Abstract
OBJECTIVES
To assess the extended feasibility of a telerehabilitation program and its effects on physical performance in older adults who have recently undergone transcatheter aortic valve implantation (TAVI).
METHODS
In this single-center feasibility study, patients underwent an eight-week telerehabilitation program, involving web-based home exercise training twice weekly, an activity tracker, access to an informative website, and one online session with a nurse, starting one-week postoperative. Data collection was performed before surgery and three months postoperative. The feasibility of the intervention was based on recruitment and adherence to the program. As a secondary outcome, we evaluated the change in six-minute walk distance from before surgery to three months postoperative.
RESULTS
Forty-one patients scheduled for TAVI were assessed for eligibility; 15 patients (37%) were enrolled. Of these, eight were excluded after surgery due to tiredness (n = 2), non-cardiac related hospital readmission (n = 2), fluctuating health (n = 1), death during hospital stay (n = 1), and reduced cognition (n = 2). Seven patients completed the eight-week web-based intervention and were evaluated three months postoperative. Their median (IQR) age was 83 [81, 87] years, and the sample comprised three men and four women. Their walked distance improved from median (IQR) 262 [199, 463] before surgery, to 381 [267, 521] meters three months postoperative. No adverse events were reported.
CONCLUSION
Web-based telerehabilitation, including supervised exercise training, in older adults who have recently undergone TAVI was feasible for a small number of patients who completed the eight-week intervention. This was reflected in an improvement in their walked distance three months after the surgery. However, the low recruitment and retention rates do question the overall feasibility of this intervention in a frail, older population of post-TAVI patients.
Transcatheter aortic valve implantation (TAVI) is the standard of care for treatment of severe aortic valve stenosis, especially in patients of old age or who are deemed to be at high perioperative mortality risk.[1,2] The benefits of TAVI are reduced mortality[3,4] and improved quality of life,[5–7] particularly in terms of increased mobility and usual activities of daily living.[5,8]
Cardiac rehabilitation (CR) is recommended following TAVI to improve functional capacity and quality of life,[9–11] while reducing mortality.[12] However, referral to or participation in CR in this particular population is low.[13–15] Cardiac telerehabilitation (CTR), defined as the use of information and communication technologies to support rehabilitation,[16,17] has proven to be as effective as center- or hospital-based CR in improving functional outcomes and reducing morbidity and mortality,[18,19] as well as improving patient activation and health literacy,[20] while being cost-effective.[21] Thus, CTR can be an effective and viable alternative to traditional CR programs, particularly for older adults who may face barriers to accessing rehabilitation services. Meanwhile, the effectiveness of CTR following TAVI has been poorly investigated, possibly because the use of modern technology to enhance postsurgical outcomes in an elderly population is still limited.[22–24] We have previously reported results from a mixed-methods feasibility study of a CTR for TAVI patients, the TeleTAVI program, where each patient tested the prototype for a period of two weeks.[25] We found that the TeleTAVI program was highly appreciated by the participants due to the web-based setting with provision of supervised exercise training with real-time feedback, without need of transportation. Meanwhile, we were challenged by a 60% study dropout rate due to unstable data coverage at patients´ homes as well as participants´ limited IT skills.[25] Consequently, we adapted the TeleTAVI program to last twelve weeks post-TAVI and provided additional IT support throughout the intervention period.
The aim of this study was to examine whether the adapted TeleTAVI program was feasible in older adults who have recently undergone TAVI. We hypothesized that an intervention period of twelve weeks and the possibility of receiving extended IT support during the project period would increase adherence and compliance to the program.
MATERIAL AND METHODS
Design
This was a single-center feasibility study performed in preparation for a randomized controlled trial, targeting older adults who have undergone TAVI. We describe the recruitment process and explore the practicability of delivering telerehabilitation, including real-time web-based supervised exercise training twice weekly for a period of eight weeks, followed by four weeks self-training. To determine adherence and compliance to the program, we assessed: (1) recruitment, retention, and training adherence rates; (2) patients´ needs for IT support; and (3) changes in outcome measures from before surgery to three months post-surgery. As part of the present study, we also performed individual interviews with patients who completed the TeleTAVI program and with the health professionals involved in the program in order to gain knowledge of their experiences with using heath technologies and in participating in the program.[26] The study was reported in accordance with the CONSORT (Consolidated Standards of Reporting Trials) extended guidelines for feasibility and pilot studies.[27]
Setting
Participants were recruited at the Department of Cardiology, Aalborg University Hospital, Denmark between March and May 2021. The hospital performs 120 TAVI procedures annually. The Danish National Health Service provides tax-supported health care for all inhabitants, guaranteeing free access to family physicians, public hospitals, and municipality-based care as well CR after hospital discharge.
Ethics
The study was registered at the hospital´s research board (registration 2020-054) and complied with the General Data Protection Regulations. The Regional Ethics Committee stated that no approval was required for the study. Informed written consent was obtained from all participants before inclusion.
Inclusion and Exclusion Criteria
Eligible participants were adults scheduled for elective TAVI who were capable of reading and understanding Danish. Indications for TAVI in the present patient cohort were patients with symptomatic aortic stenosis deemed to be at high surgical risk and/or at an age of ≥ 80 years. Exclusion criteria were physical deficits adversely influencing physical performance, known decreased cognitive functioning, and serious hearing or seeing impairments. Furthermore, we excluded patients who did not have internet at home or with poor data coverage at their home address, based on the results of the prototype testing as reported previously.[25]
Telerehabilitation Program
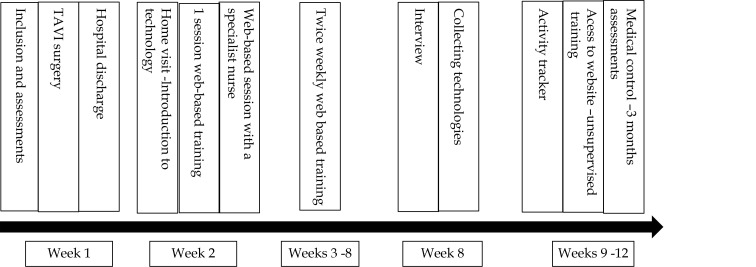
The TeleTAVI program comprised home web-based exercise training, an activity tracker to register daily steps, access to an informative website,[28] and one web-based session with a cardiac nurse specialist. Figure 1 illustrates the timeline of the TeleTAVI program from intervention through rehabilitation and clinical follow-up.
Figure 1.
Timeline of the TeleTAVI.
Telerehabilitation Technologies
The technologies used in the study are presented as supplementary material (Figure 1S), and have previously been comprehensively described.[25,26] Shortly, the technologies consisted of a tablet (Apple iPad) for use during the exercise sessions and for access to the informative website, an activity tracker (Beurer AS97), and a booklet in paper form with text information on how to use the technologies. The booklet also contained information regarding the scheduled online training sessions and could be used as a training diary. The technologies and training equipment for the home-based training sessions were delivered to the participants' homes one week after hospital discharge. We used the Open Telehealth application (OTH) [29] installed on the tablet to collect data on daily steps.
Exercise Training
The exercise training was web-based, individualized, and took place at patients’ homes. It was delivered in groups, started one week after TAVI, and lasted a total of twelve weeks. The first eight weeks were supervised. The program followed the national recommendations for CR with a combination of aerobic and strength training twice weekly, each session lasting 30–45 min.[30] The target intensity was Borg CR10 dyspnea 6–8.[31] Patients were also instructed to take a 30-min walk daily at a moderate intensity level. An additional unsupervised training program was available through access to the project´s website installed on the tablets and illustrated in the booklet,[28] for use during the first eight-week project period.
A summary of the sessions’ design and examples of exercises is provided in Table 1. Each participant was provided with training equipment, consisting of dumbbells (1, 2, 3 kg), a step bench, a training matt, and a tube grip elastic band. For resistance training, patients progressed to a heavier weight whenever they effortlessly managed to perform 10 repetitions with their previous weight.[32] The change to a heavier weight took place during the supervised training sessions.
Table 1. Summary of session design and example of exercises.
| Varm up | Aerobic training | Resistance training | Cool down/streching | |
| *Borg 0-10 exertion scale. For resistance training: target 3x10 repetitions for each exercise. Free weight exercises with dumbbells: 1 kg; 2 kg or 3 kg. The choice of a higher weight was based on Borg 5 at the 3. Set repetition. Aerobic exercises were intercalated with resistance training exercises. | ||||
| Time allocated during the sessions | 5–8 min | 20 min | 10–15 min | 10 min |
| Example of exercises performed | Stretching arms and trunk; standing trunk rolling Thoracic rotation Dynamic side bend Pursed lip breathing Walking |
Walking Walking with high knee rise Up and down - step bench Jumping jacks |
Standing or sitting pull with Tubi grip Arm curl – dumbbells Heel raise Sit to stand Abdominal muscles Upper truncus |
Static dynamic range of motion exercises targeting the thoracic column and core Pursed lip breathing Chest opening stretches Neck and shoulder stretches |
| *Target intensity | 3–4 | 6–9 | 6–8 | 2–3 |
To facilitate individualization of the exercise sessions, we scheduled two different groups, both taking place on the same weekday, as it seemed appropriate to group the patients with similar physical performance levels. The different time schedules also enabled patients to join another session should they miss their scheduled session on a given day. Two physiotherapists were present during the training sessions, the first to provide IT support before or during the sessions and the second to guide patients through the training program.
At the end of the first eight weeks, patients were given the choice to continue wearing the activity tracker for a further four weeks, registering the daily number of steps in their training diary placed in the booklet. Also, they could follow the exercise videos available at the project´s website, should they have a tablet or a computer of their own. Alternatively, they could perform the exercises as illustrated in their booklets.
Technology Management and IT Support
The introduction to the technology took place at patients’ homes one week after hospital discharge. Patients were thoroughly instructed on the use of the technologies and on how to connect to the web-based sessions. Furthermore, patients tried out the different exercises and equipment to be used during the training sessions.
The first author (BCB) provided IT support by telephone calls and, if necessary, using the application TeamViewer [33] to help participants to log in to the web-based sessions or for uploading data regarding their daily steps to the project’s database.
Recruitment of Participants, Data Collection, and Analysis
Eligible patients were approached for inclusion on the last working day before the surgery. The baseline assessments took place after written consent was obtained. The 12-week follow-up took place at the hospital clinic on the same day as the scheduled clinical post-TAVI follow-up. The assessments were performed by experienced physiotherapists.
Assessments
Demographic and perioperative data were collected from patients´ medical records. The following assessments were performed the day before surgery and again at the 3-month follow-up: a 6-min walk test (6MWT);[34] 30STS test (30-seconds sit-to-stand test) to assess functional lower extremity muscle strength [35]; and 4-m walk test to assess gait speed.[36] A gait speed < 0.7 m/s following TAVI is defined as frailty.[36] For hand grip strength we used the DHD-1 digital hand dynamometer.[35] For health-related quality of life, we used the HeartQol,[37] which is a disease-specific questionnaire validated for patients who have undergone cardiac valve replacement surgery.[37,38] Higher HeartQol values are related to worse quality-of-life. The Visual Analogue Scale (EQ-VAS) was used for responders’ self-evaluated health.[39] EQ-VAS values range from 0 (the worst imaginable health) to 100 (the best imaginable health). The MCID for the EQ-VAS in cardiac patients has not yet been established, though it has been reported to be eight points in stable COPD patients.[40] At baseline, we also assessed the cognitive function via the Mini-Mental Scale Evaluation (MMSE).[41] For frailty, we used the Tilburg Frailty Indicator (TFI). The TFI is a validated self-administered instrument for assessing multidimensional frailty in community-dwelling older people.[42] The cut-off value for frailty according to the TFI is ≥ 5 points.[43] Data were stored using the Redcap electronic data capture tool (Redcap Consortium, Vanderbilt University Medical Centre, Nashville, USA), hosted by the North Denmark Region.
Feasibility and Secondary Data
We registered process data consisting of number of home visits, need and type of IT support, and compliance with the training sessions. We also collected information on patients´ self-reported exertion level during the supervised web-based training sessions. At the twelve-week clinical control, patients were asked about their exercise habits after the initial 8-weeks supervised training. Data on individual daily steps during the eight-week supervised intervention period were collected from the OTH platform or by patients´ own entries in their personal diaries. The maximum possible number of entries for the first eight-week period was 56 days.
Data Analysis
Descriptive statistics were used to describe the study population. We used non-parametric statistics to analyze baseline differences between patients completing the three-month follow-up and those who did not complete the study. Due to the small number of participants and subsequent skewed data, we present results as median and interquartile range (IQR) or as numbers (frequency and percentage) when appropriate. Differences in “3 months minus baseline value” were thus expressed as absolute values. A 2-sided P value < 0.05 was considered statistically significant. Analyses were performed with SPSS statistical software (IBM Analytics, NY, USA).
Based on results from the first phase of the study,[25] we set the compliance with the web-based training sessions to 60% (10 out of 16 possible sessions). No formal sample size calculation was performed due to the explorative character of the study and because no efficacy testing was to be performed.[44]
RESULTS
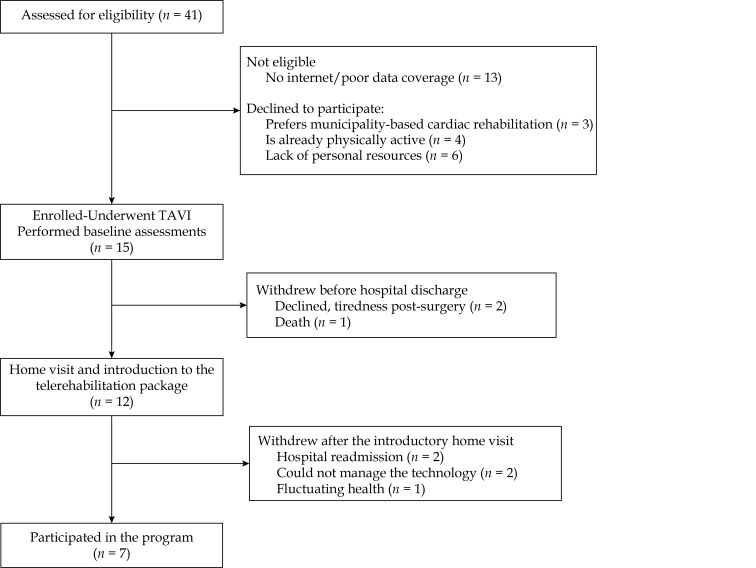
In total, 41 patients were screened for eligibility and 15 (36%) were enrolled in the study (Figure 2). Reasons for exclusion before the surgery were not meeting the inclusion criteria (n = 13) or declining to participate (n = 13). Of the 15 patients enrolled, eight were excluded after the surgery due to tiredness post-surgery (n = 2), non-cardiac related hospital readmission (n = 2), fluctuating health (n = 1), death during hospital stay (n = 1), and finally, two patients were excluded after the introductory home visit due to problems with managing the tablet. Both patients had a MMSE score of 24 points, with difficulties in the areas of spatial visualization and short-term memory, among others (individual MMSE results are not shown). Thus, seven patients completed the study, corresponding to a retainment rate of 47% (7/15). Table 1 depicts the demographic and surgical characteristics of all included patients, where the median [IQR] age is 84 years [82; 87]. Frailty according to the TFI was present in four of the patients completing the study, while a single patient not completing the study was deemed frail. A gait speed < 7 m/s was detected in three patients (two completing the study and one not completing the study).
Figure 2.
Study flow-chart.
Table 2 depicts compliance with the supervised training sessions and daily steps. Six out of the seven patients completing the study reached the 60% pre-established success rate for adherence to the web-based supervised training sessions. Patients’ exertion levels during the exercise sessions varied from five to ten. After the completion of the first 8-week program, three patients reported returning to usual community-based training sessions. None accessed the training videos at the project´s website.
Table 2. Demographics and surgical characteristics of participants.
| Variables | Included (n = 15) |
Completed the study (n = 7) |
Did not complete the study (n = 8) |
P-value |
| Data are presented as median (IQR) or n (%). *Comorbidities: arterial hypertension; ischemic heart disease; diabetes mellitus; atrial fibrillation; **Includes operative day, n = 14; #Patient or next of kin. ASA: American Society Anesthesiology Score; BMI: Body mass index; EF: ejection fraction; EQ VAS: EuroQol visual analog scale; FEV1: Forced expiratory value first second %; MRC: medical research council score; MMSE: mini mental state examination; NYHA: New York Heart Academy functional class; 6MWT: 6-minute walk test; HeartQol: quality of life questionnaire; TFI: tilburg frailty indicator. | ||||
| Age, yrs | 84 (82–87) | 83 (81–87) | 84 (82–89) | 0.61 |
| Male gender | 6 (40%) | 3 (43%) | 3 (37%) | 1.0 |
| BMI, kg/m2 | 27 (22–30) | 27 (22–30) | 26 (22–31) | 1.0 |
| *Two or more comorbidities | 6 (40%) | 3 (43%) | 3 (37%) | 1.0 |
| EF | 60 (50%–60%) | 60 (45%–60%) | 60 (55%–60%) | 0.54 |
| NYHA | 0.45 | |||
| I | 1 (7%) | 0 | 1 (13%) | |
| II | 5 (33%) | 2 (28%) | 3 (37%) | |
| III | 8 (53%) | 5 (72%) | 3 (37%) | |
| IV | 1 (7%) | 0 | 1 (13%) | |
| ASA | 0.57 | |||
| 3 | 4 (27%) | 1 (14%) | 3 (37%) | |
| 4 | 11 (73%) | 6 (86%) | 5 (63%) | |
| FEV1 % | 71 (65%-108%) | 68 (65%–120%) | 71 (48%–102%) | 0.62 |
| Hæmoglobin | 7.4 (5.9–9.0) | 7.9 (6.0-9.4) | 6.1 (5.8–6.1) | 0.40 |
| **Length of hospital stay, days | 4 (3–6) | 3 (3–7) | 4 (3–5) | 0.81 |
| Aortic peak gradient | 69 (60–93) | 64 (60-93) | 70 (61–101) | 0.61 |
| Postoperative pacemaker implantation | 9 (60%) | 4 (57%) | 5 (63%) | 1.0 |
| Measurements | ||||
| 6MWT, m | 400 (199–457) | 262 (199–463) | 308 (234–449) | 0.96 |
| 6MWT, % expected | 97 (58%-109%) | 61 (58%-104%) | 81 (61%-111%) | 0.78 |
| Gait speed 4 meter, seconds, median (IQR) | 3.90 (3.94–5.50) | 4.02 (3.85–5.82) | 4.73 (4.25–5.24) | 0.69 |
| Sit-to-stand (30 s) | 10 (8–12) | 10 (8–12) | 12 (8–12) | 0.20 |
| Hand strength % expected, median (IQR) | 123 (100%–123%) | 110 (100%–128%) | 110 (83%–121%) | 0.96 |
| MRC | 3 (2–4) | 4.0 (2–5) | 3 (2–3) | 0.07 |
| MMSE | 29 (28–29) | 29 (29–29) | 28 (24–30) | 0.15 |
| HeartQol | 1.36 (0.79–2.07) | 2.07(1.07–2.43) | 1.43 (0.67–1.51) | 0.12 |
| EQ VAS | 60 (50–70) | 50 (20-90) | 75 (53–88) | 0.28 |
| Socio demographics | ||||
| Living alone | 7 (46%) | 3 (43%) | 4 (50%) | 1.0 |
| Educational level | 0.03 | |||
| Public school or short education | 6 (40%) | 1 (14%) | 5 (63%) | |
| Medium or vocational education | 9 (60%) | 6 (64%) | 3 (37%) | |
| Information technology skills | 0.569 | |||
| Novice | 4 (27%) | 1 (14 %) | 3 (37%) | |
| #Acquainted with tablet or PC | 11 (73%) | 6 (86%) | 5 (63%) | |
| TFI, total score, median (IQR) | 4 (2–6) | 6 (2;9) | 4 (2–4) | 0.15 |
| Not frail | 10 (66%) | 4 (80%) | 6 (75%) | 0.06 |
| Frail (≥ 5 points) | 5 (34%) | 4 (57%) | 1 (13%) | |
Process and Delivery of IT Support
Home visits for introduction of the technology took place for twelve patients (Figure 2), lasting from 90 to 150 min. The distance traveled for the health professional (HP) varied from 3 to 56 km. All in all, the HP spent between 150 and 240 min in total on each home visit. Additional home visits for technical support were necessary three times and were delivered to two patients. Among the patients who attended the web-based exercise sessions, seven needed IT support to connect to the web-based sessions. The time spent by the HP for IT support varied from 10 to 30 min per session. The uploading of data on daily steps to the OTH platform installed on the tablet fluctuated a lot, mostly due to missing Bluetooth connections between the activity tracker and the platform, which resulted in incomplete records for all patients.
Changes in Outcome Measurements
We found a median (IQR) improvement in the 6MWT of 82.5 [26, 97] meters, P = 0.03, measured before surgery and three months postoperative. Also, the percentwise of expected handgrip strength was significantly increased, median (IQR) of 4% [-1, +8], P = 0.03. Changes in the MRC scores, HeartQol, EQ-VAS, gait speed, and 30STS were all non-significant (Table 3).
Table 3. Differences in outcome measurements in patients completing the TeleTAVI program.
| Variable | Before surgery (n = 7) |
3 months after surgery (n = 7) |
Difference (3 mths min baseline) |
P-value |
| Data are presented as median [IQR]; P-value based om Mann Whitney test. *P ≤ 0.05. N = 6 at 3 months after surgery llIncludes modified STT. EQ VAS: EuroQol visual analog scale; HeartQol: quality of life questionnaire; MRC: Medical Research Council Score; 6MWT: 6-minute walk test; 30STT: sit-to -stand 30 s test. | ||||
| 6MWT, m | 262 [199, 463] | 381 [267, 521] | 82.5 (+26, +97) | 0.03* |
| Handgrip % expected | 110 [80, 128] | 118 [99, 137] | 4 [-1, +8] | 0.03* |
| MRC dyspnea | 4 [2,5] | 2 [1,5] | -1 (-1, +3) | 0.14 |
| EQ5D VAS | 50 [20, 90] | 70 [50, 90] | 0.0 [-10, +44] | 0.22 |
| HeartQol | 2.07 [1.07, 2.43] | 1.64 [0.29, 1.79] | -0.58 [-0.28, 0.64] | 0.31 |
| Gait speed 4 m, s | 04.02 [03.85, 05.82] | 04.06 [03.65, 04.65] | -00.32 [-01.29, +00.21] | 0.17 |
| ll30STT | 10 [8,12] | 11 [7,14] | 2.5 [-1, +3] | 0.19 |
Daily Steps
The median [IQR] number of steps per day during the first 8-week program period was 1509 [933, 2635] steps (Table 4). Four patients reached over 5,000 steps per day at least once. The number of entries in either the OTH application or manual registration in personal diaries varied from 12 to 43 (21% to 77%). Six patients preferred to register their daily number of steps in their personal diary, while a single patient uploaded data to the OTH application. Two patients agreed to continue wearing the activity tracker after the first eight weeks. Since both patients did not use the activity tracker in the meantime, no data on daily steps was available at twelve weeks.
Table 4. Compliance to the intervention.
| ID | Age | Supervised training sessions |
Borg exertion min; max |
Daily steps Median (min, max) |
Activity tracker Number of days |
| Overall daily steps median (IQR): 1509 [933, 2635] steps. | |||||
| 1 | 85 | 12 (75%) | 5–9 | 3222 (1698; 5043) | 13 |
| 2 | 90 | 12 (75%) | 6–9 | 1062 (462; 6247) | 35 |
| 3 | 84 | 11 (69%) | 5–8 | 3250 (1732; 5452) | 36 |
| 4 | 87 | 07 (43%) | 6–10 | 353 (216; 520) | 12 |
| 5 | 82 | 10 (62%) | 6–9 | 1236 (424; 4569) | 25 |
| 6 | 74 | 10 (62%) | 6–9 | 2096 (772; 5068) | 25 |
| 7 | 81 | 13 (81%) | 6–9 | 1368 (556; 2564) | 43 |
Adverse Events
No adverse events associated with the web-based exercise intervention occurred.
DISCUSSION
This feasibility study emphasizes the practical difficulties in recruiting and implementing a web-based, supervised telerehabilitation program in the elderly population who has undergone TAVI. The TeleTAVI program was feasible in its current form and in the population as included in the study. In the small number of patients completing the intervention, we found a significant increase in the walked distance of 82.5 m, measured before and three months post-surgery. Meanwhile, a low recruitment rate of 36% (15/41), compounded by a low retainment rate of 47% (7/15), were major barriers for running the study. No access to internet at home or poor local data coverage were the main causes for patients not meeting the inclusion criteria; tiredness post-surgery and hospital readmission were common reasons for patient withdrawal after the surgery. Thus, the optimal set-up, target population, and effectiveness of telerehabilitation in the elderly population who has undergone TAVI still needs to be established.
Cardiac rehabilitation is recommended following TAVI to improve functional capacity, quality of life,[9–11] and to reduce mortality.[12] Since referral to or participation in center- or community-based CR in this particular population is low, reported to be 20%–30%,[13–15] different settings for CR need to be considered. In this context, home-based CR, including CTR, may be a useful strategy to improve recruitment and adherence to CR.[24,45–47] Six out of the seven patients completing the study reached the pre-established adherence rate to the program of 60%, showing a significant improvement in the walked distance (median 82.5 m) measured by the 6MWT from before to three months post-surgery. This improvement is beyond the 14.0 to 30.5 m clinically relevant change described in the literature for older adults with pathology.[48] Of note, the walked distance in our cohort at baseline was higher (median 400 m) than the distance reported in studies investigating the effects of CR following TAVI, probably because we assessed 6MWT prior to the surgery, while the assessment in most studies was performed after the surgery.[7,9-10,15,49–51] Assessing the walked distance after the surgery may be negatively influenced by inactivity or tiredness post-surgery.
In a previous research from our group, we found that the TeleTAVI program was highly appreciated by the participants,[25] due to the web-based setting with provision of supervised exercised training with real-time feedback, without need of transportation. Meanwhile, we were challenged by a 60% study dropout rate due to unstable data coverage at patients´ homes as well as participants' limited IT skills.[25] Thus, for the present study, we added no internet at home or poor data coverage at patients´ home as an exclusion criterion. Furthermore, we adapted the TeleTAVI program to last twelve weeks post-TAVI, with provision of additional IT support throughout the intervention period, to ensure adherence to the program. Previous research has shown that, although the majority of older patients (i.e., aged 80 years and over) are information and communication technology users, they have limited use of eHealth.[52] This is in line with findings from our parallel qualitative study that was conducted to gain knowledge of patients´ experiences with using heath technologies and in participating in the program.[26] Although CTR has been proven to be cost effective in cardiac disease in general,[21] there are particular challenges regarding its use in the aged population. In Denmark, the group of “digitally challenged” persons aged 65 years or older is 18%, and 80% among those aged 75 or older. Further, 20% of persons aged 75 years or older still do not have access to the internet at their homes.[53] This poses a challenge to offering digital home-based rehabilitation at present in the elderly and frail population who has undergone TAVI.
Future Perspectives
The challenge of securing continuous rehabilitation after TAVI is not yet solved and the knowledge from our feasibility study is an important contribution to understanding the challenges that older patients with complex health issues face, in particular regarding the use of telerehabilitation and general eHealth solutions to improve outcomes after surgery.
Efforts to increase referral to and enrollment in phase 2 rehabilitation after TAVI are still needed, in particular for patients who are deemed frail, and a multidisciplinary approach is recommended.[54] In this light, different delivery modalities of rehabilitation, tailored to patients´ individual interests and needs are necessary.[46] CTR may be an alternative to optimize access to different components of CR, including the delivery of exercise training,[55,56] provided that the patients have the required IT skills. Furthermore, future research should consider when to start CR, along with the appropriate setting.
Strengths and Limitations
Several limitations of the present feasibility study need to be addressed. First, the study was performed in a single-center study with a small number of participants included and completing the intervention. Meanwhile, we screened all patients scheduled for TAVI at our unit, which resulted in a recruitment rate similar to the study published by Rogers, et al.[57] Furthermore, our cohort is comparable to studies investigating the effects of CR after TAVI regarding age, the presence of comorbidities, ejection fraction, and NYHA.[51,57–61] Second, since we had no control group, we cannot exclude a natural improvement in outcomes due to benefits from the surgery. However, the findings of this study can inform researchers and clinicians on important issues to be addressed in future studies investigating the effects of CTR in the population who has undergone TAVI.
Conclusion
Web-based telerehabilitation including supervised exercise training in older adults who have recently undergone TAVI was feasible for a small number of patients who completed the eight-week intervention. This was reflected in significant and clinically relevant improvement in their walked distance three months after the surgery. However, the low recruitment and retention rates do question the overall feasibility of this intervention in a frail older population of post-TAVI patients. Further research is needed to find a way of securing CR in prefrail or frail patients following TAVI so they can benefit the most from the surgery in their daily life.
DISCLOSURE
Funding
This study was funded by the Danish Heart Foundation (19-R136-A9035-22130), AP Møller Fund, North Denmark Region's Research Fund, North Denmark Region´s Innovation Fund, and Jørgen Moellers Fund.
We thank all the clinical personnel involved in the clinical management of the patients.
Declaration of Conflicts
None.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgements
We thank all the clinical personnel involved in the clinical management of the patients.
References
- 1.Lytvyn L, Guyatt GH, Manja V, et al Patient values and preferences on transcatheter or surgical aortic valve replacement therapy for aortic stenosis: a systematic review. BMJ Open. 2016;6:e014327. doi: 10.1136/bmjopen-2016-014327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arsalan M, Szerlip M, Vemulapalli S, et al Should transcatheter aortic valve replacement be performed in nonagenarians? insights from the STS/ACC TVT Registry. J Am Coll Cardiol. 2016;67:1387–1395. doi: 10.1016/j.jacc.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thyregod HG, Steinbruchel DA, Ihlemann N, et al Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–2194. doi: 10.1016/j.jacc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Thourani VH, Edelman JJ, Holmes SD, et al The international society for minimally invasive cardiothoracic surgery expert consensus statement on transcatheter and surgical aortic valve replacement in low- and intermediate-risk patients: a meta-analysis of randomized and propensity-matched studies. Innovations (Phila) 2021;16:3–16. doi: 10.1177/1556984520978316. [DOI] [PubMed] [Google Scholar]
- 5.Lange R, Beckmann A, Neumann T, et al Quality of Life After Transcatheter Aortic Valve Replacement: Prospective Data From GARY (German Aortic Valve Registry) JACCCardiovascular Interv. 2016;9:2541–2554. doi: 10.1016/j.jcin.2016.09.050. [DOI] [PubMed] [Google Scholar]
- 6.Astin F, Horrocks J, McLenachan J, et al The impact of transcatheter aortic valve implantation on quality of life: A mixed methods study. Hear Lung J Acute Crit Care. 2017;46:432–438. doi: 10.1016/j.hrtlng.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Kleczynski P, Trebacz J, Stapor M, et al Inpatient cardiac rehabilitation after transcatheter aortic valve replacement is associated with improved clinical performance and quality of life. J Clin Med. 2021;10:2125. doi: 10.3390/jcm10102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CA, Rasania SP, Afilalo J, et al Functional status and quality of life after transcatheter aortic valve replacement: a systematic review. Ann Intern Med. 2014;160:243–254. doi: 10.7326/M13-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro GS, Melo RD, Deresz LF, et al Cardiac rehabilitation programme after transcatheter aortic valve implantation versus surgical aortic valve replacement: Systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:688–697. doi: 10.1177/2047487316686442. [DOI] [PubMed] [Google Scholar]
- 10.Völler H, Salzwedel A, Nitardy A, et al Effect of cardiac rehabilitation on functional and emotional status in patients after transcatheter aortic-valve implantation. Eur J Prev Cardiol. 2015;22:568–574. doi: 10.1177/2047487314526072. [DOI] [PubMed] [Google Scholar]
- 11.Abraham LN, Sibilitz KL, Berg SK, et al Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane database Syst Rev. 2021;5:CD010876. doi: 10.1002/14651858.CD010876.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butter C, Gross J, Haase-Fielitz A, et al Impact of rehabilitation on outcomes after TAVI: a preliminary study. J Clin Med. 2018;7:326. doi: 10.3390/jcm7100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen TB, Zwisler AD, Berg SK, et al Cardiac rehabilitation patients’ perspectives on the recovery following heart valve surgery: a narrative analysis. J Adv Nurs. 2016;72:1097–1108. doi: 10.1111/jan.12904. [DOI] [PubMed] [Google Scholar]
- 14.Imran HM, Baig M, Mujib M, et al Comparison of phase 2 cardiac rehabilitation outcomes between patients after transcatheter versus surgical aortic valve replacement. Eur J Prev Cardiol. 2018;25:1577–1584. doi: 10.1177/2047487318792099. [DOI] [PubMed] [Google Scholar]
- 15.Ashikaga K,Doi S, Yoneyama K, et al. Efficacy and safety of home-based cardiac telemonitoring rehabilitation in patients after transcatheter aortic valve implantation: single-center usability and feasibility study. JMIR Rehabil Assist Technol 2023; 10: e45247.
- 16.Parmanto B, Saptono A Telerehabilitation: state-of-the-art from an informatics perspective. Int J Telerehabilitation. 2009;1:73–84. doi: 10.5195/ijt.2009.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan DM, Tindall L, Theodoros D, et al A blueprint for telerehabilitation guidelines. Telemed e-Health. 2011;17:662–665. doi: 10.1089/tmj.2011.0036. [DOI] [PubMed] [Google Scholar]
- 18.Rawstorn JC, Gant N, Direito A, et al Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102:1183–1192. doi: 10.1136/heartjnl-2015-308966. [DOI] [PubMed] [Google Scholar]
- 19.Huang K, Liu W, He D, et al Telehealth interventions versus center-based cardiac rehabilitation of coronary artery disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22:959–971. doi: 10.1177/2047487314561168. [DOI] [PubMed] [Google Scholar]
- 20.Knudsen M V, Petersen AK, Angel S, et al Tele-rehabilitation and hospital-based cardiac rehabilitation are comparable in increasing patient activation and health literacy: A pilot study. Eur J Cardiovasc Nurs. 2020;19:376–385. doi: 10.1177/1474515119885325. [DOI] [PubMed] [Google Scholar]
- 21.Scherrenberg M, Falter M, Dendale P Cost-effectiveness of cardiac telerehabilitation in coronary artery disease and heart failure patients: systematic review of randomized controlled trials. Eur Hear J - Digit Heal. 2020;1:20–29. doi: 10.1093/ehjdh/ztaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heart T, Kalderon E. Older adults: are they ready to adopt health-related ICT? Int J Med Inform 2013; 82: e209–e231.
- 23.Jørgensen BB, Gregersen M, Pallesen SH, et al A group-based real-time videoconferencing telerehabilitation programme in recently discharged geriatric patients: a feasibility study. Eur Geriatr Med. 2021;12:801–808. doi: 10.1007/s41999-020-00444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcin T, Bengel C, Goldberg T, et al Patient interest in mHealth as part of cardiac rehabilitation in Switzerland. Swiss Med Wkly. 2021;151:w20510. doi: 10.4414/smw.2021.20510. [DOI] [PubMed] [Google Scholar]
- 25.Brocki CB, Andreasen JJ, Aaroe J, et al Exercise-based real-time telerehabilitation for older adult patients recently discharged after transcatheter aortic valve implantation: mixed methods feasibility study. JMIR Rehabil Assist Technol. 2022;9:e34819. doi: 10.2196/34819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorup CB, Villadsen A, Andreasen JJ, et al Perspectives on participation in a feasibility study on exercise-based cardiac telerehabilitation after transcatheter aortic valve implantation: qualitative interview study among patients and health professionals. JMIR Form Res. 2022;6:e35365. doi: 10.2196/35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldridge SM, Chan CL, Campbell MJ, et al CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot feasibility Stud. 2016;2:64. doi: 10.1186/s40814-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forløb ved indsættelse af stentklap i hjertet. 2020. https://aalborguh.rn.dk/teletavi (accessed on 14 Oct 2021).
- 29.Cichosz SL, Udsen FW, Hejlesen O. The impact of telehealth care on health-related quality of life of patients with heart failure: Results from the Danish TeleCare North heart failure trial. J Telemed Telecare 2019; 1357633X19832713.
- 30.National Klinisk Retningslinje for Hjerterehabilitering. doi: 978-87-7104-527-7.
- 31.Borg GA Perceived exertion. Exerc Sport Sci Rev. 1974;2:131–153. [PubMed] [Google Scholar]
- 32.Fragala MS, Cadore EL, Dorgo S, et al Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J strength Cond Res. 2019;33:2019–2052. doi: 10.1519/JSC.0000000000003230. [DOI] [PubMed] [Google Scholar]
- 33.Team Wiewer. https://www.teamviewer.com/en/?utm_source=google&utm_medium=cpc&utm_campaign=nordics%7Cb%7Cpr%7C22%7Caug%7Ctv-core-brand-only-sn%7Cnew%7Ct0%7C0&utm_content=Exact&utm_term=teamviewer&gad=1&gclid=EAIaIQobChMI1fX5nMPW_gIVLgWiAx0rdwrFEAAYASAAEgJUaPD_BwE (accessed on 9 Oct 2022).
- 34.American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med An Off J Am Thorac Soc Med Sect Am Lung Assoc. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 35.Thorborg, Kristian; Beyer NMP. Målemetoder i forebyggelse, behandling og rehabilitering. 2nd ed. Copenhagen (Denmark): Munksgaard Danmark 2010.
- 36.Hinterbuchner L, Strohmer B, Hammerer M, et al Frailty scoring in transcatheter aortic valve replacement patients. Eur J Cardiovasc Nurs. 2016;15:384–397. doi: 10.1177/1474515115596640. [DOI] [PubMed] [Google Scholar]
- 37.Gronset CN, Thygesen LC, Berg SK, et al Measuring HRQoL following heart valve surgery: the HeartQoL questionnaire is a valid and reliable core heart disease instrument. Qual Life Res. 2019;28:1245–1253. doi: 10.1007/s11136-018-02098-1. [DOI] [PubMed] [Google Scholar]
- 38.Oldridge N, Hofer S, McGee H, et al The HeartQoL: part II. Validation of a new core health-related quality of life questionnaire for patients with ischemic heart disease. Eur J Prev Cardiol. 2014;21:98–106. doi: 10.1177/2047487312450545. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen J, Davidsen M, Gudex C, et al Danish EQ-5D population norms. Scand J Public Health. 2009;37:467–474. doi: 10.1177/1403494809105286. [DOI] [PubMed] [Google Scholar]
- 40.Zanini A, Aiello M, Adamo D, et al Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respir Care. 2015;60:88–95. doi: 10.4187/respcare.03272. [DOI] [PubMed] [Google Scholar]
- 41.Folstein MF, Folstein SE, McHugh PR ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 42.Andreasen J, Sorensen EE, Gobbens RJJ, et al. Danish version of the Tilburg Frailty Indicator--translation, cross-cultural adaption and validity pretest by cognitive interviewing. Arch Gerontol Geriatr 2014; 59: 32–38.
- 43.Gobbens RJJ, van Assen MALM, Luijkx KG, et al The tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. 2010;11:344–355. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Macefield R How to specify the participant group size for usability studies: a practitioner’s guide. J Usability Stud. 2009;5:34–45. [Google Scholar]
- 45.Frederix I, Vanhees L, Dendale P, et al A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2014;21:45–53. doi: 10.1177/1357633X14562732. [DOI] [PubMed] [Google Scholar]
- 46.Beckie TM Utility of home-based cardiac rehabilitation for older adults. Clin Geriatr Med. 2019;35:499–516. doi: 10.1016/j.cger.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Brouwers RWM, van Exel HJ, van Hal JMC, et al Cardiac telerehabilitation as an alternative to centre-based cardiac rehabilitation. Neth Heart J. 2020;28:443–451. doi: 10.1007/s12471-020-01432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bohannon RW, Crouch R Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23:377–381. doi: 10.1111/jep.12629. [DOI] [PubMed] [Google Scholar]
- 49.Anayo L, Rogers P, Long L, et al Exercise-based cardiac rehabilitation for patients following open surgical aortic valve replacement and transcatheter aortic valve implant: a systematic review and meta-analysis. Open Hear. 2019;6:e000922. doi: 10.1136/openhrt-2018-000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oz A, Tsoumas I, Lampropoulos K, et al Cardiac rehabilitation after TAVI-a systematic review and meta-analysis. Curr Probl Cardiol. 2023;48:101531. doi: 10.1016/j.cpcardiol.2022.101531. [DOI] [PubMed] [Google Scholar]
- 51.Penati C, Incorvaia C, Mollo V, et al Cardiac rehabilitation outcome after transcatheter aortic valve implantation. Monaldi Arch Chest Dis. 2021;91:2. doi: 10.4081/monaldi.2021.1621. [DOI] [PubMed] [Google Scholar]
- 52.Terp R, Kayser L, Lindhardt T Older patients’ competence, preferences, and attitudes toward digital technology use: explorative study. JMIR Hum factors. 2021;8:e27005. doi: 10.2196/27005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tassy A, Thornfelt C. IT anvendelse i befolkningen. Danmarks Stat. 2022. https://www.dst.dk/Site/Dst/Udgivelser/GetPubFile.aspx?id=44692&sid=itbef2022 (accessed 10 May 2023).
- 54.Ha FJ, Bissland K, Mandrawa C, et al Frailty in patients with aortic stenosis awaiting intervention. Intern Med J. 2021;51:319–326. doi: 10.1111/imj.14737. [DOI] [PubMed] [Google Scholar]
- 55.Scherrenberg M, Wilhelm M, Hansen D, et al. The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020; 2047487320939671.
- 56.Nikolov H, Hubbard J, Hubbard J A critical review of cardiac rehabilitation in a digital era. Br J Card Nurs. 2021;16:1–9. [Google Scholar]
- 57.Rogers P, Al-Aidrous S, Banya W, et al Cardiac rehabilitation to improve health-related quality of life following trans-catheter aortic valve implantation: a randomised controlled feasibility study. Pilot Feasibility Stud. 2018;4:185. doi: 10.1186/s40814-018-0363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russo N, Compostella L, Tarantini G, et al Cardiac rehabilitation after transcatheter versus surgical prosthetic valve implantation for aortic stenosis in the elderly. Eur J Prev Cardiol. 2014;21:1341–1348. doi: 10.1177/2047487313494029. [DOI] [PubMed] [Google Scholar]
- 59.Tarro Genta F, Tidu M, Bouslenko Z, et al Cardiac rehabilitation after transcatheter aortic valve implantation compared to patients after valve replacement. J Cardiovasc Med (Hagerstown) 2017;18:114–120. doi: 10.2459/JCM.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 60.Eichler S, Salzwedel A, Reibis R, et al Multicomponent cardiac rehabilitation in patients after transcatheter aortic valve implantation: Predictors of functional and psychocognitive recovery. Eur J Prev Cardiol. 2017;24:257–264. doi: 10.1177/2047487316679527. [DOI] [PubMed] [Google Scholar]
- 61.Pressler A, Christle JW, Lechner B, et al Exercise training improves exercise capacity and quality of life after transcatheter aortic valve implantation: A randomized pilot trial. Am Heart J. 2016;182:44–53. doi: 10.1016/j.ahj.2016.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.