Abstract
Mercury (Hg) is a widespread element and persistent pollutant, harmful to fish, wildlife, and humans in its organic, methylated form. The risk of Hg contamination is driven by factors that regulate Hg loading, methylation, bioaccumulation, and biomagnification. In remote locations, with infrequent access and limited data, understanding the relative importance of these factors can pose a challenge. Here, we assessed Hg concentrations in an apex predator fish species, lake trout (Salvelinus namaycush), collected from 14 lakes spanning two National Parks in southwest Alaska, U.S.A. We then examined factors associated with the variation in fish Hg concentrations using a Bayesian hierarchical model. We found that total Hg concentrations in water were consistently low among lakes (0.11–0.50 ng L−1). Conversely, total Hg concentrations in lake trout spanned a thirty-fold range (101–3046 ng g−1 dry weight), with median values at 7 lakes exceeding Alaska’s human consumption threshold. Model results showed that fish age and, to a lesser extent, body condition best explained variation in Hg concentration among fish within a lake, with Hg elevated in older, thinner lake trout. Other factors, including plankton methyl Hg content, fish species richness, volcano proximity, and glacier loss, best explained variation in lake trout Hg concentration among lakes. Collectively, these results provide evidence that multiple, hierarchically nested factors control fish Hg levels in these lakes.
Keywords: Lake trout, Sockeye salmon, Volcano proximity, Glacier loss, Bayesian model
1. Introduction
National Parks in the state of Alaska are among the most remote and ostensibly pristine locations in the United States, containing intact habitat for iconic species and offering valuable ecosystem services (Reynolds et al., 2018; Vynne et al., 2021). However, previous studies found high concentrations of mercury (Hg) in fish within park boundaries, posing health risks for nearby Indigenous communities that rely on subsistence fishing. For example, fish Hg concentrations from lakes in five Alaskan parks were among the highest of the 23 western parks measured and exceeded both ecological and human health impairment benchmarks (Eagles-Smith et al., 2014; Landers et al., 2008). This raises the question: what is driving the high Hg concentrations observed in these lake fish?
Lakes integrate water, energy, nutrients, sediments, and pollutants from their surrounding watersheds and airsheds (Schindler, 2009). Therefore, lake fish often serve as biological indicators of persistent pollutants, like Hg (Gutiérrez and Agudelo, 2020; Łuczyńska et al., 2018). However, understanding pollution in fish from remote lakes is frequently challenged by a lack of basic ecological data. In the case of Hg, the challenge is exacerbated by the complexity of the Hg cycle, which includes natural and anthropogenic sources, multiple chemical species, and both newly released and remobilized legacy pools that may have differing bioavailability.
The list of potential factors regulating Hg concentrations in lake food webs is extensive, and no single conceptual model is universally applicable (Branfireun et al., 2020). Nevertheless, most factors can be grouped into four broad categories: (1) factors influencing Hg loading; (2) factors regulating methylmercury production via methylation; (3) factors controlling methylmercury bioaccumulation in organisms, and (4) factors governing methylmercury biomagnification in food webs (Wiener et al., 2003).
Distant anthropogenic emissions, followed by atmospheric transport and deposition, are key sources of Hg loading in many remote lakes (Durnford et al., 2010; Streets et al., 2011). However, three additional sources in southwest Alaska—our region of interest—warrant consideration. First, this region contains numerous volcanoes (Miller et al., 1998), and both actively erupting and passively degassing volcanoes are a known source of geogenic Hg to the atmosphere (Bagnato et al., 2011). Second, this region is undergoing rapid glacial melt due to climate change (Roberts-Pierel et al., 2022), and glaciers may contain latent reservoirs of atmospherically deposited Hg (Schuster et al., 2002). Third, this region supports the world’s largest fishery for sockeye salmon (Oncorhynchus nerka; Schindler et al., 2010), and the annual return of spawning sockeye salmon imports not only marine-derived nutrients but also pollutants like Hg to lake food webs (Zhang et al., 2001).
Regardless of the sources of Hg loading, methylation of inorganic Hg to methylmercury (MeHg) by native microbial communities increases the bioavailability and toxicity of Hg and, therefore, the exposure and risk to fish and other organisms, including humans (Wiener et al., 2003). Factors regulating the net production of MeHg are well studied. Results highlight two main types of factors: those that affect the activity of microbial communities capable of methylation, and those that affect the amount of inorganic Hg available to the microbes (Hsu-Kim et al., 2013). For example, the amount of available inorganic Hg is influenced by basin characteristics (e.g., type of land use and abundance of wetlands (Hurley et al., 1995; St. Louis et al., 1996)) and water quality conditions (e.g., pH, sulfate, and dissolved organic matter concentration and composition (Miskimmin et al., 1992; Wiener et al., 2006; Chiasson-Gould et al., 2014)). Dissolved organic matter concentration and composition also regulate the activity of microbes involved in MeHg production (Bravo et al., 2017), as does water temperature (Bodaly et al., 1993).
Following production, MeHg bioaccumulates at lower trophic levels (e.g., primary producers) then biomagnifies to higher trophic levels (e. g., fish) within aquatic food webs. Various factors can affect MeHg bioaccumulation in lake fish, including age, size, diet, growth rate, and trophic position (Wiener et al., 2003). Other factors can affect MeHg biomagnification between organisms, such as trophic structure and MeHg concentration at the base of the food web. MeHg at the base of the food web is informative because the bioconcentration between water and primary producers is orders of magnitude greater than the biomagnification between successive trophic levels (Ogorek et al., 2021). Therefore, MeHg concentrations in primary producers are often strongly related to concentrations in piscivorous fish (Pickhardt et al., 2002; Sandheinrich and Weiner, 2011).
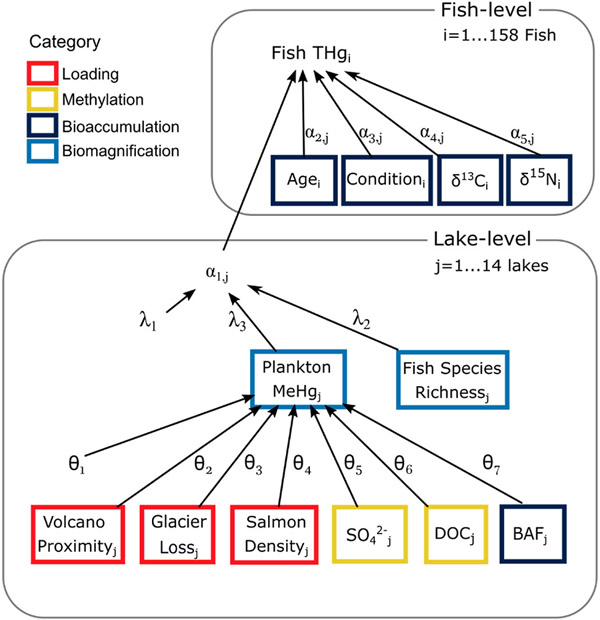
Our conceptual model, with four broad categories of Hg-regulating factors, can be parsed by the scale at which the factors function: either at the level of an individual fish or at the level of the lake or basin where fish reside (henceforth, fish-level and lake-level, respectively). Factors affecting Hg bioaccumulation operate at the fish level, while factors influencing Hg loading, methylation, and biomagnification operate at the lake level. As such, the categories in this conceptual model represent hierarchically nested processes.
In this study, we assessed Hg concentrations of resident fish from two National Parks in southwest Alaska, focusing on lake trout (Salvelinus namaycush), a long-lived, slow-growing, top-predator species in many southwest Alaska lakes. Our goals were (1) to evaluate the magnitude of Hg contamination in fish within a subset of lakes in these parks, and (2) to determine the relative importance of factors pertaining to loading, methylation, bioaccumulation, and biomagnification in controlling variability of fish Hg concentrations among lakes. Further, because the factors are hierarchically nested, we introduce a modeling framework that enables an examination of both fish-level and lake-level factors concurrently.
2. Materials and methods
2.1. Study sites
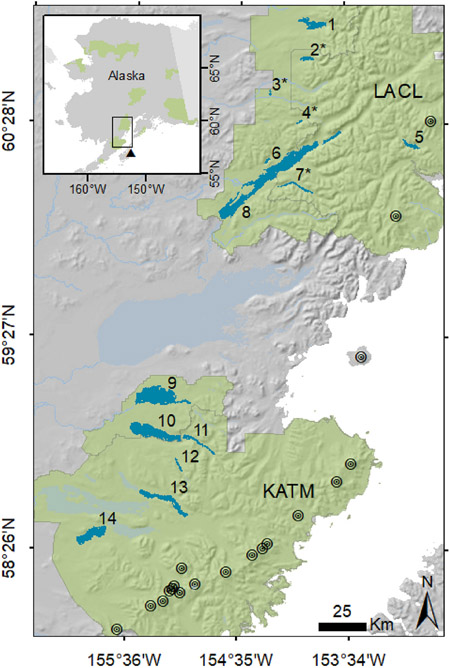
We selected study sites in Katmai National Park and Preserve (KATM) and Lake Clark National Park and Preserve (LACL), which are located at the northern end of the Alaska Peninsula. Together, these parks encompass 32,943 km2 (Fig. 1), comprising almost 10% of National Park Service holdings nationwide. Their landscapes have been shaped by dynamic geologic processes, particularly glaciation and volcanism. Both parks were glaciated during the Pleistocene but have experienced widespread glacial retreat since the Holocene (Hamilton et al., 1986), with rates of retreat accelerating due to climate change (Loso et al., 2014). The parks also sit along the Aleutian volcanic arc; 18 volcanoes exist within KATM and LACL boundaries, all but 2 occurring in KATM (Table A1).
Fig. 1.
Map of 14 study lakes sampled in 2 Alaska park units: Lake Clark (LACL) and Katmai (KATM) National Parks and Preserves. Numbers correspond to lake names (1 = Telaquana, 2 = Turquoise, 3 = Snipe, 4 = Lachbuna, 5 = Crescent, 6 = Kijik, 7 = Kontrashibuna, 8 = Clark, 9 = Kukaklek, 10 = Non-vianuk, 11 = Kulik, 12 = Hammersly, 13 = Grosvenor, 14 = Brooks) and asterisks indicate lakes without sockeye salmon, due to barriers. Park unit boundaries are shaded green, and volcanoes are depicted by black circles. The location of the Mercury Deposition Network station at Kodiak Island (AK98) is denoted by a black triangle in the inset map.
We selected 14 lakes as study sites in KATM and LACL according to three criteria: presence of lake trout; inclusion in ongoing monitoring efforts; and gradients in potential drivers such as glacier cover and salmon density. While these lakes vary in area and depth (Table A2), all are characterized as well-oxygenated, dimictic or cold polymictic, and oligotrophic, with low concentrations of nutrients and major ions, and low to moderate acid buffering capacity (Brabets and Ourso, 2006a, 2006b; Chamberlain, 1989; LaPerriere, 1997; Wilkens, 2002). Where lake trout and sockeye salmon coexist in these lakes, lake trout are known to eat sockeye salmon eggs and fry (Hartman and Burgner, 1972; Russell, 1980).
Atmospheric deposition of Hg at these lakes is unknown. However, data for years 2007–2015 from a nearby Mercury Deposition Network station (AK98) indicate that cumulative precipitation is higher than at four other stations in Alaska (2249 mm yr−1 vs. 363–1642 mm yr−1), while Hg concentration in precipitation is generally lower (2.17 ng L−1 vs. 1.89–6.15 ng L−1). Of the stations in Alaska, AK98 consistently recorded the highest annual Hg deposition loads (4.80 μg m−2 vs. 2.11–4.52 μg m−2), however these values ranked in the lowest 5% of stations nationwide (Pearson et al., 2019).
2.2. Field and Laboratory Methods
To quantify factors known to influence Hg loading, methylation, bioaccumulation, and biomagnification, we collected water, plankton, and fish in the field, and then analyzed the resulting samples in the lab for Hg and other constituents. Those constituents ranged from dissolved organic carbon (DOC) and sulfate () in water to carbon and nitrogen stable isotope ratios (δ13C and δ15N) in fish. Details regarding methods are included in Appendix A.
2.2.1. Water
We determined vertical patterns in water chemistry near each lake’s midpoint during the summer of 2016, using a YSI EXO2 multiparameter sonde that recorded water depth, pH, temperature, conductance, dissolved oxygen, and turbidity from the lake surface to the bottom or 50 m depth (whichever was shallower). Using this information, water samples were collected from three depths per site (above, within, and below the thermocline) via a Niskin sampler and a 2-L bottle. Because filter-passing inorganic Hg is the aqueous species primarily used for MeHg production (Hsu-Kim et al., 2013), water samples were filtered and stored for later analysis. Total Hg (THg), MeHg, and DOC concentrations were analyzed at the United States Geological Survey Mercury Research Laboratory (USGS-MRL) using modified versions of USEPA Methods 1631, 1630, and 9060a (USEPA, 1998a, 2002; 2004). Sulfate concentrations were analyzed at the University of Wisconsin, Madison using USEPA Method 300 (USEPA, 1997). All analyses passed established lab quality control criteria.
2.2.2. Plankton
We collected bulk plankton from the top 20 m of the water column near each lake’s midpoint using a 1-m diameter, 63-μm mesh net during the summer of 2016. Due to funding and capacity constraints, we did not assess plankton species composition. Instead, the collected plankton were sieved with size-sequential Nitex screens (500, 243, 118, and 63 μm) and frozen. Frozen plankton captured on the screens were transported to the USGS-MRL, lyophilized, and weighed. Plankton were then removed from the screens and their MeHg concentrations were determined using atomic fluorescence spectroscopy. Analyses of plankton met USGS-MRL quality control criteria.
Plankton MeHg concentrations reported here generally pertained to a single bulk sample per lake, with each size fraction (>500, 243–500, 118–243, and 63–118 μm) normalized relative to its mass contribution to the bulk sample. However, plankton MeHg concentrations used to report lake-specific bioaccumulation factors (BAF) differed, as:
where included concentrations for the smallest size fraction (63–118 μm) and included averaged concentrations from the epilimnion and thermocline.
2.2.3. Fish
We collected a total of 158 adult lake trout (n = 10–27 per lake) via angling or gillnetting during summer months (June–September) of 2011–2016. Given this time span, a temporal disconnect exists between fish collections (2011–2016) and water and plankton collections (2016) in some lakes which, in turn, may impact the comparability of constituents sampled in different years. Seasonal and inter-annual variability exists for some constituents but, for the purposes of this study, we assumed the measured values are best estimates for the entire study window.
Upon collection, fish were euthanized, and total length, weight, and sex were determined. Length (, in mm), weight (, in g), and a scaling factor of 105 () were later used to calculate fish body condition ():
as described by Ricker (1975). Sagittal otoliths were removed for age determination using standard sectioning methods (Campana et al., 2008), and axial muscle tissue samples were removed for constituent analysis. Prior to analysis, samples were lyophilized, homogenized, and weighed for moisture content.
We analyzed THg concentrations in the muscle samples as a proxy for MeHg, because most Hg in the muscle of top predator fish is MeHg (Bloom, 1992). THg analysis was conducted at the USGS-MRL or USGS Contaminant Ecology Research Lab using USEPA Method 7473 (USEPA, 1998b). As with plankton, analyses of fish met established quality control criteria. We also analyzed the muscle samples for δ13C and δ15N, which serve as indicators of foraging habitat and trophic position, respectively (Perga and Gerdeaux, 2005). In freshwater food webs containing anadromous salmon, δ15N values of resident fish can also track marine-derived nitrogen (Mathisen et al., 1988). Analysis of δ13C and δ15N was conducted at the University of California Davis Stable Isotope Facility, and results met lab quality control standards. To account for δ13C fractionation associated with lipid formation in fish, mathematical corrections approximated from sample C:N were performed on fish δ13C and labeled as δ13Clipid-free (Hoffman et al., 2015).
2.3. Other datasets
In addition to the samples collected and analyzed for this study, we quantified several factors from pre-existing data. For example, volcano proximity and glacier cover were derived from remote sensing data using GIS. Salmon density and fish species richness were derived from monitoring data and inventory reports using other methods. Select methods are summarized below; for details, see Appendix A.
We quantified volcano proximity for each lake as a unitless index, similar to that developed by Gustafson and Parker (1992). The index used the distance () between all volcano () and lake () pairs, filtered to include only distances <100 km, then summed by lake:
so higher proximity index values signified more volcanoes nearby. We determined glacier cover for each lake basin using shapefiles of glacial area during current (2000’s) and historical (1950’s) time periods (Arendt and Rich, 2013). Glacier loss was calculated as the difference between current and historical glacier cover in a basin.
We quantified salmon density for each lake as the mean annual escapement of sockeye salmon, divided by the lake perimeter (NPS, 2006). Time series of escapement, defined as the number of salmon that escape commercial harvest to spawn, included various methods for counting salmon (aerial, tower, weir, and sonar counts), and were limited to years on record from 1987 to 2016, the earliest birth year and latest death year of lake trout in this study. Escapements included salmon spawning within each lake’s watershed, not solely within each lake. We tallied fish species richness for each lake by summing the number of species identified in fish inventory reports (Jones et al., 2005; Russell, 1980), where available. Where reports were unavailable or incomplete, local knowledge from biologists and fishing guides was used as a supplement. This tally was intended as a proxy for trophic structure or complexity, given reported correlations between fish species richness and food web length (Vander Zanden et al., 1999).
2.4. Model methods
Our analyses of water, plankton, and fish samples, and the summaries of pre-existing datasets, produced many potential predictors with substantial redundancy. We reduced this list to 12 factors selected as covariates: 4 at the fish level and 8 at the lake level (Table 1), none with a variance inflation factor greater than 4 (Neter et al., 1990). The number of fish-level covariates was reduced by omitting those with many undetermined values or no clear link to a particular process. To reduce the number of lake-level covariates, we performed separate principal component analyses (PCA) for each of three processes: loading, methylation, and biomagnification. Using the PCA biplots, we then selected non-redundant covariates that best explained the two main axes, based mainly on PCA loadings. For additional details, see Appendix A.
Table 1.
Fish-level and lake-level factors included in the Bayesian hierarchical model.factors are listed by process-based category, expected effect on fish mercury concentration (positive, negative), and data source.
| Level a | Category | Factor | Units | Effect | Source |
|---|---|---|---|---|---|
| Fish | Bioaccumulation | Age | years | + | b |
| Fish | Bioaccumulation | Body condition | g·mm−3 | − | b |
| Fish | Bioaccumulation | δ13Clipid-free | ‰ | + | b |
| Fish | Bioaccumulation | δ15N | ‰ | + | b |
| Lake | Bioaccumulation | Bioaccumulation factor | log (L·kg−1) | + | b |
| Lake | Biomagnification | Plankton MeHg concentration | ng·g−1 (dw) | + | b |
| Lake | Biomagnification | Fish species richness | fish species | + | c |
| Lake | Methylation d | Water DOC concentration | mg·L−1 | + | b |
| Lake | Methylation | Water concentration | mg·L−1 | + | b |
| Lake | Loading | Volcano proximity index | unitless | − | e |
| Lake | Loading | Glacier loss | % | + | f |
| Lake | Loading | Salmon density | salmon·m−1 | + | g |
Sample sizes of fish-level and lake-level data are n = 158 lake trout and n = 14 lakes, respectively.
This study and Lepak et al., 2022a.
DOC also mediates MeHg bioaccumulation (French et al., 2014) but is limited to a single category for simplicity.
ADFG, 2021a, 2021b; Clark (2005); additional data from Steve Morstad (Alaska Department of Fish and Game, 907-246-3341) and Dan Young (Lake Clark National Park and Preserve, 907-781-3018).
To assess the relative importance of factors in controlling variability of fish Hg concentrations, we constructed a Bayesian hierarchical mixed-effects model that matched the structure of our conceptual model (Fig. 2) and data collection, while also accounting for imbalanced sample sizes. The model hierarchy captured both fish-level and lake-level influences on Hg, and reflected our understanding that loading and methylation effects should be evident in plankton MeHg concentrations. We modeled total Hg concentrations in individual fish as a lognormal variable predicted by a linear function of fish-level covariates that captured an individual’s predisposition to accumulate Hg. We modeled a lake-varying slope random effect for each fish-level covariate, and a lake-varying intercept random effect to account for among-lake variation in available Hg. The lake-varying intercept was, in turn, modeled as a function of fish species richness and plankton MeHg concentration. Plankton MeHg concentration was modeled as a function of lake-level variables influencing loading and methylation.
Fig. 2.
Schematic diagram of the Bayesian hierarchical model used to evaluate fish-level and lake-level factors potentially affecting observed total mercury (THg) concentrations in lake trout. Greek symbols represent estimated regression parameters (α, λ, θ). Colored boxes represent observed quantities, divided into four process-based categories (loading, methylation, bioaccumulation, biomagnification)
We used indicator variable selection (IVS) within our model to identify lake-level covariates that maximized model fit (Cunningham et al., 2018; Kuo and Mallick, 1998). For each covariate, IVS generated a variable inclusion probability (VIP), which ranged from 0 to 1 and was used to assess covariate strength. We fit our model with JAGS (Plummer, 2017), via the JagsUI package (Kellner and Meredith, 2021) in R Statistical Software (R Core Team, 2019). We reported posterior medians and 95% credible intervals (CrI) for a 1-SD change in the predictor variable, based on iterations where IVS included the variable in the model. For a detailed model description, see Appendix A.
3. Results
Concentrations of THg in filtered lake water ranged from 0.11 to 0.50 ng L−1 throughout the water column (; N = 42 samples) and were similar between parks (KATM and LACL; F1,36 = 1.68, p = 0.20) and depths (epilimnion, thermocline, hypolimnion; F2,36 = 0.05, p = 0.95). MeHg concentrations in filtered lake water reached 0.19 ng L−1 and were often at or below the method detection limit (0.010 ng L−1), hence we viewed the MeHg results as approximations, along with the BAFs that utilized them (Table A3).
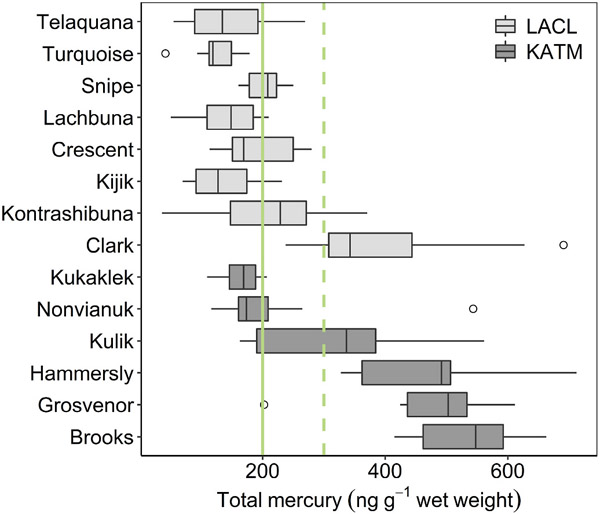
Although THg concentrations in water were low and relatively uniform, THg concentrations in fish were variable and, in some lakes, high. Lake trout spanned a thirty-fold range in THg concentrations (101–3046 ng g−1 dry weight (dw); N = 158 fish; Table 2), with distributions differing among parks (Wilcoxon W = 4072, p < 0.001). Within individual lakes, THg concentrations of lake trout spanned ranges as small as 359 ng g−1 dw (Kukaklek) or as large as 2222 ng g−1 dw (Kulik). When converted to wet weight (ww) using moisture content data, median THg concentrations in five lakes exceeded the USEPA MeHg criterion for protection of human health (300 ng g−1 ww; Borum et al., 2001), and seven lakes exceeded a similar criterion developed for Alaska (200 ng g−1 ww; Hamade, 2014) (Fig. 3). Here, we assumed THg did not vary between years during summer, as fish were collected in different years within and across lakes.
Table 2.
Summaries of the response variable (lake trout total mercury (THg)) and fish-level covariates used in the Bayesian hierarchical model. Summaries include mean (), minimum, and maximum values for the lake trout sampled in each lake.
| Park a |
Lake name |
n
b |
THg (ng·g −1 dw) |
Age (yrs) |
Condition (g·mm−3) |
δ13Clipid-free (‰) |
δ15N (‰) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | ||||||||
| LACL | Telaquana | 10 | 726 | 248 | 1403 | 15 | 10 | 20 | 0.71 | 0.55 | 0.88 | −22.09 | −24.73 | −18.95 | 12.67 | 11.12 | 14.91 |
| LACL | Turquoise | 11 | 571 | 191 | 914 | 17 | 7 | 26 | 0.82 | 0.68 | 0.91 | −23.62 | −25.69 | −21.29 | 10.73 | 10.15 | 11.25 |
| LACL | Snipe | 10 | 907 | 678 | 1200 | 10 | 7 | 13 | 0.79 | 0.71 | 0.87 | −22.71 | −23.69 | −22.04 | 10.89 | 10.34 | 11.31 |
| LACL | Lachbuna | 10 | 666 | 260 | 985 | 15 | 7 | 23 | 0.89 | 0.71 | 1.16 | −24.93 | −27.47 | −21.73 | 10.63 | 9.06 | 13.30 |
| LACL | Crescent | 10 | 796 | 474 | 1200 | 17 | 10 | 23 | 0.92 | 0.79 | 1.16 | −22.51 | −27.44 | −19.03 | 13.39 | 12.08 | 15.45 |
| LACL | Kijik | 10 | 612 | 297 | 1026 | 12 | 9 | 15 | 0.91 | 0.72 | 1.07 | −24.61 | −28.50 | −21.97 | 13.61 | 12.89 | 14.57 |
| LACL | Kontrashibuna | 10 | 953 | 151 | 1671 | 12 | 7 | 18 | 0.86 | 0.73 | 1.02 | −18.62 | −21.85 | −14.97 | 10.50 | 9.50 | 11.35 |
| LACL | Clark | 27 | 1692 | 1066 | 3039 | 14 | 10 | 21 | 0.81 | 0.59 | 1.04 | −24.36 | −27.27 | −17.97 | 11.88 | 11.06 | 12.70 |
| KATM | Kukaklek | 10 | 678 | 462 | 821 | 11 | 7 | 17 | 0.87 | 0.76 | 0.98 | −22.84 | −24.63 | −21.59 | 15.45 | 14.85 | 16.14 |
| KATM | Nonvianuk | 10 | 894 | 470 | 2563 | 11 | 8 | 15 | 0.80 | 0.58 | 0.97 | −24.10 | −25.14 | −22.74 | 12.67 | 11.14 | 13.45 |
| KATM | Kulik | 10 | 1428 | 641 | 2863 | 16 | 13 | 24 | 0.83 | 0.49 | 0.96 | −22.42 | −25.06 | −15.51 | 12.66 | 11.41 | 13.48 |
| KATM | Hammersly | 10 | 1942 | 1400 | 2930 | 14 | 10 | 22 | 0.91 | 0.78 | 1.00 | −22.94 | −24.11 | −21.12 | 10.01 | 9.62 | 11.20 |
| KATM | Grosvenor | 10 | 2129 | 1341 | 2634 | 15 | 10 | 22 | 0.87 | 0.76 | 0.97 | −25.74 | −28.52 | −22.20 | 14.58 | 13.81 | 15.84 |
| KATM | Brooks | 10 | 2201 | 1534 | 3046 | 19 | 14 | 24 | 0.84 | 0.78 | 0.92 | −25.42 | −28.91 | −23.36 | 13.36 | 9.29 | 14.24 |
LACL = Lake Clark National Park and Preserve; KATM = Katmai National Park and Preserve.
Total number of lake trout sampled per lake.
Fig. 3.
Boxplots of raw total mercury (THg) concentrations in lake trout, displayed by lake along a latitudinal gradient from north (top) to south (bottom). The midline of each box signifies the median value of 10–27 lake trout per lake. The lower and upper bounds correspond to the 25th and 75th percentiles, and the lower and upper whiskers extend to the smallest and largest values within 1.5x the inter-quartile range. Data beyond the whiskers are plotted as outliers. The green lines represent alternative thresholds for protection of human health: 200 and 300 ng·g−1 wet weight, designated by state and federal agencies, respectively.
3.1. Fish-level factors
Four fish-level factors were included in the model as covariates: lake trout age, body condition, δ13C, and δ15N (Table 2, Fig. 2). Lake trout varied in age from 7 to 26 yrs, with the youngest fish from Snipe Lake and the oldest from Lake Brooks, on average ( and 19 yrs, respectively; Table 2). Body condition in lake trout ranged from 0.49 to 1.16, where larger values indicated heavier fish for a given length. Lipid-corrected δ13C ranged from −28.91‰ to −14.97‰ and varied widely within some lakes (e.g., a 9.55‰ range at Kulik Lake). Finally, δ15N spanned values from 9.06‰ to 16.14‰ and, in most lakes, varied by one trophic level or less (i.e., ≤3.4‰; Minagawa and Wada, 1984).
3.2. Lake-level factors
Of the eight lake-level factors included in the model as covariates (Table 3, Fig. 2), four were quantified with new data (MeHg in plankton, and DOC in water, and BAF). MeHg concentrations in plankton ranged from 8.35 to 59.01 ng g−1 dw among lakes, and concentrations in water ranged from 1.89 to 13.46 mg L−1. DOC concentrations were low (0.75 ± 0.58 mg L−1), relative to values reported previously for other northern lakes (Seekell et al., 2015; Stolpmann et al., 2021).
Table 3.
Summaries of the lake-level covariates used in the Bayesian hierarchical model.
| Park A | Lake name | MeHg in plankton (ng·g−1) B |
Fish species richness |
Volcano proximity index D |
Glacier loss (%) |
Salmon density (salmon· m−1) |
in water (mg·L−1) |
DOC in water (mg·L−1) |
BAF (log (L·kg−1)) c |
|---|---|---|---|---|---|---|---|---|---|
| LACL | Telaquana | 8.67 | 11 | 0.4 | 2.32 | 1 | 13.46 | 0.34 | 5.34 |
| LACL | Turquoise | 8.35 | 7 | 0.3 | 4.37 | 0 | 4.62 | 0.19 | 5.89 |
| LACL | Snipe | 20.62 | 9 | 0.3 | 0 | 0 | 4.88 | 2.33 | 5.39 |
| LACL | Lachbuna | 18.82 | 3 | 0.4 | 0.62 | 0 | 11.79 | 0.44 | 5.03 |
| LACL | Crescent | 22.05 | 7 | 5.4 | 4.56 | 2.3 | 1.89 | 0.27 | 5.40 |
| LACL | Kijik | 20.83 | 10 | 0.3 | 0 | 0.6 | 9.76 | 0.55 | 5.88 |
| LACL | Kontrashibuna | 23.67 | 5 | 0.8 | 1.11 | 0 | 8.63 | 0.28 | 6.24 |
| LACL | Clark | 22.79 | 21 | 0.6 | 1.03 | 1.6 | 6.60 | 0.81 | 6.19 |
| KATM | Kukaklek | 21.04 | 9 | 1.6 | 0.01 | 3.5 | 7.06 | 0.83 | 6.26 |
| KATM | Nonvianuk | 18.59 | 13 | 2.5 | 1.05 | 0.8 | 7.69 | 0.87 | 5.98 |
| KATM | Kulik | 31.70 | 8 | 3.6 | 1.70 | 1.3 | 7.23 | 0.45 | 6.90 |
| KATM | Hammersly | 36.92 | 7 | 3.9 | 0 | 0.2 | 3.17 | 0.61 | 6.44 |
| KATM | Grosvenor | 59.01 | 18 | 8.8 | 0.04 | 2 | 4.16 | 1.09 | 5.92 |
| KATM | Brooks | 33.96 | 19 | 5.1 | 0 | 1 | 3.00 | 1.47 | 5.75 |
LACL = Lake Clark National Park and Preserve; KATM = Katmai National Park and Preserve.
Methylmercury (MeHg) concentrations pertain to a single bulk sample per lake, inclusive of all size fractions (range in mass = 0.27–2.1 g dw).
Bioaccumulation factor (BAF) calculations rely on concentrations of MeHg in water and the smallest size fraction of plankton (63–118 μm).
Volcano proximity index is unitless, as described in the Methods. Here it is multiplied by 1000 for ease of viewing.
Conversely, BAF values were high (log 5.90 ± 0.50 L·kg−1), compared with values reported elsewhere in the literature (Schartup et al., 2018; Tsui and Finlay, 2011).
3.3. Model results
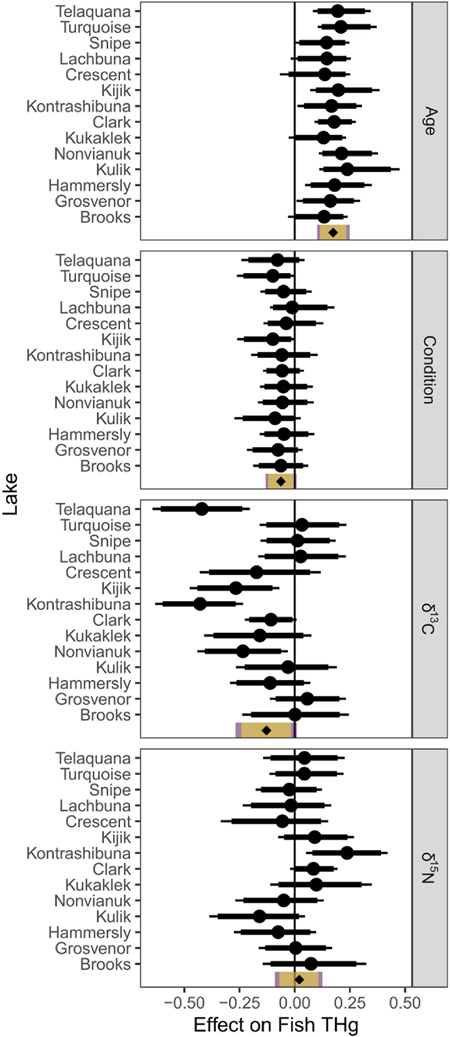
Model results highlighted the relative influence of both fish-level and lake-level factors in explaining differences in lake trout THg concentration. For the fish-level factors, age and body condition had the most consistent effects on THg concentration, while the effects of δ13C and δ15N differed substantially among lakes (Fig. 4). Specifically, THg concentration at an average lake increased by 17% for each 1-SD increase of age, 95% CrI = [10,25]. We found weaker evidence of increased THg concentration in fish with lower body condition, suggesting a 6% [13,−1] increase at an average lake for each 1-SD decrease in body condition. We found strong evidence of a negative relationship between THg concentration and δ13C in fish from four lakes, but weaker or no evidence of such a pattern in other lakes. Finally, δ15N had no consistent relationship with fish THg concentration across lakes, although a positive relationship was apparent in one lake (Kontrashibuna; Fig. 4).
Fig. 4.
Parameter estimates for four fish-level covariates from the Bayesian hierarchical model, showing the effect of a 1-SD change in the covariate on fish total mercury (THg) concentration in each study lake (points and error bars), and the estimated population average effect (point and shaded rectangles at bottom). Estimates are presented as posterior medians and 90% credible intervals (thick error bars, beige rectangles) and 95% credible intervals (thin error bars, purple rectangles).
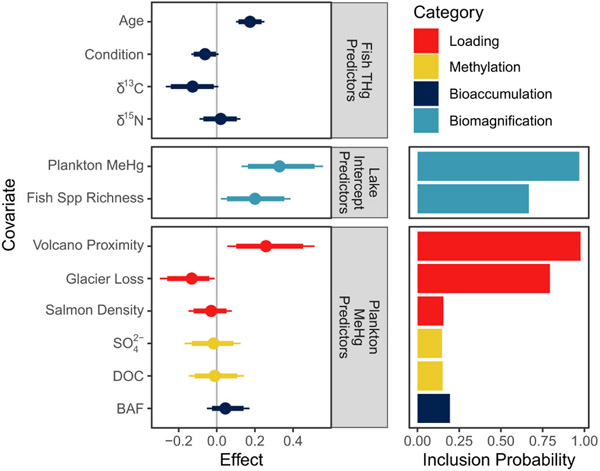
Lake trout THg concentration was also explained by several lake-level factors, including fish species richness (VIP = 0.61) and plankton MeHg concentration (VIP = 0.83; Fig. 5). Plankton MeHg concentration, in turn, was predicted by volcano proximity (VIP = 0.98) and glacier loss (VIP = 0.80; Fig. 5). Fish THg concentration increased 20% [3,39] with each 1-SD increase in fish species richness and 32% [12,53] with each 1-SD increase in plankton MeHg concentration. MeHg concentrations in plankton were higher in lakes with a greater density of nearby volcanoes, and lower in lakes with a greater loss of basin glacier cover. This translated to a 26% [7,46] increase in fish THg with increased volcano proximity, and a 13% [−26,−2] decrease in fish THg with increased glacier loss. We found only weak evidence for increased plankton MeHg concentration with increased BAF, and this covariate was not favored by indicator variable selection (IVS). Additionally, we found no evidence of an influence of DOC, , or salmon density on plankton MeHg concentration.
Fig. 5.
Parameter estimates (left) and inclusion probabilities (right) from the Bayesian hierarchical model, color coded by process-based category as in Fig. 2. Parameter estimates are presented as posterior medians, 90% credible intervals (thick error bars), and 95% credible intervals (thin error bars), and show the effect that a 1-SD change in the predictor has on fish total mercury (THg) concentration. For plankton MeHg predictors, we show the ultimate effect that a 1-SD change in the predictor has on fish THg, rather than the immediate effect it has on plankton MeHg. Parameter inclusion probabilities (right) are derived from indicator variable selection (IVS) within the Bayesian hierarchical model. IVS was only used to select lake intercept and plankton MeHg predictors.
4. Discussion
Recent findings of elevated fish Hg concentrations in some of the most remote and ostensibly pristine lakes in North American parklands have raised questions about the factors driving those concentrations. In our study of 14 remote lakes, with gradients in potential drivers of Hg, we found that THg concentrations in 59 of 158 lake trout exceeded the USEPA human consumption threshold in multiple lakes spanning both parks examined. These concentrations varied widely both within and among lakes, despite the relatively low and uniform THg concentrations observed in water. Results from a Bayesian hierarchical model indicated that fish-level and lake-level factors, related to loading, bioaccumulation, and biomagnification, explained the observed variation in lake trout THg concentrations. In particular, age and, to a lesser extent, body condition best explained variation in THg among fish, while plankton MeHg, fish species richness, volcano proximity, and glacier loss explained variation in THg among lakes.
4.1. Fish-level factors
We found strong evidence that THg concentrations are higher in older lake trout and moderate evidence that concentrations are higher in lake trout with lower body condition, indicative of poorer nutritional status. Increases in Hg concentration with fish age are well-established (Wiener et al., 2007), especially in cold high latitude lakes, where growth rates are slow and life spans are long, relative to warmer regions (Kahilainen et al., 2017). Inverse relationships between Hg concentration and body condition are also well-supported. For example, studies of both small alpine and large lowland lakes generally found lower Hg concentrations in fish with higher condition scores (Cizdziel et al., 2002; Eagles-Smith et al., 2016a). Fish condition and average growth rate are positively correlated via bioenergetics (Ricker, 1979), while average growth rate and Hg concentration are negatively correlated via growth dilution (Stafford et al., 2004). In our study, the effect of condition on THg concentration was small, negative, and consistent across lakes (Fig. 4).
We also found evidence that THg concentrations are higher in lake trout with more depleted δ13C values, but this relationship varied among lakes. More depleted δ13C values in fish typically indicate greater reliance on pelagic plankton-derived C, than on benthic or littoral algae-derived C (Hecky and Hesslein, 1995). Greater reliance on pelagic C is often associated with higher Hg concentrations in fish (Kahilainen et al., 2017; Power et al., 2002), because pelagic plankton tend to have higher Hg concentrations and lower caloric contents than benthic or littoral prey taxa (Karimi et al., 2016). In our study, model results for δ13C suggest that habitat-specific foraging by lake trout occurs in several lakes, while generalist foraging from multiple habitats occurs in others.
Notably absent in our modeling results was a consistent relationship between δ15N and THg concentration in lake trout (Fig. 4). Instead, the relationship differed across the landscape and was relatively strong in only one lake, suggesting that trophic position was not a dominant driver of variation in fish Hg. Both δ15N and THg typically increase with trophic position (Cabana and Rasmussen, 1994; Kidd et al., 2012). However, the range of lake trout δ15N within most of our study lakes was narrow (Table 2), which could explain the lack of relationship with THg. Furthermore, δ15N can serve as an indicator of marine-derived N from salmon—and by extension marine-derived Hg from salmon—which complicates the interpretation of the δ15N results. If salmon-borne Hg is negligible, or Hg from other sources is more bioavailable, then elevated Hg in lake trout would not necessarily follow from elevated δ15N levels.
4.2. Lake-level factors
After accounting for fish-level factors, our model results suggest that four lake-level factors (plankton MeHg, fish species richness, volcano proximity, and glacier loss), indicative of two key processes (biomagnification and loading), drive lake trout Hg concentrations. Our analysis provides no evidence that other lake-level factors (salmon density, , DOC, and BAF) control variation in fish Hg concentrations between lakes, despite their roles as drivers in past studies (Chiasson-Gould et al., 2014; Gilmour et al., 1992; Ogorek et al., 2021).
4.2.1. Plankton MeHg
Concentrations reported here for MeHg in plankton are similar to or lower than concentrations reported for other northern lakes (e.g., Kidd et al., 2012; Westcott and Kalff). However, cross-study comparisons are complicated by differences in plankton sampling and processing techniques, taxonomy, and relative trophic position. Nevertheless, a pattern observed in other studies—of a positive relationship between Hg levels in plankton and top-predator fish species (Kidd et al., 2012)—is evident here as well. This pattern emphasizes the importance of bottom-up transfer of Hg through the pelagic food web in these lakes.
4.2.2. Fish species richness
While Hg levels at the bottom of the food web clearly influence Hg levels at the top in our study lakes, food web structure may also play a role, evidenced by the positive relationship between fish species richness and THg concentration in lake trout (Fig. 5). This result aligns with previous findings that Hg concentrations in conspecific fish can vary greatly among lakes with similar physical characteristics and Hg deposition rates, but contrasting food web lengths and complexities (Cabana et al., 1994; Futter, 1994). In our study, we use fish species richness as a proxy for food web length, based on the findings of Vander Zanden et al. (1999). This proxy seems reasonable given that lake trout in Alaska are known to eat most of the species counted in our fish species richness tally (Hartman and Burgner, 1972; Hershey et al., 1999; Russell, 1980). Lake trout in Alaska are also known to feed opportunistically, with diets determined more by food availability than preference (Redick, 1967).
4.2.3. Volcano proximity
Volcanoes are a major source of geogenic Hg, accounting for 60% of the natural atmospheric Hg budget and 7% of the total budget, globally, according to recent estimates (Li et al., 2020). In our study, volcano proximity has the highest inclusion probability of the lake-level factors included in the model (Fig. 5). This result provides evidence that proximity to volcanoes explains variation in MeHg concentrations of plankton in our study lakes, ultimately influencing THg concentrations in lake trout. It also supports a recent study using Hg stable isotopes and a similar dataset to find that volcanism was a likely contributor to Hg loading in southwest Alaska lakes (Lepak et al., 2022a,b).
Southwest Alaska contains 53 of the 54 historically active volcanoes in the state (Cameron et al., 2018). Consequently, soils in the region often include one or more ash layers from past eruptions (Wells et al., 2013, 2021). However, there is no evidence that ash layers in or near our study lake basins contain elevated concentrations of Hg, relative to other soil layers (Lepak et al., 2022a; Figure A1). Indeed, Hg levels measured in ash shortly after volcanic eruptions in Alaska and elsewhere are consistently below detection limits (Fruchter et al., 1980; Lepel et al., 1978).
Unlike ash, emissions from passively degassing and actively erupting volcanoes contain elevated concentrations of Hg. For example, average concentrations of gaseous elemental Hg in the plumes of 9 passively degassing volcanoes ranged from 18 to 373 ng m−3, exceeding background levels of ~10 ng m−3 (Bagnato et al., 2011), and plumes sampled shortly after active eruptions reached concentrations 1-2 orders of magnitude higher (Lepel et al., 1978; Varekamp and Buseck, 1981). These concentrations, along with the abundance of volcanoes in southwest Alaska, align with our model results pointing to volcanoes as a strong driver of lake-level variation in Hg.
Sediment cores also contain evidence of a link between volcanism and high Hg levels in Alaska lakes. An analysis by Munk et al. (2010) of trace elements in sediment cores from five lakes in KATM and LACL (including three lakes from our study) found that the highest Hg concentrations and accumulation rates occurred in Lake Brooks—the lake with the highest mean and maximum fish THg concentrations in our study (Table 2). Average Hg accumulation rates reported by Munk et al. (7–236 μg m−2•y−1) exceeded those reported for lake sediment cores from three parks further north in Alaska (1–5 μg m−2•y−1; Landers et al., 2008). Munk et al. attributed this difference to higher sediment accumulation and recent volcanic activity in and near the lakes. Volcanoes may therefore be an underappreciated natural source of Hg in lacustrine systems.
4.2.4. Glacier loss
Atmospherically transported Hg can be trapped in glacial ice following wet or dry deposition (Faïn et al., 2009; Zheng et al., 2009). As glaciers melt from seasonal or long-term warming, Hg stored in ice is released. Glacial recession may also expose previously concealed Hg-rich sediment or bedrock (Hawkings et al., 2021). This newly released or exposed, primarily inorganic, Hg may be transported downstream to aquatic sites with potential for Hg methylation and bioaccumulation (Nagorski et al., 2021). Given recent declines in glacial cover in these parks (Loso et al., 2014), we hypothesized that glacial loss might be a significant positive driver of Hg in fish downstream. However, our results suggested the opposite: lakes with greater losses of glacier cover in their basins tended to have lower plankton MeHg concentrations, which were related, in turn, to lower fish THg concentrations.
While this result seems counterintuitive, it aligns with findings from a similar study in southeast Alaska, where streams draining basins with high glacial cover or recent glacial loss had lower THg levels in water, macroinvertebrates, and fish than streams draining basins with high wetland cover (Nagorski et al., 2014). The difference in THg was attributed to glacier-fed basins having more exposed bedrock, less weathered soil, and lower DOC, all of which seemed to limit Hg methylation. The same rationale could apply at our sites (Figure A2) and would highlight the importance of Hg methylation, even if no methylation-related factors were strong drivers in our model.
4.2.5. Salmon density
In addition to transporting nutrients upstream, returning sockeye salmon have been shown to import pollutants such as dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs), and Hg (Ewald et al., 1998; Krümmel et al., 2003; Zhang et al., 2001). Although the pollutant concentrations in individual sockeye salmon are low (e.g., 14–58 ng g−1 ww THg; Lepak et al., 2022a; Zhang et al., 2001), the annual influx may be considerable given their range in size (2–7 kg; ADFG, 2022) and escapement (6–26 million fish per year since 2001 in rivers entering Bristol Bay, southwest Alaska; (Elison et al., 2022). In the case of DDT and PCBs, pollutant concentrations in rainbow trout increased with sockeye salmon spawner density (Gregory-Eaves et al., 2007). We hypothesized that Hg concentrations in lake trout would behave similarly.
Contrary to expectations, we found no evidence that sockeye salmon drive Hg concentrations in lake trout at our study sites. Instead, variation in Hg concentration was explained by neither δ15N at the fish level nor spawner density at the lake level. It is possible that sockeye salmon are a key biovector for Hg in lake trout, but the low quality of the spawner density data (i.e., the differences in method and timing among lakes) masks the relationship. However, two other explanations are plausible.
First, Hg import by adult salmon may be partly offset through Hg export by out-migrating juvenile salmon, so freshwater systems might not experience the Hg amplification expected under assumed one-way transport, from the ocean upstream (Baker et al., 2009). Second, Hg concentrations in adult sockeye salmon are relatively low, and concentrations in eggs and smolts—both of which are prey items for lake trout—are lower or equivalent (4–5 and 14–47 ng g−1 ww THg for eggs and smolts, respectively; Zhang et al., 2001; Baker et al., 2009). Therefore, if lake trout feed on sockeye eggs and smolts with lower THg concentrations than other food sources, then the net effect of this consumption on lake trout THg concentrations could be neutral or even negative (Cyr et al., 2017; Gerig et al., 2018). This could also occur if the caloric content of salmon eggs and smolts exceeded that of other food sources, boosting growth and diluting THg concentrations in lake trout.
5. Conclusion
We created a simple conceptual model with four broad categories of factors, representing various processes whereby Hg reaches piscivorous lake fish. We then used the conceptual model to parameterize a statistical model, enabling us to identify key drivers of Hg concentrations in lake trout, despite having a large pool of potential drivers and a small sample size of lakes. Some results were expected but several were not, in terms of their sign (glacier loss), strength (volcano proximity) or lack thereof (salmon density).
Our study did not include all drivers of fish Hg, partly due to a lack of baseline data on metrics like lake volume, flushing rate, and inlet discharge. Also, our study did not identify the pathway by which Hg from volcanoes reaches lakes or lake fish. Nor did our data show a positive relationship between proximity to volcanoes and Hg levels in water (Figure A3). This pathway involves numerous biogeochemical steps not characterized here because, while fish samples integrated THg concentrations over time, water samples captured ephemeral concentrations at only one time point, thus requiring additional study. Measuring gaseous volcanic Hg at nearby volcanoes would be another way to explore the link between volcanoes and freshwater food webs, as would determining the chemical forms of Hg released and the distance those forms travel before deposition.
Importantly, inorganic Hg concentrations often correlate poorly with aqueous or biological MeHg concentrations (Eagles-Smith et al., 2016b; Fleck et al., 2016), because MeHg production is frequently decoupled from inorganic Hg loading, and Hg is not the limiting factor. Our findings suggest that, in some instances, volcanic Hg may be associated with biogeochemical processes or may be more bioavailable for methylation, compared with other sources.
Teasing apart sources of Hg is especially important in U.S. National Parks, where the core mission is to preserve unimpaired resources within parklands for current and future generations. Without an understanding of Hg sources—what is natural versus manmade—it is difficult to discern impaired from unimpaired resources. It also raises the question of how to manage for impairment that occurs, at least in part, naturally. A good first step would be clear communication with local communities that use fish resources for food security, given the percentages of lake trout that exceeded state (58%) and national (37%) Hg consumption thresholds in this study.
Supplementary Material
Acknowledgements
This work was funded, in part, by the USGS-NPS Natural Resources Preservation Program, the USGS Toxics Substances Hydrology Program, and the USGS Contaminant Biology Program. Data collected for this study are available at https://doi.org/10.5066/P9UEP9C5. Postdoctoral support was provided by the National Science Foundation Postdoctoral Fellowships for Research in Biology – Collection Program 2018 (award no. 1812211). We thank Steve Chipps and two anonymous reviewers for comments on this manuscript, and Evan Booher, Katie Junghans, and Jeff Nelson for their critical field support. Any use of trade, firm, or product names in this publication is for descriptive purposes only and does not imply endorsement by the U. S. Government. All animals were collected following protocols in the approved National Park Service Institutional Animal Care and Use Committee plan, under protocol number AKR_SWAN_Bartz_Fish_2019. A3.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2023.121678.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This paper has been recommended for acceptance by Michael Bank.
Data availability
Data collected for this study are available at https://doi.org/10.5066/P9UEP9C5.
References
- Alaska Department of Fish and Game (ADFG), 2021a. Fish Count Data Search. https://www.adfg.alaska.gov/sf/FishCounts/index.cfm?adfg=main.home. (Accessed 8 September 2021).
- Alaska Department of Fish and Game (ADFG), 2021b. Escapement Monitoring Inseason and Historical Data. https://www.adfg.alaska.gov/index.cfm?adfg=commercialbyareakuskokwim.emihd. (Accessed 8 September 2021).
- Alaska Department of Fish and Game (ADFG), 2022. Sockeye Salmon (Oncorhynchus nerka): Species Profile. https://www.adfg.alaska.gov/index.cfm?adfg=sockeyesalmon.main. (Accessed 1 May 2022).
- Alaska Department of Natural Resources (AKDNR), 2011. Alaska Volcanoes. https://data-soa-dnr.opendata.arcgis.com/maps/56a6b26082304fdbb78d5a6e49f18894/about.
- Arendt A, Rich JL, 2013. Randolf Glacier Inventory. University of Alaska Fairbanks, Alaska USA. https://irma.nps.gov/DataStore/Reference/Profile/2221653. [Google Scholar]
- Bagnato E, Aiuppa A, Parello F, Allard P, Shinohara H, Liuzzo M, Giudice G, 2011. New clues on the contribution of Earth’s volcanism to the global mercury cycle. Bull. Volcanol 73, 497–510. 10.1007/s00445-010-0419-y. [DOI] [Google Scholar]
- Baker MR, Schindler DE, Holtgrieve GW, St Louis VL, 2009. Bioaccumulation and transport of contaminants: migrating sockeye salmon as vectors of mercury. Environ. Sci. Technol 43, 8840–8846. 10.1021/es901798f. [DOI] [PubMed] [Google Scholar]
- Bloom NS, 1992. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can. J. Fish. Aquat. Sci 49, 1010–1017. 10.1139/f92-113. [DOI] [Google Scholar]
- Bodaly RA, Rudd JWM, Fudge RJP, Kelly CA, 1993. Mercury concentrations in fish related to size of remote Canadian Shield lakes. Can. J. Fish. Aquat. Sci 50, 980–987. 10.1139/f93-113. [DOI] [Google Scholar]
- Borum D, Manibusan M, Schoeny R, Winchester E, 2001. Water Quality Criterion for the Protections of Human Health: Methylmercury. U.S. Environmental Protection Agency, p. 303. EPA-823-R-01-001. https://www.epa.gov/sites/default/files/2020-01/documents/methylmercury-criterion-2001.pdf. [Google Scholar]
- Brabets TP, Ourso RT, 2006a. Water Quality, Physical Habitat, and Biology of the Kijik River Basin, Lake Clark National Park and Preserve, Alaska, 2004-2005. U.S. Geological Survey, p. 60. 10.3133/sir20065123. Scientific Investigations Report 2006-5123. [DOI] [Google Scholar]
- Brabets TP, Ourso RT, 2006b. Water Quality of the Crescent River Basin, Lake Clark National Park and Preserve, Alaska, 2003-2004. U.S. Geological Survey, p. 48. 10.3133/sir20065151. Scientific Investigations Report 2006-5151. [DOI] [Google Scholar]
- Branfireun BA, Cosio C, Poulain AJ, Riise G, Bravo AG, 2020. Mercury cycling in freshwater systems - an updated conceptual model. Sci. Total Environ 745 10.1016/j.scitotenv.2020.140906. [DOI] [PubMed] [Google Scholar]
- Bravo AG, Bouchet S, Tolu J, Björn E, Mateos-Rivera A, Bertilsson S, 2017. Molecular composition of organic matter controls methylmercury formation in boreal lakes. Nat. Commun 8, 1–9. 10.1038/ncomms14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana G, Rasmussen JB, 1994. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature 372, 255–257. 10.1038/372255a0. [DOI] [Google Scholar]
- Cabana G, Tremblay A, Kalff J, Rasmussen JB, 1994. Pelagic food chain structure in Ontario lakes: a determinant of mercury levels in lake trout (Salvelinus namaycush). Can. J. Fish. Aquat. Sci 51, 381–389. 10.1139/f94-039. [DOI] [Google Scholar]
- Cameron CE, Schaefer JR, Muliken KM, 2018. Historically Active Volcanoes of Alaska. Alaska Volcano Observatory, p. 1. Miscellaneous Publication 133; version 3.0. https://avo.alaska.edu/images/dbimages/display/1546466677.jpg. [Google Scholar]
- Campana SE, Casselman JM, Jones CM, 2008. Bomb radiocarbon chronologies in the Arctic, with implications for the age validation of lake trout (Salvelinus namaycush) and other Arctic species. Can. J. Fish. Aquat. Sci 65, 733–743. 10.1139/F08-012. [DOI] [Google Scholar]
- Chamberlain DM, 1989. Physical, and Biological Characteristics, and Nutrients Limiting Primary Productivity, Lake Clark, Alaska. In: Thesis MS (Ed.). Michigan Technological University, p. 135. [Google Scholar]
- Chiasson-Gould SA, Blais JM, Poulain AJ, 2014. Dissolved organic matter kinetically controls mercury bioavailability to bacteria. Environ. Sci. Technol 48, 3153–3161. 10.1021/es4038484. [DOI] [PubMed] [Google Scholar]
- Cizdziel JV, Hinners TA, Pollard JE, Heithmar EM, Cross CL, 2002. Mercury concentrations in fish from Lake Mead, USA, related to fish size, condition, trophic level, location, and consumption risk. Arch. Environ. Contam. Toxicol 43, 309–317. 10.1007/s00244-002-1191-6. [DOI] [PubMed] [Google Scholar]
- Clark JH, 2005. Abundance of Sockeye Salmon in the Alagnak River System of Bristol Bay Alaska. Alaska Department of Fish and Game, p. 34. Fishery Manuscript No. 05-01. https://www.adfg.alaska.gov/fedaidpdfs/fms05-01.pdf. [Google Scholar]
- Cunningham CJ, Westiey PAH, Adkison MD, 2018. Signals of large scale climate drivers, hatchery enhancement, and marine factors in Yukon River Chinook salmon survival revealed with a Bayesian life history model. Global Change Biol. 24, 4399–4416. 10.1111/gcb.14315. [DOI] [PubMed] [Google Scholar]
- Cyr A, Sergeant CJ, Lopez JA, O’Hara T, 2017. Assessing the influence of migration barriers and feeding ecology on total mercury concentrations in Dolly Varden (Salvelinus malma) from a glaciated and non-glaciated stream. Sci. Total Environ 580, 710–718. 10.1016/j.scitotenv.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Durnford D, Dastoor A, Figueras-Nieto D, Ryjkov A, 2010. Long range transport of mercury to the Arctic and across Canada. Atmos. Chem. Phys 10, 6063–6086. 10.5194/acp-10-6063-2010. [DOI] [Google Scholar]
- Eagles-Smith CA, Willacker JJ, Flanagan Pritz CM, 2014. Mercury in fishes from 21 national parks in the Western United States – inter- and intra-park variation in concentrations and ecological risk. U.S. Geological Survey 54. 10.3133/ofr20141051. Open-File Report 2014-1051. [DOI] [Google Scholar]
- Eagles-Smith CA, Herring G, Johnson B, Graw R, 2016a. Conifer density within lake catchments predicts fish mercury concentrations in remote subalpine lakes. Environ. Pollut 212, 279–289. 10.1016/j.envpol.2016.01.049. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Wiener JG, Eckley CS, Willacker JJ, Evers DC, Marvin-DiPasquale M, Obrist D, Fleck JA, Aiken GR, Lepak JM, Jackson AK, Webster JP, Stewart AR, Davis JA, Alpers CN, Ackerman JT, 2016b. Mercury in western North America: a synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci. Total Environ 568, 1213–1226. 10.1016/j.scitotenv.2016.05.094. [DOI] [PubMed] [Google Scholar]
- Elison T, Tiernan A, Sands T, Head J, Vega S, 2022. 2021 Bristol Bay Annual Management Report. Alaska Department of Fish and Game, p. 95. Fishery Management Report No. 22–14. https://www.adfg.alaska.gov/FedAidPDFs/FMR22-14.pdf. [Google Scholar]
- Ewald G, Larsson P, Linge H, Okla L, Szarzi N, 1998. Biotransport of organic pollutants to an Inland Alaska lake by migrating sockeye salmon (Oncorhynchus nerka). Arctic 51, 40–47. 10.14430/arctic1043. [DOI] [Google Scholar]
- Faïn X, Ferrari CP, Dommergue A lien, Albert MR, Battle M, Severinghaus J, Arnaud L, Barnola J-M, Cairns W, Barbante C, Boutron C, 2009. Polar firn air reveals large-scale impact ofanthropogenic mercury emissions during the 1970s 106, 16114–16119. 10.1073/pnas.0905117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck JA, Marvin-DiPasquale M, Eagles-Smith CA, Ackerman JT, Lutz MA, Tate M, Alpers CN, Hall BD, Krabbenhoft DP, Eckley CS, 2016. Mercury and methylmercury in aquatic sediment across western North America. Sci. Total Environ 568, 727–738. 10.1016/j.scitotenv.2016.03.044. [DOI] [PubMed] [Google Scholar]
- French TD, Houben AJ, Desforges J-PW, Kimpe LE, Kokelj SV, Poulain AJ, Smol JP, Wang X, Blais JM, 2014. Dissolved organic carbon thresholds affect mercury bioaccumulation in Arctic lakes. Environ. Sci. Technol 48, 3162–3168. 10.1021/es403849d. [DOI] [PubMed] [Google Scholar]
- Fruchter JS, Robertson DE, Evans JC, Olsen KB, Lepel EA, Laul JC, Abel KH, Sanders RW, Jackson PO, Wogman NS, Perkins RW, Van Tuyl HH, Beauchamp RH, Shade JW, Daniel JL, Erikson RL, Sehmel GA, Lee RN, Robinson AV, Moss OR, Briant JK, Cannon WC, 1980. Mount St. Helens ash from the 18 May 1980 eruption: chemical, physical, mineralogical, and biological properties. Science 209, 1116–1125. 10.1126/science.209.4461.1116. [DOI] [PubMed] [Google Scholar]
- Futter MN, 1994. Pelagic food-web structure influences probability of mercury contamination in lake trout (Salvelinus namaycush). Sci. Total Environ 145, 7–12. 10.1016/0048-9697(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Gerig BS, Chaloner DT, Janetski DJ, Moerke AH, Rediske RR, O’Keefe JP, de Alwis Pitts DA, Lamberti GA, 2018. Environmental context and contaminant biotransport by Pacific salmon interact to mediate the bioaccumulation of contaminants by stream-resident fish. J. Appl. Ecol 55, 1846–1859. 10.1111/1365-2664.13123. [DOI] [Google Scholar]
- Gilmour CC, Henry EA, Mitchell R, 1992. Sulfate stimulation of mercury methylation in freshwater sediments. Environ. Sci. Technol 26, 2281–2287. 10.1021/es00035a029. [DOI] [Google Scholar]
- Gregory-Eaves I, Demers MJ, Kimpe L, Krümmel EM, Macdonald RW, Finney BP, Blais JM, 2007. Tracing salmon-derived nutrients and contaminants in freshwater food webs across a pronounced spawner density gradient. Environ. Toxicol. Chem 26, 1100–1108. 10.1897/06-402R.1. [DOI] [PubMed] [Google Scholar]
- Gustafson EJ, Parker GR, 1992. Relationships between landcover proportion and indices of landscape spatial pattern. Landsc. Ecol 7, 101–110. 10.1007/BF02418941. [DOI] [Google Scholar]
- Gutierrez B.F. de P., Agudelo CAR, 2020. Fish as bioindicators: coal and mercury pollution in Colombia’s ecosystems. Environ. Sci. Pollut. Res 27, 27541–27562. 10.1007/s11356-020-09159-4. [DOI] [PubMed] [Google Scholar]
- Hamade AK, 2014. Fish Consumption Advice for Alaskans: A Risk Management Strategy to Optimize the Public’s Health. State of Alaska, Section of Epidemiology, p. 78. https://health.alaska.gov/dph/Epi/eph/Documents/fish/FishConsumptionAdvice2014.pdf. [Google Scholar]
- Hamilton TD, Reed KM, Thorson RM, 1986. Glaciation in Alaska – introduction and overview. In: Hamilton TD, Reed KM, Thorson RM (Eds.), Glaciation in Alaska: the Geologic Record. Alaska Geological Society, Anchorage, pp. 1–8. [Google Scholar]
- Hartman WL, Burgner RL, 1972. Limnology and fish ecology of sockeye salmon nursery lakes of the world. J. Fish. Res. Board Can 29, 699–715. 10.1139/f72-116. [DOI] [Google Scholar]
- Hawkings JR, Linhoff BS, Wadham JL, Stibal M, Lamborg CH, Carling GT, Lamarche-Gagnon G, Kohler TJ, Ward R, Hendry KR, Falteisek L, Kellerman AM, Cameron KA, Hatton JE, Tingey S, Holt AD, Vinšová P, Hofer S, Bulínová M, Větrovský T, Meire L, Spencer RGM, 2021. Large subglacial source of mercury from the southwestern margin of the Greenland Ice Sheet. Nat. Geosci 14, 496–502. 10.1038/s41561-021-00753-w. [DOI] [Google Scholar]
- Hecky RE, Hesslein RH, 1995. Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. North Am. Benthol. Soc 14, 631–653. [Google Scholar]
- Hershey AE, Gettel GM, Mcdonald ME, Miller MC, Mooers H, Brien WJO, Pastor J, Richards C, Schuldt JA, 1999. A geomorphic-trophic model for landscape control of arctic lake food webs. Bioscience 49, 887–897. http://www.jstor.org/stable/10.1525/bisi.1999.49.11.887. [Google Scholar]
- Hoffman JC, Sierszen ME, Cotter AM, 2015. Fish tissue lipid-C:N relationships for correcting δ13C values and estimating lipid content in aquatic food-web studies. Rapid Commun. Mass Spectrom 29, 2069–2077. 10.1002/rcm.7367. [DOI] [PubMed] [Google Scholar]
- Hsu-Kim H, Kucharzyk KH, Zhang T, Deshusses MA, 2013. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ. Sci. Technol 47, 2441–2456. 10.1021/es304370g. [DOI] [PubMed] [Google Scholar]
- Hurley JP, Benoit JM, Babiarz CL, Shafer MM, Andren AW, Sullivan JR, Hammond R, Webb DA, 1995. Influences of watershed characteristics on mercury levels in Wisconsin rivers. Environ. Sci. Technol 29, 1867–1875. 10.1021/es00007a026. [DOI] [PubMed] [Google Scholar]
- Jones TM, Bennett L, Hamon TR, 2005. Baseline Inventory of Freshwater Fishes of the Southwest Alaska Inventory and Monitoring Network. National Park Service, p. 120. Natural Resource Technical Report NPS/AKRSWAN/NRTR-2005/04. https://www.arlis.org/docs/vol1/71090809.pdf. [Google Scholar]
- Kahilainen KK, Thomas SM, Nystedt EKM, Keva O, Malinen T, Hayden B, 2017. Ecomorphological divergence drives differential mercury bioaccumulation in polymorphic European whitefish (Coregonus lavaretus) populations of subarctic lakes. Sci. Total Environ 599, 1768–1778. 10.1016/j.scitotenv.2017.05.099. –600. [DOI] [PubMed] [Google Scholar]
- Karimi R, Chen CY, Folt CL, 2016. Comparing nearshore benthic and pelagic prey as mercury sources to lake fish: the importance of prey quality and mercury content. Sci. Total Environ 565, 211–221. 10.1016/j.scitotenv.2016.04.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner K, Meredith M, 2021. Package ‘jagsUI’: A Wrapper Around “Rjags” to Streamline “JAGS” Analyses. https://cran.r-project.org/web/packages/jagsUI/jagsUI.pdf. [Google Scholar]
- Kidd KA, Muir DCG, Evans MS, Wang X, Whittle M, Swanson HK, Johnston T, Guildford S, 2012. Biomagnification of mercury through lake trout (Salvelinus namaycush) food webs of lakes with different physical, chemical and biological characteristics. Sci. Total Environ 438, 135–143. 10.1016/j.scitotenv.2012.08.057. [DOI] [PubMed] [Google Scholar]
- Krümmel EM, Macdonald RW, Kimpe LE, Gregory-Eaves I, Demers MJ, Smol JP, Finney B, Blais JM, 2003. Delivery of pollutants by spawning salmon. Nature 425, 255–256. 10.1038/425255a. [DOI] [PubMed] [Google Scholar]
- Kuo L, Mallick B, 1998. Variable selection for regression models. Sankhyā Indian J. Stat. Ser. B 60, 65–81. http://www.jstor.org/stable/25053023. [Google Scholar]
- Landers D, Simonich S, Jaffe D, Geiser L, Campbell D, Schwindt A, Schreck C, Kent M, Hafner W, Taylor H, Hageman K, Usenko S, Ackerman L, Schrlau J, Rose N, Blett T, Erway M, 2008. The Fate, Transport, and Ecological Impacts of Airborne Contaminants in Western National Parks (USA). U.S. Environmental Protection Agency, p. 350. EPA/600/R-07/138. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100EJ34.txt. [Google Scholar]
- LaPerriere JD, 1997. Limnology of two lake systems of Katmai National Park and Preserve, Alaska: physical and chemical profiles, major ions, and trace elements. Hydrobiologia 354, 89–99. 10.1023/a:1003067525783. [DOI] [Google Scholar]
- Lepak RF, Ogorek JM, Bartz KK, Janssen SE, Tate MT, Runsheng Y, Hurley JP, Young DB, Eagles-Smith CA, Krabbenhoft DP, 2022a. Using carbon, nitrogen, and mercury isotope values to distinguish mercury sources to Alaskan lake trout. Environ. Sci. Technol. Lett 9, 312–319. 10.1021/acs.estlett.2c00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepak RF, Bartz KK, Ogorek JM, Tate MT, DeWild JF, Janssen SE, 2022b. Assessment of Mercury Sources in Alaskan Lake Food Webs: U.S. Geological Survey data release. 10.5066/P9UEP9C5. [DOI] [Google Scholar]
- Lepel EA, Stefansson KM, Zoller WH, 1978. The enrichment of volatile elements in the atmosphere by volcanic activity: augustine volcano 1976. J. Geophys. Res 83, 6213–6220. 10.1029/jc083ic12p06213. [DOI] [Google Scholar]
- Li C, Sonke JE, Le Roux G, Piotrowska N, Van der Putten N, Roberts SJ,Daley T, Rice E, Gehrels R, Enrico M, Mauquoy D, Roland TP, De Vleeschouwer F, 2020. Unequal anthropogenic enrichment of mercury in earth’s Northern and Southern Hemispheres. ACS Earth Sp. Chem 4, 2073–2081. 10.1021/acsearthspacechem.0c00220. [DOI] [Google Scholar]
- Loso MG, Arendt A, Larsen C, Rich J, Murphy N, 2014. Alaskan National Park Glaciers – Status and Trends: Final Report. National Park Service, p. 188. Natural Resource Technical Report NPS/AKRO/NRTR—2014/922. https://irma.nps.gov/DataStore/DownloadFile/512577. [Google Scholar]
- St Louis VL, Rudd JWM, Kelly CA, Beaty KG, Flett RJ, Roulet NT, 1996. Production and loss of methylmercury and loss of total mercury from boreal forest catchments containing different types of wetlands. Environ. Sci. Technol. 30, 2719–2729. 10.1021/es950856h. [DOI] [Google Scholar]
- Łuczyńska J, Paszczyk B, Łuczyński MJ, 2018. Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol. Environ. Saf. 153, 60–67. 10.1016/j.ecoenv.2018.01.057 [DOI] [PubMed] [Google Scholar]
- Mathisen OA, Parker PL, Goering JJ, Kline TC, Poe PH, Scanlan RS, 1988. Recycling of marine elements transported into freshwater by anadromous salmon. Verhandlungen der Int. Vereinigung für Theor. und Angew. Limnol 23, 2249–2258. [Google Scholar]
- Miller TP, McGimsey RG, Richter DJ, Riehle JR, Nye CJ, Yount ME, Dumoulin JA, 1998. Catalog of the Historically Active Volcanoes of Alaska. U.S. Geological Survey, p. 104. Open File Report 98-582. https://pubs.usgs.gov/of/1998/0582/report.pdf. [Google Scholar]
- Minagawa M, Wada E, 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochem. Cosmochim. Acta 48, 1135–1140. 10.1016/0016-7037(84)90204-7. [DOI] [Google Scholar]
- Miskimmin BM, Rudd WM, Kelly CA, 1992. Influence of dissolved organic carbon, pH, and microbial respiration rates on mercury methylation and demethylation in lake water. Can. J. Fish. Aquat. Sci 49, 17–22. [Google Scholar]
- Munk LA, Cohn B, Finney B, 2010. Historical Trace Element Trends Recorded in Lake Sediment Cores from the Southwest Alaska Network of Parks. National Park Service, p. 40. Natural Resource Technical Report NPS/SWAN/NRTR—2010/395. https://irma.nps.gov/DataStore/Reference/Profile/2166026. [Google Scholar]
- Nagorski SA, Engstrom DR, Hudson JP, Krabbenhoft DP, Hood E, Dewild JF, Aiken GR, 2014. Spatial distribution of mercury in southeastern Alaskan streams influenced by glaciers, wetlands, and salmon. Environ. Pollut 184, 62–72. 10.1016/j.envpol.2013.07.040. [DOI] [PubMed] [Google Scholar]
- Nagorski SA, Vermilyea AW, Lamborg CH, 2021. Mercury export from glacierized Alaskan watersheds as influenced by bedrock geology, watershed processes, and atmospheric deposition. Geochem. Cosmochim. Acta 304, 32–49. 10.1016/j.gca.2021.04.003. [DOI] [Google Scholar]
- National Park Service (NPS), 2006. Southwest Alaska Network Lakes, https://irma.nps.gov/DataStore/Reference/Profile/2216048.
- Neter J, Wasserman W, Kutner MH, 1990. Applied Linear Statistical Models, Third, ed. CRC Press, Homewood, Illinois. [Google Scholar]
- Ogorek JM, Lepak RF, Hoffman JC, DeWild JF, Rosera TJ, Tate MT, Hurley JP, Krabbenhoft DP, 2021. Enhanced susceptibility of methylmercury bioaccumulation into seston of the Laurentian Great Lakes. Environ. Sci. Technol 55, 12714–12723. 10.1021/acs.est.1c02319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C, Howard D, Moore C, Obrist D, 2019. Mercury and trace metal wet deposition across five stations in Alaska: controlling factors, spatial patterns, and source regions. Atmos. Chem. Phys 19, 6913–6929. 10.5194/acp-19-6913-2019. [DOI] [Google Scholar]
- Perga ME, Gerdeaux D, 2005. Are fish what they eat” all year round? Oecologia 144, 598–606. 10.1007/s00442-005-0069-5. [DOI] [PubMed] [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD, 2002. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc. Natl. Acad. Sci. USA 99, 4419–4423. 10.1073/pnas.072531099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M., 2017. JAGS Version 4.3.0 User Manual, https://people.stat.sc.edu/hansont/stat740/jags_user_manual.pdf. [Google Scholar]
- Power M, Klein GM, Guiguer KRRA, Kwan MKH, 2002. Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. J. Appl. Ecol 39, 819–830. 10.1046/j.1365-2664.2002.00758.x. [DOI] [Google Scholar]
- R Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.r-project.org/. [Google Scholar]
- Redick RR, 1967. A Review of Literature on Lake Trout Life History with Notes on Alaskan Management. Alaska Department of Fish and Game, p. 21. Informational Leaflet 111. https://www.adfg.alaska.gov/fedaidpdfs/afrbIL.111.pdf. [Google Scholar]
- Reynolds JH, Trammell EJ, Taylor JJ, 2018. Migration’s foundation: ecological intactness of Alaska’s ecosystems. Alaska Park Sci. 17, 85–91. https://www.nps.gov/artides/aps-17-1-13.htm. [Google Scholar]
- Ricker WE, 1975. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can 191, 1–382. [Google Scholar]
- Ricker WE, 1979. Growth rates and models. In: Hoar WS, Randall DJ, Brett JR (Eds.), Fish Physiology. Academic Press, New York, pp. 677–743. [Google Scholar]
- Roberts-Pierel BM, Kirchner PB, Kilbride JB, Kennedy RE, 2022. Changes over the last 35 years in Alaska’s glaciated landscape: a novel deep learning approach to mapping glaciers at fine temporal granularity. Rem. Sens 14 10.3390/rs14184582. [DOI] [Google Scholar]
- Russell R., 1980. A Fisheries Inventory of Waters in the Lake Clark National Monument Area. Alaska Department of Fish and Game, p. 197. https://www.arlis.org/docs/vol1/I/8205765.pdf. [Google Scholar]
- Sandheinrich MB, Weiner JG, 2011. Methylmercury in freshwater fish recent advances in assessing toxicity of environmentally relevant exposures. In: Beyer WN, Meador JP (Eds.), Environmental Contaminants in Biota: Interpreting Tissue Concentrations. CRC Press, Boca Raton, pp. 169–189. [Google Scholar]
- Schartup AT, Qureshi A, Dassuncao C, Thackray CP, Harding G, Sunderland EM, 2018. A model for methylmercury uptake and trophic transfer by marine plankton. Environ. Sci. Technol 52, 654–662. 10.1021/acs.est.7b03821. [DOI] [PubMed] [Google Scholar]
- Schindler DW, 2009. Lakes as sentinels and integrators for the effects of climate change on watersheds, airsheds, and landscapes. Limnol. Oceanogr 54, 2349–2358. 10.4319/lo.2009.54.6_part_2.2349. [DOI] [Google Scholar]
- Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS, 2010. Population diversity and the portfolio effect in an exploited species. Nature 465, 609–612. 10.1038/nature09060. [DOI] [PubMed] [Google Scholar]
- Schuster PF, Krabbenhoft DP, Naftz DL, Cecil LD, Olson ML, Dewild JF, Susong DD, Green JR, Abbott ML, 2002. Atmospheric mercury deposition during the last 270 years: a glacial ice core record of natural and anthropogenic sources. Environ. Sci. Technol 36, 2303–2310. 10.1021/es0157503. [DOI] [PubMed] [Google Scholar]
- Seekell DA, Lapierre J-F, Ask J, Bergström A-K, Deininger A, Rodríguez P, Karlsson J, 2015. The influence of dissolved organic carbon on primary production in northern lakes. Limnol. Oceanogr 60, 1276–1285. 10.1002/lno.10096. [DOI] [Google Scholar]
- Stafford CP, Hansen B, Stanford JA, 2004. Mercury in fishes and their diet items from Flathead Lake, Montana. Trans. Am. Fish. Soc 133, 349–357. 10.1577/02-156. [DOI] [Google Scholar]
- Stolpmann L, Coch C, Morgenstern A, Boike J, Fritz M, Herzschuh U, Stoofleichsenring K, Dvornikov Y, Heim B, Lenz J, Larsen A, Anthony KW, Jones B, Frey K, Grosse G, 2021. First pan-Arctic assessment of dissolved organic carbon in lakes of the permafrost region. Biogeosciences 18, 3917–3936. 10.5194/bg-18-3917-2021. [DOI] [Google Scholar]
- Streets DG, Devane MK, Lu Z, Bond TC, Sunderland EM, Jacob DJ, 2011. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol 45, 10485–10491. 10.1021/es202765m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui MTK, Finlay JC, 2011. Influence of dissolved organic carbon on methylmercury bioavailability across Minnesota stream ecosystems. Environ. Sci. Technol 45, 5981–5987. 10.1021/es200332f. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA), 1997. Method 300.1:Determination of Inorganic Anions in Drinking Water by Ion Chromatography. U.S. Environmental Protection Agency, p. 40. https://www.epa.gov/sites/default/files/2015-06/documents/epa-300.1.pdf. [Google Scholar]
- United States Environmental Protection Agency (USEPA), 1998a. Method 1630: Methyl Mercury in Water by Distillation, Aqueous Ethylation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. U.S. Environmental Protection Agency, p. 55. https://www.epa.gov/sites/default/files/2015-08/documents/method_1630_1998.pdf. [Google Scholar]
- United States Environmental Protection Agency (USEPA), 1998b. Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. U.S. Environmental Protection Agency, p. 17. https://www.epa.gov/sites/default/files/2015-12/documents/7473.pdf. [Google Scholar]
- United States Environmental Protection Agency (USEPA), 2002. Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. U.S. Environmental Protection Agency, p. 45. https://www.nemi.gov/methods/method_summary/9628/. [Google Scholar]
- United States Environmental Protection Agency (USEPA), 2004. Method 9060A: Total Organic Carbon. U.S. Environmental Protection Agency, p. 5. https://www.epa.gov/hw-sw846/sw-846-test-method-9060a-total-organic-carbon. [Google Scholar]
- Vander Zanden MJ, Shuter BJ, Lester N, Rasmussen JB, Leibold AEM, 1999. Patterns of food chain length in lakes: a stable isotope study. Am. Nat 154, 406–416. 10.1086/303250. [DOI] [PubMed] [Google Scholar]
- Varekamp JC, Buseck PR, 1981. Mercury emissions from mount St helens during September 1980. Nature 293, 555–556. [Google Scholar]
- Vynne C, Dovichin E, Fresco N, Dawson N, Joshi A, Law BE, Lertzman K, Rupp S, Schmiegelow F, Trammell EJ, 2021. The importance of Alaska for climate stabilization, resilience, and biodiversity conservation. Front. For. Glob. Chang 4 10.3389/ffgc.2021.701277. [DOI] [Google Scholar]
- Wells A, Macander M, Jorgenson T, Christopherson T, Baird B, Trainor E, 2013. Ecological Land Survey and Soil Landscapes Map for Lake Clark National Park and Preserve, Alaska, 2011. National Park Service, p. 376. Natural Resource Technical Report NPS/LACL/NRTR—2013/693. https://irma.nps.gov/DataStore/Reference/Profile/2193315. [Google Scholar]
- Wells AF, Frost GV, Macander MJ, Ives SL, McNown RW, 2021. Ecological Land Survey and Soils Inventory for Katmai National Park and Preserve, 2016–2017. National Park Service, p. 416. 10.36967/nrr-2287466. [DOI] [Google Scholar]
- Natural Resource Report NPS/KATM/NRR—2021/2298. Westcott K, Kalff J, 1996. Environmental factors affecting methyl mercury accumulation in zooplankton. Can. J. Fish. Aquat. Sci 53, 2221–2228. 10.1139/f96-178. [DOI] [Google Scholar]
- Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM, 2003. Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr., Cairns J Jr (Eds.), Handbook of Ecotoxicology. CRC Press, Boca Raton, pp. 407–461. [Google Scholar]
- Wiener JG, Knights BC, Sandheinrich MB, Jeremiason JD, Brigham ME,Engstrom DR, Woodruff LG, Cannon WF, Balogh SJ, 2006. Mercury in soils, lakes, and fish in Voyageurs National Park (Minnesota): importance of atmospheric deposition and ecosystem factors. Environ. Sci. Technol 40, 6261–6268. 10.1021/es060822h. [DOI] [PubMed] [Google Scholar]
- Wiener JG, Bodaly RA, Brown SS, Lucotte M, Newman MC, Porcella DB, Reash RJ, Swain EB, 2007. Monitoring and evaluating trends in methylmercury accumulation in aquatic biota. In: Harris RC, Krabbenhoft DP, Mason R, Murray MW, Reash RJ, Saltman T (Eds.), Ecosystem Responses to Mercury Contamination: Indicators of Change. CRC Press/Taylor and Francis, Boca Raton, pp. 87–122. [Google Scholar]
- Wilkens AX, 2002. The Limnology of Lake Clark, Alaska. In: Thesis MS (Ed.). University of Alaska Fairbanks, p. 148. https://irma.nps.gov/DataStore/Reference/Profile/552342. [Google Scholar]
- Zhang X, Naidu AS, Kelley JJ, Jewett SC, Dasher D, Duffy LK, 2001. Baseline concentrations of total mercury and methylmercury in salmon returning via the Bering Sea (1999-2000). Mar. Pollut. Bull 42, 993–997. 10.1016/S0025-326X(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Zheng J, Fisher D, Koerner R, Zdanowicz C, Bourgeois C, Hall G, Pelchat P, Shotyk W, Krachler M, Ke F, 2009. Temporal studies of atmospheric Hg deposition with ice cores and snow in the Canadian High Arctic. In: Smith S, Stow J, Edwards J (Eds.), Synopsis of Research Conducted under the 2008–2009 Northern Contaminants Program. Indian and Northern Affairs Canada, Ottawa, pp. 221–225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for this study are available at https://doi.org/10.5066/P9UEP9C5.