Abstract
Introduction
The concept of allostatic load encompasses the cumulative effects of both ordinary daily life events as well as major challenges, and also includes related health-damaging behavior. Allostatic overload ensues when environmental challenges exceed the individual’s ability to cope. Identification of allostatic load is carried out through the use of biomarkers and clinimetric criteria. Studies are increasingly reported on allostatic load in younger populations, yet a systematic review is missing.
Objective
The aim of the present systematic review was to summarize the current knowledge on allostatic load/overload among children and adolescents.
Methods
PubMed, PsycINFO, Web of Science, and the Cochrane Library were searched from inception to April 2023. A manual search of the literature was also performed. We considered only studies in which allostatic load or overload were adequately described and assessed in either clinical or non-clinical populations younger than 18 years.
Results
A total of 38 original investigations were included in this systematic review. Studies reported an association between allostatic load and sociodemographic characteristics (e.g., poverty, ethnicity, perceived discrimination, adverse childhood experiences) and environmental factors, as well as consequences of allostatic load on both physical and mental health among children and adolescents.
Conclusions
The findings indicate that greater allostatic load is associated with poorer health outcomes in both clinical and non-clinical pediatric populations, with possible enduring effects. The results support the clinical utility of the transdiagnostic identification of allostatic load and overload in children and adolescents across a variety of settings, with a number of potential clinical implications.
Keywords: Allostatic load, Allostatic overload, Biomarkers, Clinimetrics, Diagnostic criteria for psychosomatic research, Childhood, Adolescence
Introduction
The concept of allostatic load [1] provides a conceptual framework for better understanding the relationship between stress and the processes leading to disease based on allostasis, the ability of the organism to achieve stability through change [2]. It refers to the cost of chronic exposure to fluctuating or heightened neural and neuroendocrine responses, resulting from repeated or chronic environmental challenges that an individual reacts to as being particularly stressful [1], and to the long-term effects of the wear and tear that result from either too much stress or from inefficient management of allostasis [3, 4].
The definition of allostatic load reflects the cumulative effects of experiences in daily life that involve ordinary events (subtle and long-standing life situations that may be experienced by the individual as taxing or exceeding his/her coping skills) as well as major challenges (life events), and also includes the physiological consequences of the resulting health-damaging behavior (poor sleep and circadian disruption, lack of exercise, smoking, alcohol consumption, and unhealthy diet) [5, 6]. When environmental challenges exceed the individual’s ability to cope, allostatic overload may ensue [4–6], as a transition to an extreme state, where the body stress response systems are repeatedly activated in the absence of adequate buffering factors (i.e., toxic stress) [7].
Identification of allostatic load has been carried out by two different approaches. One is concerned with the use of biomarkers [8, 9] reflecting alterations in neuroendocrine and immune systems [3, 4], hypothalamic-pituitary-adrenal axis activity [4, 10], brain architecture and neurochemical functions [11, 12], cardiovascular and gastrointestinal systems, endocrine-metabolic balances and sleep [4, 10, 13–15]. However, the biological perspective does not allow for a comprehensive understanding of the underlying individual causes. Further, high heterogeneity exists across studies as to the type and number of biological parameters to be considered [16, 17].
A substantial contribution to the determination of allostatic overload has come from clinimetrics [18–21]. Specific clinimetric criteria were introduced in 2010 [22] and subsequently incorporated in the Diagnostic Criteria for Psychosomatic Research [23] under a diagnostic rubric. Accordingly, a semi-structured interview was developed [5, 24]. It can be supplemented by a short self-rated questionnaire, the Psychosocial Index (PSI) [25–27], for assessing the degree of allostatic load and possibly identifying allostatic overload according to cut-off points and algorithms [6].
The majority of studies on allostatic load and overload have been carried out in adults as summarized in a recent systematic review [28] indicating that higher allostatic load was associated with poorer health outcomes in both general and clinical populations. A systematic review on allostatic load and its consequences among children and adolescents is still missing, even though studies are being increasingly reported on allostatic load in younger populations [17]. Indeed, the concept of allostatic load can provide an integrative framework for a better understanding of the impact of adverse childhood experiences and chronic stress in children and adolescents and can be used to broaden approaches to assessment and treatment in younger populations [29].
It is conceivable that some physiological systems are more likely to be disrupted by stress during sensitive periods of development such as adolescence, which involves changes across several physiological systems [30] and increased stress reactivity [31, 32]. Allostatic load may impact on brain and body development and functioning in childhood, partially through epigenetic changes mediated by the neuroendocrine system [33]. During critical periods of brain developments characterized by elevated neuroplasticity and enhanced sensitivity to epigenetic effects as in childhood and adolescence, allostatic load may exert long-lasting effects on particular neuronal networks that lead to enduring neuroendocrine alterations [4, 34]. The multi-systemic nature of allostatic load may allow to understand how major challenges and chronic stress during developmental stages can affect future patterns of physical health and psychological functioning later in life [35].
The aim of the present systematic review was to summarize the current knowledge on allostatic load/overload among children and adolescents (i.e., up to 18 years of age). A previous systematic review on measures of allostatic load among adolescents and young adults (age 10–24 years) [17] was specifically concerned with the range and variability of biomarkers of allostatic load used across different populations.
Methods
Search Strategy
A systematic review of the literature was performed according to the PRISMA guidelines [36]. Published articles concerning allostatic load/overload in either clinical or non-clinical pediatric and/or adolescent populations were identified by searching in PubMed, Web of Science, PsycINFO, and the Cochrane Library from inception to April 2023. Searched terms included “allostatic load” or “allostatic overload.” Only published articles in the English language and involving human subjects younger than 18 years were considered for inclusion. Reference lists of the retrieved articles were examined for further studies that are not yet identified and a manual search of the literature was also performed.
Study Selection
We considered only those studies in which allostatic load or overload were adequately described and assessed in either clinical or non-clinical pediatric and/or adolescent populations. Identification of allostatic load should be based on at least 3 biological markers, whereas assessment of allostatic overload was performed according to clinimetric criteria. Studies involving adult populations (i.e., older than 18 years) were excluded.
Data Extraction
Both authors (M.L. and J.G.) independently carried out the search, screened titles and abstracts, selected studies, assessed the full text of potentially relevant articles, and extracted data from studies meeting the eligibility criteria. In case of disagreement, a consensus was reached through discussion.
Data Synthesis
All selected original studies were reported in the systematic review. The most relevant reviews on allostatic load or overload among children and adolescents were cited in the introduction and in the discussion. Other studies (e.g., not primarily focused on allostatic load/overload or not involving pediatric or adolescent populations) were not included.
Results
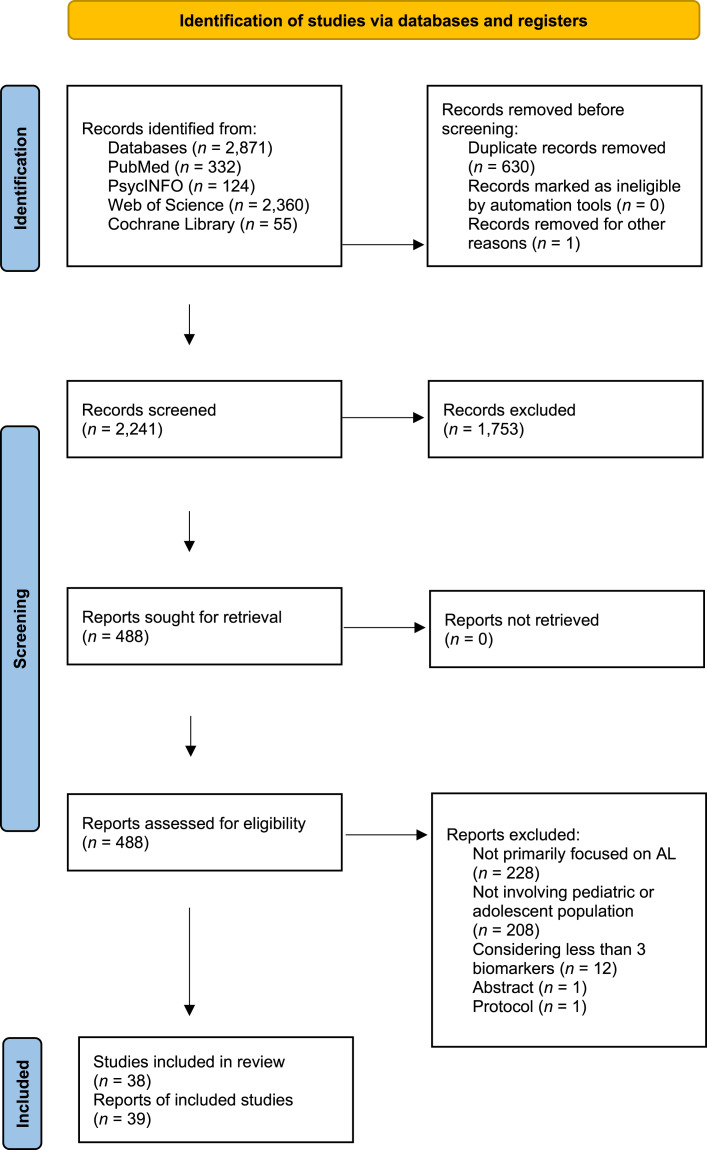
The initial search identified 2,871 published articles. After removing the duplicates, 2,241 studies were screened for potential inclusion in the review (Fig. 1). A total of 488 full-text articles were assessed for eligibility, and 38 were included in the systematic review, with one article [37] reporting data on two separate studies. The main characteristics (e.g., study design, number of participants, allostatic load assessment) of included studies are provided in the online supplementary material (Table S1) (for all online suppl. material, see https://doi.org/10.1159/000533424).
Fig. 1.
PRISMA flowchart of the systematic search.
Sociodemographic Characteristics
A number of studies addressed the association between sociodemographic variables and allostatic load in children and adolescents [38–52]. In a longitudinal study [42] conducted on a sample of young adults, childhood poverty was found to be a significant predictor of allostatic load. Further, its duration was associated with high levels of allostatic load during childhood [44]. Three longitudinal studies [39, 41, 43] reported a prospective significant relationship between childhood poverty and increased levels of allostatic load later in life. Further, the degree of increase in allostatic load over time appeared to be accelerated among those who spent longer periods of early childhood in poverty [39, 41]. Similarly, Evans and Kim [43] reported that the longer the children lived in poverty from birth to age 9, the higher was the increase in allostatic load levels between ages 9 and 17. These studies [39, 41, 43] support the idea that early life economic adversities may have long-term effects leading to elevated risk of allostatic load in emerging adulthood. Homeless children and youths living in rural villages [52] or impoverished neighborhoods [51] displayed high scores of allostatic load. Further, Theall and colleagues [49] showed that adolescents living in medium- or high- and very high-risk neighborhoods were more likely to report high levels of allostatic load compared to youths living in low-risk areas.
Several studies [38, 48, 51, 53] reported the association between ethnicity and allostatic load. In a multiethnic cohort of children, Hispanic Americans showed significantly higher levels of allostatic load when compared to their African and European American counterparts [38]. Wallace and colleagues [51] analyzed data from the Bogalusa Heart Study with the purpose of examining the relationships between maternal preconception allostatic load, race, and adverse birth outcomes, within the context of neighborhood-level poverty [54]. Findings showed that African American young women living in more impoverished neighborhoods displayed higher allostatic load than white Americans [51]. Data from the U.S. National Health and Nutrition Examination Survey (NHANES), including several adolescent ethnic groups, displayed that non-Hispanic blacks and Mexican Americans had higher scores of allostatic load compared to white Americans [48, 53]. Nonetheless, the degree of increase in allostatic load by year of age was significantly lower for black Americans than whites, supporting converging values of predicted allostatic load among ethnic groups [48].
In two investigations [46, 50], perceived discrimination was found to be positively associated with allostatic load among adolescents. Particularly, Tian and colleagues [50] found a stronger association between adolescents’ and parents’ allostatic load among adolescents reporting higher perceived discrimination. However, the adverse effects of parents’ allostatic load and perceived discrimination on adolescents’ allostatic load levels were moderated by social support [50]. Fuller-Rowell and colleagues [46] reported that among adolescents, perceived discrimination explained 13% of the effect of poverty on allostatic load, suggesting the role of social class discrimination on the association between low socioeconomic status and health.
In a study [55] conducted in a sample of 7- to 12-year-old participants to evaluate the association of prolonged separation from both parents early in life and allostatic load, significantly elevated allostatic load levels were found among children separated from both parents during early childhood or persistently into adolescence. Furthermore, De la Rosa and colleagues [56] reported that adverse childhood experiences were significantly associated with higher allostatic load scores in a pediatric population, supporting the biological response to adversities in early to mid-childhood according to the AL framework.
Environment
Two cross-sectional studies [57, 58] investigated the association between both indoor and natural environment and allostatic load among children. The accessibility to green spaces near schools was found to be important: the greater the distance from school to the nearest green space, the higher was the allostatic load in children [57]. Further, high interior density and other environmental stressors, such as noise, were found to elevate allostatic load in children, whereas bedroom ceiling height was found to attenuate the impact of perceived home crowding on allostatic load among those reporting higher perceived home crowding [58].
Consequences on Physical Health
Some studies reported the association between allostatic load and poor physical health outcomes [59–61]. High levels of dysregulated biological parameters were found to be present in perinatally HIV-infected children [62], whereas elevated levels of allostatic load were found to be related to increased likelihood of asthma [59] as well as to somatic complaints [60] among adolescents. Nonetheless, two observational studies [51, 63] reported a lack of association between preconception maternal allostatic load and adverse birth outcomes.
Both malnutrition and overweight were found to be positively associated with high levels of allostatic load in both clinical and non-clinical populations [38, 56, 64, 65]. Among children and adolescents, high body mass index, indicating an overweight status or obesity, were linked to high levels of allostatic load [38, 64, 65], as well as other related parameters such as fat mass [38, 64, 65], waist circumference [38, 65], and hepcidin concentrations [66]. As suggested by King et al. [67], body mass index and waist circumference may be considered as some of the earliest clinical signs of elevated allostatic load among adolescents. In one study [68] of adolescents engaging in binge drinking, excessive alcohol consumption was found to be associated with significantly higher levels of stress and hostility, but not with allostatic overload based on clinimetric criteria [5, 6].
Consequences on Psychological Functioning and Mental Health
The association between negative emotionality, an aspect of temperament which predispose the individual to express more negative emotions, and allostatic load among children has been reported by several studies [69–71]. Negative emotionality was associated with increased allostatic load in children displaying low self-reported maternal responsiveness [69] or low self-regulation [70]. Nonetheless, negative emotionality was linked to low allostatic load in children with high self-reported maternal responsiveness [69], which seems to modulate the effects of high cumulative risk on allostatic load levels [71]. Similarly, Ming and colleagues [72] showed that low levels of positive affect were negatively associated with AL, and marginally moderated the link between social mobility belief and AL among migrant adolescents.
Self-regulation, defined as the ability to control emotions and to inhibit or initiate behaviors in order to achieve goals [73, 74], may attenuate the impact of negative emotionality on behavioral problems in both children and adolescents [75–77]. However, research findings on the association between allostatic load and self-regulation appear to be rather controversial [42, 70]. In two longitudinal studies, Buss et al. [37] reported a significant positive association between allostatic load and internalizing problems in children. Further, allostatic load was found to interact with children fearfulness to predict greater future anxiety [37], supporting a model of risk for internalizing problems based on the interaction of both biological and environmental stressors, where individual differences as well as environmental factors play a role in determining allostatic load during developmental changes. In a study conducted by Doan and colleagues [78], the cumulative risk was correlated with allostatic load, but only in male adolescents with high scores of externalizing behaviors the association was attenuated.
Allostatic load was also linked to several cognitive functions [42, 44, 60, 79]. Rogosch et al. [60] reported a significant association between allostatic load and attention and thought problems among maltreated children. Further, a significant positive association was found between allostatic load and working memory deficits [42, 44, 79]. Doan and Evans [79] showed that early chronic allostatic load was significantly associated with working memory deficit in adolescents. Furthermore, in a study carried out by Evans and Schamberg [44], allostatic load during childhood seems to mediate the association between childhood poverty and working memory reduction in adulthood, suggesting the role of early allostatic load in predicting future working memory impairments.
As to mental health, studies suggested a relationship between allostatic load and mental health symptoms among adolescents [80] and maltreated children [60]. Particularly, depressed mothers’ offspring reporting psychiatric symptoms showed higher values on several allostatic load biological parameters compared to nondepressed mothers’ offspring [80].
Discussion
Findings from this systematic review are influenced by the fact that research on allostatic load among children and adolescents substantially relies on biomarkers that, however, do not provide information on the underlying individual causes. Further, high heterogeneity was found across studies as to the type and number of biological parameters to be considered in determining allostatic load. Nonetheless, the findings indicate that greater allostatic load is associated with poorer health outcomes in both clinical and non-clinical pediatric populations. The joint assessment of allostatic load based on clinimetric principles, which would set the use of biological parameters in a clinical context, may increase the number of children and adolescents screened and path the way for the development and the administration of specific interventions to prevent or decrease the detrimental effects of allostatic load on health later in life.
Studies conducted in general populations show that allostatic load is significantly associated with poverty [41–44] and living in impoverished neighborhoods [49, 51], ethnicity [38, 48, 51, 53], perceived discrimination [46, 50], prolonged separation from both parents during early stages of life [55] and adverse childhood experiences [56], with possible long-lasting consequences on health. Indeed, adverse childhood experiences were found to be associated with enduring changes in the nervous, endocrine, and immune systems [81]. Adversities may induce significant biological changes in children, modifying the development and the operating balance of allostatic systems. Their chronic activation may lead to allostatic load or overload, with possible long-term effects on biological aging and health [29, 81, 82]. Nonetheless, individual psychological well-being and coping styles may modulate the association between sociodemographic variables and allostatic load [83] since protective factors may counteract the long-term detrimental effects of childhood adversities on health. For instance, interventions targeting euthymia and psychological well-being in individuals exposed to childhood maltreatment, which – likely through buffering the effects of chronic stress and allostatic load – foster resilience and improve mental and physical health, represent promising clinical applications in this context [84].
Environmental factors such as availability of green spaces [57] and indoor living [58] were also found to play an important role in modulating allostatic load, shading some light on the dynamic interaction between the growing individual and the surrounding environment.
The results also indicate that higher levels of allostatic load experienced during childhood and adolescence are likely to be related to poorer health outcomes later in life in both clinical and non-clinical populations. Indeed, allostatic load was found to be associated with worse physical health. In particular, abnormal nutritional status (either malnutrition or overweight) was found to be significantly linked with greater allostatic load in both children and adolescents [38, 64, 65, 67]. Similarly, in adult populations, allostatic load was found to be associated with health-damaging lifestyle habits, such as lack of physical activity, unhealthy diet, alcohol consumption, and poor sleep [6, 28]. The long-term effects of unhealthy lifestyles in contributing to illnesses development and the importance of promoting lifestyle modifications are increasingly recognized in clinical medicine [27, 85] and should represent specific therapeutic targets of health-promoting interventions, particularly in the early stages of life. However, health promotion entails considerable difficulties in its application [27, 85, 86] since the success rate of enduring changes is exceedingly low, and psychological distress and low levels of well-being represent important obstacles to behavioral change.
As to consequences on psychological functioning, both negative emotionality [69–71] and self-regulation [70] were found to be related to allostatic load in pediatric populations. Allostatic load also appeared to negatively impact on cognitive functions such as attention and working memory [42, 44, 60, 79]. Further, psychiatric symptoms [80] were found to be related to higher allostatic load among adolescents.
The role of biosocial factors in the pathogenesis of medical disorders is increasingly recognized in adults [87]. The findings provide support to the role of allostatic load in the balance between health and disease during early stages of life, with important consequences on both physical health and psychological functioning [33–35]. These results support the clinical utility of the transdiagnostic identification of allostatic load and overload in children and adolescents across a variety of settings, with a number of potential clinical implications. Psychotherapeutic strategies aimed at improving coping with stressful situations, such as mindfulness-based approaches [88, 89], as well as cognitive-behavioral approaches [90], may be of value. It is also conceivable, yet to be tested that promoting lifestyle modifications among children and adolescents through the pursuit of euthymia, whose importance is increasingly recognized in clinical medicine [85] and psychiatry [24, 91], may provide enduring effects.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on a published literature.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no sources of funding for this work.
Author Contributions
Marcella Lucente and Jenny Guidi searched, screened, and selected studies. Jenny Guidi conceived this work. Both authors extracted data, drafted, and finalized this paper.
Funding Statement
There were no sources of funding for this work.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–101. 10.1001/archinte.153.18.2093. [DOI] [PubMed] [Google Scholar]
- 2. Sterling P. Allostasis: a model of predictive regulation. Physiol Behav. 2012;106(1):5–15. 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 3. McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840(1):33–44. [DOI] [PubMed] [Google Scholar]
- 4. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 5. Fava GA, McEwen BS, Guidi J, Gostoli S, Offidani E, Sonino N. Clinical characterization of allostatic overload. Psychoneuroendocrinology. 2019;108:94–101. 10.1016/j.psyneuen.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 6. Fava GA, Sonino N, Lucente M, Guidi J. Allostatic load in clinical practice. Clin Psychol Sci. 2023;11(2):345–56. 10.1177/21677026221121601. [DOI] [Google Scholar]
- 7. McEwen BS, Wingfield JC. What is in a name? Integrating homeostasis, allostasis and stress. Horm Behav. 2010;57(2):105–11. 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation – allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259–68. 10.1001/archinte.157.19.2259. [DOI] [PubMed] [Google Scholar]
- 9. Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98(8):4770–5. 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–81. 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 11. McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–63. 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gray JD, Kogan JF, Marrocco J, McEwen BS. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat Rev Endocrinol. 2017;13(11):661–73. 10.1038/nrendo.2017.97. [DOI] [PubMed] [Google Scholar]
- 13. McEwen BS. Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism. 2015;64(3 Suppl 1):S2–10. 10.1016/j.metabol.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 14. Agorastos A, Pervanidou P, Chrousos GP, Baker DG. Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front Psychiatry. 2019;10:118. 10.3389/fpsyt.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sonino N, Fava GA, Lucente M, Guidi J. Allostatic load and endocrine disorders. Psychother Psychosom. 2023;92(3):162–9. 10.1159/000530691. [DOI] [PubMed] [Google Scholar]
- 16. Liu SH, Juster RP, Dams-O’Connor K, Spicer J. Allostatic load scoring using item response theory. Compr Psychoneuroendocrinol. 2021;5:100025. 10.1016/j.cpnec.2020.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whelan E, O’Shea J, Hunt E, Dockray S. Evaluating measures of allostatic load in adolescents: a systematic review. Psychoneuroendocrinology. 2021;131:105324. 10.1016/j.psyneuen.2021.105324. [DOI] [PubMed] [Google Scholar]
- 18. Carrozzino D, Patierno C, Guidi J, Berrocal Montiel C, Cao J, Charlson ME, et al. Clinimetric criteria for patient-reported outcome measures. Psychother Psychosom. 2021;90(4):222–32. 10.1159/000516599. [DOI] [PubMed] [Google Scholar]
- 19. Fava GA, Tomba E, Sonino N. Clinimetrics: the science of clinical measurements. Int J Clin Pract. 2012;66(1):11–5. 10.1111/j.1742-1241.2011.02825.x. [DOI] [PubMed] [Google Scholar]
- 20. Fava GA. Forty years of clinimetrics. Psychother Psychosom. 2022;91(1):1–7. 10.1159/000520251. [DOI] [PubMed] [Google Scholar]
- 21. Feinstein AR. Clinimetrics. New Haven (CT): Yale University Press; 1987. [Google Scholar]
- 22. Fava GA, Guidi J, Semprini F, Tomba E, Sonino N. Clinical assessment of allostatic load and clinimetric criteria. Psychother Psychosom. 2010;79(5):280–4. 10.1159/000318294. [DOI] [PubMed] [Google Scholar]
- 23. Fava GA, Cosci F, Sonino N. Current psychosomatic practice. Psychother Psychosom. 2017;86(1):13–30. 10.1159/000448856. [DOI] [PubMed] [Google Scholar]
- 24. Guidi J, Fava GA. The clinical science of euthymia: a conceptual map. Psychother Psychosom. 2022;91(3):156–67. 10.1159/000524279. [DOI] [PubMed] [Google Scholar]
- 25. Sonino N, Fava GA. A simple instrument for assessing stress in clinical practice. Postgrad Med J. 1998;74(873):408–10. 10.1136/pgmj.74.873.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piolanti A, Offidani E, Guidi J, Gostoli S, Fava GA, Sonino N. Use of the Psychosocial Index: a sensitive tool in research and practice. Psychother Psychosom. 2016;85(6):337–45. 10.1159/000447760. [DOI] [PubMed] [Google Scholar]
- 27. Fava GA. Patients as health producers: the psychosomatic foundation of lifestyle medicine. Psychother Psychosom. 2023;92(2):81–6. 10.1159/000529953. [DOI] [PubMed] [Google Scholar]
- 28. Guidi J, Lucente M, Sonino N, Fava GA. Allostatic load and its impact on health: a systematic review. Psychother Psychosom. 2021;90(1):11–27. 10.1159/000510696. [DOI] [PubMed] [Google Scholar]
- 29. Katz DA, Sprang G, Cooke C. The cost of chronic stress in childhood: understanding and applying the concept of allostatic load. Psychodyn Psychiatry. 2012;40(3):469–80. 10.1521/pdps.2012.40.3.469. [DOI] [PubMed] [Google Scholar]
- 30. Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, et al. Allostasis and the development of internalizing and externalizing problems: changing relations with physiological systems across adolescence. Dev Psychopathol. 2011;23(4):1149–65. 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- 31. Ordaz S, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37(8):1135–57. 10.1016/j.psyneuen.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sumter SR, Bokhorst CL, Miers AC, Van Pelt J, Westenberg PM. Age and puberty differences in stress responses during a public speaking task: do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology. 2010;35(10):1510–6. 10.1016/j.psyneuen.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 33. McEwen CA, McEwen BS. Social structure, adversity, toxic stress, and intergenerational poverty: an early childhood model. Annu Rev Sociol. 2017;43(1):445–72. 10.1146/annurev-soc-060116-053252. [DOI] [Google Scholar]
- 34. McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doan SN. Allostatic load: developmental and conceptual considerations in a multi-system physiological indicator of chronic stress exposure. Dev Psychobiol. 2021;63(5):825–36. 10.1002/dev.22107. [DOI] [PubMed] [Google Scholar]
- 36. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buss KA, Davis EL, Kiel EJ. Allostatic and environmental load in toddlers predicts anxiety in preschool and kindergarten. Dev Psychopathol. 2011;23(4):1069–87. 10.1017/S0954579411000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cedillo YE, Murillo AL, Fernandez JR. The association between allostatic load and anthropometric measurements among a multiethnic cohort of children. Pediatr Obes. 2019;14(6):e12501. 10.1111/ijpo.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De France K, Evans GW, Brody GH, Doan SN. Cost of resilience: childhood poverty, mental health, and chronic physiological stress. Psychoneuroendocrinology. 2022;144:105872. 10.1016/j.psyneuen.2022.105872. [DOI] [PubMed] [Google Scholar]
- 40. Doan SN, Dich N, Evans GW. Childhood cumulative risk and later allostatic load: mediating role of substance use. Health Psychol. 2014;33(11):1402–9. 10.1037/a0034790. [DOI] [PubMed] [Google Scholar]
- 41. Evans GW, De France K. Childhood poverty and psychological well-being: the mediating role of cumulative risk exposure. Dev Psychopathol. 2022;34(3):911–21. 10.1017/S0954579420001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Evans GW, Fuller-Rowell TE. Childhood poverty, chronic stress, and young adult working memory: the protective role of self-regulatory capacity. Dev Sci. 2013;16(5):688–96. 10.1111/desc.12082. [DOI] [PubMed] [Google Scholar]
- 43. Evans GW, Kim P. Childhood poverty and young adults’ allostatic load: the mediating role of childhood cumulative risk exposure. Psychol Sci. 2012;23(9):979–83. 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- 44. Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci USA. 2009;106(16):6545–9. 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Dev Psychol. 2007;43(2):341–51. 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 46. Fuller-Rowell TE, Evans GW, Ong AD. Poverty and health: the mediating role of perceived discrimination. Psychol Sci. 2012;23(7):734–9. 10.1177/0956797612439720. [DOI] [PubMed] [Google Scholar]
- 47. Gallo LC, Roesch SC, Bravin J, Savin KL, Perreira KM, Carnethon MR, et al. Socioeconomic adversity, social resources, and allostatic load among Hispanic/Latino youth: the Study of Latino Youth. Psychosom Med. 2019;81(3):305–12. 10.1097/PSY.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rainisch BKW, Upchurch DM. Sociodemographic correlates of allostatic load among a national sample of adolescents: findings from the National Health and Nutrition Examination Survey, 1999–2008. J Adolesc Health. 2013;53(4):506–11. 10.1016/j.jadohealth.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Theall KP, Drury SS, Shirtcliff EA. Cumulative neighborhood risk of psychosocial stress and allostatic load in adolescents. Am J Epidemiol. 2012;176(Suppl 7):S164–74. 10.1093/aje/kws185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian Y, Ming H, Huang S, Zhang H. Discrimination increases the association between parental and adolescent allostatic load in Chinese rural-to-urban migrants. J Adolesc Health. 2020;66(4):499–505. 10.1016/j.jadohealth.2019.11.303. [DOI] [PubMed] [Google Scholar]
- 51. Wallace M, Harville E, Theall K, Webber L, Chen W, Berenson G. Neighborhood poverty, allostatic load, and birth outcomes in African American and white women: findings from the Bogalusa Heart Study. Health Place. 2013;24:260–6. 10.1016/j.healthplace.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Worthman CM, Panter-Brick C. Homeless street children in Nepal: use of allostatic load to assess the burden of childhood adversity. Dev Psychopathol. 2008;20(1):233–55. 10.1017/S0954579408000114. [DOI] [PubMed] [Google Scholar]
- 53. Park L, Gomaa N, Quinonez C. Racial/ethnic inequality in the association of allostatic load and dental caries in children. J Public Health Dent. 2022;82(2):239–46. 10.1111/jphd.12470. [DOI] [PubMed] [Google Scholar]
- 54. Chen W, Srinivasan SR, Berenson GS. Amplification of the association between birthweight and blood pressure with age: the Bogalusa Heart Study. J Hypertens. 2010;28(10):2046–52. 10.1097/HJH.0b013e32833cd31f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun Y, Fang J, Xu Y, Xu L, Su P, Zhang Z, et al. Association between prolonged separation from parents and allostatic load among children in China. Psychoneuroendocrinology. 2020;118:104715. 10.1016/j.psyneuen.2020.104715. [DOI] [PubMed] [Google Scholar]
- 56. de la Rosa R, Zablotny D, Ye M, Bush NR, Hessler D, Koita K, et al. Biological burden of adverse childhood experiences in children. Psychosom Med. 2023;85(2):108–17. 10.1097/PSY.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ribeiro AI, Tavares C, Guttentag A, Barros H. Association between neighbourhood green space and biological markers in school-aged children. Findings from the Generation XXI birth cohort. Environ Int. 2019;132:105070. 10.1016/j.envint.2019.105070. [DOI] [PubMed] [Google Scholar]
- 58. Rollings KA, Evans GW. Design moderators of perceived residential crowding and chronic physiological stress among children. Environ Behav. 2019;51(5):590–621. 10.1177/0013916518824631. [DOI] [Google Scholar]
- 59. Bahreinian S, Ball GDC, Vander Leek TK, Colman I, McNeil BJ, Becker AB, et al. Allostatic load biomarkers and asthma in adolescents. Am J Respir Crit Care Med. 2013;187(2):144–52. 10.1164/rccm.201201-0025OC. [DOI] [PubMed] [Google Scholar]
- 60. Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: consequences for physical and mental health in children from low-income families. Dev Psychopathol. 2011;23(4):1107–124. 10.1017/S0954579411000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Evans GW. A Multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39(5):924–33. 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- 62. Ezeamama AE, Zalwango SK, Tuke R, Pad RL, Boivin MJ, Musoke PM, et al. Toxic stress and quality of life in early school-aged Ugandan children with and without perinatal human immunodeficiency virus infection. New Dir Child Adolesc Dev. 2020;2020(171):15–38. 10.1002/cad.20355. [DOI] [PubMed] [Google Scholar]
- 63. Wallace M, Harville E, Theall K, Webber L, Chen W, Berenson G. Preconception biomarkers of allostatic load and racial disparities in adverse birth outcomes: the Bogalusa Heart Study. Paediatr Perinat Epidemiol. 2013;27(6):587–97. 10.1111/ppe.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Calcaterra V, Cena H, de Silvestri A, Albertini R, De Amici M, Valenza M, et al. Stress measured by allostatic load in neurologically impaired children: the importance of nutritional status. Horm Res Paediatr. 2017;88(3–4):224–30. 10.1159/000477906. [DOI] [PubMed] [Google Scholar]
- 65. Calcaterra V, Vinci F, Casari G, Pelizzo G, De Silvestri A, De Amici M, et al. Evaluation of allostatic load as a marker of chronic stress in children and the importance of excess weight. Front Pediatr. 2019;7:335. 10.3389/fped.2019.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Augustine LF, Nair KM, Rao SF, Rao MVV, Ravinder P, Laxmaiah A. Exploring the bio-behavioural link between stress, allostatic load & micronutrient status: a cross-sectional study among adolescent boys. Indian J Med Res. 2016;144(3):378–84. 10.4103/0971-5916.198675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. King AL, Garnier-Villarreal M, Simanek AM, Johnson NL. Testing allostatic load factor structures among adolescents: a structural equation modeling approach. Am J Hum Biol. 2019;31(4):e23242. 10.1002/ajhb.23242. [DOI] [PubMed] [Google Scholar]
- 68. Gostoli S, Fantini L, Casadei S, De Angelis VA, Rafanelli C. Binge drinking in 14-year-old Italian students is correlated with low or high psychological well-being: a cross-sectional study. Drugs. 2021;28(2):190–9. 10.1080/09687637.2020.1799942. [DOI] [Google Scholar]
- 69. Dich N, Doan SN, Evans GW. Children’s emotionality moderates the association between maternal responsiveness and allostatic load: investigation into differential susceptibility. Child Dev. 2015;86(3):936–44. 10.1111/cdev.12346. [DOI] [PubMed] [Google Scholar]
- 70. Dich N, Doan S, Evans G. Children’s negative emotionality combined with poor self-regulation affects allostatic load in adolescence. Int J Behav Dev. 2015;39(4):368–75. 10.1177/0165025414544232. [DOI] [Google Scholar]
- 71. Dich N, Doan SN, Evans GW. In risky environments, emotional children have more behavioral problems but lower allostatic load. Health Psychol. 2017;36(5):468–76. 10.1037/hea0000459. [DOI] [PubMed] [Google Scholar]
- 72. Ming H, Zuo C, Zhang F, Ren Y, Zhang H, Huang S. Positive affect decreases the negative association between social mobility belief and physical health among Chinese rural-to-urban migrant adolescents. Psychoneuroendocrinology. 2022;143:105846. 10.1016/j.psyneuen.2022.105846. [DOI] [PubMed] [Google Scholar]
- 73. Eisenberg N, Champion C, Ma Y. Emotion-related regulation: an emerging construct. Merrill-Palmer Q. 2004;50(3):236–59. 10.1353/mpq.2004.0016. [DOI] [Google Scholar]
- 74. Mischel W, Ayduk O. Willpower in a cognitive affective processing system: the dynamics of delay of gratification. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: research, theory, and applications. New York (NY): Guilford; 2004. [Google Scholar]
- 75. Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: their role in predicting quality of social functioning. J Pers Soc Psychol. 2000;78(1):136–57. 10.1037//0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- 76. Oldehinkel AJ, Hartman CA, Ferdinand RF, Verhulst FC, Ormel J. Effortful control as modifier of the association between negative emotionality and adolescents’ mental health problems. Dev Psychopathol. 2007;19(2):523–39. 10.1017/S0954579407070253. [DOI] [PubMed] [Google Scholar]
- 77. Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology. Social, emotional, and personality development. New York (NY): Wiley; 2006. Vol. 3. [Google Scholar]
- 78. Doan SN, Dich N, Fuller-Rowell TE, Evans GW. Externalizing behaviors buffer the effects of early life adversity on physiologic dysregulation. Sci Rep. 2019;9(1):13623. 10.1038/s41598-019-49461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Doan SN, Evans GW. Maternal responsiveness moderates the relationship between allostatic load and working memory. Dev Psychopathol. 2011;23(3):873–80. 10.1017/S0954579411000368. [DOI] [PubMed] [Google Scholar]
- 80. Nelson BW, Sheeber L, Pfeifer J, Allen NB. Psychobiological markers of allostatic load in depressed and nondepressed mothers and their adolescent offspring. J Child Psychol Psychiatry. 2021;62(2):199–211. 10.1111/jcpp.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 82. Finlay S, Roth C, Zimsen T, Bridson TL, Sarnyai Z, McDermott B. Adverse childhood experiences and allostatic load: a systematic review. Neurosci Biobehav Rev. 2022;136:104605. 10.1016/j.neubiorev.2022.104605. [DOI] [PubMed] [Google Scholar]
- 83. Chen E, Miller GE, Lachman ME, Gruenewald TL, Seeman TE. Protective factors for adults from low-childhood socioeconomic circumstances: the benefits of shift-and-persist for allostatic load. Psychosom Med. 2012;74(2):178–86. 10.1097/PSY.0b013e31824206fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pfaltz MC, Halligan SL, Haim-Nachum S, Sopp MR, Åhs F, Bachem R, et al. Social functioning in individuals affected by childhood maltreatment: establishing a research agenda to inform interventions. Psychother Psychosom. 2022;91(4):238–51. 10.1159/000523667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rippe JM. Are we ready to practice lifestyle medicine? Am J Med. 2019;132(1):6–8. 10.1016/j.amjmed.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 86. Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–9. 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 87. Horwitz RI, Singer BH, Hayes-Conroy A, Cullen MR, Mawn M, Colella K, et al. Biosocial pathogenesis. Psychother Psychosom. 2022;91(2):73–7. 10.1159/000521567. [DOI] [PubMed] [Google Scholar]
- 88. Perry-Parrish C, Copeland-Linder N, Webb L, Sibinga EM. Mindfulness-based approaches for children and youth. Curr Probl Pediatr Adolesc Health Care. 2016;46(6):172–8. 10.1016/j.cppeds.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 89. Roque-Lopez S, Llanez-Anaya E, Álvarez-López MJ, Everts M, Fernández D, Davidson RJ, et al. Mental health benefits of a 1-week intensive multimodal group program for adolescents with multiple adverse childhood experiences. Child Abuse Negl. 2021;122:105349. 10.1016/j.chiabu.2021.105349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Korotana LM, Dobson KS, Pusch D, Josephson T. A review of primary care interventions to improve health outcomes in adult survivors of adverse childhood experiences. Clin Psychol Rev. 2016;46:59–90. 10.1016/j.cpr.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 91. Guidi J, Fava GA. The emerging role of euthymia in psychotherapy research and practice. Clin Psychol Rev. 2020;82:101941. 10.1016/j.cpr.2020.101941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.