Abstract
Sulfur dioxide (SO2) is naturally synthesized by glutamate–oxaloacetate transaminase (GOT) from l-cysteine in mammalian cells. We found that SO2 may have a protective effect on acute lung injury (ALI) induced by limb ischemia/reperfusion (I/R) in rats. The PI3K/Akt, p38MAPK, and JAK2/STAT3 pathways are crucial in cell signaling transduction. The present study aims to verify the role of SO2 on limb I/R-induced ALI, and investigate whether PI3K/Akt, p38MAPK, and JAK2/STAT3 pathways were involved, as well as the relationship among the three pathways; we used specific inhibitors (LY294002, SB03580, and Stattic) to block them, respectively. The experimental methods of Western, ELISA, TUNEL, etc., were used to test the results. In the I/R group, the parameters of lung injury (MDA, MPO, TUNEL, cytokines) increased significantly, but the administration of Na2SO3/NaHSO3 attenuated the damage in the lung. The Western results showed that the rat’s lung exist expression of P-STAT3, P-AKT, and P-p38 proteins. After I/R, P-STAT3, P-Akt, and P-p38 proteins expression all increased. After using Na2SO3/NaHSO3, P-Akt, and P-p38 proteins expression increased, but P-STAT3 protein expression decreased. We also found a strange phenomenon; compared to the I/R + SO2 group, the administration of stattic, P-p38 protein expression showed no change, but P-Akt protein expression increased (p < 0.05). In conclusion, SO2 has a protective effect on rats with limb I/R-induced ALI. The JAK2/STAT3, PI3K/Akt, and p38MAPK pathways are likely all involved in the process, and the JAK2/STAT3 pathway may have an impact on the P13K/Akt pathway.
Keywords: Sulfur dioxide, Acute lung injury, Ischemia/reperfusion, Na2SO3/NaHSO3, JAK2/STAT3, PI3K/Akt, p38 MAPK, Inhibitor
Introduction
Limb ischemia is a common clinical pathological sign. Restoring its blood circulation is necessary to save the body, but it may aggravate local tissue ischemia/reperfusion (I/R) injury, cause systemic inflammatory response syndrome when serious, or even distant multiple-organ dysfunction syndrome. The lungs are the target organ that is easily affected, characterized by acute lung injury (ALI), and acute respiratory distress syndrome (ARDS), with and extremely high case fatality rate (25–40 %) [1]. As is well known, sulfur dioxide (SO2) is a very common gaseous pollutant in the atmosphere, and it has been generally regarded as an environmental toxin. Meng et al. [16, 17] reported that SO2 and its derivatives, sulfite and bisulfite, can act as systemic toxic substances capable of affecting multiple organs in mammals. There are many reports of organ damage, such as lipid peroxidative damage, chromosome variation, DNA damage, gene mutation, and changes of some enzyme activities [18–20, 32]. However, SO2 is also produced endogenously by sulfur-containing amino acids [25]. A recent study [12] suggests that endogenous SO2 can change the heart rate, lower blood pressure, effect blood vessels, and so on. They speculated that it could be a new kind of messenger molecule. Besides, we already found that SO2 might play a protective role by regulating the production of inflammatory and anti-inflammatory cytokines in plasma and in the lung during limb I/R-induced ALI [28]. Unfortunately, the mechanism is not fully understood.
The Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) pathway, p38 mitogen-activated protein kinase (MAPK) pathway, and phosphoinositide 3-kinase/Akt (PI3K/Akt) pathway are crucial in cell signaling transduction. They play an important role in many physiological and pathological process, such as inflammation, stress, apoptosis, cell cycle, and cell growth [3, 6, 27, 30]. Some researchers report that the activation of Akt, which is downstream of PI3K, may ameliorate I/R injury [5]. The inhibition of the JAK2/STAT3 signaling pathway can reduce I/R intestinal cells apoptosis [26], renal interstitial fibrosis [10], and cardiomyocyte hypertrophy [31]. The carbon monoxide (CO) can protect rat lung transplants from I/R injury via a mechanism involving the p38 MAPK pathway [11].
On the basis of the above evidence, the aim of the present study was to investigate the role of SO2 on limb I/R-induced ALI and investigate whether PI3K/Akt, p38MAPK, and JAK2/STAT3 signaling pathways were involved, as well as the relationships among the three signaling pathways.
Materials and methods
Materials
MDA and MPO kits were purchased from Nanjing Jiancheng (Nanjing, China). TUNEL kit, Bradford kit, and SB203580 inhibitor were purchased from Beyotime (Jiangsu, China). Stattic and LY294002 inhibitors were purchased from Selleck.cn (Shanghai, China). The antibody of STAT3, Akt, p38MAPK, GAPDH, P-STAT3, P-Akt, and P-p38MAPK were purchased from EnoGene (Nanjing, China). Secondary antibodies were purchased from Shanghai Yanhui Biotech Company (Shanghai, China). Protease inhibitor cocktail (50×) and a protein phosphatase inhibitors mixture (100×) were purchased from Applygen (Beijing, China); 5 % BSA were purchased from Solarbio (Beijing, China). TNF-α, IL-6, IL-10, and IL-1βELISA kits were purchased from Dakewe Biotech Company (Shenzhen, China). Na2SO3 and NaHSO3 of analytical purity, were purchased from Beijing Kang Puhui technology company (Beijing, China).
Animal model of induced ALI
Pathogen-free, adult male Sprague–Dawley (SD) rats (180–230 g) were used in the study. They were provided by the Experimental Animal Center of Chinese Academy of Sciences (Beijing, China). The Animal Ethics Committee of the Capital Medical University of China approved the study design, and all experiments were carried out in accordance with the established guiding principles for animal research. The rats were raised at a controlled ambient temperature of 23 ± 2 °C with 50 ± 10 % relative humidity and at a 12-h light–dark cycle (lights on at 8:00 AM and off at 8:00 PM). Standard rat chow and water ad libitum were provided to all rats. Forty-eight adult male SD rats were randomly divided into the following six groups with eight animals per group: control group, I/R group, I/R + SO2 group, I/R + SO2 + LY294002 group, I/R + SO2 + SB03580 group, and I/R + SO2 + Stattic group.
The administration of Na2SO3/NaHSO3 [(0.54 mmol/kg)/(0.18 mmol/kg), ip], an SO2 donor, or the same volume of saline, was performed at 20 min before reperfusion in the hind limbs of those in the I/R and control groups. Besides, the administration of JAK2/STAT3, PI3K/Akt, and p38MAPK signaling pathway inhibitors, stattic (3 mg/kg, iv), LY294002 (40 mg/kg, iv), and SB03580 (1 mg/kg, iv) respectively, or the same volume of saline, were performed at 1 h before reperfusion in the hind limbs of those in the I/R and control group.
Based on Cohen [4] provides methods to copy Limb I/R-induced ALI animal models. The SD rats were anesthetized via the intraperitoneal (ip) administration of sodium pentobarbital (40 mg/kg body weight). An additional one-third dose of sodium pentobarbital was given hourly to maintain anesthesia. The left external jugular vein and the right carotid artery were cannulated for drug and fluid administration (Ringer’s lactate, 2 ml/h) and blood sample collection, respectively. A rubber tourniquet was used to bind the double hind legs root to cause limbs ischemia. After 4 h, the tourniquet was loosened to allow for reperfusion. Application of laser Doppler blood flow detection (PeriFlux 5001, Perimed, Sweden) was used to ensure limb ischemia and reperfusion. Two hours after the reperfusion, the eight animals in each group were euthanized using an ip injection of a lethal dose of sodium pentobarbitone (90 mg/kg). Blood samples (n = 8 animals/group) were drawn from the right ventricles using heparinized syringes and centrifuged (4000 rpm, 10 min, 0–4 °C). Thereafter, plasma was aspirated and stored at −80 °C for the subsequent measurement of the cytokines (IL-1β, IL-6, IL-10, TNF-α) expression. Lung tissue samples (n = 8 animals/group) were stored at −80 °C for the subsequent measurement of tissue myeloperoxidase (MPO) activity, malondialdehyde (MDA) activity, cytokines(IL-1β, IL-6, IL-10, and TNF-α) expression, and STAT3, Akt, p38, P-STAT3, P-Akt, and P-p38 protein expressions. The extent of lung injury was determined via hematoxylin and eosin (H&E) staining. Apoptotic cells in the paraffin sections were identified using the one-step TUNEL apoptosis assay kit.
Histopathologic analyses and lung coefficient
Lung tissue samples were fixed in 4 % (vol/vol) neutral formalin and dehydrated through a graded ethanol series. After being embedded in paraffin wax, tissue samples were sectioned (4–5 μm) and stained with HE for examination under a light microscope.
Lung coefficient = lung wet weight (g)/body weight (kg). We cut off the trachea between fifth and sixth cartilage ring above the tracheal bifurcation and removed the lung tissue intact. We used filter paper to blot up the bloodstains on the surface, and then weighed it.
MDA and MPO activity estimations
Tissue samples were thawed, weighted, and homogenized, making them into 10 % tissue homogenization. The activities of MDA and MPO were assayed using the MDA and MPO activity quantitative detection kit according to the manufacturer’s instructions.
TUNEL
Lung tissue samples were fixed in 4 % (vol/vol) neutral formalin and then embedded in paraffin. Apoptotic cells in the paraffin sections were identified using the one-step TUNEL apoptosis assay kit according to the manufacturer’s instructions. A double-staining technique was used. TUNEL staining (green fluorescence) was used to quantitate apoptotic cell nuclei and DAPI staining (blue fluorescence) was used to quantitate the total cell nuclei, as Omura [22] described. The stained samples were observed under a fluorescent microscopy (OLYMPUS IX51, Tokyo, Japan) by using 488-nm excitation and 530-nm emission. The cells with green fluorescence were defined as apoptotic cells. The index of apoptotic was calculated as the ratio of the number of TUNEL-positive cells to the total number of cells. Five visual fields were selected randomly for each specimen.
The levels of IL-1β, IL-6, IL-10, and TNF-α in plasma and lung tissue
At various points of time, lung tissue samples were thawed, homogenized, and centrifuged. The liquid supernatants were collected for testing IL-1β, IL-6, IL-10, and TNF-α concentrations. Blood samples (4 ml from each rat) from each group were collected in heparinized tubes through jugular vein catheterization and centrifuged (3000 rpm). Cytokines levels were assayed using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions. Samples (100 μl) and IL-1βstandards (2000, 1000, 500, 250, 125, 62.5, and 31.3 pg/ml) were added to the ELISA plate wells. Each was tested in duplicate. Anti-rat IL-1βbiotin (50 μl) was added to each well of the plates and allowed to react for 90 min at 37 °C. After the incubation at 37 °C, samples were removed and the plates were washed once with washing buffer, followed by soaking in the same buffer for 1 min and three consecutive washes. Blotted plates were dried by tapping upside down on filter paper. After three additional washing steps, 100 μl of Streptavidin-HRP was added to the wells and allowed to react for 30 min at 37 °C. The plates were washed again four times, and 100 μl of TMB substrate was added to each well and coated by gentle shaking for 10 s. The mixture was then incubated in a dark environment for 30 min at room temperature. The reaction was terminated by adding 100 μl of stop solution to each well, and the optical density (OD) value at 450 nm was measured by Varioskan Flash (Thermo Scientific, Waltham, MA, USA). The standard curve of the OD value vs the concentration of IL-1βwas obtained. The sample data was then plotted along the standard curve and read off the sample IL-1β concentration. The IL-6, IL-10, and TNF-α contents of samples were assessed in the same assay.
Western-blot analysis of STAT3, P-STAT3, Akt, P-Akt, p38, and P-p38 protein expression in lung tissues
Lung tissue samples were homogenized in a lysis buffer (50 mM Tris–HCL, pH 7.5, 150 mM NaCl, 1 % Nonidet P-40, 0.5 % sodium deoxycholate) with protease inhibitors and a phosphatase inhibitor. After centrifugation at 13,000 × g, the supernatants were collected. The Bradford Protein Assay Kit was used to determine the concentration of total protein. After the protein was quantified, the supernatants were boiled for 5 min in a loading buffer, and then separated by SDSPAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5 % BSA for 1 h at room temperature, using TBST washing three times, 10 min each time, and then incubated with primary antibody at 4 °C overnight. The membranes were again washed with TBST three times, then fluorescent labeling secondary antibodies (corresponding with primary antibody) were added and incubated for another 1 h at room temperature. The blots were then washed as described above. The developed signal was visualized and quantified using an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
All data was expressed as the mean ± SD. Significant differences were evaluated by a one-way ANOVA followed by a post hoc test (least significant difference, LSD test). Statistical significance was set as p < 0.05. All analyses were performed using SPSS 17.0 (Chicago, IL, USA).
Results
Histopathologic analyses and lung coefficient
The morphological changes in the lung suggested inflammatory damage after limb ischemia 4 h then reperfusion 2 h under the light microscope. The lung pathological sections from rats with ALI following limb I/R exhibited interstitial edema, alveolar thickening, and severe leukocyte infiltration in the interstitium and alveoli (Fig. 1b). The administration of Na2SO3/NaHSO3 attenuated the histological damage in the lung (Fig. 1c). In the SO2 + Stattic group, the histological damage also attenuated significantly (Fig. 1f), but in the SO2 + LY294002 and SO2 + SB03580 groups, there was less attenuation in interstitial edema and leukocyte infiltration (Fig. 1d, e).
Fig. 1.
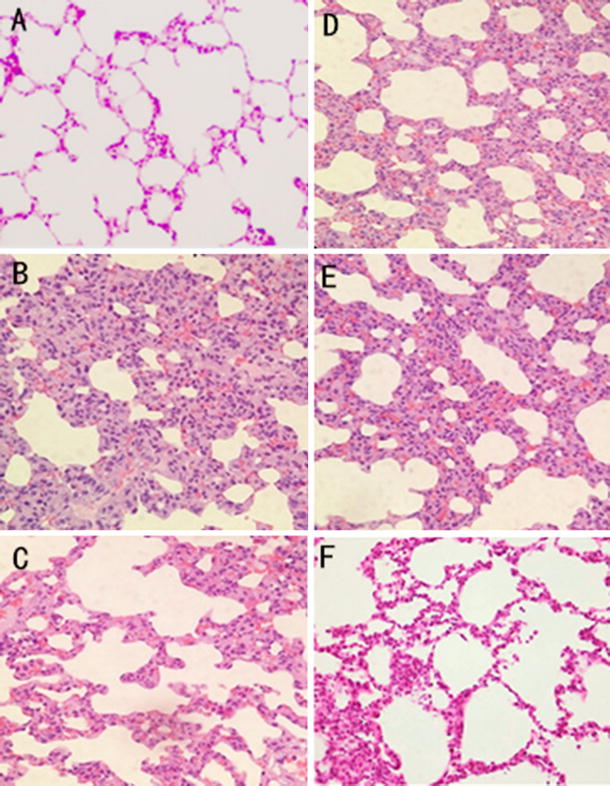
Lung morphological changes in limb I/R-induced ALI rats in different experiment group. a control group. b I/R group. c I/R + SO2 group. d I/R + SO2 + LY294002 group. e I/R + SO2 + SB03580 group. f I/R + SO2 + stattic group
Compared to the control group, the lung coefficient increased significantly in the I/R group (p < 0.01). Compared to the I/R group, the lung coefficient in the I/R + S02 group decreased significantly (p < 0.01). Compared to the I/R + S02 group, the lung coefficient in the I/R + SO2 + LY294002 and I/R + SO2 + SB03580 groups increased, while it decreased in the I/R + SO2 + stattic group (p < 0.05) (Fig. 2C).
Fig. 2.
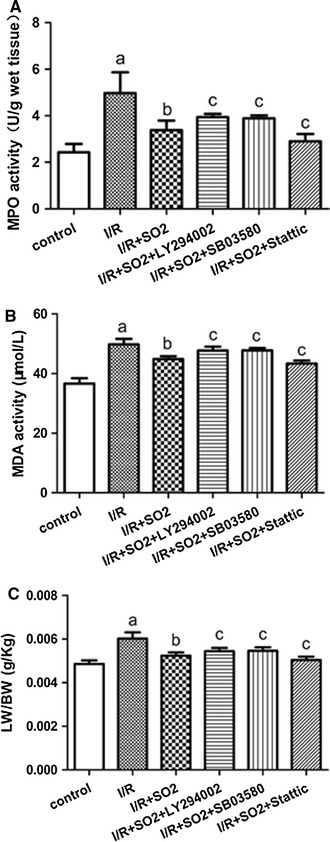
MPO activity, MDA activity, and lung coefficient changes in the lung of limb I/R-induced ALI rats in different experiment group. a MPO activity. b MDA activity. c LW/BW. All data are expressed as mean ± SD (n = 8 animals/group). Significant differences are indicated as: a p < 0.01 vs. control group, b p < 0.01 vs. I/R group, c p < 0.05 vs. I/R + SO2 group
The activation of MDA and MPO in lung tissues
Compared to the control group, the activation of MDA and MPO increased significantly in the I/R group (p < 0.01). Compared to the I/R group, the activation of MDA and MPO in the I/R + SO2 group decreased significantly (p < 0.01). Compared to the I/R + SO2 group, the activation of MDA and MPO in the I/R + SO2 + LY294002 and I/R + SO2 + SB03580 groups increased, while it decreased in the I/R + SO2 + Stattic group (p < 0.05) (Fig. 2a, b).
TUNEL results
There were very few apoptotic cells in the control group; the apoptotic index was 1.45 % for this group. The percentage of apoptotic cells in the I/R group was obviously increased, and the apoptotic index increased to 17.2 %. Such an effect was significantly attenuated by adding Na2SO3/NaHSO3, which cause the apoptotic index to decrease to 6.5 %. In the SO2 + Stattic group, the apoptotic cells also attenuated significantly, and the apoptotic index decreased to 5.5 %. However, in the SO2 + LY294002 and SO2 + SB03580 groups, the apoptotic cells attenuated less; their apoptotic indexes were 10.33 and 10.08 %, respectively (Fig. 3a, b).
Fig. 3.
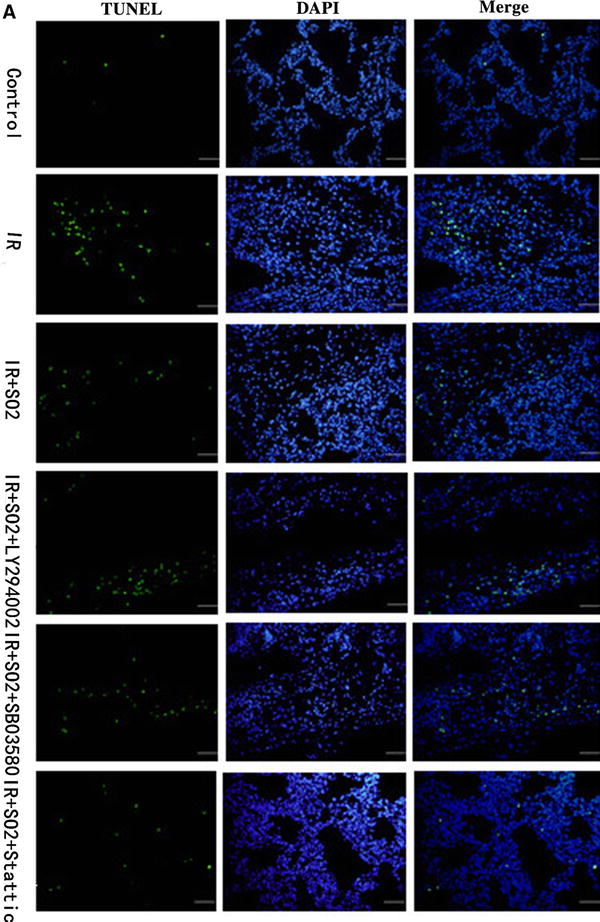
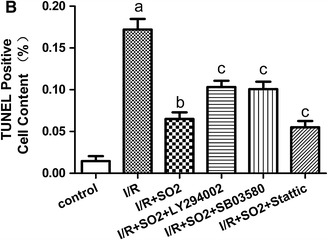
Apoptosis analysis about rat lungs of limb I/R-induced ALI in different experiment group. a DAPI staining and TUNEL assay. Lung tissue nuclei appear light blue, TUNEL-positive nuclei appear green (n = 8, five fields for each specimen). b The index of apoptotic about rat lungs in different experiment group. Results are given as mean ± SD (n = 8 animals/group). Significant differences are indicated as: a p < 0.01 vs. control group, b p < 0.01 vs. I/R group, c p < 0.05 vs. I/R + SO2 group
The Levels of IL-1β, IL-6, IL-10, and TNF-α in plasma and lung tissue
Compared to the control group, the levels of IL-1β, IL-6, IL-10, and TNF-α in plasma and lung tissue increased significantly in the I/R group (p < 0.01). Compared to the I/R group, the I/R + SO2 exhibited significant decreases in IL-1β, IL-6, and TNF-α levels but increases in IL-10 levels, in both the plasma and lung tissues (p < 0.01). Compared to the I/R + S02 group, the I/R + SO2 + LY294002 and I/R + SO2 + SB03580 groups had increases in IL-1β, IL-6, and TNF-α levels but decreases in IL-10 levels, in both the plasma and lung tissues (p < 0.05), while I/R + SO2 + Stattic group showed a decrease in IL-1β, IL-6, and TNF-α levels but a significant increase in IL-10 levels, in both the plasma and lung tissues (p < 0.05) (Fig. 4).
Fig. 4.
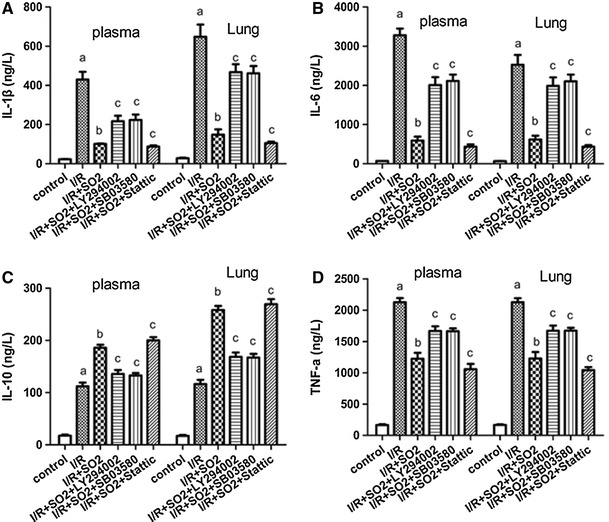
The expression of IL-1β, IL-6, IL-10, and TNF-α in the plasma and lung from limb I/R-induced ALI rats in different experiment groups. a IL-1β protein. b IL-6 protein. c IL-10 protein. d TNF-α protein. Results are expressed as mean ± SD (n = 8 animals/group). Significant differences are indicated as: a p < 0.01 vs. control group, b p < 0.01 vs. I/R group, c p < 0.05 vs. I/R + SO2 group
Akt, P-Akt, p38, P-p38, STAT3, and P-STAT3 protein expression in lung tissues
The Western-blot test results show that the rat’s lungs secreted 36.22, 39.12, and 36.23 % of the P-STAT3, P-Akt, and p-p38 proteins, respectively. Compared to the control group, P-STAT3, P-Akt, and P-p38 protein expressions increased to 82.27, 61.60, and 52.83, respectively, in the I/R group (p < 0.01). Compared to the I/R group, P-Akt, and P-p38 protein expression increased to 82.56 and 79.08 %, respectively, but P-STAT3 protein expression decreased to 52.56 % in the I/R + SO2 group (p < 0.01). Compared to the I/R + SO2 group, the I/R + SO2 + LY294002 group showed a significant decrease in P-Akt protein expression (p < 0.01). The P-STAT3 and P-p38 protein expression did not change in this group (p > 0.05). The I/R + SO2 + SB03580 group showed a significant decrease in P-p38 protein expression (p < 0.01), while the P-STAT3 and P-Akt protein expressions did not change (p > 0.05). In the I/R + SO2 + Stattic group, P-STAT3 protein expression significantly decreased (p < 0.01), and P-p38 protein expression did not significantly change (p > 0.05), but P-Akt protein expression increased by 92.13 % (p < 0.05) (Fig. 5).
Fig. 5.
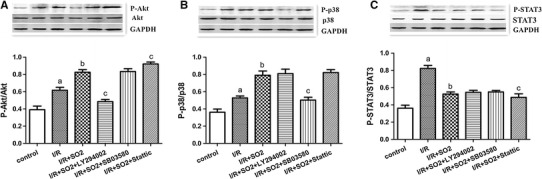
Western-blot analysis of Akt, P-Akt, p38, P-p38, STAT3, and P-STAT3 proteins expression in lung tissues of limb I/R-induced ALI rats. Top representative bands from Western blots of a P-Akt and total Akt, b P-p38 and total p38, c P-STAT-3 and total STAT-3. Bottom bar graphs show mean ± SD of the densitometry of a P-Akt-to-Akt ratio, b P-p38-to-p38 ratio, and c P-STAT-3-to-STAT-3 ratio. All data are expressed as mean ± SD (n = 8 animals/group). Significant differences are indicated as: a p < 0.01 vs. control group, b p < 0.01 vs. I/R group, c p < 0.05 vs. I/R + SO2 group
Discussion
I/R is an important pathophysiological process in clinical practice and was first described by Jennings in 1960 [13]. It not only exists in different species and organs, but is involved in various pathological processes, such as multi-organ failure, shock, and heart failure [9, 29]. An acute inflammatory process in the lung parenchyma characterizes ALI. In our animal model of ALI, limb I/R caused a significant inflammatory response and organ damage in the lung, as evidenced by distinct changes in biochemical processes (MDA, MPO activity) and histological features (severe leukocyte infiltration). MDA is one of the most important products of the membrane lipid peroxidation. By detecting it, we can determine the extent of the membrane lipid peroxidation and, thus, indirectly determine the damage of the membrane system. MPO activity is a marker of tissue neutrophil infiltration that is characteristic of histological changes. The present study results show that lung MDA and MPO activity were significantly increased after limb I/R, compared to the control group (p < 0.01, Fig. 2a, b). The administration of Na2SO3/NaHSO3 attenuated the lung MDA and MPO activity when compared to the I/R group (p < 0.01, Fig. 2a, b). Furthermore, the lung pathological sections from rats with ALI following limb I/R exhibited interstitial edema, alveolar thickening, and severe leukocyte infiltration in the interstitium and alveoli (Fig. 1b). However, the administration of Na2SO3/NaHSO3 attenuated the histological damage in the lung (Fig. 1c), and the lung coefficient decreased significantly (p < 0.01, Fig. 2c). We also found that the injection of Na2SO3/NaHSO3 at 20 min before limb I/R led to a significant decrease in IL-1β, IL-6, and TNF-α levels but an increase in IL-10 levels, in both the plasma and lung tissues (Fig. 4). IL-1β, IL-6, and TNF-α are pro-inflammatory cytokines, IL-10 is an anti-inflammatory cytokine.
As a consequence, with the use of limb I/R-induced ALI, a well-established and clinically relevant animal model for ALI, we discovered that SO2, a newly characterized gaseous messenger molecule, might play a protective role by regulating the production of inflammatory and anti-inflammatory cytokines in plasma and lung tissue during limb I/R-induced ALI. The present study further verified our former experimental results [28]. However, the mechanism by which SO2 regulates ALI pathogenesis remains unclear.
The JAK2/STAT3, p38MAPK, and PI3K/Akt pathways are crucial in cell signaling transduction. They play an important role in many physiological and pathological processes, such as inflammation, stress, apoptosis, cell cycle, and cell growth [3, 6, 27, 30].
JAK2/STAT3 signaling pathway is a signal transduction pathway. In recent years, JAK2/STAT3 has been the subject of much research. It can directly transfer the signals felt by cell membranes into nuclei, and then activate the transcription of target genes. Many cytokines (IFN, IL-2, IL-4, IL-6, etc.) and growth factors (EGF, PDGF, CSF, etc.) use the JAK2/STAT3 signal transduction pathway to induce cell proliferation, differentiation, and apoptosis. The JAK2/STAT3 signaling pathway plays a special and pleiotropic biological function in immune function regulation and the development of hematopoietic stem cells, cancer, nerves, and embryonic. One study [18] showed that AG490, another STAT3 signaling pathway inhibitor, can reduce myocardial ischemia/reperfusion injury. Macchi L. et al. [15] report that the STAT-3 pathway is involved in the FDX-induced cardioprotection effects.
The P38MAPK pathway was found by Brewster et al. [2] in 1993. It is composed of 360 amino acid residues, with a relative molecular mass of 38 × 103. Because the p38MAPK pathway is a member of MAPKs, it can change the level of gene expression through the phosphorylation of transcription factor, and it is involved in intracellular information transfer. P38MAPK activation can not only promote the mononuclear macrophages produce inflammatory factors (TNF-α, IL-1, IL-4, IL-6, IL-8, etc.) but also mediate the activation of neutrophils. The Application p38MAPK inhibitors can inhibit the neutrophils chemotaxis and superoxide generation and thus reduce inflammation reactions [21]. Oxide produced by I/R can make p38MAPK activate and, therefore, cause an increase in cytokines. Scholars from Japan [8, 14] found that though many animal experiments, adding p38MAPK specific inhibitors (FR167653) into the cold preservation solution (UW liquid or CELSIOR liquid) can reduce I/R injury in transplanted organs (heart, lung, and liver).
The protein kinase Akt is a serine/threonine kinase, which is also called protein kinase B (PKB), based on its homology to protein kinase A and protein kinase C. The protein kinase Akt acts as the major downstream target of PI3K and regulates the biological function of cells. PI3K belongs to the phosphorylation of the phosphatidyl inositol phospholipids kinase family. It is located in the D3 inositol ring. The primary structure of PI3K participates in extracellular signal transduction; its reaction product is a second messenger. The target genes of the second messenger is Akt, a proto-oncogene serine/threonine kinase. When extracellular stimuli activate PI3K, Akt from cytoplasm is transferred to the cytoplasm membrane, two residues (serine308 and threonine473) of Akt on cytoplasm membrane are phosphorylated, and Akt is completely activated. Akt can promote protein synthesis and glycogen transportation. Akt has emerged as a crucial regulator of widely divergent cellular processes including apoptosis, proliferation, and differentiation. Zhao et al. [33] report that the PI3K/Akt pathway mediates the protection of SO2 preconditioning against myocardial ischemia/reperfusion injury in rats. Another study [23] reports that insulin upregulates mRNA expression of NKCC and CFTR through the activation of PI3K.
Therefore, in order to explore whether JAK2/STAT3, PI3K/Akt, and p38MAPK signaling pathways were involved in the protection of SO2 against limb I/R-induced ALI in rats, as well as the relationship among the three signaling pathways, we used specific inhibitors, Stattic, LY294002, and SB03580, to block them, respectively.
Compared to the I/R + SO2 group, when Stattic was administered, P-STAT3 protein expression decreased significantly in the I/R + SO2 + Stattic group (p < 0.05, Fig. 5c). The reaction of lung injury index was also reduced. The results show that the inhibition of the JAK2/STAT3 signaling pathway can enhance the protection role of SO2 in rats with limb I/R-induced ALI. In the I/R + SO2 + LY294002 group and the I/R + SO2 + SB03580 group, when LY294002 or SB03580 was administered, P-Akt or P-p38 protein expression decreased significantly (p < 0.05, Fig. 5a, b), but the reaction of lung injury index increased. The results show that the inhibition of PI3K/Akt and p38MAPK signaling pathways can decrease the protection role of SO2 in rats with limb I/R-induced ALI. To sum up, the JAK2/STAT3, PI3K/Akt, and p38MAPK signaling pathways are likely all involved in the process of SO2 inhibition of limb I/R-induced ALI in rats. The activation of PI3K/Akt and p38MAPK signaling pathways can effectively reduce I/R injury. Conversely, the activation of the JAK2/STAT3 signaling pathway can increase I/R injury. The Western-blot test results show that the rat’s lung existed expression of P-STAT3, P-AKT, and P-p38 proteins. After I/R, P-STAT3, P-Akt, and P-p38 proteins expression, all increased. After using Na2SO3/NaHSO3, P-Akt, and P-p38 proteins, expression increased, but P-STAT3 protein expression decreased (Fig. 5). The results indicate that the high expression of the P-STAT3 protein can aggravate lung injury, while the high expression of the P-Akt and P-p38 proteins have lung protective effects through attenuated inflammation and inhibited apoptosis. Therefore, we speculate that SO2 might activate PI3K/Akt and p38MAPK signaling pathways, promoting the expression of P-Akt and P-p38, while inhibiting JAK2/STAT3 signaling channel, reducing P-STAT3 expression, to protect the limb I/R-induced ALI.
We also found a strange phenomenon. Compared to the I/R + SO2 group, the administration of the LY294002 inhibitor did not cause P-p38 and P-STAT3 protein expression to change (p > 0.05, Fig. 5a). The administration of SB03580 inhibitor did not cause P-Akt and P-STAT3 protein expressions to change (p > 0.05, Fig. 5b). With the administration of Stattic inhibitor, P-p38 protein expression did not change (p > 0.05, Fig. 5c), but P-Akt protein expression increased (p < 0.05, Fig. 5a). Compared to the I/R + SO2 group, the parameters of lung injury decreased in the I/R + SO2 + Stattic group (Fig. 2). The results indicate that the JAK2/STAT3 signaling pathway and the PI3K/Akt signaling pathway may have cross-talk. We speculate that the JAK2/STAT3 signaling pathway can affect the PI3K/Akt signaling pathway but that the PI3K/Akt signaling pathway cannot affect the JAK2/STAT3 signaling pathway. Stattic inhibitors, by inhibiting the JAK2/STAT3 signaling pathway, increased the expression of P-Akt protein, which has a protective effect on rats with limb I/R-induced ALI. In fact, the cross-talk between JAK2/STAT3 and PI3K/Akt signaling pathway has already been reported in myocardial I/R injury. Suleman et al. [24] found that the Akt signaling pathway inhibitor Wortmannin can decrease P-STAT3 protein expression, and STAT3 signaling pathway inhibitor AG490 can decrease P-Akt protein expression. The results indicate that JAK2/STAT3 and PI3K/Akt signaling pathways may have cross-talk. The research of Goodman et al. [7] shows that under the condition of ischemic postconditioning, the JAK/STAT signaling pathway can activate the PI3K/Akt signaling pathway, thereby providing a RISK (Akt is key components of RISK) pathway upstream initiating factor. However, this is the first report of the cross-talk between the JAK2/STAT3 and PI3K/Akt signaling pathways in limb I/R-induced ALI.
Furthermore, we found that when P-STAT3 protein expression is high, while P-Akt and P-p38 protein expression is low, cell apoptosis is increased, and vice versa. In the I/R group, the P-STAT3/STAT3 was 82.27 %, the P-p38/p38 was 52.83 %, the P-Akt/Akt was 61.60 %, and the apoptotic index was 17.2 %, while in the I/R + SO2 group, the P-STAT3/STAT3 was 52.56 %, the P-p38/p38 was 79.08 %, the P-Akt/Akt was 82.56 %, and the apoptotic index was 6.5 % (Figs. 3b, 5). It is well known that macrophage plays a very important role in ALI. Therefore, as the next step, we will explore the role of exogenous SO2 on alveolar macrophage apoptosis and the molecular mechanisms with limb I/R-induced ALI in rats.
In conclusion, SO2 has a protective effect on rats with limb I/R-induced ALI. The JAK2/STAT3, PI3K/Akt, and p38MAPK signaling pathways are all likely involved in the process of SO2 protection against limb I/R-induced ALI in rats. The activation of PI3K/Akt and p38MAPK signaling pathways can effectively reduce I/R injury. Conversely, the activation of the JAK2/STAT3 signaling pathway can increase I/R injury. The JAK2/STAT3 signaling pathway has an impact on the PI3K/Akt signaling pathway. In terms of the stattic inhibitor, by inhibiting the JAK2/STAT3 signaling pathway, and increasing P-Akt protein secretion, it plays a protective role in ALI. Nevertheless, further studies are needed in order to determine the exact mechanisms.
Acknowledgments
This research program was supported by the Natural Science Foundation of Beijing, Beijing, People’s Republic of China. The Contract Grant Number is 7152061.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Funding
The Natural Science Foundation of Beijing has no role in this study.
References
- 1.Atabai K, Matthay MA. The pulmonary physician in critical care-5: acute lung injury and the acute respiratory distress syndrome: definitions and epidemiology [J] Thorax. 2002;57:452–458. doi: 10.1136/thorax.57.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewster JL, Valoir T, Dwyer N, et al. An osmosensing signal transduction pathway in yeast [J] Science. 1993;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- 3.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen SM, Siddiqi FA, Darakchiev B, et al. Attenuation of acute lung injury caused by hind-limb ischemia-reperfusion injury by butyrolactone anti-inflammatory agent FLl003 [J] J Trauma. 1997;43(2):247–252. doi: 10.1097/00005373-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Forster K, Paul I, Solenkova N, Staudt A, Cohen MV, Downey JM, et al. NECA at reperfusion limits infarction and inhibits formation of the mitochondrial permeability transition pore by activating p70S6 kinase. Basic Res Cardiol. 2006;101:319–326. doi: 10.1007/s00395-006-0593-4. [DOI] [PubMed] [Google Scholar]
- 6.Gerits N, Kostenko S, Moens U. In vivo functions of mitogen-activated protein kinases: conclusions from knock-in and knock-out mice. Transgenic Res. 2007;16:281–314. doi: 10.1007/s11248-006-9052-0. [DOI] [PubMed] [Google Scholar]
- 7.Goodman MD, Koch SE, Fuller-Biter GA, et al. Regulating RISK: a role for JAK/STAT signaling in postconditioning [J] Am J Physiol Hcart Circ Physiol. 2008;295(4):H1649–H1656. doi: 10.1152/ajpheart.00692.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto N, Takeyoshi I, Yoshinari D, et al. Effects of a p38 mitogen-activated protein kinase inhibitor as an additive to Euro-Collins solution on reperfusion injury in canine lung transplantation [J] Transplantation. 2002;74(3):320–326. doi: 10.1097/00007890-200208150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, et al. Translating novel strategies for cardioprotection: the hatter workshop recommendations. Basic Res Cardiol. 2010;105:677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang JS, Chuang LY, Guh JY, et al. Effect of nitric oxide-cGMP-dependent protein kinase activation on advanced glycation end-product-induced proliferation in renal fibroblasts. J Am Soc Nephrol. 2005;16:2318–2329. doi: 10.1681/ASN.2005010030. [DOI] [PubMed] [Google Scholar]
- 11.Kohmoto J, Nakao A, Stolz DB, et al. Carbon Monoxide Protects Rat Lung Transplants From Ischemia-Reperfusion Injury via a Mechanism Involving p38 MAPK Pathway. Am J Transplant. 2007;7:2279–2290. doi: 10.1111/j.1600-6143.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- 12.Jin HF, Du SX, Zhao X, et al. Effects of endogenous sulfur dioxide on monocrotaline induced pulmonary hypertension in rats [J] Acta Pharmaeol Sin. 2008;29(10):1157–1166. doi: 10.1111/j.1745-7254.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaltenbach JP, Jennings RB. Metabolism of ischemic cardiac muscle. Circ Res. 1960;8:207–213. doi: 10.1161/01.RES.8.1.207. [DOI] [PubMed] [Google Scholar]
- 14.Koike N, Takeyoshi I, Ohki S, et al. Effects of adding p38 mitogen-activated protein kinase inhibitor to CELSIOR solution in canine heart transplantation from non-heart-beating donors [J] Transplantation. 2004;77(2):286–292. doi: 10.1097/01.TP.0000101039.12835.A4. [DOI] [PubMed] [Google Scholar]
- 15.Macchi Laurent, Moussa Walid Ben, Guillou Sophie, et al. The synthetic pentasaccharide fondaparinux attenuates myocardial ischemia-reperfusion injury in rats via STAT-3. Shock. 2014;41(2):166–171. doi: 10.1097/SHK.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 16.Meng Z, Liu Y. Cell morphological ultrastructural changes in various organs from mice exposed by inhalation to sulfur dioxide. Inhal Toxicol. 2007;19(6–7):543–551. doi: 10.1080/08958370701271373. [DOI] [PubMed] [Google Scholar]
- 17.Meng Z, Nie A. Effects of sodium metabisulfite on potassium currents in acutely isolated CA1 pyramidal neurons of rat hippocampus. Food Chem Toxicol. 2005;43(2):225–232. doi: 10.1016/j.fct.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Meng Z, Qin G, Zhang B, Bai J. DNA damaging effects of sulfur dioxide derivatives in cells from various organs of mice. Mutagenesis. 2004;19(6):465–468. doi: 10.1093/mutage/geh058. [DOI] [PubMed] [Google Scholar]
- 19.Meng Z, Zhang H. The vasodilator effect and its mechanism of sulfur dioxide derivatives on isolated aortic rings of rats. Inhal Toxicol. 2007;19(11):979–986. doi: 10.1080/08958370701515175. [DOI] [PubMed] [Google Scholar]
- 20.Mitsuhashi H, Ikeuchi H, Yamashita S, Kuroiwa T, Kaneko Y, Hiromura K, Ueki K, Nojima Y. Increased levels of serum sulfite in patients with acute pneumonia. Shock. 2004;21(2):99–102. doi: 10.1097/01.shk.0000105501.75189.85. [DOI] [PubMed] [Google Scholar]
- 21.Nick JA, Young SK, Arndt PG, et al. Selective suppression of neutrophil accumulation in ongoing pulmonary inflammation by systemic inhibition of p38 mitogen-activated protein kinase [J] J Immunol. 2002;169(9):5260–5269. doi: 10.4049/jimmunol.169.9.5260. [DOI] [PubMed] [Google Scholar]
- 22.Omura T, Yoshiyama M, Ishikura F, Kobayashi H, Takeuchi K, Beppu S, Yoshikawa J. Myocardial ischemia activates the JAK-STAT pathway through angiotensin II signaling in in vivo myocardium of rats. J Mol Cell Cardiol. 2001;33:307–316. doi: 10.1006/jmcc.2000.1303. [DOI] [PubMed] [Google Scholar]
- 23.Sun H, Niisato N, Inui T, Marunaka Y. Insulin is involved in transcriptional regulation of NKCC and the CFTR Cl(-) channel through PI3K activation and ERK inactivation in renal epithelial cells. J Physiol Sci. 2014;64(6):433–443. doi: 10.1007/s12576-014-0338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suleman N, Somers S, Smith R, et al. Dual activation of STAT3 and Akt is required during the trigger phase of isehaemic preconditioning [J] Cardiovasc Res. 2008;79(1):127–133. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 25.Ubuka T, Yuasa S, Ohta J, Masuoka N, Yao K, Kinuta M. Formation of sulfate from l-cysteine in rat liver mitochondria. Acta Med Okayama. 1990;44(2):55–64. doi: 10.18926/AMO/30442. [DOI] [PubMed] [Google Scholar]
- 26.Wen SH, Li Y, Li C, et al. Ischemic postconditioning during reperfusion attenuates intestinal injury and mucosal cell apoptosis by inhibiting JAK/STAT signaling activation. Shock. 2012;38(4):411–419. doi: 10.1097/SHK.0b013e3182662266. [DOI] [PubMed] [Google Scholar]
- 27.Wymann MP, Zvelebil M, Laffargue M. Phosphoinositide 3-kinase signalling-which way to target? Trends Pharmacol Sci. 2003;24:366–376. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 28.Huang Xin-Li, Liu Yang, Zhou Jun-Lin, et al. Role of Sulfur Dioxide in Acute Lung Injury Following Limb Ischemia/Reperfusion in Rats [J] J Biochem Mol Toxicol. 2013;27(8):389–397. doi: 10.1002/jbt.21492. [DOI] [PubMed] [Google Scholar]
- 29.Yavuz S. Surgery as early revascularization after acute myocardial infarction. Anadolu Kardiyol Derg. 2008;8:84–92. [PubMed] [Google Scholar]
- 30.Yu H, Jove R. The STATs of cancer–new molecular targets come of age. Nat Rev Cancer. 2004;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 31.Abe Yukiko, Ono Koh, Kawamura Teruhisa, et al. Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am J Physiol Heart Circ Physiol. 2007;292:H2387–H2396. doi: 10.1152/ajpheart.00579.2006. [DOI] [PubMed] [Google Scholar]
- 32.Zhang B, Nie A, Bai W, Meng Z. Effects of aluminum chloride on sodium current, transient outward potassium current and delayed rectifier potassium current in acutely isolated rat hippocampal CA1 neurons. Food Chem Toxicol. 2004;42(9):1453–1462. doi: 10.1016/j.fct.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Zhao MM, Yang JY, Wang XB, et al. The PI3K/Akt pathway mediates the protection of SO2 preconditioning against myocardial ischemia/reperfusion injury in rats. Acta Pharmacol Sin. 2013;34:501–506. doi: 10.1038/aps.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]


