Key Points
Question
Does metformin added to insulin for the treatment of preexisting type 2 diabetes or diabetes identified early in pregnancy reduce the risk of adverse neonatal outcomes?
Findings
In a randomized clinical trial of 794 pregnant adults (18-45 years), compared with placebo, metformin added to insulin for treatment of preexisting diabetes or diabetes identified in early pregnancy did not reduce a composite adverse neonatal outcome (71% vs 74%) but resulted in fewer large-for-gestational-age infants.
Meaning
Using metformin plus insulin to treat preexisting type 2 or gestational diabetes diagnosed early in pregnancy did not reduce a composite neonatal adverse outcome. The reduction in odds of a large-for-gestational-age infant observed after adding metformin to insulin warrants further investigation.
Abstract
Importance
Insulin is recommended for pregnant persons with preexisting type 2 diabetes or diabetes diagnosed early in pregnancy. The addition of metformin to insulin may improve neonatal outcomes.
Objective
To estimate the effect of metformin added to insulin for preexisting type 2 or diabetes diagnosed early in pregnancy on a composite adverse neonatal outcome.
Design, Setting, and Participants
This randomized clinical trial in 17 US centers enrolled pregnant adults aged 18 to 45 years with preexisting type 2 diabetes or diabetes diagnosed prior to 23 weeks’ gestation between April 2019 and November 2021. Each participant was treated with insulin and was assigned to add either metformin or placebo. Follow-up was completed in May 2022.
Intervention
Metformin 1000 mg or placebo orally twice per day from enrollment (11 weeks -<23 weeks) through delivery.
Main Outcome and Measures
The primary outcome was a composite of neonatal complications including perinatal death, preterm birth, large or small for gestational age, and hyperbilirubinemia requiring phototherapy. Prespecified secondary outcomes included maternal hypoglycemia and neonatal fat mass at birth, and prespecified subgroup analyses by maternal body mass index less than 30 vs 30 or greater and those with preexisting vs diabetes early in pregnancy.
Results
Of the 831 participants randomized, 794 took at least 1 dose of the study agent and were included in the primary analysis (397 in the placebo group and 397 in the metformin group). Participants’ mean (SD) age was 32.9 (5.6) years; 234 (29%) were Black, and 412 (52%) were Hispanic. The composite adverse neonatal outcome occurred in 280 (71%) of the metformin group and in 292 (74%) of the placebo group (adjusted odds ratio, 0.86 [95% CI 0.63-1.19]). The most commonly occurring events in the primary outcome in both groups were preterm birth, neonatal hypoglycemia, and delivery of a large-for-gestational-age infant. The study was halted at 75% accrual for futility in detecting a significant difference in the primary outcome. Prespecified secondary outcomes and subgroup analyses were similar between groups. Of individual components of the composite adverse neonatal outcome, metformin-exposed neonates had lower odds to be large for gestational age (adjusted odds ratio, 0.63 [95% CI, 0.46-0.86]) when compared with the placebo group.
Conclusions and Relevance
Using metformin plus insulin to treat preexisting type 2 or gestational diabetes diagnosed early in pregnancy did not reduce a composite neonatal adverse outcome. The effect of reduction in odds of a large-for-gestational-age infant observed after adding metformin to insulin warrants further investigation.
Trial Registration
ClinicalTrials.gov Identifier: NCT02932475
This randomized clinical trial compares the effect of insulin plus metformin vs insulin and placebo in reducing the odds of adverse neonatal outcomes in pregnant adults with preexisting type 2 diabetes or diabetes identified in early pregnancy.
Introduction
Type 2 diabetes is the most prevalent form of diabetes in the US1 and accounts for most of the preexisting diabetes in pregnancy. Pregnancies with preexisting type 2 diabetes or gestational diabetes identified early in pregnancy are at risk for adverse pregnancy outcomes, intrapartum complications, and neonatal hypoglycemia at birth.2,3,4,5,6,7 Maintaining maternal euglycemia is the mainstay of treatment.
Management of diabetes in pregnancy to optimize perinatal outcome includes maternal blood glucose monitoring, dietary management, and insulin therapy to achieve maternal euglycemia.3,8 Metformin use has been reported for pregnancies complicated by preexisting type 2 diabetes;9,10,11 however, these studies are limited by their study design, smaller sample size, and common need for adjuvant insulin. The American College of Obstetricians and Gynecologists12 and the American Diabetes Association13 both recommend insulin as first-line pharmacotherapy for preexisting type 2 diabetes in pregnancy, with metformin reserved for those who cannot use insulin or decline to do so. The primary objective of the Medical Optimization of Management of Overt Type 2 Diabetes in Pregnancy (MOMPOD) trial was to test the hypothesis that among US pregnant adults with diabetes (preexisting type 2 diabetes or diabetes identified early in gestation) in pregnancy, the addition of metformin to insulin lowers the odds of composite adverse neonatal outcome of perinatal death or severe neonatal complications compared with insulin alone, without increasing risk for adverse perinatal events.
Methods
Study Design
The MOMPOD Study was a multicenter, double-blinded randomized clinical trial of metformin vs placebo added to insulin for the treatment of preexisting type 2 diabetes or gestational diabetes diagnosed in early pregnancy. The trial design and protocol were previously published (Supplement 1),14 and the statistical analysis plan is available in Supplement 2.
The trial was conducted at 17 US clinical sites with US Food and Drug Administration oversight (investigational new drug No. 125594) and institutional review board approval at each site; all participants provided written informed consent. An independent data monitoring and safety board provided oversight and an independent medical monitor was available for acute serious adverse events that might require unmasking.
Trial Population
Participants were enrolled from June 2017 to November 2021, and the last follow up study visit was completed in May 2022. Enrollment was suspended from April 2020 to September 2020 due to the COVID-19 pandemic. Pregnant adults aged 18 to 45 years with a singleton gestation between 10 weeks 0 days and 22 weeks 6 days, confirmed by ultrasound with either preexisting type 2 diabetes or with diabetes identified prior to 23 weeks requiring insulin, were eligible to participate.14 Because of the relationship between maternal race, ethnicity, and pregnancy outcomes, participant race and ethnicity were collected by chart abstraction and if missing from the chart, by participant self-report.
The diagnosis of preexisting type 2 diabetes prior to pregnancy was confirmed by medical record review. Diabetes diagnosed early in pregnancy was defined by either 1-step testing (75-g oral glucose tolerance testing with ≥1 abnormal value)15 or 2-step testing (positive 50-g oral glucose tolerance testing followed by 100-g glucose tolerance testing with ≥2 abnormal values by Carpenter-Coustan criteria16); or by a glycated hemoglobin result of at least 6.5%, a fasting capillary blood glucose level greater than 126 mg/dL; or a random capillary blood glucose level of at least 200 mg/dL17,18; or any combination of testing that the treating clinician felt required insulin treatment.
Trial Procedures
All enrolled participants were treated with insulin. Study sites used weight-based insulin dosing, split as 2 or 3 injections using a combination of rapid/short and intermediate or long-acting insulin. Participants performed daily capillary blood glucose monitoring as fasting and either at 1 or 2 hours postprandial. Study sites were instructed to increasingly titrate insulin dosing to achieve glycemic goals of fasting 70 to 95 mg/dL; 1-hour postprandial level below 140 mg/dL, or 2-hour postprandial level below 120 mg/dL.
After enrollment, participants were randomly assigned in a 1:1 ratio to either metformin or an identical appearing placebo to be taken orally twice a day. The data coordinating center prepared the randomization sequence using permuted blocks,19 with stratification by clinical site, gestational age at randomization (<18 weeks vs ≥18 weeks) and timing of diabetes diagnosis (preexisting or during early pregnancy) using the web-based data management system Carolina Data Acquisition and Reporting Tool, developed by the data coordinating center.
Participants and study staff were masked to group allocation. Pylant Village Pharmacy (Village Compounding Pharmacy/Acclaim Allergy Solutions) provided metformin 500 mg or placebo in identical-appearing capsules and bottles. Participants were instructed to ingest 1 capsule twice a day for 1 week, then if tolerated, to increase to 2 capsules twice a day.14
Study staff made monthly visits in person or by telephone to query adverse effects, assess adherence, and dispense the study agent either in person or by mail. Chart abstraction included maternal and delivery data from enrollment until hospital discharge and neonatal data until 30 days of age or hospital discharge, whichever occurred first. If neonates were discharged prior to 30 days, then follow-up was obtained by telephone at 30 days of age. Adherence with the study agent was assessed at each study visit via self-report. At conclusion of participation, participants were asked to return any remaining empty bottles and any unused study agent. Study staff collected neonate anthropometric measurements within 72 hours of birth using previously published methods20; neonates born before 28 weeks’ gestation or who were medically unstable were not measured.
Outcomes
The primary outcome was a composite of 1 or more of the following: fetal or neonatal death (fetal loss between 10 and 20 weeks, stillbirth ≥20 weeks, or neonatal death within 28 days of birth); neonatal hypoglycemia (capillary blood glucose <40 mg/dL or any hypoglycemia that required intravenous glucose replacement); umbilical artery pH less than 7.05; shoulder dystocia with brachial plexus injury, clavicular or humeral fracture, or 3 or more maneuvers to relieve; hyperbilirubinemia requiring phototherapy within the first 72 hours after birth; delivery before 37 weeks’ gestation; large-for-gestational-age infant; small-for-gestational-age infant; and/or low birth weight of less than 2500 g. The Fenton growth chart was used to determine birthweight percentile21 and defined large for gestational age as birthweight above the 90th percentile and small for gestational age as birthweight below the 10th percentile. Reviewers masked to group assignment adjudicated primary outcomes in a random subset of participants (n = 60).
Prespecified secondary outcomes were the occurrence of clinically relevant maternal hypoglycemia, defined as capillary blood glucose of less than 60 mg/dL, and neonatal fat mass. Prespecified subgroup analyses for the primary outcome included maternal body mass index (BMI, calculated as weight in kilograms divided by height in meters squared; <30 vs ≥30 [obese]), those with preexisting type 2 diabetes vs those with diabetes identified early in pregnancy, and gestational age at randomization (<18 weeks vs 18-<23 weeks).
Statistical Analysis
The target sample size was 1200 participants to support adequate power to detect a reduction in odds of the composite adverse neonatal outcome by 40% for those randomized to metformin vs placebo, assuming an overall incidence of the primary outcome as 25% and 10% loss to follow-up. An ad hoc masked review of 102 completed participants found a higher than anticipated rate for the primary event (70%) and for loss to follow-up (20%); thus, the data and safety monitoring board approved a final enrollment sample size of 950 (428 per group completed) to provide 80% power to detect a 40% reduction in the primary outcome, assuming a baseline event rate of 50% and a 2-sided α = .044, accounting for planned interim analyses.
The primary analysis included randomized participants who took at least 1 dose of the study drug. Odds ratios (ORs) and 95% CIs for the proportions of participants meeting the primary outcome were calculated using logistic regression, adjusting for study site, timing of diabetes diagnosis (preexisting vs early in pregnancy), gestational age at randomization stratified at 18 weeks, and baseline maternal BMI. An unadjusted logistic model was generated by removing all baseline covariates to evaluate whether stratification at randomization adequately mitigated treatment differences due to differences in covariates at baseline. Where applicable, values for participants with missing maternal baseline BMI were imputed. Imputation was performed using PROC MI in SAS 9.4 (SAS Institute), with the fully conditional specification option and predictive mean matching with 20 imputed data sets and 50 burn in iterations.22 The statistical analysis plan (Supplement 2) describes planned interim and safety analyses, subgroup analyses, and sensitivity analyses evaluating the effect of missing data on the effect of metformin on the primary outcome (eTable 1 in Supplement 3).
Using similar procedures, groups were compared for secondary outcomes using logistic regression and analysis of covariance. Treatment groups were also compared for proportions of large- and small-for-gestational-age infants, mean birthweight, proportion of participants experiencing excessive weight gain as defined by the Institute of Medicine,23 and other birth outcomes using the χ2 test or a 2-sample t test, as appropriate. Continuous data were tested for skewness, and if skewed, geometric means were compared.
SAS version 9.3 or later software was used for statistical analyses. All programming and data analysis was conducted by the MOMPOD data coordinating center, which is housed in the Collaborative Studies Coordinating Center at the University of North Carolina at Chapel Hill.
Results
Participant Characteristics
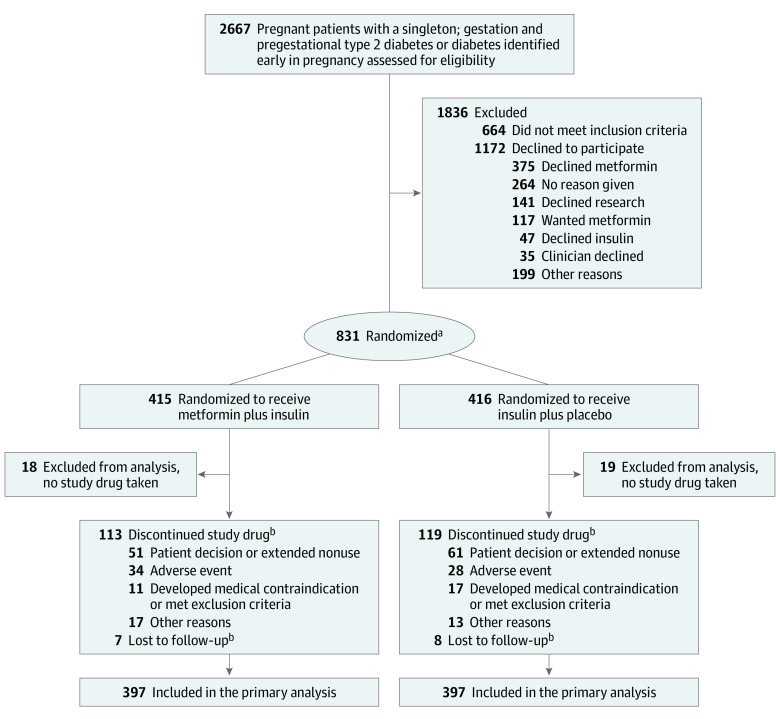
Screening for eligibility among 2667 pregnant adults took place from June 2017 to December 2021. After 695 participants had given birth and completed follow-up, the data and safety monitoring board recommended cessation of the study due to futility, and enrollment was halted in November 2021. Of the 2003 eligible participants, 835 (42%) provided written informed consent and 831 were randomized. Overall, 794 participants who reported taking at least 1 dose of the study agent were included in the final analysis (Figure). The primary analysis included 397 participants in each group, and the primary outcome was ascertained from 768 participants (386 of those randomized to metformin and 382 randomized to placebo). Of the participants who were randomized and took at least 1 dose of the study agent in the primary analysis, 768 (97%) had follow-up through delivery, and 656 (83%) neonates had follow-up through 30 days of life. Anthropometric measurements were completed on 437 (55%) neonates, which was lower than anticipated due to restrictions during the early COVID-19 pandemic. Table 1 shows participant baseline characteristics. The metformin group had more participants with obesity, medically treated chronic hypertension, and previous metformin use, which is likely due to chance.
Figure. MOMPOD Study Flow Diagram.
aRandomization was stratified by clinical site, gestational age at randomization (<18 weeks vs ≥18 weeks) and timing of diabetes diagnosis (pregestational or during pregnancy).
bPatients who discontinued the study drug or who were lost to follow-up were included in the primary analysis.
Table 1. Study Participant Baseline Characteristics.
| No. (%)a | ||
|---|---|---|
| Metformin plus insulin (n = 397) | Insulin plus placebo (n = 397) | |
| Demographics | ||
| Age, mean (SD), y | 32.8 (5.5) | 33.1 (5.7) |
| Raceb | ||
| American Indian or Alaska Native | 3 (1) | 1 (0) |
| Asian | 10 (3) | 13 (3) |
| Black or African American | 119 (30) | 115 (29) |
| Native Hawaiian or Other Pacific Islander | 2 (1) | 2 (1) |
| White | 55 (14) | 57 (14) |
| ≥ 2 Races | 8 (2) | 2 (1) |
| Not reported or declined to report | 95 (24) | 97 (24) |
| Hispanic ethnicityb | ||
| No | 194 (49) | 188 (47) |
| Yes | 203 (51) | 209 (53) |
| Type of insurance | n = 396 | n = 395 |
| Medicaid, federal, or state insurance | 290 (73) | 292 (74) |
| Private | 77 (19) | 79 (20) |
| Military | 0 | 3 (1) |
| None | 29 (7) | 21 (5) |
| First pregnancy | 66 (17) | 55 (14) |
| Prior birth ≥20 wk | 302 (76) | 296 (75) |
| Diabetes characteristics | ||
| Preexisting type 2 diabetes requiring medical therapy prior to pregnancy | 311 (78) | 313 (79) |
| Diagnosis of diabetes early in pregnancy | 86 (22) | 84 (21) |
| Use of diabetes medication prior to pregnancy | 174 (44) | 181 (46) |
| Metformin used earlier in pregnancy | 162 (41) | 175 (44) |
| Criteria to diagnose diabetes early in pregnancyc | n = 86 | n = 84 |
| HbA1c ≥6.5%d | 25 (29) | 22 (26) |
| Fasting blood glucose ≥126 mg/dL | 5 (6) | 5 (6) |
| 1-h OGTT≥200 mg/dLd | 18 (21) | 24 (29) |
| 2-Step method positive | 25 (29) | 20 (24) |
| 1-Step method positive | 12 (14) | 12 (14) |
| Other clinical characteristics and laboratory results | ||
| Chronic hypertension requiring medication | 103 (26) | 86 (22) |
| Smoking during pregnancy | 28 (7) | 32 (8) |
| Body mass index at enrollmente | n = 383 | n = 379 |
| Mean (SD) | 36.4 (8.0) | 36.3 (8.9) |
| Median (IQR) | 35.2 (31.2-41.1) | 34.4 (29.6-41.5) |
| Distribution | ||
| <30 | 76 (19) | 105 (26) |
| 30 to <40 | 198 (50) | 164 (41) |
| ≥40 | 109 (27) | 110 (28) |
| Gestational age at enrollment, wkf | ||
| 18 to <23 | 162 (41) | 165 (42) |
| <18 | 235 (59) | 232 (58) |
| HbA1c % at enrollmentd | n = 361 | n = 361 |
| Mean (SD) | 7.7 (2.02) | 7.7 (1.97) |
| Median (IQR) | 7.2 (6.0-9.1) | 7.3 (6.1-9.1) |
| Gestational age at HbA1c measurement, mean (SD), wk | 11.1 (4.4) | 10.6 (5.4) |
| Gestational age at HbA1c measurement, median (IQR), wk | 10.3 (7.9-14.1) | 10.3 (7.4-13.7) |
Abbreviations: HbA1c, hemoglobin A1c; OGTT, oral glucose tolerance test.
SI conversion: for glucose to mmol/L, multiply by 0.0555.
Data are presented as No. (%) unless otherwise indicated.
Race and ethnicity were determined by medical record abstraction or if not in the record, by participant self-report.
Subcategories do not sum because data were missing for 2 participants.
Reported as %, to convert mmol/mol use (10.93 × HbA1c) − 23.50.
Calculated as weight in kilograms divided by height in meters squared.
Gestational age determined by menstrual date confirmed by first or second trimester ultrasound, and if menstrual date was missing, by ultrasound alone.
Reported adherence was comparable between groups. Of those randomized to metformin, 244 (61%) reported adherence all or almost all the time (90%-100%) and 13 (3%) less than half of the time, compared with 236 (59%) of those randomized to placebo reporting adherence all or almost all the time and 15 (4%) reporting adherence less than half of the time. At study conclusion, less than 10% of participants remembered to bring in empty study bottles, so this data were not used to report adherence.
Primary and Secondary Outcomes
The occurrence of the primary outcome (Table 2) was higher than expected and not significantly different between groups (280 [71%] of 397 in the metformin group and 292 [74%] of 397 in the placebo group; adjusted OR, 0.86 [95% CI, 0.63-1.19]). Results from an unadjusted model and the per-protocol population were similar. Of individual components of the composite primary outcome, the metformin group had a lower proportion of large-for-gestational-age infants compared with placebo (100 [26%] of 386 live births vs 137 [36%] of 384 live births; OR, 0.63 [95% CI, 0.46-0.86]). Proportions of small-for-gestational age infants were similar. Analysis of prespecified secondary outcomes showed that clinically relevant maternal hypoglycemia was also similar between the groups, occurring in 87 (22%) of 397 assigned to the metformin group and 85 (21%) of 397 assigned to placebo (adjusted OR, 1.02 [95% CI, 0.72–1.46]). Among the 437 neonates with anthropometric measurements, mean (SD) neonatal body fat mass was comparable between those exposed to metformin vs placebo (0.46 [0.30] kg vs 0.50 [0.24] kg).
Table 2. Primary Composite Neonatal Outcome and Components.
| Metformin plus insulin (n = 397)a | Insulin plus placebo (n = 397)a | Unadjusted absolute difference (95% CI) | Adjusted odds ratio (95% CI)b | |
|---|---|---|---|---|
| Composite primary outcomec | 280 (71) | 292 (74) | −3.95 (−11.49 to 3.99) | 0.86 (0.63 to 1.19) |
| Live birthsd | 386 (97) | 384 (97) | ||
| Fetal and neonatal death | 11 (3) | 13 (3) | −4.30 (−24.54 to 15.95) | 0.83 (0.36 to 1.89) |
| Miscarriage <20 wk | 7 (2) | 4 (1) | ||
| Stillbirth ≥20 wk | 3 (1) | 7 (2) | ||
| Neonatal death <28 d | 1 (<1) | 2 (1) | ||
| Preterm birth <37 wk | 130 (34) | 143 (37) | −3.89 (−11.27 to 3.49) | 0.86 (0.64 to 1.16) |
| Neonatal hypoglycemia | 152 (39) | 162 (42) | −2.91 (−10.09 to 4.28) | 0.89 (0.67 to 1.19) |
| Birth trauma | 16 (4) | 16 (4) | −0.14 (−17.83 to 17.56) | 1.02 (0.50 to 2.07) |
| Umbilical artery pH <7.05 | 9 (2) | 9 (2) | ||
| Shoulder dystocia | 7 (2) | 7 (2) | ||
| Hyperbilirubinemia requiring phototherapy | 87 (23) | 92 (24) | −1.99 (−10.35 to 6.37) | 0.93 (0.66 to 1.30) |
| Large for gestational age (>90th percentile) | 100 (26) | 137 (36) | −11.46 (−19.04 to −3.88) | 0.63 (0.46 to 0.86) |
| Small for gestational age (<10th percentile) | 30 (8) | 26 (7) | 3.71 (−9.86 to 17.28) | 1.17 (0.68 to 2.02) |
| Low birth weight (<2500 g) | 81 (21) | 73 (19) | 3.08 (−5.73 to 11.90) | 1.14 (0.80 to 1.63) |
Eleven participants in the metformin plus insulin group and 15 in the insulin plus placebo group had missing data, and as prespecified in the statistical analysis plan, are assumed to have the composite primary outcome.
Adjusted by study site, timing of diabetes diagnosis (pregestational vs diagnosed early in pregnancy), gestational age at randomizations stratified at 18 weeks, and baseline maternal body mass index as a continuous variable.
Preterm birth before 37 weeks’ gestation, neonatal hypoglycemia, birth trauma, hyperbilirubinemia, large for gestational age, small for gestational age, and low birth weight odds ratios are reported for live births (observed and assumed) and are adjusted for all baseline characteristics noted in Table 1, except study site as some sites had too few events.
Percent values for the following categories: preterm birth, neonatal hypoglycemia, birth trauma, umbilical artery pH, shoulder dystocia, hyperbilirubinemia requiring phototherapy, large for gestational age, small for gestational age, and low birth weight are calculated from live birth denominators.
Birth outcomes are shown in Table 3. The proportion of live births, gestational age at delivery, proportion of male neonates, and cesarean deliveries (primary vs repeat and following labor or in absence of labor), were comparable between groups. However, compared with the placebo group, mean (SD) neonatal birthweight (3089 [74] vs 3244 [78] g) and mean (SD) birthweight z score (0.49 [1.12] vs 0.81 [1.18]) were lower among patients in the metformin group.
Table 3. Study Participant Birth Outcomes.
| Metformin plus insulin | Insulin plus placebo | % Unadjusted difference median, or mean (95% CI)a | |
|---|---|---|---|
| Gestational age at birth, median (IQR), wkb | 37.4 (36.1-38.6) | 37.3 (35.8-38.3) | 0.14 (−0.14 to 0.29) |
| Cesarean delivery, No. (%)c | 237/377 (63) | 236/376 (63) | 0.11 (−7.28 to 7.50) |
| Primary, no labor | 101 (43) | 108 (46) | |
| Primary, after labor | 11 (5) | 19 (8) | |
| Repeat, no labor | 51 (22) | 33 (14) | |
| Repeat, after labor | 74 (31) | 76 (32) | |
| Macrosomia as indication | 11 (5) | 21 (9) | |
| Birthweight, mean (SD), gb | 3089 (74) | 3244 (78) | −155 (−265 to −45) |
| Birthweight z score, mean (SD)b,d | 0.49 (1.12) | 0.81 (1.18) | −0.32 (−0.48 to −0.15) |
| Neonatal fat mass, mean (SD), kge | 0.46 (0.30) | 0.50 (0.24) | −0.04 (−0.09 to 0.01) |
| Infant male sex, No. (%) | 185/379 (48) | 204/375 (53) | −5.59 (−12.72 to 1.54) |
Unadjusted difference for the median is reported using the Hodges-Lehmann estimator and Moses confidence limits using PROC NPAR1WAY in SAS 9.4.
Gestational age at birth and birthweight is only reported for live births having delivery data, 376 participants in the metformin plus insulin group and 370 participants in the insulin plus placebo group.
Denominators were 377 for the metformin plus insulin group and 376 for the insulin plus placebo group. Cesarean delivery and its subcategory percentages are calculated from these denominators, and subcategory percentages may not sum to 100% due to rounding.
Birthweight z score is calculated using z score for gestational age by weeks = (birthweight – mean)/standard deviation.
Neonatal fat mass was only collected on a proportion of neonates, 227 in the metformin plus insulin group and 210 in the insulin plus placebo group. Neonatal fat mass determined by anthropometric measurements using the formula: fat mass = 0.39055(birthweight) +0.0453(flank skinfold) – 003237(length) +0.54657 and calculated on 422 (55%) neonates.
Subgroup Analyses
Prespecified subgroup analyses of the primary outcome showed no significant differences in composite adverse neonatal outcome in subgroup analyses by maternal BMI of 30 or above, timing of diagnosis of diabetes, or gestational age at randomization (eTable 2 in Supplement 3).
Exploratory Outcomes
Participants randomized to metformin vs placebo had comparable daily insulin dosing (geometric mean [SD]: 68.9 [2.1] vs 73.7 [2.04] units) and dosing by maternal weight (mean [SD]: 0.90 U/kg [0.61] vs 1.11 U/kg [3.10]). There were also comparable between-group proportions with excessive maternal weight gain (metformin group, 185 [52%] of 357 vs 195 [55%] of 353 in the placebo group). Of 794 participants, 308 (39%) had the third-trimester hemoglobin A1c (HbA1c) level measured (155 in the metformin group; mean [SD], 31.5 [3.5] weeks and 153 in the placebo group; mean [SD], 31.7 [3.3] weeks). The geometric mean [SD] level of HbA1c was lower in the metformin group compared with the placebo group (5.97 [0.08] vs 6.22 [0.08]; geometric mean ratio, 0.96 [95% CI, 0.93-1.00]).
The prevalence of fetal intolerance to labor (32 [8%] vs 31 [8%]) and failed induction of labor (38 [10%] vs 37 [10%]) was comparable between metformin and placebo groups. The prevalence of preeclampsia (metformin group, 122 [35%] vs 109 [30%] in the placebo group; adjusted OR, 1.31 [95% CI, 0.96-1.78]) was comparable between groups; however, there were more patients with chronic hypertension requiring medication in the metformin group (103 [26%] of 397) vs the placebo group (86 [22%] of 397). After adjusting for chronic hypertension requiring medication, the adjusted OR for preeclampsia in the metformin group was 1.24 (95% CI, 0.9-1.71).
Infants in the metformin and placebo groups had comparable rates of neonatal intensive care unit (NICU) admission (152 [40%] of 376, vs 165 [45%] of 370), as well as comparable percentages requiring a NICU stay longer than 2 days (124 [33%] of 376 vs 131 [35%] of 370). The median length of NICU stay was 7.5 days for neonates in the metformin group (median IQR, 3-22 days) and 7.0 days for neonates in the placebo group (median IQR, 3-17 days). The most common neonatal complication other than hypoglycemia or hyperbilirubinemia was respiratory distress syndrome, which occurred in 61 (16%) of 376 neonates exposed to metformin and 64 (17%) of 370 of those exposed to placebo.
Adverse Events and Serious Adverse Events
The most common adverse events of metformin use were nausea, vomiting, and diarrhea.24 The proportions of participants randomized to metformin vs placebo reporting nausea (36% vs 39%) and vomiting (25% vs 23%) were comparable, while a higher proportion of participants randomized to metformin reported diarrhea (28% vs 12%; P < .01). Serious adverse event data are provided in eTables 3 and 4 in Supplement 3. Unadjusted rate differences in maternal and fetal/neonatal serious adverse events were not statistically significant.
Discussion
Among pregnant adults with preexisting type 2 diabetes or diabetes diagnosed prior to 23 weeks’ gestation, metformin added to insulin did not reduce the frequency of the composite adverse neonatal outcome. Further, no significant differences were observed in prespecified secondary outcomes, including maternal hypoglycemia and neonatal fat mass. Findings were similar regardless of maternal BMI (<30 or ≥30), timing of diabetes diagnosis, or timing of enrollment (<18 weeks’ gestation or ≥18 weeks’ gestation). However, compared with those randomized to placebo, participants randomized to metformin were less likely to deliver a large-for-gestational-age infant.
To our knowledge, this is the largest trial of metformin added to insulin to treat preexisting type 2 diabetes or diabetes identified early in pregnancy. Study strengths include the randomized, blinded, placebo-controlled design and enrollment of a diverse study population with a large proportion of patients of Hispanic ethnicity. These strengths contribute to generalizability of our findings to a US pregnant population.
The current trial’s finding of no significant reduction in composite neonatal morbidity aligns with that of the MiTy trial, a previous randomized clinical trial of insulin plus metformin or placebo in pregnant participants with diabetes.25 However, in contrast to the current study, those randomized to metformin in the MiTy trial were found to have improved maternal glycemic control, less maternal weight gain, less neonatal adiposity, fewer cesarean deliveries, and more small-for-gestational-age neonates.25 The differences in results may be explained by differences in study populations. More than 50% of the current study’s participants were Hispanic, compared with 2% to 3% in the MiTy trial, and Hispanic persons tend to have greater insulin resistance compared with non-Hispanic African American and non-Hispanic White persons.26 In the current trial, approximately 20% were enrolled with diabetes diagnosed early in pregnancy, compared to approximately 10% in the MiTy trial, which may also reflect differences in insulin resistance. Compared with the MiTy trial, this trial had lower prevalence of small-for-gestational-age infants and was not powered to determine a difference for this outcome. Other factors, including regional differences in health care access and type and dosing of insulin, could also contribute to observed trial differences.
Although this trial observed a modest reduction in large-for-gestational-age infants in the metformin group, there were no differences in neonatal outcomes nor delivery by cesarean. The cesarean delivery rate in this trial was higher than the national rate and the rate among states where the study was conducted,1 but it was comparable to the rate in the MiTy study,25 which reflects the complexity of intrapartum care for patients with type 2 diabetes and diabetes identified early in pregnancy. Decisions about cesarean delivery are complex and not solely made on estimates of fetal weight, but the proportion of participants delivered by primary and repeat cesarean and in the presence and absence of labor were comparable between groups in this study.
Limitations
Some study limitations exist. First, enrollment was suspended for as many as 9 months due to the COVID-19 pandemic, and the study was halted at 75% for futility, both of which limit the ability to measure metformin effect on less common outcomes.
Second, while maternal BMI was comparable between groups, there were more participants with obesity in the metformin group compared with the placebo group, which could skew our findings toward the null because of increased insulin resistance.
Third, adherence was only measured by self-report; thus, the negative findings could be explained by poor adherence that was underreported, although using patient report for adherence is more generalizable than pill counts because it mimics what is done in clinical practice.
Fourth, HbA1c data were only collected if done as part of clinical care and only in a small percentage of participants (≈39%). Given the proportion of untested participants, definitive conclusions about effects of metformin on glycemic control cannot be drawn.
Conclusions
Using metformin plus insulin to treat preexisting type 2 or gestational diabetes diagnosed early in pregnancy did not reduce a composite neonatal adverse outcome. The effect of reduction in odds of a large for gestational age infant observed after adding metformin to insulin warrants further investigation.
Trial Protocol
Statistical Analysis Plan
eTables
eTable 1. MOMPOD Study Sensitivity Analyses
eTable 2. Prespecified Subgroup Analyses by Maternal Baseline Variables
eTable 3. Counts of Maternal Serious Adverse Events
eTable 4. Fetal/Neonatal Severe Adverse Events
Data Sharing Statement
References
- 1.Centers for Disease Control and Prevention . National Diabetes Statistics Report website. Accessed June 1, 2023. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 2.Harper LM, Jauk V, Longo S, Biggio JR, Szychowski JM, Tita AT. Early gestational diabetes screening in obese women: a randomized controlled trial. Am J Obstet Gynecol. 2020;222(5):495 e1-495 e8. doi: 10.1016/j.ajog.2019.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons D, Immanuel J, Hague WM, et al. ; TOBOGM Research Group . Treatment of gestational diabetes mellitus diagnosed early in pregnancy. N Engl J Med. 2023;388(23):2132-2144. doi: 10.1056/NEJMoa2214956 [DOI] [PubMed] [Google Scholar]
- 4.Dunne F, Brydon P, Smith K, Gee H. Pregnancy in women with type 2 diabetes: 12 years outcome data 1990-2002. Diabet Med. 2003;20(9):734-738. doi: 10.1046/j.1464-5491.2003.01017.x [DOI] [PubMed] [Google Scholar]
- 5.Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melamed N, Chen R, Soiberman U, Ben-Haroush A, Hod M, Yogev Y. Spontaneous and indicated preterm delivery in pregestational diabetes mellitus: etiology and risk factors. Arch Gynecol Obstet. 2008;278(2):129-134. doi: 10.1007/s00404-007-0541-z. [DOI] [PubMed] [Google Scholar]
- 7.Knight KM, Pressman EK, Hackney DN, Thornburg LL. Perinatal outcomes in type 2 diabetic patients compared with non-diabetic patients matched by body mass index. J Matern Fetal Neonatal Med. 2012;25(6):611-615. doi: 10.3109/14767058.2011.587059. [DOI] [PubMed] [Google Scholar]
- 8.ElSayed NA, Aleppo G, Aroda VR, et al. ; on behalf of the American Diabetes Association . 15. Management of diabetes in pregnancy: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S254-S266. doi: 10.2337/dc23-S015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickman MA, McBride R, Boggess KA, Strauss R. Metformin compared with insulin in the treatment of pregnant women with overt diabetes: a randomized controlled trial. Am J Perinatol. 2013;30(6):483-490. doi: 10.1055/s-0032-1326994 [DOI] [PubMed] [Google Scholar]
- 10.Refuerzo JS, Gowen R, Pedroza C, Hutchinson M, Blackwell SC, Ramin S. A pilot randomized, controlled trial of metformin versus insulin in women with type 2 diabetes mellitus during pregnancy. Am J Perinatol. 2015;30(2):163-170. doi: 10.1055/s-0034-1378144 [DOI] [PubMed] [Google Scholar]
- 11.Ainuddin JA, Karim N, Zaheer S, Ali SS, Hasan AA. Metformin treatment in type 2 diabetes in pregnancy: an active controlled, parallel-group, randomized, open label study in patients with type 2 diabetes in pregnancy. J Diabetes Res. 2015;2015:325851. doi: 10.1155/2015/325851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics . ACOG practice bulletin No. 201: pregestational diabetes mellitus. Obstet Gynecol. 2018;132(6):e228-e248. doi: 10.1097/AOG.0000000000002960 [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Professional Practice Committee . 15. Management of diabetes in pregnancy: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(suppl 1):S232-S243. doi: 10.2337/dc22-S015 [DOI] [PubMed] [Google Scholar]
- 14.Berry DC, Thomas SD, Dorman KF, et al. Rationale, design, and methods for the Medical Optimization and Management of Pregnancies with Overt Type 2 Diabetes (MOMPOD) study. BMC Pregnancy Childbirth. 2018;18(1):488. doi: 10.1186/s12884-018-2108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. doi: 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 16.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768-773. doi: 10.1016/0002-9378(82)90349-0 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Professional Practice Committee . Summary of revisions: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(suppl 1):S4-S7. doi: 10.2337/dc22-Srev [DOI] [PubMed] [Google Scholar]
- 18.ElSayed NA, Aleppo G, Aroda VR, et al. ; on behalf of the American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S19-S40. doi: 10.2337/dc23-S002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matts JP, Lachin JM. Properties of permuted-block randomization in clinical trials. Control Clin Trials. 1988;9(4):327-344. doi: 10.1016/0197-2456(88)90047-5 [DOI] [PubMed] [Google Scholar]
- 20.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 1995;173(4):1176-1181. doi: 10.1016/0002-9378(95)91348-3 [DOI] [PubMed] [Google Scholar]
- 21.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219-242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21(6):521-526. doi: 10.1097/GCO.0b013e328332d24e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab. 2017;19(4):473-481. doi: 10.1111/dom.12854 [DOI] [PubMed] [Google Scholar]
- 25.Feig DS, Donovan LE, Zinman B, et al. ; MiTy Collaborative Group . Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8(10):834-844. doi: 10.1016/S2213-8587(20)30310-7 [DOI] [PubMed] [Google Scholar]
- 26.Raygor V, Abbasi F, Lazzeroni LC, et al. Impact of race/ethnicity on insulin resistance and hypertriglyceridaemia. Diab Vasc Dis Res. 2019;16(2):153-159. doi: 10.1177/1479164118813890 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTables
eTable 1. MOMPOD Study Sensitivity Analyses
eTable 2. Prespecified Subgroup Analyses by Maternal Baseline Variables
eTable 3. Counts of Maternal Serious Adverse Events
eTable 4. Fetal/Neonatal Severe Adverse Events
Data Sharing Statement