Abstract
Objective
This study aimed to identify risk factors associated with the development of pulmonary arterial hypertension (PAH) in patients with systemic lupus erythematosus (SLE).
Methods
We conducted a systematic literature review of studies focusing on adult patients classified as having SLE‐related PAH by searching the electronic databases Embase, Medline, Medline in‐progress, Wanfang, China National Knowledge Infrastructure, Ichushi Web, Kmbase, and KoreaMed. Based on the findings, we conducted a Delphi survey to build expert consensus on issues related to screening for PAH in patients with SLE and on the importance and feasibility of measuring the identified factors in clinical practice.
Results
We included 21 eligible studies for data synthesis. Sixteen factors were associated with an increased risk of SLE‐PAH: pericardial effusion, serositis, longer duration of SLE, arthritis, acute and subacute cutaneous lupus, scleroderma pattern on nailfold capillaroscopy, diffusion capacity of carbon monoxide in the lungs (dlco) <70% predicted, interstitial lung disease, thrombocytopenia, and seven serological factors. Six factors were associated with a decreased risk of SLE‐PAH: malar/acute rash, hematologic disorder, renal disorder, higher Systemic Lupus Erythematosus Disease Activity Index score, and two serological factors. Among these, there were six risk factors on which the panelists reached strong or general consensus (peak tricuspid regurgitation velocity on echocardiography >2.8 m/s, pericardial effusion, DLco <70% predicted, scleroderma pattern on nailfold capillaroscopy, brain natriuretic peptide >50 ng/l, and N‐terminal pro–brain natriuretic peptide >300 ng/l). The Delphi panel confirmed the need for a screening tool to identify patients with SLE at high risk of developing PAH and provided consensus on the importance and/or practicality of measuring the identified factors.
Conclusion
The risk factors we identified could be used in a screening algorithm to identify patients with SLE with a high risk of developing PAH to facilitate early diagnosis, which could improve prognosis and management of these patients.
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare, progressive, and severe cardiopulmonary disorder (1). In patients with PAH, the small pulmonary arteries are obstructed because of cellular proliferation, fibrosis, and vascular remodeling, leading to premature death from right‐sided heart failure (2, 3, 4). PAH differs from other forms of pulmonary hypertension (PH) in that it is characterized by the presence of precapillary PH and it excludes PH induced by lung diseases, left‐sided heart failure, chronic thromboembolism, and other diseases (5). Positive diagnosis of PAH is based on hemodynamic measures obtained via right heart catheterization (RHC) (6), specifically, mean pulmonary arterial pressure higher than 20 mm Hg, pulmonary capillary wedge pressure less than or equal to 15 mm Hg, and pulmonary vascular resistance above 2 Wood units (5).
PAH tends to develop in association with connective tissue diseases (CTDs), including systemic lupus erythematosus (SLE) and systemic sclerosis (SSc) (5), which overall account for 15% to 30% of PAH cases (4). Though SLE‐PAH and SSc‐PAH are both common subtypes of CTD‐PAH, their prevalence varies geographically. SSc‐PAH is more common than SLE‐PAH (62% vs. 17%) among patients with CTD‐PAH in North America according to data from the Registry to Evaluate Early and Long‐term Pulmonary Arterial Hypertension Disease Management (7). In contrast, studies from China, Japan, and Taiwan suggest that SLE‐PAH is more common than SSc‐PAH in Asia (8, 9, 10, 11, 12). SLE‐PAH and SSc‐PAH are reported to account for 49% to 70% and 6% to 26% of CTD‐PAH cases, respectively, in China (8, 11, 12); 57% and 30% in Taiwan (9); and 29% and 19% in Japan (10).
The risk factors for SSc‐PAH have been well elucidated, and the DETECT algorithm has been developed to facilitate screening for PAH in patients with SSc, thereby allowing for earlier detection and treatment in this patient group (13). This algorithm includes a total of eight risk factors across a two‐step process. Depending on the risk score obtained in step one and the two additional echocardiographic variables, patients may then be referred for RHC if necessary.
The DETECT algorithm represents a sensitive noninvasive tool that helps to minimize missed diagnoses, identify milder disease, and address resource usage (13). However, there is currently no such screening algorithm for SLE‐PAH. Such a tool could be of great benefit in Asia Pacific (APAC) given that SLE‐PAH is more prevalent than SSc‐PAH in this region (8, 9, 10, 11, 12). As a first step toward the development of such an algorithm, we conducted a systematic literature review (SLR) to identify the risk factors associated with the development of SLE‐PAH in the APAC region. We then sought to build consensus on the risk factors with the greatest clinical utility via a Delphi panel.
MATERIALS AND METHODS
SLR methodology
The following electronic databases were searched (see Supplementary Material for the search strategy, available on the ACR Open Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611) up to October 14, 2021: Embase, Medline, Medline in‐progress, Wanfang, China National Knowledge Infrastructure (CNKI), Ichushi Web, Kmbase, and KoreaMed. Supplementary searches of conference proceedings, gray literature, and Google Scholar were also conducted.
Observational studies including adult patients who met the classification criteria of SLE according to internationally accepted criteria (eg, European Alliance of Associations for Rheumatology [formerly European League Against Rheumatism] or American College of Rheumatology classification criteria for SLE) and had undergone investigation for PAH with RHC or screening with echocardiography were eligible. Studies only reporting outcomes for patients with SLE as part of a mixed group of patients (eg, all patients with CTD) or studies that did not report how SLE was classified were excluded (14). Studies were excluded if PAH was caused by comorbid diseases, including disease of the left side of the heart, lung disease, and chronic thromboembolism (Supplementary Table 8, available at http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611). Eligibility was further restricted to studies that reported the odds ratio (OR), relative risk, or hazard ratio (HR) and 95% confidence interval (CI) for the risk factors associated with the development of SLE‐PAH and that were published in English, Chinese, Japanese, or Korean.
Articles were screened by two independent reviewers, with conflicts resolved by discussion or a third reviewer. Study quality was assessed using the Newcastle‐Ottawa Scale (15), and quality rating was converted to Agency for Health Research and Quality standards (good, fair, or poor) according to published thresholds (Supplementary Material, available at http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611) (16).
Results from the SLR were analyzed descriptively; no statistical analysis was performed. Data synthesis focused on risk factors significantly (P < 0.05) associated with the development of PAH in patients with SLE in good‐quality studies of patients diagnosed with PAH following RHC.
Delphi panel methodology
Based on the findings from our SLR, we conducted a Delphi panel survey in adherence to ethical requirements from the Ethical Delphi Manual (17) to build expert consensus on issues related to screening patients with SLE for PAH and on the feasibility of measuring the identified risk factors in clinical practice. We added several key criteria for diagnosis of PAH from international clinical guidelines (5), namely peak tricuspid regurgitation velocity (TRV) on echocardiography >2.8 m/second, brain natriuretic peptide (BNP) >50 ng/l, and N‐terminal pro–brain natriuretic (NT‐proBNP) >300 ng/l. The Delphi panel consisted of eight panelists (rheumatologists and cardiologists) from the APAC region experienced in the diagnosis and management of PAH in SLE, including two each from China, Japan, and Australia; one from South Korea; and one from Taiwan.
The Delphi questionnaire was administered in three rounds (Supplementary Material, available at http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611). Round one included a questionnaire regarding panelists’ experience in SLE‐PAH and their insights on the importance of early diagnosis of PAH in patients with SLE and important factors related to screening and investigation. Panelists were also presented with the risk factors identified in the SLR and were asked to select the ones important in the development of SLE‐PAH and the ones practical to measure in the clinic. Finally, panelists were asked whether there were any additional risk factors to be considered.
In round two, free‐text responses provided in round one were formulated into statements, and panelists were asked to rate their agreement with the statement on a Likert scale of 1 to 5. This exercise was repeated for the factors rated as important or practical to measure as well as the additional risk factors suggested by the panelists.
In round three, panelists were presented with the median ratings for every item in the questionnaire, alongside the range in ratings to indicate the current level of consensus on each item. Following the third round of the Delphi panel, the final results were circulated to all panelists for review.
RESULTS
SLR study characteristics
A total of 1254 records identified via databases after deduplication were screened based on titles and abstracts, of which 66, as well as 13 additional records identified via other methods, were retrieved for full‐text review. Following screening, 21 studies met the inclusion criteria (Figure 1) (see the Supplementary Material, available at http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611, for characteristics of included studies).
Figure 1.
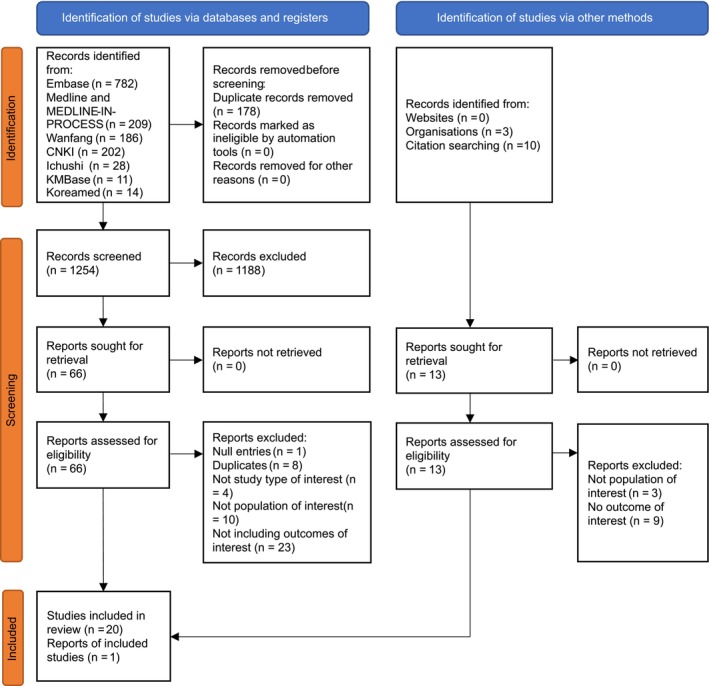
The study selection process based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. 2020 statement. China National Knowledge Infrastructure (CNKI).
All included studies were published between 2009 and 2021, from which 14 were in English and seven were in Chinese. The majority were single‐center studies (n = 15) (18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32), three were multicenter studies (33, 34, 35), and three were registry studies (36, 37, 38). All registry studies were based on the Chinese SLE Treatment and Research group (CSTAR) registry. Most included studies were from China (n = 16), followed by South Korea (n = 3) (24, 25, 34), one study from Egypt (23) and one from Brazil (21).
In total, 6 of 21 studies included patients with SLE diagnosed with PAH following RHC (21, 22, 27, 33, 37, 38), whereas 15 of 21 included patients with SLE screened for PAH with echocardiography (18, 19, 20, 23, 24, 25, 26, 28, 29, 30, 31, 32, 34, 35, 36). Patient groups screened with echocardiography might have included patients with an erroneous PAH diagnosis because pulmonary arterial systolic pressure can be substantially underestimated or overestimated when using echocardiography (39). Therefore, RHC‐based studies are the focus of this review, and their data were synthesized separately from echocardiography‐based studies, which are presented in the Supplementary Material (http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611). Additionally, patients with other autoimmune diseases was a common exclusion criterion across the six RHC‐based studies (Supplementary Table 8, available at http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611).
In terms of study quality, five of six RHC‐based studies were rated as being good quality and were included in the main data synthesis (21, 22, 33, 37, 38). The study by Lian et al (27) was the only one rated as poor quality, and its results are included in the tables. The 22 statistically significant risk factors reported in the five good‐quality studies fell into two main categories, namely clinical risk factors (13 of 22) and serological risk factors (9 of 22).
Clinical risk factors
A total of 13 clinical factors were significantly associated with the development of SLE‐PAH. Of these, nine were susceptibility factors and four were protective factors (Table 1).
Table 1.
Clinical and serological risk factors in studies of SLE‐PAH confirmed by RHC
| N | n | Trend for significant studies | Trend for non‐significant studies | Lowest OR/HR | Highest OR/HR | Lowest sample size | Highest sample size | Overall assessment | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical risk factor | |||||||||
| Acute/malar rash | 4 | 2 | Associated with decreased risk for PAH | Not significant but trend for decreased risk | 0.5 | 3.3 | 65 | 2278 | One study found that presence of malar rash significantly decreased risk of SLE‐PAH in univariate analysis, whereas a further two studies found that this did not significantly decrease risk of PAH in univariate analysis, but the trend was the same. One study found that patients with SLE without acute rash were at significantly increased risk of PAH. All four studies were good quality. |
| Acute/subacute cutaneous lupus | 1 | 1 | Associated with increased risk for PAH | 2.06 | 2.13 | N/A | 3624 | One good‐quality study found that presence of acute/subacute cutaneous lupus increased the risk for SLE‐PAH in both univariate and multivariate analyses. | |
| Alopecia | 1 | 0 | Not significant but trend for increased risk | N/A | 1.47 | N/A | 555 | One good‐quality study found that alopecia was associated with a nonsignificant trend for increasing the risk of patients with SLE developing PAH in multivariate analysis. | |
| Arthritis | 3 | 1 | Associated with increased risk for PAH | Not significant but trend for decreased risk a | 0.14 | 2.01 | 65 | 3624 | One large good‐quality study found that arthritis was associated with an increased risk for SLE‐PAH in both univariate and multivariate analyses. However, two further good‐quality studies (one large, one small) found that arthritis was not significantly associated with development of PAH in univariate analysis (though the trend in both studies was toward arthritis as a protective factor). |
| DLCO predicted <70% | 1 | 1 | Associated with increased risk for PAH | 10.02 | 14.47 | N/A | 2278 | One large good‐quality study found that a predicted DLco of less than 70% was significantly associated with SLE‐PAH in both univariate and multivariate analyses. | |
| Hematologic disorder | 1 | 1 | Associated with decreased risk for PAH | N/A | 0.70 | N/A | 2278 | One large good‐quality study found that hematological disorders were negatively associated with PAH among patients with SLE in univariate analysis, indicating they are a protective factor. | |
| Interstitial lung disease | 2 | 2 | Associated with increased risk for PAH | 4.7 | 17.0 | 555 | 3624 | Two large good‐quality studies found that presence of interstitial lung disease significantly increased risk of SLE‐PAH, one in both univariate and multivariate analyses, the other in multivariate analysis. | |
| Low serum complement level | 1 | 0 | Not significant but trend for increased risk | N/A | 1.09 | N/A | 2278 | One good‐quality study with a large sample found that a low serum complement level was not significantly associated with increased risk of patients with SLE developing PAH in univariate analysis. | |
| Lupus nephritis | 1 | 0 | Not significant but trend for increased risk | N/A | 1.67 | N/A | 555 | One good‐quality study found that lupus nephritis was not significantly associated with increased risk of patients with SLE developing PAH in multivariate analysis. | |
| Mucosal ulcers | 1 | 0 | Not significant but trend for decreased risk | N/A | 0.93 | N/A | 2278 | One large‐sample good‐quality study found that mucosal ulcers were not significantly associated with decreased risk of developing SLE‐PAH. | |
| Neurological disorder | 1 | 0 | Not significant but trend for decreased risk | N/A | 0.76 | N/A | 2278 | One large‐sample good‐quality study found that neurological disorder was not significantly associated with decreased risk of developing SLE‐PAH. | |
| Neuropsychiatric involvement | 1 | 0 | Not significant but trend for decreased risk | N/A | 0.56 | N/A | 65 | One small good‐quality study found that neuropsychiatric involvement was not significantly associated with a decreased risk for developing SLE‐PAH. | |
| Pericardial effusion | 3 | 3 | Associated with increased risk for PAH | 9.71 | 33.57 | 106 | 2278 | One large‐sample good‐quality study found that pericardial effusion significantly increased risk of SLE‐PAH in univariate analysis. Another good‐quality study found a significantly increased risk of SLE‐PAH in both univariate and multivariate analyses. A third good‐quality study found a significantly increased risk of SLE‐PAH in multivariate analysis. | |
| Photosensitivity | 1 | 0 | Not significant but trend for decreased risk | N/A | 0.31 | N/A | 65 | One small good‐quality study found that photosensitivity was not significantly associated with a lower risk of developing SLE‐PAH in univariate analysis. | |
| Raynaud's phenomenon | 2 | 1 | Associated with increased risk for PAH | Not significant but trend for increased risk | 3.05 | 3.23 | 65 | 147 | One poor‐quality study showed a significant association between the presence of Raynaud's phenomenon and increased risk of SLE‐PAH in both univariate and multivariate analyses. However, one small good‐quality study found that Raynaud's phenomenon was not significantly associated with SLE‐PAH, but the trend of the nonsignificant result was also for increased risk. |
| Renal disorder/involvement | 4 | 2 | Associated with decreased risk for PAH | Not significant but trend for decreased risk a | 0.58 | 1.07 | 65 | 3624 | Two large‐sample good‐quality studies found that renal disorder/involvement significantly decreased risk of SLE‐PAH in multivariate analysis, though it was nonsignificant in univariate analysis in one study. A third good‐quality study found that renal disorder/involvement was not significantly associated with SLE‐PAH in univariate analysis, but the trend of the nonsignificant result was for decreased risk. However, one further small good‐quality study also found that renal disorder/involvement was not significantly associated with a development of SLE‐PAH in univariate analysis, but the trend of the nonsignificant result was for increased risk. |
| Scleroderma pattern on capillaroscopy | 1 | 1 | Associated with increased risk for PAH | 6.39 | 6.80 | N/A | 65 | One small good‐quality study found that scleroderma pattern on nailfold capillaroscopy significantly increased risk of SLE‐PAH in both univariate and multivariate analyses. | |
| Serositis | 5 | 3 | Associated with increased risk for PAH | Not significant but trend for increased risk | 1.45 | 6.43 | 65 | 2278 | One large‐sample good‐quality study found that serositis significantly increased risk of SLE‐PAH in both univariate and multivariate analyses. This significant relation was also shown in another good‐quality study and a poor‐quality study in univariate analysis. Two further good‐quality studies found that serositis was not significantly associated with development of SLE‐PAH (one in univariate and one in multivariate analysis), but the trend of the nonsignificant results was for increased risk. |
| SLEDAI | 4 | 2 | Associated with decreased risk for PAH | Not significant but trend for decreased risk a | 0.89 | 26.43 b | 65 | 2278 | One large‐sample good‐quality study found that a higher SLEDAI score significantly decreased risk of SLE‐PAH in both univariate and multivariate analyses, whereas a further good‐quality study found that an SLEDAI score ≤9 significantly increased risk of SLE‐PAH in multivariate analysis. Two further studies found that SLEDAI score was not significantly associated with SLE‐PAH but with seemingly contradictory trends in the results. A third good‐quality study found that a higher SLEDAI score was associated with a nonsignificant trend for decreasing the risk of SLE‐PAH in univariate analysis. The fourth good‐quality study found that a higher SLEDAI score nonsignificantly increased risk of SLE‐PAH in univariate analysis. However, there was little difference in the SLEDAI score of the patients with and without SLE‐PAH at baseline (4 [IQR 2–6] vs. 3 [IQR 2–6], respectively; P = 0.746). |
| SLE duration | 2 | 1 | Associated with increased risk for PAH | Not significant but trend for increased risk | 1.05 | 1.12 | 106 | 555 | One good‐quality study found that longer SLE duration significantly increased risk of SLE‐PAH in multivariate analysis, whereas another good‐quality study found that longer SLE duration was associated with a nonsignificant trend for increasing the risk of SLE‐PAH in univariate analysis. |
| Thrombocytopenia | 1 | 1 | Associated with increased risk for PAH | 1.66 | 1.97 | N/A | 3624 | One large‐sample good‐quality study found that thrombocytopenia significantly increased risk of SLE‐PAH in univariate and multivariate analyses. | |
| Venous thrombosis | 1 | 0 | Not significant but trend for increased risk | N/A | 1.65 | N/A | 65 | One good‐quality study found that venous thrombosis was associated with a nonsignificant trend for increasing the risk of SLE‐PAH in univariate analysis. | |
| Miscarriage | 1 | 0 | Not significant but trend for increased risk | N/A | 4 | N/A | 65 | One good‐quality study found that a history of miscarriage was not significantly associated with the risk for developing SLE‐PAH in univariate analysis (though the trend was for increased risk). | |
| Pregnancy | 1 | 0 | Not significant but trend for increased risk | N/A | 1.385 | N/A | 65 | One good‐quality study found that being pregnant was not significantly associated with the risk for developing SLE‐PAH in univariate analysis (though the trend was for increased risk). | |
| Serological risk factor | |||||||||
| ACL antibodies | 2 | 1 | Associated with increased risk for PAH | Not significant but trend for increased risk | 1.03 | 3.75 | 65 | 147 | One poor‐quality study showed a significant association between presence of ACL antibodies and increased risk of SLE‐PAH in both univariate and multivariate analyses. One further good‐quality study found that IgM antibodies to ACL were not significantly associated with SLE‐PAH (though the trend was for increased risk), whereas there was virtually no difference in the proportions of patients with SLE with and without PAH with IgG antibodies to ACL. |
| Anti‐dsDNA antibodies | 4 | 1 | Associated with decreased risk for PAH | Not significant but trend for decreased risk a | 0.41 | 2.24 | 65 | 2278 | One good‐quality study found that presence of anti‐dsDNA antibodies significantly decreased risk of SLE‐PAH in univariate analysis. Two good‐quality studies found that anti‐dsDNA antibodies were not significantly associated with a decreased risk for PAH in univariate analysis. However, although one further good‐quality study also found that anti‐dsDNA antibodies were not significantly associated with SLE‐PAH in multivariate analysis, the trend was for increased risk. |
| Anti‐La/SSB antibodies | 2 | 1 | Associated with increased risk for PAH | Not significant but trend for decreased risk a | 1.04 | 2.04 | 65 | 3624 | One large good‐quality study found a significant association between presence of anti‐La/SSB antibodies and increased risk for SLE‐PAH in univariate analysis, but this was not significant in multivariate analysis (though the trend remained the same). In one further small good‐quality study, anti‐La/SSB antibodies were not significantly related to risk for SLE‐PAH but were (nonsignificantly) more common in the non‐PAH group. |
| APL | 1 | 1 | Associated with decreased risk for PAH | N/A | 0.557 | N/A | 2278 | One large good‐quality study found a significant association between presence of APL antibodies and decreased risk for SLE‐PAH in univariate analysis. | |
| Anti‐RNP antibodies | 6 | 5 | Associated with increased risk for PAH | Not significant but trend for increased risk | 2.41 | 15.10 | 65 | 3532 | Four good‐quality studies and one poor‐quality study found that presence of anti‐RNP antibodies was significantly associated with development of SLE‐PAH in both univariate and multivariate analyses. One small good‐quality study found that anti‐RNP antibodies were not significantly associated with SLE‐PAH in univariate analysis (but the trend was for increased risk). |
| Anti‐rRNP antibodies | 1 | 0 | Not significant but trend for increased risk | N/A | 1.72 | N/A | 555 | One good‐quality study found that anti‐rRNP antibodies were not significantly associated with PAH in multivariate analysis. | |
| Anti‐Ro/SSA | 3 | 1 | Associated with increased risk for PAH | Not significant but trend for increased risk | 1.25 | 4.84 | 65 | 3624 | Two large good‐quality studies found that positive anti‐Ro/SSA antibodies significantly increased risk of SLE‐PAH, one in univariate analysis only and one in multivariate analysis. A further small good‐quality study found that positive anti‐Ro/SSA antibodies were not significantly associated with increased risk of SLE‐PAH in univariate analysis (but the trend was for increased risk). |
| Anti‐Sm antibody | 2 | 0 | Not significant but trend for increased risk | 1.05 | 2.09 | 65 | 555 | Two good‐quality studies found that positive anti‐Sm antibody was not significantly associated with development of SLE‐PAH, one in univariate and one in multivariate analysis. The trend in both studies was for decreased risk. | |
| C2 | 1 | 0 | Not significant but trend for increased risk | N/A | 1.01 | N/A | 65 | One good‐quality study found that the C2 level did not significantly increase the risk of SLE‐PAH in univariate analysis. | |
| C3 | 1 | 0 | Not significant but trend for increased risk | N/A | 1.09 | N/A | 106 | One good‐quality study found that the C3 level did not significantly increase the risk of SLE‐PAH in univariate analysis. | |
| C4 | 1 | 0 | Not significant but trend for increased risk | N/A | 1.03 | N/A | 106 | One good‐quality study found that the C4 level did not significantly increase the risk of SLE‐PAH in univariate analysis. | |
| C100 | 1 | 0 | Not significant but trend for increased risk | N/A | 1.00 | N/A | 65 | One good‐quality study found that the complement level did not significantly increase the risk of SLE‐PAH in univariate analysis. | |
| Cysteine‐rich protein 61 | 1 | 1 | Associated with increased risk for PAH | 8.05 | 20.84 | N/A | 106 | One good‐quality study found that a cysteine‐rich protein 61 level ≥140.7 pg/ml significantly increased risk of SLE‐PAH in both univariate analysis and multivariate analyses. | |
| RDW‐CV | 1 | 1 | Associated with increased risk for PAH | 1.31 | 1.34 | N/A | 106 | One good‐quality study found that greater RDW‐CV was associated with a significant increase in the risk of SLE‐PAH in both univariate analysis and multivariate analysis. | |
| CRP | 1 | 0 | Not significant but trend for increased risk | N/A | 1.01 | N/A | 65 | One good‐quality study found that a higher CRP level was not significantly associated with the risk for developing SLE‐PAH in univariate analysis (though the trend was for increased risk). | |
| Erythrocyte sedimentation rate ≤20 mm/h | 1 | 1 | Associated with increased risk for PAH | N/A | 12.07 | N/A | 555 | One good‐quality study found that an erythrocyte sedimentation rate ≤20 mm/h significantly increased the risk of developing SLE‐PAH in multivariate analysis. | |
| Hemoglobulin >150 g/l | 1 | 0 | Not significant but trend for increased risk | N/A | 6.112 | N/A | 555 | One good‐quality study found that a hemoglobulin level >150 g/l was not significantly associated with the risk for developing SLE‐PAH in multivariate analysis (though the trend was for increased risk). | |
| Uric acid | 2 | 2 | Associated with increased risk for PAH | 3.8 | 9.67 | 106 | 555 | One good‐quality study found that a uric acid level ≥360 μmol/l significantly increased risk of SLE‐PAH in univariate analysis, and another good‐quality study found that a uric acid level >357 μmol/l significantly increased risk of SLE‐PAH in multivariate analysis. |
Note: If a single study reported only one OR/HR for the risk factor in question, OR/HR is listed under “Highest OR/HR,” with “N/A” under “Lowest OR/HR.” If the study reported several ORs or HRs for the risk factor (ie, from both univariate and multivariate analyses), the ORs or HRs are listed under highest and lowest effect size accordingly. If a single study reported on the risk factor in question, the sample size is listed under “Highest sample size.” The overall assessment takes into account study sample size, study quality, the type of analysis conducted (univariate, bivariate, multivariate, etc), the significance of the results, and the trend of results (ie, whether the factor increased the risk for PAH or was protective against PAH). “N” refers to the number of studies in which the risk factor was assessed; “n” refers to the number of studies in which the risk factor was significant.
Abbreviations: ACL, anticardiolipin; APL, antiphospholipid; CRP, C‐reactive protein; DLCO, diffusion capacity of carbon monoxide in the lungs; dsDNA, double‐stranded DNA; HR, hazard ratio; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; N/A, not applicable; OR, odds ratio; RDW‐CV, red blood cell distribution width; RHC, right‐sided heart catheterization; rRNP, ribosomal ribonucleoprotein; RNP, ribonucleoprotein ; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLE‐PAH, systemic lupus erythematosus–related pulmonary arterial hypertension.
Conflicting results from one or more studies.
One of the four studies evaluated the factor “SLEDAI score ≤9,” which was associated with an increased risk for SLE‐PAH, explaining why this OR is >1 but <1 in the other studies. However, the trend across the significant studies (ie, higher SLEDAI score is associated with lower SLE‐PAH risk).
Susceptibility clinical factors
Three studies found an association between pericardial effusion and SLE‐PAH, one in univariate analysis (UVA) (OR 21.3, 95% CI 7.2–12.8) (38) and two in multivariate analysis (MVA) (HR 33.6 [95% CI 4.9–141.7] and OR 9.6 [95% CI 4.1–110.6], respectively) (22, 33). Serositis was also associated with SLE‐PAH in two studies, one in UVA (HR 6.4, 95% CI 12.5–16.8) and one in MVA (OR 5.5, 95% CI 3.6–8.5) (33, 38).
Several aspects of immunological conditions were found to increase the risk of SLE‐PAH in MVA. One study found an association between longer duration of SLE and development of PAH (OR 1.12, 95% CI 1.03–1.21) (22), whereas two studies found that arthritis and acute or subacute cutaneous lupus (HR 1.9 [95% CI 1.2–3.2] and HR 2.1 [95% CI 1.3–3.2], respectively) (37), as well as scleroderma pattern on nailfold capillaroscopy (OR 6.4, 95% CI 1.5–26.7), were associated with SLE‐PAH (21).
Some lung disorders, including pulmonary insufficiency and interstitial lung disease, were found to be associated with an increased risk for developing SLE‐PAH in MVA. Diffusion capacity of carbon monoxide in the lungs (dlco) <70% predicted was associated with an increased risk for SLE‐PAH in one study (OR 10.0, 95% CI 6.6–15.2) (38), whereas two studies reported an association with interstitial lung disease (OR 1.1 [95% CI 1.0–1.2] and HR 4.75 [95% CI 1.5–15.3], respectively) (22, 37).
Finally, although thrombocytopenia was found to increase the risk for SLE‐PAH in MVA in one study (HR 1.97, 95% CI 1.21–3.19), the authors noted that this finding should be further investigated because the association could be influenced by other factors (37).
Protective clinical factors
Malar rash was found to be associated with lower risk of SLE‐PAH in UVA (OR 0.5, 95% CI 0.4–0.7) in one study (38). Similarly, another study found that patients with SLE without acute rash had an increased risk of SLE‐PAH in MVA (OR 3.26, 95% CI 1.2–8.7), indicating that the presence of rash may be protective (22).
Hematologic disorder and renal disorder were reported to decrease the risk for SLE‐PAH, but the studies did not specify the criteria for defining these two types of disorders; thus, it is not clear which conditions could be protective. Hematologic disorder was associated with lower risk for SLE‐PAH in one study (UVA; OR 0.7, 95% CI 0.6–0.9) (38), and two studies reported decreased risk for SLE‐PAH in patients with renal disorders, one in UVA (OR 0.6, 95% CI 0.4–0.7) (38) and one in MVA (HR 0.6, 95% CI 0–0.9) (37).
The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score was significantly lower among patients with SLE with PAH compared to those without PAH according to two studies using MVA, suggesting that a lower SLEDAI score should be regarded as a marker of SLE‐PAH. One study showed that a lower risk of SLE‐PAH was associated with a higher SLEDAI score (OR 0.89, 95% CI 0.86–0.91) (38), and the other found that an SLEDAI score ≤9 was associated with an increased risk for SLE‐PAH (OR 26.426, 95% CI 6.619–105.497), indicating that a higher SLEDAI score is protective (22).
Serological factors
A total of nine serological factors were reported to be significantly associated with SLE‐PAH, of which seven were susceptibility factors and two were protective factors (Table 1).
Susceptibility serological factors
Increased risk of SLE‐PAH was associated with the presence of three types of antibodies, namely anti‐RNP antibodies, anti‐Sjögren's syndrome–related antigen A (anti‐Ro/SSA) antibodies, and anti‐Sjögren's syndrome type B (anti‐La/SSB) antibodies. Four studies reported an association between anti‐RNP antibodies and SLE‐PAH in MVA: two reported HRs (HR 8.3 [95% CI 1.8–39.0] and HR 4.6 [95% CI 2.9–7.4]) (33, 37), whereas the other two reported ORs (OR 13.3 [95% CI 9.5–18.7] and OR 12.4 [95% CI 3.6–42.9]) (22, 38). Anti‐Ro/SSA antibodies were associated with SLE‐PAH in two studies. Whereas one study reported a significant association in MVA (OR 4.8, 95% CI 1.7–14.0) (22), the other found that this association was only significant in UVA (HR 2.5, 95% CI 1.6–3.9) but not in MVA (37). Anti‐La/SSB antibodies were associated with SLE‐PAH in UVA (HR 2.0, 95% CI 1.3–3.3) in one study, but this was not significant in MVA (37).
The remaining four serological factors were reported in two studies (22, 33). An erythrocyte sedimentation rate of ≤20 mm/hour was associated with a greater risk for SLE‐PAH in MVA (OR 12.1, 95% CI 3.6–40.2) (22). An association between greater red blood cell distribution width coefficient of variation and SLE‐PAH was found in MVA (HR 1.3, 95% CI 1.0–1.8) (33). A cysteine‐rich protein 61 level ≥140.7 pg/ml was associated with SLE‐PAH in MVA (OR 20.8, 95% CI 3.3–132.9) (33). Finally, the serum uric acid level was associated with SLE‐PAH in both studies. In one study, a serum uric acid level >357 μmol/l was found to increase the risk for SLE‐PAH in MVA (OR 9.7, 95% CI 3.2–29.2) (33), whereas the other study found that a serum uric acid level ≥360 μmol/l was significantly associated with SLE‐PAH in UVA (HR 3.8, 95% CI 1.4–10.3) (22).
Protective serological factors
According to one study, the incidence of antiphospholipid (APL) antibody positivity was significantly lower (12.0% vs. 19.6%; P = 0.002) in the SLE‐PAH group than that in the SLE without PAH group. This study found that increased positivity of anti–double‐stranded DNA (anti‐dsDNA) and APL antibodies was significantly associated with decreased risk of SLE‐PAH in UVA (OR 0.4 [95% CI 0.3–0.6, P < 0.001] and OR 0.6 [95% CI 0.4–0.8, P = 0.002], respectively) (38).
Comparison between studies using RHC and echocardiography
There was little similarity in the statistically significant risk factors identified in RHC‐ and echocardiography‐based studies. The four clinical factors that increased the risk of SLE‐PAH in both types of studies were serositis, pericardial effusion, interstitial lung disease, and duration of SLE. SLEDAI score was significantly associated with the development of SLE‐PAH in both types of studies, but whereas two RHC‐based studies found that higher SLEDAI scores were associated with a decreased risk in MVA, two echocardiography‐based studies found that higher SLEDAI scores were associated with a greater risk of developing the disease (Supplementary Material, available at http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611). Regarding serological factors, the serum uric acid level was the only one that significantly altered the risk for SLE‐PAH in both RHC (Table 1) and echocardiography studies (Supplementary Material, available at http://onlinelibrary.wiley.com/doi/10.1002/acr2.11611), increasing the risk for developing the disease in all studies in which it was assessed.
Delphi panel
Of the eight panelists, six were rheumatologists and two were cardiologists. Although all panelists noted that they treated PAH in patients with SLE themselves, two noted that they sometimes referred patients to other physicians for treatment. All panelists participated in rounds one and three, and seven panelists participated in round two. Results regarding the importance and consensus status of all factors are provided in Table 2.
Table 2.
Importance of all 33 factors (22 from our SLR, three from the ERC/ERS guidelines, and eight proposed by experts) indicated by median rating from the panelists
| Factors | Median rating of importance a | Consensus after three rounds of Delphi panel |
|---|---|---|
| NT‐proBNP >300 ng/l b | 1 | Consensus |
| Positive anti‐RNP antibodies b | 1 | No |
| Peak TRV on echocardiography >2.8 m/s c | 1 | Strong consensus |
| DLCO predicted <70% c | 1 | Strong consensus |
| BNP >50 ng/l b | 2 | Consensus |
| Pericardial effusion c | 2 | Consensus |
| Serositis c | 2 | No |
| Interstitial lung disease c | 2 | No |
| Scleroderma pattern on nailfold capillaroscopy c | 2 | Consensus |
| Anti‐topoisomerase I antibodies d | 2 | No |
| Anticentromere antibodies d | 2 | Strong consensus |
| Pulmonary artery enlargement on chest radiograph d | 2 | No |
| History of pulmonary vasculitis d | 2 | No |
| Positive anti‐La/SSB antibodies b | 3 | No |
| Positive anti‐Ro/SSA antibodies b | 3 | No |
| Serum uric acid level >357 μmol/l b | 3 | No |
| Positive APL antibodies b | 3 | No |
| SLE duration (long disease duration) c | 3 | No |
| P wave on electrocardiogram d | 3 | No |
| Cysteine‐rich protein 61 level ≥140.7 pg/ml b | 3.5 | No |
| Positive anti‐dsDNA antibodies b | 4 | No |
| Acute/subacute cutaneous lupus c | 4 | No |
| Thrombocytopenia c | 4 | No |
| Hematologic disorder c | 4 | No |
| Renal disorder/involvement c | 4 | No |
| SLEDAI score >9 c | 4 | No |
| Total IgG d | 4 | No |
| Systemic hypertension d | 4 | No |
| Elevation of C‐reactive protein level d | 4 | No |
| Increased red blood cell distribution width b | 4.5 | No |
| Arthritis c | 4.5 | No |
| Rash c | 4.5 | No |
| Erythrocyte sedimentation rate ≤ 20 mm/h b | 5 | No |
Abbreviations: APL, antiphospholipid; BNP, brain natriuretic peptide; DLCO, diffusion capacity of carbon monoxide in the lungs; dsDNA, double‐stranded DNA; ERC/ERS, XXX; IgG, immunoglobulin G; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLR, systematic literature review; TRV, tricuspid regurgitation velocity.
Ratings were given on a scale of 1 to 5, with 1 being very important, 2 being somewhat important, 3 being neither important nor unimportant, 4 being somewhat unimportant, and 5 being completely unimportant.
Serological factors.
Clinical factors.
The eight additional factors suggested by panelists during the Delphi process.
First round of Delphi panel
All panelists agreed that early diagnosis of PAH in patients with SLE is important. Specifically, six of the eight panelists suggested that patients with SLE should be screened for PAH upon presentation of signs and symptoms, three of eight suggested that the screening should be conducted after confirmation of SLE, three of eight suggested that the screening should be done regularly, and one of eight mentioned that patients with SLE planning for pregnancy should be screened for PAH. Regarding the symptoms that prompt investigation of PAH, seven of eight mentioned that dyspnea (shortness of breath) is a typical characteristic of probable PAH. Other signs were also raised by one or two panelists, such as edema, Raynaud's phenomenon, and presence of anti‐RNP antibodies. Panelists also provided opinions on the development of a screening tool for SLE‐PAH. One panelist suggested that those at higher risk of developing PAH should be prioritized; another believed that the practicality, accuracy, cost, and availability of such a tool is the priority.
Regarding the clinical factors, all eight rated peak TRV on echocardiography >2.8 m/second, pericardial effusion, and dlco <70% predicted as important factors, and seven of eight rated them as feasible to measure in clinics. Besides, seven of eight rated scleroderma pattern on nailfold capillaroscopy and six of eight rated interstitial lung disease and serositis as important factors, with five of eight rating these three factors to be feasible to measure in clinics.
For the serological factors, all eight panelists rated a NT‐proBNP level >300 ng/l as being important and easy to measure. Presence of anti‐RNP antibodies was also rated by all panelists to be easy to measure, but not all of them (seven of eight) rated it as important. A BNP level >50 ng/l was rated as an important factor by six of eight panelists, with five of eight indicating that it is easy to measure in clinics.
Second round of Delphi panel
All seven panelists participating in this round agreed that patients with SLE should be screened for PAH upon presentation of signs and symptoms. More than half (four of seven) of the panelists somewhat agreed that patients with SLE should be screened for PAH every 2 years, but the rest (three of seven) somewhat disagreed. This discrepancy might be related to the fact that these three panelists agreed on only screening patients with SLE at high risk of PAH. Besides, five of seven panelists agreed that patients with SLE planning a pregnancy should be screened for PAH.
All panelists agreed that dyspnea and presence of anti‐RNP antibodies should prompt investigation of PAH, with moderate consensus reached on Raynaud's phenomenon and heart failure. For the better development of a screening tool for PAH in patients with SLE, almost all panelists (six of seven) agreed that there is a need to ensure good coordination between rheumatologists and cardiologists, easy availability of echocardiography and support from cardiologists for catheterization, careful screening during childbearing age, precise definition and identification of risk factors for the development of PAH in SLE, and the practicality, accuracy, cost, and availability of tests. Only one panelist suggested that only symptomatic patients with SLE should be screened, especially those who become breathless and have new‐onset reduced exercise capacity.
Regarding the importance and measurability of the factors, all panelists reinforced pericardial effusion as a risk factor, which was rated by all panelists in round one as important and feasible to measure in clinics. In round one, in addition to the factors provided in the Delphi panel, the panelists nominated eight additional factors: total immunoglobulin G level, systemic hypertension, elevation of C‐reactive protein level, antitopoisomerase antibodies, anticentromere antibodies, P wave on electrocardiogram, pulmonary artery enlargement on chest radiograph, and history of pulmonary vasculitis. Panelists in round two were presented these factors and asked to rate their importance and practicality. However, only the anticentromere antibodies received moderate consensus (five of seven agreed, two of seven neither agreed nor disagreed) on its importance and ease of measurability in clinics.
Third round of Delphi panel
After three rounds of the Delphi panel, consensus was reached on several statements. Specifically, all panelists strongly agreed that early diagnosis and treatment of PAH in patients with SLE is important because it improves prognosis. Early treatment with immunosuppressants and PAH‐specific therapy (eg, endothelin receptor anatagonist (ERA) and phosphodiesterase‐5 inhibitor (PDE5i)) is also important for improving outcomes in patients with SLE‐PAH; as a feature of active SLE, the early stage of PAH would respond well to immunosuppressive treatment prescribed for SLE, thus improving the prognosis of SLE‐PAH. Development of a screening tool for identifying patients with SLE at high risk of PAH should be prioritized to optimize screening for SLE‐PAH. More importantly, high‐risk patients should be identified for annual or urgent screening and provided with access to further investigation in a center of excellence. The panelists further noted that screening for PAH upon SLE confirmation is not necessary; patients should be investigated for PAH upon presentation of relevant symptoms (eg, dyspnea and Raynaud's phenomenon). Despite these agreements, there remains a lack of consensus on whether only patients with SLE at high risk of PAH should be screened, whether patients with SLE should be screened for PAH every 2 years, and whether a screening algorithm used for screening patients with SSc for PAH should be used to screen patients with SLE for PAH.
In addition, there was consensus on four clinical risk factors that were important, but only two were practical to measure (pericardial effusion and dlco) (Table 3). Additionally, the panelists agreed on the practicality of measuring two additional clinical factors (interstitial lung disease and duration of SLE), though there was no consensus regarding their importance (Table 3). The panelists agreed that BNP and NT‐proBNP were serological factors important and practical to measure in the clinic (Table 4). Two additional serological factors were also practical to measure (anti‐RNP and APL antibodies), but there was no consensus regarding their importance (Table 4). Finally, anticentromere antibodies was the only additional factor proposed by one panelist that had received strong consensus on its importance in the diagnosis of PAH in patients with SLE, but not all panelists (six of eight) rated it as practical to measure (Table 4).
Table 3.
Clinical risk factors that panelists agreed were important and/or practical to measure
| Factor | Importance | Measurability | ||
|---|---|---|---|---|
| Median rating | Consensus level | Median rating | Consensus level | |
| Pericardial effusion | 2 | Consensus | 1 | Consensus |
| DLCO predicted <70% | 1 | Strong consensus | 1 | Consensus |
| Peak TRV on echo >2.8 m/s | 1 | Strong consensus | 1 | No consensus |
| Scleroderma pattern on nailfold capillaroscopy | 2 | Consensus | 2.5 | No consensus |
| Interstitial lung disease | 2 | No consensus | 2 | Consensus |
| Duration of SLE | 3 | No consensus | 2 | Consensus |
Note: Ratings were given on a scale of 1 to 5, with 1 being very important/practical to measure, 2 being somewhat important/practical to measure, 3 being neither important nor unimportant and/or neither practical nor impractical to measure, 4 being somewhat unimportant/impractical to measure, and 5 being completely unimportant/impractical to measure.
Abbreviations: DLco, diffusion capacity of carbon monoxide in the lungs; SLE, systemic lupus erythematosus; TRV, tricuspid regurgitation velocity.
Table 4.
Serological risk factors that panelists agreed were important and/or practical to measure
| Factor | Importance | Measurability | ||
|---|---|---|---|---|
| Median rating | Consensus level | Median rating | Consensus level | |
| BNP >50 ng/l | 2 | Consensus | 2 | Consensus |
| NT‐proBNP >300 ng/l | 1 | Consensus | 1 | Consensus |
| Positive anti‐RNP antibodies | 1 | No consensus | 1 | Consensus |
| Positive APL antibodies | 3 | No consensus | 2 | Consensus |
| Positive anticentromere antibodies | 2 | Strong consensus | 2 | No consensus |
Note: Ratings were given on a scale of 1 to 5, with 1 being very important/practical to measure, 2 being somewhat important/practical to measure, 3 being neither important nor unimportant and/or neither practical nor impractical to measure, 4 being somewhat unimportant/impractical to measure, and 5 being completely unimportant/impractical to measure.
Abbreviations: APL, antiphospholipid; BNP, brain natriuretic peptide; NT‐proBNP, N‐terminal pro–brain natriuretic peptide.
DISCUSSION
We conducted a Delphi panel to build expert consensus on risk factors for SLE‐PAH with greatest clinical utility, informed by a comprehensive SLR that focused on patients diagnosed with PAH via RHC.
In therapeutic areas where evidence on disease management is lacking or is very limited, such as for PAH, the Delphi methodology is a valuable qualitative research tool that fulfills evidence gaps (40, 41, 42). Because high expert consensus must be achieved, outcomes from this kind of structured approach are robust and are often readily transferable to clinical practice.
Our Delphi panel comprised a group of eight experts and validated the findings of the SLR. The response rate from experts was very high, with all panelists actively participating in the Delphi panel in all rounds, except for one panelist who did not participate in round two. In addition to responding to the Delphi questionnaire, some panelists also provided advice based on experience in clinical practice. For example, a panelist from Japan noted that delay of PAH treatment even up to 3 months can affect long‐term outcomes, supporting the importance of early diagnosis and treatment with a reliable screening tool. Another panelist from Australia suggested that not all centers can do good echocardiology tests, especially those assessing the right side of the heart. Therefore, although peak TRV is a good screening marker for PAH, its estimation is not always reliable, and peak TRV cannot be estimated if tricuspid regurtitation jet is absent. This is where NT‐proBNP, especially if combined with a pulmonary function test, has value. It is also noteworthy that anticentromere was nominated by a panelist and received strong consensus among panelists, but it was not reported in the included studies as a risk factor for SLE‐PAH. This could be because although anticentromere antibody is strongly associated with risk of developing PAH, it is more specific for the autoimmune disease SSc and is seldom detectable in SLE, unless there is serologic or clinical overlap with SSc.
To our knowledge, our SLR is the first to assess both clinical and serological risk factors for the development of PAH diagnosed via RHC in patients with SLE. Wang et al (43) conducted a meta‐analysis on serological risk factors for SLE‐PAH based on 12 studies, in which patients were categorized as having PH or PAH following RHC or echocardiography. In addition, serological risk factors for SLE‐PAH in patients diagnosed with PAH following RHC were reported based on subgroup analyses. In agreement with our SLR, Wang et al (43) reported that anti‐RNP and anti‐SSA antibodies significantly increased the risk for SLE‐PAH. In contrast, anti‐dsDNA and anti‐Sm antibodies significantly increased the risk for SLE‐PAH, and anti‐SSB antibody was not a significant risk factor, whereas our SLR found that anti‐dsDNA antibody was a significant protective factor, anti‐Sm antibody was not significantly associated with the disease, and anti‐SSB was significantly associated with an increased risk for SLE‐PAH. Another SLR and meta‐analysis by Xu and Wei (44) included 15 studies in which patients with SLE were diagnosed with PAH via RHC or echocardiography. However, results for RHC and echocardiography were not reported separately, a relevant aspect we considered in our study given that echocardiography‐based studies may include misdiagnosed patients despite being a useful screening tool (39). Therefore, we based our conclusion on the RHC‐based studies.
There were three CSTAR registry–based studies. Concerns could be raised regarding sample overlap. Nonetheless, this is relaxed by the fact that no meta‐analysis was conducted to generate a statistical result of the risk factors; the potential sample overlap among the three registry studies will not have a significant impact on our qualitative analysis. Besides, no discrepancies were found among these three studies, and the two RHC‐based studies (37, 38) were aligned with each other in the trend and significance of the risk factors they both investigated (ie, renal disorder or involvement, positive anti‐RNP antibodies).
Our study has several limitations. Firstly, the studies included in the SLR used the less sensitive thresholds for diagnosing PAH reported in the 2015 European Society of Cardiology and European Respiratory Society guidelines (45), which were revised in 2022 (5) after our work was conducted. Secondly, the sample size varied considerably (from 65 to 3624) across studies included in the SLR. The SLE‐PAH group was smaller than the SLE without PAH group (21–292 cases vs. 44–3532 controls). Consequently, we could have missed some meaningful factors that were not statistically significant but were showing a trend approaching the cutoff (ie, P < 0.05) for significance because of sample size insufficiency. To minimize this limitation, a Delphi panel was introduced to incorporate the clinical experience of experts and provide additional factors that could have been neglected (eg, anticentromere). Thirdly, it was counterintuitive that malar rash, hematologic disorder, renal disorder, and higher SLEDAI score were found to be protective clinical factors for SLE‐PAH. A possible reason could be that patients with SLE presenting these clinical manifestations could usually receive early treatment, thus reducing the risk for development of PAH afterward. Further studies are required to validate this finding. Similarly, the statement that early treatment with immunosuppressants and PAH‐specific therapy (eg, ERA and PDE5i) could improve outcomes in patients with SLE‐PAH was based on expert consensus and would require further clinical investigation. In addition, although we aimed to focus on the APAC region, the generalizability of the findings within the region may be limited because 16 of 21 studies were conducted in China. In the Delphi panel, though consensus was reached on the need for screening patients with SLE for PAH and on the importance and/or practicality of measuring several factors (10 of 22), there were different opinions on other relevant factors (12 of 22). Further studies are needed to raise awareness among physicians about risk factors shown to be strongly associated with increased risk for SLE‐PAH. Lastly, the Delphi panel also indicated that there is a need to distinguish between risk factors and biomarkers of PAH. Risk factors for SLE‐PAH refer to factors that increase the lifetime risk of developing PAH in patients with SLE, whereas some indicators fall better into the category of biomarkers that indicate presence of PAH rather than clinical risk factors and therefore may be useful when screening for PAH. Factors such as increased TRV and reduced dlco are markers of the presence of PAH and could be used to screen for PAH. Similarly, pericardial effusion is a feature of severe and established PAH that is picked up on echocardiography examinations, meaning that these examinations should be repeated yearly in every patient with SLE should it be considered a clinical risk factor. Further studies are needed to establish consensus on which indicators should be considered clinical and serological risk factors and which should be considered markers of PAH so that the role of each type of factor can be determined appropriately. Moreover, the identified clinical and serological risk factors and biomarkers for SLE‐PAH should ideally be tested in additional cohorts to confirm their utility.
Overall, our work reinforces the importance of identifying and measuring factors that facilitate early diagnosis of PAH in patients with SLE, which consequently improves prognosis and management of these patients.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Makanji had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Atanasov, Jiang, Wilson, Wu, Makanji.
Acquisition of data
Atanasov, Jiang, Wilson
Analysis and interpretation of data
Atsumi, Bae, Gu, Huang, Li, Nikpour, Okada, Prior, Atanasov, Jiang, Wilson, Bloomfield, Wu, Makanji.
ROLE OF THE STUDY SPONSOR
Janssen Asia Pacific internal scientific protocol committee approved the study design and protocol. All authors provided input into study design, analysis, data interpretation and jointly made the decision to submit the manuscript for publication.
ADDITIONAL DISCLOSURES
Authors Atanasov, Jiang, and Wilson were employees of Amaris Consulting, a paid consultant to Janssen Pharmaceuticals to support the conduct of this study.
Supporting information
Disclosure form
Appendix S1: Supplementary Information
This study was sponsored and fully funded by Janssen Pharmaceuticals. Dr. Bae's work was supported in part by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (NRF‐2021R1A6A1A03038899). Dr. Nikpour's work was supported by an Australian National Health and Medical Research Council Emerging Leadership Investigator Grant (GTN1176538).
Drs. Atsumi, Bae, Gu, Huang, Li, Nikpour, Okada, and Prior contributed equally to this work.
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11611.
Contributor Information
David Bin‐chia Wu, Email: dwu45@its.jnj.com.
Yogeshwar Makanji, Email: ymakanji@its.jnj.com.
REFERENCES
- 1. Lau EM, Giannoulatou E, Celermajer DS, et al. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol 2017;14:603–14. [DOI] [PubMed] [Google Scholar]
- 2. Lai YC, Potoka KC, Champion HC, et al. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 2014;115:115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schlueter M, Beaudet A, Davies E, et al. Evidence synthesis in pulmonary arterial hypertension: a systematic review and critical appraisal. BMC Pulm Med. 2020;20(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 2018;360:j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension [published erratum appears in Eur Heart J 2023;44:1312]. Eur Heart J 2022;43:3618–731. [DOI] [PubMed] [Google Scholar]
- 6. Simonneau G, Hoeper MM. The revised definition of pulmonary hypertension: exploring the impact on patient management. Eur Heart J Suppl 2019;21 Suppl K:K4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhala A. Pulmonary arterial hypertension in systemic lupus erythematosus: current status and future direction. Clin Dev Immunol 2012;2012:854941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease‐associated pulmonary arterial hypertension in Chinese patients. Eur Respir J 2014;44:963–72. [DOI] [PubMed] [Google Scholar]
- 9. Lin CY, Ko CH, Hsu CY, et al. Epidemiology and mortality of connective tissue disease‐associated pulmonary arterial hypertension: a national cohort study in Taiwan. Semin Arthritis Rheum 2020;50:957–62. [DOI] [PubMed] [Google Scholar]
- 10. Shirai Y, Yasuoka H, Okano Y, et al. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a single‐centre cohort. Rheumatology (Oxford) 2012;51:1846–54. [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Liu X. Clinical characteristics and factors associated with disease progression in Chinese patients with connective tissue disease and pulmonary arterial hypertension. Aktuelle Rheumatologie 2020;45:475–9. [Google Scholar]
- 12. Zhao J, Wang Q, Liu Y, et al. Clinical characteristics and survival of pulmonary arterial hypertension associated with three major connective tissue diseases: a cohort study in China. Int J Cardiol 2017;236:432–7. [DOI] [PubMed] [Google Scholar]
- 13. Coghlan JG, Denton CP, Grünig E, et al. Evidence‐based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014;73:1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. URL: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 16. Shamsrizi P, Gladstone BP, Carrara E, et al. Variation of effect estimates in the analysis of mortality and length of hospital stay in patients with infections caused by bacteria‐producing extended‐spectrum β‐lactamases: a systematic review and meta‐analysis. BMJ Open 2020;10:e030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Millar K, Tomkins S, Thorstensen E, et al. Ethical Delphi manual. The Hague (Netherlands): LEI, part of Wageningen UR; 2006.
- 18. Chen D, Liang L, Wang X, et al. Clinical correlation factors of systemic lupus erythematosus combined with pulmonary hypertension. International Journal of Internal Medicine 2009;36(1):9–11. [Google Scholar]
- 19. Chen X, Que W, Zheng X, et al. Clinical features and risk factors of pulmonary hypertension in Chinese patients with systemic lupus erythematosus. Arch Rheumatol 2019;34:88–95. [Google Scholar]
- 20. Chen Y, Xu J, Wang F, et al. Analysis of the relative risk factors between systemic lupus erythematosus and pulmonary artery hypertension. Acta Universitatis Medicinalis Anhui 2018;53(2): 258–262. [Google Scholar]
- 21. Donnarumma JF, Ferreira EV, Ota‐Arakaki J, et al. Nailfold capillaroscopy as a risk factor for pulmonary arterial hypertension in systemic lupus erythematosus patients. Adv Rheumatol 2019;59:1. [DOI] [PubMed] [Google Scholar]
- 22. Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine (Baltimore) 2016;95:e2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamel SR, Omar GM, Darwish AF, et al. Asymptomatic pulmonary hypertension in systemic lupus erythematosus. Clin Med Insights Arthritis Musculoskelet Disord 2011;4:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim KJ, Baek IW, Park YJ, et al. High levels of uric acid in systemic lupus erythematosus is associated with pulmonary hypertension. Int J Rheum Dis 2015;18:524–32. [DOI] [PubMed] [Google Scholar]
- 25. Lee JH, Im Cho K. Arterial stiffness, antiphospholipid antibodies, and pulmonary arterial hypertension in systemic lupus erythematosus. J Cardiol 2014;64:450–5. [DOI] [PubMed] [Google Scholar]
- 26. Lei Y, Zhang X, Feng Y, et al. Risk factors of pulmonary arterial hypertension in patients with systemic lupus erythematosus. Cardiol Young 2021;31:1619–24. [DOI] [PubMed] [Google Scholar]
- 27. Lian F, Chen D, Wang Y, et al. Clinical features and independent predictors of pulmonary arterial hypertension in systemic lupus erythematosus. Rheumatol Int 2012;32:1727–31. [DOI] [PubMed] [Google Scholar]
- 28. Luo H. Analysis of related factors of pulmonary hypertension in systemic lupus erythematosus. Journal of Community Medicine 2017;15(21):11–13. [Google Scholar]
- 29. Zhou Q, Qi B, Zhou P. Clinical analysis of 44 cases of systemic lupus erythematosus complicated with pulmonary hypertension. Journal of Clinical Internal Medicine 2020;37:125–6. [Google Scholar]
- 30. Xia E, Gao C, Liang W, et al. Relevant risk factors of systemic lupus erythematosus complicated with pulmonary arterial hypertension. Henan Medical Research 2021;30:613–6. [Google Scholar]
- 31. Si H, Zhang K, Lu L, et al. Analysis of risk factors of systemic lupus erythematosus complicated with pulmonary hypertension. Zhejiang Practical Medicine 2015;20:18–20. [Google Scholar]
- 32. Yang J, Ren Z, Luo H, et al. Analysis of risk factors of systemic lupus erythematosus complicated with pulmonary hypertension. Chin J Dermato Venerol Integ Trad W Med 2021;20:251–5. [Google Scholar]
- 33. Fan Y, Zhao J, Qian J, et al. Cysteine‐rich protein 61 as a novel biomarker in systemic lupus erythematosus‐associated pulmonary arterial hypertension. Clin Exp Rheumatol 2019;37:623–32. [PubMed] [Google Scholar]
- 34. Kim KJ, Baek IW, Yoon CH, et al. Association of anemic hypoxia and increased pulmonary artery systolic pressure in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2015;67:1702–11. [DOI] [PubMed] [Google Scholar]
- 35. Xu SZ, Liang Yan, Li XP, et al. Features associated with pulmonary arterial hypertension in Chinese hospitalized systemic lupus erythematosus patients. Clin Rheumatol 2018;37:1547–53. [DOI] [PubMed] [Google Scholar]
- 36. Li M, Wang Q, Zhao J, et al. Chinese SLE Treatment and Research group (CSTAR) registry: II. Prevalence and risk factors of pulmonary arterial hypertension in Chinese patients with systemic lupus erythematosus. Lupus 2014;23:1085–91. [DOI] [PubMed] [Google Scholar]
- 37. Qu J, Li M, Wang Y, et al. Predicting the risk of pulmonary arterial hypertension in systemic lupus erythematosus: a Chinese systemic lupus erythematosus treatment and research group cohort study. Arthritis Rheumatol 2021;73:1847–55. [DOI] [PubMed] [Google Scholar]
- 38. Zhang N, Li M, Qian J, et al. Pulmonary arterial hypertension in systemic lupus erythematosus based on a CSTAR‐PAH study: baseline characteristics and risk factors. Int J Rheum Dis 2019;22:921–8. [DOI] [PubMed] [Google Scholar]
- 39. Augustine DX, Coates‐Bradshaw LD, Willis J, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Res Pract 2018;5:G11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khanna D, Gladue H, Channick R, et al. Recommendations for screening and detection of connective tissue disease‐associated pulmonary arterial hypertension. Arthritis Rheum 2013;65:3194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McLaughlin VV, Channick R, de Marco T, et al. Results of an expert consensus survey on the treatment of pulmonary arterial hypertension with oral prostacyclin pathway agents. Chest 2020;157:955–65. [DOI] [PubMed] [Google Scholar]
- 42. Group of Pulmonary Vascular and Interstitial Diseases Associated with Rheumatic Diseases, Chinese Association of Rheumatology and Immunology Physicians, Chinese Rheumatism Data Center, National Clinical Research Center for Dermatologic and Immunologic Diseases . 2020. Chinese expert‐based consensus statement regarding the diagnosis and treatment of connective tissue disease associated pulmonary arterial hypertension. Zhonghua Nei Ke Za Zhi 2021;60:406–20. In Chinese. [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Qian J, Wang Y, et al. Serological biomarkers as risk factors of SLE‐associated pulmonary arterial hypertension: a systematic review and meta‐analysis. Lupus 2017;26:1390–400. [DOI] [PubMed] [Google Scholar]
- 44. Xu J, Wei Q. Risk factors for pulmonary hypertension in patients with systemic lupus erythematosus: a meta‐analysis. Chinese General Practice 2016;19:2078–83. [Google Scholar]
- 45. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Appendix S1: Supplementary Information


