Abstract
Background
Recently, a newly programmed cell death has been discovered, namely cuproptosis. It is considered a novel copper-dependent cell death model. Long non-coding RNA (lncRNA) influence the prognosis of bladder cancer. In this study, we established a scoring system based on 7 cuproptosis-related lncRNA to predict the prognosis and immune landscape of bladder cancer (BCa).
Method
Gene expression and clinical data of 431 tissues were downloaded from The Cancer Genome Atlas (TCGA), including 19 normal samples and 419 cancer samples. All samples were randomly categorized into train and test cohorts. Cuproptosis-related lncRNA were distinguished. Then we conduct univariate COX and multivariate COX regression, paralleled with LASSO regression to cultivate a cuproptosis-related lncRNA risk model. Kaplan-Meier curves, scatter diagram, C-index, ROC curves, nomogram, PCA analysis and univariate and multivariate Cox regression were used to test the accuracy of risk model and to predict patient survival. Additional, gene mutation status between high- and low-risk groups was calculated.
GO and KEGG were used to access the DEGs (different expression genes)-related pathway.
The ssGSEA and ESTIMATE algorithms were used to assess the immune function in different tumor samples. Besides, patient's response to immunotherapy and drug susceptibility were also been estimated.
Results
7 cuproptosis-related lncRNA (LINC01184, LINC00513, LINC02443, SMARCA5-AS1, BDNF-AS, SOD2-OT1, HYI-AS1) were selected to construct the risk model in the train cohort. This model can well predict the overall survival (OS) in test group and entire cohort with different stage. Despite no significant different is observed in gene mutation between high- and low-risk group, different immune infiltration, different survival and sensitivity to drugs are discovered.
Conclusion
We established a novel cuproptosis-related lncRNA risk model which can predict the outcome and immunotherapy response with satisfactory predictive effects. This risk model can provide a new insight into prognostic evaluation and may have potential to guide comprehensive treatment in bladder cancer.
Keywords: Cuproptosis, Bladder, lncRNA, Prognosis, Immune response
1. Introduction
Bladder cancer (BCa) is regarded as the most common cancer in urinary system and one of the most common malignant cancer in the world [1]. Every year, more than 500,000 patients are diagnosed as BCa and about 200,000 people are died for BCa-related disease [2]. BCa is divided into two major types: non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC). Generally, NMIBC has a better prognosis as the 5-year survival rate is about 90 %. Unfortunately, more than a half NMIBC patients will relapse and about 15 % patients will develop into muscle invasive stage [3]. When diagnosed as muscle invasive bladder cancer, patients are more likely to develop into metastasis stage which has a poorer outcome [4]. As for treatment, transurethral bladder tumor resection (TURBt) and surgery are first-line treatment option. During these years, more and more studies indicated the chemotherapy and immunotherapy can benefit advanced BCa patients. Even so, high recurrence rate and drug-resistance are disturbing problems in clinical treatment [5]. Therefore, it is urgent to find a novel predictive marker of BCa to estimate the recurrence rate and provide a new insight for BCa treatment.
Like any other element like Calcium and Iron, copper also play a vital role in every organism and many biological processes. The concentration of copper can cause intracellular dysfunction and induce cell death eventually. However, with the development of material science, copper chelators have anticancer effects [6]. Originally, this copper-induced cell death did not draw much attention and been regarded as apoptosis by many scholars [7]. But the latest study find that copper-induced regulatory cell death, namely cuproptosis, is distinct from other known cell death mechanisms, such as necrosis, apoptosis, autophagy and ferroptosis [8]. Cuproptosis occurs through direct binding of copper to lipoylated components of the tricarboxylic acid (TCA) cycle, causing lipoylated protein aggregation and iron-sulfur cluster protein loss. This process can result in proteotoxic stress and cell death ultimately. As a new cell death model, this study caused quite a stir internationally, and many studies have proved cuproptosis has great potential and prospects in the personalized treatment in various cancers. Cai and his colleagues have conducted research on the role of copper-mediated cell death across a wide range of cancers, highlighting the significance of this phenomenon in various tumor types. Xin, Zhang, and other researchers have similarly investigated the role of copper-mediated cell death in renal clear cell carcinoma and hepatocellular carcinoma. Their studies suggest that predictive models based on copper death-related genes exhibit a strong discriminatory ability in patient prognosis and immune infiltration. As a result, we aim to further explore the role of copper-mediated cell death in bladder cancer [[9], [10], [11]].
Initially, noncoding RNA (ncRNAs) is thought to be “useless”, but in fact, this kind of “transcription noise” is found to be a regulator in internal cell signals which control various levels of gene expression, playing a vital part in maintaining cellular homeostasis and cell deregulation [12]. The ncRNAs family is very large, including tranfer RNAs (tRNA), ribosomal RNAs (rRNAs) and small nucleolar RNAs (snoRNAs), including long noncoding RNAs (lncRNAs) and micro RNA (miRNAs), which have been very hot recently.
LncRNAs is an RNA transcript with a length of more than 200 nucleotides which does not encode protein [13]. LncRNAs has a close relationship with numerous cancer, it can regulate cancer-related biological process and play an important role in cancer diagnosis and prognosis [[14], [15], [16]], licluding bladder cancer [17]. But the relationship between bladder cancer and lncRNAs has not been illustrated clearly. Herein, we built a cuproptosis-related lncRNAs signature to evaluate the clinical prognosis and immune function of BCa patients. In the end, it was confirmed that different group divided by cuproptosis-related lncRNAs model is accurate and reliable to be an independent prognostic factor.
2. Materials and methods
2.1. Data collection
The transcriptome data and corresponding clinical and prognostic data of bladder cancer patients were downloaded from the Cancer Genome Atlas (TCGA) bladder cancer database up to September 18, 2022 (https://portal.gdc.cancer.gov/). Patients without complete clinical data (including age, gender, pathological stage, TNM stage, survival status and survival time) were excluded. The cuproptosis-related genes were obtained from previous study7 and their expression in different patients were selected. The approval of ethics committee in this study was not need as the entire data was downloaded from TCGA database and complied with TCGA guideline strictly.
2.2. Identification and expression of cuproptosis-related lncRNAs
After the expression levels of miRNA and lncRNA were isolated, the expression of cuproptosis-related lncRNAs (CRLs) were determined with |Cor| >0.4 and p-value<0.01. The “limma”R package was employed to build this co-expression network. A total of 263 cuproptosis-related lncRNAs were selected and their expression in different patients were obtained. Cytoscape software (version 3.9.1, http://www.cytoscape.org/) was used to show the correlation of the CRLs and their corresponding mRNAs through lncRMA-mRNA coexpression network. Besides, Sankey diagram was used to illustrate the correlation degree between CRLs and their corresponding mRNAs.
2.3. Construction of cuproptosis-related lnRNAs prognostic signature
Potential prognositic CRLs (p < 0.05) was filtered by univariate COX analysis based on “survival”R package. 404 bladder cancer patients were randomly divided into two cohorts (train cohort or test cohort) at 1:1 ratio. A total of 11 CRLs were selected, and their hazard ratio was visualized by forest plot. Then, least absolute shrinkage and selection operator (LASSO) Cox regression (“gemnet”R pakage) and multi COX analysis were conducted to these candidate CRLs. Finally, a cuproptosis-related lnRNAs risk score model containing 7 CRLs was obtained by multiplying the results for the expression of each lncRNA and its corresponding coefficient. After that, according to the median value of the model, patients in the train cohort were divided into a low-risk group and a high-risk group. Using “survminer”R package, the overall survival (OS) between low-risk and high-risk group in train cohort was shown in Kaplan-Meier curve. And a receiver operating characteristic curve (ROC) was generated by “timeROC”R package. To assess the model feasibility and accuracy, the C-index curve and principal component analysis (PCA) were employed. Then, the risk score model was validated in test cohort and all TCGA cohort based on the same formula, and the same methods were conducted.
2.4. Clinicopathological correlation of cuproptosis-related lncRNAs signature
The CRLs risk model in clinicopathological subgroups of the TCGA-BCLA cohort was differentially analyzed. The different expression of 7 prognositic CRLs of patients in different clinical subtypes (T, N, M, stage, gender, age) was illustrated by heatmap. In addition, survival analysis was conducted between the two groups and compared in accordance with different clinical characteristics, including age (<60 or ≥60), gender (male or female), T stage (T2 or T3-4), N stage (N0-1 or N2-3) and clinical stage (stages I–II or stages III–IV). A nomogram model containing CRLs risk model and selected parameters was built with the “rms” R package to predict the 1-, 3-, and 5-years survival of BCa patients. We used the calibration curve to test whether the predicted survival rate was consistent with the actual survival rate.
2.5. Functional analysis and gene set enrichment analysis
The different expressed genes (DEGs) between low-risk group and high-risk were also explored defining false discovery rate (FDR) < 0.05 and |log2FC|≥1. (FC: fold change) Then we use “clusterProfiler”R package to explore functional analysis including Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. (adjusted p value < 0.05)GSEA was implemented to determine the molecular and biological differences between low-risk and high-risk cohorts based on KEGG and HALLMARK gene sets from the molecular signature database [18] (https://www.gsea-msigdb.org/gsea/msigdb).
Estimation of the immune cell infiltration, immune microenvironment and genetic alterations analysis.
The immune cell infiltration in different BCa patients was estimated by ESTIMATE algorithm [19]. CIBERSORT algorithm was used to assess the differences in immune cell infiltration in the two different cohorts. CIBERSORT is a preprocess-based gene expression profiling tool that uses expression data to represent the cellular composition of complex tissues. The immune cell abundance in two cohorts were calculated also by TIMER, QUANTISEQ, MCPCOUNTER, XCELL and EPIC. TMB was defined as the total number of somatic mutations per million bases and analyzed by the “maftools”R package. Besides, immuno-checkpoint was also calculated.
2.6. Drug sensitivity estimation
In order to assess the role of predictive signals in the prediction of BC treatment response, we calculated the clinical treatment of BC commonly used chemotherapy drugs half maximum inhibitory concentration (IC50). We use Wilcoxon signed-rank test to compare the IC50 values between high- and low-risk cohorts.
2.7. Statistical analysis
Quantitative variables were analyzed by independent sample t-test. Using ROC curve analysis and Kaplan Meier survival analysis the effectiveness of the R software (version 4.1.3) to predict survival outcome. Cox scale models were used to investigate associations between prognostic classification and survival outcomes, as well as other clinical parameters.
3. Results
3.1. Identification of cuproptosis-related lncRNAs
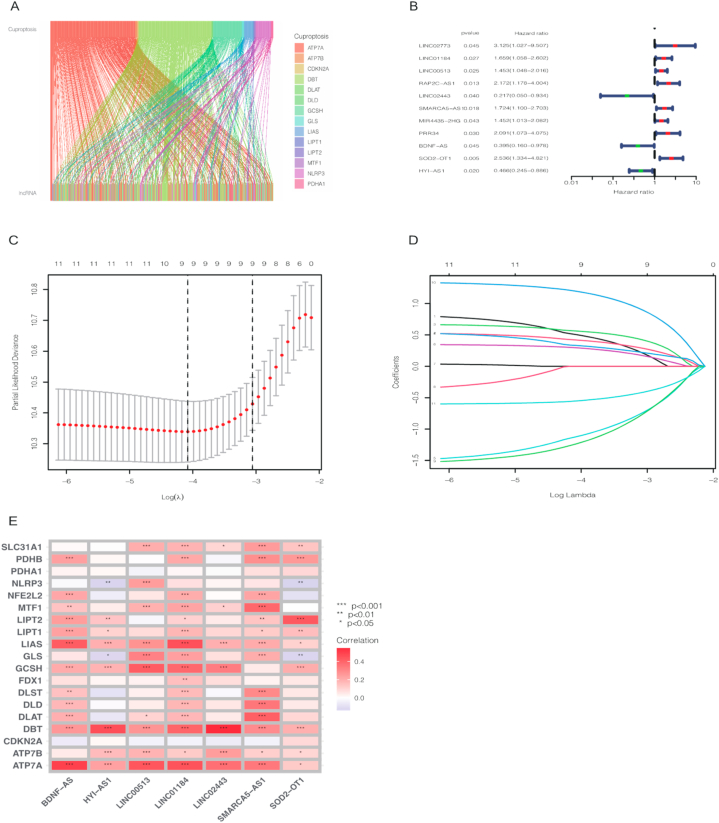
After we downloaded the gene expression matrix, we distinguished the expression of mRNA and lncRNA respectively. The expression of 19 cuproptosis-related genes was collected according to previous studies, 263 cuproptosis-related lncRNAs (CRLs) were screened out via Pearson correlation analysis (Cor>0.4 and p-value<0.01). lncRNA-mRNA co-expression network was illustrated by Cytoscape software (Supplementary Fig. 1) and Sankey diagram showed the correlation degree between CRLs and corresponding mRNAs (Fig. 1A).
Fig. 1.
Sankey diagram revealed the relationship between cuproptosis genes and cuproptosis-related lncRNAs (A). 11 candidate cuproptosis-related lncRNAs after univariate COX regression analysis (B). Distribution of LASSO coefficients of cuproptosis-associated lncRNAs (C). The cross-validation of variable selection in the LASSO analysis (D). Correlation of cuproptosis-related lncRNAs with cuproptosis-related genes in risk models (E).
3.2. Construction of cuproptosis-related lncRNA prognosis predictive model
BCa patients were randomly divided into train and test cohort. As shown in Table 1, the clinical features including age, gender, grade, stage, T, M and N between train and test cohort were not statistically significant (all p value > 0.05). Then 11 potential prognostic CRLs (LINC02773, LINC01184, LINC00513, RAP2C-AS1, LINC02443, SMARCA5-AS1, MIR4435-2 H G, PRR34, BDNF-A, SOD2-OT, HYI-AS1) were selected by univariate COX regression analysis as shown by forest plot in Fig. 1B. LASSO regression model was used to screen these 11 candidate CRLs; the lambda in best model containing 9 CRLs (Fig. 1C,D). Afterwards, these 9 CRLs were further included in multivariate COX regression analysis. In the end, a predictive model containing 7 CRLs was established; these 7 CRLs were (Supplementary Table 2). The risk score was obtained according to the multivariate COX formula LINC01184 × 0.493868850973786+LINC00513 × 0.763639968510251- LINC02443 × 1.45998184708857+ SMARCA5-AS1* 0.408686597520773- BDNF-AS* 1.46419044365778+ SOD2-OT1* 1.36930346926972- HYI-AS1* 0.663223507068694. A nomogram constructed from 7 CRLs were employed to analyze the correlation of cuproptosis-related genes (Fig. 1E).
Table 1.
Different clinical and pathological features in train and test cohorts.
| Covariates | Type | Total | Test | Train | Pvalue |
|---|---|---|---|---|---|
| Age | ≤60 | 106 (26.24 %) | 58 (28.71 %) | 48 (23.76 %) | 0.3088 |
| Age | >60 | 298 (73.76 %) | 144 (71.29 %) | 154 (76.24 %) | |
| Gender | FEMALE | 106 (26.24 %) | 55 (27.23 %) | 51 (25.25 %) | 0.7344 |
| Gender | MALE | 298 (73.76 %) | 147 (72.77 %) | 151 (74.75 %) | |
| Grade | High Grade | 381 (94.31 %) | 186 (92.08 %) | 195 (96.53 %) | 0.2375 |
| Grade | Low Grade | 20 (4.95 %) | 13 (6.44 %) | 7 (3.47 %) | |
| Grade | unknow | 3 (0.74 %) | 3 (1.49 %) | 0 (0 %) | |
| Stage | Stage I | 2 (0.5 %) | 0 (0 %) | 2 (0.99 %) | 0.0539 |
| Stage | Stage II | 128 (31.68 %) | 71 (35.15 %) | 57 (28.22 %) | |
| Stage | Stage III | 140 (34.65 %) | 74 (36.63 %) | 66 (32.67 %) | |
| Stage | Stage IV | 132 (32.67 %) | 55 (27.23 %) | 77 (38.12 %) | |
| Stage | unknow | 2 (0.5 %) | 2 (0.99 %) | 0 (0 %) | |
| T | T0 | 1 (0.25 %) | 1 (0.5 %) | 0 (0 %) | 0.7528 |
| T | T1 | 3 (0.74 %) | 1 (0.5 %) | 2 (0.99 %) | |
| T | T2 | 117 (28.96 %) | 58 (28.71 %) | 59 (29.21 %) | |
| T | T3 | 193 (47.77 %) | 99 (49.01 %) | 94 (46.53 %) | |
| T | T4 | 57 (14.11 %) | 26 (12.87 %) | 31 (15.35 %) | |
| T | unknow | 33 (8.17 %) | 17 (8.42 %) | 16 (7.92 %) | |
| M | M0 | 194 (48.02 %) | 103 (50.99 %) | 91 (45.05 %) | 0.1748 |
| M | M1 | 11 (2.72 %) | 3 (1.49 %) | 8 (3.96 %) | |
| M | unknow | 199 (49.26 %) | 96 (47.52 %) | 103 (50.99 %) | |
| N | N0 | 235 (58.17 %) | 125 (61.88 %) | 110 (54.46 %) | 0.3239 |
| N | N1 | 46 (11.39 %) | 20 (9.9 %) | 26 (12.87 %) | |
| N | N2 | 75 (18.56 %) | 33 (16.34 %) | 42 (20.79 %) | |
| N | N3 | 6 (1.49 %) | 2 (0.99 %) | 4 (1.98 %) | |
| N | unknow | 42 (10.4 %) | 22 (10.89 %) | 20 (9.9 %) |
3.3. The accuracy of cuproptosis-related lncRNA prognosis predictive model
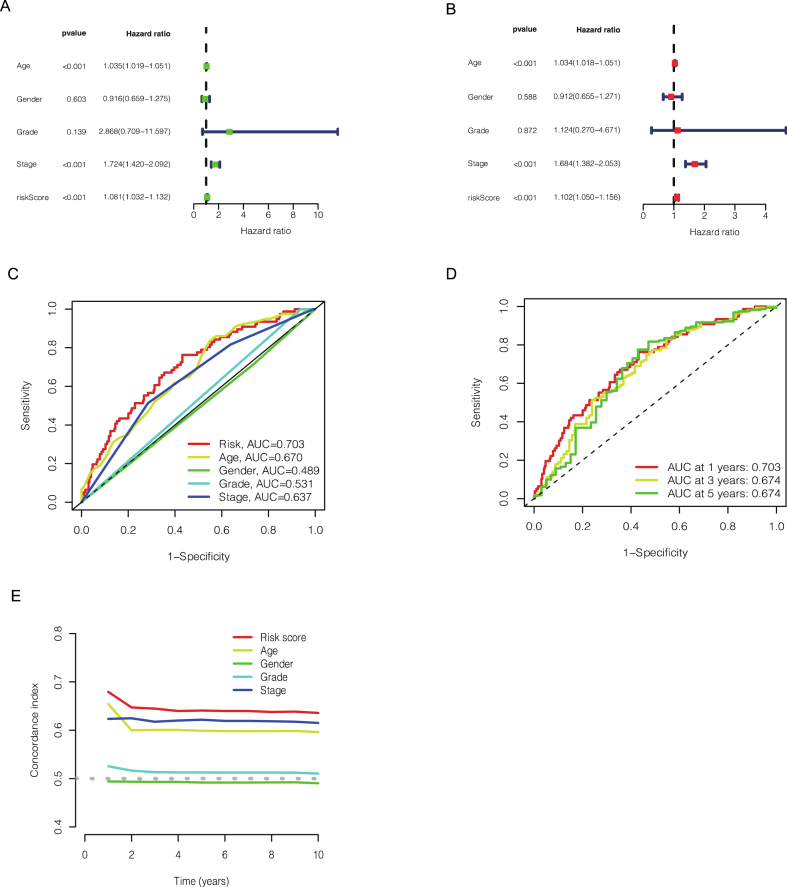
As shown in Fig. 2A, the results of univariate COX regression showed that our risk model (p < 0.001), age (p < 0.001), gender (p = 0.603), grade (p = 0.139), stage (p < 0.001) were important prediction for BCa prognosis. Furthermore, the multivariate COX regression analysis indicated that our risk model (p < 0.001, hazard ratio HR = 1.102, 95 % CI = 1.050−1.156), age (p < 0.001, hazard ratio HR = 1.034, 95 % CI = 1.018−1.051) and stage (p < 0.001, hazard ratio HR = 1.684, 95 % CI = 1.382−2.053) would be independent risk factors for bladder cancer prognosis (Fig. 2B). Besides, we also used the ROC curve analysis to estimate the accuracy of this model. As shown in Fig. 2C, the AUC value of risk model of all patients was 0.703, while the AUC value of age was 0.670, the gender was 0.489, the grade was 0.531 and the stage was 0.637. In addition, the AUC value of the ROC curve of all patients was 0.703, 0.674, 0.674 at 1, 3, and 5 years respectively (Fig. 2D). And 10-years C-index in the risk score model was also higher than other clinicopathological features, indicating that the risk model we developed has good accuracy in patient survival and is higher than predicted by other clinical measures. This would also provide clinicians with strong evidence for the prognosis assessment of patients. (Fig. 2E). All these tests demonstrate that this risk model based on seven cuproptosis-related lncRNA has a high value in bladder cancer prognostic prediction.
Fig. 2.
The different factors (age, gender, grade, stage and riskScore) for BCa prognosis prediction in univariate COX regression (A). The different factors (age, gender, grade, stage and riskScore) for BCa prognosis prediction in multivariate COX regression (B). Predictive accuracy of the risk score model compared with other factors (C). Based on 1 year, 3 years and 5 years of the receiver-operating characteristic curve to predict the accuracy of the risk characteristics of the whole group (D). C-index curve of the risk model (E).
3.4. Prognostic estimation of cuproptosis-related lncRNA risk model
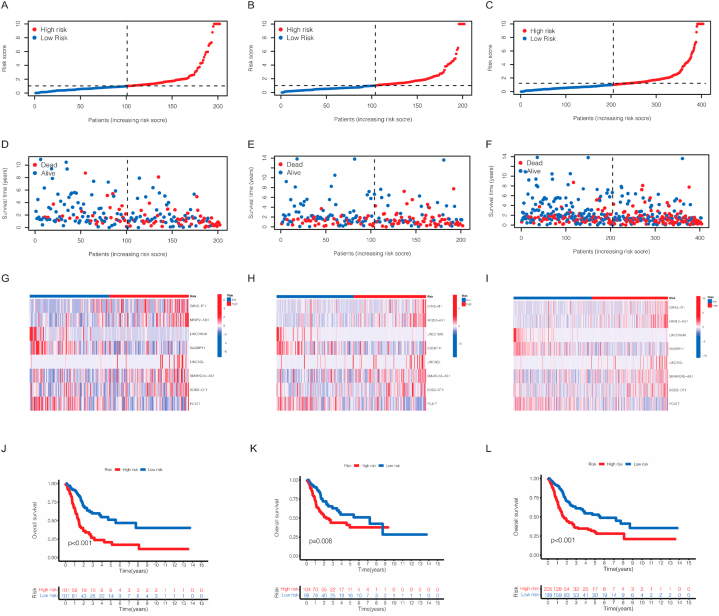
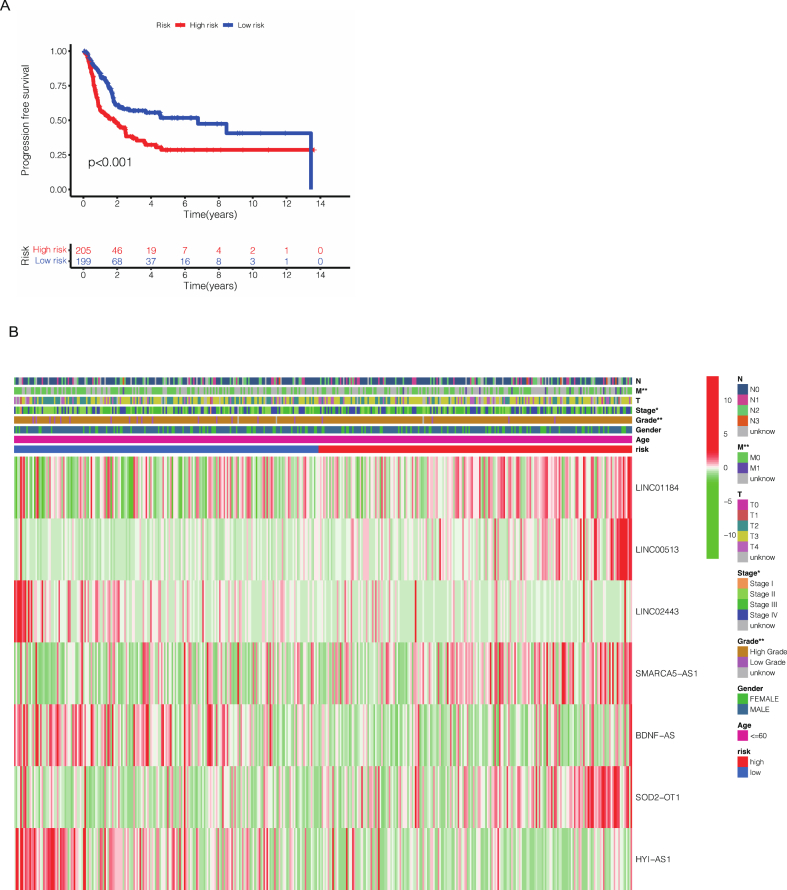
After we obtained this risk model based on seven cuproptosis-related lncRNAs in the previous period, the risk score of each patient in TCGA cohort was calculated. According to the median risk score, patients were divided into high-risk cohort and low-risk cohort. As observed in Fig. 3A–F, more patients were dead in high-risk cohort than low-risk cohort from the scatter plots of the risk score distribution in train cohort, test cohort and all patients. Besides, the expression of seven CRLs associated with risk score model in high- and low-risk cohort were demonstrated in a heatmap (Fig. 3G–I). In the meantime, to evaluate the accuracy of this model, K-M survival curve was conducted to show the overall survival (OS) in different BCa patients cohorts. Our results showed that the model has high accuracy, as p = 0.008 in test cohort and p < 0.001 both in train and all patients (Fig. 3J-L). In order to further study the applicability of the model, we also assessed the progression-free survival (PFS) in all patients. We found the p value < 0.001, indicating that our risk model had a high accuracy not only in OS prediction but also PFS prediction (Fig. 4A).
Fig. 3.
Prognosis of the risk model in different train, test and all cohorts. The distribution of overall survival risk scores (A–C), survival time and survival status (D–F), the expression of 7 cuproptosis-related lncRNAs in heatmaps (G–I). The Kaplan–Meier survival curves of overall survival in different cohorts.
Fig. 4.
The Kaplan–Meier survival curves of progression-free survival of all bladder cancer patients (A). The different expression of these seven cuproptosis-related lncRNAs in different clinical and pathological characteristics (B).
3.5. The relationship between different clinical features and risk model
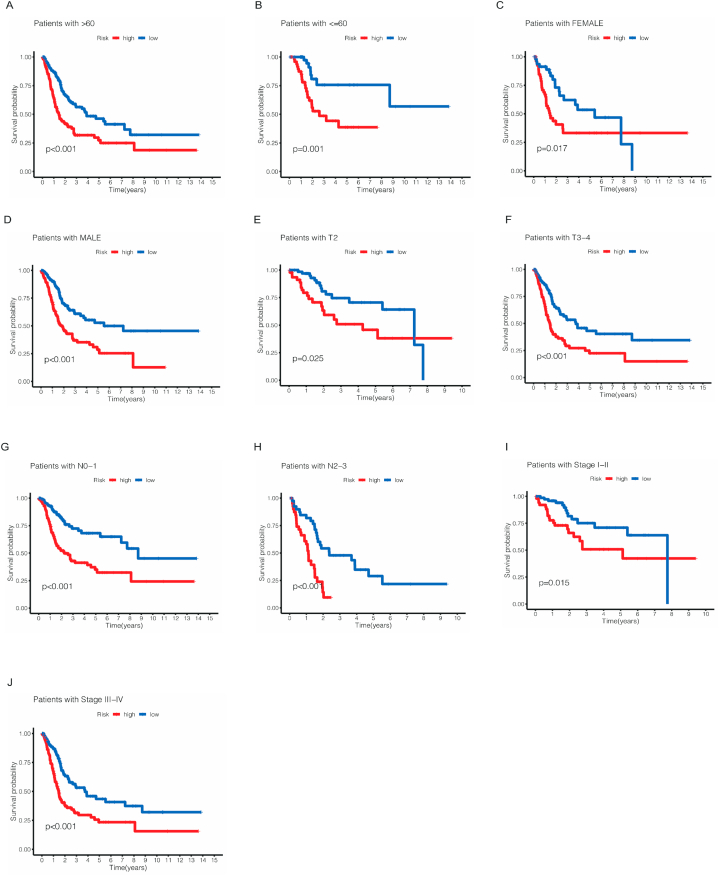
The results above showed the importance of this risk model, but the correlation between prognostic model and specific clinicopathological features needs further study. So, we performed a heatmap to show the different expression of these seven cuproptosis-related lncRNAs in different clinical and pathological features (Fig. 4B). Furthermore, we also conducted a K-M survival analysis for different subtype in clinicopathological features. As shown in Fig. 5A–J, the subtypes were separated by age, gender, stage, T and N. To our delight, the survival probability in low-risk cohort was significantly higher than high-risk group regardless of subtypes of age, gender, stage, T and N.
Fig. 5.
The Kaplan–Meier survival curves for low- and high-risk cohorts by different clinical and pathological features including age (A,B), gender (C,D), T stage (E,F), N stage (G,H) and stage (I,J).
3.6. The establish of cuproptosis-related lncRNA nomogram
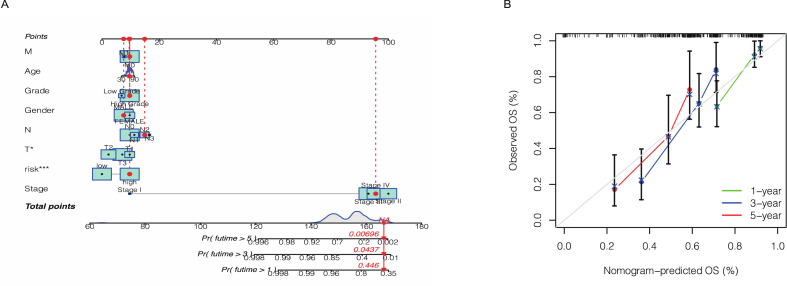
In order for our prognostic risk model to be better used in the clinic, we established a nomogram based on the risk score model and clinicopathological features which can predict the overall survival rates at 1, 3, and 5 years in bladder cancer patients. As shown in Fig. 6A, the higher risk score mean poorer prognosis, and the lower the patient's survival rate. Calibration curve in Fig. 6B showed that our nomogram had a good correlation between nomogram and predicted results.
Fig. 6.
Combined with clinical pathological variables and risk score of nomogram prediction BCa patients 1 year, 3 years and 5 years of overall survival (A). Calibration curves show the agreement between actual and predicted outcomes at 1, 3, and 5 years (B).
3.7. Principal component analysis and biological pathway analysis
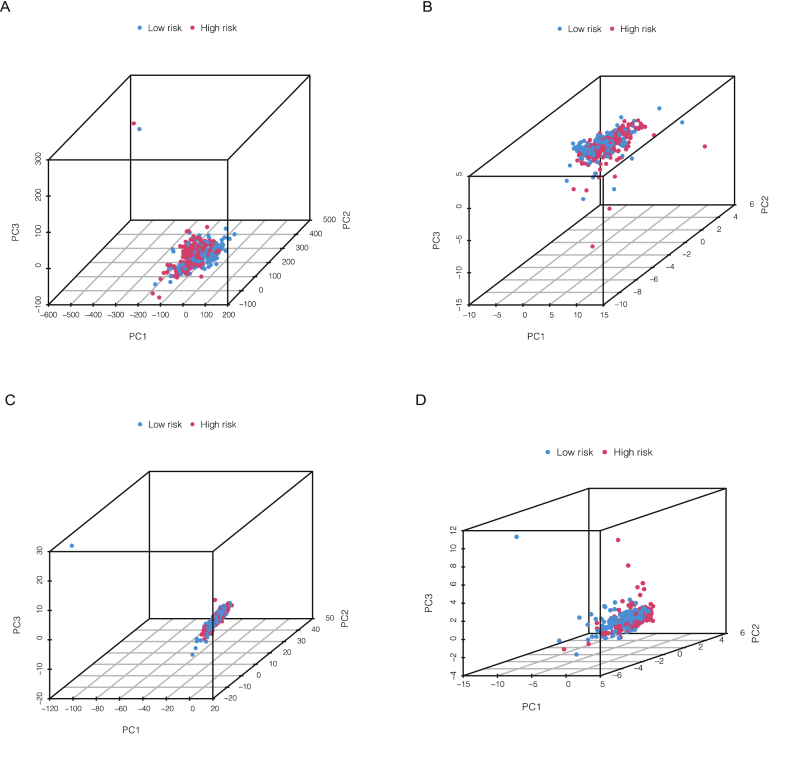
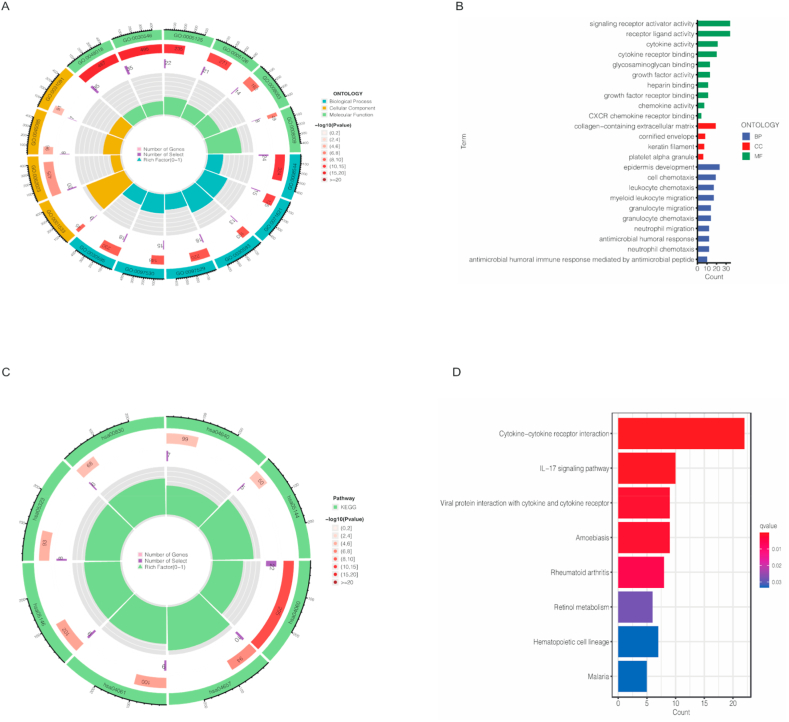
Principal component analysis (PCA) was employed to verify the discrimination degree of the model in different cohorts. Four different cohorts including total gene expression, cuproptosis genes, cuproptosis-related lncRNAs and risk lncRNAs. The results in Fig. 7A–D indicates that our risk score model have a desirable ability to discriminate different patients. Besides, the GO analysis showed that the different expressed genes (DEGs) were associated with many biological processes, including many immune responses (Fig. 8A–B). The results of KEGG showed the cytokine−cytokine receptor interaction was significantly enriched (Fig. 8C–D). We also used GSEA to analysis the different biological pathways between high- and low-risk, the results is in Supplementary Fig. 2.
Fig. 7.
Principal component analysis of total gene expression (A), cuproptosis genes (B), cuproptosis-related lncRNAs (C) and risk lncRNAs (D).
Fig. 8.
Gene Ontology (GO) analysis showed the richness of molecular biological processes (BP), cellular components (CC), and molecular functions (MF) in high- and low-risk cohorts (A–B). KEGG pathway analysis showed the significantly enriched pathways between high- and low-risk cohorts (C–D).
3.8. Analysis of immune-related function of risk model
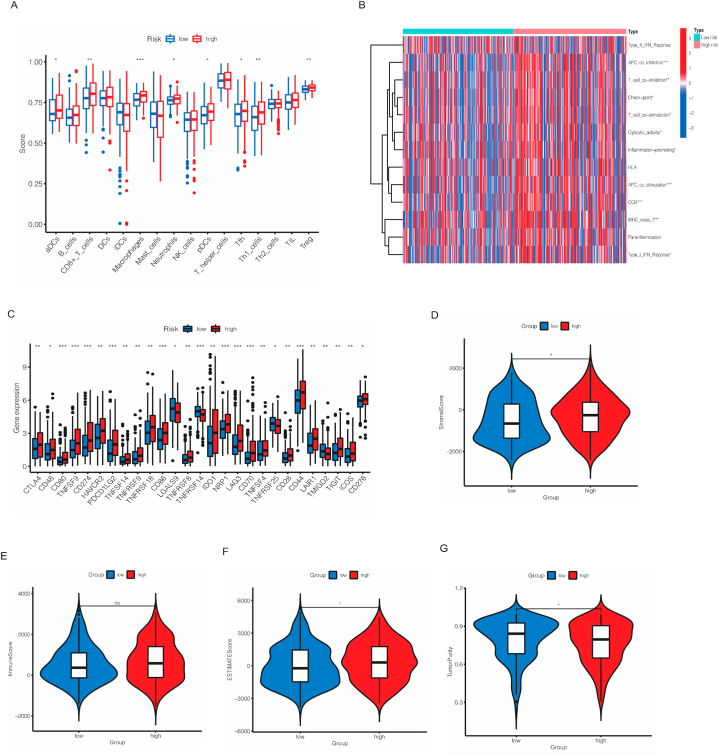
Next, we performed ssGSEA analysis, the results in Fig. 9A showed the different expression of immune cells between high- and low-risk cohorts. As we can see, ADCs, CD8+_T_cells, macrophages, neutrophils, pDCs, Tfh, Th1_cells and Treg were significantly higher in high-risk cohort, indicating these cells might result in poorer outcomes. Subsequently, the relationship between two cohorts and immune-related activities was also estimated. As shown in Fig. 9B, the heatmap showed that APC co-inhibition, T cell co-stimulation, check point, T cell co−stimulation, cytolytic activity, Inflammation−promoting, APC co-stimulation, CCR, MHC class-I and Type I IFN response were dramatically differ from two cohorts. According to these results, immune checkpoint was also been checked. Fig. 9C showed that CD80, TNFSF9, CD274, PDCD1LG2, CD86, TNFRSF14, NRP1, LAG3, CD70 and CD44 (p < 0.01) were significantly expressed differently, indicating these genes might associated to the prognosis of bladder cancer. In TME scores, stromal scores, ESTIMATE scores were higher in high-risk cohort, while tumor purity was higher in low-risk cohort and no difference in immune scores between two cohorts (Fig. 9D–G).
Fig. 9.
Different immune cell infiltration between high- and low-risk cohorts (A). The heatmap demonstrated the different immune function in the two risk groups (B). Different expression of common immune checkpoints in the risk groups (C). The violin plot comparing StromalScore, ImmuneScore, ESTIMATEScore and TumorPurity between the high- and low-risk cohorts, respectively (D–G). *p < 0.05, **p < 0.01, and ***p < 0.001.
3.9. Tumor mutation burden and drug sensitivity
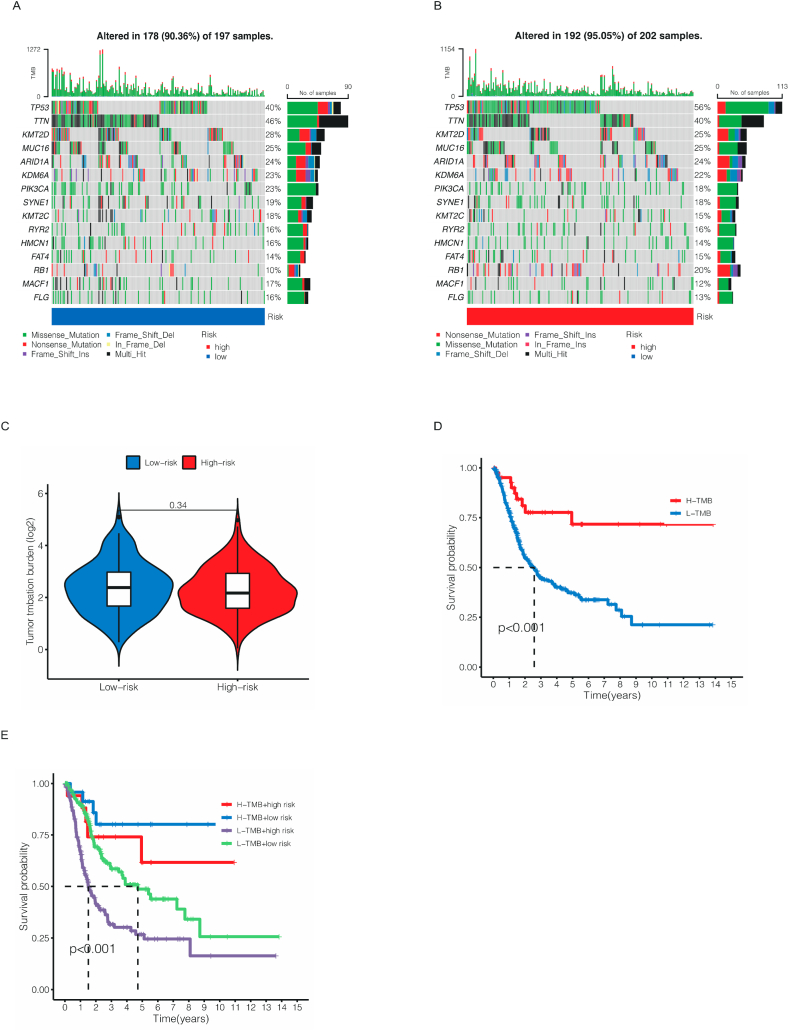
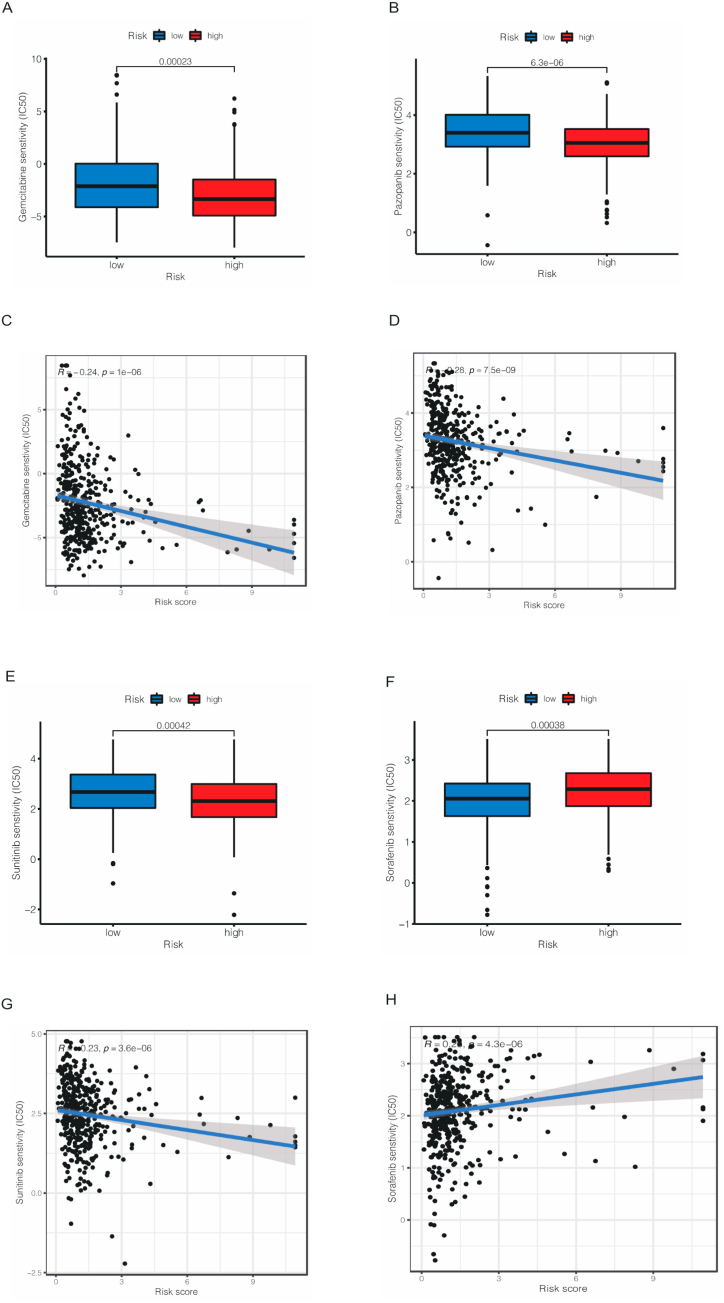
The somatic mutation data was downloaded from TCGA database and the differences between high- and low-risk cohorts. As shown in Fig. 10A–B, the most frequently mutated genes were TP53 in both two cohorts, as 56 % patients in high cohort was mutated while only 40 % patients had TP53 mutated. Nevertheless, no significant different was observed in TMB between two cohorts (Fig. 10C). To our delight, patients in low TMB and high-risk cohort had the worst outcome in four cohorts (Fig. 10D–E). As for drug sensitivity, we used IC50 to assess patients’ susceptibility to different drugs. The results were shown in Supplementary File 1. As a commonly used chemotherapy drug for bladder cancer, Gemcitabine was more effective in low-risk cohort than high-risk cohort (Fig. 11A,C). To our surprise, targeted drugs including Pazopanib (Fig. 11B,D) and Sunitinib (Fig. 11E,G) that had been widely used in other urological tumors, such as kidney cancer, had same effect while Sorafenib showed better therapeutic outcomes in high-risk cohort (Fig. 11F,H). Sorafenib is a novel multi-targeted anti-tumor drug that can simultaneously act on tumor cells and tumor blood vessels, providing dual anti-tumor effects. It achieves this by directly inhibiting tumor cell proliferation through blocking the RAF, MEK, and ERK-mediated cell signaling pathways. Furthermore, it can indirectly hinder tumor cell growth by inhibiting VEGFR and platelet-derived growth factor receptors, thereby preventing the formation of new tumor blood vessels. These results indicated that in clinical drug selection, our risk model could offer help for individual treatment of BCa patients.
Fig. 10.
Waterfall plots of somatic mutation characteristics in the low- and high-risk cohorts (A–B). The different TMB between the low- and high-risk cohorts (C). The Kaplan–Meier survival curves between high- and low-TMB cohorts (D). The Kaplan–Meier survival curves between the four different groups (E).
Fig. 11.
The results of different drug sensitivity including Gemcitabine (A, C), Pazopanib (B, D), Sunitinib (E, G) and Sorafenib (F,H) between low- and high-risk groups.
4. Discussion
Cancer is a life-threatening disease which results in thousands of death all over the world. Among the malignant tumors of urinary system, the incidence of bladder cancer is very high [20]. There are different treatments for different types of bladder cancer. The role of cuproptosis in cancer is complex and has not been understood thoroughly. Increasing number of researchers found that cuproptosis has a close relation with cancer [[21], [22], [23]]. But the relation between cuproptosis and bladder cancer has rarely studied. Our study established a novel cuproptosis-related lncRNAs risk model to predict the patients’ outcomes and provide more individual treatment, which offer a new perspective in bladder cancer therapy.
Previous studies have shown lncRNAs play an important role in the development and prognosis of bladder cancer. Xi etc. found that lncRNA RMRP was highly expressed in bladder cancer tissue and could promote the proliferation, migration and invasion via regulating miR-206 in different cell lines [24]. Depending on PROM2-activated iron export by sponging miR-129–5p, lncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance in bladder cancer [25]. Professor Lin noted that exosomal long noncoding RNA LNMAT2 can promote lymphatic metastasis and identify LNMAT2 as a therapeutic target in lymphatic metastasis bladder cancer [26].
Based on these studies, we assume that cuproptosis-related lcRNAs have a high value in bladder cancer diagnosis and therapy. So, we obtained the expression of cuproptosis-related lncRNAs expression from TCGA database, then the correlation between them was also analyzed. After univariate COX regression, LASSO analysis and multivariate COX regression, 7 prognosis-related CRLs was selected, which were LINC01184, LINC00513, LINC02443, SMARCA5-AS1, BDNF-AS, SOD2-OT1 and HYI-AS1.
LINC01184 was thought to be associated with tumor-infiltrating B lymphocytes, which might related to prognosis and immunotherapy of bladder cancer [27]. Other study also found LINC01184 enhanced the proliferation and invasion of colorectal Cancer by acting as a ceRNA through the linc01184-miR-331-HER2-p-Akt/ERK1/2 pathway regulatory network [28]. In this research, we also found that LINC01184 had different expression between normal and tumor tissues. Our model indicated it was a prognosis-related lncRNA and had therapy potential.
According to latest research, LINC00513 was regarded as a novel robust regulator of type I IFN pathway and had an important role in the pathogenesis of systemic lupus erythematosus (SLE) [29,30]. LINC02443 is positively correlated with Dihydrolipoamide Branched Chain Transacylase E2 (DBT). DBT is one of enzymes, which occurred protein lipoylation, a highly conserved lysine post-translational modification, involves regulation of carbon entry points to the TCA cycle [31,32]. DBT were associated with various diseases, including renal hypoxia, motor dysfunction and models maple syrup urine disease [33,34]. BDNF-AS was considered to be an oncogenic factor and was found in various types of cancer, such as breast cancer, brain cancer and gastric cancer [[35], [36], [37]]. In prostate and breast cancers, down-regulation of BDNF-AS has been associated with poor clinical outcome. HYI-AS1 is also a factor in prognostic and therapeutic prediction of endometrial Carcinoma [38]. The other lncRNAs were first publicly available. Delightfully, these newly discovered cuproptosis-related lncRNAs can help us have a better understand on the prognosis of bladder cancer and find novel therapy targets for individual therapy.
After we divided patients into low-risk and high-risk cohorts according to median values, ROC and C-index curve were employed to evaluate the prognostic accuracy of risk model. According to our results, we could induce that our risk model could be used as a criterion to predict the prognosis of bladder cancer. Then we established a nomogram to predict prognosis for particular patient. The calibration curves illustrated our prediction and actual outcome matched excellent. PCA analysis showed that our risk model based on seven cuproptosis-related lncRNAs could distinguish satisfied between high- and low-cohorts.
GO analysis suggested that multiple process including many immune-related activities were associated with different cohorts. KEGG analysis indicated cytokine−cytokine receptor interaction and IL−17 signaling pathway were active. IL−17 signaling pathway can be a double-edged sword. Although IL - 17 proinflammatory properties is the key to its host protection ability, but not by the inhibition of IL - 17 signal transduction and immune associated pathology, autoimmune diseases and cancer progression [39]. Generally, in the different stage of cancer, immune cell infiltration in the tumor microenvironment is also different [40,41]. Macrophages are the most abundant stromal cells associated with the immune system in the tumoral area and have been suggested to play a central role in tumor progression [42,43]. Accumulating evidence suggests that macrophages can enhance tumor invasion and metastasis by regulating host adaptive immunity [44,45]. Besides, a recent study found more tumor stroma could result in tumor growth, angiogenesis, and treatment resistance in bladder cancer [46]. In our study, Macrophages was found higher in high-risk cohort via ssGSEA. Thus, the expression of MHC class-I and CD 8 + T cell were also higher. These results indicated that our risk model had a close relationship with immune microenvironment and macrophages might had a crucial role in the progression of BCa. As for immune scores, stromal scores, tumor purity and ESTIMATE scores on different cohorts, we found that high-risk cohort had higher stromal scores and ESTIMATE scores, while the tumor purity was lower and had no difference in immune scores, indicating our risk model were highly consistent with the aforementioned study and showed the importance role of stroma componence in BCa.
In the treatment of bladder cancer, gemcitabine has important significance in the chemotherapy of advanced bladder cancer [47,48]. Some patients might have gemcitabine-resistance which results in metastasis and poorer OS [49]. Our results can provide better guidance for bladder cancer patients when choosing drugs. Through these methods, we constructed a seven cuproptosis-related lncRNAs model of BCa patients. However, our study still had some drawbacks and shortcomings. First, our data was downloaded only from TCGA database. Even though we tried to find data in GEO and GTeX, due to the differences in sequencing platforms and methods, the results could not be compared satisfactorily. Besides, the particular mechanism between stroma and bladder cancer needs further study and validation.
Author contribution statement
Haoran Wang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zhengtong Lv: Haoran Xia: Runhua Tang: Performed the experiments; Analyzed and interpreted the data.
Ming Liu: Contributed reagents, materials, analysis tools or data.
Jianlong Wang: Jianye Wang: Conceived and designed the experiments.
Data availability statement
Data will be made available on request.
Funding
This work was supported by Project of Chinese Academy of Medical Sciences,BJ-2022-237.
Declaration of competing interest
The authors state that they have no known competing financial interests or personal relationships that could influence the work reported in this article.
Acknowledgements
The authors would like to thank TCGA databases for data availability.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21153.
Contributor Information
Jianlong Wang, Email: wjlspplaaa@sina.com.
Jianye Wang, Email: wangjybjh@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Dobruch J., Oszczudłowski M. Bladder cancer: current challenges and future directions. Med. Kaunas Lith. Jul. 2021;57(8):749. doi: 10.3390/medicina57080749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenis A.T., Lec P.M., Chamie K., Mshs M.D. dder Cancer: A Review,” JAMA. Nov. 2020;324(19) doi: 10.1001/jama.2020.17598. 1980–1991. [DOI] [PubMed] [Google Scholar]
- 3.Rübben H., et al. Natural history and treatment of low and high risk superficial bladder tumors. J. Urol. Feb. 1988;139(2):283–285. doi: 10.1016/S0022-5347(17)42387-1. [DOI] [PubMed] [Google Scholar]
- 4.Patel V.G., Oh W.K., Galsky M.D. Treatment of muscle‐invasive and advanced bladder cancer in 2020. Ca - Cancer J. Clin. Sep. 2020;70(5):404–423. doi: 10.3322/caac.21631. [DOI] [PubMed] [Google Scholar]
- 5.Chen X., et al. Urine DNA methylation assay enables early detection and recurrence monitoring for bladder cancer. J. Clin. Invest. Dec. 2020;130(12):6278–6289. doi: 10.1172/JCI139597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng L., et al. Copper-catalyzed phosphorylation of N,N-disubstituted hydrazines: synthesis of multisubstituted phosphorylhydrazides as potential anticancer agents. J. Org. Chem. May 2022;87(9):6224–6236. doi: 10.1021/acs.joc.2c00452. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt B.S., Gandhi D.H., Vaidya F.U., Pathak C., Patel T.N. Cell apoptosis induced by ciprofloxacin based Cu(II) complexes: cytotoxicity, SOD mimic and antibacterial studies. J. Biomol. Struct. Dyn. Aug. 2021;39(12):4555–4562. doi: 10.1080/07391102.2020.1776641. [DOI] [PubMed] [Google Scholar]
- 8.Tsvetkov P., et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. Mar. 2022;375(6586):1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Y., et al. Comprehensive analysis of the potential cuproptosis-related biomarker LIAS that regulates prognosis and immunotherapy of pan-cancers. Front. Oncol. Aug. 2022;12 doi: 10.3389/fonc.2022.952129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin S., et al. A cuproptosis-related lncRNA signature identified prognosis and tumour immune microenvironment in kidney renal clear cell carcinoma. Front. Mol. Biosci. Sep. 2022;9 doi: 10.3389/fmolb.2022.974722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z., Zeng X., Wu Y., Liu Y., Zhang X., Song Z. Cuproptosis-related risk score predicts prognosis and characterizes the tumor microenvironment in hepatocellular carcinoma. Front. Immunol. Jul. 2022;13 doi: 10.3389/fimmu.2022.925618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amorim M., Salta S., Henrique R., Jerónimo C. Decoding the usefulness of non-coding RNAs as breast cancer markers. J. Transl. Med. Sep. 2016;14:265. doi: 10.1186/s12967-016-1025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridges M.C., Daulagala A.C., Kourtidis A. LNCcation: lncRNA localization and function. J. Cell Biol. Jan. 2021;220(2) doi: 10.1083/jcb.202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y. The novel regulatory role of lncRNA‐miRNA‐mRNA axis in cardiovascular diseases. J. Cell Mol. Med. Dec. 2018;22(12):5768–5775. doi: 10.1111/jcmm.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng W.-X., Koirala P., Mo Y.-Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. Oct. 2017;36(41):5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y.-H., Deng J.-L., Wang G., Zhu Y.-S. Long non-coding RNAs in prostate cancer: functional roles and clinical implications. Cancer Lett. Nov. 2019;464:37–55. doi: 10.1016/j.canlet.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Chen X., et al. Long noncoding RNA LBCS inhibits self-renewal and chemoresistance of bladder cancer stem cells through epigenetic silencing of SOX2. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. Feb. 2019;25(4):1389–1403. doi: 10.1158/1078-0432.CCR-18-1656. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. Oct. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T., et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. Nov. 2017;77(21) doi: 10.1158/0008-5472.CAN-17-0307. e108–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidl C. Targets for therapy of bladder cancer. Semin. Nucl. Med. Mar. 2020;50(2):162–170. doi: 10.1053/j.semnuclmed.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Lv H., et al. Comprehensive analysis of cuproptosis-related genes in immune infiltration and prognosis in melanoma. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.930041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yun Y., Wang Y., Yang E., Jing X. Cuproptosis-related gene - SLC31A1, FDX1 and ATP7B - polymorphisms are associated with risk of lung cancer. Pharmacogenomics Personalized Med. 2022;15:733–742. doi: 10.2147/PGPM.S372824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C., et al. Pan-cancer analyses confirmed the cuproptosis-related gene FDX1 as an immunotherapy predictor and prognostic biomarker. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.923737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao H.-L., Liu Z.-J., Huang P.-L., Yue Y.-L., Xi J.-N. lncRNA-RMRP promotes proliferation, migration and invasion of bladder cancer via miR-206. Eur. Rev. Med. Pharmacol. Sci. Feb. 2019;23(3):1012–1021. doi: 10.26355/eurrev_201902_16988. [DOI] [PubMed] [Google Scholar]
- 25.Luo W., et al. LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging miR-129-5p in bladder cancer. Cell Death Dis. Nov. 2021;12(11):1043. doi: 10.1038/s41419-021-04296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C., et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Invest. Jan. 2020;130(1):404–421. doi: 10.1172/JCI130892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou M., et al. Computational recognition of lncRNA signature of tumor-infiltrating B lymphocytes with potential implications in prognosis and immunotherapy of bladder cancer. Briefings Bioinf. May 2021;22(3):bbaa047. doi: 10.1093/bib/bbaa047. [DOI] [PubMed] [Google Scholar]
- 28.Sui Y.-X., Zhao D.-L., Yu Y., Wang L.-C. The role, function, and mechanism of long intergenic noncoding RNA1184 (linc01184) in colorectal cancer. Dis. Markers. 2021;2021 doi: 10.1155/2021/8897906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taheri M., Eghtedarian R., Dinger M.E., Ghafouri-Fard S. Exploring the role of non-coding RNAs in the pathophysiology of systemic lupus erythematosus. Biomolecules. Jun. 2020;10(6):E937. doi: 10.3390/biom10060937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue Z., et al. Identification of LncRNA Linc00513 containing lupus-associated genetic variants as a novel regulator of interferon signaling pathway. Front. Immunol. 2018;9:2967. doi: 10.3389/fimmu.2018.02967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solmonson A., DeBerardinis R.J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. May 2018;293(20):7522–7530. doi: 10.1074/jbc.TM117.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowland E.A., Snowden C.K., Cristea I.M. Protein lipoylation: an evolutionarily conserved metabolic regulator of health and disease. Curr. Opin. Chem. Biol. Feb. 2018;42:76–85. doi: 10.1016/j.cbpa.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedrich T., Lambert A.M., Masino M.A., Downes G.B. Mutation of zebrafish dihydrolipoamide branched-chain transacylase E2 results in motor dysfunction and models maple syrup urine disease. Dis. Model. Mech. Mar. 2012;5(2):248–258. doi: 10.1242/dmm.008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn S.H., et al. Interaction of peroxiredoxin V with dihydrolipoamide branched chain transacylase E2 (DBT) in mouse kidney under hypoxia. Proteome Sci. 2015;13:4. doi: 10.1186/s12953-014-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin X., et al. Enhancer-driven lncRNA BDNF-AS induces endocrine resistance and malignant progression of breast cancer through the RNH1/TRIM21/mTOR cascade. Cell Rep. Jun. 2020;31(10) doi: 10.1016/j.celrep.2020.107753. [DOI] [PubMed] [Google Scholar]
- 36.Colucci-D’Amato L., Speranza L., Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. Oct. 2020;21(20):E7777. doi: 10.3390/ijms21207777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang G., et al. The lncRNA BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer peritoneal metastasis by regulating VDAC3 ubiquitination. Int. J. Biol. Sci. 2022;18(4):1415–1433. doi: 10.7150/ijbs.69454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X.-Y., Dai H.-Y., Zhang H., Zhu J.-L., Hu H. Ferroptosis-related lncRNA for the establishment of novel prognostic signature and therapeutic response prediction to endometrial carcinoma. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/2056913. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Amatya N., Garg A.V., Gaffen S.L. IL-17 signaling: the yin and the yang. Trends Immunol. May 2017;38(5):310–322. doi: 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q., et al. ETV4 mediated tumor-associated neutrophil infiltration facilitates lymphangiogenesis and lymphatic metastasis of bladder cancer. Adv. Sci. Weinh. Baden-Wurtt. Ger. Apr. 2023;10(11) doi: 10.1002/advs.202205613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang M., et al. HSF1 facilitates the multistep process of lymphatic metastasis in bladder cancer via a novel PRMT5-WDR5-dependent transcriptional program. Cancer Commun. Lond. Engl. May 2022;42(5):447–470. doi: 10.1002/cac2.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantovani A., Schioppa T., Porta C., Allavena P., Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. Sep. 2006;25(3):315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A., Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. Apr. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Wang J., Li D., Cang H., Guo B. Crosstalk between cancer and immune cells: role of tumor‐associated macrophages in the tumor microenvironment. Cancer Med. Jun. 2019;8(10):4709–4721. doi: 10.1002/cam4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J., Tang Z., Gao S., Li C., Feng Y., Zhou X. Tumor-associated macrophages: recent insights and therapies. Front. Oncol. Feb. 2020;10:188. doi: 10.3389/fonc.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyake M., et al. CXCL1-Mediated interaction of cancer cells with tumor-associated macrophages and cancer-associated fibroblasts promotes tumor progression in human bladder cancer. Neoplasia N. Y. N. Sep. 2016;18(10):636–646. doi: 10.1016/j.neo.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coen J.J., et al. Bladder preservation with twice-a-day radiation plus fluorouracil/cisplatin or once daily radiation plus gemcitabine for muscle-invasive bladder cancer: NRG/RTOG 0712-A randomized phase II trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. Jan. 2019;37(1):44–51. doi: 10.1200/JCO.18.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfister C., et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. Jun. 2022;40(18) doi: 10.1200/JCO.21.02051. 2013–2022. [DOI] [PubMed] [Google Scholar]
- 49.Xiong Y., et al. KNSTRN promotes tumorigenesis and gemcitabine resistance by activating AKT in bladder cancer. Oncogene. Mar. 2021;40(9):1595–1608. doi: 10.1038/s41388-020-01634-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.