Abstract
Nonischemic cardiomyopathies are a frequent occurrence. The understanding of the mechanism(s) and triggers of these cardiomyopathies have led to improvement and even recovery of LV function. While chronic right ventricular pacing-induced cardiomyopathy has been recognized for many years, left bundle branch block (LBBB) and pre-excitation have been recently identified as potential reversible causes of cardiomyopathy. These cardiomyopathies share a similar abnormal ventricular propagation that can be recognized by a wide QRS duration with LBBB pattern and thus we coin the term “abnormal conduction-induced cardiomyopathies”. Such abnormal propagation results in an abnormal contractility that can only be recognized by cardiac imaging as ventricular dyssynchrony. Appropriate diagnosis and treatment will not only lead to improved LVEF and functional class but may also reduce morbidity and mortality. This manuscript presents an update of the mechanisms, prevalence, incidence, risk factors, as well as their diagnosis and management, while highlighting current gaps of knowledge.
Keywords: Left Bundle Branch block, Pre-excitation syndrome, RV pacing, Cardiomyopathy, Left ventricular dysfunction, Heart failure
Condensed Abstract
Chronic right ventricular pacing-induced cardiomyopathy has been recognized for many years, while left bundle branch block (LBBB) and pre-excitation have been recently identified as potential reversible causes of cardiomyopathy. These cardiomyopathies share a similar abnormal ventricular propagation recognized by ECG with LBBB pattern and thus we coin the term “abnormal conduction-induced cardiomyopathies”. This abnormal propagation results in an abnormal contractility (dyssynchrony) that can only be recognized by cardiac imaging. Appropriate diagnosis and treatment will not only lead to improved LVEF and functional class but may also reduce morbidity and mortality. This manuscript presents an update and overview of these cardiomyopathies.
An increasing emphasis exists to identify reversible causes of non-ischemic cardiomyopathies such as stress, chronic right ventricular (RV) pacing and arrhythmias1–3. Appropriate recognition of these triggers is paramount as treatment is expected to improve or in some cases fully restore LV systolic function while potentially decreasing morbidity and mortality. More recently, pre-excitation syndromes, left bundle branch block (LBBB) and RV pacing have been recognized as additional etiologies of cardiomyopathy. LBBB, pre-excitation syndromes and chronic RV pacing (RVP) are all entities that have an abnormal ventricular activation resulting in an abnormal ventricular contraction, referred to as dyssynchrony. While their common trigger is LV dyssynchrony, which can only be identified by cardiac imaging, they share a common etiology of abnormal conduction that can be easily identified by wide QRS with a LBBB pattern. Thus, we propose the new term “abnormal conduction-induced cardiomyopathies” as this should assist primary care providers with recognizing these entities and referring patients at risk. This review article presents an update of abnormal conduction-induced cardiomyopathies including common and distinctive features and management.
Pathophysiology & Mechanism
RVP-CM has been recognized for several decades as a frequent etiology of CM in those with a pacemaker and a high percentage of RV pacing. Animal studies have demonstrated that RV pacing causes worse abnormal electrical activation, acute reduction in LV function and hemodynamics than septal or LV pacing2, 4. However, the mechanism of RVP-CM remained unclear for many years. Population-based studies of patients with LBBB demonstrated that HF and CM develop several years after diagnosis of LBBB5, 6. LBBB-CM was later recognized as another cause of non-ischemic CM due to the complete recovery of LV function after implanting an LV coronary sinus or epicardial lead for cardiac resynchronization therapy (CRT). The resolution of LBBB-CM with CRT brought to attention the consequences of persistent LV dyssynchrony.
Clinical and pre-clinical studies have consistently demonstrated that LBBB and RVP are associated with abnormal LV mechanics7, 8 (Central Figure). Typical LBBB is often accompanied by persistent ventricular dyssynchrony, characterized by an early basal or mid-ventricular peak septal systolic contraction (septal flash) with a distension of the basal and mid-ventricular lateral segment followed by a late (after aortic valve closure) peak contraction of the basal lateral wall segments9. The delay in contraction of the basal lateral wall results in delayed relaxation and shortening of diastolic phase and LV filling, frequently seen in the mitral valve inflow by fusion of the E and A wave in patients with LBBB9. Similarly, an atypical LBBB masquerading as an interventricular conduction delay could have similar LV mechanics with subsequent risk of CM and HF10. Unfortunately, animal models of short-term LBBB- and RVP-CM have failed to reproduce cardiomyopathy11. This is likely in part due to the need for a longer duration of LBBB and RVP to develop CM and HF3, 9. Similarly, manifest right accessory pathway (AP) conduction typically shows an iatrogenic LBBB pattern due to RV pre-excitation, resulting in a LBBB-like LV dyssynchrony pattern. Thus, it is likely that the development of cardiomyopathy depends on the degree of LV dyssynchrony, which can vary significantly based on location of RV pacing lead or AP insertion, respectively. This concept is supported by an elegant prospective study in patients with chronic RV pacing after AV nodal ablation, where septal mechanical propagation delay predicted the development of RVP-CM12.
Central Figure.
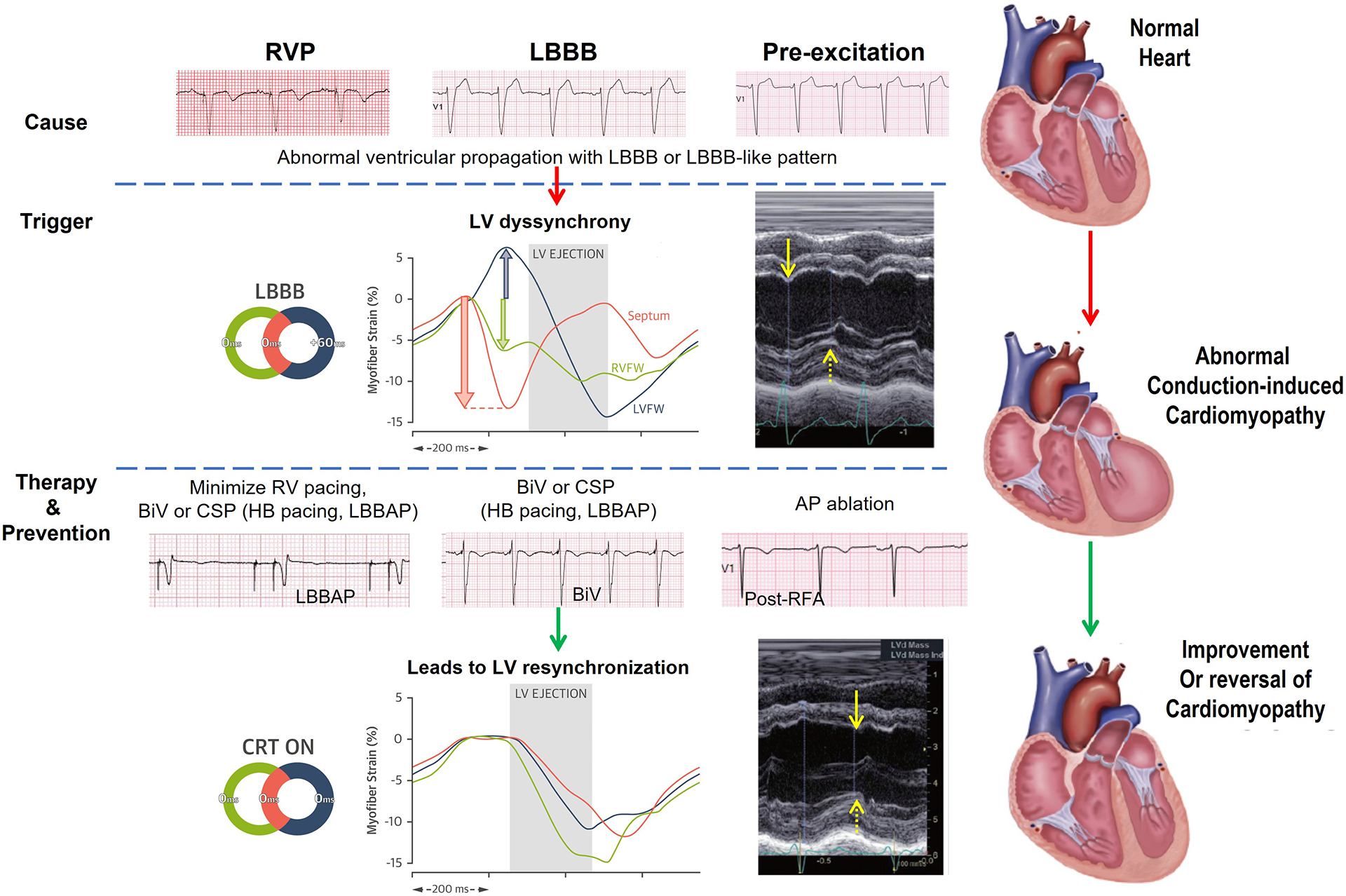
Abnormal Conduction-induced Cardiomyopathy: Causes, Triggers and Therapy and Prevention.
Note: See text for abbreviations. Solid and dash yellow arrows denote LV septum and free wall peak contraction, respectively. These arrows expose LV dyssynchrony between opposite segments, which can be restored with CRT. Illustrations and m-mode images of dyssynchrony and response to CRT reproduced with permission47, 48.
It is speculated that LV dyssynchrony (ventricular segments contracting while opposing segments over-stretching) triggers molecular changes responsible for contractile dysfunction. This hypothesis is supported by other cardiomyopathy models which are characterized by intermittent LV dyssynchrony. An example is PVC-induced cardiomyopathy, where LV dyssynchrony appears to be a marker for the development of LV dysfunction13, 14. Several mechanisms have been postulated to explain these pathophysiologic changes. A study using PET CT in patients with LBBB and dilated CM reported abnormal septal glucose metabolism without change in myocardial perfusion at baseline that improved after biventricular (BiV) pacing15. While septal hypoperfusion in LBBB has been reported, more recent studies have demonstrated that this is due to a relative lateral hyper-perfusion rather than an absolute decrease in septal blood flow16. Moreover, it is speculated that over-stretched tissue may result in molecular changes. In a mixed model of RVP (dyssynchrony) and tachycardia induced heart failure17, Spragg et.al. demonstrated that late contracting lateral free wall had a 30%, 80% and 60% reduction in SERCA2, phospholamban and connexin43, respectively, compared to the opposing wall. Moreover, this model also demonstrated changes in key mitochondrial metabolism enzymes that were reversed by BiV pacing8. Consistent with the canine data, CRT super-responder patients, likely with a LBBB-CM diagnosis, had an increase in α-myosin heavy chain, SERCA2 and SERCA2/PLN ratio after CRT implantation18. While these changes may explain the contractile dysfunction, it is not clear how LV dyssynchrony induces such changes. Another hypothesis points to the stretch activation of cardiac mechanoreceptors during LV dyssynchrony, which results in activation of myofibroblast and release of cytokines with subsequent contractile dysfunction19. Further pre-clinical and clinical studies are needed to further delineate the mechanism(s) in order to find preventive or alternative therapies.
Chronic RV pacing – induced Cardiomyopathy
Definition and prevalence
Chronic RV pacing – induced cardiomyopathy (RVP-CM) is defined as an LV systolic dysfunction that is solely due to frequent RV pacing. While the definition of LV systolic dysfunction varies between studies3, a decline of 10% or absolute value <50% in LVEF is the most sensible parameter since it allows for an early diagnosis and treatment. RVP-CM was initially suspected by subgroup analysis of the DAVID trial3. Patients with ICD indication programmed with a DDD pacing mode and frequent RV pacing (>40%) had increased HF admissions and mortality when compared to single-chamber pacing mode with minimal RV pacing. Interestingly, pre-existing LBBB was a marker of increased morbidity in patients with high RV pacing in the DAVID trial, which can only be explained by a pre-existing dyssynchrony that preceded long time before the initiation of chronic RV pacing.
The prevalence of RVP-CM is uncertain, but the incidence is estimated between 12–20% after 1 to 15 years depending on definition and follow-up20–22.
Clinical presentation, diagnosis, and imaging features
RVP-CM should be suspected in patients with LV systolic dysfunction (LVEF <50%) with or without heart failure symptoms with RV-pacing >20%3, 23. High RV pacing burden is commonly seen in patients with 1) DDD pacing mode if the AV delay is programmed shorter than intrinsic AV conduction or 2) VVI pacing mode with intrinsic rate below the programmed lower rate limit. Moreover, pacemaker’s features may inadvertently increase RV pacing (e.g., mode switch for AF with higher lower rate limit, rate smoothing algorithms, etc.). The MADIT II trial showed that age >65 years, advanced NYHA class, LVEF <25%, first degree AV block and bundle branch block and amiodarone use was associated with frequent RVP24.
RVP-CM should be a diagnosis of exclusion after ruling out other reversible etiologies. Its phenotype is indistinguishable from other dilated cardiomyopathies with global hypokinesis and increased LV systolic and diastolic dimensions and volumes21. Some studies have reported new or worsening MR in 36% of cases20. Thus, diagnosis can only be confirmed after eliminating or minimizing RV pacing. However, elimination of RV pacing is frequently not feasible in patients with complete AV block, thus CRT with either an LV lead or a conduction pacing system (CSP) may be the only approach to validate the diagnosis25.
RVP-CM should also be suspected especially in patients with atrial fibrillation and slow ventricular response requiring frequent RV pacing. However, these patients may have a combined RVP-CM and AF-induced CM and a stepwise treatment of each one could identify the specific etiology based on full or partial response.
Several risk factors for the development of RVP-CM have been reported with the most consistent being high percentage and duration of RVP, wider QRS during RVP and prior cardiomyopathy (Table 1). For instance, a study with RVP of 20–39%, 40–59%, 60–79% and >80% was associated with RVP-CM incidence of 13%, 16.7%, 26.1% and 19.8%, respectively20. While 40% RVP is considered the threshold needed to develop RVP-CM, other studies suggest that RVP as low as 20% can also trigger RVP-CM20. Duration of RVP to develop PVC-CM is variable from months to years. One of the largest studies (87 patients) showed a median time to develop RVP-CM of 4.7 year and baseline LBBB (but not RBBB), QRS duration >155ms and RVP >86% were independent predictors (adjusted HR 8.62, 2.6 and 2.4, respectively)21. Moreover, the combination of these 3 predictors estimated a 15-fold risk to develop RVP-CM (HR 15.9, 95% CI 5.87–43.3). A prospective study including patients with complete AV block showed that paced QRS<160ms, 160–189ms and >190ms had a 3-year HF incidence of 9.4%, 27.8 and 56.8%, respectively (p<0.001)26. Moreover, decrease in LVEF was directly correlated with the paced QRS duration (RR 0.423), whereas a paced QRS duration >165ms was an effective predictor for long-term risk of HF events with a sensitivity of 79%. Another study including patients with RV pacing following AV nodal ablation demonstrated that LV dyssynchrony predicted a decline in LVEF with 81% sensitivity and 88% specificity and indirectly correlated with decline in LVEF12. Other factors such as age, prior LV systolic dysfunction, global longitudinal strain, scar burden, and sex may play a role although the literature is less definitive3, 20, 27, 28. Finally, non-apical RV pacing has not shown to have a lower incidence of RVP-CM when compared to apical RV pacing22. A summary of risks factors that have been associated with RVP-CM are outlined in Table 1.
Table 1.
Summary of conduction abnormality-induced CM
| RVP-CM | LBBB - CM | Pre-Excitation-CM | |
|---|---|---|---|
| Incidence | Unknown | 17–38% after 4 years of LBBB diagnosis5, 29 | Unknown |
| Prevalence | 12–20% after 1 to 15 years20–23. | 2–20% in patients with CM and LBBB referred for CRT (4–11 years)30, 31, 42. | 65% in patients with pre-excitation and LV dysfunction37 |
| Risk Factors / Predictors | Time with high RVP (month-years) RVP burden > 40% (Min 20%)22, 23, 27 Older age27 Intrinsic QRS duration20 Paced QRS duration (>160ms)21, 26, 27 Prior LV systolic dysfunction20 LV dyssynchrony12, 22 Global longitudinal strain28 Male gender3, 20 Higher myocardial scar27 |
Time from LBBB diagnosis >4 yrs. Older population |
Right-sided and septal AP36, 37 Younger population (children)35, 37 Must rule out incessant or recurrent tachycardia |
| Treatment | CRT (LBBAP preferred over BiV or His pacing) | CRT (LBBAP preferred over BiV or His pacing) | Radiofrequency ablation36, 37
Antiarrhythmic (flecainide)47 |
| Predictors of recovery | (?) Shortening QRS duration, QRS duration >150ms, body mass index < 30kg/m2, LA volume, female39 | LV diastolic diameter and mild LV systolic dysfunction (>42%)37, 39 (?) Shortening QRS duration, QRS duration >150ms, body mass index < 30kg/m2, LA volume, female39 |
Age < 6 year of age37 |
| Outcomes | Improves LV function, NYHA class and probably outcomes (no data). | Improves LVEF, NYHA class. Decrease mortality and HF admissions. | Improves LV function and dimensions37; Unknown outcomes (no data). |
Notes:
Data derived from overall super-responders CRT data likely representing RVP-CM and LBBB
Interestingly, some patients do not develop RVP-CM despite many years of high burden of RVP. Thus, it is speculated that genetic variations can either predispose or protect against RVP-CM. Nevertheless, close surveillance should be part of the management of patients with high RVP to identify early development of RVP-CM.
Several retrospective studies have shown that RVP-CM is associated with a 10 to 15% combined increase mortality and HF admissions when compared to those without LV dysfunction despite chronic RV pacing3, 20, 21, 26. Similarly, the MADIT II trial showed that patients with frequent RVP >50% had worse outcomes (HF events HR 1.9; VT/VF requiring ICD therapies, HR 1.5)24. Thus, it is paramount to properly recognize and treat this cardiomyopathy to prevent the increased morbidity and mortality associated with RVP-CM.
Left Bundle Branch Block – induced Cardiomyopathy
Definition and Prevalence
LBBB is infrequent in the general population (<1% prevalence), but its prevalence increase with age, reported up to 5% in octogenarians3. Population-based studies have identified that patients with a normal LV function who developed HF and CM few years after the identification of the LBBB. In contrast to RBBB, LBBB portends an increased risk to develop CM and HF syndrome, and increased mortality5, 6 leading to the recognition of LBBB-CM. LBBB-CM is defined as a cardiomyopathy caused by persistent / chronic LBBB which frequently responds to CRT (BiV or CSP) by a near or complete resolution of CM. Incidence of CM in patients with LBBB and previously preserved LVEF, likely representative of LBBB-CM, has been reported between 17–38% after more than 4 years of development of LBBB5, 29. Prevalence has been reported from 2 to 20% in patients with CM and LBBB referred for CRT9, 30, 31.
Clinical presentation and diagnosis
LBBB-CM should be suspected in patients with recent CM diagnosis but with a chronic LBBB. The time of presentation of LBBB-CM has been reported greater than 4 years after the diagnosis of LBBB9. If a concomitant diagnosis of CM and LBBB is recently made, clinical suspicion for LBBB-CM should be low and work up for ischemia or infiltrative disease should be performed since LBBB could be a sign of disease progression.
Studies reporting LBBB-CM have not described any distinctive feature that can distinguish this CM from other dilated CM. Similarly, risk factors associated with LBBB-CM are scarce. Some reports have referred initial LVEF 45–60% and LVESD ≥ 2.9 cm as predictors of LBBB-CM29. Whilst mild LV dysfunction likely points toward an early marker of LBBB-CM, a severely dilated LV could identify CM where LBBB is not a cause, but rather a marker of disease progression and poor outcomes. Therefore, future studies are needed to identify patients with LBBB and preserved LV function at risk to develop LBBB-CM and better identify phenotype of LBBB-CM.
LBBB alone has been recognized as an independent predictor of 1-year HF admissions and mortality (HR 1.7)32, 33. Patients with concomitant LBBB and cardiomyopathy have been recognized at increased risk of mortality with subsequent indication for CRT-Defibrillator implantation (HR 1.5)9, 34.
It is paramount to be aware that patients with LBBB and preserved LV function should undergo imaging surveillance, particularly if they develop signs or symptoms of HF. This has become more relevant with the addition of contemporary transaortic valve replacement (TAVR) with reported incidence of iatrogenic LBBB between 12–25%9. This risk is likely to continue after TAVR since some patients frequently receive a dual-chamber pacemaker and increased RV pacing resulting in iatrogenic LBBB and potential risk to develop RVP-CM.
Pre-excitation syndrome – induced Cardiomyopathy
Definition and Prevalence
Cardiomyopathy associated with pre-excitation syndrome (without tachyarrhythmia) was first reported in 2004 and has gained recognition due to several case reports35–37. Pre-excitation-CM is defined as an LV systolic dysfunction solely due to the presence of a manifest AP that recovers upon ablation. The term of accessory pathway-induced cardiomyopathy should be avoided since concealed AP lack pre-excitation and LV dyssynchrony and if cardiomyopathy is present, it is more likely related to tachycardia or other etiology. Pre-excitation-CM (PE-CM) should not be confused with tachycardia- or AF-induced CM since these 2 (tachycardia and AF) are not uncommon in patients with manifest AP. Thus, to diagnose PE-CM, one should exclude the presence of AF and SVT as these are more likely the primary cause of LV dysfunction even though the treatment for these 3 entities involves ablation of the AP1.
The incidence and prevalence of PE-CM are unknown. While RVP-CM and LBBB-CM are mostly seen in the adult and older population, pre-excitation-CM is an entity that is most frequently reported in the children and young adults with only 2 case reports in patients above 50 years of age35, 36. One of the largest pediatric studies (n=49; median age 2.9 years) demonstrated that 65% of children with AP and LV dysfunction (without tachyarrhythmias) had full LV recovery after AP ablation37. Moreover, manifest APs are frequently ablated at early ages and if not, manifest conduction of AP frequently decreases with age, which may explain its rare presentation in the elderly.
Clinical presentation and diagnosis
Clinical presentation can be variable, from asymptomatic to heart failure syndrome and even cardiac arrest35–37. This diagnosis should only be considered in persistent pre-excitation without recurrent or incessant supraventricular tachycardia, congenital heart disease or other cardiomyopathies. Unfortunately, PE-CM diagnosis is frequently misdiagnosed, resulting in delayed therapy36, 37.
PE-CM appears to be mild with the largest study reporting LV dilatation with a mean LVEF of 41.1 ± 13.2% and a mean QRS duration of 130 ± 22.4ms37. This study reported that 47%, 30% and 23% of patients had mild (LVEF 45–55%), moderate CM (LVEF 30–45%) and severe CM (LVEF <30%), respectively. Few patients have been reported to have significant mitral regurgitation associated with PE-CM, which resolved after AP ablation35.
It is speculated that a critical factor for the development and severity of PE-CM is the degree of manifest pre-excitation, which it is frequently determined by AP location and intrinsic AV nodal conduction35, 37. However, 2 studies with the largest number of cases (n=25 and n=49) have not found any correlation between pre-excited QRS duration severity of LV dysfunction35, 37. A challenge is that the degree of pre-excitation can vary dynamically with autonomic tone due to competitive AV nodal conduction. Most reports of PE-CM include right-sided and septal AP, while there are no case reports of left-sided AP resulting in CM36, 37. Right-sided AP are closest to the sinus node and frequently activated before the AV node. Thus, right-sided AP have more pronounced ventricular preexcitation and depolarization abnormality compared to left-sided AP. Echocardiographic studies have shown that only right-sided AP cause an early septal motion and late peak contraction of the lateral free wall similar to RV pacing and LBBB35, 36. Moreover, left-sided AP are less likely to manifest pre-excitation since they are the farthest from the AV node allowing most intrinsic conduction via His-Purkinje system by the time AP depolarization occurs. However, this could change with age and autonomic tone as the AV nodal conduction may decrease allowing greater manifest pre-excitation with subsequent abnormality in ventricular propagation. It is not clear if left AP are less likely to induce LV dysfunction because of their location resulting in LV free wall activation with lesser degree of LV dyssynchrony or because lesser degrees of pre-excitation2, 8.
Treatment
Guideline-directed medical therapy should be initiated promptly and optimized as for any diagnosis of HF even though its clinical benefit may be limited3, 9, 37. The mainstay therapy is restoring or improving LV propagation through the normal His-Purkinje system, thereby eliminating or improving LV dyssynchrony (Central Figure, Table 1).
RVP-CM is reversible or partially reversible if RV pacing can be avoided or eliminated. This can be achieved by either enabling algorithms to minimize RV pacing38 or reprogramming to a single chamber pacing mode with a lower rate limit below intrinsic rate when feasible in an attempt to resolve CM . However, this is not an alternative in patients with complete AV block. Patients with permanent AF and slow ventricular response at 50–60 bpm pose a particular challenge as RV pacing maybe necessary for chronotropic incompetence.
If RV pacing is unavoidable, LBBB-like QRS morphology with LV dyssynchrony will persist for which treatment is similar to LBBB-CM. CRT with either BiV or CSP including His-bundle pacing, LBBAP alone or LBBAP-optimized CRT is the indicated procedure to improve LV function in RVP-CM and LBBB-CM. A recent study treating 69 patients RVP-CM with CRT upgrade demonstrated that 71% of patients had an improvement of >10% LVEF (mean: 15% points) with most improvement taking place after 12 months25. It is important to recognize that 29% of patients did not improve or recover LV function after upgrade to CRT. Predictors of response have been reported for patients undergoing CRT (CM and wide QRS) but not been specifically reported for RVP-CM or LBBB-CM. Besides LBBB and NICM, analysis of super-responders to CRT (likely representing RVP-CM and LBBB-CM), showed that baseline QRS duration >150ms, body mass index <30kg/m2, female sex and smaller left atrial volume index are predictors of LV recovery after CRT39. It is possible that the closer proximity between RV and LV leads in smaller hearts (i.e., smaller BMI and female sex) allow for better LV resynchronization and clinical response.
Lack of response to CRT should lead one to suspect another diagnosis or the inability of BiV pacing to significantly restore LV mechanics. Even though CRT may not improve or restore LV function in RVP-CM, early intervention may play a role in preventing further deterioration in LV contractility. Thus, CRT (BiV or LBBAP) should be strongly considered in patients at risk to develop RVP-CM. A prospective, double-blind multicenter study including patients with bradycardia, demonstrated patients with RV pacing alone had a significantly lower LVEF, and higher LV volumes compared to biventricular pacing (LVEF 54.8 vs. 62%; LVESV 35.7 vs. 27mL, P<0.0001) at 12 months follow-up40. Together with the BLOCK-HF study, AHA/ACC guidelines now recommend CRT to prevent the development of RVP-CM in those who are expected to be pacemaker dependent with a documented CM (LVEF <50%)41.
CRT with BiV pacing has shown to improve CM associated with LBBB even without normalization of the QRS duration31, 42. Besides BiV, CSP with LBBAP and HB pacing are more favorable options to recruit the left bundle as demonstrated by improvement in LV strain and significant shortening of QRS duration9, 30. A recent observational study of 247 patients referred for CRT implant (52% with LBBB) demonstrated that CSP with either HB or LBBAP pacing had a significantly better pacing thresholds (0.8 ± 0.4 vs. 1.3 ± 0.6V, p=0.01), narrower paced QRS duration (133 ± 21ms vs. 152 ± 24ms, p<0.001), greater normalization of LVEF (27.6% vs. 14.4%, p=0.005), greater improvement in LVEF in those with LBBB (41.4 ± 12.1% vs. 33.5 ± 11.7%, p < 0.001), and better outcomes (death or heart failure admissions 28% vs. 38%, p=0.01) compared to BiV pacing43. Similarly, a small study demonstrated that CSP with His-bundle pacing in RVP-CM significantly decreased QRS duration (from 177 ± 17ms to 114 ± 20ms, p<0.001), improved LVEF (34.3 ± 9.6% to 48.2 ± 9.8%, p <0.001) and NYHA class (2.8 to 1.9, p<0.01)44. This early evidence indicates potential superiority of CSP over BiV pacing. However, His-bundle pacing has fallen out of favor due to unacceptable higher thresholds and early battery depletion, requiring frequent interventions45. Moreover, several studies have demonstrated that LBBAP is superior to BiV with lower pacing thresholds, shorter QRS duration and a greater improvement in LV function and NYHA functional class, although these trials include small numbers of patients and limited follow-up durations30, 46. Contemporary studies are in progress to corroborate the superiority of LBBAP to biventricular pacing.
In contrast, the mainstay therapy to restore systolic function in PE-CM consists of radiofrequency ablation of AP. The time to recover LV function after AP ablation will depend on the severity and has been reported from 1 to up to 17 months36, 37. Moreover, severe LV dysfunction appears to more likely recover in children younger than 6 years old36. Antiarrhythmics can be an alternative to reverse PE-CM when ablation is delayed (i.e., infants) or is not feasible47. However, it is unclear if LV recovery after AP ablation in PE-CM should results in decreased morbidity and mortality. Future studies are needed to better understand the clinical benefits besides improvement in LV function in patients with abnormal conduction-induced CM.
In conclusion, health care providers caring for patients with these conduction abnormalities should be aware of potential to develop cardiomyopathy. It is important to understand the time to develop CM in patients with high percentage of RV pacing, presence of LBBB and pre-excitation since these patients should undergo a close evaluation and systematic surveillance with repeated assessment of LV function, especially in the presence of HF signs or symptoms. Patients at high-risk of developing abnormal conduction-induced CM should be identified and treated to prevent LV systolic dysfunction. Finally, early detection is important since CRT (BiV or LBBAP) can reverse LV systolic dysfunction and potentially reduce morbidity and mortality in this population. Future studies should also focus on identifying genotypes and/or phenotypes that provide resiliency to develop these cardiomyopathies as these could provide an insight to prevention and better treatments of abnormal conduction induced cardiomyopathies.
Bullet points:
Abnormal Conduction Induced-Cardiomyopathies refers to LV systolic dysfunction caused solely by either RV pacing, left bundle branch block or pre-excitation syndrome, all of which are reversible if properly treated.
Abnormal Conduction Induced-Cardiomyopathies share a similar abnormal ventricular propagation that can be recognized by a wide QRS duration with LBBB pattern.
Abnormal ventricular propagation results in an abnormal contractility that can only be recognized by cardiac imaging as ventricular dyssynchrony.
Appropriate diagnosis and treatment of these cardiomyopathies will not only lead to improved LVEF and functional class but may also reduce morbidity and mortality.
Funding:
This work was supported by the NIH [grant number 1R01HL139874-01 (PI: Huizar); 1R34HL138110-01 (PI: Huizar)] and VA Merit grants [BX-004861-01 (PI: Huizar)].
Disclosures:
Huizar JF - Research support from Abbott. Kaszala K - Research support from Boston Scientific Corp (BS) and St. Jude Medical (SJM); Tan A - Research support from BS, MDT and Biotronik, Inc. (BTK); Koneru K - Consultant for MDT, Biosense Webster (BW), Honoraria from Abbott, Baylis; Ellenbogen KA - Research support from BS, BW, MDT, SJM, NIH, Consultant for BS, SJM, Atricure, Medtronic, Honoraria from MDT, BS, BTK, BW, and Atricure; Drs. Mankad and Kron have no disclosures.
Abbreviations
- BiV
Biventricular pacing, refers to pacing from both ventricles (RV endocardial pacing and LV free wall epicardial pacing).
- CRT
Cardiac Resynchronization Therapy, refers to pacing strategies that restore or improve LV dyssynchrony including biventricular pacing (BiV) and conduction system pacing (CSP).
- CSP
Conduction system pacing, refers to either His-bundle pacing (HBP) or left bundle branch area pacing (LBBAP)
- HBP
His bundle Pacing, refers to pacing through an RV endocardial lead placed in the His-bundle region with a narrow QRS that is identical to intrinsic AV nodal conduction.
- LBBAP
Left bundle branch area pacing, refers to an endocardial lead perforating the septum to partially capture the left bundle branch of the His-Purkinje system.
- LBBB-CM
Left bundle branch block-induced Cardiomyopathy, which is defined as an LV systolic dysfunction that is solely due to chronic LBBB.
- PE
Pre-excitation
- PE-CM
Pre-excitation induced-Cardiomyopathy, which is defined as an LV systolic dysfunction that is solely due to chronic pre-excitation syndrome (manifest accessory pathway).
- RVP
Right ventricular pacing, refers to pacing through an RV endocardial lead typically placed in the apical region.
- RVP-CM
Right ventricular pacing-induced Cardiomyopathy, which is defined as an LV systolic dysfunction that is solely due to chronic RV pacing
References:
- 1.Huizar JF, Ellenbogen KA, Tan AY and Kaszala K. Arrhythmia-Induced Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2328–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prinzen FW, Auricchio A, Mullens W, Linde C and Huizar JF. Electrical management of heart failure: from pathophysiology to treatment. Eur Heart J. 2022;43:1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khurshid S and Frankel DS. Pacing-Induced Cardiomyopathy. Card Electrophysiol Clin. 2021;13:741–753. [DOI] [PubMed] [Google Scholar]
- 4.Prinzen FW and Peschar M. Relation between the pacing induced sequence of activation and left ventricular pump function in animals. Pacing Clin Electrophysiol. 2002;25:484–98. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, McCulloch C, Mangat I, Foster E, De Marco T and Saxon LA. Isolated bundle branch block and left ventricular dysfunction. J Card Fail. 2003;9:87–92. [DOI] [PubMed] [Google Scholar]
- 6.Schneider JF, Thomas HE Jr., Sorlie P, Kreger BE, McNamara PM and Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol. 1981;47:931–40. [DOI] [PubMed] [Google Scholar]
- 7.Potfay J, Kaszala K, Tan AY, Sima AP, Gorcsan J 3rd, Ellenbogen KA and Huizar JF. Abnormal Left Ventricular Mechanics of Ventricular Ectopic Beats: Insights Into Origin and Coupling Interval in Premature Ventricular Contraction-Induced Cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8:1194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirk JA and Kass DA. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ Res. 2013;113:765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auffret V, Martins RP, Daubert C, Leclercq C, Le Breton H, Mabo P and Donal E. Idiopathic/Iatrogenic Left Bundle Branch Block-Induced Reversible Left Ventricle Dysfunction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:3177–3188. [DOI] [PubMed] [Google Scholar]
- 10.Kaszala K and Ellenbogen KA. When right may not be right: right bundle-branch block and response to cardiac resynchronization therapy. Circulation. 2010;122:1999–2001. [DOI] [PubMed] [Google Scholar]
- 11.Spragg DD, Akar FG, Helm RH, Tunin RS, Tomaselli GF and Kass DA. Abnormal conduction and repolarization in late-activated myocardium of dyssynchronously contracting hearts. Cardiovasc Res. 2005;67:77–86. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed M, Gorcsan J 3rd, Marek J, Ryo K, Haugaa K, D RL and Schwartzman D. Right ventricular apical pacing-induced left ventricular dyssynchrony is associated with a subsequent decline in ejection fraction. Heart Rhythm. 2014;11:602–8. [DOI] [PubMed] [Google Scholar]
- 13.Huizar JF, Tan AY, Kaszala K and Ellenbogen KA. Clinical and translational insights on premature ventricular contractions and PVC-induced cardiomyopathy. Prog Cardiovasc Dis. 2021;66:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowlgi GN, Tan AY, Kaszala K, Kontos MC, Lozano P, Ellenbogen KA and Huizar JF. Left ventricular dyssynchrony as marker of early dysfunction in premature ventricular contraction-induced cardiomyopathy. Front Cardiovasc Med. 2022;9:978341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neri G, Zanco P, Zanon F and Buchberger R. Effect of biventricular pacing on metabolism and perfusion in patients affected by dilated cardiomyopathy and left bundle branch block: evaluation by positron emission tomography. Europace. 2003;5:111–5. [DOI] [PubMed] [Google Scholar]
- 16.Koepfli P, Wyss CA, Gaemperli O, Siegrist PT, Klainguti M, Schepis T, Namdar M, Bechir M, Hoefflinghaus T, Duru F and Kaufmann PA. Left bundle branch block causes relative but not absolute septal underperfusion during exercise. Eur Heart J. 2009;30:2993–9. [DOI] [PubMed] [Google Scholar]
- 17.Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF and Kass DA. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003;108:929–32. [DOI] [PubMed] [Google Scholar]
- 18.Vanderheyden M, Mullens W, Delrue L, Goethals M, de Bruyne B, Wijns W, Geelen P, Verstreken S, Wellens F and Bartunek J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51:129–36. [DOI] [PubMed] [Google Scholar]
- 19.Blythe NM, Muraki K, Ludlow MJ, Stylianidis V, Gilbert HTJ, Evans EL, Cuthbertson K, Foster R, Swift J, Li J, Drinkhill MJ, van Nieuwenhoven FA, Porter KE, Beech DJ and Turner NA. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J Biol Chem. 2019;294:17395–17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE and Frankel DS. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11:1619–25. [DOI] [PubMed] [Google Scholar]
- 21.Cho SW, Gwag HB, Hwang JK, Chun KJ, Park KM, On YK, Kim JS and Park SJ. Clinical features, predictors, and long-term prognosis of pacing-induced cardiomyopathy. Eur J Heart Fail. 2019;21:643–651. [DOI] [PubMed] [Google Scholar]
- 22.Bansal R, Parakh N, Gupta A, Juneja R, Naik N, Yadav R, Sharma G, Roy A, Verma SK and Bahl VK. Incidence and predictors of pacemaker-induced cardiomyopathy with comparison between apical and non-apical right ventricular pacing sites. J Interv Card Electrophysiol. 2019;56:63–70. [DOI] [PubMed] [Google Scholar]
- 23.Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, Kanj M, Wazni OM, Saliba WI, Varma N, Wilkoff BL and Cantillon DJ. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272–2278. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg JS, Fischer A, Wang P, Schuger C, Daubert J, McNitt S, Andrews M, Brown M, Hall WJ, Zareba W, Moss AJ and Investigators MI. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol. 2005;16:359–65. [DOI] [PubMed] [Google Scholar]
- 25.Khurshid S, Obeng-Gyimah E, Supple GE, Schaller R, Lin D, Owens AT, Epstein AE, Dixit S, Marchlinski FE and Frankel DS. Reversal of Pacing-Induced Cardiomyopathy Following Cardiac Resynchronization Therapy. JACC Clin Electrophysiol. 2018;4:168–177. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Yin Y, Lan X, Liu Z, Ling Z, Su L, Kiuchi MG, Li X, Zhong B, Krucoff MW and group PR-HFsi. Paced QRS duration as a predictor for clinical heart failure events during right ventricular apical pacing in patients with idiopathic complete atrioventricular block: results from an observational cohort study (PREDICT-HF). Eur J Heart Fail. 2013;15:352–9. [DOI] [PubMed] [Google Scholar]
- 27.Lee SA, Cha MJ, Cho Y, Oh IY, Choi EK and Oh S. Paced QRS duration and myocardial scar amount: predictors of long-term outcome of right ventricular apical pacing. Heart Vessels. 2016;31:1131–9. [DOI] [PubMed] [Google Scholar]
- 28.Chin JY, Kang KW, Park SH, Choi YJ, Jung KT, Lee S and Youn HJ. Pre-implant global longitudinal strain as an early sign of pacing-induced cardiomyopathy in patients with complete atrioventricular block. Echocardiography. 2021;38:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma S, Barot HV, Schwartzman AD, Ganatra S, Shah SP, Venesy DM and Patten RD. Risk and predictors of dyssynchrony cardiomyopathy in left bundle branch block with preserved left ventricular ejection fraction. Clin Cardiol. 2020;43:1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponnusamy SS and Vijayaraman P. Left Bundle Branch Block-Induced Cardiomyopathy: Insights From Left Bundle Branch Pacing. JACC Clin Electrophysiol. 2021;7:1155–1165. [DOI] [PubMed] [Google Scholar]
- 31.Vaillant C, Martins RP, Donal E, Leclercq C, Thebault C, Behar N, Mabo P and Daubert JC. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol. 2013;61:1089–95. [DOI] [PubMed] [Google Scholar]
- 32.Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, Campana C, Perini G, Deorsola A, Masotti G, Tavazzi L, Maggioni AP and Italian Network on Congestive Heart Failure I. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398–405. [DOI] [PubMed] [Google Scholar]
- 33.Sze E, Samad Z, Dunning A, Campbell KB, Loring Z, Atwater BD, Chiswell K, Kisslo JA, Velazquez EJ and Daubert JP. Impaired Recovery of Left Ventricular Function in Patients With Cardiomyopathy and Left Bundle Branch Block. J Am Coll Cardiol. 2018;71:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witt CM, Wu G, Yang D, Hodge DO, Roger VL and Cha YM. Outcomes With Left Bundle Branch Block and Mildly to Moderately Reduced Left Ventricular Function. JACC Heart Fail. 2016;4:897–903. [DOI] [PubMed] [Google Scholar]
- 35.Guo B, Dai C, Li Q, Li W and Han L. Hazards of ventricular pre-excitation to left ventricular systolic function and ventricular wall motion in children: analysis of 25 cases. Cardiol Young. 2019;29:380–388. [DOI] [PubMed] [Google Scholar]
- 36.Ko J. Left ventricular dysfunction and dilated cardiomyopathy in infants and children with wolff-Parkinson-white syndrome in the absence of tachyarrhythmias. Korean Circ J. 2012;42:803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Li XM, Jiang H, Cui J, Ge HY, Liu HJ and Li MT. Association between severity of cardiac dysfunction caused by ventricular pre-excitation-led dyssynchrony and cardiac function recovery after ablation in children. J Cardiovasc Electrophysiol. 2020;31:1740–1748. [DOI] [PubMed] [Google Scholar]
- 38.Huizar JF. Pacemaker Timing Cycles and Special Features. In: Ellenbogen KA and Kaszala K, eds. Cardiac pacing and ICDs. Seventh edition. ed. Hoboken, NJ: John Wiley & Sons,; 2020: 1 online resource. [Google Scholar]
- 39.Ellenbogen KA and Huizar JF. Foreseeing super-response to cardiac resynchronization therapy: a perspective for clinicians. J Am Coll Cardiol. 2012;59:2374–7. [DOI] [PubMed] [Google Scholar]
- 40.Yu CM, Chan JY, Zhang Q, Omar R, Yip GW, Hussin A, Fang F, Lam KH, Chan HC and Fung JW. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123–34. [DOI] [PubMed] [Google Scholar]
- 41.Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A and Varosy PD. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:e51–e156. [DOI] [PubMed] [Google Scholar]
- 42.Blanc JJ, Fatemi M, Bertault V, Baraket F and Etienne Y. Evaluation of left bundle branch block as a reversible cause of non-ischaemic dilated cardiomyopathy with severe heart failure. A new concept of left ventricular dyssynchrony-induced cardiomyopathy. Europace. 2005;7:604–10. [DOI] [PubMed] [Google Scholar]
- 43.Vijayaraman P, Zalavadia D, Haseeb A, Dye C, Madan N, Skeete JR, Vipparthy SC, Young W, Ravi V, Rajakumar C, Pokharel P, Larsen T, Huang HD, Storm RH, Oren JW, Batul SA, Trohman RG, Subzposh FA and Sharma PS. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm. 2022;19:1263–1271. [DOI] [PubMed] [Google Scholar]
- 44.Vijayaraman P, Herweg B, Dandamudi G, Mittal S, Bhatt AG, Marcantoni L, Naperkowski A, Sharma PS and Zanon F. Outcomes of His-bundle pacing upgrade after long-term right ventricular pacing and/or pacing-induced cardiomyopathy: Insights into disease progression. Heart Rhythm. 2019;16:1554–1561. [DOI] [PubMed] [Google Scholar]
- 45.Teigeler T, Kolominsky J, Vo C, Shepard RK, Kalahasty G, Kron J, Huizar JF, Kaszala K, Tan AY, Koneru JN, Ellenbogen KA and Padala SK. Intermediate-term performance and safety of His-bundle pacing leads: A single-center experience. Heart Rhythm. 2021;18:743–749. [DOI] [PubMed] [Google Scholar]
- 46.Hua J, Wang C, Kong Q, Zhang Y, Wang Q, Xiong Z, Hu J, Li J, Chen Q and Hong K. Comparative effects of left bundle branch area pacing, His bundle pacing, biventricular pacing in patients requiring cardiac resynchronization therapy: A network meta-analysis. Clin Cardiol. 2022;45:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekine M, Masutani S, Imamura T, Iwamoto Y, Muraji S, Yoshiba S, Ishido H and Sumitomo N. Improvement in Dyssynchrony with Pharmacological Ablation of Right-Sided Accessory Pathway-Induced Cardiomyopathy in Infants. Int Heart J. 2019;60:1201–1205. [DOI] [PubMed] [Google Scholar]
- 48.Lumens J, Willemen E and Prinzen FW. Does the Right Go Wrong During Cardiac Resynchronization Therapy? JACC Cardiovasc Imaging. 2020;13:1485–1488. [DOI] [PubMed] [Google Scholar]


