Abstract
Introduction
Body composition comprising low-skeletal muscle mass (LSMM) and subcutaneous and visceral adipose tissue (SAT and VAT) can be assessed by using cross-sectional imaging modalities. Previous analyses suggest that these parameters harbor prognostic relevance in various diseases. Aim of this study was to analyze possible associations of body composition parameters on mortality in patients with clinically suspected acute mesenteric ischemia (AMI).
Methods
All patients with clinically suspected AMI were retrospectively assessed between 2016 and 2020. Overall, 137 patients (52 female patients, 37.9%) with a median age of 71 years were included in the present analysis. For all patients, the preoperative abdominal computed tomography (CT) was used to calculate LSMM, VAT, and SAT.
Results
Overall, 94 patients (68.6%) of the patient cohort died within 30 days within a median of 2 days, range 1–39 days. Of these, 27 patients (19.7%) died within 24 h. According to the CT, 101 patients (73.7%) were classified as being visceral obese, 102 patients (74.5%) as being sarcopenic, and 69 patients (50.4%) as being sarcopenic obese. Skeletal muscle index (SMI) was lower in non-survivors compared to survivors (37.5 ± 12.4 cm2/m2 vs. 44.1 ± 13.9 cm2/m2, p = 0.01). There were no associations between body composition parameters with mortality in days (SMI r = 0.07, p = 0.48, SAT r = −0.03, p = 0.77, and VAT r = 0.04, p = 0.68, respectively). In Cox regression analysis, a nonsignificant trend for visceral obesity was observed (HR: 0.62, 95% CI: 0.36–1.05, p = 0.07).
Conclusion
SMI might be a valuable CT-based parameter, which could help discriminate between survivors and non-survivors. Further studies are needed to elucidate the associations between body composition and survival in patients with AMI.
Keywords: Acute mesenteric ischemia, Computed tomography, Body composition
Introduction
Acute mesenteric ischemia (AMI) is a possible life-threatening cardiovascular disease with reported 30‐day mortality rates of 31–50% [1–4]. The correct clinical diagnosis is crucial to initiate earliest possible multimodal therapy comprising interventional thrombectomy, surgical exploration and resection of necrotic bowel segments, and even conservative treatment [1–4]. Therefore, immediate risk stratification is important at the time of presentation.
Regarding diagnostic imaging, contrast-enhanced computed tomography (CT) is established as the clinical gold standard in diagnosis of AMI with a reported pooled sensitivity of 93.3% (95% confidence interval: 82.8%, 97.6%) and a pooled specificity of 95.9% (95% confidence interval: 91.2%, 98.2%) [5]. Important CT findings include the vessel occlusion of the superior mesenteric artery and the pneumatosis intestinalis with a reported odds ratio of 2.86 for short-term mortality in a recent study [6].
Besides imaging, prognosis prediction and guidance of therapy depend on many risk factors including age, serum lactate level, white blood cell counts, underlying etiology of AMI, and presence of comorbidities [1, 4, 7]. Universally accepted methods that can objectively and precisely predict clinical outcomes after surgery for patients with AMI are not available. Thus, there is a need for novel biomarkers, which could guide these procedures.
Body composition is an emerging research field to assess different fat areas (visceral and subcutaneous adipose tissue (VAT and SAT) and skeletal muscle areas (SMAs) [8–11]. The prognostic value of these parameters may be of great importance in several fields of medicine. In most studies, one single slice of the abdomen is used, preferably on lumbar level L3 [8–11]. Although there are promising reports regarding the use of body composition for risk stratification in other conditions, the possible benefit of body composition assessment in patients with AMI is still unclear. Presumably, body composition assessment could add a novel prognostic role of CT imaging to the existing necessity for diagnostic purposes in the acute care setting. Thus, the purpose of the present study was to analyze the prognostic role of CT-defined body composition in patients with AMI for prediction of mortality.
Materials and Methods
Patient Acquisition
This retrospective study was approved by the Institutional Ethics Committee of the University of Leipzig. Informed consent was waived due to the retrospective nature of the study.
Patients of our tertiary referral hospital (University Hospital Leipzig, Germany) with clinically suspected AMI were retrospectively assessed within the time period 2016–2020. All patients undergoing surgical exploration were analyzed during this period to provide a surgical patient cohort. Inclusion criteria were clinical suspicion of AMI by clinical or imaging findings. The investigated CT was performed within 24 h before the surgery, in most cases immediately before surgery. The serum markers were also obtained within 24 h prior to surgery. Patients were excluded, when imaging or clinical data were not within the reported time frame and CT imaging had been carried out without contrast media application.
Clinical Parameters
Etiology of AMI, analysis of comorbidities in patient´s history included arterial hypertension, smoking, diabetes mellitus, dyslipidemia, and history of cardiovascular disorder. The following serum markers were obtained: white blood cell count (GPT), venous lactate (mmol/L), C-reactive protein (mg/dL), hemoglobin (g/dL), lactate dehydrogenase (U/L). Mortality was assessed in days after surgical exploration. All-cause mortality was assessed over a time period of 24 h and 30 days.
Surgical Exploration and Bowel Segment Resection
Patients with suspected AMI in CT scans defined by lack of bowel wall enhancement, bowel loop dilatation, and pneumatosis intestinal were scheduled for therapy according to clinical presentation and radiologic findings. If signs of bowel necrosis were present in CT scans, timely surgery was undertaken. During surgery, subjective bowel vitality assessment was undertaken, considering bowel color, movement, and mesenteric pulse. Surgery was usually performed according to the damage control principles with discontinuous resection and intra-abdominal negative pressure dressing in order to take patients to the operating room for a scheduled second look procedure with further resections or anastomosis placement.
Imaging Technique
Contrast-enhanced CT was performed in a clinical setting on a 128-slice CT scanner (Ingenuity 128, Philips, Hamburg, Germany). Intravenous administration of an iodine-based contrast medium (90 mL Imeron 400 MCT, Bracco Imaging Germany GmbH, Konstanz, Germany) was given at a rate of 4.0 mL/s via a peripheral venous line. Automatic bolus tracking was performed in the descending aorta with a trigger of 100 Hounsfield units (HU). CT was performed in arterial and portal venous phase in every case. Typical imaging parameters were 100 kVp; 125 mAs; slice thickness, 1 mm; pitch, 0.9.
Low-Skeletal Muscle Mass Calculation
Body composition parameters were semiautomatically measured with the freely available ImageJ software 1.48v (National Institutes of Health Image program). SMA was calculated on the level L3 including psoas muscle, paraspinal muscles, and the abdominal wall muscles. The muscle area was semiautomatically measured using the HU threshold levels of −29 and 150 HU, as proposed in similar studies [12, 13]. The SMA was divided by the height squared to calculate the skeletal muscle index (SMI). For sarcopenia definition, the SMI threshold proposed by Prado et al. [14] was used: 52.4 cm2/m2 for male and 38.5 cm2/m2 for female.
Fat Area Quantification
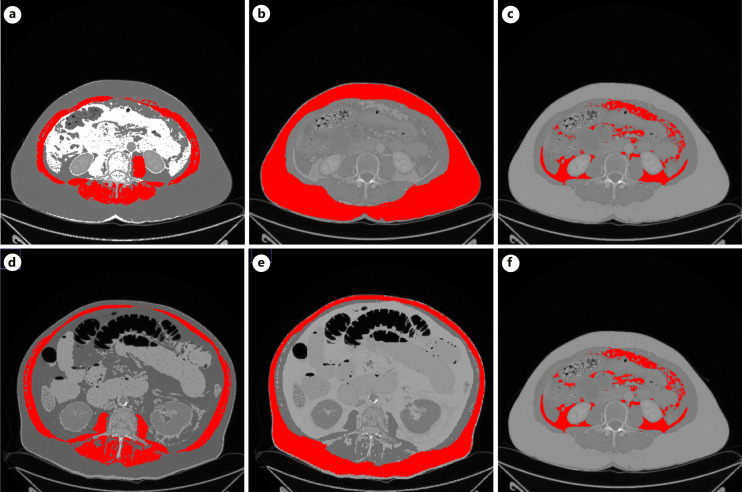
VAT and SAT were calculated on the same level L3 as the muscle areas. The fat areas were semiautomatically measured using the HU threshold levels of −190 and −30 HU [15]. VAT quantifies the visceral fat area and SAT the subcutaneous fat area. The previously proposed threshold value of 100 cm2 was used as a cutoff value to determine visceral obesity [15]. At last, sarcopenic obesity was defined for patients with sarcopenia and visceral obesity. Figure 1 displays 2 representative patients of the study to show the body composition quantifications.
Fig. 1.
a Representative case of the patient sample with regular amount of muscle and fat areas. The red area shows the measured SMA. b The red area shows the subcutaneous fat area. c The red area shows the measured visceral fat area. d Another representative case of the patient sample with sarcopenia and visceral obesity. The red shows the calculated SMA. e The red area demonstrates the subcutaneous fat area. f The red area demonstrates the calculated visceral fat area.
Statistical Analysis
Collected data were evaluated by means of descriptive statistics (absolute and relative frequencies). Spearman’s correlation coefficient (r) was used to analyze associations between the investigated parameters after testing for normality distribution. Group differences were calculated with Mann-Whitney test and Fisher’s exact test, when suitable. Multivariable Cox regression analysis was used to test for the effect of body composition parameters on mortality. In all instances, p values <0.05 were considered statistical significant. The statistical analysis and graphics creation were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) and SPSS Statistics (IBM, Version 25.0; Armonk, NY, USA).
Results
One hundred and thirty-seven patients (52 female patients, 37.9%) with a median age of 71 years, range 31–97 years, were identified in the clinical data base and included in the present study. Overall, 94 patients (68.6%) of the patient cohort died within 30 days with a median of 2 days, range 1–39 days. Of those, 27 patients (19.7%) died within 24 h. One hundred and twelve patients (81.7%) underwent bowel resection during the surgical exploration. Table 1 gives an overview of the demographics of the patient sample.
Table 1.
Demographic characteristics of the patient sample according to 30-day mortality
| Parameter | Non-survivors (n = 94) | Survivors (n = 43) | p value |
|---|---|---|---|
| Age, years | 69.8±12.9 | 69.4±10.9 | 0.56 |
| Sex | |||
| Female, n (%) | 40 (42.6) | 12 (27.9) | 0.28a |
| BMI, kg/m2 | 28.2±8.2 | 28.5±9.7 | 0.89 |
| History of hypertension, n (%) | 71 (75.5) | 28 (65.1) | 0.66a |
| History of dyslipidemia, n (%) | 40 (42.6) | 20 (46.5) | 0.86a |
| History of cardiovascular disease, n (%) | 63 (67.0) | 26 (60.4) | 0.77a |
| Diabetes mellitus, n (%) | 33 (35.1) | 13 (30.2) | 0.85a |
| Smoker, n (%) | 17 (18.1) | 7 (16.3) | 0.98a |
| Bowel resection, n (%) | 80 (85.1) | 30 (69.7) | 0.57a |
| C-reactive protein | 187.3±148.8 | 111.4±128.1 | 0.03 |
| Venous lactate, mmol/L | 7.1±5.3 | 2.7±1.9 | <0.001 |
| Hemoglobin, g/dL | 8.9±2.7 | 11.8±3.0 | <0.001 |
| Lactate dehydrogenase, U/L | 7.3±3.4 | 5.9±2.8 | 0.07 |
| White blood cell count, G/L | 19.5±10.9 | 15.5±9.4 | 0.03 |
| VAT, cm2 | 176.7±103.7 | 216.0±122.7 | 0.09 |
| SAT, cm2 | 207.0±120.6 | 216.2±143.9 | 0.86 |
| SMI, cm2/m2 | 37.5±12.4 | 44.1±13.9 | 0.01 |
| Sarcopenia according to Prado et al. [14], n (%) | 72 (76.5) | 28 (65.1) | 0.66a |
| Visceral obesity, n (%) | 67 (71.3) | 34 (79.0) | 0.78a |
| Sarcopenic obesity, n (%) | 48 (51.1) | 21 (48.8) | 0.99a |
Significant p values are highlighted in bold.
BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
aFisher’s exact test.
The mean VAT was 186.8 ± 111.6 cm2, range 12.2–505.3 cm2, mean SAT was 209.8 ± 128.0 cm2, range 6.9–793.2 cm2, mean SMI was 39.5 ± 13.2 cm2/m2, range 11.4–79.6 cm2/m2. According to the threshold value of 100 cm2, 101 patients (73.7%) were classified as being visceral obese. According to the sarcopenia threshold, 102 patients (74.5%) were classified as being sarcopenic. Overall, 69 patients (50.4%) were classified as being sarcopenic obese.
Association between Body Composition Parameters and Mortality
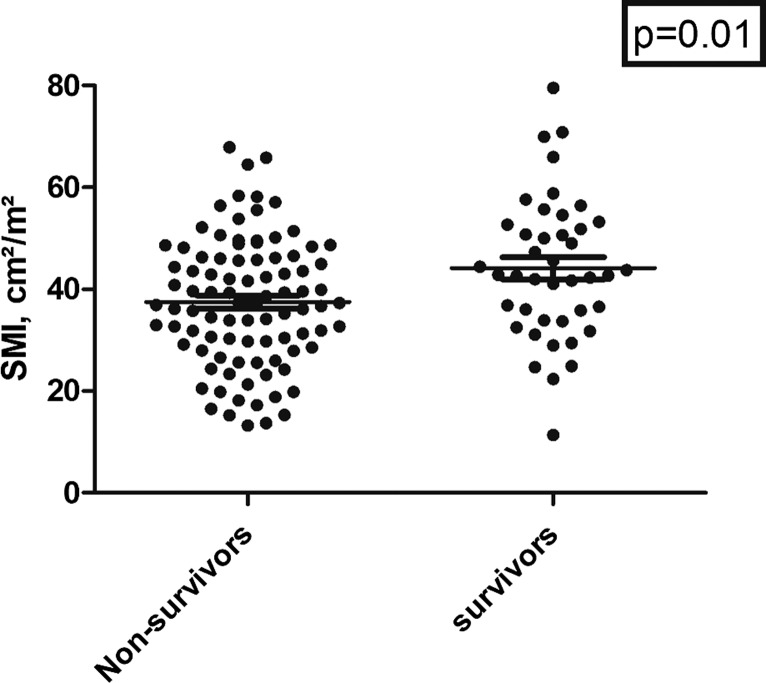
In discrimination analysis, SMI was lower in non-survivors compared to survivors (37.5 ± 12.4 cm2/m2 vs. 44.1 ± 13.9 cm2/m2, p = 0.01) (Fig. 2; Table 1). The other body composition parameters did not differ between these groups. Laboratory parameters including serum lactate level (p < 0.001), hemoglobin level (p < 0.001), C-reactive protein level (p < 0.03), and white blood cell count (p = 0.03) were statistically significant between non-survivors and survivors, whereas lactate dehydrogenase levels were comparable (p = 0.07). There was no association between body composition parameters with mortality in days (for SMI r = 0.07, p = 0.48, for SAT r = −0.03, p = 0.77, and for VAT r = 0.04, p = 0.68, respectively).
Fig. 2.
Scatter plot of the SMI values between survivors and non-survivors. The survivor group showed statistically higher SMI values (p = 0.01).
This was supported by the Cox regression analysis, which could not identify statistically significant associations between body composition parameters with 24-h or 30-day mortality (Tables 2, 3). There was only a statistical trend for visceral obesity with a HR of 0.62 (95% CI: 0.36–1.05), p = 0.07. Lactate serum level reached statistically significance with a HR of 1.07 (95% CI: 1.02–1.12), p = 0.003.
Table 2.
Cox regression analysis for 30-day mortality
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| SMI | 0.99 (0.98–1.02) | 0.88 |
| SAT | 0.99 (0.99–1.0) | 0.93 |
| VAT | 0.99 (0.99–1.0) | 0.75 |
| Sarcopenia according to Prado et al. [14] | 1.09 (0.60–1.99) | 0.76 |
| Visceral obesity | 0.62 (0.36–1.05) | 0.07 |
| Sarcopenic obesity | 0.74 (0.45–1.22) | 0.24 |
| Age | 1.01 (1.00–1.03) | 0.053 |
| Sex | 1.37 (0.91–2.06) | 0.13 |
| BMI | 1.00 (0.97–1.02) | 0.96 |
| Hypertension | 0.81 (0.50–1.31) | 0.38 |
| Dyslipidemia | 1.09 (0.72–1.64) | 0.66 |
| Diabetes | 0.91 (0.60–1.39) | 0.67 |
| Smoking | 0.97 (0.57–1.65) | 0.92 |
| Bowel resection | 1.11 (0.51–2.33) | 0.80 |
| Serum lactate | 1.07 (1.02–1.12) | 0.003 |
SMI, skeletal muscle index; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; CI, confidence interval; BMI, body mass index.
Table 3.
Comparison between survivors and non-survivors according to 24-h mortality
| Parameter | Non-survivors (n = 26) | Survivors (n = 111) | p value |
|---|---|---|---|
| VAT, cm2 | 165.1±94.4 | 191.9±115.0 | 0.34 |
| SAT, cm2 | 209.4±171.1 | 209.9±116.6 | 0.33 |
| SMI, cm2/m2 | 40.5±14.9 | 39.3±12.8 | 0.85 |
| Sarcopenia according to Prado et al. [14], n (%) | 18 (69.2) | 84 (75.6) | 0.88a |
| Visceral obesity, n (%) | 20 (76.9) | 81 (72.9) | 0.86a |
| Sarcopenic obesity, n (%) | 13 (50.0) | 56 (50.5) | 0.99a |
BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
aFisher’s exact test.
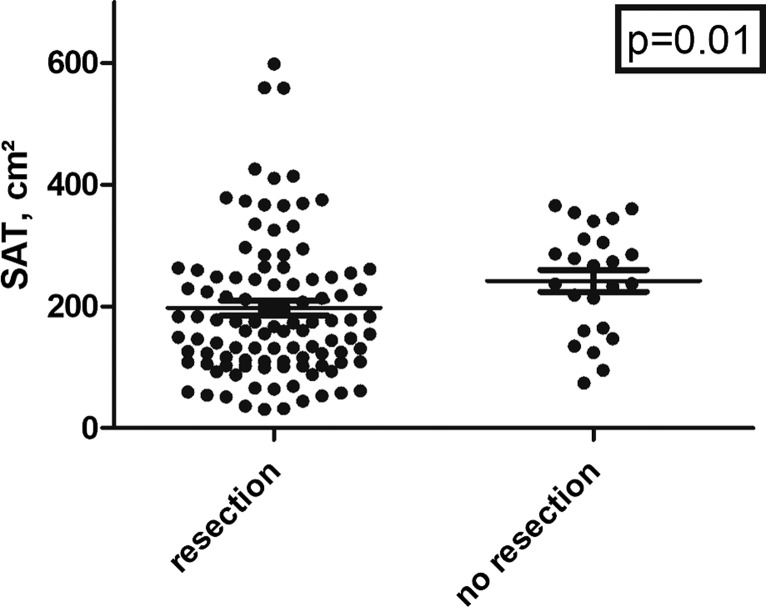
Similar results were identified in sub-analyses according to older patients over 60 years, according to gender, and of patients with an arterial occlusion of the visceral arteries. SAT was significantly lower in patients undergoing bowel segment resection compared to the patients without resection (197.4 ± 128.1 cm2 vs. 241.8 ± 87.5 cm2, p = 0.01, Fig. 3). No significant differences were identified for SMI and VAT (p = 0.67 and p = 0.33, respectively).
Fig. 3.
Scatter plot of the SAT values between patients with and without undergoing bowel resection. The group without resection showed higher SAT values (p = 0.01).
Correlations between Body Composition Parameters with Clinical and Laboratory Parameters
There were associations between all body composition parameters with BMI (SMI r = 0.35, p < 0.001; SAT r = 0.74, p < 0.001; and VAT r = 0.61, p < 0.001, respectively). There were no associations between body composition parameters with age.
For associations of the body composition parameters, SMI was not associated with SAT (r = 0.16, p = 0.06) but was moderately correlated with VAT (r = 0.47, p < 0.001). VAT was only moderately correlated with SAT (r = 0.44, p < 0.001).
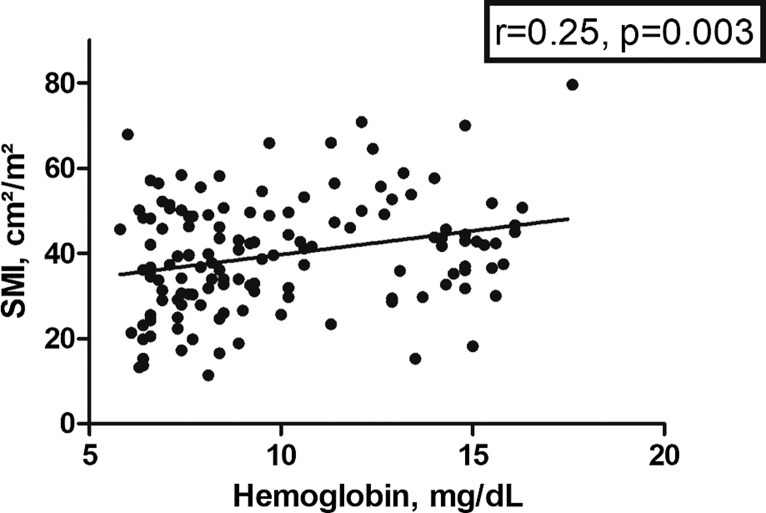
There were no correlations between body composition parameters with serum lactate or C-reactive protein. A weak association was identified between SAT and white blood cell count (r = 0.24, p = 0.005), whereas the other parameter did not correlate. There was a weak correlation between SMI with hemoglobin (r = 0.25, p = 0.003) (Fig. 4) but not with VAT and SAT. SMI showed an inverse correlation with LDH (r = −0.43, p < 0.001), whereas the other parameters did not.
Fig. 4.
Spearman’s correlations analysis between SMI and hemoglobin levels (r = 0.25, p = 0.003).
Discussion
AMI is a morbid condition with an increasing incidence and a high mortality, which needs an early diagnosis and correct treatment planning [1–4]. The diagnosis of AMI requires a variety of clinical factors. CT imaging can raise the suspicion of AMI and rule out differential diagnosis, which highlights the importance of CT in the diagnostic workup of these patients. Our aim was to add more diagnostic power to the pretreatment period by evaluating body composition as a prognostic tool in patients with the clinical suspicion of AMI.
A key finding of the present study was that body composition alone does not harbor enough prognostic relevance for these patients to provide substantial novel imaging biomarkers. The observed 30-day mortality of 68.1% seemed high, although comparable with other reported patient cohorts [2, 16], reflecting the high risk associated with this acute medical condition.
The topic of body composition is an emerging field of research with extensive studies in various disorders [8–15]. Of note, there are various possible applications and interesting prognostic implications of low-skeletal muscle mass (LSMM) and VAT quantification throughout many fields of clinical care [8–15]. Importantly, these calculations are a by-product of cross-sectional imaging and can easily be estimated in clinical routine. The present analysis identified a high frequency of visceral obesity and sarcopenia in patients with AMI.
Only few studies have investigated the possible prognostic relevance of sarcopenia assessment in patients with AMI [7, 17]. These two studies employed only the psoas muscle area as a surrogate parameter of LSMM. However, it has become the standard to employ SMI as in the present study, which utilizes the whole muscle area of the slice of level L3 and is adjusted to body height.
Nevertheless, Chen et al. [7] could show that the psoas muscle area was an important predictor of postoperative complications besides the Charlson Comorbidity Index score ≥2, and bowel resection as independent predictors. Notably, a low 30-day mortality of only 11.4% in the surgical patient group can clearly show distinctive differences between this study and the present patient sample. The difference of these cohorts is clearly an expression of the very critically ill cohort of patients with AMI. In the study by Lareyre et al. [17], 80 patients with AMI were investigated. The authors showed no significant differences between patients with sarcopenia and without for the 30-day mortality (63.2% vs. 47.5%, p = 0.297). The present analysis supports the findings of these two smaller studies that sarcopenia might not be a strong predictor of 30-day mortality, but the present analysis can add the significant relevance of sarcopenia for 24-h mortality as the sarcopenic patients had a higher 24-h mortality. Moreover, the present analysis utilizes a more standardized approach for sarcopenia assessment.
One important aspect seems to be the quality of the muscle, which was shown by the study by Miao et al. [18]. Only the psoas muscle density remained as an independent risk factor for the 30-day mortality [18].
As another key aspect of the present analysis, it provides data of the fat area assessment for patients with AMI for the first time. Visceral obesity has been identified as an important prognostic factor for surgical outcomes including postoperative complications in various oncological diseases [19–22], such as colorectal cancer [21] and other carcinomas, as well as inflammatory disorders, such as Crohn’s disease [22].
One interesting finding of the present analysis was that low SAT was significantly associated with resection of bowel segments. This could be explained by reported findings that low SAT was associated with longer duration of surgery, increased intraoperative blood loss, and longer length of bowel resected in patients with Crohn’s disease [15]. For Crohn’s disease, the importance of VAT and SAT assessment was shown in several studies, which were reported by a recent systematic review [15].
One concern of body composition assessment is the considerable variation of calculation throughout the published studies. A common approach for VAT is based on the umbilical level, which usually presents the highest amount of fat area [11]. The problem with the umbilicus is the mobility of the abdominal wall with increased tissue masses. Thus, a fixed measurement level such as the applied L3 levels seems to harvest more reproducible measurements. The present study used a validated threshold of 100 cm2 to define visceral obesity, which was employed in critically ill patients with COVID-19 [11]. Another frequently used threshold was reported by Doyle et al. [23]: 163.8 cm2 in male patients and 80.1 cm2 in female patients. Presumably, these different thresholds could result in different frequencies of visceral obesity.
Besides CT body composition parameters, we identified differences between survivors and non-survivors in some serological parameters including serum lactate level, hemoglobin level, C-reactive protein level, and white blood cell count. This is in good agreement with the reported literature that these parameters might also harbor prognostic relevance [24]. It could be interesting to incorporate serological and imaging-based parameters into prognostic scores to better stratify patients with AMI.
The present analysis has several limitations. First, retrospective single-center studies are prone for possible inherent bias. Second, the patient sample was based on a single center which may cause a selection bias. Patients with AMI but without surgery, who may have a better prognosis, were not included. Especially patients undergoing endovascular treatment should be mentioned there. These patients have a better prognosis compared to surgery alone patients. However, in the present analysis, these were not included to present a more homogenous patient sample in an already severe heterogeneous disease. Third, the body composition quantification was performed by one expert. However, the analysis was performed semiquantitatively and should not harbor relevant investigator bias. Further systematic analyses are needed to investigate the importance of body composition assessment derived from CT images in meta-analyses and multicenter analyses to overcome the limitations of the present analysis.
Conclusion
SMI might be a valuable CT-based parameter, which could help discriminate between survivors and non-survivors. Further studies are needed to elucidate the associations between body composition and survival in patients with AMI.
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Ethics Committee of the University of Leipzig, Nr.: 118/19-ck, Ethics Committee, University of Leipzig, Leipzig, Germany. Informed consent was waived due to the retrospective nature of the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors received no specific funding for this work.
Author Contributions
Study conception and design: H.J.M. and M.M.; acquisition of data: C.S., M.M., J.L., M.R., M.F.S., and S.E.; supervision: T.D. and I.G.; operation procedures and critical revision of the manuscript: all authors; analysis and interpretation of data: H.J.M. and C.S.; and drafting of the manuscript: H.J.M. All authors read and approved the final manuscript.
Funding Statement
The authors received no specific funding for this work.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007 Sep;46(3):467–74. 10.1016/j.jvs.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 2. Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: six year review. Langenbecks Arch Surg. 2008;393(2):163–71. 10.1007/s00423-007-0263-5. [DOI] [PubMed] [Google Scholar]
- 3. Andraska EA, Tran LM, Haga LM, Mak AK, Madigan MC, Makaroun MS, et al. Contemporary management of acute and chronic mesenteric ischemia: 10-year experience from a multihospital healthcare system. J Vasc Surg. 2022;75(5):1624–33.e8. 10.1016/j.jvs.2021.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim S, Halandras PM, Bechara C, Aulivola B, Crisostomo P. Contemporary management of acute mesenteric ischemia in the endovascular era. Vasc Endovascular Surg. 2019;53(1):42–50. 10.1177/1538574418805228. [DOI] [PubMed] [Google Scholar]
- 5. Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology. 2010;256(1):93–101. 10.1148/radiol.10091938. [DOI] [PubMed] [Google Scholar]
- 6. Graber SD, Sinz S, Turina M, Alkadhi H. Pneumatosis intestinalis in abdominal CT: predictors of short-term mortality in patients with clinical suspicion of mesenteric ischemia. Abdom Radiol. 2022;47(5):1625–35. 10.1007/s00261-022-03410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen FF, Ye XN, Jiang HT, Zhu GX, Miao SL, Yu GF, et al. Role of frailty and comorbidity status in predicting morbidity and mortality in patients with acute mesenteric ischemia. Ann Vasc Surg. 2020;67:105–14. 10.1016/j.avsg.2020.03.037. [DOI] [PubMed] [Google Scholar]
- 8. Saravana-Bawan B, Goplen M, Alghamdi M, Khadaroo RG. The relationship between visceral obesity and post-operative complications: a meta-analysis. J Surg Res. 2021;267:71–81. 10.1016/j.jss.2021.04.034. [DOI] [PubMed] [Google Scholar]
- 9. Vangelov B, Bauer J, Kotevski D, Smee RI. The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: a systematic review. Br J Nutr. 2022;127(5):722–35. 10.1017/S0007114521001446. [DOI] [PubMed] [Google Scholar]
- 10. Zhang X, Xie X, Dou Q, Liu C, Zhang W, Yang Y, et al. Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: a updated meta-analysis. BMC Geriatr. 2019;19(1):183. 10.1186/s12877-019-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goehler A, Hsu TMH, Seiglie JA, Siedner MJ, Lo J, Triant V, et al. Visceral adiposity and severe COVID-19 disease: application of an artificial intelligence algorithm to improve clinical risk prediction. Open Forum Infect Dis. 2021;8(7):ofab275. 10.1093/ofid/ofab275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baggerman MR, van Dijk DPJ, Winkens B, van Gassel RJJ, Bol ME, Schnabel RM, et al. Muscle wasting associated co-morbidities, rather than sarcopenia are risk factors for hospital mortality in critical illness. J Crit Care. 2020;56:31–6. 10.1016/j.jcrc.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 13. Meyer HJ, Benkert F, Bailis N, Lerche M, Denecke T, Surov A. Low skeletal muscle mass defined by thoracic CT as a prognostic marker in acute pulmonary embolism. Nutrition. 2022;98:111622. 10.1016/j.nut.2022.111622. [DOI] [PubMed] [Google Scholar]
- 14. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35. 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 15. Rowan CR, McManus J, Boland K, O’Toole A. Visceral adiposity and inflammatory bowel disease. Int J Colorectal Dis. 2021;36(11):2305–19. 10.1007/s00384-021-03968-w. [DOI] [PubMed] [Google Scholar]
- 16. Martini V, Lederer AK, Fink J, Chikhladze S, Utzolino S, Fichtner-Feigl S, et al. Clinical characteristics and outcome of patients with acute mesenteric ischemia: a retrospective cohort analysis. Langenbecks Arch Surg. 2022;407(3):1225–32. 10.1007/s00423-021-02423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lareyre F, Augène E, Chikande J, Guidi L, Ballaith A, Caradu C, et al. Evaluation of the impact of sarcopenia in patients with acute mesenteric ischemia. Ann Vasc Surg. 2020;63:170–8.e1. 10.1016/j.avsg.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 18. Miao SL, Ye XN, Lin TT, Qiu YH, Huang JY, Zheng XW, et al. The psoas muscle density as a predictor of postoperative complications and 30-day mortality for acute mesenteric ischemia patients. Abdom Radiol. 2022;47(5):1644–53. 10.1007/s00261-020-02714-0. [DOI] [PubMed] [Google Scholar]
- 19. Fehrenbach U, Wuensch T, Gabriel P, Segger L, Yamaguchi T, Auer TA, et al. CT body composition of sarcopenia and sarcopenic obesity: predictors of postoperative complications and survival in patients with locally advanced esophageal adenocarcinoma. Cancers. 2021;13(12):2921. 10.3390/cancers13122921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yue Y, Li M, Zhang X, Yu H, Song B. Prediction of clinically relevant pancreatic fistula after pancreatic surgery using preoperative CT scan: a systematic review and meta-analysis. Pancreatology. 2020;20(7):1558–65. 10.1016/j.pan.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 21. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103(5):572–80. 10.1002/bjs.10075. [DOI] [PubMed] [Google Scholar]
- 22. Grillot J, D’Engremont C, Parmentier AL, Lakkis Z, Piton G, Cazaux D, et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin Nutr. 2020;39(10):3024–30. 10.1016/j.clnu.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 23. Doyle SL, Mongan AM, Donohoe CL, Pidgeon GP, Sherlock M, Reynolds JV, et al. Impact of visceral obesity and metabolic syndrome on the postoperative immune, inflammatory, and endocrine response following surgery for esophageal adenocarcinoma. Dis Esophagus. 2017;30(6):1–11. 10.1093/dote/dox008. [DOI] [PubMed] [Google Scholar]
- 24. Otto CC, Czigany Z, Heise D, Bruners P, Kotelis D, Lang SA, et al. Prognostic factors for mortality in acute mesenteric ischemia. J Clin Med. 2022;11(13):3619. 10.3390/jcm11133619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.