Abstract
A family of mutants overexpressing the Serratia marcescens extracellular nuclease has been known for decades. A number of these alleles are characterized here at the molecular level, and the mutant genes are identified, yielding a likely model for their phenotype. The known mutations exert their effect indirectly on nucA expression by elevating the basal SOS response of the cell. Mutations have been found in xerC and uvrD, both of which result in partial SOS induction. A classic nucsu allele, that of strain W1050, is also likely to be in xerC.
The production of extracellular nuclease is one of the defining attributes of Serratia marcescens and other Serratia species. However, nuclease is only one of a number of extracellular proteins expressed by species of Serratia; others include proteases, chitinases, lipases, and a bacteriocin called marcescin. Unlike these other secreted proteins, the extracellular nuclease does not appear to be subject to substrate or catabolite regulation. Nuclease is instead regulated by growth phase (4, 16), as is the synthesis of many other extracytoplasmic degradatory proteins, toxins, and bacteriocins (1, 8, 10, 11).
Many of the extracellular proteins of Serratia nevertheless share some common regulatory pathways with nuclease, and pleiotropic regulatory mutants that exert effects on nuclease, lipase, chitinase, and marcescin have been identified (4, 12, 17, 18). One such mutant, which abolishes nuclease production and reduces chitinase and lipase activity, has been shown to be in the Serratia recA gene (2). Consistent with SOS control, a LexA binding site is located in the nuclease gene nucA promoter region (4). SOS regulation has also been shown for other extracellular proteins, such as the pectin lyase of Erwinia carotovora (14). Other alleles abolishing nuclease as well as bacteriocin production define a novel regulatory locus, nucC, which is closely related to the ogr gene of temperate phage P2 (7, 13). The nucC operon is also growth phase and SOS regulated independently of nucA.
Yet another class of pleiotropic mutants overexpresses these proteins (4, 17, 18). These overexpression mutants (called nucsu [17]) display no noticeable growth defect. They remain SOS inducible and are growth phase regulated. These alleles appear to fall into at least two categories based on their relative levels of nuclease overexpression. However, the nature of these mutations and the genes they represent have previously not been determined. The molecular characterization of these mutants is the focus of this work.
Mutant description.
We previously described (4, 12) the isolation of transposon Tn5 insertion mutations made in the wild-type S. marcescens strain SM6 that displayed altered nuclease halo sizes on DNase indicator plates. Among these were mutations that abolish expression of the nuclease (nucA) gene; these mutations map to the transcriptional activator gene nucC (13) as well as to recA. Other mutations displaying increased nuclease halo sizes were also described previously (4); these include mutants nucsu93 and nucsu95, which express nearly 50-fold more nuclease than SM6, and nucsu161, which expresses about 10-fold as much nuclease as SM6. Similar mutants had previously been described, and one of these, W1050 (U. Winkler), which we use routinely, is derived from SM6(F′ prolac) and carries a chemically induced pleiotropic mutation causing the overexpression of nuclease as well as other extracellular enzymes (17, 18).
As a preliminary characterization, Southern blot analysis of the mutants was performed after digestion of mutant strain genomic DNA with EcoRI (EcoRI does not cut in Tn5) and probed with a radiolabeled 3.3-kb HindIII fragment from Tn5. This analysis (data not presented) showed that the insertions of nucsu93 and nucsu95 were on the same-size fragment of about 25 kb and that the nucsu161 insertion was on a 9.5-kb fragment. The kanamycin resistance determinant of Tn5 allowed easy cloning of fragments spanning Tn5 from each of these mutants.
DNA sequence analysis.
Sequence data obtained with a primer to the end of Tn5 revealed that two of the mutants, nucsu93 and nucsu95, indeed have Tn5 insertions in the same gene and at essentially the same site. This gene has significant sequence identity to Escherichia coli xerC (5). XerC is a subunit of the XerCD site-specific recombinase that serves to monomerize chromosomes and plasmids prior to cell division (3). The DNA flanking the insertion in nucsu161 shows significant sequence identity to the E. coli uvrD gene. UvrD is DNA helicase II, which unwinds double-stranded DNA and is important in DNA replication, recombination, and repair (9). In E. coli, the xerC and uvrD genes are closely linked.
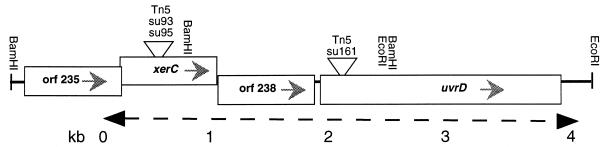
The entire region of interest was recovered as a single λ clone from a genomic SM6 λ library by probing the library with a fragment flanking the Tn5 insertion from nucsu161. Three fragments from this λ clone were subcloned, and from them, the nucleotide sequence of xerC and uvrD was determined from both strands by ABI Prizm DyeTerminator chemistry and run on the ABI 377 automated sequencer. The map of the region is shown in Fig. 1.
FIG. 1.
Map of S. marcescens xerC-uvrD region. The gene order is identical to that of E. coli, and reading frames are oriented as indicated by the gray arrowheads. Sites of Tn5 insertions are marked by inverted triangles; restriction sites are as shown. The region which has been fully sequenced is indicated by the dashed arrow with kilobase markers shown below the line.
The DNA sequence is highly similar to the E. coli sequence (5) within the open reading frames but is highly divergent outside of the reading frames. There are four open reading frames found in this region, but for one of these (Orf235), we have the sequence for only the carboxyl terminus. XerC has 74% identity to E. coli XerC, Orf238 has 65% amino acid identity to E. coli Orf238, and UvrD has 88% identity to E. coli UvrD. The predicted proteins are identical in size, except that XerC has an additional four amino acids at its start and one amino acid at its end.
Measuring SOS induction.
Why do Tn5 insertions in xerC and uvrD lead to nuclease overexpression? The simplest explanation is that these mutants are partially induced for their SOS response, resulting in induction of nucA transcription, since the nucA promoter is SOS inducible (4). E. coli strains with mutations in either xerC or uvrD show partial SOS induction (15a).
To test this, a recA-lacZ transcriptional fusion on the plasmid pMB684 was used to monitor SOS induction in the S. marcescens strains. Cultures were grown aerobically in Luria broth with 1 mg of ampicillin per ml at 30°C. In Table 1, the β-galactosidase activities of each strain harboring the tester plasmid are presented. All of the nucsu mutants show a large increase in β-galactosidase activity compared to the wild-type SM6. W1050 is lacZ+, which prevented us from accurately measuring SOS induction in this strain; however, its induction ratio is comparable to those of the strongest mutants (4). Another phenotype of SOS-induced cells is the formation of long filamentous cells. The nucsu mutants and W1050 were all examined by phase-contrast light microscopy. All strains had a number of filamentous cells that were abnormally long compared to those of the SM6 control strain.
TABLE 1.
Expression from the recA and nucA promoters
| Strainc | β-Gal activitya of pMB684d | Nuclease activityb
|
||
|---|---|---|---|---|
| No plasmid | pSD105e | pET11d-H2wtf | ||
| SM6 | 320 (10) | 1 | 1 | 0.7 |
| W1050 | NDg | 80 | 1 | 40 |
| MB984nucsu93 | 920 (40) | 70 | 1 | NDg |
| MB986nucsu95 | 1,090 (50) | 70 | 1 | NDg |
| MB1454nucsu161 | 820 (90) | 30 | 30 | 0.8 |
β-Galactosidase activity was measured according to the Miller (15) procedure, and the average of the activities from three independent stationary-phase cultures is shown along with the standard deviation in parentheses.
Nuclease activity was measured from the cell-free supernatant of an overnight culture by the microtiter dish assay (2). The assay determines the dilution factor required to show no loss of fluorescence by using DNA in the presence of ethidium bromide as substrate. The values, normalized to those of the wild-type strain SM6, represent the averages of triplicate measurements.
Wild type and nucsu mutants containing plasmids were grown in Luria broth plus 1 mg of ampicillin per ml overnight.
pMB684 Φ(recA-trp′CBA lacZYA).
Plasmid pSD105 carries the E. coli xerC gene.
Plasmid pET11d-H2wt carries the E. coli uvrD gene.
ND, not done. Strain W1050 was already Lac+ and we were unable to transform strains MB984nucsu93 and MB986nucsu95.
Suppression of the nuclease overexpression phenotype.
Since S. marcescens xerC and uvrD have a high degree of similarity to their E. coli homologs, we tested whether the E. coli genes carried on multicopy plasmids could suppress the phenotype. Plasmid pSD105 carrying E. coli xerC and pET11d-H2wt carrying E. coli uvrD (9) were transformed into the different mutant and control strains. In Table 1, nuclease activity produced by these transformants is shown. SM6 containing these plasmids had no change in nuclease activity, whereas nucsu93, nucsu95, and W1050 containing pSD105 now produced wild-type levels of nuclease. Plasmid pET11d-H2wt had no effect on nuclease activity of strain W1050. From this, we conclude that the W1050 mutation is also in xerC. We were unable to obtain stable transformants of nucsu93 or nucsu95 with this plasmid. The nucsu161 mutant containing pET11d-H2wt also produced wild-type nuclease levels, but pSD105 had no effect in this strain. These data show that the E. coli homologs specifically suppress the nuclease overexpression phenotype; therefore, the nuclease overexpression phenotype is in fact due to the Tn5 insertion mutations and not to a secondary mutation elsewhere.
Conclusions.
In this work, we demonstrate that most nuclease overexpression mutants (nucsu) are likely due to an indirect effect on transcription of nucA by the SOS system. The mutant phenotype is a result of partial induction of the SOS system caused by the mutation. Here we have sequenced two genes in which such mutations have been identified, xerC and uvrD. These genes are very similar to their E. coli homologs (5), and complementation data show that the E. coli genes are able to functionally replace the defective S. marcescens genes. The ability of E. coli XerC to repress the nuclease overexpression in W1050 suggests that it is also a xerC mutant.
We had previously suggested that these mutations may represent direct or indirect repressors of nucA expression (4, 13). Here we have demonstrated that this not correct. The nucA gene is regulated positively by the Ogr homolog NucC and negatively by LexA. Although growth phase clearly plays a role in the temporal regulation of nuclease production (16), likely due to accumulation of an extracellular factor (16a) like a homoserine lactone analog (6, 11), we do not know whether the regulatory factors responsible for this act directly at the nucA promoter, through NucC, or through the SOS system.
Nucleotide sequence accession number.
The DNA sequence has been deposited in GenBank (accession no. AF028736).
Acknowledgments
We thank David Sherratt for providing pSD105 and Steven Matson for pET11d-H2wt, Susan Hardin and Leslie Jones for the use of and assistance with their ABI 377 automated sequencer, and Ulrich Strych for many helpful discussions.
This work was supported by the Welch Foundation and grants from the Texas Advanced Research Program.
REFERENCES
- 1.Atlung T, Nielsen A, Hansen F G. Isolation, characterization, and nucleotide sequence of appY, a regulatory gene for growth-phase-dependent gene expression in Escherichia coli. J Bacteriol. 1989;171:1683–1691. doi: 10.1128/jb.171.3.1683-1691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball T K, Wasmuth C R, Braunagel S C, Benedik M J. Expression of Serratia marcescens extracellular proteins requires recA. J Bacteriol. 1990;172:342–349. doi: 10.1128/jb.172.1.342-349.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakely G, Colloms S, May G, Burke M, Sherratt D J. Escherichia coli XerC recombinase is required for chromosomal segregation at cell division. New Biol. 1991;3:789–798. [PubMed] [Google Scholar]
- 4.Chen Y C, Shipley G L, Ball T K, Benedik M J. Regulatory mutants and transcriptional control of the Serratia marcescens extracellular nuclease gene. Mol Microbiol. 1992;6:643–651. doi: 10.1111/j.1365-2958.1992.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 5.Colloms S D, Sykora P, Szatmari G, Sherratt D J. Recombination at ColE1 cer requires the Escherichia coli xerC gene product, a member of the lambda integrase family of site-specific recombinases. J Bacteriol. 1990;172:6973–6980. doi: 10.1128/jb.172.12.6973-6980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberl L, Winson M K, Sternberg C, Stewart G S, Christiansen G, Chhabra S R, Bycroft B, Williams P, Molin S, Givskov M. Involvement of N-acyl-l-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrer S, Viejo M B, Guasch J F, Enfedaque J, Regue M. Genetic evidence for an activator required for induction of colicin-like bacteriocin 28b production in Serratia marcescens by DNA-damaging agents. J Bacteriol. 1996;178:951–960. doi: 10.1128/jb.178.4.951-960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genilloud O, Moreno F, Kolter R. DNA sequence, products, and transcriptional pattern of the genes involved in production of the DNA replication inhibitor microcin B17. J Bacteriol. 1989;171:1126–1135. doi: 10.1128/jb.171.2.1126-1135.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George J W, Brosh R M, Jr, Matson S W. A dominant negative allele of the Escherichia coli uvrD gene encoding DNA helicase II. J Mol Biol. 1994;235:424–435. doi: 10.1006/jmbi.1994.1003. [DOI] [PubMed] [Google Scholar]
- 10.Givskov M, Molin S. Expression of extracellular phospholipase from Serratia liquefaciens is growth-phase-dependent, catabolite-repressed and regulated by anaerobiosis. Mol Microbiol. 1992;6:1363–1374. doi: 10.1111/j.1365-2958.1992.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 11.Givskov M, Eberl L, Molin S. Control of exoenzyme production, motility and cell differentiation in Serratia liquefaciens. FEMS Microbiol Lett. 1997;148:115–122. [Google Scholar]
- 12.Hines D W, Saurugger P N, Ihler G M, Benedik M J. Genetic analysis of extracellular proteins of Serratia marcescens. J Bacteriol. 1988;170:4141–4146. doi: 10.1128/jb.170.9.4141-4146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin S, Chen Y C, Christie G E, Benedik M J. Regulation of the Serratia marcescens extracellular nuclease: positive control by a homolog of P2 Ogr encoded by a cryptic prophage. J Mol Biol. 1996;256:264–278. doi: 10.1006/jmbi.1996.0084. [DOI] [PubMed] [Google Scholar]
- 14.McEvoy J L, Murata H, Chatterjee A K. Genetic evidence for an activator required for induction of pectin lyase in Erwinia carotovora subsp. carotovora by DNA-damaging agents. J Bacteriol. 1992;174:5471–5474. doi: 10.1128/jb.174.16.5471-5474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 15a.Sherratt, D., and S. Matson. Personal communication.
- 16.Suh Y, Jin S, Ball T K, Benedik M J. Two-step secretion of extracellular nuclease in Serratia marcescens. J Bacteriol. 1996;178:3771–3778. doi: 10.1128/jb.178.13.3771-3778.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Suh, Y., and M. J. Benedik. Unpublished data.
- 17.Winkler U. Mutants of Serratia marcescens defective or superactive in the release of a nuclease. In: Wittman H G, Schuster H, editors. Molecular genetics. Berlin, Germany: Springer; 1968. pp. 187–201. [Google Scholar]
- 18.Winkler U, Timmis K. Pleiotropic mutations in Serratia marcescens which increase the synthesis of certain exocellular proteins and the rate of spontaneous prophage excision. Mol Gen Genet. 1973;124:197–206. doi: 10.1007/BF00293091. [DOI] [PubMed] [Google Scholar]