Her blood pressure at that time was 110/69 mm Hg on metoprolol treatment. Her updated aortic CTA demonstrated a maximal descending aorta diameter of 3.4 × 3.4 cm in the mid-descending thoracic aorta without further extension of the residual descending aortic dissection. She was counseled that whereas data on the management of pregnancy with residual descending aortic dissection are scarce, a theoretical risk for adverse aortic events such as rupture and proximal progression of the dissection is of concern. The need to be followed closely throughout pregnancy and postpartum as well as meticulous blood pressure control were also emphasized. She expressed her full understanding of these risks and opted to proceed with another pregnancy. Once pregnancy was confirmed, aspirin therapy for preeclampsia prevention was initiated, and she was evaluated during each trimester at the cardio-obstetrics clinic. Whereas her echocardiograms showed stable ascending aortic diameter of ∼3 cm, her blood pressure values gradually increased from values of 105/60 mm Hg in the first trimester to 121/67 mm Hg in the early second trimester. She was transitioned from metoprolol to carvedilol at increasing doses, and hydralazine was later added for values >120/80 mm Hg. Urinalysis did not show proteinuria and preeclampsia labs were normal. During her third-trimester clinic visit, delivery options were reviewed, including vaginal delivery with an assisted second stage or a primary cesarean delivery. After careful deliberation, she elected to proceed with a primary cesarean delivery. An uncomplicated cesarean delivery was performed under spinal anesthesia at 37 + 0 weeks. Postpartum blood pressure values remained elevated at 134/62 mm Hg, and enalapril and amlodipine were added to the carvedilol and hydralazine therapy. She was monitored for 7 days and discharged to her home on as-needed hydralazine in addition to carvedilol, enalapril, and amlodipine. Repeat echocardiograms were again unchanged, showing ascending aorta measurement of 2.9 cm. At the time of the writing of this report, a repeat aortic CTA was not yet obtained. She was seen for a postpartum visit and was strongly encouraged to consider a highly effective method of birth control though ultimately, she opted to continue barrier methods. Her antihypertensives were adjusted to 50 mg of carvedilol twice daily, enalapril 20 mg daily, and spironolactone 25 mg daily.
Question 1: Are There Identified Risk Factors for Acute TBAD During Pregnancy?
Cardiovascular disease is the leading cause of death among pregnant patients in the United States, and aortic dissections are major contributors.1 TBAD occurs less frequently than type A dissection and occurs most commonly in patients with connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome, or Loeys-Dietz syndrome. Unlike type A dissections that tend to occur almost universally in patients with large-diameter aortic roots, TBAD lacks the same predictability. Narula et al2 published a large series of consecutive patients with Marfan syndrome and showed that TBAD occurred independent of the aortic root diameter. The highest risk time for both type A aortic dissection and TBAD is the postpartum period, usually within the first 2 weeks.3 This is consistent with other cardiac complications of pregnancy and is hypothesized to be related to large volume shifts after delivery, the abrupt loss of the low-resistance placental circulation, and large fluctuations in sex hormones.4
Question 2: What Is the Pathophysiology of Aortic Dissection During Pregnancy?
Many of the normal physiological changes associated with pregnancy can cause an increase in aortic wall stress and increase the risk of aortic dissections, including increases in blood volume, cardiac output, and inotropy. Some patients will also experience hypertensive disorders of pregnancy with a rise in blood pressure, which is the single most important risk factor for developing TBAD in the general population and has been linked to dissection in pregnancy.5 Finally, pregnant patients also experience large changes in sex hormones. These changes, especially increases in estradiol and progesterone, have been linked to aortic smooth muscle changes that may predispose pregnant patients to dissections.6
Question 3: What Is the Natural History of Patients With Chronic Unrepaired TBADs Who Become Pregnant?
Prior studies have shown that in nonpregnant patients, up to 40% of those with TBAD managed medically required surgical intervention on extended follow-up.7 Almost no cases of pregnancy in the setting of chronic TBAD are found in currently published reports. Residual descending aortic dissection following an ascending aortic dissection repair may have slightly different features from those of a TBAD due to the presence of graft material and having been through a prior operation, yet the management remains similar with a focus on organ perfusion, symptom management, and impulse control. Both scenarios have been rarely described in the literature; thus, little is known about outcomes in pregnant patients. In a single previously published case report, a patient is reported to have an incidentally noted chronic TBAD that likely occurred several years prior to becoming pregnant but was never diagnosed.8 Similar to the patient described here, she underwent a successful cesarean delivery, which was followed by surgical repair several days postpartum. Whereas there are reports of patients successfully completing pregnancy in the setting of prior aortic dissections, the details of their residual disease at the time of pregnancy is unknown.9 We have found no previously published reports of elective pregnancy in the setting of a known, untreated, chronic TBAD or a known residual descending aortic dissection after type A dissection repair. Currently there is insufficient information to assess the long-term outcomes or the safety of pregnancy in patients with these scenarios.
Question 4: How Should TBADs Be Managed Through Pregnancy?
The mainstay of treatment for TBAD, both acute and chronic, is careful monitoring of end-organ perfusion and of symptoms with meticulous blood pressure control. Current guidelines recommend targeting systolic blood pressure of <120 mm Hg in patients with aortic dissections.10 Typically this is done with beta blockers, which have the additional benefit of lowering heart rate and impulse amplitude and are first-line agents for hypertension in pregnant patients. Calcium-channel blockers such as nifedipine are typically second-line agents in pregnancy but are typically avoided in patients with Marfan syndrome on the basis of mouse models showing poor outcomes with long-term use.11 Beyond these agents, vasodilators such as hydralazine and central alpha agonists such as clonidine can be used in pregnancy as needed to further lower blood pressure. For pregnant patients with acute TBAD, surgery is not recommended unless needed to treat acute complications.10 If surgery is required, a nuanced multispecialty discussion around the management of the fetus should take place with a decision to proceed with delivery prior to repair based largely on viability.12
Question 5: What Is the Optimal Delivery Strategy for Patients With Chronic Unrepaired TBADs?
There are insufficient data to recommend a delivery strategy for patients with chronic untreated TBAD. Current guidelines for patients with chronic dissection, based on expert opinion level of evidence, is to undergo cesarean delivery10 based on the theoretical risk of aneurysm enlargement or rupture. In accordance with this expert opinion, and despite the absence of scientific evidence, the patients described in this report and described in a previously published case report underwent a successful cesarean delivery.8 An assisted second stage with limited Valsalva maneuvers and controlled blood pressure may be a reasonable approach for stable patients. This was offered to patient 2, but she ultimately preferred to undergo a cesarean delivery. Future similar reports to ours and pooled case series will be needed to provide more formal, evidence-based recommendations.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W., et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Narula N., Devereux R.B., Malonga G.P., Hriljac I., Roman M.J. Pregnancy-related aortic complications in women with Marfan syndrome. J Am Coll Cardiol. 2021;78(9):870–879. doi: 10.1016/j.jacc.2021.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Roman M.J., Pugh N.L., Hendershot T.P., et al. GenTAC Investigators. Aortic complications associated with pregnancy in Marfan syndrome: the NHLBI National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC) J Am Heart Assoc. 2016;5(8) doi: 10.1161/JAHA.116.004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silversides C.K., Grewal J., Mason J., et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. 2018;71(21):2419–2430. doi: 10.1016/j.jacc.2018.02.076. [DOI] [PubMed] [Google Scholar]
- 5.Sawlani N., Shroff A., Vidovich M.I. Aortic dissection and mortality associated with pregnancy in the United States. J Am Coll Cardiol. 2015;65(15) doi: 10.1016/j.jacc.2014.12.066. [DOI] [PubMed] [Google Scholar]
- 6.Manalo-Estrella P., Barker A.E. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol. 1967;83(4):336–341. [PubMed] [Google Scholar]
- 7.Tadros R.O., Tang G.H.L., Barnes H.J., et al. Optimal treatment of uncomplicated type B aortic dissection: JACC review topic of the week. J Am Coll Cardiol. 2019;74(11):1494–1504. doi: 10.1016/j.jacc.2019.07.063. [DOI] [PubMed] [Google Scholar]
- 8.Taglialegna G. de M., Katz L., de Albuquerque L.M.A., de Freitas M.M., de Lucena A.J.G., de Amorim M.M.R. Chronic aortic dissection and pregnancy: clinical case report. Arq Bras Cardiol. 2019;112(3):321–323. doi: 10.5935/abc.20190044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campens L., Baris L., Scott N.S., et al. ROPAC Investigators Group. Pregnancy outcome in thoracic aortic disease data from the Registry of Pregnancy and Cardiac disease. Heart. 2021;107(21):1704–1709. doi: 10.1136/heartjnl-2020-318183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isselbacher E.M., Preventza O., Black J.H., III, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;80(24):e223–e393. doi: 10.1016/j.jacc.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle J.J., Doyle A.J., Wilson N.K., et al. GenTAC Registry Consortium. MIVAVA Leducq Consortium A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. Elife. 2015;4 doi: 10.7554/eLife.08648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rimmer L., Mellor S., Harky A., Gouda M., Bashir M. Pernicious pregnancy: type B aortic dissection in pregnant women. J Card Surg. 2021;36(4):1232–1240. doi: 10.1111/jocs.15354. [DOI] [PubMed] [Google Scholar]