Abstract
Background
Levothyroxine is a very commonly prescribed drug, and treatment with it is often insufficient or excessive. Nonetheless, there have been only a few reports on the determinants of inadequate levothyroxine treatment.
Methods
Data from 2938 participants in the population-based Rhineland Study were analyzed. Putative determinants of inadequate levothyroxine treatment (overtreatment, thyrotropin level <0.56 mU/L; undertreatment, thyrotropin level >4.27 mU/L) were studied with logistic regression. The determinants of the levothyroxine dose were assessed with linear regression.
Results
Overall, 23% of the participants (n = 662) stated that they were taking levothyroxine. Among these participants, 18% were overtreated and 4% were undertreated. Individuals over 70 years of age and above were four times as likely to be overtreated (OR = 4.05, 95% CI [1.20; 13.72]). Each rise in the levothyroxine dose by 25 µg was associated with an increased risk of overtreatment (OR = 1.02, 95% CI [1.02; 1.03]) and of undertreatment (OR = 1.02, 95% CI [1.00; 1.03]). Well-controlled participants (normal thyrotropin levels 0.56–4.27 mU/L) received a lower levothyroxine dose (1.04 ± 0.5 µg/kg/d) than overtreated (1.40 ±0.5 µg/kg/d) or undertreated (1.37 ±0.5 µg/kg/d) participants. No association was found between sociodemographic factors or comorbidities and the levothyroxine dose. Iodine supplementation was associated with a lower daily dose (β = -0.19, 95% CI [-0.28; -0.10]), while three years or more of levothyroxine exposure was associated with a higher daily dose (β = 0.24, 95% CI [0.07; 0.41]).
Conclusion
Levothyroxine intake was high in our sample, and suboptimal despite monitoring. Our findings underscore the need for careful dosing and for due consideration of deintensification of treatment where appropriate.
Levothyroxine (LT4), the linchpin of thyroid hormone replacement therapy, is highly effective, inexpensive, and easy to administer (1, 2). LT4 use is increasing in many countries (3), most likely due to the increase in treatment of mild subclinical hypothyroidism (4, 5). In 2019, LT4 was the fourth most prescribed drug in Germany, with almost nine million prescriptions (6). A population-based study in Germany (age range 20 to > 80 years) reported a prevalence of LT4 use of 11%, while the Rhineland Study (age range 30–95 years) stated a prevalence of 24% (7–9). Studies in other European countries have reported prevalence rates of only 3–5% (10–12). These discrepancies may be attributable partly to regional differences in thyroid function parameters, thyroid diseases, or treatment protocols (13, 14).
The LT4 dosage is usually based on the serum level of thyrotropin (TSH). TSH must be monitored closely to avoid overtreatment, which causes high healthcare costs and adverse effects, or undertreatment, which has little clinical benefit (15, 16). Importantly, TSH levels outside the reference range are associated with adverse health outcomes, e.g., iatrogenic hyperthyroidism, increased cardiovascular morbidity/mortality, elevated fracture risk, and cognitive dysfunction (17–19). This is particularly true in older patients with suppressed TSH (20).
Despite the potential health risks, high rates of overtreatment (14–20%) and undertreatment (10–27%) have been described (10–12). However, these reports come from countries with a low prevalence of LT4 use compared with Germany. To date, only two studies have examined the quality of LT4 treatment in Germany. One of these (data from the period 1997–2001) reported over- and undertreatment rates of 19.5% and 10%, respectively (21), while the other (data from the years 2005–2018) reported only the cumulative risk (overtreatment 1.3%, undertreatment 3%) (22). The prevalence, however, was not reported, so the current burden in Germany remains unclear (22). A German study published in 2020 found that TSH levels are poorly monitored in LT4 users. Investigation of the current extent of over- and undertreatment is therefore needed (8).
Evidence on the determinants of over- and undertreatment and LT4 dose is also limited. Longer LT4 exposure duration and higher LT4 dose were associated with overtreatment, while men and younger persons were more likely to be undertreated (12). Age, sex, and body weight were associated with LT4 dosage, but these studies were conducted in older, obese patients or in patients who had undergone thyroidectomy (23–25).
The aim of the study described herein was to investigate the prevalence and determinants of LT4 over- and undertreatment, together with the determinants of LT4 dose, in a large-scale population-based study. Furthermore we evaluated information on the initiation, duration, and monitoring of treatment among LT4 users.
Methods
Study population
We used data from the Rhineland Study, a community-based cohort (eMethods, eTable 1). All residents (≥ 30 years) of two geographically defined areas in Bonn, Germany were invited to take part. The sole inclusion criterion was possession of sufficient German language skills to provide informed consent. The baseline data of the first 3000 participants (March 2016 to February 2020) with measured serum TSH were used. We excluded 62 participants due to incomplete TSH measurements (n = 2), missing medication data (n = 54), or because they were taking drugs that affected thyroid hormone levels (amiodarone/lithium; n = 6), so 2938 persons were included in the analyses. We also conducted a brief online survey in 2022 to obtain additional information on regular LT4 users (eMethods, eTable 2).
eMethods.
Study design
The Rhineland Study is an ongoing community-based, prospective cohort study. The participants are residents of two geographically defined areas in Bonn, Germany. Recruitment began in 2016. All residents aged ≥ 30 years were invited using contact information provided by the municipality. Participation was by invitation only, and invitations were issued regardless of the health status of those invited. The sole exclusion criterion was insufficient command of the German language to provide written informed consent. The study of (neurodegenerative) diseases and the identification of determinants and biomarkers of healthy aging is a primary objective of the Rhineland Study. Therefore, all participants underwent a standardized 8-hour in-depth phenotyping process, including cardiovascular health assessment, brain imaging, cognitive testing, metabolite profiling, and documentation of medication use. The data were collected through questionnaires, interviews, and the collection of various biomaterials such as blood, stool, urine, and hair samples. Approval to conduct the study was granted by the ethics committee of the Medical Faculty of the University of Bonn. The study protocols were conducted in accordance with the recommendations of the International Council for Harmonisation and the Good Clinical Practice standards. Written informed consent was obtained in accordance with the tenets of the Declaration of Helsinki. No financial incentives were offered to the participants.
Online survey
In addition to the data collected at baseline, we wanted to acquire more information about thyroid disease and thyroid hormone replacement therapy. Therefore, we initiated a short online survey (data collection: September 2022–March 2023). We asked all LT4 users (n = 662) to complete a questionnaire to obtain further information about the initiation, cause, duration, and monitoring of treatment and about the thyroid examinations performed. The questionnaire was completed by 456 of the 662 regular LT4 users.
TSH assessment
Venous blood was collected from participants who had fasted for at least 10 hours. The blood was transferred to S-Monovette tubes (7.5 mL) containing coagulation factor and incubated for 30 minutes for coagulation (room temperature). The tubes were centrifuged at 2000 × g and 4 °C for 15 minutes. The samples were then aliquoted (500 µL each) and transferred into 0.7-ml FluidX tubes. After aliquoting, all samples were immediately frozen at -80 °C. The TSH level in the serum samples was then measured using the Lumipulse G1200 (FujiRebio Inc., Ghent, Belgium), a non-competitive chemiluminescent enzyme immunoassay (Erasmus MC, University Medical Center, Rotterdam, The Netherlands). The TSH reference values were set by the laboratory at 0.56–4.27 mU/L. It should be noted that the measurement of TSH is instrument- and laboratory-dependent and therefore the reference values also depend on the methods, reagents, and calibration standards used. The TSH reference ranges set by laboratories therefore vary both internationally and within Germany, as noted in the current German guideline Erhöhter TSH-Wert in der Hausarztpraxis (Elevated TSH Levels in Primary Care) (39).
eTable 1. Definition of demographic and clinical characteristics.
| Characteristic | Missing | Definition | |
| General characteristics | Age group | 0.0% | Age range 30–95 years: 30–39, 40–49, 50–59, 60–69, ≥ 70 years |
| Sex | 0.0% | Women, men | |
| Education | 1.0% | Based on the International Standard Classification of Education 2011 (ISCED): low (lower secondary education or below), middle (upper secondary education to undergraduate university level), high (postgraduate university study) | |
| Smoking | 5.9% | Persons who have never smoked, formerly smoked, or currently smoke | |
| Body mass index | 0.4% | Body mass divided by square of body height (kg/m2) | |
| Comorbidities | Diabetes | 0.9% | Self-reported physician diagnosis and/or glycated hemoglobin (HbA1c) (no diabetes < 6.5%; diabetes ≥ 6.5%), fasting glucose (no diabetes < 126 mg/dl; diabetes ≥ 126 mg/dl) measured in fasting morning blood, and/or intake of antidiabetics |
| Hypertension | 1.6% | Based on the 2018 European Society of Cardiology guidelines for the management of arterial hypertension: mean systolic blood pressure ≥ 140 mmHg and/or mean diastolic blood pressure ≥ 90 mmHg and/or antihypertensive drug use, irrespective of blood pressure | |
| Cardiovascular disease | 0.4% | Based on a self-reported physician diagnosis of one or more of the following conditions: myocardial infarction, coronary artery disease, cardiac insufficiency, cardiac pacemaker, peripheral artery occlusive disease, stroke, surgery on large vessels such as aorta, carotid, or peripheral vessels | |
| Chronic kidney disease | 5.5% | Estimated glomerular filtration rate based on cystatin C (no CKD ≥ 60 mL/min/1.73 m2; CKD < 60 ml/min/1.73 m2) | |
| Medication | LT4 dosage (µg/kg/d) | 0.4% | Daily dose of LT4 consumed, expressed in relation to body weight |
| LT4 intake duration | 0.0% | 0–12 months, 13–36 months, > 36 months | |
| Iodine supplementation | 0.4% | Regular intake of iodine (ATC H03CA01) | |
| Polypharmacy | 0.0% | Regular use of ≥ 5 prescribed drugs | |
| Cognition | Global cognition (z-standardized) | 1.9% | Derived from a cognitive test battery assessing episodic verbal memory, working memory, executive function and processing speed |
CKD, Chronic kidney disease; LT4, levothyroxine
eTable 2. Prevalence of self-reported thyroid disease ever diagnosed by a doctor.
| All | LT4 treatment status | |||||
| Controlled | Overtreated | Undertreated | p*1 | p*2 | ||
| Hypothyroidism, N (%) | 310 (11.1) | 190 (40.7) | 36 (35.0) | 7 (29.2) | 0.264 | 0.270 |
| Hyperthyroidism, N (%) | 141 (5.1) | 58 (12.4) | 11 (10.7) | 6 (25.0) | 0.633 | 0.084 |
| Hashimoto, N (%) | 182 (6.5) | 129 (27.6) | 35 (34.0) | 8 (33.3) | 0.188 | 0.505 |
| Basedow, N (%) | 29 (1.0) | 17 (3.6) | 3 (2.9) | 1 (4.2) | 0.725 | 0.896 |
| Goiter, N (%) | 90 (3.2) | 41 (8.8) | 11 (10.7) | 2 (8.3) | 0.529 | 0.929 |
Group differences were calculated with logistic regression, adjusted for age and sex (age and sex were only adjusted for the other, respectively)
Treatment status controlled: TSH 0.56–4.27 mU/L; overtreated: TSH < 0.56 mU/L; undertreated: TSH > 4.27 mU/L
*1 Adjusted for age and sex (overtreated compared with controlled)
*2 Adjusted for age and sex (undertreated compared with controlled)
LT4, Levothyroxine; N, number of participants; TSH: thyrotropin
TSH assessment
Blood samples were taken in the morning after a 10-hour fast. The laboratory defined the reference range of TSH as 0.56–4.27 mU/L (26). Details of blood collection and TSH measurement/reference range can be found in the eMethods.
LT4 treatment
All participants were asked to bring the original packaging of all medications they were currently using and had taken as needed in the past year. Data were collected by interview, documenting name, dosage, and current prescription status (9, 27). LT4 treatment status was categorized by TSH levels: adequate, i.e., controlled (0.56–4.27 mU/L), or inadequate, i.e. overtreatment (< 0.56 mU/L) or undertreatment (> 4.27 mU/L).
Statistical analysis
The participants’ characteristics were summarized using descriptive statistics. Group differences were calculated using logistic regression (adjusted for age and sex). Multinomial logistic regression was performed to identify possible determinants (eTable 1) of over- and undertreatment in LT4 users (reference group: controlled participants) in a fully adjusted model. Multivariable linear regression was then used to identify predictors of LT4 dose (µg/kg/d) in a fully adjusted model. Statistical analyses were conducted using RStudio (version 4.1.1).
Results
Study population
The participants’ characteristics are presented in Table 1 and eTable 2. The persons included (n = 2938) were on average 55 ± 14.4 years old (range 30–95; 56.5% women) and did not differ significantly from those who were excluded (n = 62) in terms of age (57 ± 14.9 years, range 30–87; p = 0.187) or sex ratio (women n = 34, 54.8%; p = 0.417).
Table 1. Characteristics of the study population.
| Controlled | Overtreated | Undertreated | p*1 | p*2 | |
| Participants, N (%) | 518 (78.2) | 117 (17.7) | 27 (4.1) | < 0.001 | < 0.001 |
| Age (years), M (SD) | 58.2 (13.9) | 58.8 (13.6) | 59.4 (15.5) | 0.675 | 0.721 |
| Sex (women), N (%) | 435 (84.0) | 96 (82.1) | 21 (77.8) | 0.659 | 0.432 |
| Education, N (%) | |||||
| Low | 16 (3.1) | 2 (1.8) | 0 (0.0) | 0.474 | – |
| Middle | 276 (54.1) | 57 (50.4) | 17 (63.0) | Ref. | Ref. |
| High | 218 (42.7) | 54 (47.8) | 10 (37.0) | 0.369 | 0.457 |
| Smoking, N (%) | |||||
| Never | 216 (44.5) | 50 (45.0) | 11 (42.3) | Ref. | Ref. |
| Former | 219 (45.2) | 42 (37.8) | 10 (38.5) | 0.371 | 0.775 |
| Current | 50 (10.3) | 19 (17.1) | 5 (19.2) | 0.116 | 0.261 |
| BMI (kg/m2) | 26.5 (4.9) | 25.7 (4.7) | 25.9 (4.7) | 0.064 | 0.439 |
| TSH (mU/L), M (SD) | 1.6 (0.8) | 0.3 (0.2) | 7.3 (3.4) | 0.074 | 0.311 |
| Diabetes, N (%) | 29 (5.7) | 7 (6.0) | 2 (7.4) | 0.961 | 0.787 |
| Hypertension, N (%) | 238 (47.1) | 49 (42.2) | 14 (51.9) | 0.196 | 0.807 |
| CVD, N (%) | 54 (10.5) | 10 (8.6) | 5 (18.5) | 0.425 | 0.274 |
| CKD, N (%) | 26 (5.4) | 9 (8.0) | 4 (14.8) | 0.414 | 0.089 |
| LT4 intake duration, N (%) | |||||
| ≤ 12 months | 29 (5.6) | 8 (6.9) | 3 (11.1) | Ref. | Ref. |
| 13–36 months | 80 (15.4) | 16 (13.8) | 2 (7.4) | 0.481 | 0.277 |
| > 36 months | 409 (79.0) | 92 (79.3) | 22 (81.5) | 0.584 | 0.277 |
| Iodine supplementation, N (%) | 145 (28.5) | 31 (26.5) | 3 (11.1) | 0.610 | 0.052 |
| Polypharmacy, N (%) | 161 (31.1) | 38 (32.5) | 9 (33.3) | 0.907 | 0.956 |
| Global cognition (z-score), M (SD) | −0.1 (0.6) | −0.1 (0.7) | −0.1 (0.8) | 0.732 | 0.641 |
Treatment status “controlled” (TSH 0.56–4.27 mU/L), “overtreated” (TSH < 0.56 mU/L), “undertreated” (TSH > 4.27 mU/L). Group differences were calculated with logistic regression adjusted for age and sex (age and sex were only adjusted for the other respectively).
*1 Adjusted for age and sex (overtreated compared with controlled)
*2 Adjusted for age and sex (undertreated compared with controlled)
BMI, Body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; LT4, levothyroxine; M, mean; N, number of participants; Ref, reference group; SD, standard deviation; TSH: thyrotropin.
Overtreatment and undertreatment with LT4
Regular LT4 use was reported by 22.5% of the participants. Users were older than non-users (58.3 vs. 54.1 years; p < 0.001), and prevalence was higher in women than in men (33.3% vs. 8.6%; p < 0.001). Among LT4 users (n = 662), 78.2% were controlled (n = 518), while 21.8% (n = 144) were inadequately treated, of whom 17.7% (n = 117) were overtreated and 4.1% (n = 27) were undertreated.
Logistic regression showed that persons aged ≥ 70 years were four times more likely to be overtreated (odds ratio 4.05; 95% confidence interval [1.20; 13.72]) than those who were younger, and that increasing the LT4 dose by 25 µg/d increased the likelihood of both overtreatment (OR 1.02; [1.02; 1.03]) and undertreatment (OR 1.02; [1.00; 1.03]) (Table 2).
Table 2. Determinants of LT4 overtreatment and undertreatment (n = 557).
| Status | Determinant | OR | [95% CI] | p |
| Overtreated | Age 40–49 years (vs. 30–39 years) | 0.89 | [0.34; 2.35] | 0.810 |
| Age 50–59 years (vs. 30–39 years) | 1.87 | [0.73; 4.75] | 0.189 | |
| Age 60–69 years (vs. 30–39 years) | 2.73 | [0.96; 7.74] | 0.060 | |
| Age ≥ 70 years (vs. 30–39 years) | 4.05 | 1.20; 13.72] | 0.025 | |
| Sex (men vs. women) | 0.84 | [0.43; 1.65] | 0.616 | |
| Education (low vs. middle) | 0.29 | [0.03; 2.52] | 0.262 | |
| Education (high vs. middle) | 1.22 | [0.74; 2.00] | 0.438 | |
| Smoking (former vs. never) | 0.61 | [0.36; 1.04] | 0.068 | |
| Smoking (current vs. never) | 1.42 | [0.69; 2.92] | 0.337 | |
| BMI (kg/m2, increase per unit) | 0.95 | [0.90; 1.01] | 0.077 | |
| Diabetes (yes vs. no) | 0.63 | [0.21; 1.94] | 0.420 | |
| Hypertension (yes vs. no) | 0.79 | [0.44; 1.41] | 0.422 | |
| CVD (yes vs. no) | 0.51 | [0.19; 1.37] | 0.182 | |
| CKD (yes vs. no) | 1.50 | [0.53; 4.24] | 0.444 | |
| Iodine supplementation (yes vs. no) | 1.11 | [0.63; 1.96] | 0.725 | |
| Polypharmacy (yes vs. no) | 1.07 | [0.60; 1.92] | 0.812 | |
| LT4 dose (per 25 µg increase) | 1.02 | [1.02; 1.03] | < 0.001 | |
| LT4 intake 13–36 months (vs. 0–12 months) | 0.59 | [0.18; 1.96] | 0.389 | |
| LT4 intake > 36 months (vs. 0–12 months) | 0.50 | [0.18; 1.42] | 0.192 | |
| Global cognition (z-score, per SD) | 1.23 | [0.72; 2.09] | 0.445 | |
| Undertreated | Age 40–49 years (vs. 30–39 years) | 0.64 | [0.12; 3.53] | 0.607 |
| Age 50–59 years (vs. 30–39 years) | 1.58 | [0.34; 7.41] | 0.560 | |
| Age 60–69 years (vs. 30–39 years) | 1.42 | [0.23; 8.76] | 0.703 | |
| Age ≥ 70 years (vs. 30–39 years) | 1.72 | [0.21; 14.27] | 0.617 | |
| Sex (men vs. women) | 1.42 | [0.47; 4.32] | 0.539 | |
| Education (low vs. middle) | – | – | – | |
| Education (high vs. middle) | 0.85 | [0.35; 2.09] | 0.725 | |
| Smoking (former vs. never) | 0.78 | [0.29; 2.09] | 0.623 | |
| Smoking (current vs. never) | 1.93 | [0.56; 6.64] | 0.299 | |
| BMI (kg/m2, increase per unit) | 0.96 | [0.87; 1.06] | 0.418 | |
| Diabetes (yes vs. no) | 1.16 | [0.20; 6.63] | 0.867 | |
| Hypertension (yes vs. no) | 0.97 | [0.33; 2.81] | 0.951 | |
| CVD (yes vs. no) | 3.04 | [0.80; 11.56] | 0.103 | |
| CKD (yes vs. no) | 2.08 | [0.42; 10.39] | 0.370 | |
| Iodine supplementation (yes vs. no) | 0.27 | [0.06; 1.27] | 0.097 | |
| Polypharmacy (yes vs. no) | 0.67 | [0.21; 2.13] | 0.494 | |
| LT4 dose (per 25 µg increase) | 1.02 | [1.00; 1.03] | 0.017 | |
| LT4 intake 13–36 months (vs. 0–12 months) | 0.26 | [0.03; 2.23] | 0.220 | |
| LT4 intake > 36 months (vs. 0–12 months) | 0.42 | [0.08; 2.23] | 0.312 | |
| Global cognition (z-score, per SD) | 1.37 | [0.55; 3.46] | 0.499 |
The sample size is based on persons with complete data on all determinants.
BMI, Body mass index; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; LT4, levothyroxine; n, number of participants; OR, odds ratio; SD, standard deviation; vs., versus
LT4 dose
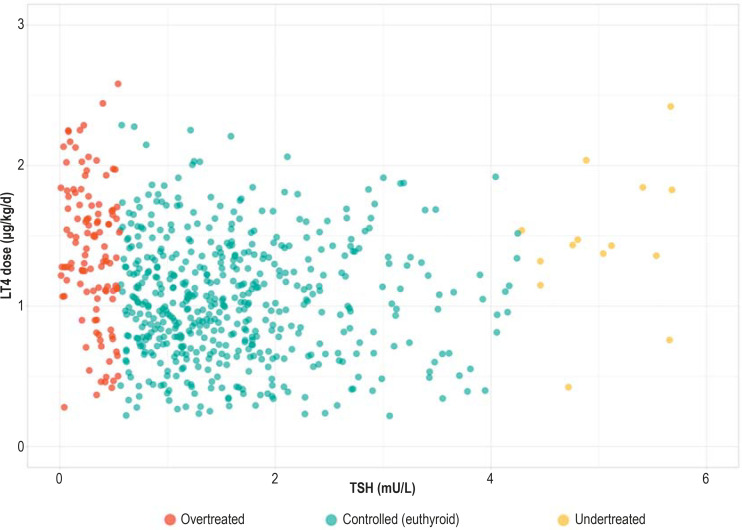
Controlled persons had lower daily doses of LT4 (1.04 ± 0.5 µg/kg/d) than overtreated (1.40 ± 0.5 µg/kg/d) and undertreated (1.37 ± 0.5 µg/kg/d) persons, but adjustment for age and sex revealed no significant differences (Table 1). Figure 1 illustrates the LT4 doses (µg/kg/d) in relation to the TSH levels (mU/L) and shows individuals with very high and very low doses in all treatment groups. We found no association of sociodemographic factors or comorbidities with the daily LT4 dose. Iodine supplementation (β = –0.19; [–0.28; –0.10], p < 0.001) was associated with lower LT4 dose, and LT4 exposure duration (β= 0.24; [0.07; 0.41), p = 0.001) of ≥ 3 years was associated with a higher dose (Table 3).
Figure.
LT4 dose (μg/kg/d) compared with TSH (mU/L), stratified by treatment status
LT4, Levothyroxine; TSH, thyrotropin
Table 3. Determinants of LT4 dose (increase per unit µg/kg/d), n = 556.
| Determinant | β | [95% CI] | p |
| Age 40–49 years (vs. 30–39 years) | 0.06 | [−0.09; 0.22] | 0.437 |
| Age 50–59 years (vs. 30–39 years) | 0.06 | [−0.09; 0.21] | 0.433 |
| Age 60–69 years (vs. 30–39 years) | −0.09 | [−0.25; 0.07] | 0.267 |
| Age ≥ 70 years (vs. 30–39 years) | −0.09 | [−0.26; 0.08] | 0.297 |
| Sex (men vs. women) | −0.02 | [−0.13; 0.10] | 0.783 |
| Education (low vs. middle) | −0.04 | [−0.30; 0.22] | 0.744 |
| Education (high vs. middle) | −0.00 | [−0.09; 0.08] | 0.941 |
| Smoking (former vs. never) | 0.05 | [−0.04; 0.13] | 0.285 |
| Smoking (current vs. never) | 0.11 | [−0.02; 0.24] | 0.106 |
| Diabetes (yes vs. no) | 0.07 | [−0.11; 0.26] | 0.431 |
| Hypertension (yes vs. no) | −0.06 | [−0.15; 0.03] | 0.209 |
| CVD (yes vs. no) | 0.03 | [−0.12; 0.17] | 0.720 |
| CKD (yes vs. no) | 0.08 | [−0.10; 0.27] | 0.371 |
| Iodine supplementation (yes vs. no) | −0.19 | [−0.28; −0.10] | <0.001 |
| Polypharmacy (yes vs. no) | 0.03 | [−0.06; 0.13] | 0.493 |
| TSH (mU/L, increase per unit) | −0.00 | [−0.03; 0.02] | 0.704 |
| LT4 intake duration 13–36 months (vs. 0–12 months) | 0.02 | [−0.17; 0.21] | 0.838 |
| LT4 intake duration > 36 months (vs. 0–12 months) | 0.24 | [0.07; 0.41] | 0.006 |
The sample size is based on individuals with complete data on all determinants.
BMI, Body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease;
LT4, levothyroxine; n, number of participants; TSH, thyrotropin; vs., versus
Online survey
The results of the survey are shown in eTable 3. The LT4 users who were included (n = 456; mean age 56.0 ± 13.0 years, range 30–94; 83.1% women) did not differ from those who were excluded (n = 206; mean age 56.0 ± 12.4 years, range 31–88; 84.4% women) in terms of age (p = 0.913) or sex (p = 0.613). Participants were predominantly long-term users (21.3 ± 12.2 years) and 60.4% reported having their TSH levels monitored every 6–12 months.
eTable 3. Results of the online survey (n=456).
| LT4 users | Missing | LT4 treatment status | |||
| Controlled | Overtreated | Undertreated | |||
| Participants, N | 456 | 361 | 82 | 13 | |
| Sex, N (%) | 0.0% | ||||
| Women | 379 (83.1) | 300 (83.1) | 66 (80.5) | 13 (100.0) | |
| Men | 76 (16.7) | 60 (16.6) | 16 (19.5) | 0 (0.0) | |
| Diverse | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) | |
| LT4 intake (years), M (SD) | 21.3 (12.2) | 9.2% | 20.6 (12.0) | 24.1 (12.9) | 22.8 (9.9) |
| Diagnosis-based initiation of LT4, N (%) | 450 | 1.2% | |||
| Hypothyroidism | 174 (38.7) | 139 (39.1) | 30 (36.6) | 5 (38.5) | |
| Benign struma | 82 (18.2) | 59 (16.6) | 22 (26.8) | 1 (7.7) | |
| Hashimoto | 122 (27.1) | 94 (26.5) | 23 (28.0) | 5 (38.5) | |
| Other diagnosis | 51 (11.3) | 44 (12.4) | 5 (6.1) | 2 (15.4) | |
| Unknown | 21 (4.7) | 19 (5.4) | 2 (2.4) | 0 (0.0) | |
| TSH monitoring frequency, N (%) | 19.3% | ||||
| Every 6 months | 61 (16.6) | 43 (14.6) | 13 (21.0) | 5 (45.5) | |
| Yearly | 161 (43.8) | 131 (44.4) | 25 (40.3) | 5 (45.5) | |
| Every 1–2 years | 77 (20.9) | 63 (21.4) | 14 (22.6) | 0 (0.0) | |
| Irregularly | 56 (15.2) | 48 (16.3) | 7 (11.3) | 1 (9.1) | |
| No monitoring | 13 (3.5) | 10 (3.4) | 3 (4.8) | 0 (0.0) | |
| Most recent LT4 dose adjustment in years, M (SD) | 6.2 (6.0) | 24.1% | 6.5 (6.1) | 5.6 (6.1) | 3.1 (1.7) |
| Thyroid examinations performed | 9.0% | ||||
| Biopsy | 54 (11.8) | 42 (11.6) | 11 (13.4) | 1 (7.7) | |
| Ultrasound | 389 (85.3) | 305 (84.5) | 72 (87.8) | 12 (92.3) | |
| Scintigraphy | 275 (60.3) | 218 (60.4) | 50 (61.0) | 7 (53.8) | |
Treatment status controlled: TSH 0.56–4.27 mU/L; overtreated: TSH < 0.56 mU/L; undertreated: TSH > 4.27 mU/L
LT4, Levothyroxine; M, mean; N, number of participants; SD, standard deviation; TSH, thyrotropin
Discussion
We investigated the prevalence and determinants of overtreatment, undertreatment, and LT4 dosage in a large population-based cohort. A high proportion of participants, mainly women (women 33%; men 9%), reported taking LT4 (23%). Of these, 18% were overtreated and 4% undertreated. Older age was associated with overtreatment, while higher LT4 dose was associated with both overtreatment and undertreatment. Iodine supplementation was associated with lower LT4 dosage, whereas longer LT4 intake (≥ 3 years) was associated with higher doses.
LT4 is the most commonly used drug in our cohort (9). The frequency of use was higher in women than in men and increased with age, which was to be expected based on the prevalence of thyroid disease in these groups (11, 17, 28, 29). Importantly, the adverse health consequences of overtreatment are most pronounced in the elderly (20).
An increase in LT4 prescriptions has been observed worldwide, apparently mainly due to increased treatment of subclinical hypothyroidism with mild TSH elevation (30). Although treating mildly elevated TSH levels (< 10 mIU/L) is not recommended in the current guidelines (31), an American study found a median TSH of 5.3 mIU/L in 9331 patients newly started on LT4 treatment (5). This is worrying and shows how remarkable it is that the prevalence of LT4 use in our study is seven times higher than in other European studies (3.1–4.4%) (10–12) and more than twice as high as in other German population-based studies (˜11%) (7, 8). Possible reasons for the differences between our study and other German studies are the period of data collection (2000–2016), different age and sex distributions, and regional variations in prescribing patterns, iodine availability, or thyroid disease (7, 8, 13, 14).
One explanation for the overall high prevalence of LT4 use in Germany may be the frequent use of TSH measurement and thyroid ultrasound (8). In Germany, there appears to be a strong focus on the thyroid both in detection of morphological changes and in drug therapy.
Indeed, the annual rate of thyroid surgery (109/100 000) is high compared with England (27/100 000) or the Netherlands (16/100 000), and thyroid hormone prescriptions increased by 40% between 2010 and 2019, when almost 9 million prescriptions were issued (6, 32). Additionally, a case-based survey found that German general practitioners were more likely to prescribe LT4 for patients with subclinical hypothyroidism than their colleagues in other European countries (33).
Approximately 18% of LT4 users were overtreated and 4% undertreated. Although the prevalence of LT4 use in our study is higher than in other studies, our results are comparable regarding overtreatment (14–20%), though not for undertreatment (10–27%) (10–12, 21). Given the high use of LT4 in our population, we expected higher rates of overtreatment. Perhaps we underestimate the prevalence of overtreatment (Figure 1), because controlled persons with low LT4 doses and low TSH levels could be overtreated as TSH levels would probably remain within the reference range after LT4 discontinuation. Whether these individuals require treatment cannot be conclusively established on the basis of our data. In some cases, e.g., patients with thyroid cancer (34), very low TSH is desirable so the levels are deliberately kept low. However, no individuals in our sample self-reported thyroid cancer, so the high prevalence of overtreatment cannot be justified in this way.
Similar to another German study (8), almost 60% of LT4 users in the Rhineland Study reported having their TSH levels monitored every 6–12 months, with no noticeable difference between controlled and inadequately treated persons (eTable 3). This demonstrates that frequent monitoring does not necessarily prevent inadequate treatment.
The likelihood of overtreatment was high in individuals ≥ 70 years, which is unsurprising as it has been reported that overtreatment is common in the elderly (35). Complex LT4 treatment regimens, with varying dosages across weekdays to achieve optimal titration, can become challenging with increasing age, especially as non-adherence rises with age (17). Among the LT4 users with suppressed TSH levels, 27% were aged ≥ 70 years. Importantly, suppressed TSH is particularly strongly associated with adverse health outcomes in the elderly (20). This makes the finding that 23% of all participants used LT4 all the more important.
Reducing the number of unnecessary LT4 prescriptions may improve health status and reduce healthcare costs. Future studies should aim to understand what factors contribute to use of LT4 by this extremely high proportion of people. In agreement with another study (12), the probability of over- and undertreatment rose with increasing LT4 dose. One can only speculate about the possible reasons for undertreatment despite high dosage. One possible explanation is lack of adherence to treatment, or reluctance on the part of physicians to increase the dose beyond a certain point for fear of adverse events. In contrast to a previous study, we did not find that men were more often undertreated than women, but we did observe a trend in that direction (12). This could be because thyroid disease is more common in women, and women more frequently receive TSH tests (8).
Both overtreated and undertreated participants had higher mean daily doses than controlled users. No associations were found between either sociodemographic factors or comorbidities and LT4 dosage. However, iodine supplementation was associated with lower daily doses, while LT4 exposure duration of ≥ 3 years was associated with higher daily doses.
Although there was no significant association between age and LT4 dose, younger persons tended to receive higher doses and older persons to receive lower doses, which is consistent with recent evidence that older persons should start with a low dose (24, 36).
Iodine, an important micronutrient, is known to control thyroid function by reducing the thyroid gland’s response to TSH. In high concentrations, iodine inhibits thyroid hormone secretion. Especially in persons with pre-existing thyroid disease, iodine can induce hypo- or hyperthyroidism (37). Therefore, correct dose adjustment in iodine supplementation is all the more important.
One possible reason for the association between the duration of LT4 intake and higher dosage is that treatment for thyroid hormone deficiency is usually started at a low dose and then increased by dose titration to achieve target TSH levels. Alternatively, thyroid function may decline progressively in patients who initially have subclinical hypothyroidism.
Strengths and limitations
One of the strengths of our study is the examination of both overtreatment and undertreatment in a large population-based cohort. Our extensive data allowed us to analyze various determinants, and self-reported medication data may better reflect actual use than secondary data. Although self-reported medication data may introduce reporting bias, we validated the reliability of our data (27).
Potential limitations include the fact that treatment adherence could not be considered. Furthermore, as is often the case in epidemiological studies (21), only one TSH measurement time point was available, so that our results cannot account for any TSH fluctuations (38). We did not have detailed information on whether and when dose adjustments were made. According to participants’ reports, however, the last dose adjustment had taken place on average 6 years earlier. Moreover, we do not have longitudinal data, so we could not follow changes in TSH levels or general health. Finally, our population may be “healthier,” which would limit the generalizability of our results. However, the prevalences of hypertension and polypharmacy and the age and sex distributions are all comparable with the German population (9, 27).
The prevalence of LT4 use in our population was very high and was suboptimal in almost a quarter of the participants despite frequent TSH monitoring. This high proportion of LT4 use is probably due to overtreatment in the vast majority of participants and, assuming that 18% of participants have suppressed TSH, will contribute to adverse health outcomes.
Conclusion
Our report suggests that the focus should be not only on intensification of treatment, but also on deintensification. Furthermore, the strategy for monitoring should be reconsidered, as it does not appear to lead to high-quality care at present.
Acknowledgments
Study funding
This work was supported by a grant provided by the Federal Institute for Drugs and Medical Devices (BfArM), Bonn, Germany (V-15646/68502/2014–2016 and V-17746/68502/2018–2020).
Data sharing
The datasets for this manuscript are not publicly available because of data protection regulations. Access to data can, however, be provided to scientists in accordance with the Rhineland Study’s Data Use and Access Policy. Requests to access the datasets should be directed to Prof. Dr. Dr. Monique M.B. Breteler, RS-DUAC@dzne.de.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550–1562. doi: 10.1016/S0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eligar V, Taylor PN, Okosieme OE, et al. Thyroxine replacement: a clinical endocrinologist’s viewpoint. Ann Clin Biochem. 2016;53:421–433. doi: 10.1177/0004563216642255. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Gutierrez R, Maraka S, Ospina NS, et al. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol. 2017;5:246–248. doi: 10.1016/S2213-8587(16)30276-5. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PN, Iqbal A, Minassian C, et al. Falling Threshold for Treatment of Borderline Elevated Thyrotropin Levels—Balancing Benefits and Risks. JAMA Intern Med. 2014;174 doi: 10.1001/jamainternmed.2013.11312. [DOI] [PubMed] [Google Scholar]
- 5.Brito JP, Ross JS, El Kawkgi OM, et al. Levothyroxine Use in the United States, 2008-2018. JAMA Intern Med. 2021;181:1402–1405. doi: 10.1001/jamainternmed.2021.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwabe U, Wolf-Dieter Ludwig. Arzneiverordnungs-Report 2020. Springer Berlin Heidelberg. 2020:825–831. [Google Scholar]
- 7.Khattak RM, Ittermann T, Nauck M, et al. Monitoring the prevalence of thyroid disorders in the adult population of Northeast Germany. Popul Health Metr. 2016;14 doi: 10.1186/s12963-016-0111-3. DOI: 10.1186/s12963-016-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiel S, Ittermann T, Völzke H, et al. Frequency of thyroid function tests and examinations in participants of a population-based study. BMC Health Serv Res. 2020;20 doi: 10.1186/s12913-020-4910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries FM, Stingl JC, Breteler MMB. Polypharmacy, potentially inappropriate medication and pharmacogenomics drug exposure in the Rhineland Study. Br J Clin Pharmacol. 2021;87:2732–2756. doi: 10.1111/bcp.14671. [DOI] [PubMed] [Google Scholar]
- 10.Wouters HJCM, Slagter SN, Muller Kobold AC, et al. Naitza S, editor. Epidemiology of thyroid disorders in the Lifelines Cohort Study (the Netherlands) PLoS One. 2020;15 doi: 10.1371/journal.pone.0242795. e0242795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janett-Pellegri C, Wildisen L, Feller M, et al. Lupattelli A, editor. Prevalence and factors associated with chronic use of levothyroxine: A cohort study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0261160. e0261160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okosieme OE, Belludi G, Spittle K, et al. Adequacy of thyroid hormone replacement in a general population. Qjm. 2011;104:395–401. doi: 10.1093/qjmed/hcq222. [DOI] [PubMed] [Google Scholar]
- 13.Girschik C, Muchalla P, Kowall B, et al. Regionale Unterschiede von Schilddrüsenfunktionsparametern: ein Vergleich europäischer Kohortenstudien. Das Gesundheitswes. 2022:1–2. doi: 10.1055/a-1806-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meisinger C, Ittermann T, Wallaschofski H, et al. Geographic variations in the frequency of thyroid disorders and thyroid peroxidase antibodies in persons without former thyroid disease within Germany. Eur J Endocrinol. 2012;167:363–371. doi: 10.1530/EJE-12-0111. [DOI] [PubMed] [Google Scholar]
- 15.Lyu H, Xu T, Brotman D, et al. Overtreatment in the United States. PLoS One. 2017;12:1–11. doi: 10.1371/journal.pone.0181970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearney M, Treadwell J, Marshall M. Overtreatment and undertreatment: time to challenge our thinking. Br J Gen Pract. 2017;67:442–443. doi: 10.3399/bjgp17X692657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Effraimidis G, Watt T, Feldt-Rasmussen U. Levothyroxine Therapy in Elderly Patients With Hypothyroidism. Front Endocrinol (Lausanne) 2021;12:1–12. doi: 10.3389/fendo.2021.641560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum MR, Bauer DC, Collet T-H, et al. Subclinical Thyroid Dysfunction and Fracture Risk. JAMA. 2015;313 doi: 10.1001/jama.2015.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collet T-H, Gussekloo J, Bauer DC, et al. Subclinical Hyperthyroidism and the Risk of Coronary Heart Disease and Mortality. Arch Intern Med. 2012;172:1–7. doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biondi B, Cooper DS. Subclinical Hyperthyroidism. N Engl J Med. 2018;378:2411–2419. doi: 10.1056/NEJMcp1709318. [DOI] [PubMed] [Google Scholar]
- 21.Hannemann A, Friedrich N, Haring R, et al. Thyroid function tests in patients taking thyroid medication in Germany: Results from the population-based Study of Health in Pomerania (SHIP) BMC Res Notes. 2010;3 doi: 10.1186/1756-0500-3-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostev K. Frequency of over- and under-treatment with levothyroxine in primary care in Germany. Eur J Endocrinol. 2022;186 doi: 10.1530/EJE-21-0916. L5. [DOI] [PubMed] [Google Scholar]
- 23.Mele C, Tagliaferri MA, Pagano L, et al. Levothyroxine Replacement in Obese Adults: The Role of Metabolic Variables and Aging on Thyroid Testing Abnormalities. J Clin Endocrinol Metab. 2019;104:6265–6274. doi: 10.1210/jc.2019-00773. [DOI] [PubMed] [Google Scholar]
- 24.Jonklaas J. Sex and Age Differences in Levothyroxine Dosage Requirement. Endocr Pract. 2010;16:71–79. doi: 10.4158/EP09257.OR. [DOI] [PubMed] [Google Scholar]
- 25.Devdhar M, Drooger R, Pehlivanova M, et al. Levothyroxine Replacement Doses Are Affected by Gender and Weight, But Not Age. Thyroid. 2011;21:821–827. doi: 10.1089/thy.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellikaan K, Snijders F, Rosenberg AGW, et al. Thyroid Function in Adults with Prader-Willi Syndrome; a Cohort Study and Literature Review. J Clin Med. 2021;10 doi: 10.3390/jcm10173804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alaeddin N, Stingl JC, Breteler MMB, et al. Validation of self-reported medication use applying untargeted mass spectrometry-based metabolomics techniques in the Rhineland study. Br J Clin Pharmacol. 2022;88:2380–2395. doi: 10.1111/bcp.15175. [DOI] [PubMed] [Google Scholar]
- 28.Canaris GJ, Manowitz NR, Mayor G, et al. The colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 29.Åsvold BO, Vatten LJ, Bjøro T. Changes in the prevalence of hypothyroidism: The HUNT study in Norway. Eur J Endocrinol. 2013;169:613–620. doi: 10.1530/EJE-13-0459. [DOI] [PubMed] [Google Scholar]
- 30.Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365 doi: 10.1136/bmj.l2006. l2006. [DOI] [PubMed] [Google Scholar]
- 31.Peeters RP. Solomon CG, editor. Subclinical Hypothyroidism. N Engl J Med. 2017;376:2556–2565. doi: 10.1056/NEJMcp1611144. [DOI] [PubMed] [Google Scholar]
- 32.Verburg FA. Is thyroid surgery performed too often in Germany? Nuklearmedizin. 2015;54:101–105. [PubMed] [Google Scholar]
- 33.den Elzen WPJ, Lefèbre van de Fliert AA, Virgini V, et al. International variation in GP treatment strategies for subclinical hypothyroidism in older adults: a case-based survey. Br J Gen Pract. 2015;65:e121–e132. doi: 10.3399/bjgp15X683569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somwaru LL, Arnold AM, Joshi N, et al. High Frequency of and Factors Associated with Thyroid Hormone Over-Replacement and Under-Replacement in Men and Women Aged 65 and Over. J Clin Endocrinol Metab. 2009;94:1342–1345. doi: 10.1210/jc.2008-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottwald-Hostalek U, Razvi S. Getting the levothyroxine (LT4) dose right for adults with hypothyroidism: opportunities and challenges in the use of modern LT4 preparations. Curr Med Res Opin. 2022;0:1–6. doi: 10.1080/03007995.2022.2071059. [DOI] [PubMed] [Google Scholar]
- 37.Leung AM, Braverman LE. Iodine-induced thyroid dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19:414–419. doi: 10.1097/MED.0b013e3283565bb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weeke J, Gundersen HJ. Circadian and 30 minutes variations in serum TSH and thyroid hormones in normal subjects. Acta Endocrinol (Copenh) 1978;89:659–672. doi: 10.1530/acta.0.0890659. [DOI] [PubMed] [Google Scholar]
- 39.Schübel J, Voigt K, Uebel T. Erhöhter TSH-Wert in der Hausarztpraxis. S2k-Leitlinie. DEGAM Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin e.V. 2023 accessed 04 August 2023. [Google Scholar]