Abstract
Exercise has an effect on the reduction of myocardial fibrosis in diabetic rats as previously reported, in which oxidative stress and the TGF-β1/Smad signaling pathway may play key roles. There is little direct experimental evidence that exercise alleviates myocardial fibrosis in type 2 diabetes mellitus (T2DM). Here we established a type 2 diabetic model by using streptozotocin and a high-fat diet. Rats were divided into groups of normal control (NC), T2DM and T2DM plus exercise (T2DME). The T2DME group received further treadmill training at moderate intensity for 8 weeks. Histological and biochemical methods were used to detect the benefits of exercise to T2DM. Results showed that the weight of rats in the T2DM group dropped dramatically, along with significant increases in blood glucose, myocardial fibrosis and oxidative stress, associated with upregulated expression of factors of myocardial fibrosis, except Smad7. Exercise largely reversed T2DM-induced alterations in factors of myocardial fibrosis, including suppressing expression of MMP-2, CTGF, TGF-β1, p-Smad2 and p-Smad3, and increased expression of TIMP–1 and Smad7. Therefore, exercise might be considered an alternative therapeutic remedy for diabetic cardiomyopathy.
Keywords: Exercise, TGF-β1/Smad, Diabetes, Myocardial fibrosis, Rat
Introduction
Diabetic cardiomyopathy (DC) is an important complication caused by diabetes, which induces myocardial fibrosis over time. Myocardial fibrosis may lead to a decrease in myocardial adaptation, resulting in cardiac contraction, systolic and diastolic dysfunction, and ultimately in heart failure [1]. Aerobic exercise has been previously reported to reduce the extent of cardiac fibrosis and dysfunction in diabetic rats [2]. However, the mechanism’s details remain unclear. Diabetes-induced oxidative stress was previously reported to be crucial to the occurrence of DC, where the reactive oxygen species (ROS)-dependent TGF-β1 activation was considered to play a key factor in assisting myocardial fibrosis, and ROS can be induced by DC–hyperglycemia [3]. The TGF-β1/Smad pathway is involved in the histogenesis of myocardial fibrosis. Therefore, effective inhibition of TGF-β1/Smad signaling is the key to prevent myocardial fibrosis [4]. TGF-β1 mainly activates the downstream Smad protein, promotes the synthesis of collagen, inhibits the degradation of collagen, leads to the imbalance between collagen synthesis and degradation, and further induces myocardial fibrosis. Following the binding between TGF-β1 and its receptor on the sarcolemma, cellular Smad2 and Smad3 are phosphorylated, then they bind with Smad4 to form a complex that translocates into the nucleus, thereby regulating the expression of related genes, while Smad7 can inhibit the phosphorylation of Smad2/3 [5]. Hence, an important strategy for the prevention and treatment of diabetic cardiomyopathy is to find an intervention inhibiting the TGF-β1/Smad signaling pathway. Exercise was known to have beneficial effect on suppressing DC, because of its effective reductions on blood glucose inflammatory response, and cellular apoptosis [6, 7]. However, few studies have reported the importance of exercise on the effect of diabetic myocardial fibrosis. Recently, long-term exercise was found to reduce myocardial collagens type I and III by DC in a STZ-induced type 1 diabetic mellitus (T1DM) protocol, through downregulating fibrosis-associated gene expression of matrix metalloproteinases (MMPs) 2 and 9, which were associated with mediation by tissue inhibitors of MMPs (TIMPs). However, it is still unclear whether the exercise induced TGF-β1/Smad signaling pathway mediates the process of myocardial fibrosis suppression. To address this question, a model of type 2 diabetic mellitus (T2DM) rats developed by high-fat diet and intraperitoneal injection of low dose streptozocorcin (STZ) was applied in the present study. We hypothesized that exercise may inhibit myocardial fibrosis in diabetic rats by increasing myocardial antioxidant capacity and suppressing the TGF-β1/Smad signaling pathway. Therefore, exercise might be considered an alternative therapeutic remedy for diabetic cardiomyopathy.
Materials and methods
Experimental animals
Healthy 8-week-old male Sprague–Dawley rats (n = 40 rats, license SCXK 2012-001, Charles River, Beijing, China) with average weights of about 296 ± 8 g were housed at constant temperature 22 ± 2 °C, humidity 40% ~ 50%, and 12-h light/dark cycle. Rats were fed with standard rodent mash for 1 week adaptation and water ad libitum, then were randomly separated into two groups of normal control (NC, n = 8) and type 2 diabetes mellitus (T2DM, n = 32). The NC group was fed with normal mash, while the T2DM group was fed with a high-fat diet of total energy 20 kJ/g, which was mixed with the basal mash and contained 28% sucrose, 20% lard, l% cholesterol and 0.25% choleate. All animal procedures were in accordance with the institutional guidelines and approved by the institutional animal care and use committee (NIH Publication No. 85-23, revised 1996), and approved by the Ethics Committee for Science Research of the Hunan Technology University.
Experimental protocol
The protocol for type 2 diabetic rats has been described elsewhere [8]. After 4 weeks of continuous high-fat diet feeding, the rats in the T2DM group were fasted for 12 h, then the 2% dilution of STZ solution was dissolved with 0.01 mM citric acid buffer at pH 4.1, and was intraperitoneally injected at 30 mg/kg BW. The rats in the NC group were given a single intraperitoneal injection of 0.01 mM citric acid buffer of equal volume. After 72 h, the blood was collected from the tail vein at a random time. Following the standard of random blood glucose that is more than 11.1 mM, the T2DM protocol was established successfully. A final total of 32 rats were successfully modeled after 1 week of reexamination and the removal of rats with substandard blood glucose. Rats with successful modeling were randomly divided into the T2DM group (n = 16) and the type 2 diabetes mellitus plus exercise group (T2DME, n = 16).
Rats in the T2DME group were subjected to 1 week of adaptive training (10 m/min, 15 min/day), then were followed by a 8 weeks of aerobic treadmill exercise at moderate intensity, which refers to the Bedford training program, speed of 15.2 m/min, slope of 3° (exercise intensity is equivalent to 58.40 ± 1.7% VO2max), 60 min per day, and 5 days per week.
Echocardiography followed a previous report [9]. In brief, after narcosis by 10% chloral hydrate at 40 mg/100 g body weight by intraperitoneal injection, and fixation in a supine position, the chests of rats were defoliated. Small animal ultrasonic probes were placed on the left anterior chest and performed transthoracic echocardiography at 22 ~ 55 MHz using SXTCH 2.0 (Ultrasonic, Gorham St, Canada). The M-mode was used to measure the cardiac structural parameters in millimeters, such as LVIDd, LVIDs, LVPWd, LVPWs, and functional parameters FS (%) and EF (%).
All rats were anesthetized by intraperitoneally injecting 10% trichloroacetaldehyde monohydrate at a dose of 400 mg/kg BW. After 0.85% saline perfusion, for myocardial detection by using western blot and RT-PCR, the heart was immediately excised and the tissue close to the apex of the left ventricle wall was collected, rapidly frozen in liquid nitrogen, and then stored at − 80 °C. For histological analysis, supplementary 4% paraformaldehyde was perfused after the saline, and the heart was excised and kept in 4% paraformaldehyde for 24 h.
Insulin resistance test
After fasting for 12 h, 8 randomly selected rats from the NC and T2DM groups had their serums collected by standing blood for 30 min, then the fasting blood glucose (FBG) was detected by using an automatic biochemistry analyzer (Hitachi 7020, Japan) via a glucose oxidase kit method (Enzyme-linked Biotechnology, Shanghai, China). ELISA was applied to test fasting plasma insulin (FINS) via a microplate reader (WellScan MK3, LabSystems, USA) kit. The insulin sensitivity index (ISI) and insulin resistance index (HOMA-IR) were calculated by using the following formulas: ISI = − log (FPG × FINS), HOMA-IR = (FPG × FINS)/22.5.
Sirius red staining
Sirius red staining was used to assess the extent of fibrosis of the myocardial interstitium. The procedure of histological handling follows our previous report [10, 11]. In brief, the tissue was dehydrated, made transparent, sliced, dewaxed to water in sequence, and then soaked in a solution of picric acid-Sirius red for 15 min. After dehydration and making transparent, the slices were sealed with neutral gum and images were taken at 400× magnification. Eight samples were randomly selected from each group, and four regions of vision were randomly selected from each sample. Image-Pro Plus 6.0 image analysis software (Media Cybernetics, Silver Springs, MD) was used by adjusting the grey value for semi-quantitative analysis to distinguish areas of collagen and non-collagen; this allowed the measurement of the ratio between collagen area and total image, that is the collagen volume fraction (CVF).
Immunofluorescence staining
Immunofluorescence staining was performed as described elsewhere [12]. In brief, frozen slices were thrown into the acetone for fixation, then washed by PBS. Fixed slices were blocked with goat serum for 30 min, and primary rabbit antibodies, collagen I (anti-rat, 1:5000, Abcam, CBG, USA), and collagen III (anti-rat, 1:5000, Abcam, CBG, USA), were add at 4 °C and incubated overnight. After washing with PBS, fluorescein-labeled secondary antibodies (FITC) were added in the dark, and washed with PBST. Glycerin was used for sealing slices. Microscopy at 200× magnification was used to capture images. Under the same acquisition parameters, 5 images were randomly taken from each sample. The fluorescence intensity of the target protein was quantified by Image-Pro Plus 6.0 image analysis software (Media Cybernetics, Silver Springs, MD). The fluorescence intensity of the negative control sample was taken as the contrast, and the fluorescence intensity was expressed by integrated optical density (IOD).
Spectrophotometric analysis
Protein quantification of the myocardium was performed using the Coomassie brilliant blue method. Spectrophotometric analysis was performed elsewhere [10]. In brief, kits used to detect malondialdehyde (MDA), T-SOD and GSH-PX were used by referring to their respective instructions (Jiancheng Bioengineering Institute, Nanjing, China). MDA content was detected by the TBA method, T-SOD activity was detected by the hydroxylamine method, and GSH-PX activity was detected by a colorimetry method. The absorbance was measured by the ELISA instrument, and the data was analyzed and calculated according to the given formula.
RT-PCR
RNA was extracted by using Trizol; 2 μg extracted RNA was used as the template for next step cDNA synthesis. The cDNA synthesis kits (RR370A, TaKaRa Bio Inc., Shiga, Japan) were used with nucleic acid amplification (GeneAmp® PCR System 9700, Applied Biosystems, CA). For cDNA reverse transcription, reaction conditions were as follows: 37 °C for 15 min; 85 °C for 5 s; then maintained at 4 °C. Synthesized cDNA was used as templates, and β-actin was set as the internal control. In 20 μl of reaction system, each sample received three repeats and samples were amplified by a real-time fluorescent quantitative PCR system, reaction conditions as follows: pre-degeneration at 95 °C for 30 s; PCR reaction at 95 °C for 5 s, 60 °C for 31 s, and 40 cycles. A fluorescence quantitative kit (RR820A, TaKaRa, Japan) was used. The relative mRNA level was calculated by the 2−∆∆CT formula, in which ∆∆CT = (the target gene of the CT test group − the internal reference gene of the CT test group) − (the target gene of the CT control group − the internal reference gene of the CT control group). All the primers used were synthesized by Sangon Biotech, Shanghai, China. Primer sequences were as follows:
β-actin primary sequences were:
F: 5ʹCATTGCTGACAGGATGCAGAAG3ʹ, R: 5ʹGAGCCACCAATCCACACAGAGT3ʹ.
CTGF (connective tissue growth factor) primary sequences were:
F: 5ʹGGAAATGCTGTGAGGAGTGG 3ʹ, R: 5ʹGTCAGGGCCAAATGTGTCTT 3ʹ.
TIMP-1 primary sequences were:
F: 5ʹCTGGTTCCCTGGCATAATCT3ʹ, R: 5ʹATCGCTCTGGTAGCCCTTCT3ʹ.
MMP-2 primary sequences were:
F: 5ʹAAGTTCCCGTTCCGCTTC3ʹ, R: 5ʹGGTCATAATCCTCGGTGGTG3ʹ.
MMP-9 primary sequences were:
F: 5ʹ- TGTATGGTCGTGGCTCAA-3ʹ, R: 5ʹ- TTGGCTTCCTCCGTGATT-3ʹ.
Western blotting
Total protein was extracted and measured by bicinchoninic acid assay (BCA) and adjusted to the same concentration. Proteins were separated by SDS-PAGE electrophoresis at a constant 120 V for 1 h. Proteins were transferred to polyvinylidene fluoride (PVDF) membranes at a constant 70 V for 70 min. The membrane was incubated in 5% milk at 4 °C overnight. After blocking, primary antibodies used to detect TGF-β1 (anti-rat, 1:1000, Abcam, USA), Smad2 (anti-rat, 1:1000, Abcam, USA), Smad3 (anti-rat, 1:1000, Abcam, USA), Smad2-phospho S467 (anti-rat, 1:500, Abcam, USA), Smad3-phospho S213 (anti-rat, 1:500, Abcam, USA), and β-actin mouse antibody (anti-rabbit, 1:5000, Abcam, USA) were added and incubation was continued at 4 °C with shaking overnight. After incubation, the primary antibodies were recycled and the membranes were washed with TBST buffer 3 times. The horseradish peroxidase (HRP) labeled secondary antibody (1:500) was added and incubated at room temperature for 1 h. The membrane was washed again with TBST buffer another 3 times, and ECL™ chemiluminescence reagent was added onto the membrane and incubated at room temperature for 2 min. The integrated optical density (IOD) of the target protein and internal loading control protein bands was calculated by Image Lab software (BIO-RAD, Hercules, CA).
Statistical analysis
Data were analyzed using SPSS 20.0 (IBM, Chicago, IL). One-way ANOVA was used to determine significant group differences based on pairwise comparisons and to determine the major effects. Post-hoc contrasts were analyzed using a Student’s t-test and Newman-Keuls test (SNK). The results were expressed as the mean ± SD, using P < 0.05 in determining statistical significance.
Results
Effects of exercise on body weight and blood glucose in diabetic rats
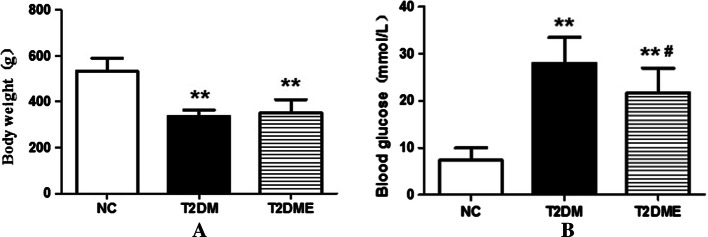
Changes in weight of rats are shown in Fig. 1a. Compared with the NC group, the weight of rats in the T2DM group and T2DME group were significantly reduced (P < 0.01). The weights of rats in the T2DME group were heavier than in the T2DM group, but there was no significant difference. Figure 1b shows the changes in levels of blood glucose. Compared with the NC group, blood glucose was significantly increased in both the T2DM group and the T2DME group (P < 0.01). However, blood glucose was significantly reduced in the T2DME group in comparison with the T2DM group (P < 0.05).
Fig. 1.
Weight and blood glucose of rats. a Changes in weight of rat. b Changes in levels of blood glucose. NC negative control, T2DM type 2 diabetes mellitus, T2DME type 2 diabetes mellitus plus exercise. **Compared with NC Group, P < 0.05; #Compared with T2DM Group, P <0.05
In Table 1, rats in the T2DM group had significantly higher FBG and FINS than those in the NC group (P < 0.01, P < 0.05, respectively), had significantly lower ISI than those in the NC group (P < 0.05), and had significantly higher HOMA-IR than in the NC group (P < 0.01). The results showed that insulin resistance was increased in the T2DM group. This indicated that the present animal model was consistent with the representations of type 2 diabetes.
Table 1.
Changes of insulin sensitivity of T2DM (n = 8 per group)
| Groups | FBG (mmol/l) | FINS (mIU/l) | ISI | HOMA-IR |
|---|---|---|---|---|
| NC | 5.08 ± 0.43 | 6.73 ± 2.66 | − 1.54 ± 0.13 | 1.52 ± 0.21 |
| T2DM | 9.47 ± 0.84** | 11.35 ± 3.17* | − 2.04 ± 0.25* | 4.78 ± 0.32** |
FBG fasting blood glucose, FINS fasting plasma insulin, ISI insulin sensitivity index, HOMA-IR insulin resistance. NC and T2DM respectively represent control group, type 2 diabetes group
*P < 0.05 vs NC
**P < 0.01 vs NC
Effects of exercise on myocardial collagen and LV functions in diabetic rats
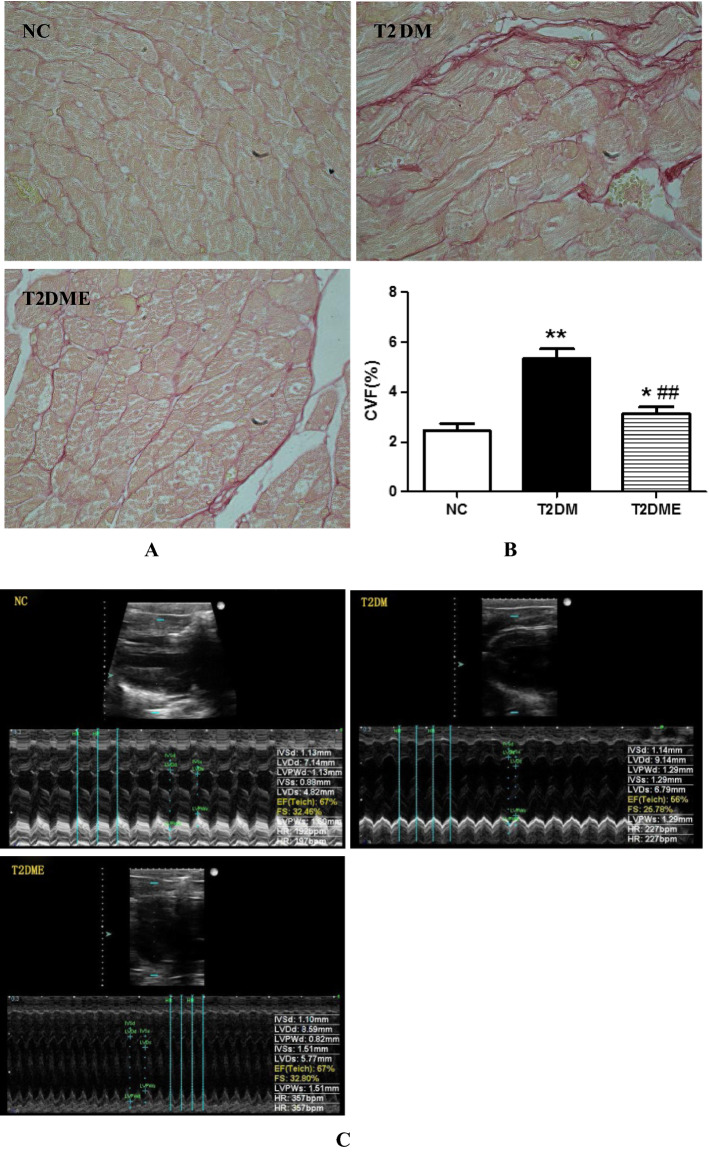
To determine whether exercise could also affect myocardial collagen formation, Sirus red staining assay was applied. Figure 2a shows the results of Sirus red staining, where the nuclei of rat cardiomyocytes was stained blue and cytoplasm was stained brown. No obvious red-stained fibrosis was shown in the NC group, but significant portion of tissue in the T2DM and T2DME groups were stained by red. Collagen Volume Fraction (CVF) was used to indicate fibrosis extension. As shown in Fig. 2b myocardial CVF was significantly increased in T2DM group (P < 0.01) and T2DME group (P < 0.05) in comparison with NC group even though T2DME group was lower than T2DM group (P < 0.01),
Fig. 2.
Sirius Red staining of rat myocardium and echocardiograms. a Images of Sirius Red staining, non-fibrosis area of myocardium stained brown, fibrosis area of myocardium stained red. Original magnification was ×200. b Percentage of Sirius Red staining–collagen volume fraction (CVF). c Analysis of left ventricular echocardiography. B-mode showing in upper and M-mode showing in lower part of each image. Green dotted lines indicate diastolic (longer) and systolic (shorter) chamber lengths in M-mode. Real-time parameters are shown in the bottom-right corner for each group. NC negative control, T2DM type 2 diabetes mellitus; T2DME type 2 diabetes mellitus plus exercise. *Compared with NC Group, P < 0.05; **compared with NC Group, P < 0.01; ##compared with T2DM Group, P <0.01
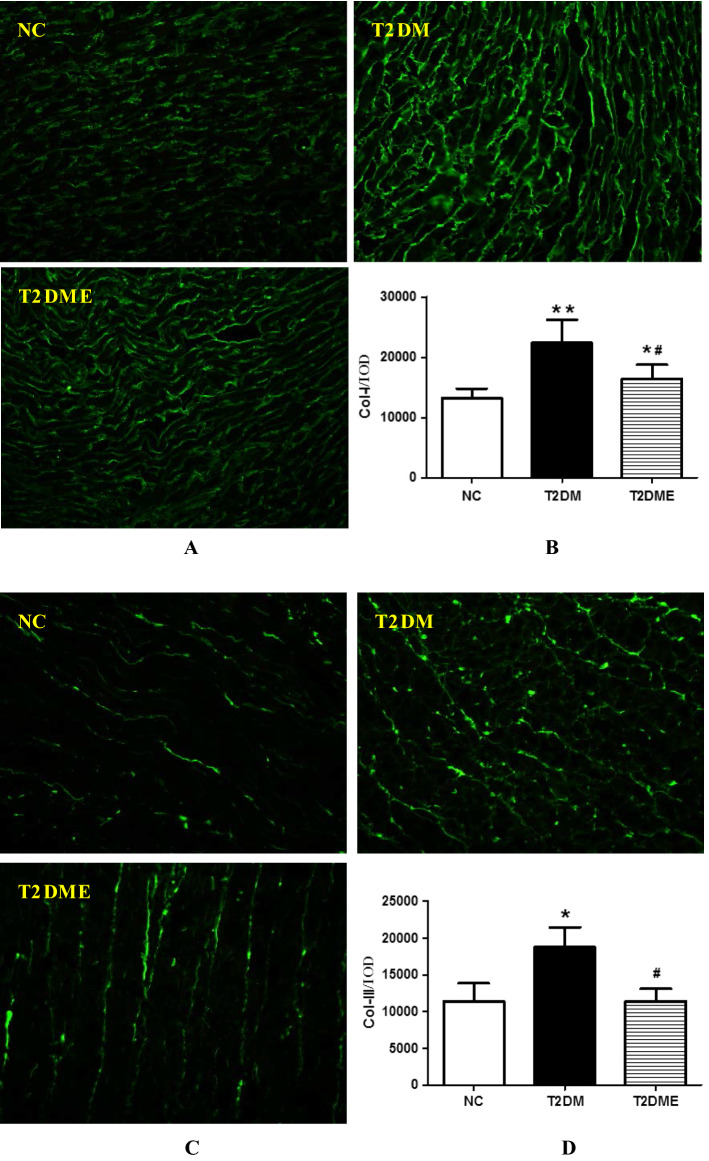
To further detect collagen type I (Col-I) and Collagen type III (Col-III) expression, immunofluorescence staining assay was performed. The scanned green immunofluorescence images of collagen type I (Col-I) were shown in Fig. 3a. Figure 3b were quantified data obtained from Fig. 3a. Results showed that T2DM has the highest expression level of myocardial Col-I among the 3 groups and myocardial Col-I expression level both in T2DM group and T2DME group were significantly increased in comparison with NC group (P <0.05) Similar to collagen type I (Col-I) expression, Collagen type III (Col-III) expression was also elevated in T2DM group in comparison with NC group (P < 0.05), While Col-III expression tended to increase in T2DME group, the difference between NC and T2DME group was not statistically significant.
Fig. 3.
Staining of collagen type-I (Col-I) and collagen type III (Col-III) in myocardium. a Scanning immunofluorescence images of collagen type I. The images showed green fluorescence. Original magnification was ×200. b IOD value of collagen type I. c Scanning immunofluorescence images of collagen type III. The images showed green fluorescence. Original magnification was ×200. d IOD value of collagen type III. NC negative control, T2DM type 2 diabetes mellitus; T2DME type 2 diabetes mellitus plus exercise. *Compared with NC Group, P <0.05; **compared with NC Group, P < 0.01; #compared with T2DM Group, P <0.05
Data of left ventricular (LV) functions were shown in Table 2 and Fig. 2c by analyzing echocardiograms. Left ventricular end diastolic diameter (LVIDd) and left ventricular end-systolic diameter (LVIDs) in the T2DM group were significantly higher than that in the NC (P < 0.01), where short axis shortening fraction (FS) and ejection fraction (EF) were correspondingly lower than that in the NC (P < 0.01, P < 0.05, respectively). These indicate T2DM was associated with LV dysfunction. Exercise significantly reversed these undesirable changes of T2DM in aspects of LVIDs, FS and EF by 19.83%, 26.09%, and 19.64%, respectively (P < 0.05).
Table 2.
Changes of the structural and functional parameters in the left ventricle (n = 8 per group)
| Index | NC | T2DM | T2DME |
|---|---|---|---|
| LVIDd (mm) | 7.51 ± 0.86 | 9.06 ± 0.74** | 8.13 ± 0.85 |
| LVIDs (mm) | 5.25 ± 0.98 | 7.26 ± 0.97** | 5.82 ± 0.63# |
| LVPWd (mm) | 1.01 ± 0.35 | 1.38 ± 0.48 | 1.28 ± 0.42 |
| LVPWs (mm) | 1.37 ± 0.34 | 1.69 ± 0.41 | 1.54 ± 0.50 |
| FS (%) | 31 ± 4.78 | 23 ± 4.18* | 29 ± 3.36# |
| EF (%) | 71 ± 9.60 | 56 ± 5.07** | 67 ± 6.19# |
LVIDd left ventricular end diastolic diameter, LVIDs left ventricular end systolic diameter, LVPWd wall thickness of left ventricular end diastole, LVPWs wall thickness of the left ventricle at the end of systolic period, FS short axis shortening fraction of the left ventricle, EF left ventricular ejection fraction. NC, T2DM and T2DME respectively represent control group, type 2 diabetes group and type 2 diabetes plus exercise group
*P < 0.05 vs NC
**P < 0.01 vs NC
#P < 0.05 vs T2DM
Effects of exercise on myocardial oxidative stress in diabetic rats
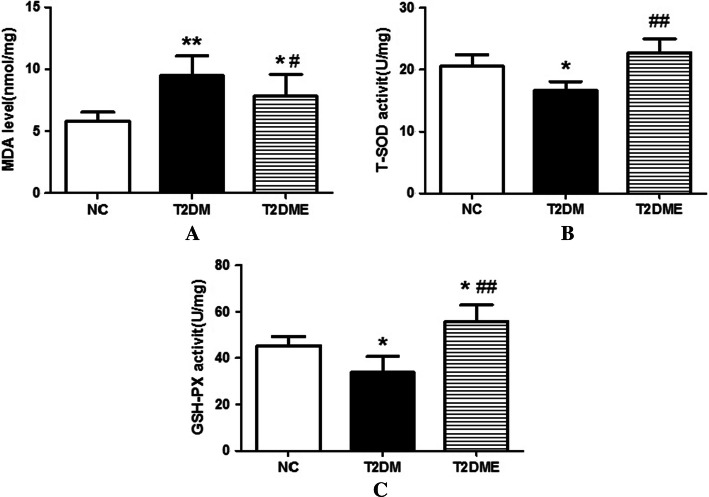
To elucidate whether exercise also affected the function of myocardial anti-oxidation system. malondialdehyde (MDA) content, T-SOD and GSH-PX activity, which were important indicators of myocardial oxidation system function were tested to measure reflect the level of myocardial oxidative stress and degree of oxidative damage.
As shown in Fig. 4a Compared with NC group, and MDA content in T2DM group was significantly increased (P < 0.01) and MDA content in the T2DME group was significantly lower than that in the T2DM group (P < 0.05), but still significantly higher than that in the NC group (P < 0.05). Figure 4b, c show changes in total (T)-SOD activity and GSH-PX activity respectively. Compared with NC group, the activity of T-SOD and GSH-PX in T2DM group was significantly reduced (P < 0.05). Compared with the T2DM group, the activity of T-SOD and GSH-PX in the T2DME group was significantly increased (P < 0.01), and the activity of GSH-PX was significantly higher than that in the NC group (P < 0.05).
Fig. 4.
Changes in myocardial oxidative stress of rats. a Changes in MDA levels. b Changes in total (T)–SOD activity. c Changes in GSH-PX activity. NC negative control, T2DM type 2 diabetes mellitus, T2DME type 2 diabetes mellitus plus exercise vs NC Group. *Compared with NC Group, P <0.05; **compared with NC Group, P <0.01; #compared with T2DM Group, P <0.05; ##compared with T2DM Group, P <0.01
Effects of exercise on myocardial fibrosis factors in diabetic rats
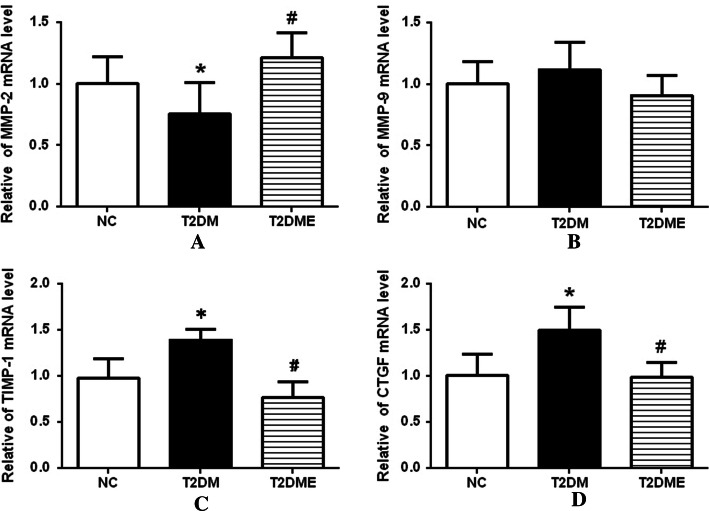
The mRNA level of myocardial fibrosis factors including matrix metalloproteinase-2 (MMP-2), connective tissue growth factor (CTGF), MMP-9 and tissue inhibitor of metalloproteinase-1 (TIMP-1) were also tested among NC, T2DM and T2DME groups. As shown in Fig. 5, compared with the NC group, MMP-2 (Fig. 5a) and CTGF expression levels (Fig. 5d) were significantly decreased in the T2DM group (P < 0.01). Also, the MMP-2 expression level was much lower in the T2DM group in comparison with the T2DME group (P < 0.05).There was no significant difference in MMP-9 expression (Fig. 5b) between all 3 groups. TIMP-1 expression (Fig. 5c) in the T2DM group was higher than that found in the NC group, or theT2DME group (P < 0.05).
Fig. 5.
mRNA changes of myocardial fibrosis factors in myocardium. a Changes in relative levels of MMP-2 mRNA. b Changes in relative levels of MMP-9 mRNA. c Changes in relative levels of TIMP-1 mRNA. d Changes in relative levels of CTGF mRNA. NC negative control, T2DM type 2 diabetes mellitus, T2DME type 2 diabetes mellitus plus exercise. *Compared with NC Group, P <0.05; #compared with T2DM Group, P <0.05
Effects of exercise on myocardial TGF-β1/Smad signaling pathway in diabetic rats
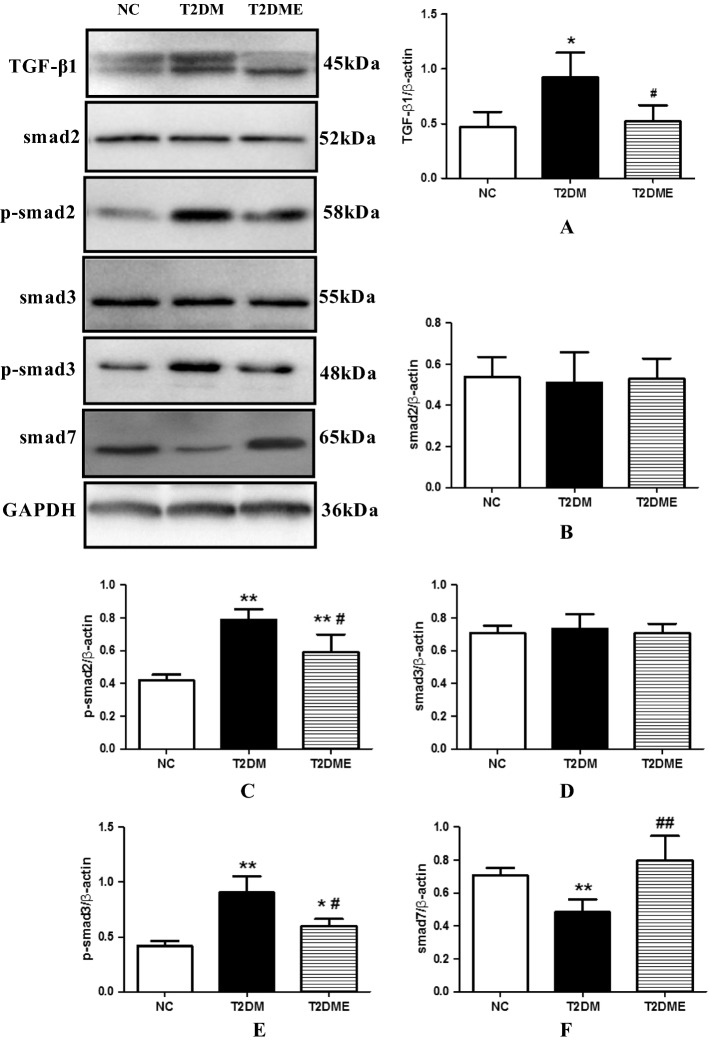
Previous studies have demonstrated that the TGF-β signaling pathway plays a key role in myocardial fibrosis formation. To test whether exercise alleviated myocardial fibrosis was mediated through regulating the TGF-β signaling pathway, TGF-β1/Smad signaling pathway related gene expression was detected. Results showed that compared with the NC group, TGF-β1 protein levels were (Fig. 6a) significantly increased in the T2DM group (P < 0.05), but no significant increase in TGF-β1 was found in the T2DME group. Also, the TGF-β1 protein level was significantly higher in the T2DM group than in the T2DME group, (P < 0.05). Although western blotting showed no significant change in the expression of Smad2 (Fig. 6b) or Smad3 (Fig. 6d) among the 3 groups, the protein level of p-Smad2 (Fig. 6c) and p-Smad3 (Fig. 6e) were significantly increased in the T2DME group and the T2DM group when compared with the NC group (P < 0.01), even though p-Smad2 and p-Smad3 in T2DM were much higher than in the T2DME group (P < 0.01). Compared with the NC group, the expression of Smad7 (Fig. 6f) in myocardium of the T2DM group was significantly reduced (P < 0.01), and the expression of Smad7 in the T2DME group was significantly higher than that in the T2DM group (P < 0.01).
Fig. 6.
Protein expression changes of TGF-β1/smad signaling in myocardium. a Changes in TGF-β1 levels. b Changes in Smad2 levels. c Changes in phosphorylated (p)–Smad2 levels. d Changes in Smad3 levels. e Changes in phosphorylated (p)–Smad3 levels. f Changes in Smad7 levels. NC negative control, T2DM type 2 diabetes mellitus, T2DME type 2 diabetes mellitus plus exercise. *Compared with NC Group, P < 0.05; **compared with NC Group, P <0.01; #compared with T2DM Group, P <0.05; ##compared with T2DM Group, P <0.01
Discussion
“Three more and one less”, that is, more drink, more food, more urine and weight loss are typical characteristics of diabetic people. Changes in weight of diabetic patients can indirectly reflect the severity of diabetes [13]. In the present study, it was found that the weight of diabetic rats was significantly reduced. However, compared with the diabetic rats, the weights of the rats were elevated after 8 weeks of treadmill training. The trends of weight loss in diabetic rats were not significantly reversed, and this may be due to the exercise-assisted degradation of substances caused by increased energy consumption. In addition, changes in body weight were the consequence of long-term changes, and this may relate to exercise intervention which did not take long enough, failing to cause significant weight improvement in diabetic rats.
We found that the blood glucose levels of diabetic rats were significantly reduced by 8 weeks of treadmill exercise, but it was still significantly higher than that of control rats, indicating that 8 weeks of aerobic exercise can partially alleviate the increase of blood glucose caused by diabetes. In patients with type 2 diabetes, increased blood glucose is mainly associated with the organism’s sensitivity to insulin; insulin in the blood is relatively insufficient, and promotes an increase in insulin sensitivity. In aerobic exercise, more Glut4 (glucose transporters 4) was transferred to the cytomembrane, which promoted the metabolism of glucose molecules into the cell, thus reducing the blood glucose [14].
Studies have shown that diabetic cardiomyopathy is one of the important complications in diabetic patients, which can more than double the mortality rate of diabetic patients [15, 16]. Human and animal experiments have shown that myocardial fibrosis may be one of the important pathologic mechanisms of diabetic cardiomyopathy [17, 18]. Myocardial fibrosis refers to the phenotype of myocardial extracellular matrix (ECM) representing its excessive increases. Where Col-III and Col-I are main components of the ECM, Col-I is more than 80%. Excessive increase of ECM can lead to increased myocardial hardness and decreased myocardial compliance [19]. Studies have found that the proportion of diabetic cardiomyopathy in patients is associated with myocardial ECM changes, and with significantly increased Col-I and Col-III [20]. Diabetes is an independent pathogenic factor leading to myocardial fibrosis. The degree of myocardial fibrosis is related to the duration of diabetes, and the degree of myocardial fibrosis gradually increases with the progression of the disease [21]. Gonzalez-Quesada et al. found that 4-month-old db/db mice showed obvious symptoms of obesity and diabetes, and gradually showed symptoms of myocardial fibrosis, accompanied by cardiomyocyte hypertrophy and myocardial dysfunction [22]. In the present study, rats generated myocardial fibrosis by one-time intraperitoneal injection of STZ and feeding with a high-fat diet for 4 weeks. After another 8 weeks of the experimental cycle, myocardial fibrosis was found by Sirius Red staining. Consistent with previous studies, during the modeling process of type 2 diabetic rats, obvious myocardial fibrosis lesions can only be observed after 4 ~ 12 weeks of high-fat dietary intervention after streptozotocin has been injected [23].
The right choice of exercise intervention is particularly important when applying non-pharmacological therapy to diabetic cardiomyopathy (DC), because excessive training intensity and volume were likely insufficient to form adaptations for T2DM-induced stresses: for example, it fails to restore imbalanced nitroso-redox, but also induces albuminuria to aggravate renal risks [24, 25]. As such, short-term exercise may aggravate diabetic myocardial oxidative stress and fibrosis [26]. Instead, recent studies have shown that long-term aerobic exercise can alleviate type 2 diabetes-induced myocardial fibrosis. For example, swimming training can reduce the degree of myocardial fibrosis in rats, which promotes myocardial compliance, and facilitate the diabetes-caused myocardial dysfunction [27]. Moderate exercise intervention has also been reported to have a better oxygen transport elevations on T2DM patients in myocardial stain rate and HbA, when relative to vigorous activity [28]. We found that 8 weeks of treadmill exercise can significantly reduce CVF and myocardial fibrosis caused by type 2 diabetes by 36%, which is concurrent with reversed T2DM-associated cardiac dysfunction. Although the immunofluorescence showed that the contents of Col-I and Col-III were significantly reduced, Sirius Red staining showed that myocardial fibrosis in diabetic rats which trained for 8 weeks was not fully inhibited, possibly due to the relatively short duration of single-bout exercise. Exercise can reduce the degree of myocardial fibrosis, which may be related to such factors as reduced oxidative stress in diabetic myocardium, attenuated activity of renin angiotensin and inhibited advanced glycosylation end products (AGEs) [29, 30]. Further, moderate exercise was reported to increase mitochondrial adaptations which potentially suppress fibrosis-related oxidative stress in T2DM; these thus preserve cardiac function from the perspective of energy metabolism [31, 32]. However, there are few reports on the intracellular signal transduction pathways of exercise-relieved diabetic myocardial fibrosis, which requires further study.
Many studies have shown that transforming growth factor-β1 (TGF-β1) is a key factor in leading to myocardial fibrosis, and plays an important regulatory role in the process of myocardial fibrosis caused by myocardial infarction and hypertension [33]. Guan et al. found that the myocardial collagen content in diabetic rats was significantly correlated with the content of TGF-β1, and TGF-β1 may mediate the fibrosis process [34]. Recently, Suematsu et al. have shown that streptozotocin induced the increase in expression of TGF-β1 in the myocardium of diabetic mice, resulting in myocardial fibrosis accompanied by myocardial dysfunction [35]. In the present study, we found that the expression of TGF-β1 and downstream Col-I, Col-III, MMP-2, and CTGF were significantly elevated in the myocardial tissue of diabetic rats. Consistent with this, Wu et al. confirmed that at 2 months after streptozotocin treatment, myocardial fibrosis can be seen by Masson staining in mice, and the expression of TGF-β1 was significantly increased [36]. After the intervention of resveratrol, the expression of TGF-β1 was downregulated and myocardial fibrosis was reversed [36]. Recent studies have reported that aerobic exercise can reduce the expression of renal TGF-β1 in rats, reducing diabetic nephrotic fibrosis, and improving renal function [37]. However, the effect of aerobic exercise on the expression of TGF-β1 in diabetic myocardium has not been reported. The results of the present study showed that 8 weeks of aerobic exercise can significantly reduce the expression of TGF-β1 and the downstream molecules in the myocardium of diabetic rats and may be involved in the inhibition of myocardial fibrosis. TGF-β1 is a restoration factor for impairments. Short-term increase of TGF-β1can be beneficial to the healing of the lesion area [38]. However, long-term high expression of TGF-β1 can lead to excessive tissue repair and further generate fibrosis [39]. Cellular TGF-β1 is regulated by adrenal hormones, and lower intensity aerobic exercise may significantly reduce the level of adrenaline in the circulation of patients with hypertension and diabetes [40, 41]. Therefore, exercise in the present study may decrease the expression of TGF-β1 in the myocardium of diabetic rats and reduce the degree of myocardial fibrosis by inhibiting the secretion of adrenaline in diabetic rats [42].
The TGF-β1/Smad signaling pathway regulates fibrosis mainly through Smad protein, including smad2/3, smad4 and smad7. In the present study, it was found that there was no significant change in the expression of Smad2 and Smad3 in diabetic rats, but both Smad2/3 were phosphorylated, and Smad7 was upregulated, indicating the activation of the TGF-β1/Smad signaling pathway in diabetic myocardium. The activity of matrix metalloproteinase (MMP) including MMP-2 and MMP-9 can be inhibited by TGF-β1/Smad via promoting the expression of tissue inhibitor of metalloproteinase (TIMP) which slows down the degradation rate of collagen [21, 43, 44]. In the present study, the low expression of TIMP and high expression of MMP signaling resulted in an imbalance between MMP and TIMP, leading to myocardial fibrosis. In accordance with this, it has been confirmed that the activation of the TGF-β1/Smad signaling pathway in diabetic rats induced by STZ injection significantly increased expression of TIMP-1, as well as decreased expression of MMP-2. The ratio of MMP-2/TIMP-1 was thus decreased, and the degradation of collagen was suppressed, forming myocardial fibrosis [45]. According to previous reports, increased MMPs play key roles in fibrosis degradation and TIMP-1 inhibits this process. On the other hand, as markers of fibrosis, increased MMPs make CTGF tend to fibrosis has been reported [46]. We noticed that Silva, et al. have reported T1DM reduce MMP2 mRNA levels, and counteracted by exercise, which is consistent with our results. We also considered that these may be due to different protocols that ours is T2DM. However, further experimental evidence in vivo about cardiac MMP mRNA expression is absent, and the present results might provide a fresh proof on exercise-alleviated T2DM-fibrosis.
Oxidative stress has been reported to promote the development of cardiac fibrosis by upregulating TGF-β1 expression [3]. ROS are largely generated from mitochondria, and ROS predominant oxidative impairment can be attenuated by various antioxidant enzymes such as superoxide-scavenger SOD and NADPH-preserver GSH-PX [10]. In the T2DM, the activities of antioxidant enzymes were reported to be suppressed by upstream SIRT1 and PGC-1α inhibition, and ROS was further generated by upregulated Ang-II [47, 48]. Based on these, uninhibited ROS exerts an important effect on the development of DC [49]. Diabetes-induced oxidative stress also leads to cardiac injury and interstitial fibrosis [3]. Ang-II inducing TGF-β1-mediated fibrosis is attenuated by Smad7 [50]. Smad7 was reported to reduce NADPH-mediated ROS production, and inhibits ROS-dependent MMP-9 activation [50]. TGF-β1 itself can also be induced by ROS via transforming from latent TGF-β1 [51]. Therefore, there exists extensive crosstalk between oxidative stress and fibrotic factors. Malondialdehyde (MDA), is a negative product of lipid peroxidation, and is thus considered to be a marker of ROS imbalance. Our data showed that the production of MDA was increased in the T2DM group, which was accompanied by reductions of SOD activity and GSH-PX activity, suggesting ROS overexpression. Conversely, the generation of MDA is significantly reduced by exercise, in which decreased MDA is strongly associated with the increased activities of SOD and GSH-PX. Beyond these, we found that the MDA levels coincided with levels of connective tissue growth factor (CTGF) mRNA. CTGF is another fibrotic factor besides TGF-β1. We speculated that CTGF expression in the T2DM group may be inhibited by exercise through attenuating ROS-mediated extracellular signal-regulated kinase (ERK) phosphorylation [52]. Taken together, these findings were in accord with a previous study, which demonstrated that left atrium dilation and cardiac dysfunction were potentially attenuated by exercise, and that reduced oxidative stress is a factor [53].
In summary, the present study demonstrates that exercise could attenuate diabetes-induced myocardial fibrosis, the mechanism of which may mostly rely on suppression of oxidative stress and the TGF-β1/Smad signaling pathway. Where the long-term moderate exercise reduce ROS generation of T2DM by preventing cardiac function, is then contributed to TGF-β1 inhibition. Therefore, the phosphorylation of Smad2/3 was attenuated by Smad7 activity, to increase transcription of MMP-2, as well as to decrease mRNA expressions of TIMP-1 and of CTGF. In consequence, collagen I/III generations were inhibited to T2DM-fibrosis [31, 51, 54].
Conclusion
Diabetic rats showed that myocardial fibrosis is associated with TGF-β1/Smad signaling pathway activation. Eight weeks of treadmill exercise reduced blood glucose in diabetic rats, partially inhibited myocardial fibrosis, decreased expression of myocardial TGF-β1 protein, and inhibited phosphorylation of Smad2/3. These results indicated that exercise may reduce myocardial fibrosis in diabetic rats by inhibiting the TGF-β1/Smad signaling pathway, in which increased activities of antioxidant enzymes play potential roles for inhibition of DC-myocardial fibrosis.
Acknowledgements
This work was supported by the Humanities and Social Sciences Foundation of the Ministry of Education, China (No. 18YJC890041), and by the Natural Science Foundation of Hunan Province, China (No. 2018JJ3118).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S, Liang M, Gao D, Su Q, Laher I. Changes in titin and collagen modulate effects of aerobic and resistance exercise on diabetic cardiac function. J Cardiovasc Transl Res. 2019 doi: 10.1007/s12265-019-09875-4. [DOI] [PubMed] [Google Scholar]
- 3.Purnomo Y, Piccart Y, Coenen T, Prihadi JS, Lijnen PJ. Oxidative stress and transforming growth factor-beta1-induced cardiac fibrosis. Cardiovasc Hematol Disord Drug Targets. 2013;13(2):165–172. doi: 10.2174/1871529X11313020010. [DOI] [PubMed] [Google Scholar]
- 4.Yue Y, Meng K, Pu Y, Zhang X. Transforming growth factor beta (TGF-beta) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract. 2017;133:124–130. doi: 10.1016/j.diabres.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Moore-Morris T, Cattaneo P, Puceat M, Evans SM. Origins of cardiac fibroblasts. J Mol Cell Cardiol. 2016;91:1–5. doi: 10.1016/j.yjmcc.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novaes RD, Goncalves RV, Penitente AR, Bozi LH, Neves CA, Maldonado IR, Natali AJ, Talvani A. Modulation of inflammatory and oxidative status by exercise attenuates cardiac morphofunctional remodeling in experimental Chagas cardiomyopathy. Life Sci. 2016;152:210–219. doi: 10.1016/j.lfs.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 7.Chengji W, Xianjin F. Treadmill exercise alleviates diabetic cardiomyopathy by suppressing plasminogen activator inhibitor expression and enhancing eNOS in streptozotocin-induced male diabetic rats. Endocr Connect. 2018;7(4):553–559. doi: 10.1530/ec-18-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metab Clin Exp. 2000;49(11):1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 9.Garza MA, Wason EA, Cruger JR, Chung E, Zhang JQ. Strength training attenuates post-infarct cardiac dysfunction and remodeling. J Physiol Sci. 2019;69(3):523–530. doi: 10.1007/s12576-019-00672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Y, Pan S-S, Wan D-F, Lu J, Huang Y. H2O2 signaling-triggered PI3K mediates mitochondrial protection to participate in early cardioprotection by exercise preconditioning. Oxid Med Cell Longev. 2018;2018:16. doi: 10.1155/2018/1916841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Pan SS. Elevated C-type natriuretic peptide elicits exercise preconditioning-induced cardioprotection against myocardial injury probably via the up-regulation of NPR-B. J Physiol Sci. 2017;67(4):475–487. doi: 10.1007/s12576-016-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Y, Pan SS, Shen YJ. Cardioprotection of exercise preconditioning involving heat shock protein 70 and concurrent autophagy: a potential chaperone-assisted selective macroautophagy effect. J Physiol Sci. 2018;68(1):55–67. doi: 10.1007/s12576-016-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bgeginski R, Ribeiro PA, Mottola MF, Ramos JG. Effects of weekly supervised exercise or physical activity counseling on fasting blood glucose in women diagnosed with gestational diabetes mellitus: a systematic review and meta-analysis of randomized trials. J Diabetes. 2016;1:2. doi: 10.1111/1753-0407.12519. [DOI] [PubMed] [Google Scholar]
- 14.Way KL, Hackett DA, Baker MK, Johnson NA. The effect of regular exercise on insulin sensitivity in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab J. 2016;40(4):253–271. doi: 10.4093/dmj.2016.40.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joubert M, Manrique A, Cariou B, Prieur X. Diabetes-related cardiomyopathy: the sweet story of glucose overload from epidemiology to cellular pathways. Diabetes Metab. 2019;45(3):238–247. doi: 10.1016/j.diabet.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Ng HH, Leo CH, Parry LJ, Ritchie RH. Relaxin as a therapeutic target for the cardiovascular complications of diabetes. Front Pharmacol. 2018;9:501. doi: 10.3389/fphar.2018.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Q, Jia TZ, Cao QC, Tian F, Ying WT. A crude 1-DNJ extract from home made Bombyx batryticatus inhibits diabetic cardiomyopathy-associated fibrosis in db/db mice and reduces protein N-glycosylation levels. Int J Mol Sci. 2018;19(6):1. doi: 10.3390/ijms19061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian J, Zhao Y, Liu Y, Liu Y, Chen K, Lyu S. Roles and mechanisms of herbal medicine for diabetic cardiomyopathy: current status and perspective. Oxid Med Cell Longev. 2017;2017:8214541. doi: 10.1155/2017/8214541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, Xue M, Li CJ, Yang W, Wang SS, Ma ZJ, Zhang XN, Wang XY, Zhao R, Chang BC, Chen LM. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J Ethnopharmacol. 2016;193:333–344. doi: 10.1016/j.jep.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Mihailovici AR, Deliu RC, Margaritescu C, Simionescu CE, Donoiu I, Istratoaie O, Tudorascu DR, Tartea EA, Gheonea DI. Collagen I and III, MMP-1 and TIMP-1 immunoexpression in dilated cardiomyopathy. Rom J Morphol Embryol. 2017;58(3):777–781. [PubMed] [Google Scholar]
- 21.Adeshara KA, Diwan AG, Tupe RS. Diabetes and complications: cellular signaling pathways, current understanding and targeted therapies. Curr Drug Targets. 2016;17(11):1309–1328. doi: 10.2174/1389450117666151209124007. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee DW, Saxena A, Frunza O, Dobaczewski M, Shinde A, Frangogiannis NG. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res. 2013;113(12):1331–1344. doi: 10.1161/circresaha.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CJ, Lv L, Li H, Yu DM. Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol. 2012;11:73. doi: 10.1186/1475-2840-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novoa U, Arauna D, Moran M, Nuñez M, Zagmutt S, Saldivia S, Valdes C, Villaseñor J, Zambrano CG, Gonzalez DR. High-intensity exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxid Med Cell Longev. 2017;2017:7921363. doi: 10.1155/2017/7921363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Climie RE, Srikanth V, Keith LJ, Davies JE, Sharman JE. Exercise excess pressure and exercise-induced albuminuria in patients with type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2015;308(9):H1136–H1142. doi: 10.1152/ajpheart.00739.2014. [DOI] [PubMed] [Google Scholar]
- 26.Laher I, Beam J, Botta A, Barendregt R, Sulistyoningrum D, Devlin A, Rheault M, Ghosh S. Short-term exercise worsens cardiac oxidative stress and fibrosis in 8-month-old db/db mice by depleting cardiac glutathione. Free Radic Res. 2013;47(1):44–54. doi: 10.3109/10715762.2012.737463. [DOI] [PubMed] [Google Scholar]
- 27.da Silva E, Natali AJ, da Silva MF, Gomes Gde J, da Cunha DN, Toledo MM, Drummond FR, Ramos RM, Dos Santos EC, Novaes RD, de Oliveira LL, Maldonado IR. Swimming training attenuates the morphological reorganization of the myocardium and local inflammation in the left ventricle of growing rats with untreated experimental diabetes. Pathol Res Pract. 2016;212(4):325–334. doi: 10.1016/j.prp.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Hordern MD, Coombes JS, Cooney LM, Jeffriess L, Prins JB, Marwick TH. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart. 2009;95(16):1343–1349. doi: 10.1136/hrt.2009.165571. [DOI] [PubMed] [Google Scholar]
- 29.Bostick B, Aroor AR, Habibi J, Durante W, Ma L, DeMarco VG, Garro M, Hayden MR, Booth FW, Sowers JR. Daily exercise prevents diastolic dysfunction and oxidative stress in a female mouse model of Western diet induced obesity by maintaining cardiac heme oxygenase-1 levels. Metabolism. 2017;66:14–22. doi: 10.1016/j.metabol.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemuriyama T, Tandai-Hiruma M, Kato K, Ohta H, Maruyama S, Sato Y, Nishida Y. Endogenous angiotensin II has fewer effects but neuronal nitric oxide synthase has excitatory effects on renal sympathetic nerve activity in salt-sensitive hypertension-induced heart failure. J Physiol Sci. 2009;59(4):275–281. doi: 10.1007/s12576-009-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veeranki S, Givvimani S, Kundu S, Metreveli N, Pushpakumar S, Tyagi SC. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J Mol Cell Cardiol. 2016;92:163–173. doi: 10.1016/j.yjmcc.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muhammad MH, Allam MM. Resveratrol and/or exercise training counteract aging-associated decline of physical endurance in aged mice; targeting mitochondrial biogenesis and function. J Physiol Sci. 2018;68(5):681–688. doi: 10.1007/s12576-017-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Lu S, Xu M, Liu P, Ren R, Ma W. Role of miR-24, furin, and transforming growth factor-beta1 signal pathway in fibrosis after cardiac infarction. Med Sci Monit. 2017;23:65–70. doi: 10.12659/MSM.898641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan J, Liu WQ, Xing MQ, Shi Y, Tan XY, Jiang CQ, Dai HY. Elevated expression of periostin in diabetic cardiomyopathy and the effect of valsartan. BMC Cardiovasc Disord. 2015;15:90. doi: 10.1186/s12872-015-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suematsu Y, Miura SI, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail. 2016;1:2. doi: 10.1002/ejhf.474. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Li GN, Xie J, Li R, Chen QH, Chen JZ, Wei ZH, Kang LN, Xu B. Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-beta/periostin pathway in STZ-induced diabetic mice. BMC Cardiovasc Disord. 2016;16:5. doi: 10.1186/s12872-015-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amaral LS, Silva FA, Correia VB, Andrade CE, Dutra BA, Oliveira MV, de Magalhaes AC, Volpini RA, Seguro AC, Coimbra TM, Soares Tde J. Beneficial effects of previous exercise training on renal changes in streptozotocin-induced diabetic female rats. Exp Biol Med (Maywood) 2016;241(4):437–445. doi: 10.1177/1535370215609696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51(4):600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ares-Carrasco S, Picatoste B, Benito-Martin A, Zubiri I, Sanz AB, Sanchez-Nino MD, Ortiz A, Egido J, Tunon J, Lorenzo O. Myocardial fibrosis and apoptosis, but not inflammation, are present in long-term experimental diabetes. Am J Physiol Heart Circ Physiol. 2009;297(6):H2109–H2119. doi: 10.1152/ajpheart.00157.2009. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto C, Hayakawa Y, Aoyama T, Komaki H, Minatoguchi S, Iwasa M, Yamada Y, Kanamori H, Kawasaki M, Nishigaki K, Mikami A, Minatoguchi S. Excessively low salt diet damages the heart through activation of cardiac (pro) renin receptor, renin-angiotensin-aldosterone, and sympatho-adrenal systems in spontaneously hypertensive rats. PLoS One. 2017;12(12):e0189099. doi: 10.1371/journal.pone.0189099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin Q, Feng J, Hu C, Chen X, Qin L, Li Y. Low-intensity aerobic exercise mitigates exercise-induced bronchoconstriction by improving the function of adrenal medullary chromaffin cells in asthmatic rats. Tohoku J Exp Med. 2014;234(2):99–110. doi: 10.1620/tjem.234.99. [DOI] [PubMed] [Google Scholar]
- 42.Zhang CD, Tian Y, Song L, Zhang CP. The influence of adrenaline on the expression of TGF-beta1, bFGF and I procollagen for hypertrophic scar. Zhonghua Zheng Xing Wai Ke Za Zhi. 2005;21(6):440–444. [PubMed] [Google Scholar]
- 43.Ma J, Ma SY, Ding CH. Curcumin reduces cardiac fibrosis by inhibiting myofibroblast differentiation and decreasing transforming growth factor beta1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinase 1. Chin J Integr Med. 2017;23(5):362–369. doi: 10.1007/s11655-015-2159-5. [DOI] [PubMed] [Google Scholar]
- 44.Silva FS, Bortolin RH, Araujo DN, Marques DES, Lima J, Rezende AA, Vieira WHB, Silva NB, Medeiros KCP, Ackermann PW, Abreu BJ, Dias FAL. Exercise training ameliorates matrix metalloproteinases 2 and 9 messenger RNA expression and mitigates adverse left ventricular remodeling in streptozotocin-induced diabetic rats. Cardiovasc Pathol. 2017;29:37–44. doi: 10.1016/j.carpath.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Lee TW, Kao YH, Lee TI, Chang CJ, Lien GS, Chen YJ. Calcitriol modulates receptor for advanced glycation end products (RAGE) in diabetic hearts. Int J Cardiol. 2014;173(2):236–241. doi: 10.1016/j.ijcard.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 46.Ramazani Y, Knops N, Elmonem MA, Nguyen TQ, Arcolino FO, van den Heuvel L, Levtchenko E, Kuypers D, Goldschmeding R. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018;68–69:44–66. doi: 10.1016/j.matbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, Hochhauser E. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1alpha’. Cardiovasc Diabetol. 2018;17(1):111. doi: 10.1186/s12933-018-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newsholme P, Homem De Bittencourt PI, De Vito G, Murphy C, Krause MS. Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide. Clin Sci (Lond) 2009;118(5):341–349. doi: 10.1042/cs20090433. [DOI] [PubMed] [Google Scholar]
- 49.Teshima Y, Takahashi N, Nishio S, Saito S, Kondo H, Fukui A, Aoki K, Yufu K, Nakagawa M, Saikawa T. Production of reactive oxygen species in the diabetic heart. Roles of mitochondria and NADPH oxidase. Circ J. 2014;78(2):300–306. doi: 10.1253/circj.CJ-13-1187. [DOI] [PubMed] [Google Scholar]
- 50.Yu H, Huang J, Wang S, Zhao G, Jiao X, Zhu L. Overexpression of Smad7 suppressed ROS/MMP9-dependent collagen synthesis through regulation of heme oxygenase-1. Mol Biol Rep. 2013;40(9):5307–5314. doi: 10.1007/s11033-013-2631-2. [DOI] [PubMed] [Google Scholar]
- 51.Richter K, Kietzmann T. Reactive oxygen species and fibrosis: further evidence of a significant liaison. Cell Tissue Res. 2016;365(3):591–605. doi: 10.1007/s00441-016-2445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan LL, Liu XH, Shen YQ, Wang NZ, Xu J, Wu D, Xiong QH, Deng HY, Huang GY, Zhu YZ. Inhibition of NADPH oxidase 4-related signaling by sodium hydrosulfide attenuates myocardial fibrotic response. Int J Cardiol. 2013;168(4):3770–3778. doi: 10.1016/j.ijcard.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Gimenes C, Gimenes R, Rosa CM, Xavier NP, Campos DH, Fernandes AA, Cezar MD, Guirado GN, Cicogna AC, Takamoto AH, Okoshi MP, Okoshi K. Low intensity physical exercise attenuates cardiac remodeling and myocardial oxidative stress and dysfunction in diabetic rats. J Diabetes Res. 2015;2015:457848. doi: 10.1155/2015/457848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu XP, Wang HJ, Wang YL, Shen HR, Tan YZ. Serelaxin inhibits differentiation and fibrotic behaviors of cardiac fibroblasts by suppressing ALK-5/Smad2/3 signaling pathway. Exp Cell Res. 2018;362(1):17–27. doi: 10.1016/j.yexcr.2017.10.004. [DOI] [PubMed] [Google Scholar]