Abstract
Objective
To assess outcomes related to Lupus Therapeutics’ Patient Advocates for Lupus Studies (LT‐PALS), a peer‐to‐peer lupus clinical trial (LCT) education program designed to improve representation of diverse groups in LCTs. Patients with lupus and clinical trial participation experience were trained as peer educators (PALs) providing trial‐agnostic education to trial‐naive patients with lupus.
Methods
We used a two‐arm, randomized pretest/posttest study design to evaluate outcomes related to LCT participation: knowledge, attitudes, self‐efficacy, and intentions to participate in an LCT. Five academic medical centers piloted the program. The intervention group (IG) individually received peer‐to‐peer education sessions with trained PALs, primarily via telephone; the control group (CG) received a 3‐week waiting period. We conducted within/between‐group t‐tests and multiple linear regressions with posttest scores as dependent variables and participation in LT‐PALS as the exposure variable.
Results
The sample (n = 136) included 64 IG and 72 CG participants, with 67.7% identifying as Black. At posttest, IG participants had higher knowledge (P < 0.01) scores than the CG participants. Regression models controlling for participant characteristics showed higher IG posttest scores for knowledge (P < 0.001) and intentions (P < 0.05). From pretest to 3‐month follow‐up, IG self‐efficacy scores increased (P < 0.01). About half (46.9%) of IG participants reported engagement with an LCT at 1‐year follow‐up. Black and Hispanic participants rated higher overall program satisfaction compared with White (P < 0.01) and non‐Hispanic (P < 0.05) participants.
Conclusion
Findings demonstrated feasibility of LT‐PALS and showed promise in increasing engagement from groups underrepresented in LCTs.
INTRODUCTION
Clinical trials (CTs) of new medical treatments for systemic lupus erythematosus (SLE) in the US have historically struggled to adequately enroll participants who are racially and ethnically diverse. This underrepresentation leads to difficulties assessing the efficacy and risks associated with new treatments and hinders the ability of the US Food and Drug Administration (FDA) to approve new drugs for diverse populations’ use (1, 2). This issue is exemplified among Black patients with SLE, who are diagnosed with SLE three times more often than White patients with SLE (3) yet have been frequently underrepresented in CTs for SLE treatments.
SIGNIFICANCE & INNOVATIONS.
Lupus Therapeutics’ Patient Advocates for Lupus Studies (LT‐PALS) is a first of its kind peer‐support program that aimed to improve minority participation in lupus clinical trials (LCTs) through partnering with patient advocates to deliver education and increase availability of information about clinical trials.
The LT‐PALS program showed promise in supporting patients living with lupus in engaging LCTs by addressing cognitive outcomes theoretically related to behavior change, including knowledge about, attitudes toward, confidence around, and intentions to participate in LCTs through peer‐to‐peer education and outreach (outcomes defined in this article).
Overall, intervention group participants had a favorable opinion about the LT‐PALS program and were associated with significantly higher posttest scores in knowledge about and intentions to participate in LCTs, and nearly half reported broad engagement in an LCT at 1‐year follow‐up.
SLE is a chronic, inflammatory autoimmune disease that disproportionately affects racial and ethnic minorities who experience greater morbidity and mortality from the disease (4). Although Black patients account for 31% to 43% of SLE cases, they make up just 14% of SLE randomized controlled trial (RCT) participants (4, 5). The issue of underrepresentation of Black participants in lupus clinical trials (LCTs) was exemplified in the pivotal trials leading to the FDA approval of belimumab (6). The confluence of lupus health disparities and CT disparities is a major challenge for the development of new lupus treatment options. Because racial and ethnic minorities have the greatest risk of developing lupus and often experience more severe manifestations of the disease, it is imperative that diverse populations are well represented in CTs.
Black and Latino patients with lupus encounter disproportionate barriers to engaging with CTs. There are access‐related barriers, including access to specialists knowledgeable about LCTs, transportation (7), child‐care (8), bilingual informational materials (9), or paid time off from work (9). Another barrier to increasing diversity and representation in LCTs is limited knowledge or awareness about LCT opportunities (10) and support services/benefits that CTs often provide (eg, reduced‐cost or free care) (11). There may also be trust barriers among minority patients, including distrust of medical systems that have historically and currently discriminated against minority patients (7, 10, 12) and fears of possible deportation among undocumented migrants (9). Additionally, patients face barriers related to health literacy, including limited knowledge (13) about their disease or how CTs may benefit them (9, 14).
Patient advocacy (PA) can improve CT representation (15). PA can be defined as someone brought into a health care team to help empower patients to engage with and navigate the health care system by facilitating patient knowledge (16). Several models of PA have been used in other fields, such as oncology, and have shown success in increasing patient participation and retention. The Increasing Minority Participation in Clinical Trials (IMPaCT) project (15) trained advocates or “patient navigators” to engage directly with Black patients with cancer. The IMPaCT project found that by addressing both patient and system level barriers to participation, minority participation in cancer CTs increased from 9% to 16% and completion rates among Black participants nearly doubled.
The Community‐based Retention Intervention Study aimed to increase CT inclusion and retention of low‐income and Black women with assistance from Community Health Advisors (17). Assignment to the intervention group (IG) was associated with a significantly higher rate of follow‐up visit completion. The Patient Partners in Arthritis program used patient teachers to train medical students on musculoskeletal examinations (18) and later evolved to directly serve peer patients with arthritis. This peer‐to‐peer communication was associated with significantly higher knowledge scores and greater ability to identify relevant resources and self‐help aids (19).
Given the critical necessity to improve diversity and representation in LCTs and the promise of PA programs, there is a clear need to adapt and evaluate such programs tailored to the unique needs and experiences of patients with lupus. Community approaches have been established that educate and use people with lupus as popular opinion leaders to perform community outreach about CTs among underrepresented populations, showing promise in the power of peers in disseminating LCT information through their communities (20).
The Lupus Research Alliance (LRA)/Lupus Therapeutics (LT) undertook formative work to develop a PA program specifically for lupus research: the LT for Lupus Studies program (LT‐PALS). The social support model has been found to be effective in addressing lupus self‐management among Black women and was used to guide LT‐PALS approach (21).
LT‐PALS peer‐support approach aims to empower underrepresented patients with lupus through peer education to make informed decisions about LCT participation. LT‐PALS aims to address several patient‐level barriers to participation in LCTs relating to lack of education/awareness about clinical research and lack of diverse representation in LCTs (2, 22, 23). LT‐PALS materials were designed to help patients better understand how CTs operate, the potential risks/benefits of participation, the level of care involved, and how LCT participation among diverse populations affected by lupus supports the development and approval of safe and efficacious treatments.
MATERIALS AND METHODS
Ethical approval for this study was obtained from the KDH Research & Communication (KDHRC) Institutional Review Board (IRB) (FWA00011177, IRB 00005850).
Study participants
The recruitment period lasted from July 16, 2019, to May 28, 2021. Recruitment took place at five university sites from LRA's Lupus Clinical Investigators Network (LuCIN) that serve a high prevalence of patients underrepresented in LCTs: Emory University (Georgia), University of North Carolina at Chapel Hill (North Carolina), Columbia University (New York), Northwestern University (Illinois), and University of Mississippi Medical Center (Mississippi).
Formative work
LT‐PALS was codesigned with input from the lupus community and LuCIN sites. The original steering committee received input from individuals with lupus who helped design the intervention to address perceived community needs.
Trial design
We used a parallel arm, randomized, pretest/posttest/follow‐up study design to evaluate the impact of LT‐PALS on four cognitive outcomes related to participation in LCTs: knowledge, attitudes, self‐efficacy, and intentions. LT‐PALS paired adults with lupus interested in learning about CTs with “PALs,” peers who have lupus, prior involvement in a CT, and received peer‐support training. PALs provided trial‐agnostic education (ie, general education not specific to one CT) about CTs to trial‐naive patients with lupus who had no prior experience participating in a CT.
In this study, we explore the following three research questions: 1) To what extent is LT‐PALS exposure associated with significant positive changes in patients’ awareness and knowledge about, attitudes toward, self‐efficacy in, and intentions to participate in an LCT relative to the control group (CG)? 2) What is the relation between demographic characteristics (eg, age, race, and ethnicity) and experimental differences in knowledge, attitudes, self‐efficacy, and intentions (ie, moderation of effects)? 3) To what extent does LT‐PALS exposure lead broadly to increased patient engagement with an LCT? We define broad LCT engagement as initiating conversation with a health care provider about an LCT, following up on an LCT referral, contacting an LCT site, participating in screening for an LCT, and/or enrolling in an LCT.
The COVID‐19 pandemic disrupted many sites’ recruitment, as many sites were no longer seeing patients in the clinic. Moreover, the pandemic altered people's schedules and impacted other aspects of the program. The ability to enroll in trials was lower than usual because of suspension of trials at sites. In response to the pandemic, the project team made real time course corrections, including allowing more time for step completion and switching from in‐person to virtual recruitment of participants and education sessions. Sites shared the study opportunity with patients during in‐person or virtual clinic visits and outreach. Research staff at each site administered consents or provided flyers with links to study information and consent forms until the switch was made to mostly virtual. Staff conducted reminders with study participants once participants began the enrollment process. Reminders included emails, texts, and/or phone calls.
The study eligibility criteria for participants were 1) age 18 or older; 2) English speaking; 3) meeting Revised American College of Rheumatology Criteria and/or Systemic Lupus International Collaborating Clinics Criteria for SLE; 4) had no prior participation in a previous drug CT (participation in biomarker or other research‐type activities were allowed); and 5) had reliable internet access.
To participate in the study, participants completed secure online eligibility and consent forms hosted in Alchemer. Site staff shared online forms through flyers and emails, which enrolled then directed participants to the pretest survey. Participants self‐reported race and ethnicity via two categorical multiple‐choice questions on the pretest survey. From July 16 to October 21, 2019, participants could elect to be in the control or IG; however, participants showed a strong bias with nine out of the ten who self‐selected choosing the IG, so we changed the protocol to random assignment. Because of the potential for bias, we also conducted all analyses without these 10 self‐selected participants and provide changes to the significance of findings (Supplementary Material 1). Upon completion of the pretest survey, research staff at KDHRC randomized participants using a 2:1 randomization weighted toward the CG until balanced groups were achieved, at which point the randomization switched to 1:1. The computer‐generated randomization allocation sequence was sequentially numbered and stored in Excel. Only KDHRC researchers had access to the sequence to assign participants to the intervention or CG, which was based on the order in which participants completed the pretest. After assigning 60 participants to the CG, we switched to 100% assignment of participants to the IG. IG participants received up to $60 in gift card incentives and CG participants received $20 in gift card incentives for participation.
PALs participant eligibility and training
PALs were identified and recruited primarily through the principal investigators (PIs) at each institution. The PIs were asked to identify individuals who were representative of the population served by the institution and who the PIs felt would make good peer educators. PALs filled out application forms to assess eligibility. Eligibility criteria for PALs were the same as for participants, except PALs were required to have had prior experience participating in clinical research with a strong emphasis on participation in a CT. PALs were recommended by their PIs initially and after a phone call between potential PALs and LT in which the program and responsibilities were discussed, PALs were asked to complete an application (Supplementary Material 2) and provide a resume. PALs were then selected to participate in the training and required to complete an after‐training assessment with a score of 80% or higher. LT‐PALS recruited 11 PALs to take part in the study, one of whom passed away during the program implementation.
As a person living with lupus who had been enrolled in a prior CT, each PAL was meant to be a peer resource for patients on the topic of CTs. PALs aimed to empower individual patients to make informed decisions regarding CTs by serving as a support for patients with lupus who wished to learn more about CTs. Although PALs worked closely with the health care team, they were not there to provide medical advice or make decisions for patients. Through their experiences and training, PALs were able to discuss the clinical research process, concerns about CTs, the importance of clinical research, and the reality of participating in a CT with individuals considering trials. PALs helped ensure that patients had the necessary tools to decide if a trial was right for them.
The subjective qualifications for being a PAL centered around themes of positive regard, patience, objectivity, and relevant professional experience. It is important that PALs understood and respected that everyone has their own unique lupus experience, respected that individuals may have different priorities with regard to their treatment, be able to connect with individuals from diverse backgrounds and treat individuals from all backgrounds with respect and integrity, listen patiently, consider different levels of knowledge and comprehension around clinical research, be empathetic while remaining objective, be capable of withholding judgment and offering support and information to individuals with whom they may not personally agree, and be willing and able to undergo training and comprehend basic medical terminology related to lupus and CTs.
PALs were compensated for training and participation in the program and expenses reimbursed. Before engaging participants, PALs received a two‐stage training (online and in‐person) of lupus and CT concepts. The first part of the PALs training was virtual and included a six‐module course using “Blackboard.” The modules topics and their descriptions are listed in Supplementary Table 1.
Part two of the PALs training was an in‐person, two‐day training (Supplementary Table 2). Attendees at the training included the PALs, LT and LRA staff, KDHRC staff, PIs, co‐PIs, and two social workers from Hospital for Special Surgery (HSS). PIs and co‐PIs led training sessions that provided more depth to the information provided in the online modules. Presenters from HSS conducted training sessions on peer counseling, which reviewed multicultural considerations in engaging patients with lupus (eg, communication skills and attending behaviors, such as active listening). PALs received an LT‐developed training manual with more in‐depth information to refer to during LT‐PALS (Supplementary Material 3). PALs also received an education session guide that they could use while planning and conducting education sessions.
Intervention
Health Insurance Portability and Accountability Act compliant software was developed for the PALs to use when communicating with participants. A portal allowed PALs and participants to securely message one another, video chat, and share a variety of resources. Each participant had the opportunity to complete an intake survey when they first logged on to the software, which was used by PALs to tailor the education to specific needs, concerns, and knowledge level of CTs. Each PAL had a caseload of no more than 12 participants throughout the intervention period and engaged participants for five to six structured education sessions to discuss key concepts.
PALs were advised to not skip sessions but were allowed to complete certain sessions more quickly than others or combine sessions based on the participant's educational goals. Education sessions lasted 20 to 45 minutes and were conducted remote/virtually (phone or video call) or, prior to the COVID‐19 pandemic, in‐person at a public place chosen by the PAL and the participant. PALs attempted to schedule and initiate structured sessions with each individual participant assigned to their caseload.
PALs were recommended to take three to twelve weeks to complete five to six education sessions with each participant, although many took longer with an average of 15 weeks. There was an optional sixth education session to address any additional questions and review topics not previously discussed. The LT‐PALS software enabled texting, video, and voice chat; however, most PALs and their participants communicated outside of the software because of software glitches and site navigation difficulties. The IG took the posttest after completing all education sessions with PALs, whereas the CG had no interaction with PALs before taking the posttest.
Measures
We developed five multiple‐choice knowledge questions and adapted validated attitudes (24, 25, 26), self‐efficacy (27), and intentions (28) scales. We conducted pilot testing for the pretest/posttest/3‐month follow‐up survey measures among members of the research team and 40 individuals living with lupus, including analyses of the quantitative data collected and assessment of qualitative feedback received. After pilot testing, we revised survey instruments to clarify wording of questions before launching the study.
Dependent variables (outcome measures)
Knowledge about, positive attitudes toward, self‐efficacy with, and intentions to participate in LCTs serve as the primary dependent variables in this study. Each outcome measure consisted of five to six questions that covered topic content from the educational course (Supplementary Material 4). The knowledge measure consisted of five multiple‐choice questions, which were scored as correct or incorrect. Likert‐type questions that ranged from zero (strongly disagree) to 10 (strongly agree) were implemented to assess attitudes, self‐efficacy, and intentions around CT participation.
We averaged survey responses into a single composite score for each outcome domain, ranging from 0 to 100 for knowledge and from 0 to 10 for attitudes, self‐efficacy, and intentions. A composite score of 100 for knowledge indicates a participant answered all knowledge questions correctly. We calculated composite scores for each outcome at pretest, posttest, and the 3‐month follow‐up. We assessed the differences between these three timepoints to measure changes associated with participation in LT‐PALS.
The IG's satisfaction with LT‐PALS is another dependent variable. Theoretically, participants are more likely to engage with and learn from materials they find enjoyable (29, 30, 31). Satisfaction was reported via 10 Likert‐type questions, which related to perceived ease and usefulness of LT‐PALS at posttest. Each satisfaction answer choice ranged from zero (strongly disagree) to 10 (strongly agree). We averaged all 10 satisfaction questions to create a single overall satisfaction score ranging from zero to 10.
Independent variable
Exposure to LT‐PALS is the study's primary independent variable, which was coded as a dichotomous (yes/no) variable.
Covariates
We collected sociodemographic data on age, sex, race, ethnicity, education, employment status, and income at pretest. Because a patient's personal experiences with lupus may affect program engagement (32), we also collected data on length of lupus diagnosis, and the number of doctor visits, hospital visits, and flares experienced in the past year.
Data collection
We asked each participant identically worded questions for each of the outcome measures at both pretest and posttest. Outcome measures were assessed again for the IG at a 3‐month follow‐up, and we inquired about engagement with LCTs at 1‐year follow‐up.
We programmed the consent forms, pretest surveys, posttest surveys, and follow‐up surveys in Alchemer. Each survey was estimated to take between 5 and 10 minutes. After completion of the evaluation, the raw pretest, posttest, and follow‐up data were downloaded into encrypted Excel spreadsheets.
Statistical analysis
We conducted power analyses for multiple regression based on the designated outcomes, a single measure of treatment exposure, and demographics modeled as control measures (33). We estimated power at 0.80, assuming each outcome measure will have the same range of explained variance. With a medium R2 value (0.30) and contribution of treatment less than 0.30, the estimated sample size for power of 0.80 is 36 (range 23‐59). A sample size of 60 participants per condition was estimated to account for adequate power while allowing for participant attrition.
We used Stata/IC 16.1 statistical software to perform all data analyses. We manually matched completed pretests and posttests for each participant and excluded participants who only completed the pretest. Only matched pairs for both the intervention and CGs were analyzed between pretest and posttest. Only matched intervention participants were analyzed in comparison with the 3‐month follow‐up. Missing data were excluded from the analyses.
Participants who completed both a pretest survey and a posttest survey were included in analyses, regardless of completion of the 3‐month follow‐up. We performed between groups t‐tests to measure differences between the intervention and CGs in posttest scores and in average increases from pretest to posttest. We performed within‐group t‐tests for the IG between pretest and posttest, pretest and 3‐month follow‐up, and posttest and 3‐month follow‐up. We used Bonferroni's adjusted significance threshold (P ≤ 0.166) to account for the number of t‐tests performed. Next, we conducted Spearman's rank correlation analyses between overall satisfaction scores and each of the composite outcome scores at posttest. We calculated Cohen's d effect sizes to indicate the standardized difference in mean outcome posttest scores between the intervention and CGs. Then, we explored multiple linear regressions on each of the dependent variable posttest scores, with group assignment as the main exposure variable. Finally, we reviewed qualitative feedback from PALs and participants and assessed frequencies of reported LCT participation among the IG at the 1‐year follow‐up.
RESULTS
In total, we recruited and consented 235 participants with 209 who completed the pretest survey and 136 who completed the posttest survey, resulting in 136 participants (64 intervention and 72 CG participants) (Figure 1).
Figure 1.
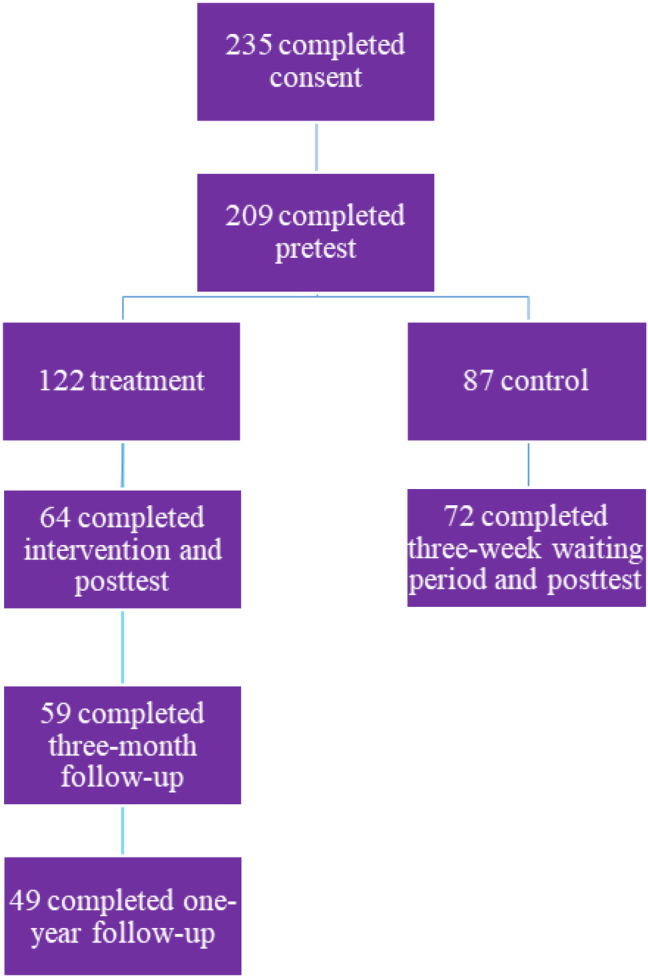
Overview of recruitment numbers.
Participants self‐reported their demographics and experiences with lupus. There were no significant differences in participant characteristics identified between participants who completed the posttest survey and participants who only completed the pretest survey, nor between intervention participants who did or did not complete the 1‐year follow‐up (Supplementary Table 3).
Most of the participants identified as Black (n = 92, 67.65%), female (n = 129, 94.85%), and not Hispanic/Latino/a (n = 115, 84.56%). Participants had a mean age of 40 years (SD = 11.86) and had been diagnosed with lupus from less than a month to 43 years prior to participation. When asked why participants had not participated in an LCT before, the most common answer given was never being offered or not having the opportunity (n = 45, 34.35%).
Eight PALs self‐identified as Black and two as White. None of the PALs self‐reported Hispanic ethnicity. Four PALs served two or more sites each, whereas six PALs served one site.
Group comparability at pretest
Age and length of lupus diagnosis were the only participant characteristics that significantly differed between the intervention and CGs. The IG was approximately 5 years older on average (P = 0.01), whereas the CG had been diagnosed with lupus approximately 2.85 years longer on average (P < 0.05) (Table 1). We found no statistically significant differences in composite scores between the intervention and CGs for the outcome variables before the IG's exposure to LT‐PALS (Table 2).
Table 1.
PALS participant demographic characteristics of the intervention and control group
| Variables | Total sample N = 136 | Intervention group n = 64 | Control group n = 72 | P value |
|---|---|---|---|---|
| Age, mean (SD)*, a | 40.18 (11.86) | 42.81 (13.17) | 37.85 (10.10) | 0.01 b |
| Sex, n (%) c | 0.82 | |||
| Male | 7 (5.15) | 3 (4.69) | 4 (5.56) | |
| Female | 129 (94.85) | 61 (95.31) | 68 (94.44) | |
| Race, n (%) d | 0.21 | |||
| Black | 92 (67.65) | 48 (75.00) | 44 (61.11) | |
| White | 19 (13.97) | 8 (12.50) | 11 (15.28) | |
| Other | 10 (7.35) | 5 (7.81) | 5 (6.94) | |
| Prefer not to answer | 6 (4.41) | 0 | 6 (8.33) | |
| Asian | 5 (3.68) | 2 (3.13) | 3 (4.17) | |
| Native American | 2 (1.47) | 1 (1.56) | 1 (1.39) | |
| More than one race | 2 (1.47) | 0 | 2 (2.78) | |
| Ethnicity, n (%) e | 0.22 | |||
| Hispanic | 18 (13.24) | 6 (9.38) | 12 (16.67) | |
| Non‐Hispanic | 115 (84.56) | 56 (87.50) | 59 (81.94) | |
| Prefer not to answer | 3 (2.21) | 2 (3.13) | 1 (1.39) | |
| Education, n (%) f | 0.37 | |||
| 5th‐8th grade | 0 | 0 | 0 | |
| 9th‐12th grade, (no diploma or GED) | 7 (5.15) | 4 (6.25) | 3 (4.17) | |
| 12th grade (received diploma or GED) | 18 (13.24) | 6 (9.38) | 12 (16.67) | |
| Some college | 38 (27.94) | 16 (25.00) | 22 (30.56) | |
| Associate degree | 15 (11.03) | 9 (14.06) | 6 (8.33) | |
| Bachelor's degree | 38 (27.94) | 16 (25.00) | 22 (30.56) | |
| Master's degree | 19 (13.97) | 12 (18.75) | 7 (9.72) | |
| Doctorate degree | 1 (0.74) | 1 (1.56) | 0 | |
| Employment, n (%) g | 0.36 | |||
| I work part‐time | 20 (14.71) | 12 (18.75) | 8 (11.11) | |
| I work full‐time | 54 (39.71) | 26 (40.63) | 28 (38.89) | |
| I do not have a job | 62 (45.59) | 26 (40.63) | 36 (50.00) | |
| Number of people in household, n (%) h | 0.15 | |||
| 1 | 26 (19.12) | 15 (23.44) | 11 (15.28) | |
| 2 | 37 (27.21) | 19 (29.69) | 18 (25.00) | |
| 3 | 32 (23.53) | 19 (29.69) | 13 (18.06) | |
| 4 | 21 (15.44) | 6 (9.38) | 15 (20.83) | |
| 5 | 11 (8.09) | 4 (6.25) | 7 (9.72) | |
| 6 | 4 (2.94) | 1 (1.56) | 3 (4.17) | |
| 7 | 3 (2.21) | 0 | 3 (4.17) | |
| 8 | 1 (0.74) | 0 | 1 (1.39) | |
| 9 | 1 (0.74) | 0 | 1 (1.39) | |
| Household income, n (%) i | 0.40 | |||
| $29,999 or less | 47 (34.56) | 20 (31.25) | 27 (37.50) | |
| $30,000‐$49,999 | 34 (25.00) | 19 (29.69) | 15 (20.83) | |
| $50,000‐$74,999 | 25 (18.38) | 13 (20.31) | 12 (16.67) | |
| $75,000‐$99,999 | 10 (7.35) | 3 (4.69) | 7 (9.72) | |
| $100,000‐$149,999 | 12 (8.82) | 4 (6.25) | 8 (11.11) | |
| $150,000‐$199,999 | 5 (3.68) | 4 (6.25) | 1 (1.39) | |
| $200,000 or more | 3 (2.21) | 1 (1.56) | 2 (2.78) | |
| Past‐year flares, n (%) j | 0.40 | |||
| 0‐3 times | 77 (56.62) | 39 (60.94) | 38 (52.78) | |
| 4‐7 times | 34 (25.00) | 17 (26.56) | 17 (23.61) | |
| 8‐11 times | 11 (8.09) | 4 (6.25) | 7 (9.72) | |
| 12 or more times | 14 (10.29) | 4 (6.25) | 10 (13.89) | |
| Past‐year doctor visits, n (%) k | 0.34 | |||
| 0‐3 times | 70 (51.47) | 32 (50.00) | 38 (52.78) | |
| 4‐7 times | 52 (38.24) | 28 (43.75) | 24 (33.33) | |
| 8‐11 times | 9 (6.62) | 2 (3.13) | 7 (9.72) | |
| 12 or more times | 5 (3.68) | 2 (3.13) | 3 (4.17) | |
| Past‐year ED visits, n (%) l | 0.25 | |||
| 0‐3 times | 123 (90.44) | 61 (95.31) | 62 (86.11) | |
| 4‐7 times | 10 (7.35) | 2 (3.13) | 8 (11.11) | |
| 8‐11 times | 1 (0.74) | 0 | 1 (1.39) | |
| 12 or more times | 2 (1.47) | 1 (1.56) | 1 (1.39) | |
| Past‐year hospital admissions, n (%) m | 0.13 | |||
| 0‐3 times | 130 (95.59) | 63 (98.44) | 67 (93.06) | |
| 4‐7 times | 4 (2.94) | 0 | 4 (5.56) | |
| 8‐11 times | 1 (0.74) | 0 | 1 (1.39) | |
| 12 or more times | 1 (0.74) | 1 (1.56) | 0 | |
| Length of lupus diagnosis, mean (SD)*, n | 18,259.35 (3078.42) | 17,707.81 (3525.06) | 18,749.61 (2544.64) | 0.05 b |
Abbreviations: ED, emergency department; GED, general education development; PALS, Patient Advocates for Lupus Studies.
Participants were asked, “How old are you?” with numeric answer choices between 18 and 100. We used age as a continuous variable.
Statistically significant at P ≤ 0.05.
Participants were asked, “What is your gender?” with answer choices including “male,” “female,” “write in option,” and “I prefer not to answer.” We created a dummy variable for sex in which one represented a participant who reported identifying as “male” and zero represented a participant who reported identifying as “female.”
Participants were asked, “Which of the following best describe you? Select all that apply.” Answer choices included “Black or African American,” “White or Caucasian,” “Asian,” “American Indian/Alaska Native,” “write‐in,” “I prefer not to answer,” and “more than one race.” We combined participants who reported “Asian,” “American Indian/Alaska Native,” and “more than one race” into “other” because of low numbers across these categories. We created a dummy variable for each race in which one represented a participant who reported being that respective race and zero represented a participant who did not. We used the most prominent category “Black or African American,” as the reference.
Participants were asked, “Do you identify as Hispanic or Latino?” with answer choices of “yes,” “no,” and “I prefer not to answer.” We created a dummy variable for gender in which one represented a participant who reported “yes” and zero represented a participant who reported “no.”
Participants were asked, “What is the highest grade level you have reached?” with answer choices of “5th‐8th grade,” “9th‐12th grade,” “no diploma or GED,” “12th grade, received diploma or GED,” “some college,” “associate degree,” “bachelor's degree,” “master's degree,” and “doctorate degree.” Because of low numbers across categories, we created a dummy variable for sex using the median as the cutoff, so that one represented a participant who reported having an “associate degree” or higher and zero represented a participant who reported “some college” or lower.
Participants were asked, “How would you describe your current job or work status?” with answer choices of “I work part‐time,” “I work full‐time,” and “I do not have a job.” Because of low representation in the part‐time category, we combined respondents who reported working and created a dummy variable for employment in which one represented a participant who reported either part‐time or full‐time work and zero represented a participant who reported being unemployed.
Participants were asked, “How many people are in your household? Your household is made of yourself, your husband, wife, or partner, your children, and any other family members living with you.” Answer choices were 1‐10. Because of low counts among the higher categories, we created a dummy variable for number of people in the household in which one represented a participant who reported more than three people and zero represented participants who reported one to three people.
3Participants were asked, “If you combined how much money everyone in your household made in 2018, how much would that be?” with answer choices of “under $29,999,” “$30,000‐$49,999,” “$50,000‐$74,999,” “$75,000‐$99,999,” “$100,000‐$149,999,” “$150,000‐$199,999,” and “$200,000 or more.” Because of low representation in the higher income categories, we created a dummy variable for income using the median as the cutoff, so that one represented a participant who reported an income of “$50,000‐$74,999,” or higher and zero represented a participant who reported “$30,000‐$49,999,” or lower.
Participants were asked, “In the past year, how many times have you had a lupus flare?” with answer choices of “0‐3 times,” “4‐7 times,” “8‐11 times,” and “12 or more times.” Because of low counts among the higher categories, we created a dummy variable for number of past‐year flares in which one represented a participant who reported more than three times in the past year and zero represented participants who reported “0‐3 times.”
In the past year, “How many times have you seen a doctor about your lupus or for a lupus flare up?” with answer choices of “0‐3 times,” “4‐7 times,” “8‐11 times,” and “12 or more times.” Because of low counts among the higher categories, we created a dummy variable for number of past‐year doctor visits in which one represented a participant who reported more than three times in the past year and zero represented participants who reported “0‐3 times.”
Participants were asked, “In the past year, how many times have you been to the ER due to your lupus or for a lupus flare up?” with answer choices of “0‐3 times,” “4‐7 times,” “8‐11 times,” and “12 or more times.” We removed this covariate from analyses because only 13 participants reported over three ER visits.
Participants were asked, “In the past year, how many times have you been admitted to the hospital for your lupus or for a lupus flare up?” with answer choices of “0‐3 times,” “4‐7 times,” “8‐11 times,” and “12 or more times.” We removed this covariate from analyses because only 15 participants reported over three hospital visits.
Participants were asked, “When were you diagnosed with lupus by a healthcare provider? Please provide the month and year (format MM/DD/YYYY).” We used the %td format in Stata, which interprets each date as the number of days since January 1, 1960; therefore, more recently diagnosed participants had a higher number than participants who were diagnosed longer ago.
P values were derived from chi‐square tests except for age and length of lupus diagnosis, which were derived from two‐group t‐test.
Table 2.
Study outcome variables between intervention and control groups
| Variables | Pretest | Posttest | ||||
|---|---|---|---|---|---|---|
| Intervention mean (SD) | Control mean (SD) | P value | Intervention mean (SD) | Control mean (SD) | P value | |
| Knowledge | 66.56 (27.62) | 63.06 (26.78) | 0.45 | 79.38 (22.53) | 63.33 (26.22) | <0.01 a |
| Attitudes | 6.10 (1.62) | 5.98 (1.32) | 0.66 | 6.34 (1.32) | 5.84 (1.17) | 0.02 |
| Self‐efficacy | 8.03 (1.64) | 7.97 (1.62) | 0.85 | 8.44 (1.34) | 7.99 (1.65) | 0.09 |
| Intentions | 5.53 (2.67) | 5.28 (2.40) | 0.56 | 5.93 (2.61) | 5.03 (2.42) | 0.04 |
Note: Max score for knowledge=100; attitudes, self‐efficacy, intentions = 10.
N = 136, intervention n = 64, control n = 72.
Statistically significant at Bonferroni's adjusted threshold P ≤ 0.016.
Between‐group differences after LT‐PALS intervention
Differences between groups at posttest
After exposure to LT‐PALS, the IG participants had significantly higher mean posttest composite score for knowledge (P < 0.01) compared with the CG. The IG also had higher scores for attitudes (P = 0.02) and intentions (P = 0.04) to participate in LCTs, although these did not meet Bonferroni's adjusted threshold of significance (Table 2).
Changes from pretest to posttest
After exposure to LT‐PALS, the IG participants had significantly higher mean gains for knowledge (P < 0.01) compared with the CG, increasing on average by nearly 13 percentage points compared with a 0.28‐point difference from pretest to posttest in the CG (Table 3).
Table 3.
Changes in outcome variables from pretest to posttest
| Variables | Intervention mean change (SD) | Control mean change (SD) | P value |
|---|---|---|---|
| Knowledge | 12.81 (26.75) | 0.28 (22.89) | <0.01 a |
| Attitudes | 0.29 (2.04) | −0.14 (1.25) | 0.15 |
| Self‐efficacy | 0.34 (1.41) | 0.06 (1.44) | 0.27 |
| Intentions | 0.36 (2.20) | −0.20 (1.98) | 0.13 |
Note: Max score for knowledge = 100; attitudes, self‐efficacy, intentions = 10. N = 136, intervention n = 64, control n = 72.
Statistically significant at Bonferroni's adjusted threshold P ≤ 0.016.
Cohen's d effect sizes for dependent variables at posttest
There was a medium effect for knowledge scores (d = 0.65), and small effects for attitudes (d=0.40), intentions (d = 0.36), and self‐efficacy (d = 0.30) scores.
Within‐group differences (IG only)
Changes from pretest to posttest
The IG had a significant gain in composite knowledge scores from pretest to posttest (P < 0.001) (Table 4).
Table 4.
Intervention group outcomes from pretest to posttest
| Variables | Pretest mean (SD) | Posttest mean (SD) | P value |
|---|---|---|---|
| Knowledge | 66.56 (27.62) | 79.38 (22.53) | <0.001 a |
| Attitudes | 6.10 (1.62) | 6.34 (1.32) | 0.28 |
| Self‐efficacy | 8.03 (1.64) | 8.44 (1.34) | 0.06 |
| Intentions | 5.53 (2.67) | 5.93 (2.61) | 0.20 |
Note: Max score for knowledge = 100; attitudes, self‐efficacy, intentions = 10. Average of 15 weeks intervention delivery between pretest and posttest. Intervention group only, n = 64.
Statistically significant at Bonferroni's adjusted threshold P ≤ 0.016.
Changes from pretest to 3‐month follow‐up
From pretest to 3‐month follow‐up, the IG had a significant increase relative to their pretest scores in self‐efficacy (P < 0.01) (Table 5).
Table 5.
Study outcome variables for intervention group at pretest, posttest, and 3‐month follow‐up
| Pretest vs. follow‐up | |||
|---|---|---|---|
| Variables | Pretest mean (SD) | 3‐month follow‐up mean (SD) | P value |
| Attitudes (n = 44) | 6.00 (1.49) | 6.19 (1.25) | 0.47 |
| Self‐efficacy (n = 45) | 7.94 (1.74) | 8.54 (1.40) | <0.01 a |
| Intentions (n = 44) | 5.27 (2.75) a | 5.37 (2.42) a | 0.75 a |
| Posttest vs. follow‐up | |||
|---|---|---|---|
| Variables | Posttest mean (SD) | 3‐month follow‐up mean (SD) | P value |
| Attitudes (n = 43) | 6.28 (1.35) | 6.23 (1.25) | 0.85 |
| Self‐efficacy (n = 44) | 8.59 (1.18) | 8.64 (1.33) | 0.72 |
| Intentions (n = 45) | 5.80 (2.61) | 5.25 (2.53) | <0.05 a |
Note: 3‐month follow‐up scores vary by comparison as a different number of participants answered all questions at pretest than at posttest and follow‐up observations were dropped if they did not have a pretest or posttest to compare against. Max score for attitudes, self‐efficacy, intentions is 10.
Statistically significant at P ≤ 0.05.
Changes from posttest to 3‐month follow‐up
Approximately 3 months after the posttest, the IG decreased in intentions scores (P < 0.05), although this did not reach Bonferroni's adjusted threshold of significance.
Findings from 1‐year follow‐up
The 1‐year follow‐up asked about participants’ engagement with LCTs since completion of LT‐PALS. Among the 49 (76.6%) intervention participants who completed the 1‐year follow‐up survey, 36.7% reported that they had spoken about LCTs with their health care provider, 12.2% had received referrals to an LCT, 26.5% had called a CT site about being in a CT, 16.7% were screened for an LCT, and 10.2% had been enrolled in an LCT (Table 6). Overall, 23 participants (46.94%) reported broad engagement in an LCT.
Table 6.
1‐year follow‐up questions
| Question | Answer | n (%) |
|---|---|---|
| “Since you finished the PALS program, have you talked about lupus clinical trials with your health care provider?” | Yes | 18 (36.73) |
| No | 31 (63.27) | |
| “About how many times did you talk to your doctor about clinical trials?” | One to three times | 17 (35.42) |
| 3 to 5 times | 1 (2.08) | |
| 6 or more times | 3 (6.25) | |
| I have not talked to my doctor about clinical trials since I finished the PALS program | 27 (56.25) | |
| “Since you finished the PALS program, has your doctor given you a referral to a lupus clinical trial?” | Yes | 6 (12.24) |
| No | 43 (87.76) | |
| “Since you finished the PALS program, have you called a clinical trial site about being in a clinical trial?” | Yes | 13 (26.53) |
| No | 36 (73.47) | |
| “Since you finished the PALS program, have you been screened for a lupus clinical trial?” | Yes | 8 (16.67) |
| No | 40 (83.33) | |
| “Since you finished the PALS program, have you enrolled in a lupus clinical trial?” | Yes | 5 (10.20) |
| No | 44 (89.80) |
Note: Intervention group only, n = 49.
Abbreviation: PALS, Patient Advocates for Lupus Studies.
Regression findings
Even when controlling for participant characteristics and experiences with lupus, we found positive and statistically significant associations between LT‐PALS exposure and posttest knowledge scores (P < 0.001) and posttest intentions to participate in LCTs (P = 0.04).
Participant satisfaction with LT‐PALS
About 75% (n = 48) of the IG provided feedback for LT‐PALS by completing all 10 satisfaction questions after the completion of the posttest. The mean overall satisfaction score across all questions for intervention participants was 7.55 (SD = 1.55) indicating a favorable opinion about LT‐PALS. Participants who identified as Black reported significantly higher overall satisfaction scores than White participants (P < 0.01), and Hispanic participants reported significantly higher overall satisfaction scores than non‐Hispanic participants (P = 0.04).
Dependent variable correlations with satisfaction
Spearman's rank correlation revealed several positive relationships between overall satisfaction scores and the dependent variables for the IG when not controlling for other variables. Higher posttest scores for attitudes (r[43] = 0.31, P = 0.037), self‐efficacy (r[43] = 0.48, P < 0.001), and intentions (r[45] = 0.35, P = 0.017) were each associated with higher overall satisfaction scores.
We interviewed PALs and site staff after program completion to better understand the challenges encountered during LT‐PALS’ recruitment and implementation, and what influenced sites’ decisions when recruiting PALs. Take‐aways from these interviews are summarized in Supplementary Table 4.
DISCUSSION
Overall, LT‐PALS received favorable ratings from participants, and participation in LT‐PALS was associated with significantly higher scores in knowledge about and intentions to participate in LCTs at posttest while controlling for participant characteristics. The IG also gained significantly in self‐efficacy from baseline to the 3‐month follow‐up. At 1‐year follow‐up, nearly half of the IG participants reported broad engagement in an LCT.
Although our findings provide optimism for moving forward, there were limitations in our assessment worth considering in terms of validity and potential for bias. For one, this study coincided with the onset of the COVID‐19 pandemic, which disrupted recruitment and altered people's schedules. Additionally, participant disease activity, challenges regarding time commitments, software issues, and scheduling between PALs and participants likely contributed to the relatively high attrition rate. Given the pilot nature of the project and that no follow‐ups were conducted with participants lost to attrition, we are unable to determine the exact reasons for variation in participation.
Changes to make LT‐PALS virtually accessible may have impacted intervention efficacy. Changes in the study protocol and attrition issues may have further biased results. The program was designed with flexibility in administration for PALs and participants. Software problems led to all but one PAL preferring to use the telephone for sessions, which could have affected participants differently. Additionally, the randomization ratios were inconsistent during recruitment and attempts to rebalance groups may impact validity.
The 3‐month and 1‐year follow‐ups only involved the IG, so between‐group comparisons were not possible. Without comparing LCT participation rates to the CG, we are limited in ascribing participation outcomes to involvement in LT‐PALS. Additionally, results may be limited by use of self‐reported measures, which are subject to response bias.
In this study, we targeted Black patients because of their underrepresentation in LCTs and successfully recruited a sample that was 68% Black. Likewise, most PALs identified as Black. None of the PALs were of Hispanic ethnicity, thus limiting our ability to assess whether congruence of ethnicity between participants and PALs affected participants’ experience with LT‐PALS. We were limited in our ability to make inferences regarding the impact of LT‐PALS on male patients as only 5.2% of participants were male. Because male patients with lupus are also underrepresented in RCTs compared with their SLE prevalence (5), future research may wish to explore whether the findings related to LT‐PALS are generalizable to male patients with lupus. Questions used may not have fully assessed the potential impact of disease burden. Further research may consider including clinician‐reported or patient‐reported SLE disease activity measures, such as the Systemic Lupus Activity Questionnaire (34).
LT‐PALS software issues further limited our ability to track frequency or content of contact. Future software improvements, adequate program testing, and training should enable more insights into the interactions between participants and PALs to help identify the themes of peer support most beneficial for participant outcomes.
Future iterations may benefit from additional practice being a PAL and time exploring PAL roles with doctors. To identify challenges early on, regular check‐ins between sites and coordinators may be useful. Sites noted a desire to adapt the program to meet participants’ needs, such as offering online, in‐person, or hybrid versions of LT‐PALS and developing short‐ and long‐term adaptations.
In conclusion, the results of LT‐PALS suggest that the peer‐support program is a promising means to educate patients with lupus about LCTs. This is an important finding because, from a theoretical perspective, knowledge leading to an improved understanding of LCTs is the first step in increasing patients’ awareness of protocols, resources, and consideration of participation in LCTs.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted. Wanty, Simkus, McNeill, and Holtz had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Sheikh, Donovan, Menezes, Roy, Simkus, Gross, Askanase, Ramsey‐Goldman, Majithia, Wanty, Holtz, Lim.
Acquisition of data
Sheikh, Donovan, Menezes, Wanty, McNeill, Holtz, Lim.
Analysis and interpretation of data
Simkus.
ROLE OF THE STUDY SPONSOR
GlaxoSmithKline, Pfizer, Genentech, and Bristol‐Meyers Squibb did not have any role in the design or development of the program nor in the interpretation or analysis of the results. Publication of this article was not contingent upon approval by GlaxoSmithKline, Pfizer, Genentech, and Bristol‐Meyers Squibb.
Supporting information
Disclosure form:
APPENDIX A. RESULTS WITHOUT SELF‐SELECTED PARTICIPANTS
APPENDIX B. PAL APPLICATION
APPENDIX C. TABLE OF CONTENTS FOR PALs TRAINING MANUAL
APPENDIX D. SURVEY MEASURES
Supplemental Table S1. Topics for part 1 of PALs trainings (virtual)
Supplemental Table S2. Topics for part 2 of PALs trainings (in person)
Supplemental Table S3. Participant characteristics based on program completion
Supplemental Table S4. Qualitative feedback from PAL and site interviews
ACKNOWLEDGMENTS
We thank the patient advocates (PALs) and the program participants for their immense contributions. The PALS Program, co‐designed and led by individuals living with lupus, was made possible by their continued efforts and leadership toward advancing lupus research.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding sponsors. This project was privately funded through philanthropic support via Lupus Research Alliance. Additional funding contributions were received from four pharmaceutical sponsors: GlaxoSmithKline, Pfizer, Genentech, and Bristol‐Meyers Squibb.
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11612.
Contributor Information
Saira Z. Sheikh, szsheikh@email.unc.edu.
S. Sam Lim, Email: sslim@emory.edu.
REFERENCES
- 1. Chandra A, Paul DP III. African American participation in clinical trials: recruitment difficulties and potential remedies. Hosp Top 2003;81:33–8. [DOI] [PubMed] [Google Scholar]
- 2. Sheikh SZ, Wanty NI, Stephens J, et al. The state of lupus clinical trials: minority participation needed. J Clin Med 2019;8:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim SS, Bayakly AR, Helmick CG, et al. The incidence and prevalence of systemic lupus erythematosus, 2002‐2004: the Georgia Lupus Registry. Arthritis Rheumatol 2014;66:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta‐analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol 2021;73:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falasinnu T, Chaichian Y, Bass MB, et al. The representation of gender and race/ethnic groups in randomized clinical trials of individuals with systemic lupus erythematosus. Curr Rheumatol Rep 2018;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheikh SZ, Englund TR, Burriss SW, et al. EMBRACE: one small story in lupus—one giant challenge in clinical trials. ACR Open Rheumatol 2022;4:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta‐analysis and systematic review of patient‐reported factors. Lancet Oncol 2006;7:141–8. [DOI] [PubMed] [Google Scholar]
- 8. Guadagnolo BA, Petereit DG, Helbig P, et al. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clin Trials 2009;6:610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health 2014;104:e16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer 2008;112:228–42. [DOI] [PubMed] [Google Scholar]
- 11. National Cancer Institute . Results from quarterly omnibus survey: clinical trials questions‐April 22, 1997. Bethesda (MD): National Institute of Health; 1997. [Google Scholar]
- 12. Harris Y, Gorelick PB, Samuels P, et al. Why African Americans may not be participating in clinical trials. J Natl Med Assoc 1996;88:630–4. [PMC free article] [PubMed] [Google Scholar]
- 13. The Society for Women's Health Research . Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials. Washington (DC): US Food and Drug Administration Office of Women's Health; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evans KR, Lewis MJ, Hudson SV. The role of health literacy on African American and Hispanic/Latino perspectives on cancer clinical trials. J Cancer Educ 2012;27:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fouad MN, Acemgil A, Bae S, et al. Patient navigation as a model to increase participation of African American in cancer clinical trials. J Oncol Pract 2016;12:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Earp JA, French EA, Gilkey MB. What is patient advocacy? In: Patient advocacy for health care quality: strategies for achieving patient‐centered care: strategies for achieving patient‐centered care. Vol 1. Sudbury (MA): Jones & Bartlett Publishers; 2007. p. 3–15. [Google Scholar]
- 17. Fouad MN, Johnson R, Nagy C, et al. Adherence and retention in clinical trials: a community‐based approach. Cancer 2014;120 Suppl 7:1106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Branch VK, Lipsky PE. Positive impact of an intervention by arthritis educators on retention of information, confidence, and examination skills of medical students. Arthritis Care Res 1998;11:32–8. [DOI] [PubMed] [Google Scholar]
- 19. Branch VK, Nieman T, Lipsky PE. Positive impact of an intervention by arthritis patient educators on knowledge and satisfaction of patients in a rheumatology practice. Arthritis Care Res 1998;12:370–5. [DOI] [PubMed] [Google Scholar]
- 20. Arneson LC, Taber KA, Williams JN, et al. Use of popular opinion leader models to disseminate information about clinical trials to Black individuals with lupus in two US cities. Arthritis Care Res (Hoboken) 2023;75:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams EM, Hyer JM, Viswanathan R, et al. Peer‐to‐peer mentoring for African American women with lupus: a feasibility pilot. Arthritis Care Res (Hoboken) 2018;70:908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamel LM, Penner LA, Albrecht TL, et al. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control 2016;23:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langford A, Bateman‐House A. Clinical trials For COVID‐19: populations most vulnerable to COVID‐19 must be included. Health Affairs Blog. URL: https://www.healthaffairs.org/content/forefront/clinical‐trials‐covid‐19‐populations‐most‐vulnerable‐covid‐19‐must‐included. [Google Scholar]
- 24. Schneider CD, Meek PM, Bell IR. Development and validation of IMAQ: Integrative Medicine Attitude Questionnaire. BMC Med Educ 2003;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Maesschalck S, Willems S, De Maeseneer J, et al. Development and validation of EMP‐3: an instrument to measure physicians' attitudes towards ethnic minority patients. Fam Med 2010;42:262–7. [PubMed] [Google Scholar]
- 26. Lam WY, Gunukula SK, McGuigan D, et al. Validated instruments used to measure attitudes of healthcare students and professionals towards patients with physical disability: a systematic review. J Neuroeng Rehabil 2010;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwarzer R, Jerusalem M, editors. Generalized self‐efficacy scale. Windsor (UK): NFER‐NELSON; 1995. [Google Scholar]
- 28. Francis JJ, Eccles MP, Johnston M, et al. Constructing questionnaires based on the theory of planned behaviour: a manual for health services researchers. Newcastle upon Tyne (UK): Centre for Health Services Research, University of Newcastle upon Tine; 2004. [Google Scholar]
- 29. Goodman MS, Si X, Stafford JD, et al. Quantitative assessment of participant knowledge and evaluation of participant satisfaction in the CARES training program. Prog Community Health Partnersh 2012;6:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Viswanathan M, Ammerman A, Eng E, et al. Community‐based participatory research: assessing the evidence. URL: https://bit.ly/2SQL0sF. [PMC free article] [PubMed]
- 31. Lucardie D. The impact of fun and enjoyment on adult's learning. Procedia Soc Behav Sci 2014;142:439–46. [Google Scholar]
- 32. Wiginton KL. Illness representations: mapping the experience of lupus. Health Educ Behav 1999;26:443–53. [DOI] [PubMed] [Google Scholar]
- 33. Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- 34. Svenungsson E, Gunnarsson I, Illescas‐Bäckelin V, et al. Quick Systemic Lupus Activity Questionnaire (Q‐SLAQ): a simplified version of SLAQ for patient‐reported disease activity. Lupus Sci Med 2021;8:e000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form:
APPENDIX A. RESULTS WITHOUT SELF‐SELECTED PARTICIPANTS
APPENDIX B. PAL APPLICATION
APPENDIX C. TABLE OF CONTENTS FOR PALs TRAINING MANUAL
APPENDIX D. SURVEY MEASURES
Supplemental Table S1. Topics for part 1 of PALs trainings (virtual)
Supplemental Table S2. Topics for part 2 of PALs trainings (in person)
Supplemental Table S3. Participant characteristics based on program completion
Supplemental Table S4. Qualitative feedback from PAL and site interviews


