Abstract
Elevated and low blood pressure (BP) may lead to poor functional outcome after ischemic stroke, which is conflicting. Hence, there must be another factor—such as cerebral small vessel disease (cSVD) -interacting with BP and thus, affecting outcome. Here, we investigate the relationship between BP and cSVD regarding outcome after stroke. Data of 423/503 stroke patients were prospectively analyzed. Diastolic (DBP) and systolic BP (SBP) were collected on hospital admission (BPad) and over the first 72 h (BP72h). cSVD-burden was determined on MR-scans. Good functional outcome was defined as a modified Rankin Scale score ≤ 2 at hospital discharge and 12 months thereafter. cSVD was a predictor of poor outcome (OR 2.8; p < 0.001). SBPad, DBPad and SBP72h were not significantly associated with outcome at any time. A significant relationship was found between DBP72h, (p < 0.01), cSVD (p = 0.013) and outcome at discharge. At 12 months, we found a relationship between outcome and DBP72h (p = 0.018) and a statistical tendency regarding cSVD (p = 0.08). Changes in DBP72h were significantly related with outcome. There was a U-shaped relationship between DBP72h and outcome at discharge. Our results suggest an individualized stroke care by either lowering or elevating DBP depending on cSVD-burden in order to influence functional outcome.
Subject terms: Neurological disorders, Cerebrovascular disorders, Stroke, White matter disease
Introduction
Increased blood pressure (BP) is found in around 80% of all patients with acute ischemic stroke (AIS) on hospital admission as well as in the acute phase of AIS1. However, the impact of BP in the acute phase of AIS on functional outcome remains an matter of debate2–4. So far, there is some evidence that raised BP early after AIS is associated with dependency, clinical deterioration and subsequent death5. In contrast, some studies revealed that both low and high BP entails poor prognosis after AIS4,6. Further studies found no effect of BP in the acute phase of AIS on functional outcome7,8. Of note, some other studies even suggested that an increase of BP in the acute phase of AIS may be a protective response to reduced cerebral perfusion and thus, may enhance decreasing blood flow to the infarcted zone and surrounding penumbra9–11. Hence, these different and partially contradictory findings suggest that additional factors such as cerebral small vessel disease (cSVD) may interfere prognosis and clinical outcome after AIS. The term cSVD refers to the dysfunction of cerebral microvessel endothelium affecting cell–cell interactions and finally resulting in brain damage12–14. Clinically, cSVD may lead to stroke, cognitive dysfunction and worsening of gait as well as balance15. On brain MRI, cSVD presents with lacunes, white matter hyperintensities (WMH), cerebral microbleeds (CMB) and perivascular spaces12,15,16. In particular, WMH are the most common cSVD lesions that are observed on brain MR scans of most people aged over 70 years17. CMB also increase with age although they occur less frequent compared to WMH and are unusual in the absence of WMH13. In a recently published study on stroke patients undergoing endovascular treatment, no signs of cSVD was observed in 61%, mild to moderate and severe degree of cSVD was seen in 20.0% and 19.7%, respectively18. Hence the question arises whether patients with AIS and no cSVD profit e.g., from a lower BP than stroke patients with a moderate or severe degree of cSVD. Of note, autoregulation of cerebral blood flow is hampered in the acute phase of AIS making cerebral perfusion directly dependent on BP19–21. Additionally, patients with severe cSVD have an even more reduced cerebral blood flow and impaired cerebral autoregulation22,23.
With these considerations in mind, we aimed to investigate whether functional outcome in the short term (at discharge) and in the long term (12 months after cerebrovascular event) differs between stroke patients with a high and low burden of cSVD depending on the level of acute-phase systolic (SBP) and/or diastolic blood pressure (DBP). Additionally, we examined whether there is an interaction between BP in the acute phase and severity of cSVD regarding functional impairment, recurrent stroke/TIA and death 12 month after index event.
Methods
Study design and setting
We conducted a prospective single-center cohort study at the University Clinic Würzburg (Stroke Unit, Department of Neurology). From October 2016 to October 2017, all consecutive patients with an ischemic stroke or transient ischemic attack (TIA) admitted to the emergency room within 24 h after onset of symptoms were included in the study. Written informed consent from the patient or patients’ next of kin was obtained before enrolment. The study was approved by the local ethics committee of Würzburg (AZ 223/16). The study protocol adheres to the established standards for the reporting of observational studies24. Anonymous data will be shared by request.
Data collection
During hospitalization, data on demographic characteristics (age, sex), vascular risk factors (hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, myocardial infarction, atrial fibrillation, previous stroke or TIA, peripheral artery occlusive disease and current cigarette smoking), comorbidities (liver and kidney dysfunction), previous medication (antihypertensives, antiplatelets, anticoagulants, statins), previous degree of disability (according to modified Rankin scale (mRS)), recanalization therapy (intravenous thrombolysis; endovascular treatment), discharge medication (antihypertensives, antiplatelets, anticoagulants, statins) and stroke/TIA characteristics were collected prospectively in a database (Table 1). Stroke characteristics included (1) stroke severity on admission and at discharge assessed by the National Institutes of Health Stroke Scale (NIHSS) score25 and (2) stroke subtype classified according to the criteria of Trial of Org 10,172 in Acute Stroke Treatment (TOAST)26.
Table 1.
Baseline characteristics of stroke patients regarding outcome at discharge. mRS, modified Rankin Scale, SBP, systolic blood pressure; DBP, diastolic blood pressure, CMB, cerebral microbleeds, WML, white matter lesions; cSVD cerebral small vessel disease, NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack; SD, standard deviation, p-values calculated with chi2-test.
| All patients | Good outcome (mRS 0–2) | Poor outcome (mRS 3–6) | p-value | |
|---|---|---|---|---|
| n | 423 | 311 | 112 | |
| Demographics | ||||
| Age, median (Q1-Q3), y, n = 423 | 73 (61–79) | 70 (59–79) | 78 (65.8–83) | < 0.0011 |
| Female, n = 423 (%) | 184 (43.5%) | 127 (40.8%) | 57 (50.9%) | 0.084 |
| Vascular risk factors, n (%) | ||||
| Hypertension, n = 422 | 359 (85.1) | 256 (82.3) | 103 (92.8) | 0.012 |
| Newly diagnosed hypertension, n = 422 | 31 (7.35) | 22 (7.07) | 9 (8.11) | 0.883 |
| Atrial fibrillation, n = 422 | 114 (27.0) | 72 (23.2) | 42 (37.8) | 0.004 |
| Current Smoking, n = 422 | 74 (17.5) | 58 (18.6) | 16 (14.4) | 0.389 |
| Hypercholesterolemia, n = 422 | 238 (56.4) | 180 (57.9) | 58 (52.3) | 0.360 |
| Diabetes mellitus, n = 422 | 97 (23.0) | 61 (19.6) | 36 (32.4) | 0.009 |
| Coronary heart disease, n = 422 | 56 (13.3) | 32 (10.3) | 24 (21.6) | 0.004 |
| Myocardial Infarction, n = 422 | 23 (5.45) | 13 (4.18) | 10 (9.01) | 0.093 |
| Prior stroke or TIA, n = 422 | 103 (24.4) | 69 (22.2) | 34 (30.6) | 0.099 |
| Positive Family history, n = 422 | 101 (23.9) | 76 (24.4) | 25 (22.5) | 0.782 |
| Peripheral artery occlusive disease, n = 422 | 23 (5.45) | 11 (3.54) | 12 (10.8) | 0.008 |
| Kidney dysfunction, n = 422 | 135 (32.0) | 86 (27.7) | 49 (44.1) | 0.002 |
| Liver dysfunction, n = 422 | 7 (1.66) | 2 (0.64) | 5 (4.5) | 0.015 |
| Clinical data | ||||
| NIHSS at entry, median (Q1-Q3), n = 423 | 3 (1–6) | 2 (0–4) | 7 (4–12.2) | < 0.0011 |
| NIHSS at discharge, median (Q1-Q3), n = 423 | 1 (0–3) | 0 (0–1) | 4 (2–7) | < 0.0011 |
| mRS at entry, mean ± SD, n = 423 | 2.37 ± 1.58 | 1.77 ± 1.35 | 4.0 ± 0.76 | < 0.001 |
| mRS at discharge, mean ± SD, n = 423 | 1.44 ± 1.43 | 0.71 ± 0.81 | 3.45 ± 0.6 | < 0.001 |
| Recanalization therapy, n (%) | ||||
| Intravenous thrombolysis, n = 423 | 91 (21.5) | 54 (17.4) | 37 (33.0) | < 0.001 |
| Endovascular treatment, n = 423 | 37 (8.75) | 15 (4.82) | 22 (19.6) | < 0.001 |
| MRI Findings, n (%) | ||||
| Lacunes, n = 412 | 172 (41.7) | 119 (39.0) | 53 (49.5) | 0.074 |
| WML (Fazekas score 2–3) , n = 414 | 228 (55.1) | 154 (50.5) | 74 (67.9) | 0.003 |
| CMB, n = 419 | 113 (27.0) | 76 (24.6) | 37 (33.6) | 0.087 |
| cSVD (CMB a./o.WML) , n = 419 | 259 (61.8) | 173 (56.0) | 86 (78.2) | < 0.001 |
| Stroke etiology, n = 423, n (%) | ||||
| Large artery disease | 79 (18.7) | 59 (19.0) | 20 (17.9) | 0.115 |
| Cardioembolism | 160 (37.8) | 107 (34.4) | 53 (47.3) | 0.221 |
| Small vessel occlusion | 30 (7.09) | 23 (7.40) | 7 (6.25) | 0.849 |
| Other determined | 9 (2.13) | 6 (1.93) | 3 (2.68) | 0.609 |
| Undetermined | 145 (34.3) | 116 (37.3) | 29 (25.9) | 0.364 |
1Calculated with Mann–Whitney-U-test.
All patients underwent brain MRI within 72 h after admission to identify acute ischemic stroke or TIA. WMH were categorized visually on fluid-attenuated inversion recovery (FLAIR) scans using the Fazekas scale and rated as mild (Fazekas 0–1) or severe (Fazekas 2–3)27. CMB were defined as small, circular or rounded, hypointense lesions within brain parenchyma ranging from 2 to 10 mm in size28. Assessment of CMB was performed on T2*-weighted gradient-recalled echo (T2*-GRE) scans or on susceptibility-weighted images (SWI). Lacunes were defined as CSF-filled cavities with a diameter of at least 3 mm29. cSVD burden was visually rated on MR scans and divided in absent/mild cSVD (Fazekas 0–1, no CMB) and moderate/severe cSVD (Fazekas 2–3 and/or ≥ 1 CMB).
Blood pressure (BP) was measured on the non-paralytic arm in the supine position by doctors or trained nurses using a noninvasive BP monitoring device according to our in-hospital guidelines for acute stroke management. BP values obtained on patients’ admission and during their stay in the stroke unit were entered in the electronic health record system. From this health record system, we retrieved the very first SBP and DBP value of each patient at the time of admission (i.e., BP at entry). SBP and DBP values measured every 8 h during the first three days after hospital admission were also extracted from the electronic health record system. To obtain the mean SBP and DBP value for each of these three days, the three SBP and DBP values of day 1, 2 and 3 respectively, were averaged. Finally, the mean values of each day were averaged resulting in the mean SBP and DBP value over the first 72 h. In addition, patients with known and treated hypertension continued antihypertensive treatment, unless BP was below 120/70 mmHg (intern guidelines). When SBP at the stroke unit was > 160 mmHg, antihypertensive treatment was adjusted accordingly and BP was measured every 15 min., until SBP was below 160 mmHg. Patients without antihypertensive treatment until admission received angiotensin-converting enzyme inhibitor as the drug of first choice when SBP was > 160 mmHg. The number of patients with newly diagnosed arterial hypertension on hospital was 7.35%. Change in SBP or DBP over the time was calculated as SBP or DBP at time point—SBP or DBP at baseline (i.e. at hospital admission)21.
In order to evaluate functional outcome of TIA and stroke patients, the mRS score was assessed prospectively on discharge (outcomedisc) and at 12 months after cerebrovascular event (outcome12mo) through a structured follow-up telephone interview by a trained stroke fellow (S.G.). During the interview, current medication and BP were collected. The mRS score on discharge as well as at 12 months was dichotomized into good (mRS score 0–2) and poor outcome (mRS score of 3–6)30.
Statistical analysis
The baseline data of all patients are demonstrated as mean ± SD or median (first quartile, Q1; third quartile, Q3) for continuous variables or frequency (percentage) for categorical variables. First, study parameters were compared in a descriptive manner regarding good and poor functional outcome at discharge and at 12 months after stroke. Metric or ordinal variables have been compared with unpaired t-test, or Mann–Whitney-U-test as appropriate. For dichotomous traits like hypertension or diabetes mellitus, Chi-square-test was used. Second, multivariable logistic regression was used to estimate the influence of the different study variables in respect to functional outcome at discharge and at 12 months thereafter. Multivariable logistic regression was adjusted for age and sex as well as for each selected parameter (i.e., cSVD, mean DBP over 72 h). DBP and age were modelled in a non-linear way using a 3-knot restricted cubic spline, as described in detail elsewhere31. Results of the regression analysis were depicted as odds ratios (OR) and 95% confidence interval (95%CI). Possible interactions between selected variables were calculated using Likelihood-ratio Chi-square-tests. P < 0.05 was regarded statistically significant. Statistical analyses were performed using R version 4.0.4 (R: a Language and Environment for Statistical Computing; Vienna, Austria, 2021-03-15).
Results
Study population and baseline characteristics
A total of 503 patients with either ischemic stroke or TIA were screened for this study. Out of them, full data set was available for 423 patients at discharge (stroke-patients 76% (n = 322); TIA-patients 24% (n = 101)), and 369 patients completed follow-up 12 months after event (stroke-patients 75% (n = 276); TIA-patients 25% (n = 93)) (Fig. 1). The baseline characteristics for the final cohort are summarized in Tables 1 and 2.
Figure 1.
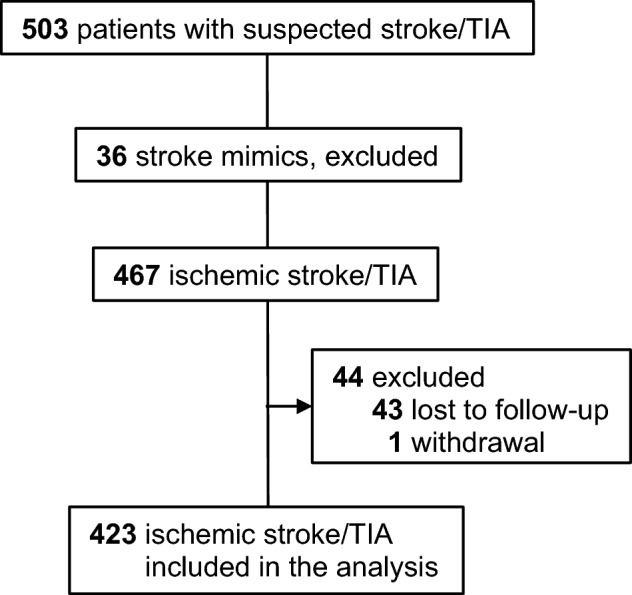
Flowchart of study participants.
Table 2.
Baseline characteristics of stroke patients regarding outcome at 12 months. mRS, modified Rankin Scale, SBP, systolic blood pressure; DBP, diastolic blood pressure, CMB, cerebral microbleeds, WML, white matter lesions; cSVD, cerebral small vessel disease, NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack; SD, standard deviation, p-values calculated with chi2-test.
| All patients | Good outcome (mRS 0–2) | Poor outcome (mRS 3–6) | p-value | |
|---|---|---|---|---|
| n | 369 | 261 | 108 | |
| Demographics | ||||
| Age, median (Q1-Q3), n = 369, y | 72 (60–79) | 68 (58–77) | 78 (69–83) | < 0.0011 |
| Female, n = 369, n (%) | 157 (42.5%) | 104 (39.8%) | 53 (49.1%) | 0.106 |
| Vascular risk factors, n (%) | ||||
| Hypertension, n = 369 | 310 (84.0) | 214 (82.0) | 96 (88.9) | 0.137 |
| Newly diagnosed hypertension, n = 369 | 25 (6.78) | 20 (7.66) | 5 (4.63) | 0.408 |
| Atrial fibrillation, n = 369 | 97 (26.3) | 50 (19.2) | 47 (43.5) | < 0.001 |
| Current Smoking, n = 369 | 66 (17.9) | 53 (20.3) | 13 (12.0) | 0.082 |
| Hypercholesterolemia, n = 369 | 211 (57.2) | 154 (59.0) | 57 (52.8) | 0.325 |
| Diabetes mellitus, n = 369 | 86 (23.3) | 51 (19.5) | 35 (32.4) | 0.012 |
| Coronary heart disease, n = 369 | 50 (13.6) | 28 (10.7) | 22 (20.4) | 0.022 |
| Myocardial Infarction, n = 369 | 20 (5.42) | 10 (3.83) | 10 (9.26) | 0.065 |
| Prior stroke or TIA, n = 369 | 93 (25.2) | 55 (21.1) | 38 (35.2) | 0.007 |
| Positive Family history, n = 369 | 93 (25.2) | 69 (26.4) | 24 (22.2) | 0.474 |
| Peripheral artery occlusive disease, n = 369 | 23 (6.23) | 7 (2.68) | 16 (14.8) | < 0.001 |
| Kidney dysfunction, n = 369 | 115 (31.2) | 76 (29.1) | 39 (36.1) | 0.232 |
| Liver dysfunction, n = 369 | 5 (1.36) | 2 (0.77) | 3 (2.78) | 0.152 |
| Clinical data | ||||
| NIHSS at entry, median (Q1-Q3) , n = 369 | 2 (1–5) | 2 (0–4) | 5 (2–9) | < 0.0011 |
| NIHSS at discharge, median (Q1-Q3), n = 369 | 1 (0–3) | 0 (0–2) | 2 (0–6) | < 0.0011 |
| mRS at entry, mean ± SD, n = 369 | 2.31 ± 1.58 | 1.87 ± 1.45 | 3.37 ± 1.38 | < 0.001 |
| mRS at discharge, mean ± SD, n = 369 | 1.38 ± 1.41 | 0.87 ± 1.06 | 2.61 ± 1.39 | < 0.001 |
| Recanalization therapy, n (%) | ||||
| Intravenous thrombolysis, n = 369 | 74 (20.1) | 49 (18.8) | 25 (23.1) | 0.417 |
| Endovascular treatment, n = 369 | 29 (7.86) | 16 (6.13) | 13 (12.0) | 0.088 |
| MRI Findings, n (%) | ||||
| Lacunes, n = 361 | 153 (42.4) | 97 (37.7) | 56 (53.8) | < 0.001 |
| WML (Fazekas score 2–3), n = 368 | 200 (54.9) | 126 (48.8) | 74 (69.8) | 0.003 |
| CMB, n = 368 | 103 (28.0) | 68 (26.1) | 35 (32.7) | 0.244 |
| cSVD (CMB a./o.WML) , n = 368 | 229 (62.2) | 145 (55.6) | 84 (78.5) | < 0.001 |
| Stroke etiology, n (%), n = 369 | ||||
| Large artery disease | 70 (19.0) | 49 (18.8) | 21 (19.4) | 0.037 |
| Cardioembolism | 137 (37.1) | 86 (33.0) | 51 (47.2) | 0.307 |
| Small vessel occlusion | 29 (7.86) | 22 (8.43) | 7 (6.48) | 0.574 |
| Other determined | 7 (1.90) | 4 (1.53) | 3 (2.78) | 0.508 |
| undetermined | 126 (34.1) | 100 (38.3) | 26 (24.1) | 0.3149 |
| Death within 1 year, n (%), n = 368 | 28 (7.61) | 0 | 28 (25.9%) | < 0.001 |
| Recurrent stroke within 1 year, n (%), n = 368 | 42 (11.4) | 25 (9.58) | 17 (15.9) | 0.122 |
1Calculated with Mann–Whitney-U-test.
Early functional outcomes at discharge
At hospital discharge, 311 (73.5%) patients had a good clinical outcome and 112 (26.5%) a poor outcome (Table 1). WMH (Fazekas score 2–3) occurred significantly more often in patients with a poor outcome (74 (67.9%) vs 154 (50.5%), p < 0.001), whereas CMB did not (37 (33.6% vs 76 (24.6%), p = 0.087). Previous lacunes were more common in patients with a poor outcome than in those with a good outcome at hospital discharge, however there was no significant difference between these two groups (119 (39%) vs 53 (49.5%), p = 0.07). When comparing the burden of cSVD among these two groups (i.e., WMH and/or CMB), patients with a poor outcome were significantly more often affected by cSVD (86 (78.2%) vs 173 (56%), p < 0.001) (Table 1).
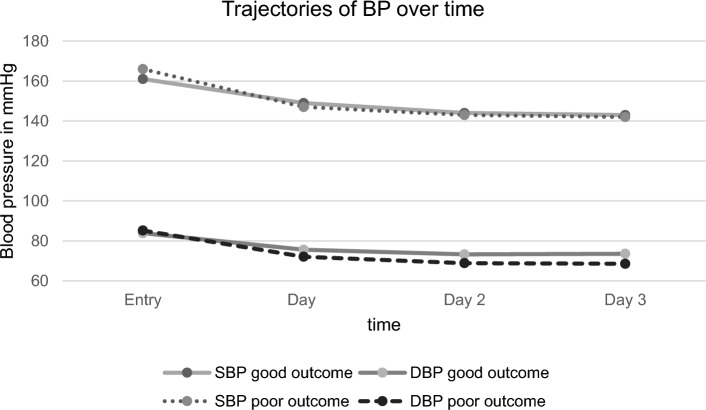
SBP and DBP detected on admission (SBPad; DBPad) did not differ significantly between the group with good and poor functional outcome (mean SBPad 161 ± 28 mmHg vs 166 ± 36 mmHg; p = 0.23; mean DBPad 84 ± 17 mmHg vs 85 ± 22 mmHg; p = 0.58)), the same was true for the mean SBP measured during the first 72 h (SBP72h) at the stroke unit (mean SBP72h 145 ± 15 mmHg vs 144 ± 18 mmHg; p = 0.55). Interestingly, mean DBP of the first 72 h (DBS72h) was significantly higher in patients with good outcome compared to those with poor outcome (mean DBP72h 74 ± 10 mmHg vs 70 ± 13 mmHg; p < 0.001) (Table 3). A trajectory of SBP and DBP of both groups during the first 72 h after admission is outlined in Fig. 2. Mean SBP decrease during the first 72 h after admission did not differ significantly between patients with good and poor functional outcome (−16 ± 22 mmHg vs −22 ± 28 mmHg; p = 0.07). In contrast, mean DBP decrease over the first 72h after admission was significantly higher in patients with poor outcome compared to those with good outcome at discharge (−10 ± 15 mmHg vs −15 ± 21 mmHg; p = 0.012) (Table 3).
Table 3.
Blood pressure characteristics of stroke patients regarding outcome at discharge. mRS, modified Rankin Scale, SBP, systolic blood pressure; DBP, diastolic blood pressure.
| All patients | Good outcome (mRS 0–2) | Poor outcome (mRS 3–6) | p-value | |
|---|---|---|---|---|
| At entry (n = 422) | ||||
| SBP mmHg ± SD | 163 ± 30 | 161 ± 28 | 166 ± 36 | 0.2291 |
| DBP mmHg ± SD | 84 ± 18 | 84 ± 17 | 85 ± 22 | 0.5761 |
| SBP > 140 mmHg n (%) | 341 (80.8) | 255 (82.0) | 86 (77.5) | 0.3702 |
| DBP > 90 mmHg n (%) | 180 (42.7) | 126 (40.5) | 54 (48.6) | 0.1692 |
| Day 1 (n = 420) | ||||
| SBP mmHg ± SD | 149 ± 20 | 149 ± 19 | 147 ± 22 | 0.3531 |
| DBP mmHg ± SD | 75 ± 13 | 76 ± 12 | 72 ± 14 | 0.0231 |
| SBP > 140 mmHg n (%) | 294 (70) | 221 (71.3) | 73 (66.4) | 0.3972 |
| DBP > 90 mmHg n (%) | 64 (15.2) | 45 (14.5) | 19 (17.3) | 0.5912 |
| Day 2 (n = 418) | ||||
| SBP mmHg ± SD | 143 ± 17 | 144 ± 16 | 143 ± 20 | 0.7421 |
| DBP mmHg ± SD | 72 ± 12 | 73 ± 12 | 69 ± 14 | 0.0021 |
| SBP > 140 mmHg n (%) | 260 (62.2) | 192 (62.3) | 68 (61.8) | 0.9992 |
| DBP > 90 mmHg n (%) | 36 (8,6) | 27 (8.8) | 9 (8.2) | 0.9992 |
| Day 3 (n = 414) | ||||
| SBP mmHg ± SD | 143 ± 17 | 143 ± 16 | 142 ± 19 | 0.8721 |
| DBP mmHg ± SD | 72 ± 12 | 74 ± 11 | 69 ± 14 | 0.0011 |
| SBP > 140 mmHg n (%) | 238 (57.5) | 175 (57.6) | 63 (57.3) | 0.9992 |
| DBP > 90 mmHg n (%) | 30 (7.3) | 22 (7.2) | 8 (7.3) | 0.9992 |
| Ø 72 h (n = 420) | ||||
| SBP mmHg ± SD | 145 ± 16 | 145 ± 15 | 144 ± 18 | 0.5481 |
| DBP mmHg ± SD | 73 ± 11 | 74 ± 10 | 70 ± 13 | 0.0011 |
| BP decrease over 72 h, reference BP at entry | ||||
| SBP mmHg ± SD | −17.6 ± 24 | −16.1 ± 22 | −21.6 ± 28 | 0.0691 |
| DBP mmHg ± SD | −11.1 ± 17 | −9.7 ± 15 | −15.3 ± 21 | 0.0121 |
1Calculated with t-Test. 2Calculated with Chi2-Test or Fishers-Exact-Test when expected frequencies were < 5.
Figure 2.
Trajectories of blood pressure over time of stroke patients regarding outcome at discharge. BP, blood pressure, SBP, systolic blood pressure; DBP, diastolic blood pressure.
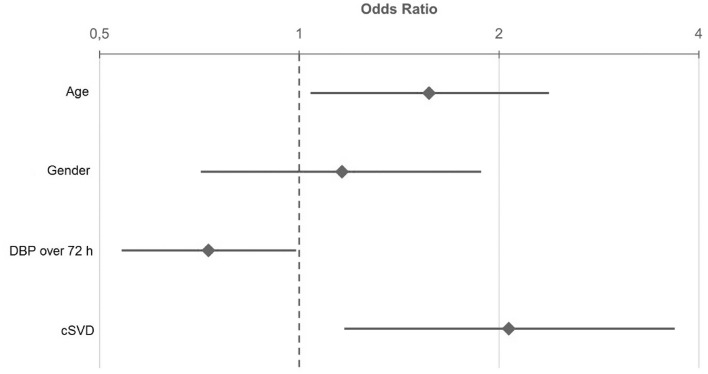
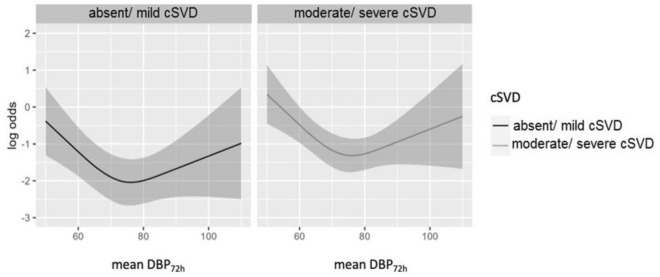
In multivariable logistic regression, significant predictors for poor clinical outcome at discharge were mean DBP measured during the first 72 h after admission (OR 0.73; 95%CI 0.54–0.99; p < 0.001) and cSVD (OR 2.07; 95%CI 1.17–3.68; p = 0.013), whereas the variable age and sex were not significantly associated with poor clinical outcome (Fig. 3). Additionally, we found a significant U-shaped dependence of the mean DBP72h and clinical outcome (Fig. 4). Mean DBP < 66 mmHg and mean DBP > 80 mmHg over the first 72 h after admission were associated with a poor outcome at discharge.
Figure 3.
Odds Ratio and 95% confidence interval for age, gender, DBP over the first 72h after admission and cSVD of stroke patients regarding functional outcome at discharge. Odds Ratio for age and DBP over 72 h indicates the ratio of the odds from the third to the first quartile. Age: 1. Quartile = 61.5 years; 3. Quartile = 80 years; DBP over 72 h: 1. Quartile = 66 mmHg; 3. Quartile = 80.3 mmHg. DBP, diastolic blood pressure, cSVD, cerebral small vessel disease.
Figure 4.
Log odds for cSVD dependent on DBP over the first 72 h after admission for patients regarding functional outcome at discharge. Adjusted for age and gender.
One year after cerebrovascular event, new ischemic stroke or TIA was reported in 11.9% of patients with a good and in 9.89% of those with a poor clinical outcome at discharge (p = 0.62). Among patients with good and poor outcome, 5.78% and 12.9%, respectively, died within one year (p = 0.035).
Late functional outcomes at 12-month follow-up
Twelve months after ischemic stroke or TIA, good functional outcome was found in 261 (70.7%) patients and poor outcome in 108 (29.3%) patients (Table 2). Among patients with a poor outcome, WMH (Fazekas score 2–3) were more frequent observed than in those with a good outcome at 12 months (74 (69.8%) vs 126 (48.8%), p < 0.001). The same was true for the presence of lacunes (56 (53.8%) vs 97 (37.7%), p = 0.007). CMB occurred in both, patients with poor and good outcome almost in the same frequency (35 (32.7%) vs 65 (26.1%), p = 0.24). Over all, the burden of cSVD was higher in patients with poor outcome compared to the group with good outcome at 12 months (84 (78.5%) vs 145 (55.6%), p < 0.001) (Table 2).
We found no significant difference between patients with good and poor functional outcome in respect to mean SBPad (161 ± 28 mmHg vs 166 ± 33 mmHg, p = 0.13) and mean DBPad (84 ± 16 mmHg vs 85 ± 22 mmHg, p = 0.73). SBP72h was similar between patients with good and poor outcome at 12 months (mean SBP72h 145 ± 16 mmHg vs 146 ± 16 mmHg; p = 0.86). In contrast, patients with a good outcome showed a significantly higher mean DBP72h when comparing to patients with a poor outcome (mean DBP72h 75 ± 10 mmHg vs 69 ± 10 mmHg; p < 0.001) (Table 4).
Table 4.
Blood pressure characteristics of stroke patients regarding outcome at 12 months. mRS, modified Rankin Scale, SBP, systolic blood pressure; DBP, diastolic blood pressure.
| All patients | Good outcome (mRS 0–2) | Poor outcome (mRS 3–6) | p-value | |
|---|---|---|---|---|
| At entry (n = 422) | ||||
| SBP mmHg ± SD | 162 ± 30 | 161 ± 28 | 166 ± 33 | 0.1331 |
| DBP mmHg ± SD | 84 ± 18 | 84 ± 16 | 85 ± 22 | 0.7251 |
| SBP > 140 mmHg n (%) | 300 (81.3) | 211 (80.8) | 89 (82.4) | 0.8382 |
| DBP > 90 mmHg n (%) | 157 (42.5) | 111 (42.5) | 46 (42.6) | 0.9992 |
| Day 1 (n = 420) | ||||
| SBP mmHg ± SD | 149 ± 20 | 149 ± 19,4 | 148 ± 20 | 0.8161 |
| DBP mmHg ± SD | 75 ± 12 | 76 ± 12 | 71 ± 12 | < 0.0011 |
| SBP > 140 mmHg n (%) | 260 (70.7) | 184 (70.8) | 76 (70.4) | 0.9992 |
| DBP > 90 mmHg n (%) | 56 (15.2) | 45 (17.3) | 11 (10.2) | 0.1162 |
| Day 2 (n = 418) | ||||
| SBP mmHg ± SD | 144 ± 17 | 144 ± 17 | 144 ± 17 | 0.6211 |
| DBP mmHg ± SD | 72 ± 12 | 74 ± 12 | 70 ± 11 | < 0.0011 |
| SBP > 140 mmHg n (%) | 260 (62.2) | 162 (62.5) | 69 (63.9) | 0.9022 |
| DBP > 90 mmHg n (%) | 36 (8.6) | 25 (9.7) | 5 (4.6) | 0.1642 |
| Day 3 (n = 414) | ||||
| SBP mmHg ± SD | 143 ± 17 | 142 ± 16 | 144 ± 16 | 0.3091 |
| DBP mmHg ± SD | 72 ± 12 | 74 ± 11 | 68 ± 12 | < 0.0011 |
| SBP > 140 mmHg n (%) | 238 (57.5) | 143 (55.9) | 68 (63.0) | 0.2552 |
| DBP > 90 mmHg n (%) | 30 (7.3) | 19 (7.4) | 7 (6.5) | 0.9242 |
| Ø 72 h (n = 420) | ||||
| SBP mmHg ± SD | 145 ± 16 | 145 ± 16 | 146 ± 16 | 0.6831 |
| DBP mmHg ± SD | 73 ± 11 | 75 ± 10 | 70 ± 10 | < 0.0011 |
| BP decrease over 72 h, reference BP at entry | ||||
| SBP mmHg ± SD | −17.3 ± 23 | −15.8 ± 22 | −20.6 ± 26 | 0.0901 |
| DBP mmHg ± SD | −11.1 ± 17 | −9.4 ± 15 | −15.5 ± 21 | 0.0071 |
1Calculated with t-Test. 2Calculated with Chiy-Test or Fishers-Exact-Test when expected frequencies were < 5.
Mean SBP decrease during the first 72 h after admission did not show a statistic difference between patients with good and poor functional outcome (−16 ± 22 mmHg vs −21 ± 26 mmHg; p = 0.09). However, mean DBP decrease over the first 72 h after admission was significantly higher in patients with poor outcome compared to those with good outcome at 12 months (−9 ± 15 mmHg vs −15 ± 21 mmHg; p = 0.007) (Table 4).
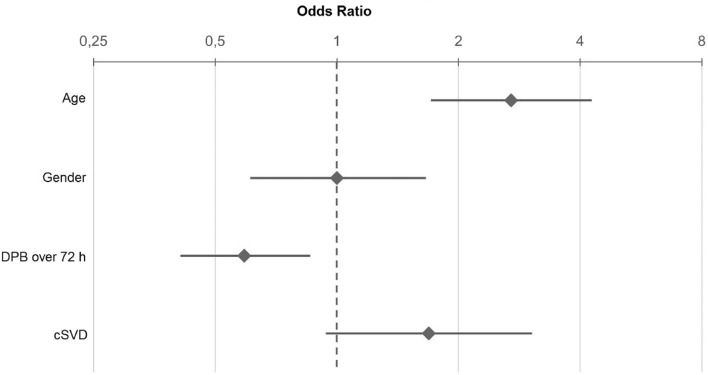
In multivariable logistic regression, significant predictors for poor clinical outcome at 12 months are mean DBP72h (OR 0.59; 95%CI 0.41–0.86; p = 0.018) and age (OR 2.7; 95%CI 1.71–4.27; p < 0.001) (Fig. 5). The burden of cSVD did not significantly predict poor outcome at 12 months; nonetheless, there was a statistical trend (OR 1.69; 95%CI 0.94–3.04; p = 0.08) (Fig. 5).
Figure 5.
Odds Ratio and 95% confident interval for age, gender, DBP over the first 72 h after admission and cSVD of stroke patients regarding outcome at 12 months. OR for age and DBP over 72 h indicates the ratio of the odds from the third to the first quartile. Age: 1. Quartile = 61.5 years; 3. Quartile = 80 years; DBP over 72 h: 1. Quartile = 66 mmHg; 3. Quartile = 80.3 mmHg. DBP, diastolic blood pressure, cSVD, cerebral small vessel disease.
Twelve months after cerebrovascular event, recurrent ischemic stroke or TIA was diagnosed in 9.58% of patients with a good outcome and in 15.9% of those with a poor clinical outcome (p = 0.09).
Discussion
This prospective study of 423 patients with an AIS or TIA showed that cSVD burden was significantly higher in patients with a poor functional outcome at discharge and 12 months after cerebrovascular event. However, neither SBPad nor mean SBP72h was significantly related to functional outcome at discharge or at 12 months after index event. An unexpected finding of the present study was a significant association between mean DBP72h and functional outcome at discharge and 12 months afterwards.
A growing number of studies suggests that cSVD in patients with AIS is independently associated with poor functional outcome in the short and long term after cerebrovascular event32–34 which is in line with our findings. The impact of cSVD on functional outcome after AIS is poorly understood. It has been shown that severe cSVD may lead to a reduction of cerebral blood flow and is associated with impaired cerebral autoregulation23,35,36. Thus, one might argue that low SBP during the acute phase of IS may result in a drop in cerebral blood flow of those patients with severe cSVD. A low SBP might lead to an even faster and more extensive damage of cerebral tissue surrounding the infarcted core than a high SBP, which finally may result in a poor functional outcome. Though, we did not find a statistically significant relationship between SBPad or SBP72h and functional outcome in stroke patients with or without cSVD. This might be due to the small sample size in this study. Additionally, we included patients with AIS as well as with TIA who were treated for hypertension; furthermore, some patients had a mRS > 0 prior to study enrollment, which might—to some extent—influence the results. Another reason for the missing effect of SBP on functional outcome in patients with cSVD could be the fact that SBP only impacts functional outcome when cerebral perfusion is severely compromised as one would expect in patients with high burden of cSVD (e.g., Fazekas grade 3, multiple lacunes and/or CMB). Importantly, we combined patients with Fazekas grade 2 and 3 in one group which could have biased the results. In addition, cSVD burden was determined by using a qualitative and not by a quantitative approach, i.e., we did not assess the volume of WMH in our patients. However, a higher Fazekas scale score is highly correlated with higher WMH volume37. Furthermore, different regional distribution of WMH load and/or locations of stroke lesions might also impact functional outcome38. Furthermore, factors independent of blood pressure such as variations in the cerebral autoregulation, especially in the acute phase as well as genetics are also of great importance in this context. On the other hand, a large randomized trial (n = 3035) has also revealed that SBP is not likely to explain a relationship between cSVD and poor functional outcome after stroke when adjusting for age39. In contrast to our study, the main focus of the aforementioned study was on SBP variability during the first 24 h after stroke.
Although SBP decreased over the first 72 h after admission, there was no statistically significant relationship between SBP decline and functional outcome at discharge and at 12 months after AIS, and thus, an interaction between these parameters and cSVD burden was not expected. Our results are in contrast with findings of a previously published study, showing that a large change of SBP over the first 24 h after stroke is related with a poor functional outcome in the short term (i.e. at day 7 after stroke) as well as in the long term (90 days after stroke)21. However, patients with cSVD burden were not particularly addressed in that study. Although all patients have been recommended to take continuously antihypertensive drugs, adherence of drug intake is low; nearly a quarter of patients are non-adherent to their cardiovascular medicaments40–42. Hence, repeated high SBP values (> 150 mmHg) during the 12 months might occur in these stroke patients and further impair cerebral tissue which has been already damaged by pre-existing cSVD. This might further bias our results.
Little is known about the impact of DBP on functional outcome in stroke survivors with and without cSVD. Here, we found that mean DBP72h was significantly lower in stroke patients with cSVD. Moreover, DBP72h and cSVD burden were associated with a poor outcome at discharge. In contrast to our findings, a previously published trial revealed no consistent association between the level of DBP measured over 24 h and functional outcome21, however, in the aforementioned trial, DBP was generally lower in patients with a poor outcome at day 7 and at day 9021. In another previously published study, DBP variability was associated with poor functional outcome at hospital discharge43. However, the aforementioned studies did not focus on stroke patients with/without cSVD. Göthel-Ezzeiani and coworkers also investigated the impact of WMH and blood pressure on functional outcome. They found a relationship between DBP and mortality but none between DBP and functional outcome at 3 months44; of note, only stroke patients undergoing mechanical recanalization were included in their study. In the present study, there was a significant linear relationship between DBP72h and late functional outcome (at 12 months) but only a statistical trend for cSVD burden when adjusting for sex and age. In contrast, a previous trial reported similar DBP measured over 24 h after stroke in patients with good and poor late functional outcome (at 3 months)45. However, that study did not specifically investigate stroke survivors presenting cSVD and enrolled only stroke patients treated with intravenous thrombolysis.22,22
Additionally, we observed a U-shaped relationship between mean DBP72h and functional outcome at discharge among stroke patients with and without cSVD meaning that only a DBP of about 60–80 mmHg was related to a better functional outcome. A U-shaped relationship between DBP on admission and early deterioration as well as functional outcome at 3 months4 or 6 months46 was provided by previous studies. In contrast to our study, the cutoff value for DBP was higher, namely at 100–110 mmHg4 or 87.8–95 mmHg, respectively46. In the latter study however, this association was no longer significant, when assessed with logistic regression. Other large studies on patients with ischemic stroke (n > 300′000 patients)47 or TIA (n > 200′000 patients)48 reported a U-shaped relationship between DBP on admission and independent ambulation at discharge, likelihood of being discharged at home, and in-hospital death such that below and above 70 mmHg the unadjusted and adjusted odds of these outcomes increased. This value is in the same range as observed in our study. In a cohort study of patients with ischemic stroke treated with endovascular therapy, DBP on admission and functional outcome at 3 months showed a J-shaped relationship with an inflection point at the median value of DBP of 81 mmHg49. In contrast to our work, cSVD was not in the scope of these studies.
The reason why there was a U-shaped relationship between DBP and functional outcome whereas this was not true for SBP in the present study remains elusive. One possible explanation of this observation could be the small number of patients. The U-shaped association between low and high DBP and poor functional outcome might be closely linked to the state of cerebral perfusion for several reasons. First of all, there is some evidence that cerebral perfusion might be lower in patients with arterial hypertension irrespective of the presence of WMH compared to healthy controls50. Patients with WMH have been reported to exhibit a reduction of cerebral blood flow of the white matter (up to 38%) compared with those without WMH51,52. There was even a decrease in CBF within normal appearing white matter surrounding the WMH53. On the other hand, levels of cerebral perfusion especially in the early phase of AIS display a reverse U-shaped curve depending on SBP and DBP levels and—in turn—impact functional outcome at 3 months after stroke54. The most favorable functional outcome was associated with a SBP between 161 to 177 mmHg and DBP ranging from 103 to 114 mmHg54. Interestingly, Park and Ovbiagele found also an independent association between DBP and vascular outcomes 2 years after non-cardioembolic stroke, showing that DBP < 70 mmHg as well as DBP > 90 mmHg was linked to an increased risk of vascular events55. Although this study revealed that patients with low DBP had higher comorbidities of diabetes mellitus, heart failure and carotid artery disease, DBP < 70 mmHg (but not DBP > 90 mmHg) was an independent predictor of vascular events, in particular recurrent stroke, after multivariable adjustment55. Notably, when the BP in the brachial artery is 117/75 mmHg in normotensive subjects, the pressure in the lenticulostriate vascular bed would be 91/58 mmHg and that in the same-size parietal arterioles 59/38 mmHg according to the calculation of Blanco and coworkers56. Given that there is a BP gradient in the brain, a decrease in peripheral DBP may result in a critical undersupply of cerebral tissue, since more than half of cerebral perfusion is during diastole57.
The present study has some limitations. First, the sample size of this work is relatively small. Second, we included patients receiving intravenous thrombolysis and/or undergoing mechanical thrombectomy as well as those with neither of these acute stroke therapies. However, this reflects the real, less than ideal clinical everyday practice. Third, the patients showed mild symptoms of stroke making a generalization of the study data difficult. Fourth, no additional cerebral MRI was performed at 12 months (except in patients with recurrent stroke or TIA) and thus, new or enlarged WMH were not able to be detected. Notably, WMH may regress over time after stroke and thus, provide potentially better functional and brain tissue outcome58. The strength of this work are the prospective study design and well-characterized patients. Furthermore, we used mean SBP and DBP over the first 72 h along with BP on admission. In contrast, a plenty of studies investigating the effect of acute-phase BP on functional outcome after AIS have used a single BP value (mainly the BP on admission) or BP values over the first 24 h as a predictor for the functional outcome. However, different mechanisms may be responsible for an elevation of BP values measured on admission. Beside a physiological response to cerebral ischemia, untreated arterial hypertension as well as increased sympathetic activation, fear of serious illness and hospitalization may contribute to increased BP values on admission59,60. Moreover, data collection and functional assessment by means of a structured telephone interview were carried out by one mRS-experienced person (S. G.).
In conclusion, this observational prospective study demonstrates that cSVD predicts a poor outcome among stroke survivors and thus corroborates findings of previous studies. We did not find a statistically significant relationship between cSVD, level of SBP, and functional outcome probably due to the fact that SBPad and SBP72h values ranged between 145 and 160 mmHg, values that have been associated with a good functional outcome after AIS. However, DBP and cSVD were associated with poor outcome depending on the level of DBP; additionally, DBP showed a U-shaped relationship with functional outcome. When comparing our work to other studies which investigate the impact of BP on stroke outcome, a lot of them only focus on the effect of SBP and reperfusion grade after mechanical thrombectomy, without including cSVD21,43–45, or refer to cSVD alone32–34, making comparisons with our study difficult. Here, we have examined the impact of BP in relating with cSVD on stroke outcome in the acute phase, that has been rarely investigated from this point of view. Furthermore, our study suggests a prognostic significance for functional outcome after stroke depending on DBP value and degree of cSVD burden, which has scarcely been addressed so far, since most studies on stroke outcome analyze only the significance of SBP. Our findings may suggest an individualized stroke care by either lowering or elevating DBP in order to influence functional outcome. However additional large randomized studies are required to further investigate an association between DBP, cSVD and functional outcome after IS.
Acknowledgements
This study was funded by the Interdisziplinäres Zentrum für Klinische Forschung (IZKF), University Hospital, Würzburg. This publication was supported by the Open Access Publication Fund of the University of Würzburg.
Author contributions
Conceived and designed the study: F.F., S.G. Performed the study: S.G., A.S., F.F. Analyzed the data: S.G., A.S., F.F. Contributed analysis tools: S.G., F.F., A.S. Wrote the paper: S.G., A.S., F.F.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qureshi AI, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am. J. Emerg. Med. 2007;25:32–38. doi: 10.1016/j.ajem.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bath, P. M. & Krishnan, K. Interventions for deliberately altering blood pressure in acute stroke. in Cochrane Database of Systematic Reviews (ed. The Cochrane Collaboration) (John Wiley & Sons, Ltd, 2014). 10.1002/14651858.CD000039.pub3. [DOI] [PMC free article] [PubMed]
- 3.Ji N, et al. A reasonable blood pressure level for good clinical outcome after the acute phase of ischemic stroke. J. Clin. Hypertens. Greenwich Conn. 2016;18:536–542. doi: 10.1111/jch.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo J, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520–526. doi: 10.1161/01.STR.0000109769.22917.B0. [DOI] [PubMed] [Google Scholar]
- 5.Willmot M, Leonardi-Bee J, Bath PMW. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertens. Dallas Tex. 2004;1979(43):18–24. doi: 10.1161/01.HYP.0000105052.65787.35. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi-Bee J, Bath PMW, Phillips SJ, Sandercock PAG. Blood pressure and clinical outcomes in the international stroke trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.STR.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. Blood pressure and clinical outcome among patients with acute stroke in Inner Mongolia. China. J. Hypertens. 2008;26:1446–1452. doi: 10.1097/HJH.0b013e328300a24a. [DOI] [PubMed] [Google Scholar]
- 8.Jusufovic M, et al. Blood pressure management in acute stroke. Curr. Hypertens. Rev. 2016;12:121–126. doi: 10.2174/1573402112666160302102711. [DOI] [PubMed] [Google Scholar]
- 9.Sandset, E. C., Murray, G. D., Bath, P. M. W., Kjeldsen, S. E. & Berge, E. Relation Between Change in Blood Pressure in Acute Stroke and Risk of Early Adverse Events and Poor Outcome. [DOI] [PubMed]
- 10.Shin HK, et al. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39:1548–1555. doi: 10.1161/STROKEAHA.107.499483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattle HP, et al. Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke. 2005;36:264–268. doi: 10.1161/01.STR.0000153052.59113.89. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw JM, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardlaw JM, Benveniste H, Williams A. Cerebral vascular dysfunctions detected in human small vessel disease and implications for preclinical studies. Annu. Rev. Physiol. 2022;84:409–434. doi: 10.1146/annurev-physiol-060821-014521. [DOI] [PubMed] [Google Scholar]
- 14.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 17.Smith EE, et al. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2017;48:e44–e71. doi: 10.1161/STR.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 18.Uniken Venema SM, et al. White matter lesions and outcomes after endovascular treatment for acute ischemic stroke: MR CLEAN registry results. Stroke. 2021;52:2849–2857. doi: 10.1161/STROKEAHA.120.033334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira RC, et al. Review of studies on dynamic cerebral autoregulation in the acute phase of stroke and the relationship with clinical outcome. J. Cereb. Blood Flow Metab. 2022;42:430–453. doi: 10.1177/0271678X211045222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins AI, et al. Recanalization modulates association between blood pressure and functional outcome in acute ischemic stroke. Stroke. 2016;47:1571–1576. doi: 10.1161/STROKEAHA.115.012544. [DOI] [PubMed] [Google Scholar]
- 21.Sare GM, Ali M, Shuaib A, Bath PMW. Relationship between hyperacute blood pressure and outcome after ischemic stroke: data from the VISTA collaboration. Stroke. 2009;40:2098–2103. doi: 10.1161/STROKEAHA.108.539155. [DOI] [PubMed] [Google Scholar]
- 22.Bakker SLM, et al. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology. 1999;52:578–578. doi: 10.1212/WNL.52.3.578. [DOI] [PubMed] [Google Scholar]
- 23.Immink RV, et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke. 2005;36:2595–2600. doi: 10.1161/01.STR.0000189624.06836.03. [DOI] [PubMed] [Google Scholar]
- 24.von Elm E, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet Lond. Engl. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 25.Brott T, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.STR.20.7.864. [DOI] [PubMed] [Google Scholar]
- 26.Adams HP, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Fazekas F, Chawluk J, Alavi A, Hurtig H, Zimmerman R. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg SM, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflanz CP, et al. Association of blood pressure lowering intensity with white matter network integrity in patients with cerebral small vessel disease. Neurology. 2022 doi: 10.1212/WNL.0000000000201018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.STR.19.5.604. [DOI] [PubMed] [Google Scholar]
- 31.Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. (Springer International Publishing, 2015). 10.1007/978-3-319-19425-7.
- 32.Ryu W-S, et al. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain. 2017;140:158–170. doi: 10.1093/brain/aww259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgakis MK, Duering M, Wardlaw JM, Dichgans M. WMH and long-term outcomes in ischemic stroke: A systematic review and meta-analysis. Neurology. 2019;92:e1298–e1308. doi: 10.1212/WNL.0000000000007142. [DOI] [PubMed] [Google Scholar]
- 34.IST-3 collaborative group. Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third International Stroke Trial (IST-3): secondary analysis of a randomised controlled trial. Lancet Neurol.14, 485–496 (2015). [DOI] [PMC free article] [PubMed]
- 35.Stewart, C. R., Stringer, M. S., Shi, Y., Thrippleton, M. J. & Wardlaw, J. M. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: an updated meta-analysis. 10.1101/2020.10.06.20207373 (2020) [DOI] [PMC free article] [PubMed]
- 36.Han H, et al. Associations between cerebral blood flow and progression of white matter hyperintensity in community-dwelling adults: a longitudinal cohort study. Quant. Imaging Med. Surg. 2022;12:4151–4165. doi: 10.21037/qims-22-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zerna C, et al. Association of white matter hyperintensities with short-term outcomes in patients with minor cerebrovascular events. Stroke. 2018;49:919–923. doi: 10.1161/STROKEAHA.117.017429. [DOI] [PubMed] [Google Scholar]
- 38.Bonkhoff AK, et al. Association of stroke lesion pattern and white matter hyperintensity burden with stroke severity and outcome. Neurology. 2022;99:e1364–e1379. doi: 10.1212/WNL.0000000000200926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickie DA, et al. Blood pressure variability and leukoaraiosis in acute ischemic stroke. Int. J. Stroke. 2018;13:473–480. doi: 10.1177/1747493017729267. [DOI] [PubMed] [Google Scholar]
- 40.Glader E-L, Sjölander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41:397–401. doi: 10.1161/STROKEAHA.109.566950. [DOI] [PubMed] [Google Scholar]
- 41.Lane, D. et al. High non-adherence rates to secondary prevention by chemical adherence testing in patients with TIA. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc.31, 106665 (2022). [DOI] [PubMed]
- 42.Tomaszewski M, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart Br. Card. Soc. 2014;100:855–861. doi: 10.1136/heartjnl-2013-305063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ningning W, et al. Blood pressure variability related to early outcome of acute ischemia stroke in a prospective observational study. Medicine (Baltimore) 2022;101:e30780. doi: 10.1097/MD.0000000000030780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Göthel-Ezzeiani A, et al. Impact of leukoaraiosis or blood pressure on clinical outcome, mortality and symptomatic intracerebral hemorrhage after mechanical thrombectomy in acute ischemic stroke. Sci. Rep. 2022;12:21750. doi: 10.1038/s41598-022-25171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed N, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from safe implementation of thrombolysis in stroke-international stroke thrombolysis register (SITS-ISTR) Stroke. 2009;40:2442–2449. doi: 10.1161/STROKEAHA.109.548602. [DOI] [PubMed] [Google Scholar]
- 46.Sprigg N, et al. Relationship between outcome and baseline blood pressure and other haemodynamic measures in acute ischaemic stroke: data from the TAIST trial. J. Hypertens. 2006;24:1413–1417. doi: 10.1097/01.hjh.0000234123.55895.12. [DOI] [PubMed] [Google Scholar]
- 47.Bangalore S, et al. Blood pressure and in-hospital outcomes in patients presenting with ischaemic stroke. Eur. Heart J. 2017;38:2827–2835. doi: 10.1093/eurheartj/ehx330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bangalore S, et al. Relation of admission blood pressure to in-hospital and 90-day outcomes in patients presenting with transient ischemic attack. Am. J. Cardiol. 2019;123:1083–1095. doi: 10.1016/j.amjcard.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 49.van den Berg SA, et al. Admission blood pressure in relation to clinical outcomes and successful reperfusion after endovascular stroke treatment. Stroke. 2020;51:3205–3214. doi: 10.1161/STROKEAHA.120.029907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parfenov, V. A. et al. Brain perfusion, cognitive functions, and vascular age in middle aged patients with essential arterial hypertension. Kardiologiia 23–31 (2018). [PubMed]
- 51.Markus HS. Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J. Neurol. Neurosurg. Psychiatry. 2000;69:48–53. doi: 10.1136/jnnp.69.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, et al. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2016;36:1653–1667. doi: 10.1177/0271678X16662891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu J, Tang J, Han J, Hong Z. The reduction of regional cerebral blood flow in normal-appearing white matter is associated with the severity of white matter lesions in elderly: A xeon-CT study. PLoS ONE. 2014;9:e112832. doi: 10.1371/journal.pone.0112832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He M, et al. Blood pressures immediately following ischemic strokes are associated with cerebral perfusion and neurologic function. J. Clin. Hypertens. 2018;20:1008–1015. doi: 10.1111/jch.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park J-H, Ovbiagele B. Post-stroke diastolic blood pressure and risk of recurrent vascular events. Eur. J. Neurol. 2017;24:1416–1423. doi: 10.1111/ene.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanco PJ, Müller LO, Spence JD. Blood pressure gradients in cerebral arteries: a clue to pathogenesis of cerebral small vessel disease. Stroke Vasc. Neurol. 2017;2:108–117. doi: 10.1136/svn-2017-000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spence J. Blood pressure gradients in the brain: their importance to understanding pathogenesis of cerebral small vessel disease. Brain Sci. 2019;9:21. doi: 10.3390/brainsci9020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wardlaw JM, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology. 2017;89:1003–1010. doi: 10.1212/WNL.0000000000004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlberg B, Asplund K, Hägg E. Factors influencing admission blood pressure levels in patients with acute stroke. Stroke. 1991;22:527–530. doi: 10.1161/01.STR.22.4.527. [DOI] [PubMed] [Google Scholar]
- 60.Harper G, Castleden CM, Potter JF. Factors affecting changes in blood pressure after acute stroke. Stroke. 1994;25:1726–1729. doi: 10.1161/01.STR.25.9.1726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.