Abstract
We demonstrated recently that dominant negative mutants of rat DNA polymerase β (Pol β) interfere with repair of alkylation damage in Saccharomyces cerevisiae. To identify the alkylation repair pathway that is disrupted by the Pol β dominant negative mutants, we studied the epistatic relationship of the dominant negative Pol β mutants to genes known to be involved in repair of DNA alkylation damage in S. cerevisiae. We demonstrate that the rat Pol β mutants interfere with the base excision repair pathway in S. cerevisiae. In addition, expression of one of the Pol β dominant negative mutants, Pol β-14, increases the spontaneous mutation rate of S. cerevisiae whereas expression of another Pol β dominant negative mutant, Pol β-TR, does not. Expression of the Pol β-14 mutant in cells lacking APN1 activity does not result in an increase in the spontaneous mutation rate. These results suggest that gaps are required for mutagenesis to occur in the presence of Pol β-14 but that it is not merely the presence of a gap that results in mutagenesis. Our results suggest that mutagenesis can occur during the gap-filling step of base excision repair in vivo.
DNA alkylation damage is repaired by the base excision repair (BER) pathway in a variety of organisms (12). During BER, the DNA lesion is first recognized and removed from the sugar-phosphate backbone by a DNA glycosylase, creating an abasic site. This abasic site is subsequently processed by an apurinic/apyrimidinic (AP) endonuclease and an exonuclease. The resulting small gap is filled in by a DNA polymerase, and the nick is sealed by a DNA ligase (12). The biochemical characteristics of DNA polymerase β (Pol β) indicate that it is the polymerase that fills in the resulting gap. Pol β has high affinity for duplex DNA containing small gaps (30, 33). Pol β is processive when filling small gaps of up to 6 nucleotides (30) and has a higher catalytic efficiency on 5′ phosphorylated single-base-pair gaps (6), and purified Pol β protein functions in BER in vitro (15, 16, 31). Evidence from numerous laboratories has implicated Pol β as playing a role in DNA repair in vivo. For example, chemical inhibitors of Pol β inhibit DNA repair in cells treated with DNA-damaging agents such as methyl-N′-nitro-N-nitrosoguanidine (MNNG), methyl methanesulfonate (MMS), bleomycin, and γ irradiation (11, 14, 19). Simian virus 40-transformed mouse fibroblasts that lack Pol β are more sensitive to MMS than are their wild-type counterparts (31). Finally, in addition to its DNA polymerase activity, Pol β is able to catalyze the removal from DNA of 5′-deoxyribose-phosphate termini that sometimes result from the action of an AP endonuclease (18). This also provides evidence that Pol β participates in BER.
In Saccharomyces cerevisiae, a putative homolog of mammalian DNA Pol β has been identified. The polymerase IV gene encodes a protein with 26% identity and 50% similarity to Pol β (4). The biochemical characteristics of Pol IV are very similar to those of mammalian DNA Pol β (23, 29). Disruption of the Pol IV gene does not result in a significantly different phenotype from that observed in Pol IV+ strains with respect to DNA repair, growth, and sporulation (17, 23); this could be due to functional redundancy in S. cerevisiae. We previously demonstrated that S. cerevisiae cells expressing dominant negative mutants of rat Pol β, a putative Pol IV homolog, are sensitive to MMS and not to UV light (10). At that time, we suggested that the dominant negative rat Pol β mutants interfered with BER in S. cerevisiae. Recently, Bhattacharyya and Banerjee showed that a variant of Pol β associated with colon cancer also acts as a dominant negative mutant (2).
Three genes in S. cerevisiae that are known to specifically repair DNA alkylation damage have been cloned. The 3-methyladenine DNA glycosylase gene product (MAG) releases 3-methyladenine (MeA) and 7-methylguanine (7MeG) bases from DNA (7–9). The APN1 gene encodes the major AP endonuclease of S. cerevisiae (22, 24) and is responsible for processing the abasic sites that remain after the action of MAG. Both of these proteins function in BER. The O6-methylguanine DNA methyltransferase (MGT) is encoded by the MGT1 gene in S. cerevisiae (26, 35, 36). This protein repairs alkylation damage by directly transferring the methyl group from the methylated base to a cysteine residue on the MGT protein itself (26, 27). Each of these enzymes functions to protect the cell from endogenous alkylation damage resulting in a low rate of spontaneous mutagenesis in S. cerevisiae (37).
To determine if the rat Pol β dominant negative mutants interfere with BER specifically, we characterized the functional relationship between our rat DNA Pol β dominant negative mutants with the MAG, APN1, and MGT1 gene products. Our results demonstrate that the DNA polymerase β mutants specifically interfere with the gap-filling step of BER and suggest that mutagenesis can occur during gap filling in vivo.
MATERIALS AND METHODS
Yeast strains and media.
RS1 (MATa his3 leu2 ura3-52 trp1-289a GAL+) and RS8 (MATa his3 leu2 ura3-52 trp1-289a GAL+) were obtained from L. Samson (Harvard School of Public Health). Both the apn1-Δ1::HIS 3 and the mag1-Δ2::LEU2 strains were obtained from L. Samson (37) and were created in the RS8 background. The mgt1-Δ1::LEU2 strain was created as described previously (35) by disrupting the endogenous MGT1 gene in RS1 with the mgt1::LEU2 in vitro cassette. Disruption at the MGT1 locus was verified by Southern hybridization (data not shown) (25) and sensitivity to MNNG (see below). Rich medium (YPD) was prepared as described previously (28). Synthetic complete (SC) medium (28) was supplemented with 2% raffinose instead of glucose and with the appropriate amino acids. Transformants of all strains were selected on SC medium–2% raffinose in the absence of uracil (for pYES2 vector).
Plasmids.
Yeast expression plasmids for pBK3 (Pol β-WT-expressing plasmid), pPol β-TR, and pPol β-14 were constructed as described previously (10) in pYES 2 with the URA3 selectable marker for transformation.
Cell survival.
Survival data were obtained by a modification of previously described methods (10). Briefly, cells were grown overnight in YPD–2% glucose. Cells from the overnight culture were then washed three times with sterile water and used to inoculate (1:50 from overnight culture) SC medium or SC medium lacking the appropriate amino acid for plasmid selection plus 2% raffinose to suppress or 2% galactose to induce Pol β protein expression and incubated at 30°C overnight to a cell density of 1 × 107 to 2 × 107 cells/ml. The cells were treated with 0.3% MMS or 30 μg of MNNG per ml at 30°C. Aliquots were removed at various times, diluted into 0.05 M potassium phosphate buffer (pH 7.0), and plated on YPD. The cells were incubated for 3 days at 30°C, and colonies were counted.
Mutagenesis.
Spontaneous mutation rates for Trp+ reversion were calculated by using fluctuation analysis by a modification of the method of von Borstel (32). Briefly, cells were grown overnight in YPD and washed three times with sterile water. These cells were used to inoculate (1:100) SC medium lacking uracil for plasmid selection and containing 2% raffinose. The cells were grown to 107 cells/ml, diluted 1:10 into fresh medium, and grown for 3 h at 30°C. The culture was induced to express Pol β protein by further incubation with 0.5% galactose for 3 h at 30°C. Then 4 × 103 cells per ml were grown to 1 × 107 cells per ml (12 days at 30°C) in 240 individual 1-ml cultures in SC medium with limiting tryptophan (1.5 μM). The number of wells with no Trp+ revertants after the incubation period (12 to 14 days at 30°C) was used to calculate the mutation rate (32).
RESULTS
To study the in vivo role of a protein, one approach is to disrupt its normal cellular function and characterize the resulting phenotype. Because disruption of both copies of the Pol β gene in mice results in death (13) and because disruption of the Pol IV gene in S. cerevisiae does not result in any significant cellular phenotype (17, 23), gene disruption studies have been inconclusive. Therefore, to begin to study the cellular role of Pol β and its S. cerevisiae Pol IV homolog, we constructed rat Pol β dominant negative mutants (10). One of these mutants, Pol β-TR, contains the first 170 amino acids of rat Pol β and has no catalytic activity. The other mutant, Pol β-14, which contains a point mutation that alters amino acid residue 265 from cysteine to tyrosine, has catalytic activity similar to that of wild-type Pol β (34). We previously demonstrated that expression of the rat DNA Pol β dominant negative mutants in S. cerevisiae resulted in cellular sensitivity specifically to the DNA alkylation agent MMS but not to UV light (10). These data suggested that Pol β dominant negative mutants interfere with BER and not nucleotide excision repair (NER) (10). However, because DNA alkylation damage is not repaired exclusively by the BER pathway, in this report we characterize the epistatic relationship of our dominant negative mutants to the functions of known enzymes of the BER pathway in S. cerevisiae.
DNA Pol β mutants are epistatic to the APN1 and MAG gene products.
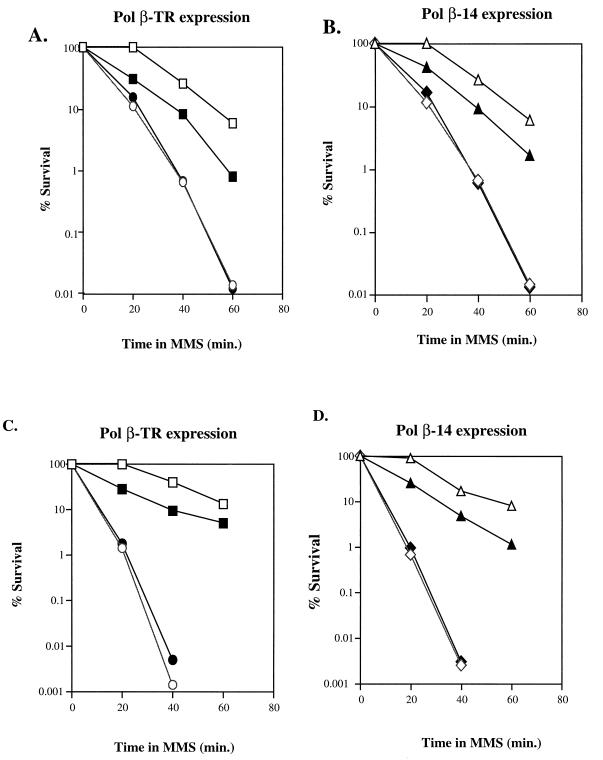
We compared the sensitivity of the wild-type, apn1-Δ1::HIS3, and mag1-Δ2::LEU2 strains to MMS in the presence and absence of Pol β-TR and Pol β-14 to determine if these proteins interfere with BER in S. cerevisiae. Figure 1 shows the MMS survival curves of the various strains with and without expression of Pol β-TR (Fig. 1A and C) or Pol β-14 (Fig. 1B and D). When expressed in the wild-type background Pol β-TR (Fig. 1A) and Pol β-14 (Fig. 1B), mutant proteins sensitize the wild-type RS8 strain to MMS. The sensitizing effect of expression of Pol β-14 or Pol β-TR in the RS8 genetic background is limited to the shoulder portion of the curve. We offer three explanations for this phenomenon. The most likely explanation is that due to the heterologous nature of the system and the methods we used to express the proteins, expression is not adequate or does not occur long enough to alter the rate of cell killing (i.e., to alter the slope). Second, we suggest that the proteins are turned over rapidly and are not present long enough to significantly alter the rate of cell killing. Third, the Pol β-TR and Pol β-14 proteins are derived from the rat Pol β enzyme and may not interact with proteins in S. cerevisiae that function to repair DNA damage. A lack of interaction with other BER proteins may not allow Pol β to gain access to sites in DNA in need of repair or to participate in a repair complex of proteins. We observed no increase in MMS sensitivity when we expressed the Pol β-TR or Pol β-14 proteins in either the apn-1-Δ1::HIS3 (Fig. 1A and B) or the mag1-Δ2::LEU2 (Fig. 1C and D) background. This result demonstrates that the Pol β mutant proteins are epistatic to the APN1 and MAG1 gene products and therefore most probably function in the same pathway. Since it is known that MAG and APN1 act sequentially in the BER pathway of yeast, our data suggest that the rat Pol β dominant negative mutants interfere with BER downstream of the action of APN1, most probably during the gap-filling step of BER.
FIG. 1.
Epistasis analysis of Pol β mutants with APN1 and MAG. Yeast strains expressing Pol β-TR (A and C) or Pol β-14 (B and D) were grown and induced to express Pol β protein as described in Materials and Methods. (A) MMS sensitivity of wild-type (open squares), Pol β-TR in wild-type (solid squares), apn1-Δ1::HIS3 (open circles), and Pol β-TR in apn1-Δ1::HIS3 (solid circles) strains. (B) MMS sensitivity of wild-type (open triangles), Pol β-14 in wild-type (solid triangles), apn1-Δ1::HIS3 (open diamonds), and Pol β-14 in apn1-Δ1::HIS3 (solid diamonds) strains. (C) MMS sensitivity of wild-type (open squares), Pol β-TR in wild-type (solid squares), mag1-Δ2::LEU2 (open circles), and Pol β-TR in mag1-Δ2::LEU2 (solid circles) strains. (D) MMS sensitivity of wild-type (open triangles), Pol β-14 in wild-type (solid triangles), mag1-Δ2::LEU2 (open diamonds), and Pol β-14 in mag1-Δ2::LEU2 (solid diamonds) strains. S. cerevisiae strains harboring the vector alone or a nondominant mutant, when induced with galactose to express protein, showed no increase in MMS sensitivity (reference 10 and data not shown). The results are representative of three independent determinations; all determinations demonstrated similar results.
Pol β mutants are epistatically distinct from the MGT1 gene product.
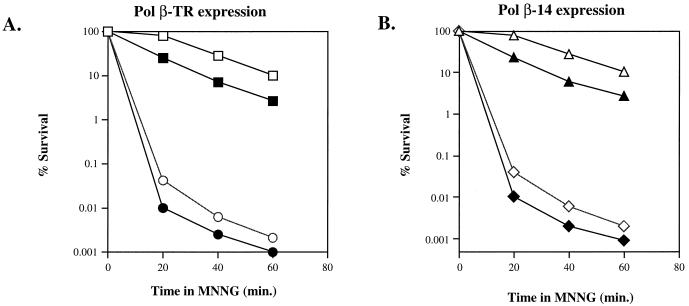
Since alkylation damage can also be repaired via a methyltransferase pathway, a pathway independent from BER in S. cerevisiae, the specificity of the Pol β mutants was assayed by investigating the epistatic relationship of Pol β-TR and Pol β-14 to the O6-methylguanine DNA methyltransferase (MGT1) gene product. Figure 2 shows the MNNG survival curves of S. cerevisiae wild-type and mgt1-Δ1::LEU2 strains with and without expression of the rat Pol β mutant proteins. Expression of Pol β-TR (Fig. 2A), or Pol β-14 (Fig. 2B) sensitizes the wild-type strain to MNNG. The mgt1-Δ1::LEU2 strain is very sensitive to MNNG, as reported previously (36, 37). However, expression of either Pol β-TR (Fig. 2A) or Pol β-14 (Fig. 2B) in the mgt1-Δ1::LEU2 strain further sensitizes this strain to MNNG. These data demonstrate that the Pol β mutant proteins do not interfere with the methyltransferase pathway of DNA alkylation damage repair.
FIG. 2.
Epistasis analysis of Pol β mutants with MGT1. Yeast cells expressing Pol β-TR (A) or Pol β-14 (B) were grown and induced to express protein as described in Materials and Methods. (A) MNNG sensitivity of wild-type (open squares), Pol β-TR in wild-type (solid squares), Δmgt1::LEU2 (open circles), and Pol β-TR in Δmgt1::LEU2 (solid circles) strains. (B) MNNG sensitivity of wild-type (open triangles), Pol β-14 in wild-type (solid triangles), Δmgt1::LEU2 (open diamonds), and Pol β-14 in Δmgt1::LEU2 (solid diamonds) strains. The results are representative of two independent determinations; all determinations demonstrated similar results.
Pol β-14 increases the spontaneous mutagenesis rate in yeast.
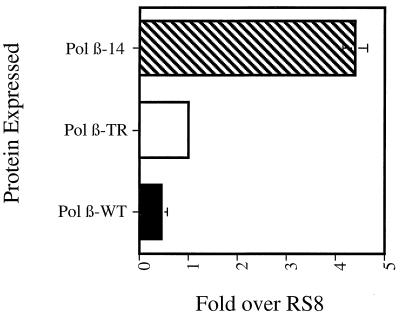
The above results demonstrate that expression of rat Pol β dominant negative mutants specifically interferes with BER in S. cerevisiae. The BER pathway is a major DNA repair pathway in S. cerevisiae for the repair of alkylation damage. Alkylation damage results from both exogenous and endogenous sources, and it has been demonstrated previously that inhibiting BER in S. cerevisiae leads to an increase in the spontaneous mutation rate (37). Therefore, enzymes that play a role in this pathway are responsible for maintaining and monitoring the genomic fidelity of the cells. To determine if expression of the dominant negative mutants results in an increase in spontaneous mutagenesis, we measured the spontaneous rate of reversion to Trp+ by using the auxotrophic allele trp1-289 in strains overexpressing either Pol β-WT, Pol β-TR, or Pol β-14. The trp 1-289 allele is the same allele as that used by Xiao and Samson (37). This allele is an amber mutation that can revert to Trp+ directly at the site of the mutation or by amber suppressor mutations, thus allowing us to measure a variety of in vivo mutational events. Figure 3 shows the fold increase of the spontaneous mutation rate over the RS8 strain in the presence of either the Pol β-WT, Pol β-TR, or Pol β-14 protein. We observed no increase over RS8 in the spontaneous mutation rate when the Pol β-WT or Pol β-TR was expressed and a fivefold increase when the Pol β-14 protein was expressed. We observed a 10-fold increase over that for Pol β-WT when we expressed Pol β-14 in the RS8 strain. This increase in the spontaneous mutation rate is similar to the increase observed in cells deficient in APN1 (37). This effect is specific for the expression of the Pol β-14 protein, since the same strain gave no increase in the mutation rate in the absence of the inducing agent (data not shown). These results suggest that interference with BER does not always result in an increase in mutagenesis.
FIG. 3.
Pol β-14 increases the spontaneous mutation rate in S. cerevisiae. Strains were grown and induced to express Pol β-14, Pol β-TR, or Pol β-WT protein as described in Materials and Methods. In the absence of the inducing agent, galactose, the spontaneous mutation rates of RS8 with either Pol β-14, Pol β-TR, or Pol β-WT were within a range of 1 to 1.2 fold over that with RS8 alone.
Pol β-14 makes mutations during the gap-filling step of BER in S. cerevisiae.
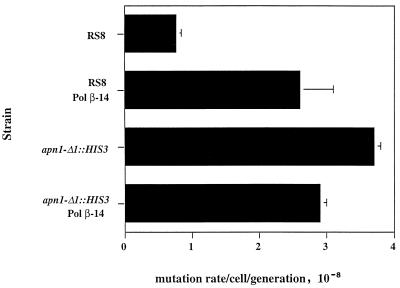
To determine if Pol β-14 functions downstream of the APN1 protein in mutagenesis, we measured the spontaneous mutation rate in the apn1-Δ1::HIS3 strain expressing Pol β-14, the protein that increased the spontaneous mutation rate of the RS8 wild-type strain. We reasoned that if gaps were required for mutagenesis in the presence of Pol β-14, we would not see an increase in the spontaneous mutation rate in cells lacking APN1 activity. Figure 4 shows a comparison of the spontaneous mutation rate of the wild-type RS8 strain and apn1-Δ1::HIS3 strain in the absence or presence of Pol β-14 expression. As noted above, the background rate of Trp+ reversion in the wild-type RS8 strain is low (0.5 × 10−8) and is increased fivefold in the presence of Pol β-14 (2.5 × 10−8). However, as shown in Fig. 4, expression of Pol β-14 in the apn1-Δ1::HIS3 background does not result in an increase in the spontaneous mutation rate compared with that of the apn1-Δ1::HIS3 strain alone (compare the apn1-Δ1::HIS3 strain alone [3.7 × 10−8] with the apn1-Δ1::HIS3 strain expressing Pol β-14 [2.9 × 10−8]). This result strongly suggests that the ability of Pol β-14 to increase the spontaneous mutation rate is dependent on the activity of the APN1 gene product and most probably requires the presence of a gap.
FIG. 4.
Spontaneous mutation rate in the apn1-Δ1::HIS3 strain expressing Pol β-14. Yeast cells were grown and induced to express protein as described in Materials and Methods. Cells were diluted into minimal medium with limiting tryptophan (1.5 μM), and Trp+ revertants were scored as described in Materials and Methods. The results are the average of two independent determinations.
DISCUSSION
Previous work from our laboratory demonstrated that rat DNA Pol β dominant negative mutants sensitized wild-type S. cerevisiae cells to MMS but not to UV light. At that time, we suggested that our rat DNA Pol β dominant negative mutants interfered with BER but not NER in S. cerevisiae (10). We hypothesized that the mutant proteins disrupted BER by binding to the DNA nonproductively, preventing the gap from being filled in a timely manner, and ultimately resulting in an increase in the cytotoxicity of the yeast cells to MMS. These results prompted us to investigate the relationship of our mutant proteins to the BER pathway. This pathway has been well studied in S. cerevisiae, and the genes known to be involved in BER have been cloned and their functional relationships have been established (for example, see reference 37). The epistasis analysis we describe here with various S. cerevisiae strains that are disrupted in known BER proteins demonstrates that the DNA Pol β dominant negative mutants function in the same DNA repair pathway as the MAG and APN1 gene products with respect to repair of DNA alkylation damage (Fig. 1). However, the Pol β dominant negative mutants are not epistatic to the MGT1 gene product (Fig. 2), an enzyme known to function in a pathway distinct from BER. We conclude that the rat Pol β-TR and Pol β-14 mutants interfere specifically with BER in S. cerevisiae.
Pol β-TR does not fill the gap.
Pol β-TR contains the DNA binding domain of the Pol β protein but lacks the aspartic acid residues that are essential for catalysis (5, 20). We suggest that Pol β-TR competes with an endogenous S. cerevisiae DNA polymerase, perhaps Pol IV or Pol δ (3), for binding to the gapped DNA. Because Pol β-TR lacks the ability to catalyze DNA synthesis, the gaps to which it is bound remain unfilled, resulting in cell death.
Pol β-14 fills gaps slowly.
Pol β-14 may also block the access of a gap to an endogenous S. cerevisiae DNA polymerase. However, Pol β-14 is only about 10 times less active than wild-type Pol β (34) in vitro, indicating that it has a reduced ability to fill in gaps (31a). We suggest that in S. cerevisiae cells, Pol β-14 most probably fills gaps slowly or fills fewer gaps in vivo, resulting in an increased sensitivity to MMS.
Pol β-TR and Pol β-14 do not act by inhibiting APN1.
Recently, it has been shown that human DNA polymerase β interacts with human Ape, the major AP endonuclease in human cells (1). Therefore, it is possible that the Pol β mutants are acting by inhibiting APN1. We do not favor this explanation for the following reasons. First, if Pol β-TR or Pol β-14 were inhibiting the action of APN1, we would expect that expression of these proteins would sensitize the cells to the same extent as would result from disrupting the APN1 gene. This is not the case. Second, in the case of mutagenesis, whereas Pol β-14 expression does result in a similar increase in the rate of spontaneous mutagenesis as that observed in APN1-containing strains, expression of Pol β-TR has no effect on mutagenesis. If expression of Pol β-TR inhibited the action of APN1, we would expect to observe an increase in spontaneous mutagenesis similar to that observed in APN1-disrupted strains. Finally, Bennett et al. demonstrated that APN1 stimulates the activity of Pol β by loading Pol β onto a gap (1). These data suggest that APN1 acts at a step prior to that of Pol β. Therefore, mutants of Pol β should not decrease the activity of APN1 but, instead, should act after APN1.
Pol β-TR and Pol β-14 may interfere with Pol IV.
Pol β-TR and Pol β-14 interfere with the gap-filling step of BER, suggesting that they interfere with the action of a DNA polymerase. The identity of the cellular polymerase whose function is inhibited by our dominant negative mutants remains to be uncovered. Pol β-TR and Pol β-14 may interfere with Pol IV. Pol IV has homology to Pol β and biochemical properties similar to Pol β (23, 29), an enzyme that has been implicated as functioning in BER. Therefore, by analogy, Pol IV may function in BER in S. cerevisiae. However, no significant cellular phenotype results from the disruption of the Pol IV gene (20, 23), suggesting either that Pol IV does not act in BER or that another polymerase substitutes for Pol IV when the cells are deficient in this enzyme. If Pol IV acts during BER, our dominant negative mutants would most probably block the action of this enzyme because the substrate specificities of Pol IV and Pol β are similar (23, 29).
Pol β-TR and Pol β-14 may interfere with Pol δ.
Blank et al. suggested that Pol δ functions in BER in S. cerevisiae because the Pol δ mutant allele cdc2-2 is sensitive to alkylating agents (3). Therefore, Pol β-TR and Pol β-14 may bind to gaps that are normally filled in by Pol δ, interfering with the action of this enzyme. Alternatively, Pol β-TR and Pol β-14 may block the action of another polymerase in S. cerevisiae.
Pol β-14 commits errors during the gap-filling step of BER.
BER is a major cellular repair pathway that acts to repair both endogenous and exogenous alkylation damage. Inhibition of the early steps of BER has been shown to result in mutagenesis (37). Our present work extends these findings by focusing on the action of the DNA Pol and the consequences to the cell if BER malfunctions at the gap-filling stage. In our study, both Pol β-TR and Pol β-14 interfere with the gap-filling step of BER, as demonstrated by our epistasis analysis (Fig. 1). However, only expression of Pol β-14 increases the spontaneous mutation rate (Fig. 3) in the wild-type strain to a level similar to that observed in an APN1-disrupted strain (37). Furthermore, the increase in the spontaneous mutation rate we observed in cells expressing Pol β-14 is dependent upon APN1, because we did not observe an increase in the spontaneous mutation rate in cells expressing Pol β-14 but lacking APN1. These data suggest that mutagenesis resulting from the expression of Pol β-14 occurs after the abasic site is removed and that it most probably requires a gapped DNA substrate. Because we observed an increase in the spontaneous mutation rate only in the presence of Pol β-14 and not Pol β-TR, our data suggest that interfering with the gap-filling step of BER does not necessarily result in mutagenesis and indicates that gaps themselves are not mutagenic intermediates. Our results with the Pol β-TR mutant demonstrate that it is not the loss of gap filling itself that is mutagenic, but the loss of correct gap filling that leads to mutations.
Our data strongly suggest that a specific property of Pol β-14 is responsible for the observed increase in the spontaneous rate. The mutator activity we observed most probably results directly from errors committed by the Pol β-14 protein during the gap-filling step of BER. This suggestion is supported by previous work from our laboratory showing that Pol β-14 is a mutator polymerase in vivo and in vitro (34) and by the fact that even though Pol β-TR inhibits BER, it does not promote spontaneous mutagenesis. An alternative explanation for the lack of mutagenesis displayed by cells expressing Pol β-TR is that this protein is missing the domain it requires to interact with the APN1 protein. If this were the case, Pol β-TR would most probably not be loaded onto the DNA by APN1, resulting in a lack of mutagenesis and no increase in MMS sensitivity compared to the wild-type strain. However, we did observe an increase in MMS sensitivity when we expressed Pol β-TR in S. cerevisiae, strongly indicating that APN1 is able to load the Pol β-TR protein onto the DNA. Therefore, we favor the explanation that Pol β-14 commits errors during the gap-filling step of BER in S. cerevisiae. In support of this suggestion, Polesky et al. have described a DNA Pol I Klenow fragment mutant that also seems to participate in mutagenic gap filling (21).
ACKNOWLEDGMENTS
We thank Leona Samson and Brian Glassner for the generous gifts of the RS1, RS8, apn1-Δ1::HIS3, and mag1-Δ2::LEU2 strains and the Δmgt1::LEU2 disruption cassette. We also thank Brian Glassner for very helpful discussions.
This work was supported by Public Health Service grant CA-64134 to J.B.S. and by NRSA postdoctoral fellowship CA68764-02 to C.A.C.
Footnotes
Dedicated to the memory of Franklin Hutchinson.
REFERENCES
- 1.Bennett R A O, Wilson III D M, Wong D, Demple B. Interaction of human apurinic endonuclease and DNA polymerase β in the base excision repair pathway. Proc Natl Acad Sci USA. 1997;94:7166–7169. doi: 10.1073/pnas.94.14.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya N, Banerjee S. A variant of DNA polymerase beta acts as a dominant negative mutant. Proc Natl Acad Sci USA. 1997;94:10324–10329. doi: 10.1073/pnas.94.19.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank A, Kim B, Loeb L A. DNA polymerase delta is required for base excision repair of DNA methylation damage in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1994;91:9047–9051. doi: 10.1073/pnas.91.19.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bork P, Ouzounis C, Sander C, Scharf M, Schneider R, Sonnhammer E. Comprehensive sequence analysis of the 182 predicted open reading frames of yeast chromosome III. Protein Sci. 1992;1:1677–1690. doi: 10.1002/pro.5560011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casas-Finet J R, Wilson S H, Karpel R L. Selective photochemical modification by trichloroethanol of tryptophan residues in proteins with a high tyrosine-to-tryptophan ratio. Anal Biochem. 1992;205:27–35. doi: 10.1016/0003-2697(92)90574-q. . (Erratum, 211:177, 1993.) [DOI] [PubMed] [Google Scholar]
- 6.Chagovetz A M, Sweasy J B, Preston B D. Increased activity and fidelity of DNA polymerase β in single-nucleotide gapped DNA. J Biol Chem. 1997;272:27501–27504. doi: 10.1074/jbc.272.44.27501. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Derfler B, Maskati A, Samson L. Cloning a eukaryotic DNA glycosylase repair gene by the suppression of a DNA repair defect in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:7961–7965. doi: 10.1073/pnas.86.20.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Derfler B, Samson L. Saccharomyces cerevisiae 3-methyladenine DNA glycosylase has homology to the AlkA glycosylase of E. coli and is induced in response to DNA alkylation damage. EMBO J. 1990;9:4569–4575. doi: 10.1002/j.1460-2075.1990.tb07910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Samson L. Induction of S. cerevisiae MAG 3-methyladenine DNA glycosylase transcript levels in response to DNA damage. Nucleic Acids Res. 1991;19:6427–6432. doi: 10.1093/nar/19.23.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clairmont C A, Sweasy J B. Dominant negative rat DNA polymerase β mutants interfere with base excision repair in Saccharomyces cerevisiae. J Bacteriol. 1996;178:656–661. doi: 10.1128/jb.178.3.656-661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dresler S, Lieberman W M. Identification of DNA polymerases involved in DNA excision repair in diploid fibroblasts. J Biol Chem. 1983;258:9990–9994. [PubMed] [Google Scholar]
- 12.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C: American Society for Microbiology; 1995. [Google Scholar]
- 13.Gu H, Marth J, Orban P, Mossmann H, Rajewsky K. Deletion of a polymerase β gene segment in T cells using cell type specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 14.Hammond R, McClung J, Miller M. Effect of DNA inhibitors on DNA repair in intact and permeabilized human fibroblasts: evidence that DNA polymerases δ and β are involved in DNA repair synthesis induced by N-methyl-N′-nitrosoguanidine. Biochemistry. 1990;29:286–291. doi: 10.1021/bi00453a039. [DOI] [PubMed] [Google Scholar]
- 15.Klungland A, Lindahl A. Second pathway for completion of human DNA base excision repair: reconstitution with purified proteins and requirement for DNase IV (FEN1) EMBO J. 1997;16:3341–3348. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubota Y, Nash R A, Klungland A, Schar P, Barnes D E, Lindahl T. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase β and the XRCC1 protein. EMBO J. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 17.Leem S H, Ropp P A, Sugino A. The yeast Saccharomyces cerevisiae DNA polymerase IV: possible involvement in double strand break DNA repair. Nucleic Acids Res. 1994;22:3011–3017. doi: 10.1093/nar/22.15.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto Y, Kim K. Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science. 1995;269:699–702. doi: 10.1126/science.7624801. [DOI] [PubMed] [Google Scholar]
- 19.Miller M, Lui L. Participation of different DNA polymerases in mammalian DNA repair synthesis is not related to patch size. Biochem Biophys Res Commun. 1982;18:1617–1682. doi: 10.1016/s0006-291x(82)80103-4. [DOI] [PubMed] [Google Scholar]
- 20.Pelletier H, Sawaya M R, Kumar A, Wilson S H, Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–903. [PubMed] [Google Scholar]
- 21.Polesky A H, Steitz T A, Grindley N D, Joyce C M. Identification of residues critical for the polymerase activity of the Klenow fragment of DNA polymerase I from Escherichia coli. J Biol Chem. 1990;265:14579–14591. [PubMed] [Google Scholar]
- 22.Popoff S, Spira A, Johnson A, Demple B. Yeast structural gene (APN1) for the major apurinic endonuclease: homology to Escherichia coli endonuclease IV. Proc Natl Acad Sci USA. 1990;87:4193–4197. doi: 10.1073/pnas.87.11.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad R, Widen S G, Singhal R K, Watkins J, Prakash L, Wilson S H. Yeast open reading frame YCR14C encodes a DNA beta-polymerase-like enzyme. Nucleic Acids Res. 1993;21:5301–5307. doi: 10.1093/nar/21.23.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramotar D, Popoff S, Gralla E, Demple B. Cellular role of yeast Apn1 apurinic endonuclease/3′-diesterase: repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol Cell Biol. 1991;11:4537–4544. doi: 10.1128/mcb.11.9.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Sassanfar M, Dosanjh M K, Essigmann J M, Samson L. Relative efficiencies of the bacterial, yeast, and human DNA methyltransferases for the repair of O6-methylguanine and O4-methylthymine. Suggestive evidence for O4-methylthymine repair by eukaryotic methyltransferases. J Biol Chem. 1991;266:2767–2771. [PubMed] [Google Scholar]
- 27.Sassanfar M, Samson L. Identification and preliminary characterization of an O6-methylguanine DNA repair methyltransferase in the yeast Saccharomyces cerevisiae. J Biol Chem. 1990;265:20–25. [PubMed] [Google Scholar]
- 28.Sherman F. Getting started with yeast. In: Guthrie C, Fink G, editors. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press, Inc.; 1991. pp. 3–21. [Google Scholar]
- 29.Shimizu K, Santocanale C, Ropp P A, Longhese M P, Plevani P, Lucchini G, Sugino A. Purification and characterization of a new DNA polymerase from budding yeast Saccharomyces cerevisiae. A probable homolog of mammalian DNA polymerase beta. J Biol Chem. 1993;268:27148–27153. [PubMed] [Google Scholar]
- 30.Singhal R K, Wilson S H. Short gap-filling synthesis by DNA polymerase beta is processive. J Biol Chem. 1993;268:15906–15911. [PubMed] [Google Scholar]
- 31.Sobol R W, Horton J K, Kuhn R, Gu H, Singhal R K, Prasad R, Rajewsky K, Wilson S H. Requirement of mammalian DNA polymerase-beta in base-excision repair. Nature. 1996;379:183–186. doi: 10.1038/379183a0. [DOI] [PubMed] [Google Scholar]
- 31a.Sweasy, J. B. Unpublished results.
- 32.von Borstel R. Measuring spontaneous mutation rate in yeast. Methods Cell Biol. 1978;20:1–24. doi: 10.1016/s0091-679x(08)62005-1. [DOI] [PubMed] [Google Scholar]
- 33.Wang T, Korn D. Specificity of the catalytic interaction of human DNA polymerase β with nucleic acid substrates. Biochemistry. 1982;21:1597–1608. doi: 10.1021/bi00536a021. [DOI] [PubMed] [Google Scholar]
- 34.Washington S L, Yoon M S, Chagovetz A M, Li S-X, Clairmont C A, Preston B D, Eckert K A, Sweasy J B. A genetic system to identify DNA polymerase β mutator mutants. Proc Natl Acad Sci USA. 1997;94:1321–1326. doi: 10.1073/pnas.94.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao W, Derfler B, Chen J, Samson L. Primary sequence and biological functions of a Saccharomyces cerevisiae O6-methylguanine/O4-methylthymine DNA repair methyltransferase gene. EMBO J. 1991;10:2179–2186. doi: 10.1002/j.1460-2075.1991.tb07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao W, Samson L. The Saccharomyces cerevisiae MGT1 DNA repair methyltransferase gene: its promoter and entire coding sequence, regulation and in vivo biological functions. Nucleic Acids Res. 1992;20:3599–3606. doi: 10.1093/nar/20.14.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao W, Samson L. In vivo evidence for endogenous DNA alkylation damage as a source of spontaneous mutation in eukaryotic cells. Proc Natl Acad Sci USA. 1993;90:2117–2121. doi: 10.1073/pnas.90.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]