Abstract
The prevalence and severity of periodontitis are increased and advanced in diabetes. Severe periodontitis elicits adverse effects on diabetes by impairing insulin actions due to systemic microinflammation. Recent studies unveil the emerging findings and molecular basis of the bidirectional relationship between periodontitis and diabetes. In addition to conventional mechanisms such as hyperglycemia, hyperlipidemia, and chronic inflammation, deficient insulin action may play a pathogenic role in the progression of periodontitis under diabetes. Epidemiologically, from the viewpoint of the adverse effect of periodontitis on diabetes, recent studies have suggested that Asians including Japanese and Asian Americans with diabetes and mild obesity (BMI <25 kg/m2) should pay more attention to their increased risk for cardiovascular diseases. In this review, we summarize recent findings on the effect of diabetes on periodontitis from the viewpoint of abnormalities in metabolism and insulin resistance with novel mechanisms, and the influence of periodontitis on diabetes mainly focused on micro-inflammation related to mature adipose tissue and discuss future perspectives about novel approaches to interrupt the adverse interrelationship.
Keywords: Periodontitis, Diabetes, Periodontal medicine, Insulin resistance, Micro-inflammation, Diabetes-related periodontitis
1. Introduction
Periodontal disease is an infectious and inflammatory disease localized in the periodontal tissue caused by gram-negative obligate anaerobes. The prevalence and severity of periodontitis in people with diabetes are significantly higher than those in healthy people [1], [2], [3], [4], [5], [6]. Hyperglycemia and hyperlipidemia may cause cellular stress and dysfunction in the periodontal tissue-constructing cells such as gingival fibroblasts, epithelial cells, endothelial cells, immune cells, and so on [7], [8], [9], [10]. Chronic inflammation in periodontal tissue, subsequently induced by hyperglycemia and hyperlipidemia, also contributes to the exacerbation of periodontitis by failure in the resolution of inflammation [11], [12], [13]. Insulin resistance, which has been considered a representative feature of diabetic complications resulting from abnormalities in glucose and lipid metabolism and chronic inflammation, might play an important role in the pathogenesis of diabetic complications such as nephropathy, atherosclerosis, non-alcoholic fatty liver disease and so on [14], [15], [16]. The emerging evidence shows that insulin resistance, in other words, the deficiency of insulin action, on the periodontal tissue-constructing cells such as gingival fibroblasts and endothelial cells contribute to the pathogenesis of diabetes-related periodontitis [17], [18].
Furthermore, severe periodontitis contributes to worsened blood glucose control by impairing insulin action, resulting from the induction of systemic micro-inflammation especially in people with obesity and diabetes. Amplification of micro-inflammation via the interaction of adipocytes with macrophages in mature visceral adipose tissue plays a pivotal role in the induction of systemic micro-inflammation [19]. Lipopolysaccharides (LPS) derived from periodontitis-related pathobionts and intestinal dysbiotic microbiome enhance the interaction between adipocytes and inflammatory immune cells, resulting in hypersecretion of inflammatory cytokines such as tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), and so on [20], [21]. CC-chemokine receptor 7 (CCR7)- chemokine (C-C motif) ligand 19 (CCL19) axis may contribute to the development and progression of amplified inflammation in the adipose tissue [22]. Additionally, adaptive immune cells are also involved in the amplification of inflammation in the adipose tissue through interleukin 17 (IL-17)-mediated chemokine (C-C motif) ligand 20 (CCL20) and IL-6 production [23]. Additionally, activated macrophages could interact with tissue-resident cells such as mesangial cells and pancreatic beta cells [24], [25].
In clinical studies, the Hiroshima study revealed that people with a body mass index (BMI) around 23–25 kg/m2 were more susceptible to systemic inflammation by peripheral periodontitis [26]. Periodontal therapy with local antibiotic reagents significantly improved hemoglobin A1c (HbA1c) in people with a BMI less than 25 kg/m2 but not in those with more than 25 kg/m2. A unique cohort of people with type 1 diabetes of more than 50 years showed the severity of periodontitis positively correlated with serum IL-6 levels and negatively correlated with the serum levels of C-peptides, indicating that endogenous insulin may contribute to the prevention of progression of periodontitis under hyperglycemia [27].
In this review, we discuss the bidirectional relationship between periodontitis and diabetes based on the molecular basis of micro-inflammation and insulin actions.
2. The effect of diabetes on the progression of periodontitis
2.1. Hyperglycemia
2.1.1. Hyperglycemia-induced oxidative stress
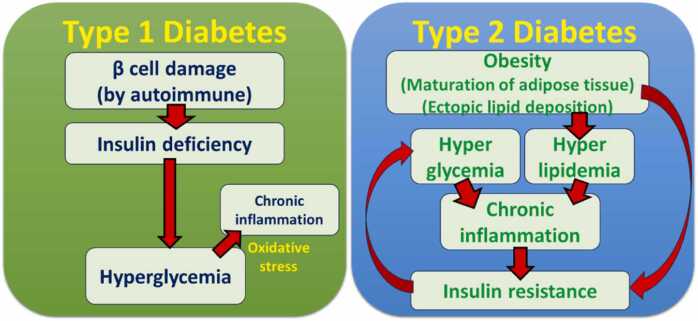
Hyperglycemia is the major feature of diabetes due to either a lack of insulin or a decrease in insulin action in both type 1 and 2 diabetes, despite the differences in the origins between them (Fig. 1). Besides, hyperglycemia is one of the most important factors in the pathogenesis of diabetic complications and diabetes-related diseases. The number of oxidative stress marker-positive cells was increased in the periodontal tissue of people with diabetes [28], [29], suggesting that the relationship between oxidative stress and the exacerbation of periodontitis under sustained exposure to high glucose. Diabetic rodents showed the induction of oxidative stress and chronic inflammation due to hyperglycemia systemically [30].
Fig. 1.
Pathogenic factors in type 1 and 2 diabetes.In type 1 diabetes, pancreatic β cell damage caused by the autoimmune system leads β cell death, resulting in hyperglycemia due to insulin deficiency. Hyperglycemia might induce oxidative stress-mediated chronic inflammation in peripheral tissue, leading to diabetic complications. In contrast, obesity induces maturation of adipose tissue and ectopic lipid deposition in organs with high metabolism such as the liver and muscle, which contribute to the development of insulin resistance. Then, hyperglycemia and hyperlipidemia occur due to a lack of insulin action and dysregulation of metabolism. Such abnormalities in metabolism could induce chronic inflammation in both peripheral and systemic manner, which also contributes to insulin resistance.
Mechanistically, basic studies have shown that excess influx of glucose into the cell may cause cellular dagame via activation of protein kinase C (PKC), leading to intracellular generation of reactive oxidative species (ROS) [31], [32]. In addition, glucotoxicity could evoke ROS production from mitochondria as well [33]. Hyperglycemia-related oxidative stress may contribute to M1 polarization in macrophages [34] which may induce excess inflammatory cytokines production and attenuation in migration of innate immune cells such as neutrophils and monocytes in response to chemokines and their bacteria-killing or clearance capacities.
2.1.2. Hyperglycemia-induced dysfunction of immune cells
Neutrophils exposed to high glucose induce oxidative stress intracellularly, subsequently resulting in attenuation of their capacity of migration and bacterial intake in response to chemokines, and delayed apoptosis [35], [36]. In humans, neutrophilic bacterial intake and migration were blunted in people with type 1 diabetes, and attenuated migration and delayed apoptosis were observed in neutrophils of people with type 2 diabetes, suggesting that hyperglycemia disrupt initial immune response to infection and, thereby, chronic inflammation is induced by sustained infiltration of proinflammatory immune cells into periodontal tissue [37].
2.1.3. Advanced glycation end products (AGEs)
Long-term exposure to high glucose could cause protein glycation mediated by the Maillard reaction. Advanced glycation end product (AGE) is the general term for these glycated products. AGEs contain dozens of types of glycated protein and may induce inflammatory response and oxidative stress through their receptors (receptor for AGEs; RAGEs) [38]. Clinically, AGEs and AGEs-induced oxidative stress were increased in inflamed periodontal tissue in people with diabetes [39]. Another study has shown that the level of blood AGEs positively correlated with the severity of periodontitis in people with type 2 diabetes [40].
2.1.4. Hyperglycemia-induced tissue abnormality
Furthermore, hyperglycemia mediates poor bone quality related to glycation, which may contribute to the increase in the incidence of bone fracture in people with diabetes [41], implying that greater absorption of alveolar bone loss in periodontitis [42]. The effect of high glucose on the osteoclastgenesis has been controversial. Many basic studies have shown that high glucose actually inhibited RANKL-induced osteoclastogenesis in osteoclast precursor cells by inhibiting redox-sensitive nuclear factor-kappa B (NF-κB) activity [43]. Other reports showed that AGEs could promote osteoclast-induced bone absorption [44] and AGEs might differently affect depending on the stage of maturation of osteoclasts [45]. In contrast, hyperglycemia also inhibited osteoblast differentiation as well [46]. However, in type 1 diabetic mice, experimental periodontitis was advanced compared to non-diabetic controls [47], [48]. Regarding wound healing, hyperglycemia impaired oral wound healing by altering proliferation and apoptosis in gingival fibroblasts [49]. Taken together, hyperglycemia may induce an abnormality in bone remodeling, wound healing and tissue repair, resulting in the exacerbation of periodontitis.
2.1.5. Diabetes-related change in oral microbiome
The oral microbiome consists of a tremendous number of species of bacteria. Many investigations have been progressively conducted to explore the potential role of oral microbiome in oral diseases since it was relatively easy to collect samples from the oral cavity.
Studies on the oral microbiome have revealed that the composition of the oral microbiome in the dental plaque from people with obesity and diabetes was differed from that of healthy people [50]. The most important point was whether the altered oral microbiome might be more pathogenic for periodontitis. A recent basic study has shown that oral microbiomes from diabetic mice exhibited more periodontitis-prone composition compared to those from lean mice [51]. These studies implied that the oral microbiome of people with diabetes shifted to higher pathogenic profiles as well.
2.2. Hyperlipidemia
People with type 2 diabetes frequently revealed obesity, accompanied by an increment in blood levels of free fatty acid and cholesterol, diagnosed as hyperlipidemia. Hyperlipidemia may prime inflammatory response in immune cells: saturated fatty acids could directly or indirectly stimulate toll-like receptors (TLRs) to induce inflammation [10].
Mice fed with a 60 % high-fat diet (HFD) were more susceptible to experimental periodontitis induced by Porphyromonas gingivalis (Pg), a representative periodontal pathogen in human, inoculation around the molars compared to normal diet-fed mice [52]. Additionally, feeding with a palmitate-rich high-fat diet also exacerbated Pg infection-induced experimental periodontitis in mice [53]. Furthermore, macrophages from HFD-fed mice exhibited less response to bacterial endotoxin, and lipopolysaccharide (LPS), compared to those from lean mice, suggesting that the immune system against bacterial infection could be impaired under obesity and diabetes [53]. Collectively, dysregulation of immune response to periodontal infection and failure in resolution of inflammation result in the exacerbation of periodontitis under obesity and diabetes.
2.3. Systemic chronic inflammation
In people with obesity and type 2 diabetes, the blood levels of inflammatory cytokines such as TNFα and interleukin 1β (IL-1β), and inflammatory marker C-reactive protein (CRP) are elevated, indicating systemic low-grade inflammation. Under such a situation, periodontitis may progress via the impairment in the regulation of inflammatory response in periodontal tissue. Systemic low-grade inflammation (micro-inflammation), mainly caused by mature adipose tissue and periodontitis could enhance through activation of monocytes/macrophages (described details in later chapter), may interrupt homeostasis in organs by metabolic dysregulation which results in progression of lipid accumulation and abnormalities in glucose metabolism [54].
In periodontitis, the resolution of inflammation is a key function to repair damaged periodontal tissue and maintain homeostasis [55]. Micro-inflammation may disrupt the series of regulations from the initiation of an inflammatory response to resolution via specific immune cell migration and excess cytokines production [56]. Failure in a resolution of inflammation may drive prolonged inflammation in periodontal tissue which contributes to more osteoclast differentiation and tissue damage. Inflammatory cytokines induce matrix metalloprotease proteins (MMPs) expression in periodontal tissue which may contribute to destructing periodontal tissue such as connective tissue, periodontal ligament, and so on [57]. Besides, inflammatory cytokines such as TNFα and IL-1β could promote osteoclast differentiation, which may elicit more alveolar bone loss [58].
2.4. Insulin resistance
Basically, insulin resistance is considered the result of hyperglycemia, hyperlipidemia, and subsequent chronic inflammation. Since insulin is the only hormone that possesses a glucose-lowering effect in the body, impairment of insulin action in the tissue with high metabolism such as the liver, adipose tissue, and muscle may cause hyperglycemia and abnormal metabolism. Recent studies have revealed that insulin resistance itself also plays an important role in the pathogenesis of diabetic complications and related disorders. Liver-specific insulin receptor depletion mice exhibited more progressed steatosis by enhancing lipid accumulation and synthesis in the liver compared to wild-type littermates under high-fat diet feeding [16]. Astrocyte-specific insulin receptor knockout mice displayed anxiety- and depressive-like behavior via decreased in exocytosis of adenosine triphosphate (ATP) from astrocytes resulting in decreased purinergic signaling on dopaminergic neurons [59], and loss of insulin signaling in astrocytes contributed to the exacerbation of Alzheimer-like phenotypes in an Alzheimer model mouse [60].
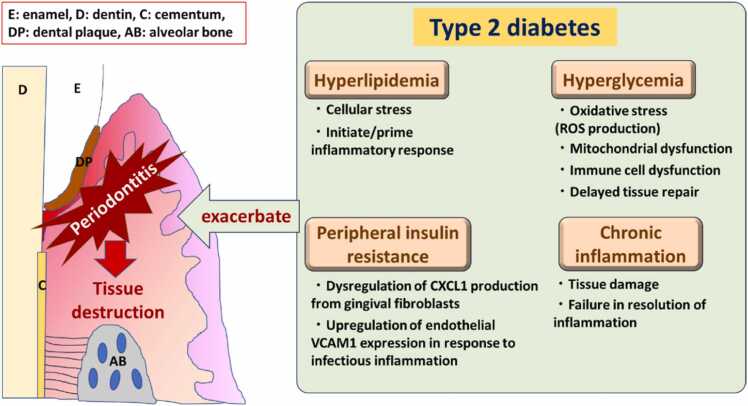
Genetically obese and mild diabetes model Zucker fatty rat showed lower insulin-induced phosphorylation of Akt, indicating insulin resistance in the gingiva [61]. In this report, PKC activation and endothelial inflammation were also observed in the gingiva from obese rats. Besides, a previous study in insulin resistance in gingival fibroblasts showed that depletion of insulin receptors in gingival fibroblasts contributed to the exacerbation of periodontitis independently from obesity and diabetes due to less production of C-X-C motif chemokine ligand 1 (CXCL1), a potent neutrophil chemoattractant, via less activation of NF-κB in response to periodontal infection and LPS [17]. Additionally, a recent study using endothelial-cell-specific insulin receptor knockout mice showed that insulin resistance in endothelial cells could impair insulin-mediated Vascular cell adhesion molecule 1 (VCAM-1) regulation, resulting in increased leukocyte infiltration in inflamed periodontal tissue through less phosphorylation of Akt/Forkhead box O1 (FoxO1) [18] (Fig. 2).
Fig. 2.
Type 2 diabetes-related contributing factors in the progression of periodontitis.
Future studies in the pathogenic role of insulin resistance in other periodontal tissue-consisting cells such as epithelial cells and periodontal ligament cells will be necessary.
3. The effect of periodontitis on diabetes
3.1. Microinflammation
Microinflammation is chronic and systemic inflammation accompanying with obesity. Mature adipose tissue shows infiltration of monocytes/macrophages due to upregulation of chemoattractant in adipocytes, thereby adipocyte and these innate inflammatory cells interacts each other to amplify inflammatory responses [19], [20], [21]. Microinflammation may contribute to exacerbation of poor glycemic control levels due to elevation of blood inflammatory cytokines such as TNFα, IL-1β and IL-6, which is known to downregulate insulin signaling via serine phosphorylation of insulin receptor substrate (IRS) [62], [63], [64]. Microinflammation is evaluated as one of the driving factors for cardiovascular diseases such as atherosclerosis and ischemic heart disease [65]. CRP is known as a marker for systemic inflammation in blood tests. Recently, minor elevations in CRP within the normal limit, recognized as high-sensitivity CRP (hs-CRP), have been shown to increase the risk of mortality related to or future onset of coronary heart disease 2–3-fold in people free from systemic disorders [66], [67]. These studies suggest that microinflammation is a factor promoting cardiovascular diseases (CVDs), as increases in hsCRP indicate the presence of microinflammation.
People with severe periodontitis show an increased level of hsCRP in blood, and periodontal treatment could decrease the hsCRP levels [68], which supports the possibility that severe periodontitis induces systemic micro-inflammation. Studies have suggested that periodontitis-induced increment in CRP was compatible with the CRP levels associated with a 2-fold increase in the risk of coronary heart disease onset or mortality in a Japanese population [66], [68]. These studies suggest that periodontitis could enhance microinflammation by affecting inflammatory responses in mature adipose tissue in type 2 diabetes.
3.2. ‘Adipocyte-macrophage interaction’
Obesity is one of the most common diseases which can induce systemic micro-inflammation. Amplification of inflammation through the interaction between adipocytes and macrophages may cause systemic micro-inflammation in obesity and type 2 diabetes [19]. TNFα could be a representative contributing factor in the adipocyte-macrophage interaction, resulting in the tremendous production of multiple inflammatory cytokines and adipokines [19]. Production of IL-6, especially, may drive hepatic CRP production which bridges cardiovascular diseases. Severe periodontitis could activate monocytes and macrophages in circulation or peripheral tissues [66], [69]. Furthermore, obesity and diabetes could enhance gut permeability, raising blood endotoxin levels [70].
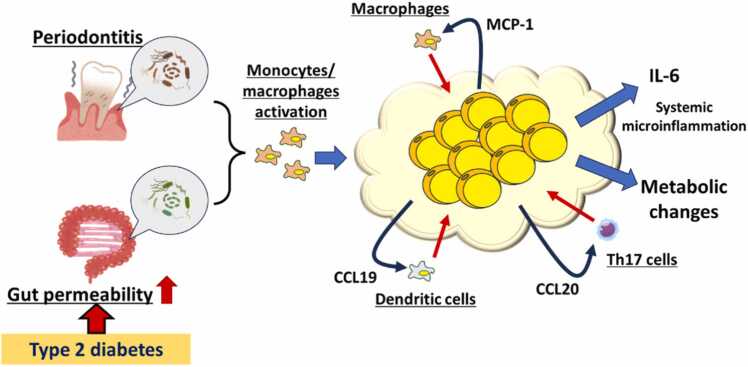
Periodontitis could be involved in the induction of systemic microinflammation as abovementioned. Low-dose of endotoxin, the assumed concentration of systemic microinflammation related to periodontitis or increased gut permeability, could enhance the number of adipokines production such as IL-6 and monocyte chemoattractant protein-1 (MCP-1) through the interaction with macrophages in both in vitro and in vivo studies [20], [21]. DNA microarray in the adipocytes co-cultured with macrophages showed remarkable changes in the genes involved in insulin resistance, increase in CVD risk, and proinflammatory cytokines [21] (Fig. 3).
Fig. 3.
The systemic impact of microinflammation related to periodontitis and gut permeability through amplification of inflammation in mature adipose tissue in type 2 diabetes.Periodontal pathogens and inflammatory mediators related to periodontitis and endotoxemia derived from increased gut permeability could activate monocytes/macrophages. Activated monocytes/macrophages migrate and infiltrate into mature adipose tissues, then interact with adipocytes to produce multiple chemoattractants such as MCP-1, CCL19, CCL20, and so on.
Inflammatory cytokines such as TNFα and IL-1β could induce insulin resistance by inhibiting insulin action via interrupting intracellular insulin signaling [62], [63], [64]. Considering into people with type 2 diabetes frequently accompany with obesity, amplification of microinflammation in mature adipose tissue based on the interaction between adipocytes and macrophages might affect glycemic control in diabetes.
3.3. The role of CCL19-CCR7 axis in mature adipose tissue
In mature adipose tissue, accompanied by microinflammation, immune cells such as monocytes and macrophages are migrated through increased production of chemokines including MCP-1. Besides, dendritic cells (DCs) may play a pivotal role in the inflammation of adipose tissue. The population of DCs was increased in inflamed adipose tissue [71]. In a comprehensive analysis of adipocytes co-cultured with macrophages in the presence or absence of LPS, in addition to MCP-1, a series of chemokines were also upregulated in adipocytes [22]. Among them, CCL19 and 21 are known to be involved in the migration of DCs into lymph nodes through its receptor CCR7. CCR7 deficient mice were prevented from HFD-induced gain of body weight and metabolic abnormalities in adipose tissue [22], [72]. In contrast, adipocyte-specific CCL19 overexpression enhanced western-diet-induced body weight gain and insulin resistance by upregulation of inflammation in visceral adipose tissue [73]. Taken together, DC infiltration via the CCL19-CCR7 axis, resulting from amplified microinflammation derived from adipocyte-macrophage interactions, might be an essential factor in the maturation and induction of microinflammation in adipose tissue (Fig. 3). These studies clearly suggest that amplification of microinflammation in mature adipose tissue via activation of CCR7-CCL19 axis may play a pivotal role of worsening glycemic control in type 2 diabetes.
3.4. The role of adaptive immune cells in mature adipose tissue
Some reports suggest that the involvement of adaptive immune cells in the inflammation of mature adipose tissue. Helper T17 cells and γδT cells population may be increased in the mature adipose tissue and IL-17 could contribute to insulin resistance in adipocytes [74], [75], [76]. IL-17 is known as a cytokine that can induce potent inflammation through NF-κB activation. IL-17 could be involved in increased insulin resistance in adipose tissue. Additionally, IL-17 may activate adipocyte-macrophage interaction and synergistically enhance TNFα-induced CCL20 and IL-6 production from adipocytes co-cultured with macrophages [23] (Fig. 3). These reports imply that the possible role of adaptive immune cells in the maturation and enhancement of microinflammation in adipose tissue.
4. Insights from clinical studies
4.1. People who are more susceptible to microinflammation in mature adipose tissue
Recent clinical studies showed that in contrast to Westerners who generally showed severe adiposity phenotypes, most Asians and Asian Americans, who display mild obesity, were at higher risk for diabetes and diabetic complications at similar BMI [77], [78]. These reports suggest that, in other words, Asians and Asian Americans were more susceptible to metabolic disorders and their related complications than Westerners. One of the possible reasons could be explained by the differences in the capacity of adiposity [79]. Most Asians and Asian Americans display mild obesity, and many of them with type 2 diabetes do not fall into the obesity category (BMI >25 kg/m2). However, patients with diabetes and a BMI of around 25 kg/m2 are considered to be most susceptible to increases in hsCRP related to periodontitis [26]. In this study, it has been shown that people with higher hsCRP (even within normal level) and showed lowering hsCRP after periodontal therapy showed BMI of 25 kg/m2 [26]. In contrast, other clinical study has reported that in people with extreme obesity (BMI > 30 kg/m2), periodontal therapy failed to significantly decrease systemic inflammation indicated as blood CRP levels, suggesting that the systemic influence of periodontitis was masked by systemic inflammation related to obesity [80].
Considering from these reports, periodontitis might clearly exhibit adverse effect on glycemic control via increase of inflammatory cytokines derived from mature adipose tissue, in where adipocytes interact with macrophages activated by periodontitis, in people with mild obesity and diabetes, especially Asian and American people with BMI of around 25 kg/m2 and diabetes.
4.2. The association of progression of periodontitis and lack of insulin action in diabetes
Investigation of the association between insulin deficiency or lack of insulin action and periodontitis may help in understanding the molecular basis of the relationship of insulin resistance with diabetes-related periodontitis. The pathogenic factor of type 1 diabetes is a lack of endogenous insulin secretion. Pancreatic beta cells are injured by inflammation in Langerhans islet mediated by autoimmune response in type 1 diabetes. An epidemiological study in people with type 1 diabetes of ≥ 50 years showed that the severity of periodontitis negatively correlated with the levels of serum C-peptide, which is simultaneously cleaved from pro-insulin when insulin is secreted at a 1:1 ratio [27]. Systemic insulin level could be calculated by using serum C-peptide level since insulin is rapidly metabolized in minutes while C-peptide is much more stable in blood. Additionally, serum C-peptide levels were negatively correlated with mean pocket depth and clinical attachment loss. Several clinic studies have reported the association of systemic insulin resistance with periodontitis. A study in the Korea National Health and Nutrition Examination Survey indicated that Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), an index for systemic insulin resistance, positively correlated with the severity of periodontitis in people without obesity [81]. Another study in obese people in Puerto Rico showed that people with the highest tertile HOMA-IR revealed significantly more increased number of points with bleeding on probing and pocket depth ≥4 mm than people with HOMA-IR in the other two tertile [82]. Taken together, these results suggested that endogenous insulin might play a role in the prevention of periodontitis, and systemic insulin resistance may contribute to the progression of periodontitis.
5. Conclusion
So far, the molecular mechanisms of both the effect of diabetes on periodontitis and the impact of periodontitis on diabetes has been investigated, thereby several key factors in the bidirectional relationship were found by basic and clinical research. In the interrelationship between periodontitis and diabetes, future studies unveiling the profound molecule basis of the pathogenesis of diabetes-related periodontitis will help in understanding the pathology and establishing novel therapeutic approaches for improving the efficacy of periodontal therapy. Elucidating how metabolic abnormalities such as glucose, lipid metabolism alterations, mitochondrial dysfunctions, and insulin resistance might greatly contribute to it. In addition, the accumulation of findings in the molecule mechanisms of how periodontitis influences systemic disorders will also contribute to the reinforcement of the cooperation between dental and medical fields.
Declaration of generative AI in scientific writing
The authors did not use generative AI for writing this article at all.
Funding
This study was supported by Japan Society for Promotion of Science (JSPS) KAKENHI Grant Numbers JP25893149 and JP20K18513 (to TS), and by JSPS KAKENHI Grant Numbers JP21390556, JP25293425, and JP16H05555 (all to FN).
Conflict of Interest
The authors have no conflicts of interests directly relevant to the content of this article.
Acknowledgements
This work was supported by the JSPS KAKENHI Grant Numbers JP25893149, JP20K18513, JP21390556, JP25293425, and JP16H05555.
Data Availability
Data related to this article are available from the corresponding author upon reasonable request.
References
- 1.Firatli E. The relationship between clinical periodontal status and insulin-dependent diabetes mellitus. Results after 5 years. J Periodontol. 1997;68:136–140. doi: 10.1902/jop.1997.68.2.136. [DOI] [PubMed] [Google Scholar]
- 2.Seppälä B., Seppälä M., Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol. 1993;20:161–165. doi: 10.1111/j.1600-051x.1993.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 3.Nelson R.G., Shlossman M., Budding L.M., Pettitt D.J., Saad M.F., Genco R.J., et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836–840. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- 4.Shlossman M., Knowler W.C., Pettitt D.J., Genco R.J. Type I.I. diabetes and periodontal disease. J Am Dent Assoc. 1990;121:532–536. doi: 10.14219/jada.archive.1990.0211. [DOI] [PubMed] [Google Scholar]
- 5.Emrich L.J., Shlossman M., Genco R.J. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62:123–131. doi: 10.1902/jop.1991.62.2.123. [DOI] [PubMed] [Google Scholar]
- 6.Tsai C., Hayes C., Taylor G.W. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–192. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 7.Bastos A.S., Graves D.T., Loureiro A.P., Rossa Júnior C., Abdalla D.S., Faulin Tdo E., et al. Lipid peroxidation is associated with the severity of periodontal disease and local inflammatory markers in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E1353–E1362. doi: 10.1210/jc.2011-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaker O., Ghallab N.A., Hamdy E., Sayed S. Inducible nitric oxide synthase (iNOS) in gingival tissues of chronic periodontitis with and without diabetes: immunohistochemistry and RT-PCR study. Arch Oral Biol. 2013;58:1397–1406. doi: 10.1016/j.archoralbio.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Zheng J., Chen S., Albiero M.L., Vieira G.H.A., Wang J., Feng J.Q., et al. Diabetes activates periodontal ligament fibroblasts via NF-κB in vivo. J Dent Res. 2018;97:580–588. doi: 10.1177/0022034518755697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancaster G.I., Langley K.G., Berglund N.A., Kammoun H.L., Reibe S., Estevez E., et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 2018;27:1096–1110.e5. doi: 10.1016/j.cmet.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Pacios S., Kang J., Galicia J., Gluck K., Patel H., Ovaydi-Mandel A., et al. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012;26:1423–1430. doi: 10.1096/fj.11-196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S.Y., Wei C.C., Shang T.T., Lian Q., Wu C.X., Deng J.Y. High glucose induces inflammatory cytokine through protein kinase C‐induced toll‐like receptor 2 pathway in gingival fibroblasts. Biochem Biophys Res Commun. 2012;427:666–670. doi: 10.1016/j.bbrc.2012.09.118. [DOI] [PubMed] [Google Scholar]
- 13.Yang X., Zhang J., Ni J., Ouyang B., Wang D., Luo S., et al. Toll-like receptor 4-mediated hyper-responsiveness of gingival epithelial cells to lipopolysaccharide in high glucose environments. J Periodontol. 2014;85:1620–1628. doi: 10.1902/jop.2014.140087. [DOI] [PubMed] [Google Scholar]
- 14.Welsh G.I., Hale L.J., Eremina V., Jeansson M., Maezawa Y., Lennon R., et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rask-Madsen C., Li Q., Freund B., Feather D., Abramov R., Wu I.H., et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michael M.D., Kulkarni R.N., Postic C., Previs S.F., Shulman G.I., Magnuson M.A., et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 17.Shinjo T., Onizuka S., Zaitsu Y., Ishikado A., Park K., Li Q. Dysregulation of CXCL1 expression and neutrophil recruitment in insulin resistance and diabetes-related periodontitis in male mice. Diabetes. 2023;72:986–998. doi: 10.2337/db22-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeze T., Shinjo T., Sato K., Nishimura Y., Imagawa M., Chen S., et al. Endothelial insulin resistance exacerbates experimental periodontitis. J Dent Res. 2023;102:1152–1161. doi: 10.1177/00220345231181539. [DOI] [PubMed] [Google Scholar]
- 19.Suganami T., Nishida J., Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita A., Soga Y., Iwamoto Y., Yoshizawa S., Iwata H., Kokeguchi S., et al. Macrophage-adipocyte interaction: marked interleukin-6 production by lipopolysaccharide. Obes (Silver Spring) 2007;15:2549–2552. doi: 10.1038/oby.2007.305. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita A., Soga Y., Iwamoto Y., Asano T., Li Y., Abiko Y., et al. DNA microarray analyses of genes expressed differentially in 3T3-L1 adipocytes co-cultured with murine macrophage cell line RAW264.7 in the presence of the toll-like receptor 4 ligand bacterial endotoxin. Int J Obes (Lond) 2008;32:1725–1729. doi: 10.1038/ijo.2008.153. [DOI] [PubMed] [Google Scholar]
- 22.Sano T., Iwashita M., Nagayasu S., Yamashita A., Shinjo T., Hashikata A., et al. Protection from diet-induced obesity and insulin resistance in mice lacking CCL19-CCR7 signaling. Obes (Silver Spring) 2015;23:1460–1471. doi: 10.1002/oby.21127. [DOI] [PubMed] [Google Scholar]
- 23.Shinjo T., Iwashita M., Yamashita A., Sano T., Tsuruta M., Matsunaga H., et al. IL-17A synergistically enhances TNFα-induced IL-6 and CCL20 production in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2016;477:241–246. doi: 10.1016/j.bbrc.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 24.Hashikata A., Yamashita A., Suzuki S., Nagayasu S., Shinjo T., Taniguchi A., et al. The inflammation-lipocalin 2 axis may contribute to the development of chronic kidney disease. Nephrol Dial Transpl. 2014;29:611–618. doi: 10.1093/ndt/gft449. [DOI] [PubMed] [Google Scholar]
- 25.Tsuruta M., Iwashita M., Shinjo T., Matsunaga H., Yamashita A., Nishimura F. Metabolic endotoxemia-activated macrophages promote pancreatic β cell death via IFNβ-Xaf1 pathway. Horm Metab Res. 2018;50:160–167. doi: 10.1055/s-0043-121467. [DOI] [PubMed] [Google Scholar]
- 26.Munenaga Y., Hiroshima Study Group, Yamashina T., Tanaka J., Nishimura F. Improvement of glycated hemoglobin in Japanese subjects with type 2 diabetes by resolution of periodontal inflammation using adjunct topical antibiotics: results from the Hiroshima Study. Diabetes Res Clin Pract. 2013;100:53–60. doi: 10.1016/j.diabres.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Shinjo T., Ishikado A., Hasturk H., Pober D.M., Paniagua S.M., Shah H., et al. Characterization of periodontitis in people with type 1 diabetes of 50 years or longer duration. J Periodontol. 2019;90:565–575. doi: 10.1002/JPER.18-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastos A.S., Graves D.T., Loureiro A.P., Rossa Júnior C., Abdalla D.S., Faulin Tdo E., et al. Lipid peroxidation is associated with the severity of periodontal disease and local inflammatory markers in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E1353–E1362. doi: 10.1210/jc.2011-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaker O., Ghallab N.A., Hamdy E., Sayed S. Inducible nitric oxide synthase (iNOS) in gingival tissues of chronic periodontitis with and without diabetes: immunohistochemistry and RT-PCR study. Arch Oral Biol. 2013;58:1397–1406. doi: 10.1016/j.archoralbio.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Zheng J., Chen S., Albiero M.L., Vieira G.H.A., Wang J., Feng J.Q., et al. Diabetes activates periodontal ligament fibroblasts via NF-κB in vivo. J Dent Res. 2018;97:580–588. doi: 10.1177/0022034518755697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang S.Y., Wei C.C., Shang T.T., Lian Q., Wu C.X., Deng J.Y. High glucose induces inflammatory cytokine through protein kinase C-induced toll-like receptor 2 pathway in gingival fibroblasts. Biochem Biophys Res Commun. 2012;427:666–670. doi: 10.1016/j.bbrc.2012.09.118. [DOI] [PubMed] [Google Scholar]
- 32.Ishii H., Koya D., King G.L. Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J Mol Med (Berl) 1998;76:21–31. doi: 10.1007/s001090050187. [DOI] [PubMed] [Google Scholar]
- 33.Russell J.W., Golovoy D., Vincent A.M., Mahendru P., Olzmann J.A., Mentzer A., et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B., Yang Y., Yi J., Zhao Z., Ye R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J Periodontal Res. 2021;56:991–1005. doi: 10.1111/jre.12912. [DOI] [PubMed] [Google Scholar]
- 35.Marhoffer W., Stein M., Schleinkofer L., Federlin K. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res Clin Pract. 1993;19:183–188. doi: 10.1016/0168-8227(93)90112-i. [DOI] [PubMed] [Google Scholar]
- 36.Gyurko R., Siqueira C.C., Caldon N., Gao L., Kantarci A., Van Dyke T.E. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol. 2006;177:7250–7256. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowey R., Iqbal A., Heller S.R., Sabroe I., Prince L.R. A bittersweet response to infection in diabetes; targeting neutrophils to modify inflammation and improve host immunity. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.678771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47(Suppl 1):3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt A.M., Weidman E., Lalla E., Yan S.D., Hori O., Cao R., et al. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. 1996;31:508–515. doi: 10.1111/j.1600-0765.1996.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 40.Takeda M., Ojima M., Yoshioka H., Inaba H., Kogo M., Shizukuishi S., et al. Relationship of serum advanced glycation end products with deterioration of periodontitis in type 2 diabetes patients. J Periodontol. 2006;77:15–20. doi: 10.1902/jop.2006.77.1.15. [DOI] [PubMed] [Google Scholar]
- 41.Lecka-Czernik B. Diabetes, bone and glucose-lowering agents: basic biology. Diabetologia. 2017;60:1163–1169. doi: 10.1007/s00125-017-4269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang P.C., Chien L.Y., Yeo J.F., Wang Y.P., Chung M.C., Chong L.Y., et al. Progression of periodontal destruction and the roles of advanced glycation end products in experimental diabetes. J Periodontol. 2013;84:379–388. doi: 10.1902/jop.2012.120076. [DOI] [PubMed] [Google Scholar]
- 43.Wittrant Y., Gorin Y., Woodruff K., Horn D., Abboud H.E., Mohan S., et al. High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone. 2008;42:1122–1130. doi: 10.1016/j.bone.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyata T., Notoya K., Yoshida K., Horie K., Maeda K., Kurokawa K., et al. Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles. J Am Soc Nephrol. 1997;8:260–270. doi: 10.1681/ASN.V82260. [DOI] [PubMed] [Google Scholar]
- 45.Li Z., Li C., Zhou Y., Chen W., Luo G., Zhang Z., et al. Advanced glycation end products biphasically modulate bone resorption in osteoclast-like cells. Am J Physiol Endocrinol Metab. 2016;310:E355–E366. doi: 10.1152/ajpendo.00309.2015. [DOI] [PubMed] [Google Scholar]
- 46.Maycas M., Portolés M.T., Matesanz M.C., Buendía I., Linares J., Feito M.J., et al. High glucose alters the secretome of mechanically stimulated osteocyte-like cells affecting osteoclast precursor recruitment and differentiation. J Cell Physiol. 2017;232:3611–3621. doi: 10.1002/jcp.25829. [DOI] [PubMed] [Google Scholar]
- 47.Mahamed D.A., Marleau A., Alnaeeli M., Singh B., Zhang X., Penninger J.M., et al. G(-) anaerobes-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes. 2005;54:1477–1486. doi: 10.2337/diabetes.54.5.1477. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q., Zhang P., Aprecio R., Zhang D., Li H., Ji N., et al. Comparison of experimental diabetic periodontitis induced by Porphyromonas gingivalis in mice. J Diabetes Res. 2016;2016 doi: 10.1155/2016/4840203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desta T., Li J., Chino T., Graves D.T. Altered fibroblast proliferation and apoptosis in diabetic gingival wounds. J Dent Res. 2010;89:609–614. doi: 10.1177/0022034510362960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casarin R.C., Barbagallo A., Meulman T., Santos V.R., Sallum E.A., Nociti F.H., et al. Subgingival biodiversity in subjects with uncontrolled type-2 diabetes and chronic periodontitis. J Periodontal Res. 2013;48:30–36. doi: 10.1111/j.1600-0765.2012.01498.x. [DOI] [PubMed] [Google Scholar]
- 51.Xiao E., Mattos M., Vieira G.H.A., Chen S., Corrêa J.D., Wu Y., et al. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe. 2017;22:120–128.e4. doi: 10.1016/j.chom.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amar S., Zhou Q., Shaik-Dasthagirisaheb Y., Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci U S A. 2007;104:20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muluke M., Gold T., Kiefhaber K., Al-Sahli A., Celenti R., Jiang H., et al. Diet-induced obesity and its differential impact on periodontal bone loss. J Dent Res. 2016;95:223–229. doi: 10.1177/0022034515609882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daryabor G., Atashzar M.R., Kabelitz D., Meri S., Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eltay E.G., Van Dyke T. Resolution of inflammation in oral diseases. Pharm Ther. 2023;247 doi: 10.1016/j.pharmthera.2023.108453. [DOI] [PubMed] [Google Scholar]
- 56.Van Dyke T.E., Sima C. Understanding resolution of inflammation in periodontal diseases: Is chronic inflammatory periodontitis a failure to resolve? Periodontol 2000. 2020;82:205–213. doi: 10.1111/prd.12317. [DOI] [PubMed] [Google Scholar]
- 57.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 58.Boyce B.F., Yamashita T., Yao Z., Zhang Q., Li F., Xing L. Roles for NF-kappaB and c-Fos in osteoclasts. J Bone Min Metab. 2005;23:11–15. doi: 10.1007/BF03026317. [DOI] [PubMed] [Google Scholar]
- 59.Cai W., Xue C., Sakaguchi M., Konishi M., Shirazian A., Ferris H.A., et al. Insulin regulates astrocyte gliotransmission and modulates behavior. J Clin Invest. 2018;128(2):2914–2926. doi: 10.1172/JCI99366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen W., Huang Q., Lazdon E.K., Gomes A., Wong M., Stephens E., et al. Loss of insulin signaling in astrocytes exacerbates Alzheimer-like phenotypes in a 5xFAD mouse model. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2220684120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizutani K., Park K., Mima A., Katagiri S., King G.L. Obesity-associated gingival vascular inflammation and insulin resistance. J Dent Res. 2014;93:596–601. doi: 10.1177/0022034514532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savage D.B., Petersen K.F., Shulman G.I. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension. 2005;45:828–833. doi: 10.1161/01.HYP.0000163475.04421.e4. [DOI] [PubMed] [Google Scholar]
- 63.Ballak D.B., Stienstra R., Tack C.J., Dinarello C.A., van Diepen J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015;75:280–290. doi: 10.1016/j.cyto.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andreozzi F., Laratta E., Procopio C., Hribal M.L., Sciacqua A., Perticone M., et al. Interleukin-6 impairs the insulin signaling pathway, promoting production of nitric oxide in human umbilical vein endothelial cells. Mol Cell Biol. 2007;27:2372–2383. doi: 10.1128/MCB.01340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arima H., Kubo M., Yonemoto K., Doi Y., Ninomiya T., Tanizaki Y., et al. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama study. Arterioscler Thromb Vasc Biol. 2008;28:1385–1391. doi: 10.1161/ATVBAHA.107.157164. [DOI] [PubMed] [Google Scholar]
- 66.Ridker P.M. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 67.Iwamoto Y., Nishimura F., Soga Y., Takeuchi K., Kurihara M., Takashiba S., et al. Antimicrobial periodontal treatment decreases serum C-reactive protein, tumor necrosis factor-alpha, but not adiponectin levels in patients with chronic periodontitis. J Periodontol. 2003;74:1231–1236. doi: 10.1902/jop.2003.74.8.1231. [DOI] [PubMed] [Google Scholar]
- 68.Madianos P.N., Bobetsis G.A., Kinane D.F. Is periodontitis associated with an increased risk of coronary heart disease and preterm and/or low birth weight births? J Clin Periodontol. 2002;29(Suppl 3):22–36. doi: 10.1034/j.1600-051x.29.s3.2.x. discussion 37-8. [DOI] [PubMed] [Google Scholar]
- 69.Noz M.P., Plachokova A.S., Smeets E.M.M., Aarntzen E.H.J.G., Bekkering S., Vart P., et al. An explorative study on monocyte reprogramming in the context of periodontitis in vitro and in vivo. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.695227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serino M., Luche E., Gres S., Baylac A., Bergé M., Cenac C., et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–553. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stefanovic-Racic M., Yang X., Turner M.S., Mantell B.S., Stolz D.B., Sumpter T.L., et al. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61:2330–2339. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hellmann J., Sansbury B.E., Holden C.R., Tang Y., Wong B., Wysoczynski M., et al. CCR7 maintains nonresolving lymph node and adipose inflammation in obesity. Diabetes. 2016;65:2268–2281. doi: 10.2337/db15-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayashi M., Iwashita M., Nishimura Y., Shinjo T., Sano T., Yamashita A., et al. Adipose-specific C-C motif chemokine ligand (CCL) 19 overexpression drives the mice to both insulin resistance and weight gain. BMJ Open Diabetes Res Care. 2021;9 doi: 10.1136/bmjdrc-2020-001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y., Tian J., Tian X., Tang X., Rui K., Tong J., et al. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One. 2014;9(18) doi: 10.1371/journal.pone.0092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mehta P., Nuotio-Antar A.M., Smith C.W. γδ T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J Leukoc Biol. 2015;97:121–134. doi: 10.1189/jlb.3A0414-211RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le Menn G., Sibille B., Murdaca J., Rousseau A.S., Squillace R., Vergoni B., et al. Decrease in αβ/γδ T-cell ratio is accompanied by a reduction in high-fat diet-induced weight gain, insulin resistance, and inflammation. FASEB J. 2019;33:2553–2562. doi: 10.1096/fj.201800696RR. [DOI] [PubMed] [Google Scholar]
- 77.Hsu W.C., Boyko E.J., Fujimoto W.Y., Kanaya A., Karmally W., Karter A., et al. Pathophysiologic differences among Asians, native Hawaiians, and other Pacific Islanders and treatment implications. Diabetes Care. 2012;35(5):1189–1198. doi: 10.2337/dc12-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.King G.L., McNeely M.J., Thorpe L.E., Mau M.L., Ko J., et al. Understanding and addressing unique needs of diabetes in Asian Americans, native Hawaiians, and Pacific Islanders. Diabetes Care. 2012;35:1181–1188. doi: 10.2337/dc12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujimoto W.Y., Bergstrom R.W., Boyko E.J., Leonetti D.L., Newell-Morris L.L., Wahl P.W. Susceptibility to development of central adiposity among populations. Obes Res. 1995;3(Suppl 2):179S–186S. doi: 10.1002/j.1550-8528.1995.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 80.Chen L., Luo G., Xuan D., Wei B., Liu F., Li J., et al. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized study. J Periodontol. 2012;83:435–443. doi: 10.1902/jop.2011.110327. [DOI] [PubMed] [Google Scholar]
- 81.Song I.S., Han K., Park Y.M., Ji S., Jun S.H., Ryu J.J., et al. Severe periodontitis is associated with insulin resistance in non-abdominal obese adults. J Clin Endocrinol Metab. 2016;101:4251–4259. doi: 10.1210/jc.2016-2061. [DOI] [PubMed] [Google Scholar]
- 82.Andriankaja O.M., Muñoz-Torres F.J., Vivaldi-Oliver J., Leroux B.G., Campos M., Joshipura K., et al. Insulin resistance predicts the risk of gingival/periodontal inflammation. J Periodontol. 2018;89:549–557. doi: 10.1002/JPER.17-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to this article are available from the corresponding author upon reasonable request.