Abstract
Purpose
This study aimed to clarify the effect of donor and recipient age combinations on the short-term survival rates of patients undergoing lung transplantation.
Patients and methods
We retrospectively reviewed the 2017–2020 data of the Affiliated Wuxi People’s Hospital of Nanjing Medical University database for all adults (≥18 years), lung transplant recipients, and their associated donors. The impact of donor and recipient ages on survival was analyzed using a multivariable Cox proportional hazards regression model. Subgroup analysis was also performed based on recipient and donor ages.
Results
Different donor and recipient age combinations affected the short-term postoperative survival rates. When recipients were ≤55 years, the survival rates of the younger donor age group were significantly higher than the older donor age group at 30 days after surgery (P = 0.040); when the donors were ≤40 years, the postoperative survival rates of the younger recipient age group were significantly higher than the older recipient age group (P = 0.031, P = 0.026, P = 0.034, and P = 0.018 for 30 days, 90 days, 180 days, and 1 year after surgery, respectively).
Conclusion
Younger recipients had a higher survival rate after transplantation than older recipients, and this benefit could be compromised by older donors. Furthermore, the influence of donor age on patient survival rate was limited and more pronounced in younger recipients and shortly after surgery.
Keywords: Lung transplantation, Age, Survival rate, Donor, Recipient
1. Introduction
Lung transplantation (LTx) is a life-saving intervention and the most effective therapy for patients with irreversible lung disease [1]. Unfortunately, morbidity and mortality rates remain high in postoperative follow-up strategies for lung transplant recipients [2]. Reasons for the high morbidity and mortality in these patients include an elevated risk of infection, development of primary chronic graft dysfunction, and chronic lung allograft dysfunction [[3], [4], [5]].
Additionally, age can substantially affect the risk of complications in patients undergoing LTx [6,7]. For example, existing studies have demonstrated that older patients receiving LTx exhibit a significant decrease in 1- and 5-year survival compared to patients under 60 years of age [8,9]. Older age serves as a marker for a complex collection of factors, such as comorbidity profiles and underlying diseases, which potentially increase the morbidity and mortality of postoperative patients [10]. At the same time, as the age of the recipient increases, the probability of serious postoperative complications such as the development of thromboembolism [11] and post-transplant lymphoproliferative disorder also increases [12].
As the number of patients requiring LTx increases, the demand for donor organs exceeds the supply. This situation has led to the progressive expansion of the criteria for donor selection in LTx, and a portion of the lungs from older donors have been transferred to recipients [13,14].
Studies on other organ transplantations have revealed that combining the age of the recipient and donor for analysis may yield different results compared to separately analyzing the impact of the age of the recipient or donor on patient outcomes [15,16]; it would be more objective to investigate the influence of age on the prognosis of lung transplantation patients if recipient and donor ages are considered together. Therefore, this retrospective study was conducted to characterize the effect of donor and recipient age combinations on the short-term survival rate of patients receiving a LTx.
2. Methods
This study was approved by the Medical Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (approval number: KY22058). Written informed consent was not required because of the retrospective nature of the study and anonymity of the patients. All procedures involving human participants were performed according to the Declaration of Helsinki (revised in 2000).
2.1. Donation statement
All grafts were obtained from volunteer donations after the death of the patients, and the next of kin provided autonomous written informed consent. The Institutional Ethics Committee of the Organ Procurement Organization approved the donation procedure. Donor lungs were allocated through the China Organ Transplant Response System (COTRS, https://www.cot.org.cn/) with a comprehensive consideration of the recipient’s body size, blood type, urgency status (Lung Allocation Score was used to measure medical urgency for recipients aged above 12 years), and time spent on the waiting list. Donated lungs were obtained according to the Technical Specifications for Lung Donor Acquisition and Protection in China (2019 Edition) (https://zhyzzz.cma-cmc.com.cn/CN/10.3877/cma.j.issn.1674-3903.2019.02.002). Under the coordination of the OPO where the donor is located, the donor lung-harvesting team of the transplant hospital actively maintains the in vivo quality of the donated lung, including anti-infective therapy, airway management, fluid management, protective ventilation, and hormone administration. After the lungs were isolated, they were preserved and maintained in vitro using an appropriate perfusion preservation solution and temperature. The criteria for lung donors were based on the Chinese Guidelines for Lung Transplantation Donor Criteria and Acquisition and Transfer (https://www.organtranspl.com/cn/article/doi/10.3969/j.issn.1674-7445.2018.05.001), and mainly included ABO compatibility, age, smoking history, mechanical ventilation time, oxygenation index, chest radiograph, bronchoscopy, infection index, and cold ischemia time (CIT).
2.2. Study design
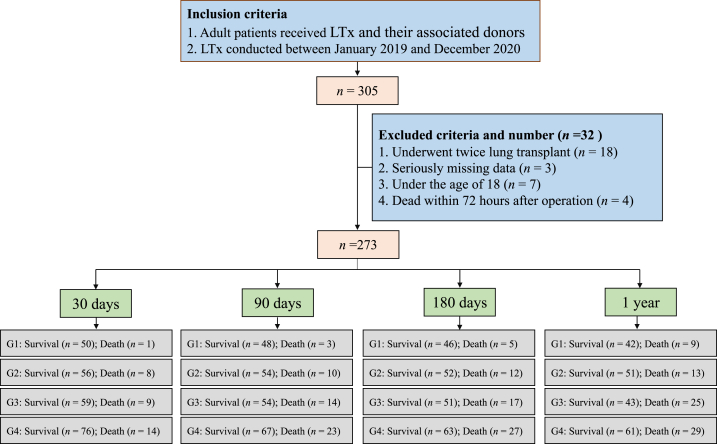
In this retrospective cohort study, we screened data from 305 patients who underwent LTx between January 2017 and December 2020 at the Affiliated Wuxi People’s Hospital of Nanjing Medical University, the largest LTx center in China. There were 32 patients excluded from this study for the following reasons: two LTx, missing data, under the age of 18 years, and within 72 h after surgery. A total of 273 patients were included in the data analysis (Fig. 1). The criteria for selecting LTx recipients were based on the Technical Specifications for the Selection and Preoperative Evaluation of Lung Transplantation Recipients in China (2019 edition) (https://zhyzzz.cma-cmc.com.cn/CN/10.3877/cma.j.issn.1674-3903.2019.02.001).
Fig. 1.
Flowchart of study inclusion. G1 indicates the recipient age is less than 55 years and the donor age is less than 40 years; G2 indicates the age of the recipient is less than 55 years old and the age of the donor is more than 40 years old; G3 indicates the age of recipient is more than 55 years old and the age of donor is less than 40 years old; G4 indicates the age of recipient is more than 55 years old and the age of donor is more than 40 years old.
2.3. Surgery
The LTx complied with standard procedures. First, whether recipients should undergo LTx was assessed by a multidisciplinary team consisting of an LTx surgeon, a pulmonologist, an intensive care unit (ICU) physician, and an anesthesiologist based on the potential benefits and risks. Second, all patients received general anesthesia with close bedside monitoring, and pulmonary and cardiovascular functions were accurately assessed during all phases of surgery using fluid and vasopressor therapy. Third, surgical techniques, including single and double LTx, were standardized, and intraoperative extracorporeal membrane oxygenation (ECMO) was adopted if respiratory or hemodynamic failure was highly expected. Patients were immediately transported to the ICU after surgery.
2.4. Immuno-suppressive therapy
Immunosuppressive therapy for LTx recipients primarily included immune-induction and maintenance. Specific protocols for immunosuppressive therapy and treatment of different types of rejection were based on China’s lung transplantation immunosuppressive therapy, rejection diagnosis, and treatment specifications (2019 edition) (https://zhyzzz.cma-cmc.com.cn/CN/10.3877/cma.j.issn.1674-3903.2019.02.004).
2.5. Data collection
The clinical characteristics of donors and recipients and early survival of recipients after LTx were retrospectively collected from the electronic medical record system and LTx data management platform of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (https://fyz.xinz.top/login).
Donor characteristics included sex, age, body mass index (BMI), blood type, total lung capacity (TLC), and graft CIT. The recipient characteristics included sex, age, BMI, blood type, TLC, and primary indications.
The predicted TLC of the donors and recipients were calculated from the height and age at transplantation as follows:
Males: predicted TLC (L) = (0.094 × height in cm) − (0.015 × age in years) − 9.167.
Females: predicted TLC (L) = (0.079 × height in cm) − (0.008 × age in years) − 7.49.
The preoperative characteristics of the recipients included the presence or absence of mechanical ventilation, presence or absence of bacterial infection, presence or absence of pan-drug resistant infection, presence or absence of fungal infection, need for hormone-dependent treatment, history of smoking, hypertension, diabetes, history of pneumothorax and/or pulmonary embolism, presence or absence of cardiovascular disease complications, history of blood transfusion, total bilirubin at baseline, levels of glutathione aminotransferase and glutathione transaminase at baseline, arterial partial pressure of oxygen (PaO2) and arterial partial pressure of carbon dioxide (PaCO2) at baseline, fraction of inspired oxygen (FiO2), and oxygenation index.
The donor and recipient combination characteristics included the donor/recipient predicted TLC ratio and ABO blood type compatibility.
Surgical characteristics included single or double LTx and the type of intraoperative ECMO. Intraoperative ECMO was divided into three types according to whether it passed through an arterial bypass: veno-venous ECMO (VV ECMO), veno-arterial ECMO (V-A ECMO), or veno-arterial-veno ECMO (V-A-V ECMO).
2.6. Follow-up
All patients were followed up using the LTx data management platform (https://fyz.xinz.top/login). The fixed follow-up time points were 30 days, 90 days, 180 days, and 1 year after LTx, and information for each recipient’s irregular visit to the hospital was also included in the management platform. In this study, all patients were followed up for at least 1 year or until death, with no loss of visits throughout the follow-up period.
2.7. Data analysis
All statistical analyses and plots were performed using R 4.0.2. and statistical significance was set at 0.05 (two-tailed test).
Continuous variables with normal or approximately normal distribution are presented as mean ± standard deviation (mean ± SD), and differences between groups were compared using independent samples t-test. Continuous variables that did not follow (approximately) normal or severely skewed distribution are presented as the median and interquartile range (IQR), and differences between groups were compared using the Wilcoxon rank sum test. Unordered categorical variables and ordered categorical variables are presented using the number of cases (n) and percentages (%), and differences between groups were compared using the chi-square (χ2) test and Wilcoxon rank sum test, respectively.
Time-dependent ROC (Time-ROC) was used to determine the best grouping threshold for recipient age and donor age using X-tile 3.6.1 software. Based on the best dichotomous threshold of recipient age and donor age, all recipients were divided into four groups: younger recipient and younger donor (group 1, G1), younger recipient and older donor (group 2, G2), older recipient and younger donor (group 3, G3), and older recipient and older donor (group 4, G4).
Log-rank tests were conducted to compare the differences in the risk of death at 365, 180, 90, and 30 days among the four groups. We then used a multivariable Cox proportional hazards regression model to correct for multiple variables (covariates), including donor characteristics and the preoperative and intraoperative characteristics of recipients, to assess the connection between donor and recipient age and the risk of death. Based on the variables in this database and expert perspectives, the corrected covariables included the donor/recipient predicted TLC ratio and ABO blood type compatibility, recipient sex, recipient BMI, primary indications, history of smoking, diabetes, hypertension, cardiovascular disease, donor BMI, and procedure type. Before fitting the Cox regression model, proportional hazard assumptions for covariates were assessed by testing the correlation between the weighted Schoenfeld residuals for each covariate and survival time.
In this study, multiple sensitivity analyses were performed to explore the effect of age on the risk of death at 365, 180, 90, and 30 days postoperatively according to recipient and donor ages.
3. Results
3.1. Description of donors and recipients
Patients were divided into four groups according to the best dichotomous threshold of recipient age and donor age: recipient age <55 years and donor age <40 years; recipient age <55 years and donor age >40 years; recipient age >55 years and donor age <40 years; and recipient age >55 years and donor age >40 years. These groups were denoted as G1, G2, G3, and G4.
The basic patient characteristics and preoperative and surgical characteristics of the patients in each group are described in Table 1. Age (P < 0.001), BMI (P = 0.017), and TLC (P < 0.001) differed across the donor groups. Sex (P < 0.001), age (P < 0.001), BMI (P = 0.024), and primary indications (P < 0.001) differed across recipients. There were also differences in the donor- and recipient predicted TLC ratios (P < 0.001) among the four groups. Several differences among the four groups existed in the preoperative characteristics of the recipients, including history of smoking (P < 0.001), diabetes (P = 0.004), hypertension (P < 0.001), and cardiovascular disease (P < 0.001). Procedure type (single or double LTx) also differed among the four groups (P < 0.001).
Table 1.
Comparison of characteristics among the different donor/recipient age-combination.
| Characteristic | Total | Group 1 (N = 51) | Group 2 (N = 64) | Group 3 (N = 68) | Group 4 (N = 90) | Statistics | P value |
|---|---|---|---|---|---|---|---|
| Characteristic of donors | |||||||
| Gender, n (%) | – | 0.225 | |||||
| Male | 233 (85.3) | 42 (82.4) | 54 (84.4) | 63 (92.6) | 74 (82.2) | ||
| Female | 40 (14.7) | 9 (17.6) | 10 (15.6) | 5 (7.4) | 16 (17.8) | ||
| Age (year), mean ± SD | 40.69 ± 11.27 | 29.82 ± 7.52 | 48.03 ± 4.74 | 30.37 ± 7.37 | 49.43 ± 5.90 | 23596.896 | <0.001 |
| BMI (kg/m2), mean ± SD | 23.32 ± 3.26 | 22.27 ± 3.47 | 23.34 ± 3.29 | 23.10 ± 3.12 | 24.05 ± 3.08 | 107.360 | 0.017 |
| Blood type, n (%) | 4.718 | 0.874 | |||||
| A | 74 (27.1) | 14 (27.5) | 15 (23.4) | 19 (27.9) | 26 (28.9) | ||
| B | 73 (26.7) | 13 (25.5) | 16 (25.0) | 21 (30.9) | 23 (25.6) | ||
| AB | 9 (3.3) | 3 (5.9) | 3 (4.7) | 2 (2.9) | 1 (1.1) | ||
| O | 117 (42.9) | 21 (41.2) | 30 (46.9) | 26 (38.2) | 40 (44.4) | ||
| TLC, mean ± SD | 5.99 ± 0.73 | 5.99 ± 0.72 | 5.84 ± 0.75 | 6.33 ± 0.65 | 5.84 ± 0.70 | 11.387 | <0.001 |
| CIT (min), mean ± SD | 8.09 ± 1.90 | 8.33 ± 1.56 | 8.47 ± 1.61 | 7.77 ± 2.37 | 7.93 ± 1.86 | 21.395 | 0.116 |
| Characteristic of recipients | |||||||
| Gender, n (%) | – | <0.001 | |||||
| Male | 227 (83.2) | 31 (60.8) | 51 (79.7) | 59 (86.8) | 86 (95.6) | ||
| Female | 46 (16.8) | 20 (39.2) | 13 (20.3) | 9 (13.2) | 4 (4.4) | ||
| Age (year), mean ± SD | 55.49 ± 12.46 | 43.67 ± 10.08 | 44.22 ± 10.02 | 64.12 ± 5.87 | 63.69 ± 4.91 | 26371.609 | <0.001 |
| BMI (kg/m2), mean ± SD | 20.37 ± 3.94 | 19.83 ± 4.37 | 19.32 ± 3.43 | 21.14 ± 3.71 | 20.85 ± 4.06 | 145.973 | 0.024 |
| Blood type, n (%) | 7.336 | 0.597 | |||||
| A | 87 (31.9) | 15 (29.4) | 19 (29.7) | 24 (35.3) | 29 (32.2) | ||
| B | 76 (27.8) | 15 (29.4) | 13 (20.3) | 21 (30.9) | 27 (30.0) | ||
| AB | 19 (7.0) | 4 (7.8) | 7 (10.9) | 5 (7.4) | 3 (3.3) | ||
| O | 91 (33.3) | 17 (33.3) | 25 (39.1) | 18 (26.5) | 31 (34.4) | ||
| TLC, mean ± SD | 5.78 ± 0.74 | 5.68 ± 0.88 | 5.91 ± 0.79 | 5.65 ± 0.71 | 5.82 ± 0.61 | 2.834 | 0.159 |
| Primary indications, n (%) | 60.921 | <0.001 | |||||
| IPF | 77 (28.2) | 10 (19.6) | 14 (21.9) | 21 (30.9) | 32 (35.6) | ||
| Secondary IPF | 59 (21.6) | 11 (21.6) | 11 (17.2) | 19 (27.9) | 18 (20.0) | ||
| COPD | 58 (21.2) | 5 (9.8) | 7 (10.9) | 15 (22.1) | 31 (34.4) | ||
| Pneumoconiosis | 36 (13.2) | 7 (13.7) | 21 (32.8) | 3 (4.4) | 5 (5.6) | ||
| Others | 43 (15.8) | 18 (35.3) | 11 (17.2) | 10 (14.7) | 4 (4.4) | ||
| Donor/recipient characteristic-combination | |||||||
| Donor/recipient predicted TLC ratio, mean ± SD | 1.05 ± 0.16 | 1.07 ± 0.15 | 1.00 ± 0.13 | 1.13 ± 0.15 | 1.01 ± 0.15 | 0.798 | <0.001 |
| ABO blood type compatibility, n (%) | – | 0.922 | |||||
| Identical | 230 (84.2) | 44 (86.3) | 55 (85.9) | 56 (82.4) | 75 (83.3) | ||
| Nonidentical | 43 (15.8) | 7 (13.7) | 9 (14.1) | 12 (17.6) | 15 (16.7) | ||
| Preoperative characteristic of recipients | |||||||
| Mechanical ventilation, n (%) | – | 0.256 | |||||
| No | 255 (93.4) | 45 (88.2) | 62 (96.9) | 65 (95.6) | 83 (92.2) | ||
| Yes | 18 (6.6) | 6 (11.8) | 2 (3.1) | 3 (4.4) | 7 (7.8) | ||
| Infection of pan-drug resistant bacterial, n (%) | – | 0.316 | |||||
| No | 255 (93.4) | 46 (90.2) | 61 (95.3) | 66 (97.1) | 82 (91.1) | ||
| Yes | 18 (6.6) | 5 (9.8) | 3 (4.7) | 2 (2.9) | 8 (8.9) | ||
| Infection of bacterial, n (%) | – | 0.668 | |||||
| No | 206 (75.5) | 37 (72.5) | 48 (75.0) | 55 (80.9) | 66 (73.3) | ||
| Yes | 67 (24.5) | 14 (27.5) | 16 (25.0) | 13 (19.1) | 24 (26.7) | ||
| Infection of fungal, n (%) | – | 0.065 | |||||
| No | 237 (86.8) | 45 (88.2) | 60 (93.8) | 53 (77.9) | 79 (87.8) | ||
| Yes | 36 (13.2) | 6 (11.8) | 4 (6.3) | 15 (22.1) | 11 (12.2) | ||
| Hormone dependency treatment, n (%) | – | 0.675 | |||||
| No | 139 (50.9) | 29 (56.9) | 32 (50.0) | 31 (45.6) | 47 (52.2) | ||
| Yes | 134 (49.1) | 22 (43.1) | 32 (50.0) | 37 (54.4) | 43 (47.8) | ||
| History of smoking, n (%) | – | 0.001 | |||||
| No | 136 (49.8) | 35 (68.6) | 34 (53.1) | 36 (52.9) | 31 (34.4) | ||
| Yes | 137 (50.2) | 16 (31.4) | 30 (46.9) | 32 (47.1) | 59 (65.6) | ||
| Diabetes, n (%) | – | 0.004 | |||||
| No | 216 (79.1) | 46 (90.2) | 55 (85.9) | 44 (64.7) | 71 (78.9) | ||
| Yes | 57 (20.9) | 5 (9.8) | 9 (14.1) | 24 (35.3) | 19 (21.1) | ||
| Hypertension, n (%) | – | <0.001 | |||||
| No | 213 (78.0) | 47 (92.2) | 59 (92.2) | 44 (64.7) | 63 (70.0) | ||
| Yes | 60 (22.0) | 4 (7.8) | 5 (7.8) | 24 (35.3) | 27 (30.0) | ||
| History of pneumothorax, n (%) | – | 0.282 | |||||
| No | 232 (85.0) | 41 (80.4) | 52 (81.3) | 62 (91.2) | 77 (85.6) | ||
| Yes | 41 (15.0) | 10 (19.6) | 12 (18.8) | 6 (8.8) | 13 (14.4) | ||
| History of pulmonary embolism, n (%) | – | 0.486 | |||||
| No | 264 (96.7) | 49 (96.1) | 63 (98.4) | 67 (98.5) | 85 (94.4) | ||
| Yes | 9 (3.3) | 2 (3.9) | 1 (1.6) | 1 (1.5) | 5 (5.6) | ||
| Cardiovascular disease, n (%) | – | <0.001 | |||||
| No | 218 (79.9) | 50 (98.0) | 61 (95.3) | 44 (64.7) | 63 (70.0) | ||
| Yes | 55 (20.1) | 1 (2.0) | 3 (4.7) | 24 (35.3) | 27 (30.0) | ||
| History of blood transfusion, n (%) | – | 0.092 | |||||
| No | 259 (94.9) | 45 (88.2) | 62 (96.9) | 67 (98.5) | 85 (94.4) | ||
| Yes | 14 (5.1) | 6 (11.8) | 2 (3.1) | 1 (1.5) | 5 (5.6) | ||
| Total bilirubin (μmol/L), median (IQR) | 11.0 (7.7–14.6) | 10.0 (7.2–13.6) | 11.1 (7.6–15.1) | 11.6 (8.6–14.9) | 11.0 (7.8–14.6) | 159.075 | 0.668 |
| Glutathione aminotransferase (U/L), median (IQR) | 20.0 (12.0–31.0) | 24.0 (15.3–33.5) | 22.0 (15.0–32.0) | 15.5 (10.8–26.3) | 19.0 (12.0–30.0) | 1161.599 | 0.365 |
| Glutathione transaminase (U/L), median (IQR) | 22.0 (16.7–30.3) | 22.5 (18.0–33.5) | 24.0 (18.0–34.0) | 20.0 (15.0–34.5) | 21.0 (15.0–27.0) | 676.963 | 0.365 |
| PaO2 (mmHg), median (IQR) | 72.0 (58.0–93.0) | 68.5 (55.3–85.5) | 69.0 (56.9–86.0) | 74.8 (59.8–95.8) | 74.0 (59.0–103.0) | 1564.831 | 0.695 |
| PaCO2 (mmHg), median (IQR) | 46.2 (40.8–55.2) | 43.3 (38.4–50.2) | 45.0 (41.0–53.5) | 47.4 (40.7–51.7) | 48.2 (41.7–59.7) | 855.472 | 0.205 |
| FiO2 (%), median (IQR) | 28.0 (21.0–37.0) | 29.0 (21.0–39.3) | 21.0 (21.0–36.0) | 29.5 (21.0–37.8) | 29.0 (21.0–33.0) | 1325.258 | 0.295 |
| Oxygenation index, median (IQR) | 271.5 (208.0–343.0) | 247.0 (199.8–328.5) | 266.0 (191.5–340.5) | 268.0 (222.3–343.0) | 290.0 (223.0–352.0) | 39950.269 | 0.365 |
| Surgical characteristics | |||||||
| Procedure type, n (%) | – | <0.001 | |||||
| SLT | 87 (31.9) | 7 (13.7) | 10 (15.6) | 31 (45.6) | 39 (43.3) | ||
| BLT | 186 (68.1) | 44 (86.3) | 54 (84.4) | 37 (54.4) | 51 (56.7) | ||
| Type of ECMO, n (%) | 6.484 | 0.370 | |||||
| V-A/V-A-V | 60 (22.0) | 11 (21.6) | 18 (28.1) | 16 (23.5) | 15 (16.7) | ||
| VV | 145 (53.1) | 28 (54.9) | 34 (53.1) | 30 (44.1) | 53 (58.9) | ||
| No | 68 (24.9) | 12 (23.5) | 12 (18.8) | 22 (32.4) | 22 (24.4) | ||
BLT, bilateral lung transplantation; BMI, body mass index; CIT, Cold ischemia time; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range, SLT, single lung transplantation; TLC, total lung capacity; V-A, veno-arterial; V-A-V, veno-arterial-veno; VV, veno-venous; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; FiO2, fraction of inspired oxygen.
3.2. Postoperative survival rate of patients
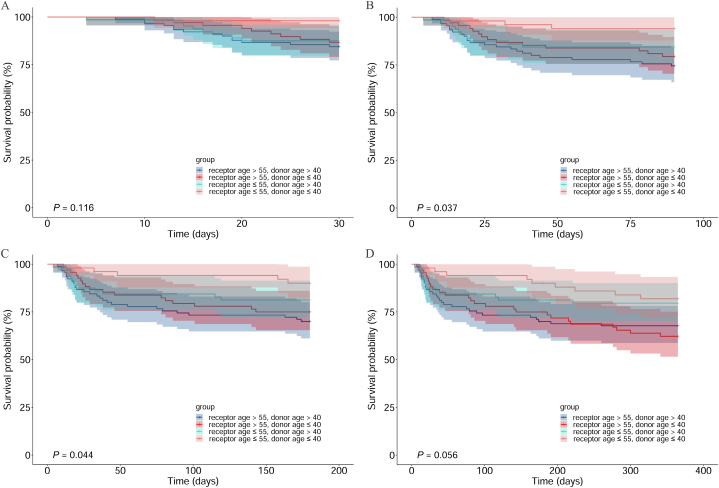
Survival outcomes are described in Table 2 and Fig. 2. A log-rank test was conducted to compare the differences in survival rates among the groups (Fig. 2). The 1-year survival rates of the four groups (G1, G2, G3, and G4) after surgery were 81.9%, 79.6%, 62.2%, and 67.7%, respectively (P = 0.056, Fig. 2D); the 180-day survival rates of the four groups were 90.1%, 81.3%, 75.0%, and 70.0%, respectively (P = 0.044, Fig. 2C); the 90-day survival rates of the four groups were 94.1%, 84.4%, 79.4%, and 74.4%, respectively (P = 0.037, Fig. 2B); and the 30-day survival rates of the four groups were 98.0%, 87.5%, 86.8%, and 84.4%, respectively (P = 0.116, Fig. 2A). Survival rates at 1 year, 180 days, and 90 days postoperatively differed among the four groups.
Table 2.
The short-term survival rate after surgery of different donor/recipient age-combination.
| age-combination | Follow up time (days) | Number of Survival | Number of deaths | Survival rate (95%CI) |
|---|---|---|---|---|
| G1 | 30 | 50 | 1 | 98.0 (94.3–100.0) |
| 90 | 48 | 3 | 94.1 (87.8–100.0) | |
| 180 | 46 | 5 | 90.1 (82.2–98.7) | |
| 365 | 42 | 9 | 81.9 (71.8–93.3) | |
| G2 | 30 | 56 | 8 | 87.5 (79.8–96.0) |
| 90 | 54 | 10 | 84.4 (75.9–93.8) | |
| 180 | 52 | 12 | 81.2 (72.2–91.4) | |
| 365 | 51 | 13 | 79.6 (70.3–90.1) | |
| G3 | 30 | 59 | 9 | 86.8 (79.1–95.2) |
| 90 | 54 | 14 | 79.4 (70.4–89.6) | |
| 180 | 51 | 17 | 75.0 (65.4–86.0) | |
| 365 | 43 | 25 | 62.2 (51.5–75.2) | |
| G4 | 30 | 76 | 14 | 84.4 (77.3–92.3) |
| 90 | 67 | 23 | 74.4 (66.0–84.0) | |
| 180 | 63 | 27 | 70.0 (61.1–80.1) | |
| 365 | 61 | 29 | 67.7 (58.7–78.1) |
Fig. 2.
Comparison of postoperative survival rate among donor/recipient age-combination at different follow up time. A: for 30 days follow-up, B: for 90 days follow-up, C: for 180 days follow-up, D: for 365 days follow-up.
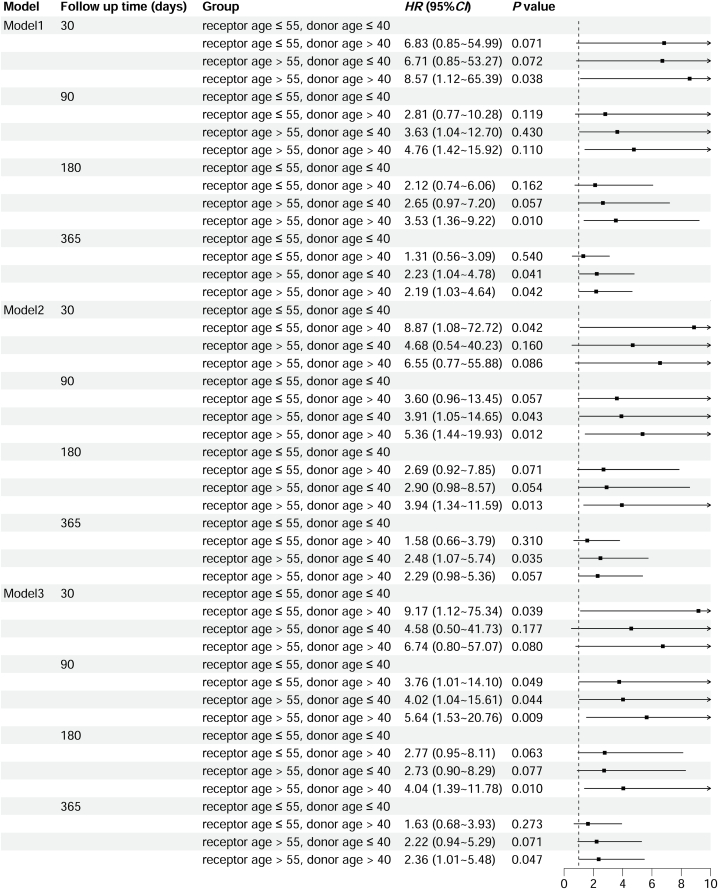
3.3. Factors influencing the 1-year survival rate following operation
Multivariate Cox regression analysis was used to analyze the effect of the donor/recipient age combination on the risk of postoperative mortality at different follow-up times (Fig. 3). In Model 1, the adjusted covariates included the donor/recipient predicted TLC ratio and ABO blood type compatibility. The influence of donor/recipient age combination on postoperative mortality can be found at 30 days, 180 days, and 1 year postoperatively. In Model 2, the adjusted covariates included recipient sex, recipient BMI, primary indications, history of smoking, diabetes, hypertension, cardiovascular disease, donor BMI, and procedure type. The influence of the donor/recipient age combination on postoperative mortality can be found at 30 days, 90 days, 180 days, and 1 year after surgery. Model 3 was adjusted for all covariates included in Models 1 and 2. The influence of the donor/recipient age combination on postoperative mortality was also observed at 30 days, 90 days, 180 days, and 1 year after surgery.
Fig. 3.
The result of multivariate Cox regression analysis about effect of donor/recipient age-combination on the risk of postoperative mortality at different follow up time. Model 1 adjusted covariates including donor/recipient predicted TLC ratio and ABO blood type compatibility; Model 2 adjusted covariates including recipient gender, recipient BMI, primary indications, history of smoking, diabetes, hypertension, cardiovascular disease, donor BMI, and procedure type; Model 3 adjusted covariates include both mode 1 and model 2.
3.4. Subgroup analysis
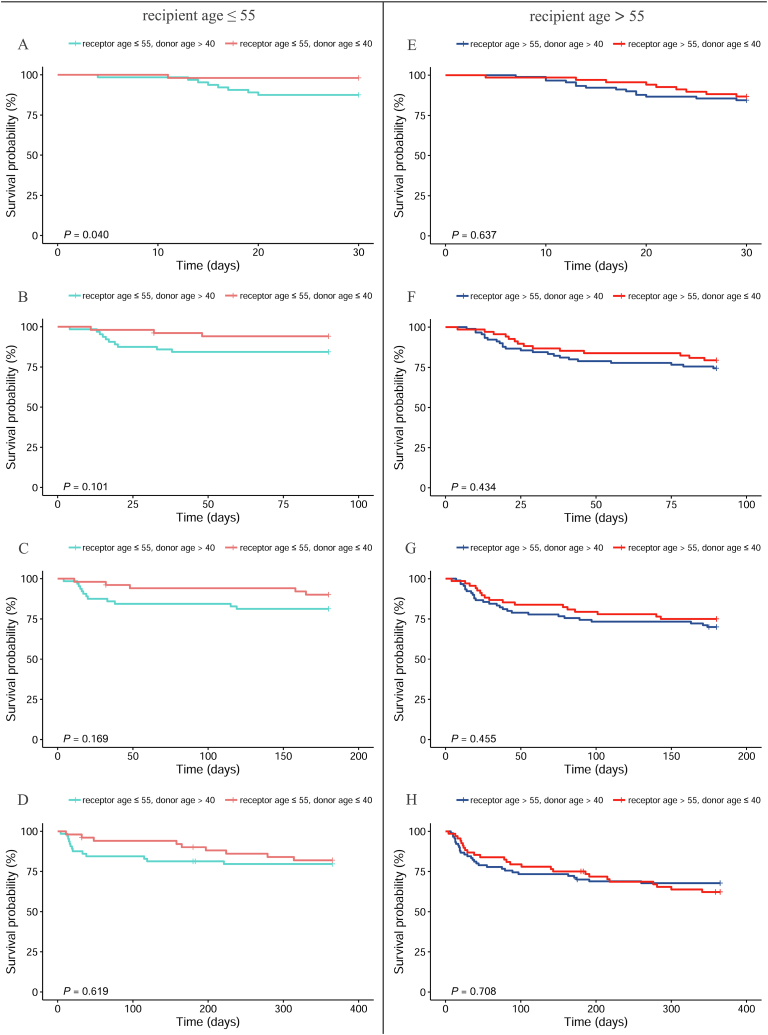
3.4.1. Subgroups by recipient age
The survival status of the two subgroups at different donor ages and time points was analyzed according to whether the recipient was younger than 55 years (Fig. 4). When the recipient was younger than 55 years, the survival rates of the younger donor group were significantly higher than those of the older donor group at 30 days after surgery (P = 0.040, Fig. 4A). Furthermore, donor age had no influence on the survival rates of recipients at 90 days (P = 0.101, Fig 4B), 180 days (P = 0.169, Fig. 4C) and 1 year (P = 0.619, Fig. 4D) after surgery.
Fig. 4.
Comparison of postoperative survival rate between different donor age by recipient age at different follow up time. A and E: for 30 days follow-up, B and F: for 90 days follow-up, C and G: for 180 days follow-up, D and H: for 365 days follow-up.
When the recipient was older than 55 years, the donor age had no influence on the survival rate of the recipient at all timepoints after surgery (P = 0.637 for 30 days after surgery, Fig. 4E; P = 0.434 for 90 days after surgery, Fig. 4F; P = 0.455 for 180 days after surgery, Fig. 4G; and P = 0.708 for 1 year, Fig. 4H).
3.4.2. Subgroups by donor age
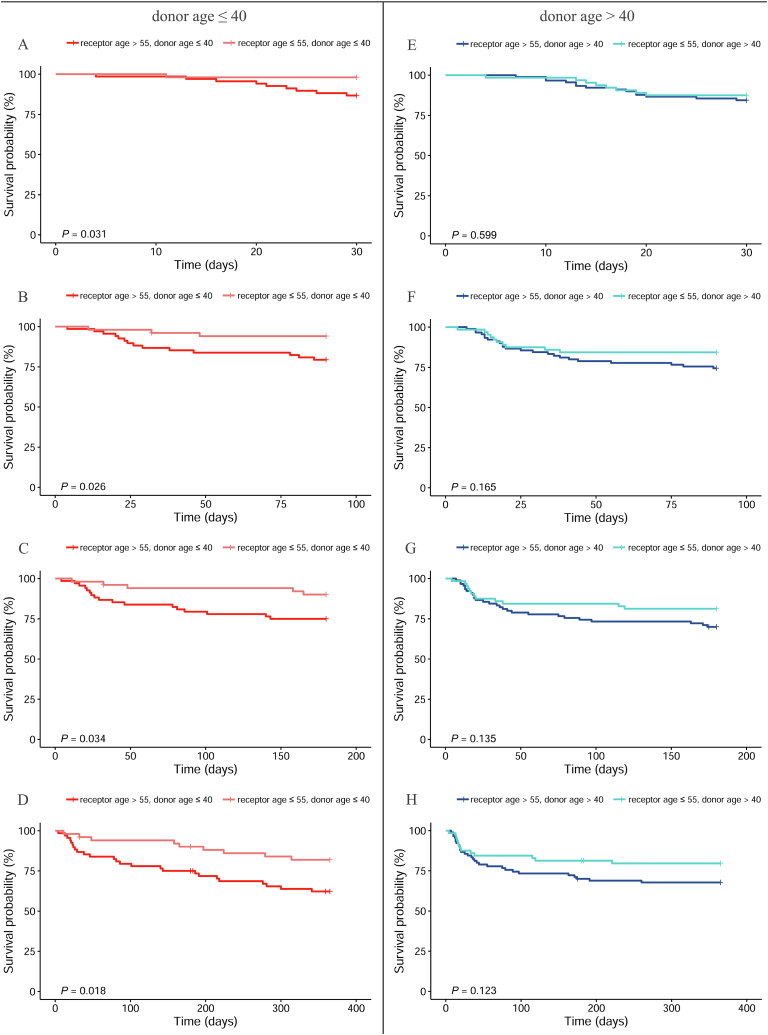
When the donors were divided into two groups according to age (<40 years), we analyzed the differences in the survival status of the donors at various time points in the subgroups and found various differences in survival rates (Fig. 5). Specifically, when the donor age was less than 40 years, at 30 days, 90 days, 180 days, and 1 year after surgery, the survival rates of the younger recipient age group were significantly higher than those of the older recipient age group (P = 0.031 for 30 days after surgery, Fig. 5A; P = 0.026 for 90 days after surgery, Fig. 5B; P = 0.034 for 180 days after surgery, Fig. 5C; and P = 0.018 for 1year after surgery, Fig. 5D). Furthermore, when the age of the donor was >40 years, the recipient age had no influence on the survival rates of patients at all timepoints after surgery (P = 0.599 for 30 days after surgery, Fig. 5E; P = 0.165 for 90 days after surgery, Fig. 5F; P = 0.135 for 180 days after surgery, Fig. 5G; and P = 0.123 for 1year after surgery, Fig. 5H).
Fig. 5.
Comparison of postoperative survival rate between different recipient age by donor age at different follow up time. A and E: for 30 days follow-up, B and F: for 90 days follow-up, C and G: for 180 days follow-up, D and H: for 365 days follow-up.
4. Discussion
In this retrospective study, the data of 273 LTx patients and their related donors collected from the Affiliated Wuxi People’s Hospital of Nanjing Medical University were analyzed to better understand the association between donor and recipient age and post-transplant recipient survival. The overall survival rates reported in our study were comparable to those reported in other countries [17]. The results indicated that the combination of donor and recipient age may influence recipient survival at 30 days, 90 days, 180 days, and 1 year postoperatively. The highest survival rate in our study was found in the group of recipients aged ≤55 and donor aged ≤40, while the highest mortality at 90 days, 180 days, and 1 year after surgery was seen in the group of recipients aged >55 and donors aged >40. The results of the current study also indicate that donor age had a limited influence on the postoperative survival rates of patients who underwent LTx, which can only be found when the recipients were younger than 55 years and shortly after surgery (30 days). Furthermore, recipient age was a critical factor in the postoperative survival rate of patients when the donor age was less than 40 years. However, the influence of recipient age on patient survival rates disappeared when the donor was aged >40 years.
As indicated by the International Society of Heart and LTx, older patients undergoing LTx exhibit a significant decrease in 1- and 5-year survival rates compared to patients under 60 years of age [18,19]. This could indicate that the mean age of recipients plays a key role in the recovery of patients receiving LTx. Therefore, it is understandable that aging is likely associated with physiological vulnerability to stressors, and may produce adverse health outcomes, including frailty [20,21]. A phenomenon was found in our study: recipients over 55 years had a higher incidence of diabetes, systemic hypertension, and cardiovascular disease. Diabetes is an independent risk factor for increased morbidity and mortality after LTx. A previous study that included 192 patients undergoing LTx demonstrated that preoperative diabetes was associated with higher mortality after transplantation and that earlier endocrine participation in LTx services is likely to lower diabetes-related morbidity and mortality [22]. Furthermore, a retrospective study reported that pre-transplant diabetes mellitus could increase the risk of cancer after LTx, and cancer is a major source of morbidity and mortality in patients with solid organ transplantations [23]. To our knowledge, diabetes also increases the morbidity of cardiovascular diseases, which are thought to increase the mortality of patients receiving LTx [[24], [25], [26]].
The results of a previous study indicated that elevated systolic pulmonary artery pressure, low cardiac index before transplantation, and history of atrial fibrillation were associated with worse survival after transplantation [27]. It is also important to note that coronary artery disease is common in older recipients. Although the results of an existing study indicated that mild-to-moderate coronary artery disease was not an independent risk factor for the overall long-term outcome of recipients [28,29], severe preoperative coronary heart disease may still impact patient survival due to unpredictable cardiovascular events [10]. A previous study that included 280 patients undergoing LTx demonstrated that patients with significant coronary artery disease had a higher annual rate of cardiovascular events than those without mild coronary artery disease [30]. In addition, chronic immunosuppression and other drug therapies are associated with accelerated atherosclerosis, which can aggravate coronary artery disease after LTx [31]. Furthermore, systemic hypertension has been reported to be a risk factor for the recovery of patients receiving LTx, as accumulated evidence suggests that systemic hypertension has increased the occurrence of atrial arrhythmias [10,32].
Our study demonstrated that younger recipients had higher postoperative survival rates than older recipients when the donor age was <40 years. This indicates that recipient age plays a critical role in determining the survival rate of patients undergoing LTx. However, it is unclear whether age can be considered a marker of the complex collection of factors that reduce long-term survival after LTx. It appears that the fundamental factor affecting survival rate is the degradation of physiological functions caused by aging [10].
With an increasing number of patients requiring LTx, donor organ availability has become a serious issue [13]. Basic and clinical research has made significant efforts to increase donor utilization [[33], [34], [35]]. One concept to overcome the shortage of donor organs is the use of lungs from “marginal” donors, and the older donor age (≥55 years) was considered as an independent factor for these “marginal” donors [36]. Currently, there is still a lack of insufficient evidence to determine the negative effect of donor age on recipient mortality after transplantation. In a retrospective study with a large sample size, outcomes of more than 20,000 LTx recipients were analyzed, and the results indicated that older donor lungs did not negatively impact survival in older recipients but had limited outcomes in younger recipients [37], which indicated the potential impact of donor age on the outcomes of recipients. The following reasons may explain the influence of donor age: decreased elastic recoil and senile emphysema may reduce forced expiratory volume in 1 s [38,39] and increased accumulation of oxidative stress and telomere shortening may lead to fibrosis [40], which is a pathological feature of chronic lung allograft dysfunction.
The results of our study demonstrated that for recipients younger than 55 years, younger donors offered a lower mortality rate 30 days after surgery, indicating that the benefits of younger donors were inadequate. However, our study found that older donors (>40 years old) compromised the benefits of younger recipients on the postoperative survival of patients receiving LTx. It should be noted that age thresholds set in our study (55 for recipients and 40 for donors) were different than those set in previous studies (60 or 65 for recipients and 50 for donors). The results obtained from our study offer limited evidence that the advantage of younger donor organs should not be dismissed, and the study of donor or recipient age independently may not be conducive to a more in-depth disclosure of potential influencing factors. Furthermore, the combination of donor and recipient age may be an important factor affecting the survival rate of patients after surgery, and our results can help to guide that understanding. Unadjusted and adjusted Cox proportional hazards modeling demonstrated that donor and recipient age combinations impacted the short-term postoperative survival rate of patients.
The maximum follow-up period for patients in this study was 1 year; therefore, we could not provide evidence on the impact of donor and recipient age matching on the long-term survival of patients. However, when the donor age was less than 40 years, the benefit of younger age can be expected in the long-term survival rate of recipients, as young recipients still achieved higher survival rates one year after surgery than older recipients, and the age of recipients was indicated as a critical factor affecting the survival rate of recipients [13,41].
The findings of this study have considerable implications, indicating that the age of the donor may not be the main factor affecting patient prognosis; though, for younger recipients, grafts from younger donors could be a better choice. However, it could be premature to generalize our findings to higher-volume transplant centers and other geographical regions because limited donors do not provide us with many options.
Our study has some limitations. The sample size of this study was small. This is because of the limited number of LTx procedures performed in China per year. In the future, we need to expand the sample size for further observation. As indicated in previous studies, most donors in the older cohort died of cerebrovascular accidents, whereas more donors in the younger cohort died from head injuries [13]. In our study, we did not analyze the causes of brain death in donors. From the perspective of neurohumoral and biochemical changes, there may be significant differences between patients whose death was caused by trauma and those whose death was the result of a cerebrovascular accident. More data on the causes of death may provide better clarity on why older donors affect the survival rate of patients receiving LTx.
In conclusion, our study revealed that younger recipients had a higher survival rate than older recipients after transplantation, and that this benefit could be compromised by older donors. Furthermore, the influence of donor age on patient survival rate was limited and more pronounced in younger recipients and shortly after surgery.
Data availability statement
The datasets generated and/or analyzed in the current study are available from the corresponding authors upon reasonable request.
Ethics declarations
This study was approved by the Medical Ethics Committee of the Affiliated Wuxi People’s Hospital of the Nanjing Medical University (approval number: KY22058). Written informed consent was not required because of the retrospective nature of the study and anonymity of the patients. All procedures involving human participants were performed according to the Declaration of Helsinki (revised in 2000).
CRediT authorship contribution statement
Bin Mei: Writing – original draft, Funding acquisition, Data curation. Xiaoshan Li: Methodology, Formal analysis. Juntao Weng: Writing – original draft. Jing Wang: Investigation, Formal analysis, Data curation. Feng Liu: Methodology, Investigation, Data curation. Jingyu Chen: Writing – review & editing, Supervision, Conceptualization. Xuesheng Liu: Writing – review & editing, Supervision, Formal analysis, Conceptualization. Chunxiao Hu: Writing – review & editing, Resources, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant No. 82172136), The Innovation and Entrepreneurship Project for returned overseas students in Anhui Province (Grant No. 2022LCX023), and the Special Funds of Science and Technology Program in Jiangsu Province (Key R&D Program Social Development, Grant No. BE2022697). The authors would like to thank Xiaoxia Xu, MBBS., from the Department of Anesthesiology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China, for her work on table and figures formatting.
Contributor Information
Xuesheng Liu, Email: liuxuesheng@ahmu.edu.cn.
Chunxiao Hu, Email: huchunxiao91211@163.com.
References
- 1.Capuzzimati M., Hough O., Liu M. Cell death and ischemia-reperfusion injury in lung transplantation. J. Heart Lung Transplant. 2022;41(8):1003–1013. doi: 10.1016/j.healun.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Rudym D., Benvenuto L., Costa J., et al. What awaits on the other side: post-lung transplant morbidity and mortality after pre-transplant hospitalization. Ann. Transplant. 2020;25 doi: 10.12659/AOT.922641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng D., Chang R., Zhu R. Analysis of nosocomial infection and risk factors in lung transplant patients: a case-control study. Ann. Transl. Med. 2022;10(14):804. doi: 10.21037/atm-22-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGinniss J.E., Whiteside S.A., Deek R.A., et al. The lung allograft microbiome associates with Pepsin, inflammation, and primary graft dysfunction. Am. J. Respir. Crit. Care Med. 2022;206(12):1508–1521. doi: 10.1164/rccm.202112-2786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McInnis M.C., Ma J., Karur G.R., et al. Chronic lung allograft dysfunction phenotype and prognosis by machine learning CT analysis. Eur. Respir. J. 2022;60(1) doi: 10.1183/13993003.01652-2021. [DOI] [PubMed] [Google Scholar]
- 6.Schaenman J.M., Diamond J.M., Greenland J.R., et al. Frailty and aging-associated syndromes in lung transplant candidates and recipients. Am. J. Transplant. 2021;21(6):2018–2024. doi: 10.1111/ajt.16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dugger D.T., Calabrese D.R., Gao Y., et al. Lung allograft epithelium DNA methylation age is associated with graft chronologic age and primary graft dysfunction. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.704172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inci I., Schuurmans M., Ehrsam J., et al. Lung transplantation for emphysema: impact of age on short- and long-term survival. Eur. J. Cardio. Thorac. Surg. 2015;48(6):906–909. doi: 10.1093/ejcts/ezu550. [DOI] [PubMed] [Google Scholar]
- 9.Kwon J.H., Hardy W.A., Shorbaji K., et al. Risk of recipient age on 1-year mortality after simultaneous heart-lung transplantation. J. Card. Surg. 2022;37(12):4437–4440. doi: 10.1111/jocs.17009. [DOI] [PubMed] [Google Scholar]
- 10.Ehrsam J.P., Benden C., Seifert B., et al. Lung transplantation in the elderly: influence of age, comorbidities, underlying disease, and extended criteria donor lungs. J. Thorac. Cardiovasc. Surg. 2017;154(6):2135–2141. doi: 10.1016/j.jtcvs.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Moneke I., Ogutur E.D., Kalbhenn J., Hettich I., Passlick B., Jungraithmayr W., Senbaklavaci O. Independent risk factors for an increased incidence of thromboembolism after lung transplantation. J. Thromb. Thrombolysis. 2023;55(2):252–262. doi: 10.1007/s11239-022-02748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaffiri L., Long A., Neely M.L., Cherikh W.S., Chambers D.C., Snyder L.D. Incidence and outcome of post-transplant lymphoproliferative disorders in lung transplant patients: analysis of ISHLT Registry. J. Heart Lung Transplant. 2020;39(10):1089–1099. doi: 10.1016/j.healun.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer S., Gohrbandt B., Struckmeier P., et al. Lung transplantation with lungs from donors fifty years of age and older. J. Thorac. Cardiovasc. Surg. 2005;129(4):919–925. doi: 10.1016/j.jtcvs.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 14.Chaney J., Suzuki Y., Cantu E., 3rd, et al. Lung donor selection criteria. J. Thorac. Dis. 2014;6(8):1032–1038. doi: 10.3978/j.issn.2072-1439.2014.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerbase-DeLima M., de Marco R., Monteiro F., Tedesco-Silva H., Medina-Pestana J.O., Mine K.L. Impact of combinations of donor and recipient ages and other factors on kidney graft outcomes. Front. Immunol. 2020;11:954. doi: 10.3389/fimmu.2020.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bittermann T., Goldberg D.S. Quantifying the effect of transplanting older donor livers into younger recipients: the need for donor-recipient age matching. Transplantation. 2018;102(12):2033–2037. doi: 10.1097/TP.0000000000002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dotan Y., Vaidy A., Shapiro W.B., Zhao H., Dass C., Toyoda Y., Marchetti N., Shenoy K., Cordova F.C., Criner G.J., Mamary A.J. Effect of acute exacerbation of idiopathic pulmonary fibrosis on lung transplantation outcome. Chest. 2018;154(4):818–826. doi: 10.1016/j.chest.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Yusen R.D., Christie J.D., Edwards L.B., et al. The registry of the international society for heart and lung transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. J. Heart Lung Transplant. 2013;32(10):965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Yusen R.D., Edwards L.B., Dipchand A.I., et al. The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J. Heart Lung Transplant. 2016;35(10):1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Schaenman J.M., Diamond J.M., Greenland J.R., et al. Frailty and aging-associated syndromes in lung transplant candidates and recipients. Am. J. Transplant. 2021;21(6):2018–2024. doi: 10.1111/ajt.16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proietti M., Cesari M. Frailty: what is it? Adv. Exp. Med. Biol. 2020;1216:1–7. doi: 10.1007/978-3-030-33330-0_1. [DOI] [PubMed] [Google Scholar]
- 22.Fazekas-Lavu M., Reyes M., Malouf M., et al. High prevalence of diabetes before and after lung transplantation: target for improving outcome? Intern. Med. J. 2018;48(8):916–924. doi: 10.1111/imj.13963. [DOI] [PubMed] [Google Scholar]
- 23.Kirov H., Moschovas A., Caldonazo T., et al. Diabetes is an independent risk factor for cancer after heart and/or lung transplantation. J. Clin. Med. 2022;11(14):4127. doi: 10.3390/jcm11144127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvador D.B., Jr., Gamba M.R., Gonzalez-Jaramillo N., et al. Diabetes and myocardial fibrosis: a systematic review and meta-analysis. JACC Cardiovasc. Imaging. 2022;15(5):796–808. doi: 10.1016/j.jcmg.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Baldissera S., Minardi V., Masocco M., et al. Cardiovascular risk and protective factors in adults with and without diabetes mellitus (Italy, 2016-19) Eur. J. Publ. Health. 2022;32(4):617–623. doi: 10.1093/eurpub/ckac037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato T.S., Armstrong H.F., Schulze P.C., et al. Left and right ventricular functional dynamics determined by echocardiograms before and after lung transplantation. Am. J. Cardiol. 2015;116(4):652–659. doi: 10.1016/j.amjcard.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plantier L., Skhiri N., Biondi G., et al. Impact of previous cardiovascular disease on the outcome of lung transplantation. J. Heart Lung Transplant. 2010;29(11):1270–1276. doi: 10.1016/j.healun.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Choong C.K., Meyers B.F., Guthrie T.J., et al. Does the presence of preoperative mild or moderate coronary artery disease affect the outcomes of lung transplantation? Ann. Thorac. Surg. 2006;82(3):1038–1042. doi: 10.1016/j.athoracsur.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 29.Zanotti G., Hartwig M.G., Castleberry A.W., et al. Preoperative mild-to-moderate coronary artery disease does not affect long-term outcomes of lung transplantation. Transplantation. 2014;97(10):1079–1085. doi: 10.1097/01.TP.0000438619.96933.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaikriangkrai K., Jyothula S., Jhun H.Y., et al. Impact of pre-operative coronary artery disease on cardiovascular events following lung transplantation. J. Heart Lung Transplant. 2016;35(1):115–121. doi: 10.1016/j.healun.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowska M., Oldakowska-Jedynak U., Wojtaszek E., et al. Potential effects of immunosuppression on oxidative stress and atherosclerosis in kidney transplant recipients. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/6660846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan J., Zhou K., Li S., et al. Incidence, risk factors and prognosis of postoperative atrial arrhythmias after lung transplantation: a systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2016;23(5):790–799. doi: 10.1093/icvts/ivw208. [DOI] [PubMed] [Google Scholar]
- 33.Carr B.D., Poling C.J., Hala P., et al. A model of pediatric end-stage lung failure in small lambs <20 kg. Am. Soc. Artif. Intern. Organs J. 2020;66(5):572–579. doi: 10.1097/MAT.0000000000001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyun S., Haam S. Donation after circulatory death in lung transplantation. J. Chest. Surg. 2022;55(4):283–287. doi: 10.5090/jcs.22.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu W.S., Son J. Donor selection, management, and procurement for lung transplantation. J. Chest. Surg. 2022;55(4):277–282. doi: 10.5090/jcs.22.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierre A.F., Sekine Y., Hutcheon M.A., et al. Marginal donor lungs: a reassessment. J. Thorac. Cardiovasc. Surg. 2002;123(3):421–428. doi: 10.1067/mtc.2002.120345. [DOI] [PubMed] [Google Scholar]
- 37.Hayes D., Jr., Black S.M., Tobias J.D., et al. Influence of donor and recipient age in lung transplantation. J. Heart Lung Transplant. 2015;34(1):43–49. doi: 10.1016/j.healun.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Colebatch H.J., Greaves I.A., Ng C.K. Exponential analysis of elastic recoil and aging in healthy males and females. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979;47(4):683–691. doi: 10.1152/jappl.1979.47.4.683. [DOI] [PubMed] [Google Scholar]
- 39.Knudson R.J., Kaltenborn W.T. Evaluation of lung elastic recoil by exponential curve analysis. Respir. Physiol. 1981;46(1):29–42. doi: 10.1016/0034-5687(81)90066-9. [DOI] [PubMed] [Google Scholar]
- 40.Faust H.E., Golden J.A., Rajalingam R., et al. Short lung transplant donor telomere length is associated with decreased CLAD-free survival. Thorax. 2017;72(11):1052–1054. doi: 10.1136/thoraxjnl-2016-209897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inci I., Schuurmans M., Ehrsam J., Schneiter D., Hillinger S., Jungraithmayr W., Benden C., Weder W. Lung transplantation for emphysema: impact of age on short- and long-term survival. Eur. J. Cardio. Thorac. Surg. 2015;48(6):906–909. doi: 10.1093/ejcts/ezu550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in the current study are available from the corresponding authors upon reasonable request.