Abstract
The iviVII gene of Vibrio cholerae was previously identified by a screen for genes induced during intestinal infection. In the present study, nucleotide sequence analysis revealed that iviVII is a 1,659-bp open reading frame, herein designated vieB, that is predicted to be last in a tricistronic operon (vieSAB). The deduced amino acid sequence of VieS exhibited similarity to the sensor kinase component, and those of VieA and VieB were similar to the response regulator components, respectively, of the two-component signal transduction family. Analysis of transcriptional fusions to a site-specific DNA recombinase reporter, tnpR, revealed that vieS and vieA are transcribed during in vitro growth in a vieAB-independent and vieA-dependent manner, respectively. In contrast, transcription of vieB occurred exclusively during infection and was not dependent upon VieB. We conclude that the vieSAB genes are differentially regulated, at least during laboratory growth. Use of a V. cholerae strain harboring a vieB::tnpR transcriptional fusion allowed the kinetics and location of vieB expression within the intestine to be determined. We found that vieB transcription is induced shortly after infection of the proximal and mid-small intestine.
Vibrio cholerae is a highly motile gram-negative bacterium and is the causative agent of epidemic cholera. Since an aquatic environmental reservoir exists for this intestinal pathogen, it is reasonable to hypothesize that the expression of genes endowing the appropriate physiology and virulence attributes for human infection is up-regulated upon entry of the bacteria into the human host. We know for example, that in El Tor biotype strains, which cause almost all cholera in the world at present (26), it is difficult to detect the expression of genes within the ToxR/ToxT virulence gene regulon during growth in standard laboratory media; however, upon entry into the host intestinal environment, this regulon undergoes significant induction (3, 18). This regulon includes many of the known virulence factors of V. cholerae such as the phage-encoded cholera toxin structural genes and the toxin-coregulated pilus biosynthetic operon (15, 24).
To learn more about the physiology and virulence of V. cholerae during infection, we recently developed a genetic screen that utilized gene fusions to a site-specific DNA recombinase to identify transcription units that were specifically induced during infection in an infant mouse model of cholera (5). Among the transcripts identified was iviVII, whose transcription was silent during growth of the bacteria in a rich medium but was induced during infection. Initial DNA sequence characterization of iviVII revealed an open reading frame (ORF) that lacked amino acid similarity to known proteins. An insertion mutation within this ORF resulted in a slight reduction in colonization ability in an infant-mouse competition assay (5). These initial results suggested that iviVII encoded a polypeptide(s) that was expressed only during infection and that played a role in intestinal colonization.
In the present study, we have extended our characterization of iviVII to include a complete nucleotide sequence analysis and comparisons of the transcriptional activity of this gene during in vitro growth and during infection of the infant-mouse intestinal tract. iviVII was found to be the last gene, herein renamed vieB, of a putative tricistronic operon that encodes a sensor kinase (VieS) and two distinct response regulatory proteins (VieA and VieB). Evidence for differential transcriptional regulation of the vie genes during in vitro growth was obtained. vieB was found to be transcribed exclusively within the intestine and not in vitro. Finally, a recombinase gene fusion to vieB was used to localize its initial transcriptional induction to the proximal and mid-small intestine at an early time in the infectious process.
MATERIALS AND METHODS
DNA sequencing and analysis.
Using the previously determined DNA sequence of an internal portion of vieB (iviVII in reference 5), inverse PCR (16) was used to amplify adjacent regions for sequence determination (data not shown). Cycle sequencing with fluorescent dideoxynucleotides was performed and analyzed on an ABI 373A automatic sequencer as specified by the manufacturer (PE Applied Biosystems).
DNA sequences were analyzed for ORFs with DNA STRIDER version 1.2 (C. Marck, Cedex, France). Start codons within ORFs were assigned based on visual inspection for appropriately spaced ribosome-binding sequences. Putative amino acid sequences were used to search for similar polypeptide sequences contained in the National Center for Biotechnology Information nonredundant protein database on 9 October 1997 with the BLAST algorithm (1). Multiple alignments of conserved protein domains were performed with the PILEUP program (Genetics Computer Group, version 9.0).
Plasmid constructions.
All plasmids used are mobilizable suicide plasmids and are listed in Table 1. Transcriptional fusions to vie genes were constructed by integration of pIVET5 (5) derivatives within the vie locus. Plasmid pAC301 is a derivative of pIVET5 used as an intermediate in some plasmid constructions. A 1-kbp kanamycin resistance (Kmr) gene flanked on both sides by both SfiI and BamHI restriction endonuclease recognition sequences was ligated into the unique BglII site in pIVET5 to generate pAC301. pAC301 was digested with SfiI, and the vector backbone DNA fragment was band purified on an agarose gel. This fragment was subsequently used in cloning some vie gene DNA fragments containing SfiI adapters on each end. The SfiI adapter used was 5′-CGTGGCCGCAC-3′, with the last three bases at the 3′ end unpaired. A 1,105-bp vieS′ fragment (bases 1089 to 2193 in Fig. 1 [see below] and in the GenBank submission) was amplified by PCR with primers 5′-GCAACAACAGAGTGGTTTG-3′ and 5′-GGCAAAGGGTTTTTCTTCCAT-3′ with Pfu DNA polymerase (Stratagene). SfiI adapters were ligated onto the PCR product ends, and the ligation product was purified away from unincorporated primers and adapters and subsequently ligated into the SfiI-digested pAC301 backbone to generate pAC303. This SfiI cloning method has the advantage that intramolecular ligations of vector and insert are prohibited (data not shown). pAC258 is a derivative of pCRII (Invitrogen) containing a 555-bp inverse PCR product of vieA (bases 5637 to 6148 and 6194 to 6236) generated with AflIII-digested AC-V51 chromosomal DNA and the primers 5′-ATGTATGGCGTTGGGCTATCG-3′ and 5′-CACCTCATGCTCCGTCATCTC-3′. Plasmid pAC258 was digested with Sau3AI to release a 403-bp internal fragment of vieA (bases 5669 to 6071), which was subsequently ligated into the unique BglII site of pIVET5 to generate pAC298. Plasmid pSL116 is a derivative of pIVET5. A 4,783-bp vieSA′ fragment (bases 755 to 5537) was amplified by PCR with the primers 5′-GCTCTAGACGATAACGCTCCGCATTGAT-3′ and 5′-GCTCTAGACCATCTGCGGCATTCGAATA-3′, which contained the restriction endonuclease recognition site for XbaI within their 5′ termini. The PCR-amplified product was digested with XbaI and subsequently ligated into the unique XbaI site of pIVET5.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and phenotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| SM10λpir | thi recA thr leu tonA lacY supE RP4-2-Tc::Mu λ::pir | Laboratory strain |
| DH5αλpir | F− Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 λ::pir | 10, 12 |
| AC-E298 | Sm10λpir (pAC298) | This work |
| AC-E303 | Sm10λpir (pAC303) | This work |
| AC-E355 | Sm10λpir (pSL116) | This work |
| V. cholerae strains | ||
| AC-V51 | C6709-1, El Tor biotype, Smr | 19 |
| AC-V66 | AC-V51 lacZ::res1-tet-res1, Tcr | 5 |
| AC-V186 | AC-V51 iviVII::pGP704 | 5 |
| AC-V232 | AC-V66 iviVII::pIVET5 | 5 |
| AC-V296 | AC-V66 vieA::pIVET5 | This work |
| AC-V311 | AC-V66 vieS::pIVET5 | This work |
| AC-V336 | AC-V66 ΔvieAB vieS::pIVET5 | This work |
| AC-V279 | AC-V66 ΔvieAB | This work |
| AC-V282 | AC-V51 ΔvieSAB | This work |
| AC-V323 | AC-V51 ΔvieB | This work |
| AC-V354 | AC-V66 vieSA::pIVET5 | This work |
| Plasmids | ||
| pIVET5 | oriR6K mobRP4 tnpR-lacZY, Apr | 5 |
| pCVD442 | oriR6K mobRP4 sacB, Apr | 7 |
| pAC301 | pIVET5::SfiI-Neor-SfiI | This work |
| pAC298 | pIVET5::′vieA′ | This work |
| pAC303 | pIVET5::vieS′ | This work |
| pAC274 | pCVD442::ΔvieAB | This work |
| pAC275 | pCVD442::ΔvieSAB | This work |
| pSL108 | pCVD442::ΔvieB | This work |
| pSL116 | pIVET5::vieSA′ | This work |
FIG. 1.
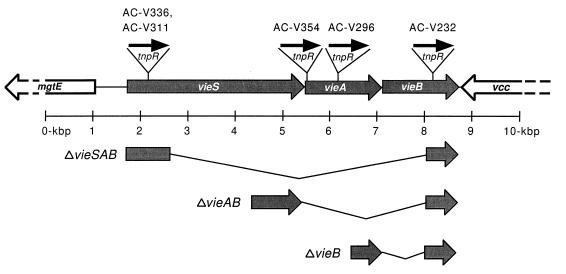
Genetic organization of the V. cholerae vie genes. The vieSAB genes are flanked by a partially sequenced, divergently transcribed ORF designated mgtE and a partially sequenced, convergently transcribed ORF designated vcc. The sites of the transcriptional fusions in strains AC-V311, AC-V336, AC-V354, AC-V296, and AC-V232 are shown at the top. The flanking sequences used to construct in-frame deletion mutations are shown at the bottom along with the corresponding deleted regions, designated by the thin angled lines.
Plasmids used for generating in-frame deletion mutations were constructed in pCVD442 which contains the counterselectable marker sacB (7). Plasmid pAC274 (ΔvieAB) was constructed by ligating a 997-bp ′vieSA′ PCR-generated fragment (bases 4393 to 5389) and a 603-bp ′vieB PCR-generated fragment (bases 8162 to 8764) into pCVD442. The "vieSA" fragment was amplified with the primers 5′-GCGGTCGACGTGAAGAGTGACTTTGAGC-3′ and 5′-CGAGCTCGCTCATCTTCTACTATCATTATT-3′, which contained restriction endonuclease recognition sequences for SalI and SacI within their 5′ ends, respectively. The ′vieB fragment was amplified with the primers 5′-GCGAGCTCATAAAGGCCAGCATGAAGATC-3′ and 5′-TGTAAACGATAGCGACTACGA-3′, where the former primer contained the recognition sequence for SacI within its 5′ end. The PCR-amplified products were digested with SalI and SacI and ligated at their SacI cohesive termini. This ligation product was subsequently ligated into pCVD442 digested with SalI and SmaI. To construct pAC275 (ΔvieSAB), PCR was used to generate a 1,046-bp vieS′ fragment (bases 1592 to 2637) with the primer 5′-GCTCTAGATGCTTGGGGTTGAATAAAATA-3′, which contained an XbaI recognition sequence within its 5′ end, and 5′-GCCCGCATGCCAATATGACATCGGAAATAA-3′, which contained an SphI recognition sequence within its 5′ end. Next, PCR was used to generate a 603-bp ′vieB fragment (bases 8163 to 8765) with the primer 5′-GCCCGCATGCTAAAGGCCAGCATGAAGATC-3′, which contained an SphI recognition sequence within its 5′ end, and the primer 5′-TGTAAACGATAGCGACTACGA-3′. Both PCR products were digested with XbaI and SphI, ligated, and then ligated into pCVD442-digested with XbaI and SmaI. Plasmid pSL108 (ΔvieB) was a derivative of pAC274. The 997-bp ′vieSA′ fragment (bases 4393 to 5389) was removed from pAC274 and replaced with a 633-bp ′vieAB′ fragment (bases 6509 to 7141) that was generated by PCR with primers 5′-GCTCTAGAATCGTCAGTGTTTAGG-3′ and 5′-CGGAGCTCTAGGTACAGCCATAACTCT-3′ containing recognition sequences for XbaI and SacI within their 5′ ends, respectively. The PCR product was digested with XbaI and SacI and then ligated into the pAC274 plasmid backbone after the removal of the ′vieSA′ fragment by prior digestion with XbaI and SacI and band purification on an agarose gel.
Construction of bacterial strains.
The bacterial strains used in this study are listed in Table 1. The transcriptional gene fusion strains AC-V296, AC-V311, and AC-V354 were constructed by mating V. cholerae AC-V66 with E. coli AC-E298, AC-E303, and AC-E355, respectively. Plasmids pAC298, pAC303, and pSL116 were first moved into SM10λpir by electroporation with selection on Luria-Bertani (LB) agar supplemented with 50 μg of ampicillin per ml. SM10λpir has transfer functions for mobilizing the mobRP4-containing plasmids used in this study (Table 1). Each resultant E. coli donor strain was mixed at approximately a 1:1 ratio with the streptomycin-resistant (Smr) recipient strain AC-V66 and mated on LB agar for 4 h at 37°C. Each mating mixture was subsequently streaked onto LB agar supplemented with 50 μg of ampicillin per ml and 100 μg of streptomycin per ml and then incubated overnight at 37°C to select exconjugates in which the suicide plasmid had integrated into the recipient chromosome. Integration occurred by single-crossover homologous recombination between the chromosome and plasmid-containing vie gene sequences. Exconjugates were colony purified and stored at −75°C in 50% glycerol.
Strains harboring in-frame deletion mutations in vie genes were constructed by allelic exchange in AC-V51, the virulent Smr V. cholerae strain C6709-1 (El Tor biotype). The ΔvieSAB, ΔvieAB, and ΔvieB deletion strains were constructed with pAC275, pAC274, and pSL108, respectively. Each of these plasmids was conjugated into AC-V51 by bacterial mating as described above. Approximately 10 Apr and Smr exconjugate colonies were pooled and passaged for approximately 20 generations in LB broth at 37°C to allow allelic exchange to take place. The final culture was diluted 1/10,000, 100 μl was plated on 2× YT (1.6% tryptone, 1% yeast extract, 0.5% [wt/vol] NaCl) plates supplemented with 10% sucrose, and the plates were incubated overnight at 30°C. Several sucrose-resistant colonies were colony purified and subsequently screened by PCR and Southern blot analyses for loss of the integrated plasmid and retention of the deletion allele on the chromosome.
In vitro transcription assays.
V. cholerae transcriptional fusion strains were grown to stationary phase at 37°C with aeration in LB broth containing 100 μg of streptomycin per ml, 30 μg of ampicillin per ml, and 1 μg of tetracycline per ml. These cultures were diluted 1/2,000 into the following media and grown at 37°C with aeration until stationary phase unless indicated otherwise. For strain AC-V232, dilutions were made into the following: LB broth; M9 minimal medium–0.2% glucose–0.05% (wt/vol) Casamino Acids; LB–0.2% or 0.4% (wt/vol) horse bile (Sigma); LB–1, 10, 20, or 50% (vol/vol) 5-day-old CD-1 mouse (Charles River Breeding Laboratories) intestinal homogenate (made by mechanically shearing one small intestine in 0.8 ml of LB broth); syncase broth (8); AKI broth (11); low-iron LB broth containing 0.1 mM dipyridyl (Sigma); low-magnesium M9 minimal medium containing 0.2% (wt/vol) glucose, 0.05% (wt/vol) Casamino Acids, and 50 μM MgCl2; low-nitrogen-source M9 minimal medium containing 0.2% glucose and 0.05% Casamino Acids but lacking NH4Cl; LB broth buffered with HCl to pH 8, pH 7, pH 6, or pH 5.7; and LB broth containing 5 mM glutathione. All other V. cholerae fusion strains were diluted in LB broth. Serial dilutions of the resulting stationary-phase cultures were plated on LB agar containing 100 μg of streptomycin per ml. Strain AC-V232 was also grown extracellularly on two intestinal cell lines as follows. Overnight cultures of AC-V232 were washed with fresh LB broth, and 105 CFU was used to infect semiconfluent monolayers of CaCo-2 (20) and the mucus-producing HCT-8 (25) cell lines (kindly provided by D. W. Acheson) grown in modified Eagle’s medium (Sigma) at 37°C in a 5% CO2 atmosphere. After 30 min, the monolayers were washed with phosphate-buffered saline to remove nonadherent V. cholerae, fresh medium was added, and the incubation was continued for another 2 h at 37°C in 5% CO2. The monolayers were disrupted with Triton X-100, and serial dilutions were plated on LB agar containing 100 μg of streptomycin per ml. After each of the above treatments, the percentage of colonies that were Tcs was determined by replica plating colonies onto LB agar containing 2 μg of tetracycline per ml and incubating the replica plates at 37°C for 8 h. Loss of Tcr resulted from TnpR-mediated excision of the res1-tet-res1 cassette from the chromosome and was a measure of the transcriptional activity of the corresponding vie gene fusion to tnpR-lacZY (4). For some strains, the β-galactosidase activity of the vie gene fusion to tnpR-lacZY was measured visually after growth on LB agar containing 50 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml.
In vivo transcription assays.
V. cholerae transcriptional fusion strains were grown overnight to stationary phase at 30°C with aeration in LB broth containing 100 μg of streptomycin per ml, 30 μg of ampicillin per ml, and 1 μg of tetracycline per ml. Approximately 106 CFU of each overnight culture diluted in 50 μl of LB broth was used to intragastrically inoculate 5-day-old CD-1 mice as previously described (4). After 24 h, the bacteria were recovered from the small intestines by homogenization as previously described (4) and approximately 200 CFU was grown on LB agar containing 100 μg of streptomycin per ml. In addition, the overnight cultures used as inocula were serially diluted and approximately 200 CFU was grown on LB agar containing 100 μg of streptomycin per ml. The percent Tcs CFU was determined for both inocula and intestinally grown bacteria by replica plating as described above.
To determine the temporal transcription pattern of vieB during infection of the mouse small intestine, AC-V232 was grown to stationary phase and used to intragastrically inoculate 5-day-old CD-1 mice as above. At 1, 2, 3, 4, 5, 7.5, 10, and 14 h postinfection, the small intestines were removed from three animals and homogenized separately in 5 ml of LB broth containing 15% glycerol. Approximately 200 CFU was plated on LB agar containing 100 μg of streptomycin per ml and incubated overnight at 37°C. For each time point, the total number of bacteria was determined, as well as the percent Tcs CFU.
For spatial determination of vieB transcription during infection of the mouse small intestine, AC-V232 was grown to stationary phase and used to intragastrically inoculate infant mice as above. At 3.5 h postinfection, the stomach and small intestine together with the cecum were removed. The small intestine and cecum were laid out straight and dissected into 10 equally spaced segments (approximately 1.5 cm per segment). Each segment was then homogenized in 4.5 ml of LB broth containing 15% glycerol. The total number of bacteria and the percent Tcs CFU were determined for each segment by plating serial dilutions and replica plating as above.
Competition assays of mutant strains.
Each Lac+ V. cholerae mutant test strain and the Lac− derivative of wild-type strain C6709-1 (AC-V66) were grown to stationary phase at 30°C in LB broth containing 100 μg of streptomycin per ml and then mixed at a 1:1 ratio and diluted 1/2,000 in LB broth. Approximately 106 CFU of each mixture was used to intragastrically inoculate eight 5-day-old CD-1 mice, and the infections were allowed to proceed for 24 h. Each in vivo competition was accompanied by an in vitro competition assay with the same inoculum. The in vitro competition assay was done by diluting a portion of the original inoculum 1/100 in LB broth and incubating it for 24 h at 37°C with aeration. Finally, the precise ratio of test strain (Lac+) to virulent strain (Lac−) was determined for each inoculum, in vitro competition, and in vivo competition by plating serial dilutions on LB agar containing 100 μg of streptomycin per ml and 50 μg of X-Gal per ml as previously described (5). The plates were incubated overnight at 37°C, and the numbers of Lac+ and Lac− colonies were counted. The ratios for in vitro and in vivo competitions were corrected for deviations in the inoculum ratio from a value of 1:1.
Nucleotide sequence accession number.
The vieSAB genes and flanking sequences have been deposited in GenBank under accession no. AF031552.
RESULTS
Sequence analysis of the V. cholerae vieSAB locus.
In an earlier study, the sequence of a portion of vieB (originally referred to as iviVII) was determined (5). The deduced amino acid sequence had no similarity to known proteins. To determine the complete amino acid sequence of VieB and to identify flanking genes which might be cotranscribed with vieB during infection, we determined the nucleotide sequence upstream and downstream of vieB on both strands. vieB was found to lie downstream of and in the same transcriptional orientation as two genes designated vieS and vieA (Fig. 1). The absence of identifiable factor-independent transcriptional terminators within or between the three vie genes, as well as the presence of nearly overlapping stop and start codons, suggested that these three genes may be cotranscribed as a tricistronic operon. Upstream of vieSAB was a divergently transcribed ORF designated mgtE, and downstream was a convergently transcribed ORF designated vcc (Fig. 1 and see below).
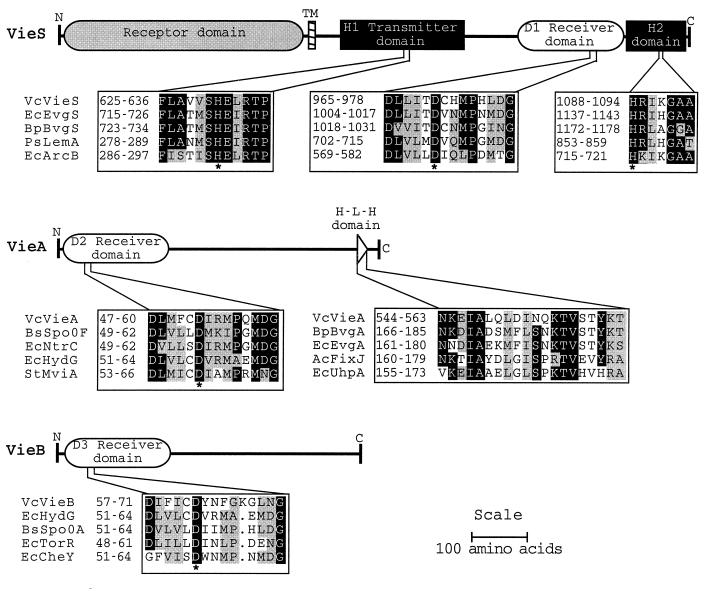
Similarity searches with the deduced amino acid sequences revealed that vieSAB codes for three proteins of the two-component signal transduction family. VieS is predicted to be a 1,147-amino-acid protein belonging to the subclass of complex sensor kinases such as ArcB and BvgS that contain three cytosolic domains, a transmitter (H1 with a conserved H631), a medial receiver domain (D1 with conserved D970), and a C-terminal transmitter (H2 with conserved H1088) (Fig. 2). The most similar protein to VieS from this subclass was BvgS from Bordetella pertussis, which regulates a set of virulence genes in response to sulfate anion, nicotinic acid, and temperature (2, 14). VieA is predicted to be a 584-amino-acid protein having a high degree of similarity to response regulators such as CheY (21) containing an N-terminal receiver domain (D2 with conserved D52) and C-terminal helix-turn-helix DNA-binding motif (Fig. 2). VieB is predicted to be a 553-amino-acid protein also having a high degree of similarity to response regulators such as CheY containing an N-terminal receiver domain (D3 with conserved D62) but lacking any apparent DNA-binding motif. The portions of VieB outside the receiver domain lacked significant similarity to other known proteins nor to VieA.
FIG. 2.
Schematic representations of VieS, VieA, and VieB, and multiple alignments of conserved domains characteristic of two-component signal transduction proteins. Identical residues are indicated by black squares, and functionally similar residues are indicated by gray squares. Conserved phosphorylated histidine and aspartate residues are indicated below by an asterisk. Protein domains in the alignments are numbered and are designated by the abbreviated species name joined to the gene name. Abbreviations: Vc, V. cholerae; Ec, E. coli; Bp, B. pertussis; Ps, Pseudomonas syringae; Bs, Bacillus subtilis; St, Salmonella typhimurium.
The partial gene sequence upstream of vieSAB encoded a putative polypeptide having 45% identity to amino acids 1 to 280 of the magnesium transporter MgtE of Providencia stuartii (23) and was thus referred to as mgtE. Likewise, the partial gene sequence downstream of vieSAB encoded a putative polypeptide having 75% identity to amino acids 471 to 562 of the secreted collagenase protein Vcc of Vibrio parahaemolyticus (13) and was thus referred to as vcc.
Transcriptional activity of vie genes in vitro and during infection.
vieB was previously shown to be transcriptionally induced during infection in an infant-mouse model of cholera (5). To test whether vieS and vieA, which may form a tricistronic operon with vieB, were also induced during infection, we constructed transcriptional fusions of each gene to the promoterless synthetic operon tnpR-lacZY. tnpR codes for the site-specific DNA recombinase enzyme, resolvase, from Tnγδ (17). When tnpR is expressed in a genetic background containing the artificial substrate cassette res1-tet-res1, excision of the tet gene occurs, resulting in a Tcs phenotype of daughter cells (4). Loss of Tcr in this system has been shown to be a sensitive ex post facto measure of transcription of the gene fusion (4). The sites of the vie gene fusions to tnpR-lacZY are shown at the top in Fig. 1. Plasmids containing 5′ fragments of vieS, vieA, or vieSA fused to tnpR-lacZY were transferred by conjugation into a V. cholerae strain which contained the res1-tet-res1 cassette integrated into the endogenous lacZ gene. These suicide plasmids integrated into the V. cholerae vie locus by homologous recombination (data not shown), resulting in generation of the merodiploid transcriptional fusion strains AC-V311, AC-V296, and AC-V354, respectively. The plasmid containing a 5′ fragment of vieS was also integrated into a lacZ::res1-tet-res1 strain in which the vieAB genes were deleted, to generate strain AC-V336. The vieB::tnpR-lacZY transcriptional fusion strain AC-V232 was constructed previously (5). The genotype and the predicted phenotype (based on polarity effects of the integrated plasmid) of each fusion strain are listed in Table 2.
TABLE 2.
Transcriptional activity of vie genes
| Strain | Genotype | Predicted phenotype | In vitroa β-gal activity | % Tcs CFUb
|
|
|---|---|---|---|---|---|
| In vitroc | In vivod | ||||
| AC-V311 | vieS::tnpR-lacZY | VieS+A+B+ | ++ | >80 | NDe |
| AC-V336 | vieS::tnpR-lacZY ΔvieAB | VieS+A−B− | ++ | >80 | ND |
| AC-V354 | vieSA::tnpR-lacZY | VieS+A+B+ | + | ∼50 | ND |
| AC-V296 | vieA::tnpR-lacZY | VieS+A−B− | ± | 0 | 0 |
| AC-V232 | vieB::tnpR-lacZY | VieS+A+B− | ± | 0 | 90 |
The β-galactosidase activity of colonies was observed visually on LB agar supplemented with 50 μg of X-Gal per ml; ± represents background levels, and ++ represents transcriptional activity.
% Tcs CFU was determined by replica plating colonies onto LB agar supplemented with 2 μg of tetracycline per ml and is a measure of the transcriptional activity of the corresponding fusion to tnpR-lacZY.
Cultures were grown in LB broth.
Bacteria recovered from intestinal infection of 5-day-old CD-1 mice.
ND, not determined.
The transcriptional activity of each vie gene fusion was measured qualitatively by assaying both β-galactosidase activity and loss of Tcr from the strains listed in Table 2. When grown on LB agar supplemented with X-Gal, colonies of AC-V232 (vieB::tnpR-lacZY in a vieB background) and AC-V296 (vieA::tnpR-lacZY in a vieA background) failed to show β-galactosidase activities above background levels. Likewise, both strains retained Tcr after in vitro growth in LB broth, indicating transcriptional silence of their respective vie gene fusions. Colonies of AC-V354 (vieSA::tnpR-lacZY in a vieS+A+B+ background) showed β-galactosidase activities equal to or slightly above background levels, and the more sensitive tnpR reporter revealed a 50% loss of Tcr in the cell population after growth in vitro. These results suggested that vieB was transcriptionally silent during in vitro growth while vieA was transcriptionally active during in vitro growth and its expression was autoregulatory. In contrast, colonies of AC-V311 (vieS::tnpR-lacYZ) and AC-V336 (vieS::tnpR-lacZY ΔvieAB) exhibited both significant levels of β-galactosidase expression and loss of Tcr after in vitro growth (Table 2). These results showed that vieS was transcriptionally active during in vitro growth and, furthermore, that this expression was independent of VieA and VieB. The sum of these results do not support our original hypothesis based on nucleotide sequence analysis that the vieSAB genes are cotranscribed. However, it remains possible that vieA and vieB, or perhaps all three genes, are cotranscribed during infection.
To extend our analysis of transcription of the vie genes to the intestinal environment, V. cholerae fusion strains lacking detectable expression during growth in vitro were intragastrically inoculated into 5-day-old mice and the bacteria recovered from the small intestine after 24 h were tested for TnpR-mediated loss of Tcr. AC-V296 (vieA::tnpR-lacZY) showed no loss of Tcr after infection (Table 2). In contrast, AC-V232 (vieB:: tnpR-lacZY) exhibited approximately 90% loss of Tcr during growth in the intestine (Table 2). These latter results confirmed our previous observation (5) that vieB is an infection-induced gene and, furthermore, showed that this expression does not require VieB. In contrast, since we were unable to detect the activity of a vieA::tnpR-lacZY transcriptional fusion either in vitro or in vivo but were able to detect transcription in a vieA+ background in vitro, it is likely that VieA is required for its own expression and perhaps for that of vieB as well.
Transcription assays of vieB under various in vitro growth conditions.
Thus far, transcriptional induction of vieB has been observed only during infection of the infant-mouse intestinal tract. To better understand the intestinal signal(s) necessary for induction of vieB transcription and to facilitate promoter mapping and Northern blot analysis of vieB transcripts, we sought to identify in vitro growth conditions that would induce vieB transcription. First, we tested whether simple manipulations of in vitro growth conditions might induce the transcription of vieB. Strain AC-V232 (vieB::tnpR-lacZY) was grown at 37°C in a minimal medium and under growth-limiting conditions for iron, magnesium, and nitrogen source. Limiting magnesium was chosen as a growth condition because of the close linkage of the vie operon to a putative magnesium transporter (mgtE in Fig. 1). None of these growth conditions induced transcription of the vieB::tnpR-lacZY fusion, as shown by no observed loss of Tcr (Table 3). Note that in prior studies we have shown that the sensitivity of this recombination assay is exquisite compared to that of traditional transcriptional fusion reporters such as phoA (4) or lacZ (unpublished results). Next, we tested a series of growth conditions thought to mimic one or more intestinal parameters. Strain AC-V232 was grown microaerophilically or aerobically in LB broth supplemented with bile salts, mouse intestinal homogenate, or glutathione (a reducing condition). None of the conditions described resulted in loss of Tcr (Table 3). To mimic the acid shock that V. cholerae may experience within the host stomach before passage into the small intestine, AC-V232 was grown in LB broth at growth-limiting acidic pH (Table 3). V. cholerae is acid sensitive and does not grow below a pH of 5.7 unless preshocked with acid (data not shown). These conditions also failed to induce vieB transcription. To ascertain whether ToxR/ToxT, the major known virulence regulators in V. cholerae (6), play a role in the transcriptional induction of vieB, strain AC-V232 was grown microaerophilically in AKI medium, a growth condition known to activate the ToxR/ToxT regulon in El Tor biotype strains (11). We observed no loss of Tcr, suggesting that vieB transcription is not induced by growth conditions known to induce the ToxR/ToxT regulon. Finally, AC-V232 was used to infect two intestinal cell lines, CaCo-2 and the mucus-producing cell line HCT-8. Although V. cholerae adhered well to both cell lines and multiplied extensively (data not shown), neither growth condition resulted in loss of Tcr (Table 3). The above results suggest that the parameter(s) which induce transcription of vieB is specific to the host intestinal environment.
TABLE 3.
Transcriptional assays of vieB
| Growth medium and conditiona | % Tcs CFUb |
|---|---|
| LB broth | 0 |
| M9 salts–0.2% (wt/vol) glucose–0.05% (wt/vol) CAA | 0 |
| Low-iron LB broth | 0 |
| Low-magnesium M9 salts–0.2% (wt/vol) glucose–0.05% CAA | 0 |
| M9 salts lacking NH4+–0.2% (wt/vol) glucose–0.05% CAA | 0 |
| Syncase broth (microaerophilic) | 0 |
| LB broth–0.2 or 0.4% (wt/vol) bile salts | 0 |
| LB broth–1, 10, 20, or 50% (vol/vol) intestinal homogenate | 0 |
| LB broth–5 mM glutathione | 0 |
| Acid shock at pH 8, 7, 6, or 5.7 | 0 |
| AKI (microaerophilic) (ToxR/ToxTON) | 0 |
| CaCo-2 and HCT-8 intestinal cell lines | 0 |
| Mouse intestinal infection | 80 |
Growth conditions are described in Materials and Methods. CAA, Casamino Acids.
The percent Tcs CFU was determined by replica plating colonies onto LB agar supplemented with 2 μg of tetracycline per ml and is a measure of the transcriptional activity of the vieB::tnpR-lacZY fusion.
Spatiotemporal studies of vieB transcription during infection.
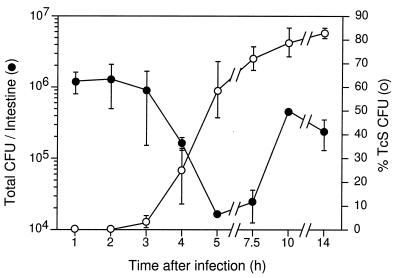
Our inability to induce vieB transcription during in vitro growth, coupled with our lack of knowledge of where the vieB promoter lies, greatly limits the experimental tools we can use to further characterize this gene. However, the resolvase gene fusion reporter system facilitated the further characterization of vieB expression during intestinal infection. Specifically, this reporter system allowed us to determine the spatial and temporal transcriptional induction patterns of vieB during the course of infection. To determine the earliest time of vieB transcriptional induction, we inoculated strain AC-V232 into several infant mice. Then, at various times after inoculation a small intestine was harvested, bacteria were recovered, and loss of Tcr was measured by replica plating. Both the total CFU and percent Tcs CFU were determined at each time point (Fig. 3). Transcriptional induction of the vieB::tnpR-lacZY fusion occurred as early as 3 h postinoculation and the percent Tcs CFU began to level off at approximately 80% after 5 h. The total CFU in the small intestine decreased approximately 20-fold by 5 h but then increased ∼15-fold over the next 5 h. These results demonstrate that only ∼5% of the inoculum was able to colonize the small intestine and initiate growth. In addition, these results suggest that the majority of the colonizing bacteria experienced the microenvironment(s) necessary to signal transcriptional induction of vieB.
FIG. 3.
Kinetics of transcriptional induction of vieB during infection. V. cholerae AC-V232 (vieB::tnpR-lacZY) was used to intragastrically inoculate infant CD-1 mice. At the postinoculation times indicated on the x axis, the small intestines were removed and homogenized. Total CFU per intestine, shown on the left axis, was determined by plating serial dilutions on agar medium. The percent Tcs CFU per intestine, shown on the right axis, was determined by replica plating. Each time point was investigated in triplicate, i.e., three animals were used per time point, and the means and standard deviations are shown.
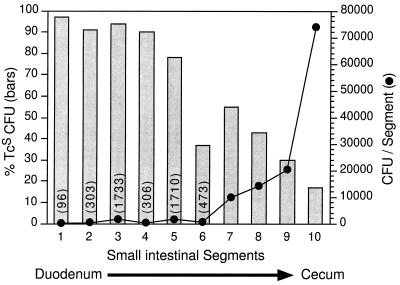
The results above indicated that vieB transcription was first induced at an early time during infection, i.e., 3 h postinoculation. We therefore selected 3.5 h postinoculation as an optimal time to determine the anatomic site of transcriptional induction of vieB within the intestinal tract. Strain AC-V232 was intragastrically inoculated into 5-day-old mice, and at 3.5 h postinoculation the stomach and the small intestine together with the cecum were removed. The tissue was sectioned into 10 segments of equal length, and bacteria were recovered from each segment. No cultivatable bacteria were recovered from the stomach; however, bacteria were recovered from each segment derived from the small intestine and cecum. The total CFU and percent Tcs CFU were determined for each segment (Fig. 4). Interestingly, the greatest percent loss of Tcr was seen in segments 1 through 5, corresponding to the proximal small intestine (duodenum and jejunum). In contrast, the majority of bacteria were found to reside in segments 7 through 10, corresponding to the distal small intestine (ileum) and cecum. These results show that although the proximal small intestine supports the colonization of only a fraction of the inoculum, it provides an inducing environment for vieB transcription.
FIG. 4.
Localization of the intestinal segment where vieB transcription is induced. V. cholerae AC-V232 (vieB::tnpR-lacZY) was used to intragastrically inoculate three infant CD-1 mice. At 3.5 h postinoculation, the small intestines and cecum were removed and dissected into 10 segments of equal length. The total CFU, shown on the right axis, and percent Tcs CFU (bars), shown on the left axis, were determined for each segment. The total CFU for segments 1 to 6 are shown in parentheses above each data point. The data in this figure are from one animal and are representative of the results found with the other two animals.
Role of the vieSAB genes in colonization.
We found previously that a V. cholerae strain in which vieB was disrupted by a plasmid insertion exhibited a slight but reproducible reduction in colonization ability as assessed by an infant-mouse competition assay (5). The possibility of negative effects on virulence associated with plasmid sequences being present in the V. cholerae chromosome was controlled for in those experiments by using a virulent isogenic competing strain that contained the same plasmid inserted into the endogenous lacZ gene. These results indicated a possible role for vieB in colonization. To test this possibility more rigorously, we constructed a nonpolar in-frame deletion mutation in vieB, as well as in vieAB and vieSAB, and then tested each mutant strain in the infant-mouse competition assay. In each case, allelic exchange was used to delete the majority of the coding region of each gene (Fig. 1). For the triple and double gene deletion mutations, a fusion of the remaining coding regions of vieS and vieB and of vieA and vieB, respectively, was generated as a result. Each Lac+ mutant strain was competed against a fully virulent isogenic Lac− strain, both in vitro in LB broth and in vivo in infant mice. Then bacteria recovered from the small intestines and from the in vitro competitions were plated on LB agar supplemented with X-Gal to allow enumeration of each strain. A ratio of test strain to virulent strain (the competitive index) that is less than 1 indicates a decreased colonization ability of the former. All three mutant strains had competitive indices of approximately 1 for both the in vitro and mouse competitions (Table 4). These results show that the vieSAB genes do not play a detectable role in growth in LB broth or in colonization of the infant-mouse small intestine by this competition assay. These results further suggest that the colonization defect observed in the original vieB mutant strain, which contained a plasmid insertion within vieB, may have been due to production of a truncated form of VieB during infection, since amino acids 1 to 350 of VieB is expected to be produced by that strain (data not shown).
TABLE 4.
Effects of vie gene deletions on in vitro growth and colonization of infant mice
| Strain | Genotype | Ratio of mutant to wild typea
|
|
|---|---|---|---|
| In vitro competitionb | In vivo competitionc | ||
| AC-V323 | ΔvieB | 1.3 | 1.1 |
| AC-V279 | ΔvieAB | 1.0 | 1.0 |
| AC-V282 | ΔvieSAB | 1.0 | 1.1 |
Ratio of test strain to virulent strain corrected for deviations in inoculum ratio from a value of 1:1.
Cultures were grown in LB broth at 37°C for 24 h.
Bacteria were recovered from the small intestines of infant CD-1 mice after 24 h. Each ratio represents the mean for eight animals.
DISCUSSION
In this study, we have determined the complete nucleotide sequence of the vieSAB genes from Vibrio cholerae. The deduced amino acid sequences revealed that vieSAB codes for three proteins which are predicted to be members of the two-component signal transduction family. This represents the first such system described for this intestinal pathogen. VieS belongs to a subclass of complex sensor kinases such as BvgS of Bordetella pertussis and ArcB of E. coli, which contain two transmitter domains surrounding a central receiver domain. A recent study (9) suggests an intramolecular phosphorelay model for this class of sensor kinases, whereby appropriate stimulation of the sensor domain results in autophosphorylation at the N-terminal transmitter domain followed by transphosphorylation of the medial receiver domain and then of the C-terminal transmitter domain; the last of these can then transfer the phosphate to its cognate response regulator, i.e., BvgA or ArcA in the examples above. In the case of the V. cholerae VieS sensor kinase, an additional level of complexity is introduced by the presence of two distinct response regulators, VieA and VieB, which are encoded by genes in an apparent operon with vieS. VieA and VieB both contain highly conserved N-terminal phosphoreceiver domains, but only VieA appears to have a C-terminal DNA-binding domain. Thus, the role of VieB is not clear. It may modulate the phosphorylation state of VieA indirectly by competing for phosphate from VieS. Alternatively, but not exclusively, VieB may serve as an effector protein having an activity unrelated to regulation of transcription.
In a previous study, we showed that vieB was an infection-induced gene that was transcriptionally silent during in vitro growth (5). In this study, transcriptional fusions of vieS and vieA to reporter genes encoding the site-specific DNA recombinase, resolvase, and the E. coli LacZY proteins revealed that vieS is transcribed during in vitro growth in a VieAB-independent manner. In contrast, vieA was transcriptionally active during in vitro growth only in the presence of VieA. Because vieA was found to be transcriptionally silent during infection in a vieA mutant background, vieA is also autoregulatory during infection or is simply not transcribed in the host. These data, coupled with our finding that transcription of vieB occurs only during infection and is VieB independent, lead to several potential regulatory schemes for vieS, vieA, and vieB. A likely scenario is that each of the three vie genes has its own distinct promoter, where that for vieS is constitutively active, that for vieA is autoregulatory both in vitro and during infection, and that for vieB is active only during infection and may rely on VieS and VieA for its induction. A second possibility is that vieA and vieB are cotranscribed via a single promoter which is VieA dependent and VieB independent but that transcription terminates prior to reaching vieB during in vitro growth. Because vieB is transcribed during infection, this latter scenario requires either antitermination during infection or a sufficient level of readthrough if vieA transcription was fully induced. Experiments are in progress to distinguish between these and other possible regulatory schemes. The differential transcription patterns of vieSAB observed thus far reveal that the vie genes are not cotranscribed as a tricistronic operon during in vitro growth.
In the course of infection, V. cholerae cells must encounter a variety of biochemical and nutritional parameters that constitute the microenvironments of the small intestine. Since vieB is an infection-induced gene, its transcription must be modulated by one or more of these parameters. Our attempts to induce transcription of vieB by mimicking some of these parameters in vitro were wholly unsuccessful. Transcription of vieB was not modulated by growth in a minimal medium, limiting divalent cations or nitrogen source, acid shock, or reducing conditions. Transcription of vieB was also not induced during growth under conditions known to induce genes within the ToxR/ToxT virulence gene regulon. We were also unable to induce transcription of vieB by introducing factors endogenous to the small intestine such as bile salts and intestinal homogenate. Growth of V. cholerae on two intestinal cell lines, CaCo-2 and the mucus-producing cell line HCT-8, also failed to induce transcription of vieB. These results suggest that the host intestinal parameter(s) which induces the transcription of vieB may be complex, i.e., requiring multiple signals for induction, and/or cryptic, i.e., requiring an unknown or simply untested parameter(s) for induction. To our knowledge, vieB represents the first bacterial gene characterized for which an in vitro growth condition capable of inducing its transcription has not been identified. Indeed, the identification and characterization of such a gene would be exceedingly difficult without a reporter system like that used in the present study (resolvase gene fusions), which provides a means to assay transcription during bacterial growth in complex environments such as the intestinal tract of an intact animal.
In this and in a previous study (5), vieB transcription was measured during infection by using a resolvase gene fusion reporter. In this report, we have expanded the use of this reporter technology to include anatomic and temporal determinations of vieB transcription during a bona fide infection. First, we determined that vieB transcription was induced sharply at an early time of infection (3 h). Then we made use of this result to determine the spatial pattern of vieB transcription at 3.5 h postinoculation, and we found that the proximal and mid-small intestine (duodenum and jejunum) contain an inducing environment for vieB.
A second result of these experiments was the finding that only 5% of the initial infecting population of V. cholerae colonizes the small intestine and then multiplies. This is in contrast to the result previously reported by Skorupski and Taylor (22), who found no drop in the number of bacteria recoverable from the small intestine at any time during the infection. This discrepancy may be due to the use in that study of classical biotype strains of V. cholerae, which may colonize the small intestine more efficiently than the El Tor biotype strains like that used in the present study. Thus, in our experiments, it is unclear what happens to the other 95% of the bacteria. Given that the number of bacteria increases greatly toward the distal small intestine and cecum (at 3.5 h postinoculation), it is likely that these “missing” bacteria simply passed through the lumen of the small intestine to the large intestine. Interestingly, induction of vieB transcription at this early time of infection occurred predominantly among the minor population of bacteria that colonized the proximal and mid-small intestine. We predict from these results that transcriptional induction of vieB occurs soon after colonization of the small intestine and that the majority of bacteria inoculated do not colonize this site and are thus not exposed to the inducing environment. Our data do not preclude the existence of other vieB-inducing environments further down the intestinal tract or at later times during infection. Our identification of the time and site of vieB transcriptional induction within the intestinal tract may aid in the identification of the exact host inducing parameter(s). This may require further dissection of the inducing environment, for example by recovering bacteria from different locations within the inducing region (lumen, mucus layer, epithelial surface) and assaying vieB expression with the resolvase or other reporter system.
There are several possible ramifications for the transcriptional induction of an infection-specific gene in relation to colonization ability of V. cholerae. One is the requirement of the gene product to assist attachment to host surfaces, and a second is to support survival or growth within the host. For example, mutations disrupting the V. cholerae toxin-coregulated pilus major subunit gene tcpA have been shown to cause a severe reduction in colonization (3, 18), presumably because the bacteria can no longer attach to mucosal surfaces in the small intestine. A plasmid insertion mutation in vieB caused a reduction in colonization but had no effect on in vitro growth (5). However, deletion mutations in vieB as well as in vieAB and vieSAB do not cause a detectable reduction in colonization by the same assay. The discrepancy between these two results may be due to production (during infection) of a truncated peptide of VieB in the plasmid insertion strain that is somewhat toxic to the bacteria. These results lead to yet a third ramification for the transcriptional induction of an infection-induced gene in V. cholerae, which is that such a gene, as is the case for the vieSAB genes, may not play a detectable, or any, role during colonization. Such a result may be due to a limitation(s) of the animal model used or may be due to the presence of redundant regulatory and/or effector functions.
ACKNOWLEDGMENTS
This work was supported by NIH training grant AI 07422 (S.H.L.), NIH grants AI 26289 (J.J.M.) and AI 40262 (A.C.), and Pew Scholars Award P0168SC (A.C.).
We are grateful to Daniel Steiger for performing the automated DNA sequencing and John Tobias for sharing his SfiI adapter method. We thank our colleague Matthew Waldor for helpful discussions and an excellent critical review of our manuscript.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Arico B, Miller J F, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attridge S R, Voss E, Manning P A. The role of toxin-coregulated pili in the pathogenesis of Vibrio cholerae O1 El Tor. Microb Pathog. 1993;15:421–431. doi: 10.1006/mpat.1993.1091. [DOI] [PubMed] [Google Scholar]
- 4.Camilli A, Beattie D, Mekalanos J. Use of genetic recombination as a reporter of gene expression. Proc Natl Acad Sci USA. 1994;91:2634–2638. doi: 10.1073/pnas.91.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilli A, Mekalanos J J. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol Microbiol. 1995;18:671–683. doi: 10.1111/j.1365-2958.1995.mmi_18040671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiRita V J. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol Microbiol. 1992;6:451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 7.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes P B, Smith H L. The effect of anaerobiosis and bile salts on the growth and toxin production by Vibrio cholerae. J Gen Microbiol. 1977;98:77–86. doi: 10.1099/00221287-98-1-77. [DOI] [PubMed] [Google Scholar]
- 9.Georgellis D, Lynch A S, Lin E C C. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J Bacteriol. 1997;179:5429–5435. doi: 10.1128/jb.179.17.5429-5435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 11.Iwanaga M, Yamamoto K. New medium for the production of cholera toxin by Vibrio cholerae biotype El Tor. J Clin Microbiol. 1985;22:405–408. doi: 10.1128/jcm.22.3.405-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolter R, Inuzuka M, Helinski D R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978;15:1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee C Y, Su S C, Liaw R B. Molecular analysis of an extracellular protease gene from Vibrio parahaemolyticus. Microbiology. 1995;141:2569–2576. doi: 10.1099/13500872-141-10-2569. [DOI] [PubMed] [Google Scholar]
- 14.Melton A R, Weiss A A. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J Bacteriol. 1989;171:6206–6212. doi: 10.1128/jb.171.11.6206-6212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller V L, Mekalanos J J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci USA. 1984;81:3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochman H, Gerber A S, Hartl D L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed R R. Transposon-mediated site-specific recombination: a defined in vitro system. Cell. 1981;25:713–719. doi: 10.1016/0092-8674(81)90178-1. [DOI] [PubMed] [Google Scholar]
- 18.Rhine J A, Taylor R K. TcpA pilin sequences and colonization requirements for O1 and O139 Vibrio cholerae. Mol Microbiol. 1994;13:1013–1020. doi: 10.1111/j.1365-2958.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 19.Roberts A, Pearson G D, Mekalanos J J. Proceedings of the 28th Joint Conference, U. S.-Japan Cooperative Medical Science Program on Cholera and Related Diarrheal Diseases. 1992. Cholera vaccine strains derived from a 1991 Peruvian isolate of Vibrio cholerae and other El Tor strains. [Google Scholar]
- 20.Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986;68:1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- 21.Sanders D A, Gillece-Castro B L, Stock A M, Burlingame A L, Koshland D E., Jr Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J Biol Chem. 1989;264:21770–21778. [PubMed] [Google Scholar]
- 22.Skorupski K, Taylor R K. Cyclic AMP and its receptor protein negatively regulate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci USA. 1997;94:265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith R L, Thompson L J, Maguire M E. Cloning and characterization of MgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J Bacteriol. 1995;177:1233–1238. doi: 10.1128/jb.177.5.1233-1238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tompkins W A F, Watrach A M, Schmale J D, Schultz R M, Harris J A. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Natl Cancer Inst. 1974;52:1101–1110. doi: 10.1093/jnci/52.4.1101. [DOI] [PubMed] [Google Scholar]
- 26.Wachsmuth I K, Blake P A, Olsvik O. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. [Google Scholar]