Abstract
Background
Despite the increasing use of biologics in severe asthma, there is limited research on their use in asthma-chronic obstructive pulmonary disease overlap (ACO). We compared real-world treatment responses to biologics in ACO and asthma.
Methods
We conducted a multicenter, retrospective, cohort study using data from the Precision Medicine Intervention in Severe Asthma (PRISM). ACO was defined as post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7 and a smoking history of >10 pack-years. Physicians selected biologics (omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab) based on each United States Food & Drug Administration (FDA) approval criteria.
Results
After six-month treatment with biologics, both patients with ACO (N = 13) and asthma (N = 81) showed positive responses in FEV1 (10.69 ± 17.17 vs. 11.25 ± 12.87 %, P = 0.652), Asthma Control Test score (3.33 ± 5.47 vs. 5.39 ± 5.42, P = 0.290), oral corticosteroid use (−117.50 ± 94.38 vs. −115.06 ± 456.85 mg, P = 0.688), fractional exhaled nitric oxide levels (−18.62 ± 24.68 vs. −14.66 ± 45.35 ppb, P = 0.415), sputum eosinophils (−3.40 ± 10.60 vs. −14.48 ± 24.01 %, P = 0.065), blood eosinophils (−36.47 ± 517.02 vs. −363.22 ± 1294.59, P = 0.013), and exacerbation frequency (−3.07 ± 4.42 vs. −3.19 ± 5.11, P = 0.943). The odds ratio for exacerbation and time-to-first exacerbation showed no significant difference after full adjustments, and subgroup analysis according to biologic type was also showed similar results.
Conclusions
Biologics treatment response patterns in patients with ACO and asthma were comparable, suggesting that biologics should be actively considered for ACO patients as well.
Keywords: Asthma-COPD overlap, Asthma, Biologics, Monoclonal antibodies, Treatment response
Introduction
Asthma-chronic obstructive pulmonary disease (COPD) overlap (ACO) does not refer to a single disease entity.1 It is a heterogeneous condition that shares some features of both asthma and COPD, which are chronic inflammatory airway diseases.2 Clinical features of ACO include asthma with fixed airflow obstruction, eosinophilic COPD, COPD with a significant bronchodilator response, and smoking asthmatics.3,4 ACO is reported to have worse clinical outcomes and higher mortality rates than either asthma or COPD alone, but its pathophysiology remains unclear, and there is no effective and specific treatment for ACO.2 The current uncertainty regarding ACO primarily results from the lack of consensus about its definition or diagnostic criteria, which would enable a more standardized approach to diagnosis and management.1
In 2014, the Global Initiative for Asthma (GINA) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) issued a joint statement describing ACO as a condition with features of both asthma and COPD, with persistent airflow limitation.5 The diagnostic criteria for ACO vary depending on whether it is approached from the asthma or COPD perspective.1,6 A definition of ACO derived from COPD cohorts typically includes a diagnosis of COPD with a post-bronchodilator (BD) forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) < 0.7 and a history of asthma before the age of 40 years, or a great BD response of ≥15 % in FEV1.1,7 However, in asthma cohorts, a definition of ACO was proposed based on a diagnosis of asthma with a significant smoking history, plus a post-BD FEV1/FVC <0.7.8 The lack of a standardized definition also makes it difficult to compare outcomes across studies on ACO.
Importantly, most clinical trials for drug development have only enrolled patients with pure asthma or pure COPD, excluding those with suspected ACO, resulting in limited research on the treatment of ACO. Although precision medicine and biologics have been highlighted in recent years for asthma, ACO has been left behind in this regard. Therefore, real-world studies on the use of biologics in ACO are important. This study aimed to generate new evidence on the use of biologics in patients with ACO by comparing clinical outcomes, including inflammatory markers, lung function, symptom control, and exacerbation rates, between patients with asthma and ACO undergoing treatment with biologics for 6 months or more, using multicenter, real-world adult severe asthma cohort data from Korea.
Methods
Study population
The Precision Medicine Intervention in Severe Asthma (PRISM) project is a prospective, observational, multicenter cohort study involving patients with severe asthma attending asthma clinics in Korea (38 centers) and the United Kingdom (3 centers), which has been conducted since May 2020.9 This study enrolled adult patients between 18 and 80 years who were diagnosed with asthma by allergy or pulmonology experts and were classified as having severe asthma according to the European Respiratory Society/American Thoracic Society (ERS/ATS) criteria published in 2014.10 Accordingly, patients required GINA Step 4–5 treatment or systemic corticosteroids for more than 50 % of the past year or were still uncontrolled despite these treatments.10
Subsequently, the subjects were classified into type 2 (T2)-high or T2-low asthma based on skin prick tests, blood eosinophils, induced sputum eosinophils, and fractional exhaled nitric oxide (FeNO) levels. T2-high asthma was defined as meeting 1 or more of the following criteria: blood eosinophils ≥150/μL, sputum eosinophils ≥2 %, FeNO levels ≥20 ppb, and suspected allergic asthma with sensitivity to inhalant allergen.9,11 Patients with T2-high asthma were newly treated with biologics such as omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab according to United States Food and Drug Administration (FDA) approval criteria or continued with conventional treatment according to the usual clinical protocols. Follow-up evaluations were performed at baseline, 1 month, 6 months, and 12 months.
From the PRISM database, we selected only Korean biologic users aged ≥40 years who completed at least 6 months of follow-up. Biologics were administered on the day of enrollment after screening, in accordance with the recommended dose and treatment interval protocols specific to each pharmaceutical agent using FDA-approved criteria, which are applied to patient care in Korea. This study was approved by the Institutional Review Board of Ewha Womans University Medical Center (SEUMC 2020-04-014-023) and Asan Medical Center (2019–1676).
Variable definitions
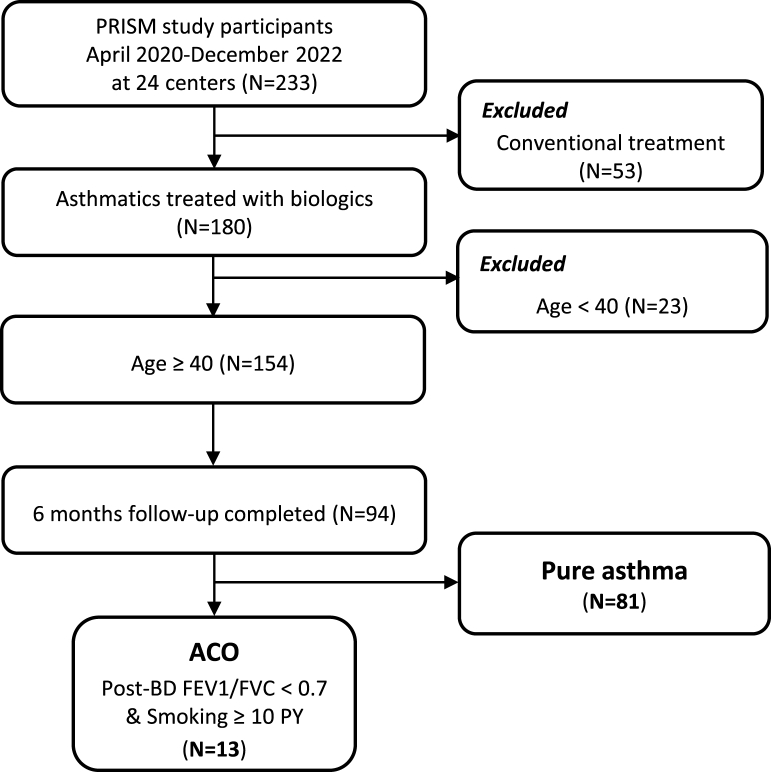
ACO was defined as asthma with post-BD airway flow limitation (FEV1/FVC <0.7) and smoking history of ≥10 pack-years (Fig. 1).12 A total of 94 subjects were enrolled, including 13 patients with ACO and 81 with pure asthma. Baseline clinical and laboratory characteristics were obtained at enrollment, and pulmonary function, blood and sputum eosinophils, FeNO levels, and asthma control test (ACT) were measured at every follow-up visit. A bronchodilator reversibility test was performed after stopping short-acting β2-agonist ≥4 h and LABA ≥24 h, and positive BD response was defined as the increase in FEV1 of >12 % and >200 mL from baseline after 10–15 min of inhalation of salbutamol 200–400 μg.11 Atopy was identified as positive for inhalant allergens by skin prick test or multiple allergen simultaneous test (MAST). Asthma exacerbation was defined as positive if subjects experienced at least 1 of the following: an unscheduled outpatient visit, an emergency room (ER) visit, hospitalization, or corticosteroid burst, identified through monthly telephone surveys. Corticosteroid burst was defined as the use of a prednisolone dose greater than 30 mg/day or its equivalent for more than 3 consecutive days.
Fig. 1.
Study population flow chart. Abbreviations: ACO, asthma-chronic obstructive pulmonary disease overlap; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PY, pack-year.
Statistical analyses
Descriptive statistics are presented as number (%) for categorical variables or a mean ± standard deviation for continuous variables. Demographic, clinical, and asthma characteristics were assessed between the pure asthma and the ACO groups using the Student's t-test or Wilcoxon rank sum test for normally or non-normally distributed continuous variables and the Chi-square test or Fisher's exact test for categorical variables. A univariate and multivariate logistic regression model was used to identify the association between the presence of asthma exacerbation and ACO among study groups. Adjusting variables to identify potential confounders included age, sex, body mass index, smoking pack-year, blood eosinophils (cells/μL), and FeNO (ppb) levels at baseline. To model time-to-first exacerbation, the Kaplan-Meier time-to-event analysis was plotted in the ACO and pure asthma groups. A P-value <0.05 was considered statistically significant. All statistical analyses were performed with SAS (SAS Institute v. 9.4, Cary, NC) and R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
General characteristics
A total of 94 patients with severe asthma treated with biologics were enrolled, of which 81 had pure asthma and 13 had ACO. Patient baseline characteristics are presented in Table 1. There were more males in the ACO group than in the pure asthma group (100 % vs 44.4 %, P < 0.001) and mean smoking pack-year was 29.6 ± 16.1 and 12.6 ± 14.9, in the ACO group and pure asthma group, respectively (P = 0.001). Patients with ACO exhibited lower blood eosinophil counts (453.3 ± 304.5 vs 730.9 ± 588.3/μL, P = 0.015) and less atopy (18.2 % vs 65.5 %, P = 0.007) than patients with pure asthma, and lower post-BD FEV1 (47.8 ± 18.8 vs. 67.6 ± 19.0 %, P = 0.001) and FEV1/FVC ratio (0.50 ± 0.13 vs 0.68 ± 0.12, P < 0.001). Patients with ACO had a higher budesonide equivalent inhaled corticosteroid (ICS) dose than patients with pure asthma (1027.7 ± 626.4 vs 685.5 ± 467.5 mcg/day, P = 0.023).
Table 1.
Characteristics of study participants (N = 94).
| Characteristics | ACO |
Pure asthma |
P-value |
|---|---|---|---|
| (n = 13) | (n = 81) | ||
| Male, n (%) | 13 (100) | 36 (44.4) | <.0001 |
| Age (years) | 54.5 ± 9.3 | 54.1 ± 9.0 | 0.886 |
| BMI (kg/m2) | 25.3 ± 3.2 | 24.9 ± 3.3 | 0.669 |
| Onset of asthma (age) | 44.2 ± 12.1 | 42.6 ± 11.9 | 0.663 |
| Duration of asthma (years) | 10.7 ± 5.8 | 11.3 ± 8.5 | 0.791 |
| Smoking status, n (%) | 0.001 | ||
| Nonsmoker | 0 | 45 (55.6) | |
| Past smoker | 12 (92.3) | 31 (38.3) | |
| Current smoker | 1 (7.7) | 5 (6.2) | |
| Smoking history (pack-years) | 29.6 ± 16.1 | 12.6 ± 14.9 | 0.001 |
| Comorbidities, n (%) | |||
| Allergic rhinitis | 8 (61.5) | 63 (77.8) | 0.295 |
| Chronic rhinosinusitis | 5 (38.5) | 44 (54.3) | 0.374 |
| Nasal polyp | 2 (15.4) | 19 (23.5) | 0.726 |
| NERD | 0 | 2 (2.5) | 1.000 |
| Atopic dermatitis | 3 (23.1) | 10 (12.4) | 0.381 |
| Bronchiectasis | 0 | 3 (3.7) | 1.000 |
| Obstructive sleep apnea | 1 (7.7) | 3 (3.7) | 0.454 |
| Gastroesophageal reflux disease | 6 (46.2) | 21 (25.9) | 0.186 |
| Diabetes mellitus | 2 (15.4) | 9 (11.1) | 0.646 |
| Hypertension | 4 (30.8) | 27 (33.3) | 1.000 |
| Family history of allergic disease, n (%) | 9 (69.2) | 57 (70.4) | 1.000 |
| Laboratory findings | |||
| WBC (103/μL) (n = 94) | 8.7 ± 2.5 | 8.1 ± 2.5 | 0.444 |
| Blood eosinophil (%) (n = 94) | 5.6 ± 3.9 | 9.5 ± 6.9 | 0.007 |
| Blood eosinophil (/μL) (n = 94) | 453.3 ± 304.5 | 730.9 ± 588.3 | 0.015 |
| C-reactive protein (mg/dL) (n = 67) | 0.4 ± 0.4 | 0.4 ± 0.7 | 0.836 |
| Serum total IgE (IU/mL) (n = 72) | 838.0 ± 834.5 | 439.2 ± 593.9 | 0.078 |
| Sputum eosinophil (%) (n = 66) | 12.3 ± 23.8 | 24.3 ± 30.8 | 0.228 |
| Sputum neutrophil (%) (n = 62) | 54.7 ± 39.7 | 48.6 ± 34.9 | 0.605 |
| Sputum eosinophil ≥2 % (n = 66) | 6 (54.6) | 36 (65.5) | 0.511 |
| Atopy (n = 90) | 2 (18.2) | 50 (63.3) | 0.007 |
| FeNO (ppb) (n = 91) | 48.7 ± 38.5 | 77.9 ± 49.6 | 0.046 |
| FeNO ≥ 20 ppb | 11 (84.6) | 72 (92.3) | 0.320 |
| Lung function test | |||
| Pre-bronchodilator FEV1 (%) (n = 94) | 49.1 ± 21.4 | 63.4 ± 18.6 | 0.013 |
| Pre-bronchodilator FEV1 (L) (n = 94) | 1.78 ± 0.80 | 1.91 ± 0.72 | 0.567 |
| Pre-bronchodilator FVC (%) (n = 94) | 70.5 ± 19.2 | 75.3 ± 15.1 | 0.316 |
| Pre-bronchodilator FVC (L) (n = 94) | 3.3 ± 1.0 | 2.9 ± 1.0 | 0.119 |
| Pre-bronchodilator FEV1/FVC (n = 94) | 0.52 ± 0.14 | 0.66 ± 0.13 | <.0001 |
| Post-bronchodilator FEV1 (%) (n = 88) | 47.8 ± 18.8 | 67.6 ± 19.0 | 0.001 |
| Post-bronchodilator FEV1 (L) (n = 88) | 1.73 ± 0.68 | 2.04 ± 0.70 | 0.154 |
| Post-bronchodilator FVC (%) (n = 88) | 71.6 ± 16.7 | 77.8 ± 16.0 | 0.164 |
| Post-bronchodilator FVC (L) (n = 88) | 3.4 ± 0.9 | 3.0 ± 0.9 | 0.215 |
| Post-bronchodilator FEV1/FVC (n = 88) | 0.50 ± 0.13 | 0.68 ± 0.12 | <.0001 |
| Positive bronchodilator response (n, %) | 1 (7.69) | 16 (19.75) | 0.225 |
| Budesonide equivalent ICS dose (mcg/day) | 1027.7 ± 626.4 | 685.5 ± 467.5 | 0.023 |
| Prednisolone equivalent OCS dose during last month (mg) | 269.0 ± 138.0 | 223.3 ± 236.0 | 0.681 |
| Annual exacerbation rate | |||
| Ever experienced AE (previous 12 months), n (%) | 10 (76.9) | 50 (61.7) | 0.364 |
| All AE (/year) | 4.2 ± 4.3 | 3.6 ± 5.2 | 0.663 |
| SCS burst use, n (%) | 6 (46.2) | 37 (45.7) | 1.000 |
| ER visit (/year) | 1.5 ± 0.6 | 1.7 ± 1.2 | 0.707 |
| Unexpected outpatient visits (/year) | 3.3 ± 3.4 | 2.5 ± 2.8 | 0.545 |
| Hospitalization (/year) | 1.3 ± 0.5 | 1.8 ± 0.8 | 0.225 |
| ACT scores (total 25) | 16.3 ± 4.2 | 15.2 ± 5.6 | 0.504 |
Data are presented as number (%) or mean ± standard deviation. P-value was calculated by Fisher's exact test for categorical variables and the t-test for continuous variables. Abbreviations: ACO, Asthma-COPD Overlap; BMI, body mass index; NERD, NSAID exacerbated respiratory disease; WBC, white blood cell; IgE, immunoglobulin E; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroids; OCS, oral corticosteroids; AE, acute exacerbation; SCS, systemic corticosteroids; ER, emergency room; ACT, Asthma Control Test.
Clinical characteristics according to biologic therapy
There was no difference in asthma medications between the ACO and pure asthma groups except for the use of biologics. Among the biologics used, dupilumab was more frequently used in the ACO group (61.5 % vs 23.5 %, P = 0.009), while omalizumab and benralizumab were not used in the ACO group (Table 2). The selection of biologics was at the discretion of the treating physician according to guidelines and each drug's FDA approval criteria, without any intervention. When examining the baseline characteristics according to the biologic agents used, patients with ACO treated with reslizumab had a lower post-BD FEV1/FVC ratio (0.53 ± 0.12 vs 0.70 ± 0.10, P = 0.038) and a higher smoking pack-year (32.3 ± 15.7 vs 10.0 ± 10.6, P = 0.042) than pure asthmatics treated with reslizumab, and patients with ACO treated with dupilumab had lower pre-BD FEV1/FVC ratio (0.50 ± 0.17 vs 0.65 ± 0.14, P = 0.037), post-BD FEV1/FVC ratio (0.47 ± 0.15 vs 0.68 ± 0.13, P = 0.012), and a higher smoking pack-year (31.1 ± 18.3 vs 14.9 ± 17.8, P = 0.019) than pure asthmatics treated with dupilumab. There were no significant differences in blood and sputum eosinophils, serum IgE, comorbidities, or other factors between the groups (Table 3). When comparing anti-interleukin (IL)-5 agents, mepolizumab and reslizumab, with dupilumab, the ACO group had lower pre- and post-BD FEV1/FVC ratios and a higher smoking pack-year compared to the pure asthma group (Supplement Table 1).
Table 2.
The use of asthma medications in ACO and pure asthma groups (N = 94).
| ACO |
Pure asthma |
P-value | |
|---|---|---|---|
| (n = 13) | (n = 81) | ||
| ICSa-LABAb only | 3 (23.1) | 26 (32.1) | 0.748 |
| ICS-LABA-LAMAc | 10 (76.9) | 54 (66.7) | 0.540 |
| Theophylline/aminophylline/doxofylline | 5 (38.5) | 26 (32.1) | 0.753 |
| Leukotriene receptor antagonist | 9 (69.2) | 58 (71.6) | 1.000 |
| Daily low dose OCSd | 5 (38.5) | 26 (32.1) | 0.753 |
| Omalizumab | 0 | 4 (4.9) | 1.000 |
| Mepolizumab | 2 (15.4) | 23 (28.4) | 0.502 |
| Reslizumab | 3 (23.1) | 32 (39.5) | 0.359 |
| Dupilumab | 8 (61.5) | 19 (23.5) | 0.009 |
| Benralizumab | 0 | 3 (3.7) | 1.000 |
Data are expressed as number (%). P-value was calculated by Fisher's exact test for categorical variables. Abbreviations: ACO, Asthma-COPD Overlap; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; OCS, oral corticosteroid.
Beclomethasone, budesonide, triamcinolone, ciclesonide, fluticasone, or flunisolide.
Salmeterol, formoterol, indacaterol, or vilanterol.
Tiotropium, aclidinium, glycopyrronium, or umeclidinium.
Methylprednisolone, prednisolone, hydrocortisone, dexamethasone, or deflazacort.
Table 3.
Baseline characteristics according to biologics type in ACO vs pure asthma groups (N = 87).
| Mepolizumab (n = 25) |
P-value | Reslizumab (n = 35) |
P-value | Dupilumab (n = 27) |
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| ACO |
Pure asthma |
ACO |
Pure asthma |
ACO |
Pure asthma |
||||
| (n = 2) | (n = 23) | (n = 3) | (n = 32) | (n = 8) | (n = 19) | ||||
| Male, n (%) | 2 (100) | 6 (26.1) | 0.093 | 3 (100) | 16 (50) | 0.234 | 8 (100) | 13 (68.4) | 0.136 |
| Age (years) | 50.5 ± 9.2 | 52.7 ± 8.6 | 0.882 | 57.3 ± 15.4 | 54.9 ± 9.5 | 0.907 | 54.4 ± 7.7 | 53.2 ± 9.8 | 0.563 |
| BMI (kg/m2) | 25.3 ± 4.9 | 24.5 ± 3.7 | 0.729 | 25.4 ± 4.5 | 24.7 ± 3.0 | 0.726 | 25.3 ± 2.8 | 25.9 ± 3.2 | 0.773 |
| Onset of asthma (age) | 43.5 ± 13.4 | 40.2 ± 12.7 | 0.787 | 47.0 ± 32.5 | 44.6 ± 12.8 | 0.971 | 43.6 ± 7.2 | 39.4 ± 9.8 | 0.213 |
| Duration of asthma (years) | 7.0 ± 4.2 | 11.7 ± 8.8 | 0.518 | 14.0 ± 12.7 | 10.3 ± 6.9 | 0.611 | 10.8 ± 4.6 | 13.8 ± 10.8 | 0.674 |
| Smoking status, n (%) | 0.050 | 0.093 | 0.234 | ||||||
| Nonsmoker | 0 | 19 (82.6) | 0 | 16 (50) | 0 | 4 (21.1) | |||
| Past smoker | 2 (100) | 4 (17.4) | 2 (66.7) | 13 (40.6) | 8 (100) | 13 (68.4) | |||
| Current smoker | 0 | 0 | 1 (33.3) | 3 (9.4) | 0 | 2 (10.5) | |||
| Smoking history (pack-years) | 19.5 ± 5.0 | 14.9 ± 22.3 | 0.518 | 32.3 ± 15.7 | 10.0 ± 10.6 | 0.042 | 31.1 ± 18.3 | 14.9 ± 17.8 | 0.019 |
| Comorbidities, n (%) | |||||||||
| Allergic rhinitis | 1 (50) | 22 (95.6) | 0.157 | 2 (66.7) | 21 (65.6) | 1.000 | 5 (62.5) | 15 (79.0) | 0.633 |
| Chronic rhinosinusitis | 1 (50) | 13 (56.5) | 1.000 | 1 (33.3) | 19 (59.4) | 0.565 | 3 (37.5) | 10 (52.6) | 0.678 |
| Nasal polyp | 2 (100) | 6 (26.1) | 0.093 | 0 | 6 (18.8) | 1.000 | 0 | 6 (31.6) | 0.136 |
| NERD | 0 | 1 (4.4) | 1.000 | 0 | 0 | N/A | 0 | 1 (5.3) | 1.000 |
| Atopic dermatitis | 0 | 4 (17.4) | 1.000 | 1 (33.3) | 3 (9.4) | 0.313 | 2 (25.0) | 1 (5.3) | 0.201 |
| Laboratory findings | |||||||||
| WBC (103/μL) | 6.6 ± 0.1 | 8.6 ± 2.4 | 0.222 | 7.4 ± 2.8 | 7.7 ± 1.7 | 0.726 | 9.7 ± 2.3 | 8.7 ± 3.7 | 0.196 |
| Blood eosinophil (%) | 11.7 ± 2.1 | 12.1 ± 8.2 | 0.804 | 8.2 ± 1.5 | 9.8 ± 6.7 | 0.770 | 3.1 ± 2.1 | 5.2 ± 3.8 | 0.204 |
| Blood eosinophil (/μL) | 773.7 ± 156.6 | 990.1 ± 698.6 | 0.961 | 629.0 ± 324.8 | 752.5 ± 605.1 | 0.977 | 307.4 ± 241.7 | 368.0 ± 207.6 | 0.335 |
| Serum total IgE (IU/mL) | N/A | 325.1 ± 329.6 | N/A | 1422.5 ± 1265.0 | 387.4 ± 500.3 | 0.116 | 671.0 ± 717.9 | 374.7 ± 352.3 | 0.677 |
| Sputum eosinophil (%) | 35 | 30.8 ± 30.1 | 1.000 | 3.7 ± 3.2 | 13.6 ± 26.8 | 0.823 | 13.4 ± 29.2 | 32.8 ± 37.5 | 0.235 |
| Sputum neutrophil (%) | 53 | 45.5 ± 31.3 | 0.776 | 39.0 ± 49.0 | 60.7 ± 36.2 | 0.521 | 61.7 ± 40.5 | 34.8 ± 35.9 | 0.117 |
| Atopy | 0 | 13 (56.5) | 0.100 | 1 (33.3) | 17 (53.1) | 0.603 | 1 (12.5) | 16 (84.2) | 0.001 |
| FeNO (ppb) | 104.5 ± 84.2 | 85.6 ± 52.1 | 0.757 | 33.7 ± 9.1 | 70.6 ± 45.3 | 0.235 | 40.4 ± 21.0 | 70.6 ± 48.4 | 0.108 |
| Lung function test | |||||||||
| Pre-bronchodilator FEV1 (%) v(n = 23) | 57.5 ± 7.8 | 57.4 ± 16.1 | 0.843 | 49.0 ± 25.2 | 66.7 ± 19.1 | 0.258 | 47.0 ± 23.8 | 65.0 ± 20.9 | 0.106 |
| Pre-bronchodilator FEV1 (L) (n = 23) | 2.36 ± 0.40 | 1.68 ± 0.63 | 0.159 | 1.61 ± 0.65 | 2.00 ± 0.73 | 0.466 | 1.70 ± 0.92 | 2.12 ± 0.83 | 0.264 |
| Pre-bronchodilator FVC (%) (n = 23) | 81.0 ± 11.3 | 72.3 ± 15.3 | 0.553 | 64.0 ± 20.5 | 77.3 ± 15.0 | 0.258 | 70.4 ± 21.0 | 77.1 ± 15.7 | 0.464 |
| Pre-bronchodilator FVC (L) (n = 23) | 4.2 ± 0.4 | 2.6 ± 0.9 | 0.063 | 2.9 ± 0.7 | 3.0 ± 1.0 | 1.000 | 3.3 ± 1.0 | 3.2 ± 0.9 | 0.733 |
| Pre-bronchodilator FEV1/FVC (n = 23) | 0.56 ± 0.04 | 0.65 ± 0.16 | 0.326 | 0.54 ± 0.13 | 0.67 ± 0.10 | 0.096 | 0.50 ± 0.17 | 0.65 ± 0.14 | 0.037 |
| Post-bronchodilator FEV1/FVC (n = 21) | 0.53 ± 0.06 | 0.63 ± 0.12 | 0.203 | 0.53 ± 0.12 | 0.70 ± 0.10 | 0.038 | 0.47 ± 0.15 | 0.68 ± 0.13 | 0.012 |
| Budesonide equivalent ICS dose (mcg/day) | 1200.0 ± 339.4 | 864.4 ± 543.8 | 0.274 | 1200.0 ± 1131.4 | 678.0 ± 499.3 | 0.595 | 910.0 ± 656.8 | 545.6 ± 243.1 | 0.218 |
| Prednisolone equivalent OCS dose during last month (mg) | 70 | 462.2 ± 443.5 | 0.349 | N/A | 148.1 ± 98.4 | N/A | 318.8 ± 94.4 | 244.4 ± 229.6 | 0.411 |
| OCS maintenance, n (%) | 1 (50) | 4 (17.4) | 0.367 | 0 | 12 (37.5) | 0.536 | 4 (50) | 8 (42.1) | 1.000 |
| Annual exacerbation rate (/year) | |||||||||
| Ever experienced AE (previous 12 months), n (%) | 2 (100) | 16 (69.6) | 1.000 | 3 (100) | 18 (56.3) | 0.259 | 5 (62.5) | 12 (63.2) | 1.000 |
| All AE (/year) | 5.5 ± 0.7 | 4.0 ± 5.4 | 0.275 | 2.3 ± 1.5 | 3.1 ± 4.0 | 0.808 | 4.6 ± 5.3 | 4.2 ± 7.3 | 0.644 |
| SCS burst use, n (%) | 2 (100) | 11 (47.8) | 0.523 | 2 (66.7) | 14 (43.8) | 0.582 | 2 (25) | 9 (47.4) | 0.405 |
| ER visit (/year) | N/A | 1.8 ± 1.0 | N/A | 2 | 1.9 ± 1.5 | 0.647 | 1.3 ± 0.6 | 1.7 ± 1.2 | 1.000 |
| Unexpected outpatient visits (/year) | 3 | 3.0 ± 2.0 | 0.847 | 1 | 1.5 ± 0.7 | 0.590 | 4.0 ± 4.1 | 3.8 ± 5.5 | 0.668 |
| ACT scores (total 25) | 14.5 ± 9.2 | 15.5 ± 5.8 | 0.766 | 14.3 ± 2.1 | 14.8 ± 5.4 | 0.977 | 17.7 ± 3.4 | 15.6 ± 6.2 | 0.476 |
Data are presented as number (%) or mean ± standard deviation. P-value was calculated by the Wilcoxon rank sum test for continuous variables. Abbreviations: ACO, Asthma-COPD Overlap; BMI, body mass index; NERD, NSAID exacerbated respiratory disease; WBC, white blood cell; IgE, immunoglobulin E; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroids; OCS, oral corticosteroids; AE, acute exacerbation; SCS, systemic corticosteroids; ER, emergency room; ACT, Asthma Control Test.
Changes in lung function and other parameters in ACO and pure asthma after treatment with biologics for 6 months
We examined changes in FEV1 (mL and % predicted), FEV1/FVC ratio, ACT score, budesonide equivalent ICS dose, prednisolone equivalent oral corticosteroid (OCS) dose, FeNO, blood eosinophil count, sputum eosinophil, and the number of exacerbations before the start of biologic treatment and 6 months later. Both the ACO and asthma groups showed positive responses with no significant difference in FEV1 improvement (10.7 ± 17.2 vs 11.3 ± 12.9 %, P = 0.652), ACT score increase (3.3 ± 5.5 vs 5.4 ± 5.4, P = 0.290), reduction in ICS dose (−66.7 ± 460.0 vs −17.9 ± 236.2 mcg/day, P = 0.912), reduction in OCS maintenance dose (−117.5 ± 94.4 vs −115.1 ± 456.9 mg, P = 0.688), reduction in FeNO levels (−18.6 ± 24.7 vs. −14.7 ± 45.4 ppb, P = 0.415), reduction in sputum eosinophils (−3.4 ± 10.6 vs −14.5 ± 24.0 %, P = 0.065), and decrease in exacerbation frequency (−0.96 ± 2.47 vs −1.40 ± 2.63, P = 0.575). The only significant difference observed was a decrease in blood eosinophil levels (−36.5 ± 517.0 vs −363.2 ± 1294.6/μL, P = 0.013) (Table 4). No significant differences were observed when comparing the 2 groups for each biologic (Supplement Table 2).
Table 4.
The changes in treatment response parameters in the ACO and pure asthma group after 6 months of treatment with biologics.
| Variables | ACO (n = 13) | Pure asthma (n = 81) | P-value |
|---|---|---|---|
| ΔFEV1 (L) | 0.38 ± 0.67 (n = 13) | 0.32 ± 0.42 (n = 81) | 0.649 |
| ΔFEV1 (%) | 10.7 ± 17.2 (n = 13) | 11.3 ± 12.9 (n = 81) | 0.652 |
| ΔFEV1/FVC | 0.03 ± 0.06 (n = 13) | 0.03 ± 0.09 (n = 80) | 0.959 |
| ΔACT | 3.3 ± 5.5 (n = 13) | 5.4 ± 5.4 (n = 79) | 0.290 |
| ΔBudesonide equivalent ICS dose (mcg/day) | −66.7 ± 460.0 (n = 13) | −17.9 ± 236.2 (n = 75) | 0.912 |
| ΔPrednisolone equivalent OCS maintenance dose (mg) | −117.5 ± 94.4 (n = 4) | −115.1 ± 456.9 (n = 7) | 0.415 |
| ΔFeNO (ppb) | −18.6 ± 24.7 (n = 13) | −14.7 ± 45.4 (n = 77) | 0.688 |
| ΔBlood eosinophil (/μL) | −36.5 ± 517.0 (n = 13) | −363.2 ± 1294.6 (n = 78) | 0.013 |
| ΔSputum eosinophil (%) | −3.4 ± 10.6 (n = 5) | −14.5 ± 24.0 (n = 31) | 0.065 |
| ΔNumber of exacerbation | −0.96 ± 2.47 | −1.40 ± 2.63 | 0.575 |
The changes (Δ) were calculated by 6 M − baseline. P-value was calculated by the Wilcoxon rank sum test for continuous variables. Abbreviations: ACO, Asthma-COPD Overlap; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ACT, Asthma Control Test; ICS, inhaled corticosteroids; OCS, oral corticosteroids; FeNO, fractional exhaled nitric oxide.
Comparison of exacerbation risk between ACO and pure asthma
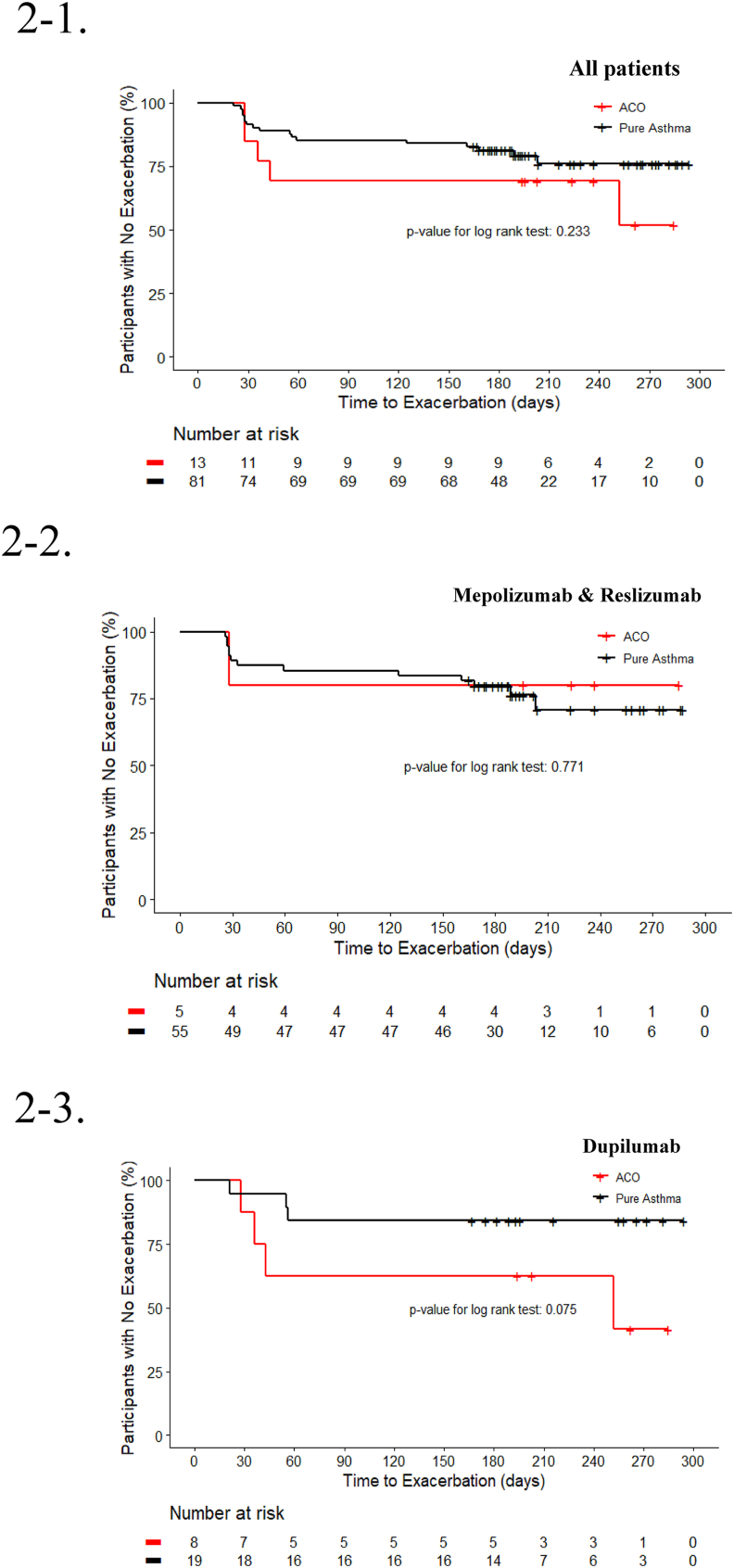
There was no significant difference in the risk of asthma exacerbation between the ACO and pure asthma groups during the six-to-nine-month follow-up period. This finding remained consistent across various adjusted models (Table 5). When grouping by anti-IL5/R vs. dupilumab, there was also no difference in the risk of exacerbation between the ACO and pure asthma groups for each medication group in the all adjusted models (Table 6). Additionally, the time-to-first asthma exacerbation (TFE) analysis using Kaplan-Meier curves did not reveal any significant differences between the ACO and pure asthma groups (Fig. 2).
Table 5.
Risk of asthma exacerbation in the ACO and pure asthma group (N = 94).
| Adjusted model | Reference |
All exacerbations |
SCS burst |
Unexpected outpatient visit |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pure asthma |
ACO |
p-value | ACO |
p-value | ACO |
p-value | ||||
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |||||
| Model 1: no adjustment | 1.0 | 2.4 | 0.7, 8.1 | 0.176 | 2.3 | 0.6, 8.7 | 0.210 | 2.8 | 0.6, 16.0 | 0.257 |
| Model 2: age, sex, BMI adjusted | 1.0 | 2.7 | 0.7, 11.0 | 0.168 | 2.2 | 0.5, 9.9 | 0.289 | 8.2 | 0.6, 111.3 | 0.113 |
| Model 3: age, sex, BMI + smoking pack-year | 1.0 | 2.1 | 0.5, 10.3 | 0.342 | 1.4 | 0.3, 7.4 | 0.689 | 7.6 | 0.4, 129.9 | 0.164 |
| Model 4: Model 3 + blood eosinophils | 1.0 | 2.2 | 0.5, 10.5 | 0.335 | 1.4 | 0.3, 7.4 | 0.689 | 10.8 | 0.5, 254.1 | 0.139 |
| Model 5: Model 3 + FeNO | 1.0 | 2.4 | 0.5, 11.9 | 0.303 | 1.8 | 0.3, 9.9 | 0.524 | 7.1 | 0.4, 131.1 | 0.188 |
| Model 6: Model 3 + blood eosinophils, FeNO | 1.0 | 2.3 | 0.5, 11.8 | 0.303 | 1.8 | 0.3, 9.9 | 0.524 | 7.3 | 0.4, 141.9 | 0.191 |
All adjusting variables were measured at baseline visit. Abbreviations: ACO, Asthma-COPD Overlap; OR, odds ratio; CI, confidence interval; SCS, systemic corticosteroid; BMI, body mass index; FeNO, fractional exhaled nitric oxide.
Table 6.
Risk of asthma exacerbation in ACO and pure asthma group according to biologics type (N = 87).
| Adjusted model | Reference |
Mepolizumab & Reslizumab (n = 60) |
Dupilumab (n = 27) |
||||
|---|---|---|---|---|---|---|---|
| Pure asthma |
ACO |
p-value | ACO |
p-value | |||
| OR | 95 % CI | OR | 95 % CI | ||||
| Model 1: no adjustment | 1.0 | 0.8 | 0.1, 7.9 | 0.854 | 5.3 | 0.8, 34.1 | 0.077 |
| Model 2: age, sex, BMI adjusted | 1.0 | 1.1 | 0.1, 13.1 | 0.919 | 3.5 | 0.5, 26.4 | 0.231 |
| Model 3: age, sex, BMI + smoking pack-year | 1.0 | 0.9 | 0.1, 13.6 | 0.924 | 1.6 | 0.1, 18.8 | 0.716 |
| Model 4: Model 3 + blood eosinophils | 1.0 | 0.9 | 0.1, 13.5 | 0.917 | 1.4 | 0.1, 17.1 | 0.806 |
| Model 5: Model 3 + FeNO | 1.0 | 1.1 | 0.1, 17.3 | 0.958 | 2.1 | 0.1, 33.6 | 0.615 |
| Model 6: Model 3 + blood eosinophils, FeNO | 1.0 | 1.1 | 0.1, 17.7 | 0.948 | 1.6 | 0.1, 27.7 | 0.765 |
| Model 7: Model 3 + blood eosinophils, FeNO, number of exacerbations at baseline | 1.0 | 0.9 | 0.1, 15.4 | 0.931 | 2.2 | 0.1, 50.1 | 0.634 |
All adjusting variables were measured at baseline visit. Abbreviations: ACO, Asthma-COPD Overlap; OR, odds ratio; CI, confidence interval; BMI, body mass index; FeNO, fractional exhaled nitric oxide.
Fig. 2.
Kaplan–Meier curves for the time-to-first asthma exacerbation, according to each group.
2-1. ACO vs. Pure asthma
2-2. ACO vs. Pure asthma among mepolizumab and reslizumab users
2-3. ACO vs. Pure asthma among dupilumab users
Abbreviations: ACO, asthma-chronic obstructive pulmonary disease overlap.
Discussion
In this study, we compared treatment responses to biologics for 6 months between patients with ACO and pure asthma, with the selection of biologics determined according to the treating physician's discretion based on the guidelines and the FDA approval criteria for asthma in a real-world practice. Both the ACO and pure asthma groups exhibited positive treatment responses, including improvements in FEV1, ACT scores; reductions in ICS/OCS doses, FeNO levels, blood and sputum eosinophil counts, and exacerbation frequency. The only significant difference observed was a further reduction in blood eosinophil count in the pure asthma group compared to the ACO group. Furthermore, when adjusting for multiple confounding factors, there was no significant difference in the risk of exacerbation between the ACO and pure asthma groups treated with biologics. Additionally, the analysis of time-to-first asthma exacerbation using the Kaplan-Meier method revealed no significant difference between the ACO and pure asthma groups. Therefore, our study demonstrates that patients with ACO exhibit similar treatment response patterns to patients with pure asthma undergoing biologic therapy, as indicated by various treatment outcome measures.
The lack of a standardized definition or diagnostic criteria for ACO is problematic. ACO is generally referred to as a condition of persistent airflow limitation with clinical features that are consistent with both asthma and COPD.13,14 Various definitions of ACO have been proposed, including conceptual definitions, combinations of major and minor criteria, and definitions from the perspective of COPD or asthma, respectively.1,7,15 Therefore, there are inevitable obstacles when conducting research or extrapolating conclusions from previous ACO study results, as different studies use different ACO definitions based on different patient populations. Variable ACO prevalence rates are reported: 0.9–11 % in the general population, 11.1–61 % in patients with asthma, and 4.2–66 % in patients with COPD.16 A meta-analysis of 19 asthma studies reported a pooled prevalence of ACO of 26.5 % (CI 19.5–33.6 %).17 The use of different diagnostic criteria can result in significantly different prevalence rates. The prevalence of ACO was 3.0 % in 1067 patients with COPD using the ATS definition, 12.9 % using GINA/GOLD criteria, and 20.7 % based on the Spanish criteria.18,19
ACO is characterized by more severe symptoms, a lower quality of life, and more frequent exacerbations than asthma or COPD alone, resulting in higher medical costs.20 However, despite the burden of ACO, there are limited treatment options available, as there is a lack of a standard definition for ACO, and there are no effective biomarkers or standard treatments.
In the era of precision medicine and the increasing use of biologics for severe asthma, there is still limited research on ACO. Currently, the biomarkers used in asthma, such as blood eosinophils, serum IgE, aeroallergen sensitization, and FeNO, are also used in ACO.21,22 These biomarkers may help differentiate ACO among patients with COPD or choose biologics for uncontrolled ACO.21,22 However, patients with uncontrolled ACO are approximately 3 times less likely to receive biologic therapy than those with asthma.23 Therefore, biologic treatment in ACO is an area of research need and interest.
A post hoc analysis of the PROSPERO study to evaluate omalizumab effectiveness in ACO, which included 737 patients with ACO treated with omalizumab for 48 weeks, showed similar improvement in asthma symptom scores and exacerbation rates in ACO and asthma groups.24 This study used the same definition of ACO as in our study, which is post-BD FEV1/FVC <0.70 and at least 10 pack-years of smoking.24 The Australian Xolair Registry reported symptom control and quality of life improvement in 177 patients with ACO treated with omalizumab, while no significant improvement in lung function compared to asthma alone was observed.25 Another study on the effect of omalizumab in patients with asthma with or without fixed airway obstruction found that both groups showed improvement in exacerbation rates, but only the non-fixed airway obstruction group showed slight improvement in FEV1.26
A study using Medicare data reported that mepolizumab treatment reduced exacerbation rates and OCS use over a 12-month follow-up period in patients with both severe asthma and COPD diagnosis.27 Another single-center, retrospective observational study in elderly patients, including 9 patients with asthma and 11 patients with ACO, showed favorable efficacy of mepolizumab in reducing blood eosinophil levels, OCS use, and exacerbation rates in both groups.28 The Australian Mepolizumab Registry also showed that mepolizumab significantly reduced exacerbations and improved symptom scores and lung function even in patients with ACO as a comorbidity among patients with severe eosinophilic asthma.29 Mepolizumab has also been shown to be effective in reducing exacerbations in eosinophilic COPD compared to placebo.30
We found no studies on the use of benralizumab in ACO. A study showed that benralizumab had a less clear effect on reducing exacerbations in patients with moderate to severe COPD with elevated blood eosinophils.31 In a phase 3 trial for eosinophilic COPD, despite a decrease in sputum and blood eosinophils, benralizumab did not significantly reduce exacerbations.31 Another study showed that the greatest effect of benralizumab was observed in patients with COPD with blood eosinophil levels ≥220 cells/μL, particularly those with severe airflow obstruction of FEV1 <40 % and frequent exacerbations.32 A phase 2 study is currently underway to evaluate the use of benralizumab in both COPD and asthma (ClinicalTrials.gov Identifier: NCT04098718). The recent clinical trial investigating the use of dupilumab in T2 high (elevated blood eosinophil counts) moderate-to-severe COPD showed better treatment response than placebo.33 No studies on the use of reslizumab in ACO or COPD were found.
At present, we cannot draw a definitive conclusion regarding the efficacy of asthma biologics in ACO, and more research is needed to determine their role in the management of ACO. Many of the previous studies were clinical trials, and ACO has been excluded from almost all clinical trials of asthma or COPD medications, making real-world research even more important. Our study is 1 of the first real-world studies to investigate which biologics are prescribed for ACO by physicians in a severe asthma registry, as well as treatment effectiveness and prognosis compared to pure asthma. We selected ACO from the severe asthma population using the ATS definition, which includes a post-BD FEV/FVC <0.7, age ≥40 years, and a smoking history of at least 10 pack-years.12
We identified a single study conducted in a similar setting, utilizing data from the GEMA-DATA register in Spain, which included 297 patients with asthma and 24 patients with ACO.34 Patients with ACO exhibited lower FEV1 values, but there were no significant differences in other T2 inflammatory biomarkers between the 2 groups.34 Omalizumab was the most commonly used biologic agent, followed by mepolizumab, benralizumab, and reslizumab.34 After a 12-month follow-up, the ACO group had a significantly higher proportion of patients experiencing exacerbations than that in the asthma group, and the percentage of controlled patients was significantly lower in the ACO group (16.5 % in ACO vs 39.7 % in asthma).34 The authors only reported proportions (%) of exacerbated or uncontrolled patients without any adjustments, whereas our study provided fully adjusted odds ratios (ORs) for exacerbation and time-to-first exacerbation.
Our study investigated the treatment responses of biologics in patients with ACO and asthma using real-world cohort data. Our findings showed no significant differences in the improvement of treatment indicators between the ACO and asthma groups following treatment with biologics. Furthermore, even after adjusting for multiple factors, there were no significant differences in the risk of exacerbation. Considering these results, this study provides evidence that treatment with biologics in patients with ACO can result in benefits similar to those observed in patients with asthma.
This study has the following limitations. The most important limitation is the relatively small sample size. If more ACOs using biologics had been included, more convincing and reliable results could have been obtained. However, considering the obstacles of using biologics in real-world settings, it is challenging to recruit a large number of participants for this type of study. Additionally, omalizumab and benralizumab were not used in the ACO group, and this need to be further studied. Another limitation is the relatively short follow-up period of 6 to 9 months. Future studies with longer treatment durations and extended follow-up periods are necessary. Lastly, the results of this study may have limited applicability to populations other than the Korean population.
In conclusion, we consider that the efficacy of biologics in patients with ACO is comparable to that in patients with pure asthma. Therefore, there is no need to refrain from using biologics in ACO patients; instead, therapeutic options can be considered for treatment based on general criteria for using biologics.
Abbreviations
ACT, asthma control test; ACO, asthma-COPD overlap; BD, bronchodilator; COPD, chronic obstructive pulmonary disease; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroid; IL, interleukin; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; OR, odds ratio; SCS, systemic corticosteroid; T2, type 2; TFE, time-to-first asthma exacerbation
Funding
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government Ministry of Science and ICT (MSIT) (2019M3E5D3073365).
Availability of data and materials
The data-sets analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
JS, MH, and TK designed the study; JS, JK, SP, SK, BK, YN, MY, MK, SK, BL, TL, SK, SP, YC, CP, JJ, HP, JK, JC, JM, MK, and TK collected the data; JS and HK analyzed the data; JS, HK, MH, and TK interpreted the data; JS drafted the manuscript; MH, TK, IA, KFC revised the manuscript critically for important intellectual content; all authors critically reviewed and approved the manuscript as submitted and agreed to be accountable for all aspects of the work.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Ewha Womans University Medical Center (SEUMC 2020-04-014-023) and Asan Medical Center (2019–1676). Informed written consent was obtained from all participants.
Authors’ consent for publication
All authors agreed to the publication of this work in the World Allergy Organization Journal.
Submission declaration
The authors confirm that this manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgement
We thank all the members participating in PRISM research group for their support and discussion of the study.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2023.100848.
Contributor Information
Min-Hye Kim, Email: mineyang81@ewha.ac.kr.
Tae-Bum Kim, Email: tbkim@amc.seoul.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Barczyk A., Maskey-Warzęchowska M., Górska K., et al. Asthma-COPD overlap-A discordance between patient populations defined by different diagnostic criteria. J Allergy Clin Immunol Pract. 2019;7(7):2326–2336.e2325. doi: 10.1016/j.jaip.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Leung C., Sin D.D. Asthma-COPD overlap: what are the important questions? Chest. 2022;161(2):330–344. doi: 10.1016/j.chest.2021.09.036. [DOI] [PubMed] [Google Scholar]
- 3.Toledo-Pons N., van Boven J.F.M., Román-Rodríguez M., et al. ACO: time to move from the description of different phenotypes to the treatable traits. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fouka E., Papaioannou A.I., Hillas G., Steiropoulos P. Asthma-COPD overlap syndrome: recent insights and unanswered questions. J Personalized Med. 2022;12(5) doi: 10.3390/jpm12050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Strategy for Asthma Management and Prevention . 2014. Global Initiative for Asthma. [Google Scholar]
- 6.Milne S., Mannino D., Sin D.D. Asthma-COPD overlap and chronic airflow obstruction: definitions, management, and unanswered questions. J Allergy Clin Immunol Pract. 2020;8(2):483–495. doi: 10.1016/j.jaip.2019.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Gibson P.G., McDonald V.M., Granchelli A., Olin J.T. Asthma and comorbid conditions-pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868–3875. doi: 10.1016/j.jaip.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Kiljander T., Helin T., Venho K., Jaakkola A., Lehtimäki L. Prevalence of asthma-COPD overlap syndrome among primary care asthmatics with a smoking history: a cross-sectional study. NPJ primary care respiratory medicine. 2015;25 doi: 10.1038/npjpcrm.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J.H., Dixey P., Bhavsar P., et al. Precision Medicine Intervention in Severe Asthma (PRISM) study: molecular phenotyping of patients with severe asthma and response to biologics. ERJ open research. 2023;9(2) doi: 10.1183/23120541.00485-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 11.Gif Asthma. Global Initiative for Asthma; 2023. Global Strategy for Asthma Management and Prevention. [Google Scholar]
- 12.Sin D.D., Miravitlles M., Mannino D.M., et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48(3):664–673. doi: 10.1183/13993003.00436-2016. [DOI] [PubMed] [Google Scholar]
- 13.Bumbacea D., Campbell D., Nguyen L., et al. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur Respir J. 2004;24(1):122–128. doi: 10.1183/09031936.04.00077803. [DOI] [PubMed] [Google Scholar]
- 14.Kaminska M., Foley S., Maghni K., et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol. 2009;124(1):45–51. doi: 10.1016/j.jaci.2009.03.049. e41-44. [DOI] [PubMed] [Google Scholar]
- 15.Godbout K., Gibson P.G. Defining asthma-chronic obstructive pulmonary disease overlap. Immunol Allergy Clin. 2022;42(3):507–519. doi: 10.1016/j.iac.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Uchida A., Sakaue K., Inoue H. Epidemiology of asthma-chronic obstructive pulmonary disease overlap (ACO) Allergol Int : official journal of the Japanese Society of Allergology. 2018;67(2):165–171. doi: 10.1016/j.alit.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Hosseini M., Almasi-Hashiani A., Sepidarkish M., Maroufizadeh S. Global prevalence of asthma-COPD overlap (ACO) in the general population: a systematic review and meta-analysis. Respir Res. 2019;20(1):229. doi: 10.1186/s12931-019-1198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jo Y.S., Hwang Y.I., Yoo K.H., et al. Effect of inhaled corticosteroids on exacerbation of asthma-COPD overlap according to different diagnostic criteria. J Allergy Clin Immunol Pract. 2020;8(5):1625–1633.e1626. doi: 10.1016/j.jaip.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Soler-Cataluña J.J., Novella L., Soler C., et al. Clinical characteristics and risk of exacerbations associated with different diagnostic criteria of asthma-COPD overlap. Arch Bronconeumol. 2020;56(5):282–290. doi: 10.1016/j.arbres.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Alshabanat A., Zafari Z., Albanyan O., Dairi M., FitzGerald J.M. Asthma and COPD overlap syndrome (acos): a systematic review and meta analysis. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama Y., Ohnishi H., Ogasawara F., Oyama K., Kubota T., Yokoyama A. Clinical utility of fractional exhaled nitric oxide and blood eosinophils counts in the diagnosis of asthma-COPD overlap. Int J Chronic Obstr Pulm Dis. 2018;13:2525–2532. doi: 10.2147/COPD.S167600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M., Yang T., He R., et al. The value of inflammatory biomarkers in differentiating asthma-COPD overlap from COPD. Int J Chronic Obstr Pulm Dis. 2020;15:3025–3037. doi: 10.2147/COPD.S273422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddel H.K., Vestbo J., Agustí A., et al. Heterogeneity within and between physician-diagnosed asthma and/or COPD: NOVELTY cohort. Eur Respir J. 2021;58(3) doi: 10.1183/13993003.03927-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanania N.A., Chipps B.E., Griffin N.M., Yoo B., Iqbal A., Casale T.B. Omalizumab effectiveness in asthma-COPD overlap: post hoc analysis of PROSPERO. J Allergy Clin Immunol. 2019;143(4):1629–1633.e1622. doi: 10.1016/j.jaci.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Maltby S., Gibson P.G., Powell H., McDonald V.M. Omalizumab treatment response in a population with severe allergic asthma and overlapping COPD. Chest. 2017;151(1):78–89. doi: 10.1016/j.chest.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 26.Hanania N.A., Fortis S., Haselkorn T., et al. Omalizumab in asthma with fixed airway obstruction: post hoc analysis of EXTRA. J Allergy Clin Immunol Pract. 2022;10(1):222–228. doi: 10.1016/j.jaip.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Casale T., Molfino N.A., Silver J., et al. Real-world effectiveness of mepolizumab in patients with severe asthma and associated comorbidities. Ann Allergy Asthma Immunol. 2021;127(3):354–362.e352. doi: 10.1016/j.anai.2021.05.021. official publication of the American College of Allergy, Asthma, & Immunology. [DOI] [PubMed] [Google Scholar]
- 28.Isoyama S., Ishikawa N., Hamai K., et al. Efficacy of mepolizumab in elderly patients with severe asthma and overlapping COPD in real-world settings: a retrospective observational study. Respir Investig. 2021;59(4):478–486. doi: 10.1016/j.resinv.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Kritikos V., Harvey E.S., Stevens S., et al. Comorbidities modify the phenotype but not the treatment effectiveness to mepolizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2023;11(3):885–895.e813. doi: 10.1016/j.jaip.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Pavord I.D., Chanez P., Criner G.J., et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 31.Criner G.J., Celli B.R., Brightling C.E., et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med. 2019;381(11):1023–1034. doi: 10.1056/NEJMoa1905248. [DOI] [PubMed] [Google Scholar]
- 32.Criner G.J., Celli B.R., Singh D., et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: analyses of GALATHEA and TERRANOVA studies. Lancet Respir Med. 2020;8(2):158–170. doi: 10.1016/S2213-2600(19)30338-8. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt S.P., Rabe K.F., Hanania N.A., et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med. 2023;389(3):205–214. doi: 10.1056/NEJMoa2303951. [DOI] [PubMed] [Google Scholar]
- 34.Pérez de Llano L., Dacal Rivas D., Marina Malanda N., et al. The response to biologics is better in patients with severe asthma than in patients with asthma-COPD overlap syndrome. J Asthma Allergy. 2022;15:363–369. doi: 10.2147/JAA.S338467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data-sets analyzed during the current study are available from the corresponding author on reasonable request.