Abstract
Conserved mitogen-activated protein kinase (MAPK) signaling pathways are major mechanisms through which cells perceive and respond properly to their surrounding environment. Such homeostatic responses maintain the life of the organism. Since errors in MAPK signaling pathways can lead to cancers and to defects in immune responses, in the nervous system and metabolism, these pathways have been extensively studied as potential therapeutic targets. Although much has been studied about the roles of MAPKs in various cellular functions, less is known regarding regulation of MAPK in living organisms. This review will focus on the latest understanding of the dynamic regulation of MAPK signaling in intact cells that was revealed by using novel fluorescence imaging techniques and advanced systems-analytical methods. These techniques allowed quantitative analyses of signal transduction in situ with high spatio-temporal resolution and have revealed the nature of the molecular dynamics that determine cellular responses and fates.
Keywords: MAPK, Fluorescence imaging, Systems analysis, Environmental response
Introduction
Intracellular signaling events in cells of living organisms regulate a vast array of cellular functions to maintain the life of the organisms. Cells must obtain proper information about the microenvironment surrounding them and adapt to this by inducing gene expression, cellular proliferation, differentiation or even cell death. Cells sense chemical or physical environments, pass this information to the inside of cells through the plasma membrane, process information according to the cellular context and induce a proper cellular response during the decision-making process.
Accumulating evidence based on genetic and molecular biological studies has proven that a large number of molecules are involved in this process. More recent progress in high-throughput analysis of cellular signaling molecules has added evidence of complex connections and regulations among molecules that comprise a huge intracellular signaling network whose regulation is far beyond our imagination. Due to the technical limitations of the previous methods used for analysis, how those molecules behave in cells of living intact animals has long been unknown.
In the last 3 decades, application of fluorescent proteins in analyzing intracellular signaling by fluorescence imaging has been steadily developed and improved. As a consequence, the spatiotemporal dynamic behaviors of these signaling molecules are finally beginning to be unraveled. The aim of this review is to survey recent advancements in our understanding of the regulation and function of the dynamically behaving signaling molecules that occur in vivo. Based on recent studies on MAPK signaling dynamics, how and why cells utilize such dynamic signaling will be described. The imaging methods indispensable for analyzing the dynamic signaling in vivo will be also introduced in this review.
Versatile MAPK signaling in eukaryotes
A conserved three-tiered cascade of kinases
Signaling by the MAPK family is a major mechanism through which cells respond to a variety of stimuli from the extracellular environment [1, 2]. Molecules that comprise the core of MAPK signaling are evolutionarily conserved among eukaryotes from yeasts to mammals. MAPKs are activated via a three-tiered cascade of kinases, which is composed of a MAPK, a MAPK kinase (MAP2K) and a MAPK kinase-kinase (MAP3K). Several subfamilies of MAPK cascades coexist in mammalian cells: the growth-promoting extracellular signal-regulated kinase (ERK) family and growth-suppressing stress-activated protein kinase (SAPK) families, namely the c-jun N-terminal kinase (JNK) and p38 families. The relatively recently discovered ERK5 is also ubiquitously expressed in mammalian cells. In addition, atypical MAPK families (ERK3/4, ERK7/8) that have distinct regulation and functions were also discovered. Although we mainly focus on conventional MAPKs in this review, interested readers are encouraged to consult comprehensive reviews [3, 4].
Regulation of MAPK subfamilies
Of these MAPK family cascades, the ERK family cascade is the best studied. In the ERK cascade, a cell surface receptor is first stimulated by a growth factor (i.e., a mitogen). The activated receptor (typically a tyrosine kinase-type receptor such as epidermal growth factor receptor, fibroblast growth factor receptor or platelet-derived growth factor receptor) then induces activation of the small G protein Ras, which activates the MAP3K Raf. Activated Raf then phosphorylates its cognate MAP2Ks (MEK1/2), which subsequently phosphorylate a downstream MAPKs (ERK1/2). In this manner, information from growth factors or mitogens outside the cells is transmitted to the cytoplasm and the nucleus in the form of activated ERK.
The JNK and p38 MAPK family cascades are initiated by physiological mediators such as transforming growth factor-ß (TGF-ß), tumor necrosis factor-α (TNF-α) and interleukin-1ß (IL-1ß) as well as by environmental (physical and chemical) stresses such as ultraviolet light, gamma rays, translation inhibitors, hyperosmotic stress and oxidative stress. Similarly to the ERK cascade, small GTPases act upstream of the p38 and JNK cascades, but in these cases the relevant GTPases are Cdc42, Rac and Rho. The specific stress-activated MAP2Ks are activated by diverse MAP3Ks including MEKK1/2/3, MTK1, TAK1, ASKs, MLKs and TAOs [5]. The p38 kinase is activated mainly by the MKK3 and MKK6 MAP2Ks, whereas JNK is activated by the MKK4 and MKK7 MAP2Ks. Involvement of a large number of upstream stress MAP3Ks in these cascades presumably reflects the diversity of the physiological mediators and stress stimuli that activate these cascades [6]. However, the molecular mechanisms by which various stress stimuli activate MAP3K are still largely unknown. Many of the stress MAP3Ks are shared in the JNK and p38 pathways, and some of them (MEKK2/3) are also commonly utilized in the ERK5 pathway. Actually, ERK5 can respond to several types of stresses as well as to growth factors [4] (Fig. 1).
Fig. 1.
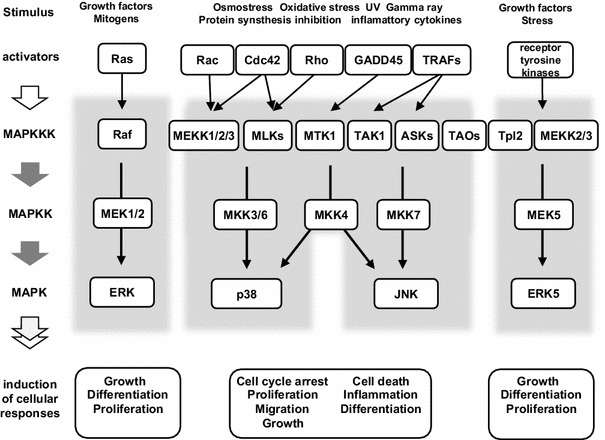
Schematic diagrams of conventional MAPK signaling cascades. Mammalian cells simultaneously express several subfamilies of MAPK cascades. They include the growth-promoting extracellular-signal regulated kinase (ERK) family and the growth-suppressing stress-activated protein kinase (SAPK) families, the JNK and p38, and the relatively recently discovered ERK5. The ERK family cascade consists of a three-tired kinase cascade of a MAP3K (Raf), MAP2K (MEK) and MAPK (ERK). Activation of a small-G protein Ras triggers the activation of Raf and thus begins the cascade. Similarly, a wide variety of stresses activates the SAPK cascades via diverse stress MAP3Ks and their activators. Although there are at least 14 stress MAP3Ks, many fewer MAP2Ks (MKK3/4/6/7) and MAPKs (p38α/ß/γ/δ and JNK1/2/3) function in the stress response pathway. The activators of stress MAP3Ks include the small-G proteins (Rac, Rho and Cdc42), TRAFs and GADD45s. The MEK5-ERK5 pathway is also activated by several types of stresses and growth factors and shares some stress MAP3Ks (MEKK2/3) with JNK and p38. Arrows indicate the activation signal. Abbreviations used in this figure are as follows: MEK MAPK/ERK kinase, MEKK MEK kinase, MLK mixed-lineage kinase, TAK1 transforming growth factor ß-activated kinase, MTK1 MAP three kinase 1, ASK apoptosis signal-regulating kinase, TAO thousand and one amino-acid kinase, Tpl tumor progression locus, TRAF tumor necrosis factor receptor-associated factor, GADD growth arrest and DNA damage-inducible protein
Specific docking domains and scaffold proteins
The specificity of a signaling cascade should be maintained throughout the cascade. It is therefore of interest to determine how the signal of an activated MAP3K is correctly transmitted to its cognate MAP2K and MAPK and how closely related (homologous) MAP2Ks and MAPKs are discriminated against. In general, the interaction between the catalytic center of a kinase and phospho-acceptor site of a substrate is an important factor for determining the specificity of their interaction. Indeed, mammalian MAPKs specifically phosphorylate a Ser/Thr-Pro motif in their substrates, and MAP2Ks phosphorylate threonine and tyrosine residues in the Thr-Glu-Tyr, Thr-Gly-Tyr or Thr-Pro-Tyr motifs of their substrates [7, 8]. In addition to the substrate site specificity, several other mechanisms ensure a specific kinase-substrate interaction in the MAPK cascades. A docking domain located at the C-terminus of MAP2Ks (the DVD domain) allows specific interaction between MAP3Ks and their cognate MAP2Ks [9]. Similarly, a conserved CD docking domain of MAPKs binds to the MAPK-binding domain (the D-domain) of the MAP2Ks, MAPK phosphatases and MAPK substrates [10]. In addition to the D-domain, ERK-MAPK recognizes another docking motif (DEF domain), and both the D-domain and DEF domain contribute to binding specificity to ERK [4]. In fact, the fidelity of the signaling cascade can be achieved by specific docking interactions between the kinases and their substrates [11–14]. Specific physical interactions between components of the cascade can also be achieved by scaffold proteins. Scaffold proteins, such as KSR-1 and ß-arrestin, bind the MAPK, MAP2K and MAP3K of a particular cascade and bring them together to form a functional signaling complex [15–17]. The scaffold complexes may also include upstream GTPases and cytoskeletal components as well as cell surface receptors [18–20].
Visualization of signal transduction
Monitoring the dynamics of signaling molecules using fluorescent proteins
Rapid advances in fluorescence technology have made a new trend in biological studies toward analysis of the behavior of a molecule in live cells, tissues and organisms under physiologically relevant conditions instead of analysis using fixed samples. In particular, the use and application of fluorescent proteins (FPs) have been significantly improved in recent years so that FPs are now indispensable tools to study intracellular signal transduction. We will therefore first describe FP-based imaging methods.
Signaling molecules often change their properties upon activation, including changes in subcellular localization, protein-protein interaction (or interaction with other molecules such as DNA or RNA) and protein conformation. In addition, changes in the status of post-transcriptional modifications (such as phosphorylation, acetylation, glycosylation or ubiquitination) are also frequent events in signal activation. For example, ERK resides in the cytoplasm in its resting state, but translocates into the nucleus upon activation by MEK. To monitor the ERK localization in cells, an approach is to exogenously express an ERK-green fluorescent protein (GFP) fusion protein in cells and to obtain time-lapse fluorescence images in real time [21, 22]. Similarly, changes in the subcellular distribution of almost any molecule can be monitored using a fairly simple method (Fig. 2a). Currently, a variety of FP colors has been developed whose range spans almost all visible wavelengths [23] as well as invisible ones near infrared [24, 25]. The wide selection of FPs allows multiplex analyses by using several differently labeled molecules simultaneously [26]. FPs with unusual photo-physical properties have also been developed. Ando and colleagues, for example, developed a reversible light-switchable FP (Dronpa) [27]. By using Dronpa, they succeeded in measuring the rates of ERK nuclear import and export separately, a difficult problem to address using previous methods. More recently, super-resolution imaging techniques using the light-switchable FP have been developed, and the techniques permit us to achieve image resolution at ~20 nm beyond the diffraction limit [28]. The invention and use of novel FPs [29] have therefore enabled much deeper understanding of how intracellular molecules are dynamically regulated in a cell.
Fig. 2.
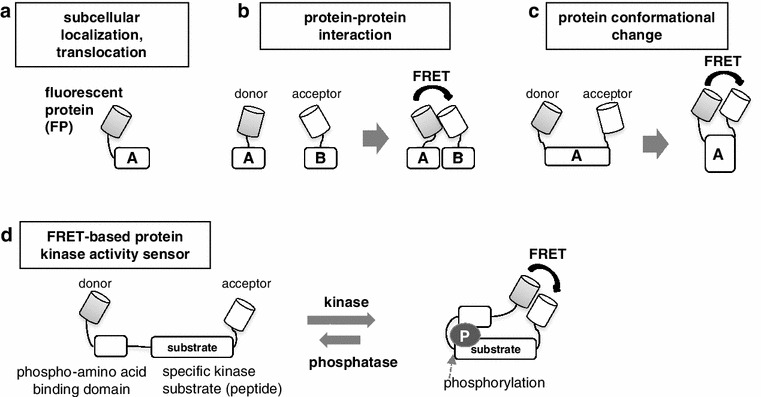
FP-based analysis of cellular signaling. The behavior of a molecule in a living cell can be analyzed using fluorescent proteins (FPs). a A subcellular localization (or translocation) of a protein can be visualized by expressing FP-fused protein in cells. b The intermolecular interaction can be optically measured as changes in the FRET efficiency between two fused FPs, respectively, to the two target proteins. c The intramolecular protein conformational change can be detected by measuring the FRET efficiency between two FPs fused to the same target protein. d Measurement of protein kinase activity by a FRET-based sensor. The sensor includes a phosphorylation site specific to the kinase and the phospho-amino acid binding domain as well as two FPs located, respectively, at the N and C termini. Phosphorylation of the substrate region by an endogenous kinase allows it to bind to the phospho-amino acid binding domain within the sensor, and the resulting intramolecular conformational change between two FPs can be detected as an increase in FRET efficiency. Similarly, other post-translational modifications, such as glycosylation, methylation and ubiquitination, can also be visualized by including a specific binding domain for the modified peptide in place of the phospho-amino acid binding domain
FP-based FRET imaging
Signaling events are often accompanied by intermolecular interactions. Thus, methods have been devised based on the Förster resonance energy transfer (FRET) to determine how protein-protein interactions occur in situ [30]. FRET is a phenomenon in which an excited donor fluorophore transmits its energy to a neighboring acceptor fluorophore without irradiative emission, thereby inducing fluorescence emission from the acceptor fluorophore. Therefore, FRET allows optical measurement of changes in the distance between two molecules. For example, when cyan FP and yellow FP are positioned close together, the light-excited cyan FP (donor), instead of emitting blue radiation, transfers the energy to the yellow FP (acceptor), which then emits yellow light. The efficiency of FRET is mainly determined by three factors under the given experimental conditions: (1) the distance between the donor and acceptor, (2) the orientation factor, which is determined by the directions of the emission transition dipole of the donor and the absorption transition dipole of the acceptor, and (3) the spectral overlap of emission and excitation wavelengths of the two fluorophores (see review [30] for details). By expressing FP-fused target proteins in living cells, changes in the distances (and orientations) of the target molecules can be optically measured as a change in FRET efficiency (Fig. 2b). Importantly, FRET efficiency is inversely proportional to the sixth power of the distance and highly sensitive to distance changes in the range of 1–10 nm. Since this distance range is also suitable for detecting conformational changes within proteins, a change in the intramolecular conformation can be detected by FRET analysis by fusing both donor and acceptor FPs to the same target protein (Fig. 2c).
Monitoring the endogenous activity of the signaling molecules using FRET biosensors
Sophisticated FRET-based biosensors have been developed that allow detection of endogenous activities of signaling molecules. Biosensors for Ca2+ [31], PKA [32] and small GTPases [33, 34] were some of the earliest sensors to be developed. Small GTPase sensors have been useful for analysis of MAPK activation. The active GTP-bound form of the small GTPase Ras binds to and activates the MAP3K Raf. Thus, activation of Ras can be detected by monitoring the Ras-Raf interaction. In 2001, Mochizuki and colleagues developed a single-molecule FRET sensor for Ras activation that consists of yellow FP, Ras, Raf and cyan FP connected in series with optimized amino acid linkers. Using this sensor, they succeeded in visualizing growth factor-dependent Ras activation in living cells [33]. Soon thereafter, biosensors for endogenous kinase activity were reported [35]. Kinase activity sensors in general include a phosphorylation site specific to the kinase linked in tandem to the phospho-amino acid binding domain and two FPs (cyan and yellow) at the N and C termini, respectively (Fig. 2d). Phosphorylation of the substrate site by endogenous kinase allows it to bind to the phospho-amino acid binding domain in the sensor, which leads to a closer association between the FPs. Thus, endogenous kinase activity can be measured as an increase in FRET efficiency. Kinase activity sensors for ERK (i.e., ERKus [36], EKAR [37] and ERKy [38]) and JNK (i.e., dJUN-FRET [39], JNKAR1 [40] and JuCKY [41] ) have been reported. A reporter for stress-responsive MAP3K (SAP3K) was also developed in a similar manner, but it included a modification to the general kinase reporter [42]. In the SAP3 K reporter, the C-lobe region of a MAP2K (MKK6) was used instead of the short substrate peptide in the MAPK reporter because inclusion of the MAP3K-specific docking site in the C-lobe region of MAP2K increases the efficiency and specificity of MAP3K-dependent phosphorylation [9]. Representative FRET-based observations on MAPKs and their related signaling are summarized in Table 1.
Table 1.
Summary of representative FRET imaging for MAPKs and related signaling
| Target | FRET sensor | Sensor type | Stimulation/application (Cells) |
Author | Year | References |
|---|---|---|---|---|---|---|
|
MEKK3, OSM (scaffold) |
– | Intermolecular FRET | Osmostress (Cos-7) | Uhlik et al. | 2003 | [67] |
| SAP3Ks | SAP3K reporter | Kinase activity sensor | Osmostress, DNA damage, UV-C, TNF-α (Cos-7, Hela) | Tomida et al. | 2009 | [42] |
| Ras, Rap1 | Raichu-Ras, Raichu-Rap1 | Intramolecular FRET | EGF, NGF (Cos-1, PC12) | Mochizuki et al. | 2001 | [33] |
| Rac, Cdc42 | Raichu-Rac, Raichu-Cdc42 | Intramolecular FRET | Cell migration (HT1080) | Itoh et al. | 2002 | [34] |
| c-Raf | Prin-c-Raf | Intramolecular FRET | EGF (Cos-1) | Terai et al. | 2005 | [68] |
| ERK | ERKus | Kinase activity sensor | EGF (MCF-7) | Sato et al. | 2007 | [36] |
| ERK | EKAR | Kinase activity sensor | Electrically evoked action potentials (hippocampal neuron) | Harvey et al. | 2008 | [37] |
| ERK (C. elegans MPK-1) | ERKy | Kinase activity sensor | In vivo imaging, NaCl-off stimulation (C. elegans sensory neuron) | Tomida et al. | 2012 | [38] |
| ERK, JNK | EKAR-EV, JNKAR1-EV | Kinase activity sensor | EGF (Hela) | Komatsu et al. | 2011 | [48] |
| ERK | EKAR-EV | Kinase activity sensor | EGF (MCF-10A) | Albeck et al. | 2013 | [104] |
| ERK | EKAREV, -NLS | Kinase activity sensor | In vivo imaging, laser ablation (mouse auricular epidermis/dermis) | Kamioka et al. | 2012 | [98] |
| ERK | EKAREV-NES, -NLS | Kinase activity sensor | In vivo imaging (mouse mammary gland epithelial cells), fetal bovine serum (MDCK, NRK-52E, Hela, etc.) | Aoki et al. | 2013 | [105] |
| JNK (Drosophila) | dJun-FRET | kinase activity sensor | RNAi screening (Drosophila BG-2) | Bakal et al. | 2008 | [39] |
| JNK | JNKAR1 | Kinase activity sensor | Anisomycin, osmostress, TNF-α (Hela) | Fosbrink et al. | 2010 | [40] |
| JNK | JuCKY | Kinase activity sensor | Anisomycin (Hela) | Suzuki et al. | 2010 | [41] |
| Ras | FRas-F | Intermolecular FRET | Electrically evoked action potentials (hippocampal neuron) | Yausda et al. | 2006 | [73] |
| Ras | FRas-F | Intermolecular FRET | 2-photon glutamate uncaging (hippocampal neuron) | Harvey et al. | 2008 | [75] |
| ERK | EKARnuc | Kinase activity sensor | 2-photon glutamate uncaging (hippocampal neuron) | Zhai et al. | 2013 | [76] |
Advanced methods for the generation and use of FRET sensors
Development of a FRET sensor is a laborious process since even a single amino-acid substitution might greatly affect its properties because of the high sensitivity of the FRET efficiency to the distance between the FPs and their relative orientation. To mitigate this difficulty, methods have been reported that rationally design FRET sensors or optimize their sensitivity. These include modification of the FPs themselves [43–47] and optimization of the arrangement of domains and linker regions in between the FPs [48–50]. An increasing number of FP-based FRET reporters now allow detection of a variety of molecular activities as well as a variety of environmental factors such as membrane potential [51–53], pH [54], temperature [55] and Redox states [56–59]. For the list of recently developed sensors, see the comprehensive reviews [60, 61].
To determine how cells recognize and properly adapt to their surrounding environment, multiparametric analysis will be helpful for understanding how environmental information is transduced from one molecule to another. For this, several methods have also been developed that allow simultaneous imaging of one FRET sensor together with a second FRET sensor [62–65] (for a review, see [66]).
It should be noted that the FRET signal might result in false negatives attributed to the inadequate orientation of the FPs even when the two FP-fused proteins are interacting and the donor and acceptor are in close proximity. Conversely, intrinsic weak affinity of FPs for each other may cause a false-positive FRET signal especially when the effective concentration of the sensor is increased, for example, by anchoring the sensor to the microdomain of the plasma membrane [43]. The FP fused protein becomes substantially larger than the corresponding endogenous protein, and the attached FP might interrupt naturally occurring protein-protein interactions. As for any other kind of sensor, it is important to confirm that the sensor selectively and accurately reports the status of endogenous protein activity. It should be additionally emphasized that the observed signal results from mutual interactions between the sensor and the endogenous signaling molecules, hence suffer from the artifact attributed to the expression of the sensor by itself. For example, the intracellular signaling might be perturbed by the stoichiometric changes due to overexpression of the sensor.
The advantages of FP-based FRET over other reporters (i.e., chemical fluorescent dyes) are that these genetically encoded sensors can be easily transferred into target cells by means of virus infection, DNA (RNA) transfection or electroporation not only in cultured cells, but also in intact animal cells, and that their expression can be precisely controlled using tissue-specific or inducible promoters.
Spatial regulation of MAPK
Stimulus-specific distinction in the MAPK activation locus
Important questions in MAPK signaling are how MAPKs discriminate between types of stimuli and how they induce a proper adaptive response. FRET studies have revealed the importance of the localization of signaling proteins to appropriate subcellular compartments for their function. Uhlik and colleagues analyzed how intracellular p38 signaling is initiated in cells during hyperosmotic sorbitol stimulation [67]. For this, they explored protein-protein interactions of signaling components in situ by FRET imaging. They showed that MEKK3 (MAP3K) forms a complex with a novel scaffold protein (OSM) in the cytoplasm and that this complex rapidly moves to actin-containing membrane ruffles upon hyperosmotic sorbitol cellular stimulation, where Rac-MEKK3–MKK3 was activated in a series upstream of p38. This study suggested that signaling proteins can be activated only at a specific locus depending on the type of stress applied to the cell. Consistent with this idea, live-cell imaging of stress-MAP3K activity using a novel FRET reporter showed that hyperosmotic stress induced MAP3K activity mainly in the plasma membrane, whereas ultraviolet light or a ribotoxic protein synthesis inhibitor induced MAP3K activation in the cytoplasm [42]. Such regulation of activity by subcellular localization seems to be a common mechanism in MAPK activation. Terai and colleagues visualized a conformational change in c-Raf (a MAP3K of the ERK pathway) upon activation by FRET [68]. They demonstrated that cytoplasmic c-Raf was recruited to the plasma membrane upon EGF stimulation, where binding to Ras induced a conformational change in c-Raf resulting in its activation. Thus, Ras binding opens up c-Raf to expose the docking site for MEK. Differences in the accessibility of regulatory proteins such as small GTPases, kinases and phosphatases, or differences in protein concentration that are due to differential cellular localization of MAPKs, might confer a different sensitivity to MAPK modules [69]. In this way, stimulation input can take place depending on the location where the MAPK module is activated. These studies clearly pointed out that MAPK signaling can be initiated from a specific subcellular locus and that stimulus-specific distinctions in MAPK activation loci would lead to different outcomes.
Visualization of Ras-MAPK activation in neuronal microcompartments
The importance of subcellular localization of signaling has also been extensively studied in neuronal cells. ERK is involved in many forms of synaptic plasticity and has therefore been proposed to be a key determinant of learning and memory [70, 71]. Various targets of the ERK kinase activity have been discovered in neuronal plasma membranes, spines, axons and nuclei. These targets include ion channels (i.e., the Kv4.2 channel and AMPA receptor), cytoskeleton regulators (i.e., focal adhesion kinase and Rho) and transcription factors [i.e., cyclic AMP-responsive element-binding protein (CREB) and Elk-1] (for details, see a comprehensive review [72]). A major difficulty in imaging analyses of neurons are their minute size, and quantification of FRET in neuronal microcompartments has been especially challenging. Instead of using conventional fluorescence intensity-based FRET measurement, Yasuda and colleagues carried out quantitative FRET imaging of Ras activity in microcompartments of a neuron using fluorescence lifetime measurements combined with two-photon excitation laser scanning microscopy (2p-FLIM FRET) [73]. Fluorescence lifetime imaging is based on the detection of time-resolved fluorescence decay after an ultra-short pulsatile excitation of the fluorophore (for details, see this review [74]). Harvey and colleagues demonstrated that Ras signaling can spread from an LTP (long-term potentiation)-induced spine along dendrites as long as ~10 µm [75]. The same group also succeeded in imaging ERK activity using 2p-FLIM FRET [37]. Zhai and colleagues further investigated the prerequisite conditions for nuclear ERK activation and found that induction of LTP in a relatively small number of dendritic spines is sufficient to maintain nuclear ERK activity as well as activation of downstream transcription factors [76]. These studies clearly demonstrated that the compartmentalized signal activation defines the extent and duration of the subsequent signaling according to the spatial parameters (i.e., length of the axon, location of the spine and distance from the nucleus). In addition to the roles of ERK in synaptic plasticity, many studies have also shown that MAPKs are involved in axonal growth, synapse development, apoptosis, degeneration and re-generation after injury [77–80] (for details, see the comprehensive reviews [81, 82]). Undoubtedly, MAPK signaling can function as a potential target in terms of medical treatment of neurological disorders (details are reviewed in [83]). Although the mechanism by which MAPK signaling contributes to those neuronal functions is still largely unknown, the increasing number of imaging tools will facilitate understanding of the dynamic nature of signal transduction in living neurons.
Temporal regulation of MAPKs
Cell fate determination by the duration of MAPK activity
Another important factor that determines MAPK signaling is timing. Many biochemical studies have pointed out that MAPK activities occur in either a transient or a sustained manner depending on the type of stimulation. One of the best known examples of this type of regulation is signaling in the neuroendocrine cell line PC12, which has distinct outcomes resulting from specific stimulation. Treatment of PC12 cells with EGF induces transient ERK activation and cell proliferation, whereas treatment with NGF causes relatively persistent ERK activation and the cells differentiate into neuron-like cells [84, 85]. A similar phenomenon has been reported for the stress MAPK pathway. TNF induces JNK in a biphasic manner with robust initial transient activation (<1 h) followed by relatively lower sustained activation (1–6 h). Ventura and colleagues hypothesized that the time course of JNK signaling may play a role in the determination of a specific outcome. They utilized a chemical genetic approach to specifically manipulate the catalytic activity of JNK at a fixed time and found that apoptotic signaling induced by JNK required sustained activation and that transient activation of JNK induced cell survival signaling [86]. Thus, the duration of MAPK activity is as important as the extent of MAPK activity in determining cellular responses.
Systems analysis of MAPK signaling based on FP imaging
Owing to recent progress in the so-called “omics” analyses, such as transcriptome, interactome and phospho-proteome analyses using mass spectroscopy and to high-throughput gene expression analysis, a tremendous number of connections between signaling molecules that are involved in their regulation have been revealed [87] (for details, see the reviews [88, 89]). This information has forced a revision of our understanding of signal transduction, suggesting that it would be more appropriate to consider that MAPKs function as part of a large signaling network that encompasses signaling of the entire cell rather than a simple straightforward modular kinase cascade. To date, mechanisms and regulation of MAPK activation have been elucidated in detail, and it is becoming possible to predict the dynamics of MAPK activation in silico [90–93]. However, explanations of MAPK dynamics that occur in actual cells under physiologically relevant conditions have not yet been elucidated.
Identification of the core regulatory mechanism of MAPK dynamics in living cells was first successfully addressed using yeast cells. Mettetal and colleagues applied a system identification method combined with real-time imaging to analyze the temporal regulation of yeast osmo-responding Hog1 MAPK signaling [94]. They stimulated the cell with repeated salt shock pulses and simultaneously measured the kinetics of nuclear translocation (activation) of a Hog1-FP fusion protein. By analysis of the stimulation-frequency dependence of the Hog1 response, the authors succeeded in identifying regulatory negative feedback loops in yeast osmo-adaptive signaling. Using similar methods, Hersen and colleagues explored the time scales of signal activation and inactivation, and they demonstrated that the kinetics of Hog1 signaling are distinctly regulated depending on the type of upstream osmolality-sensing receptors [95]. The combined application of live-cell imaging and systems analysis thus allows a knowledge of the functional topology of a signaling network. Recently, transgenic animals harboring a FRET sensor have been generated allowing visualization of signaling events in situ under physiologically relevant conditions [96–98]. Tomida and colleagues, using a combination of in vivo FRET imaging with a systems-analysis method (Fig. 3), determined how information in the external cellular environment that stimulates cells is transduced in the cell of a living animal, using the nematode Caenorhabditis elegans as a model system [38]. In that study, a FRET-based ERK-family kinase sensor was created and expressed in a sensory neuron, and the nematode was then exposed to various cyclic patterns of repetitive salt stimulation in a flow chamber. This neuronal MAPK imaging analysis demonstrated that the intensity and duration of MAPK activity are determined by the temporal pattern of input stimulation, i.e., a combination of stimulation period length, stimulation pulse length and pulse frequency. The highest MAPK response was achieved following a stimulation of modest frequency, whereas a much higher frequency or less frequent stimuli resulted in transient or low-level MAPK activation. Identification of such a nonlinear relationship in which the resulting MAPK activity is not determined by the total amount of stimulation but by the adequate timing of cyclic stimulations also provided a hint regarding its regulatory mechanism. Further in vivo Ca2+ imaging analysis using this system demonstrated that a similar nonlinear Ca2+ signaling response determined the temporal MAPK dynamics in the nematode neuron [38]. These studies pointed out that an endogenous signaling system can properly respond to the outer fluctuating environment by interpreting the temporal information (i.e., frequency) of the environmental change and adequately inducing MAPK activation according to the temporal parameter.
Fig. 3.
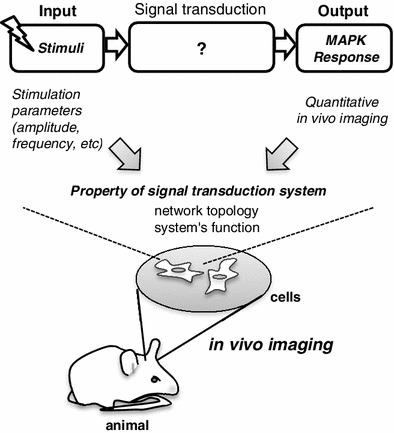
Systems analysis of signal transduction in vivo. A systems-analytical method can be combined with the in vivo imaging to study the regulation and function of the network of the signaling molecules in situ. The analysis typically consists of a defined set of input stimulations (for example, the cyclic pulsatile salt stimulation, whose parameters, such as the amplitude, frequency and duration of the stimulation pulses are varied) followed by measurement of output responses (i.e., MAPK activity) from which the system properties are deduced. An in vivo imaging of the signaling inside the target cell can be achieved using a genetically encoded sensor, which can be expressed by standard gene transferring methods (i.e., viral infection and DNA transfection)
Oscillatory MAPK activation
Recent imaging analysis further demonstrated another characteristic behavior of MAPKs. Theoretical studies of MAPK dynamics have predicted the existence of oscillatory kinase activation [99, 100]. This hypothesis was proven by studies that focused on feedback regulation of MAPKs in mammalian and yeast cells [101–105, 108]. Nakayama and colleagues investigated FGF-induced oscillatory Hes1 expression and ERK activation and found that a negative feedback phosphorylation of the guanine nucleotide exchange factor Sos (which activates Ras) by ERK [106] is required for this oscillation [101]. Shankaran and colleagues also demonstrated that EGF stimulation induces oscillatory ERK translocation between the cytosol and nucleus. They quantified temporal parameters of ERK translocation and deduced a model that suggested the involvement of a similar negative feedback loop by ERK [103]. Indeed, typical biochemical oscillators often involve a negative feedback loop with a time delay (for a review, see [107]). Albeck and colleagues [104] demonstrated that the frequency of the EGF-stimulated ERK oscillation played important roles in the regulation of cellular proliferation. Interestingly, Aoki and colleagues described another type of ERK activity oscillation that can be stochastically induced by periodic activation of Raf and that occurs under normal growth conditions. Although the detailed mechanism of how Raf is stochastically activated remains elusive, they clearly demonstrated that cellular density regulates the frequency of the stochastic ERK oscillation and, moreover, that oscillatory ERK activities induce cell proliferation signals [105]. Recently, Regot and colleagues developed the novel kinase translocation reporters (KTRs) that translocate into the nucleus upon phosphorylation by ERK, JNK and p38 MAPKs, respectively. Using these reporters, they demonstrated that ERK and JNK activities fluctuated in living cells. They further conducted multiplexed imaging analysis using KTR and other reporters that were fused to different FPs and examined the correlation and the crosstalk between different signaling pathways. Interestingly, they found that not the peak amplitude but the peak number of oscillatory ERK activities was modulated by the p38 activity [108]. Importantly, oscillatory MAPK dynamics have been difficult to address without single-cell measurement because averaging cell population responses will negate stochastic or asynchronous oscillation occurring in individual cells. Surprisingly, oscillatory activation of MAPK has also been found in the yeast Fus3 pathway [102], suggesting that some conserved regulatory mechanism may underlie the oscillatory dynamics of MAPK signaling.
Identification of temporal codes in signal transduction
As described above, analysis of the temporal regulation of MAPK signaling has suggested a universal mode of signal transduction that likely utilizes the temporal parameter as a code. The regulatory mechanisms and significance of such temporal codes of MAPK signaling under physiological circumstances are still under investigation. However, lessons from studies on other signaling systems that exhibit similar temporal behaviors, such as NF-kB, p53 and PKC signaling, would provide clues for such studies [109–112]. For example, oscillatory NF-kB determines the set of cytokine genes to be expressed according to the frequency of NF-kB activation spikes [109, 113]. When considering fluctuations in extracellular environmental signals under physiological conditions, it would be important to discriminate physiologically relevant information from surrounding noises. In this sense, temporally determined signaling (such as the frequency modulation) may be advantageous in terms of a gain in robustness against environmental noise.
Concluding remarks and perspectives
MAPK signaling functions to relay information from the outer environment in order to induce distinct cellular responses. To properly perceive and respond to fluctuating environmental changes, the signal should filter out noises, amplify physiologically relevant information, coordinate information with other incoming signals and carry this information to the proper subcellular locus where targeted molecules execute proper effector functions depending on the cellular context. As described above, recent successful quantification of molecular states under physiologically relevant conditions has revealed the roles of signaling molecules in terms of time and space in the determination of cell fates (Fig. 4). Such spatially and temporally regulated signaling would be beneficial to the cell because variable targets could be simultaneously but specifically regulated by a limited number of MAPK subtypes. Furthermore, these signals could be processed or filtered so that only physiologically relevant information is transduced. Abnormal regulation of MAPK signaling is responsible for human diseases such as cancer, autoimmune defects, neurological disorders and metabolic diseases; hence, MAPK signals are of potential importance for the diagnosis and development of therapeutic drugs. It will be beneficial to determine whether the misregulation of the spatio-temporal dynamics of the signaling is involved in various diseases and disorders using animal models. Although there have been only a few examples of in vivo signaling dynamics described to date, future advances in imaging techniques and analytical methods will accelerate the progress of research of the dynamics of the signaling within cells or within intact organs or animals.
Fig. 4.
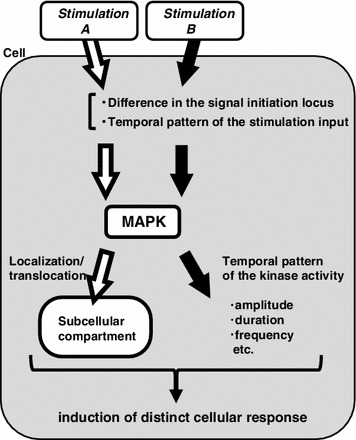
Schematic illustration of the role of dynamic MAPK signaling. The information of the outer environment (stimulation or input) can be transduced into the inside of the cells, where the network of signaling molecules transmits this information to activate the MAPK molecule. The type and location of the input stimulation, as well as its strength, duration and temporal pattern, determine the subsequent dynamics of MAPK activity. MAPK can be activated either at a specific locus or in the entire cytoplasmic region, or translocated into a specific compartment (i.e., the nucleus), to execute a proper effector function such as gene expression. Furthermore, MAPK activation can be transient, long sustained or oscillatory in manner. Subsequent cellular function can be regulated depending on the temporal parameters (i.e., oscillation frequency) of MAPK activation. Thus, a combination of a set of the spatial and temporal parameters of MAPK dynamics encodes the cellular context to induce a specific adaptive response
Acknowledgments
The author thanks Drs. Haruo Saito and Pauline O’Grady for critical reading of this review and valuable advice. The author’s work was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Conflict of interest
The author declares that there is no conflict of interest.
References
- 1.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26(22):3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 2.Avruch J. MAP kinase pathways: the first twenty years. Biochim Biophys Acta. 2007;1773(8):1150–1160. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochim Biophys Acta. 2007;1773(8):1376–1387. doi: 10.1016/j.bbamcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev: MMBR. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig EA, Stevens MV, Vaillancourt RR, Camenisch TD. MAP3Ks as central regulators of cell fate during development. Dev Dyn: Off Publ Am Assoc Anat. 2008;237(11):3102–3114. doi: 10.1002/dvdy.21750. [DOI] [PubMed] [Google Scholar]
- 6.Winter-Vann AM, Johnson GL. Integrated activation of MAP3Ks balances cell fate in response to stress. J Cell Biochem. 2007;102(4):848–858. doi: 10.1002/jcb.21522. [DOI] [PubMed] [Google Scholar]
- 7.Nakielny S, Cohen P, Wu J, Sturgill T. MAP kinase activator from insulin-stimulated skeletal muscle is a protein threonine/tyrosine kinase. EMBO J. 1992;11(6):2123–2129. doi: 10.1002/j.1460-2075.1992.tb05271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawler S, Fleming Y, Goedert M, Cohen P. Synergistic activation of SAPK1/JNK1 by two MAP kinase kinases in vitro. Curr Biol. 1998;8(25):1387–1390. doi: 10.1016/s0960-9822(98)00019-0. [DOI] [PubMed] [Google Scholar]
- 9.Takekawa M, Tatebayashi K, Saito H. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol Cell. 2005;18(3):295–306. doi: 10.1016/j.molcel.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Tanoue T, Nishida E. Molecular recognitions in the MAP kinase cascades. Cell Signal. 2003;15(5):455–462. doi: 10.1016/s0898-6568(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 11.Xia Y, Wu Z, Su B, Murray B, Karin M. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 1998;12(21):3369–3381. doi: 10.1101/gad.12.21.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SH, Galanis A, Sharrocks AD. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol Cell Biol. 1999;19(6):4028–4038. doi: 10.1128/mcb.19.6.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997;16(8):1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2(2):110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 15.Roy F, Laberge G, Douziech M, Ferland-McCollough D, Therrien M. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 2002;16(4):427–438. doi: 10.1101/gad.962902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen A, Burack WR, Stock JL, Kortum R, Chaika OV, Afkarian M, Muller WJ, Murphy KM, Morrison DK, Lewis RE, McNeish J, Shaw AS. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol Cell Biol. 2002;22(9):3035–3045. doi: 10.1128/MCB.22.9.3035-3045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science (New York) 2000;290(5496):1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 18.Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26(22):3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 19.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. TEM. 2006;17(4):159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101(8):2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 21.Rubinfeld H, Hanoch T, Seger R. Identification of a cytoplasmic-retention sequence in ERK2. J Biol Chem. 1999;274(43):30349–30352. doi: 10.1074/jbc.274.43.30349. [DOI] [PubMed] [Google Scholar]
- 22.Matsubayashi Y, Fukuda M, Nishida E. Evidence for existence of a nuclear pore complex-mediated, cytosol-independent pathway of nuclear translocation of ERK MAP kinase in permeabilized cells. J Biol Chem. 2001;276(45):41755–41760. doi: 10.1074/jbc.M106012200. [DOI] [PubMed] [Google Scholar]
- 23.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2(12):905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 24.Filonov GS, Piatkevich KD, Ting LM, Zhang J, Kim K, Verkhusha VV. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat Biotechnol. 2011;29(8):757–761. doi: 10.1038/nbt.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu X, Royant A, Lin MZ, Aguilera TA, Lev-Ram V, Steinbach PA, Tsien RY. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science (New York) 2009;324(5928):804–807. doi: 10.1126/science.1168683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kogure T, Karasawa S, Araki T, Saito K, Kinjo M, Miyawaki A. A fluorescent variant of a protein from the stony coral Montipora facilitates dual-color single-laser fluorescence cross-correlation spectroscopy. Nat Biotechnol. 2006;24(5):577–581. doi: 10.1038/nbt1207. [DOI] [PubMed] [Google Scholar]
- 27.Ando R, Mizuno H, Miyawaki A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science (New York) 2004;306(5700):1370–1373. doi: 10.1126/science.1102506. [DOI] [PubMed] [Google Scholar]
- 28.Tiwari DK, Nagai T. Smart fluorescent proteins: innovation for barrier-free superresolution imaging in living cells. Dev Growth Differ. 2013;55(4):491–507. doi: 10.1111/dgd.12064. [DOI] [PubMed] [Google Scholar]
- 29.Lippincott-Schwartz J, Patterson GH. Fluorescent proteins for photoactivation experiments. Methods Cell Biol. 2008;85:45–61. doi: 10.1016/S0091-679X(08)85003-0. [DOI] [PubMed] [Google Scholar]
- 30.Miyawaki A. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev Cell. 2003;4(3):295–305. doi: 10.1016/s1534-5807(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 31.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388(6645):882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 32.Nagai Y, Miyazaki M, Aoki R, Zama T, Inouye S, Hirose K, Iino M, Hagiwara M. A fluorescent indicator for visualizing cAMP-induced phosphorylation in vivo. Nat Biotechnol. 2000;18(3):313–316. doi: 10.1038/73767. [DOI] [PubMed] [Google Scholar]
- 33.Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, Matsuda M. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 2001;411(6841):1065–1068. doi: 10.1038/35082594. [DOI] [PubMed] [Google Scholar]
- 34.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22(18):6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato M, Ozawa T, Inukai K, Asano T, Umezawa Y. Fluorescent indicators for imaging protein phosphorylation in single living cells. Nat Biotechnol. 2002;20(3):287–294. doi: 10.1038/nbt0302-287. [DOI] [PubMed] [Google Scholar]
- 36.Sato M, Kawai Y, Umezawa Y. Genetically encoded fluorescent indicators to visualize protein phosphorylation by extracellular signal-regulated kinase in single living cells. Anal Chem. 2007;79(6):2570–2575. doi: 10.1021/ac062171d. [DOI] [PubMed] [Google Scholar]
- 37.Harvey CD, Ehrhardt AG, Cellurale C, Zhong H, Yasuda R, Davis RJ, Svoboda K. A genetically encoded fluorescent sensor of ERK activity. Proc Natl Acad Sci USA. 2008;105(49):19264–19269. doi: 10.1073/pnas.0804598105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomida T, Oda S, Takekawa M, Iino Y, Saito H. The temporal pattern of stimulation determines the extent and duration of MAPK activation in a Caenorhabditis elegans sensory neuron. Sci Signal. 2012;5(246):ra76. doi: 10.1126/scisignal.2002983. [DOI] [PubMed] [Google Scholar]
- 39.Bakal C, Linding R, Llense F, Heffern E, Martin-Blanco E, Pawson T, Perrimon N. Phosphorylation networks regulating JNK activity in diverse genetic backgrounds. Science (New York) 2008;322(5900):453–456. doi: 10.1126/science.1158739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fosbrink M, Aye-Han NN, Cheong R, Levchenko A, Zhang J. Visualization of JNK activity dynamics with a genetically encoded fluorescent biosensor. Proc Natl Acad Sci USA. 2010;107(12):5459–5464. doi: 10.1073/pnas.0909671107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki H, Sato M. Genetically encoded fluorescent indicators to visualise protein phosphorylation by c-Jun NH2-terminal kinase in living cells. Supramol Chem. 2010;22(7–8):434–439. [Google Scholar]
- 42.Tomida T, Takekawa M, O’Grady P, Saito H. Stimulus-specific distinctions in spatial and temporal dynamics of stress-activated protein kinase kinase kinases revealed by a fluorescence resonance energy transfer biosensor. Mol Cell Biol. 2009;29(22):6117–6127. doi: 10.1128/MCB.00571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science (New York) 2002;296(5569):913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 44.Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22(4):445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 45.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA. 2004;101(29):10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen AW, Daugherty PS. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat Biotechnol. 2005;23(3):355–360. doi: 10.1038/nbt1066. [DOI] [PubMed] [Google Scholar]
- 47.Vinkenborg JL, Evers TH, Reulen SW, Meijer EW, Merkx M. Enhanced sensitivity of FRET-based protease sensors by redesign of the GFP dimerization interface. Chembiochem: Eur J Chem Biol. 2007;8(10):1119–1121. doi: 10.1002/cbic.200700109. [DOI] [PubMed] [Google Scholar]
- 48.Komatsu N, Aoki K, Yamada M, Yukinaga H, Fujita Y, Kamioka Y, Matsuda M. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22(23):4647–4656. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grunberg R, Burnier JV, Ferrar T, Beltran-Sastre V, Stricher F, van der Sloot AM, Garcia-Olivas R, Mallabiabarrena A, Sanjuan X, Zimmermann T, Serrano L. Engineering of weak helper interactions for high-efficiency FRET probes. Nat Methods. 2013;10(10):1021–1027. doi: 10.1038/nmeth.2625. [DOI] [PubMed] [Google Scholar]
- 50.Fritz RD, Letzelter M, Reimann A, Martin K, Fusco L, Ritsma L, Ponsioen B, Fluri E, Schulte-Merker S, van Rheenen J, Pertz O. A versatile toolkit to produce sensitive FRET biosensors to visualize signaling in time and space. Sci Signal. 2013;6(285):rs12. doi: 10.1126/scisignal.2004135. [DOI] [PubMed] [Google Scholar]
- 51.Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat Methods. 2008;5(8):683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- 52.Lundby A, Mutoh H, Dimitrov D, Akemann W, Knopfel T. Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS One. 2008;3(6):e2514. doi: 10.1371/journal.pone.0002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akemann W, Mutoh H, Perron A, Park YK, Iwamoto Y, Knopfel T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J Neurophysiol. 2012;108(8):2323–2337. doi: 10.1152/jn.00452.2012. [DOI] [PubMed] [Google Scholar]
- 54.Esposito A, Gralle M, Dani MA, Lange D, Wouters FS. pHlameleons: a family of FRET-based protein sensors for quantitative pH imaging. Biochemistry. 2008;47(49):13115–13126. doi: 10.1021/bi8009482. [DOI] [PubMed] [Google Scholar]
- 55.Okabe K, Inada N, Gota C, Harada Y, Funatsu T, Uchiyama S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nature Commun. 2012;3:705. doi: 10.1038/ncomms1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearce LL, Gandley RE, Han W, Wasserloos K, Stitt M, Kanai AJ, McLaughlin MK, Pitt BR, Levitan ES. Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein. Proc Natl Acad Sci USA. 2000;97(1):477–482. doi: 10.1073/pnas.97.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robin E, Guzy RD, Loor G, Iwase H, Waypa GB, Marks JD, Hoek TL, Schumacker PT. Oxidant stress during simulated ischemia primes cardiomyocytes for cell death during reperfusion. J Biol Chem. 2007;282(26):19133–19143. doi: 10.1074/jbc.M701917200. [DOI] [PubMed] [Google Scholar]
- 58.Yano T, Oku M, Akeyama N, Itoyama A, Yurimoto H, Kuge S, Fujiki Y, Sakai Y. A novel fluorescent sensor protein for visualization of redox states in the cytoplasm and in peroxisomes. Mol Cell Biol. 2010;30(15):3758–3766. doi: 10.1128/MCB.00121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolossov VL, Spring BQ, Clegg RM, Henry JJ, Sokolowski A, Kenis PJ, Gaskins HR. Development of a high-dynamic range, GFP-based FRET probe sensitive to oxidative microenvironments. Exp Biol Med. 2011;236(6):681–691. doi: 10.1258/ebm.2011.011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueda Y, Kwok S, Hayashi Y. Application of FRET probes in the analysis of neuronal plasticity. Front Neural Circuits. 2013;7:163. doi: 10.3389/fncir.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiyokawa E, Aoki K, Nakamura T, Matsuda M. Spatiotemporal regulation of small GTPases as revealed by probes based on the principle of forster resonance energy transfer (FRET): implications for signaling and pharmacology. Annu Rev Pharmacol Toxicol. 2011;51:337–358. doi: 10.1146/annurev-pharmtox-010510-100234. [DOI] [PubMed] [Google Scholar]
- 62.Kawai H, Suzuki T, Kobayashi T, Sakurai H, Ohata H, Honda K, Momose K, Namekata I, Tanaka H, Shigenobu K, Nakamura R, Hayakawa T, Kawanishi T. Simultaneous real-time detection of initiator- and effector-caspase activation by double fluorescence resonance energy transfer analysis. J Pharmacol Sci. 2005;97(3):361–368. doi: 10.1254/jphs.fp0040592. [DOI] [PubMed] [Google Scholar]
- 63.H-w Ai, Hazelwood KL, Davidson MW, Campbell RE. Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat Meth. 2008;5(5):401–403. doi: 10.1038/nmeth.1207. [DOI] [PubMed] [Google Scholar]
- 64.Tomosugi W, Matsuda T, Tani T, Nemoto T, Kotera I, Saito K, Horikawa K, Nagai T. An ultramarine fluorescent protein with increased photostability and pH insensitivity. Nat Methods. 2009;6(5):351–353. doi: 10.1038/nmeth.1317. [DOI] [PubMed] [Google Scholar]
- 65.Fujii H, Inoue M, Okuno H, Sano Y, Takemoto-Kimura S, Kitamura K, Kano M, Bito H. Nonlinear decoding and asymmetric representation of neuronal input information by CaMKIIalpha and calcineurin. Cell Rep. 2013;3(4):978–987. doi: 10.1016/j.celrep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 66.Carlson HJ, Campbell RE. Genetically encoded FRET-based biosensors for multiparameter fluorescence imaging. Curr Opin Biotechnol. 2009;20(1):19–27. doi: 10.1016/j.copbio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell’Acqua ML, Johnson GL. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5(12):1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 68.Terai K, Matsuda M. Ras binding opens c-Raf to expose the docking site for mitogen-activated protein kinase kinase. EMBO Rep. 2005;6(3):251–255. doi: 10.1038/sj.embor.7400349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harding A, Tian T, Westbury E, Frische E, Hancock JF. Subcellular localization determines MAP kinase signal output. Curr Biol. 2005;15(9):869–873. doi: 10.1016/j.cub.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 70.Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 71.Davis S, Laroche S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes, Brain, Behav. 2006;5(Suppl 2):61–72. doi: 10.1111/j.1601-183X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 72.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5(3):173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 73.Yasuda R, Harvey CD, Zhong H, Sobczyk A, van Aelst L, Svoboda K. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nat Neurosci. 2006;9(2):283–291. doi: 10.1038/nn1635. [DOI] [PubMed] [Google Scholar]
- 74.Wallrabe H, Periasamy A. Imaging protein molecules using FRET and FLIM microscopy. Curr Opin Biotechnol. 2005;16(1):19–27. doi: 10.1016/j.copbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science (New York) 2008;321(5885):136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhai S, Ark ED, Parra-Bueno P, Yasuda R. Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science (New York) 2013;342(6162):1107–1111. doi: 10.1126/science.1245622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432(7019):822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- 78.Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120(3):407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 79.Lewcock JW, Genoud N, Lettieri K, Pfaff SL. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron. 2007;56(4):604–620. doi: 10.1016/j.neuron.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science (New York) 2009;323(5915):802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fulga TA, Van Vactor D. Synapses and growth cones on two sides of a highwire. Neuron. 2008;57(3):339–344. doi: 10.1016/j.neuron.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 82.Coffey ET. Nuclear and cytosolic JNK signalling in neurons. Nat Rev Neurosci. 2014;15(5):285–299. doi: 10.1038/nrn3729. [DOI] [PubMed] [Google Scholar]
- 83.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 84.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288(2):351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qui MS, Green SH. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9(4):705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- 86.Ventura JJ, Hubner A, Zhang C, Flavell RA, Shokat KM, Davis RJ. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21(5):701–710. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 87.Bandyopadhyay S, Chiang CY, Srivastava J, Gersten M, White S, Bell R, Kurschner C, Martin C, Smoot M, Sahasrabudhe S, Barber DL, Chanda SK, Ideker T. A human MAP kinase interactome. Nat Methods. 2010;7(10):801–805. doi: 10.1038/nmeth.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jensen ON. Modification-specific proteomics: characterization of post-translational modifications by mass spectrometry. Curr Opin Chem Biol. 2004;8(1):33–41. doi: 10.1016/j.cbpa.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 89.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143(7):1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schoeberl B, Eichler-Jonsson C, Gilles ED, Muller G. Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat Biotechnol. 2002;20(4):370–375. doi: 10.1038/nbt0402-370. [DOI] [PubMed] [Google Scholar]
- 91.Sasagawa S, Ozaki Y, Fujita K, Kuroda S. Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nat Cell Biol. 2005;7(4):365–373. doi: 10.1038/ncb1233. [DOI] [PubMed] [Google Scholar]
- 92.Kholodenko BN, Birtwistle MR. Four-dimensional dynamics of MAPK information processing systems. Wiley Interdiscip Rev Syst Biol Med. 2009;1(1):28–44. doi: 10.1002/wsbm.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hughey JJ, Lee TK, Covert MW. Computational modeling of mammalian signaling networks. Wiley Interdiscip Rev Syst Biol Med. 2010;2(2):194–209. doi: 10.1002/wsbm.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mettetal JT, Muzzey D, Gomez-Uribe C, van Oudenaarden A. The frequency dependence of osmo-adaptation in Saccharomyces cerevisiae. Science (New York) 2008;319(5862):482–484. doi: 10.1126/science.1151582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hersen P, McClean MN, Mahadevan L, Ramanathan S. Signal processing by the HOG MAP kinase pathway. Proc Natl Acad Sci USA. 2008;105(20):7165–7170. doi: 10.1073/pnas.0710770105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takemoto K, Kuranaga E, Tonoki A, Nagai T, Miyawaki A, Miura M. Local initiation of caspase activation in Drosophila salivary gland programmed cell death in vivo. Proc Natl Acad Sci USA. 2007;104(33):13367–13372. doi: 10.1073/pnas.0702733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, Yosida H, Miura M. Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J Cell Biol. 2011;195(6):1047–1060. doi: 10.1083/jcb.201104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamioka Y, Sumiyama K, Mizuno R, Sakai Y, Hirata E, Kiyokawa E, Matsuda M. Live imaging of protein kinase activities in transgenic mice expressing FRET biosensors. Cell Struct Funct. 2012;37(1):65–73. doi: 10.1247/csf.11045. [DOI] [PubMed] [Google Scholar]
- 99.Kholodenko BN. Negative feedback and ultrasensitivity can bring about oscillations in the mitogen-activated protein kinase cascades. Eur J Biochem/FEBS. 2000;267(6):1583–1588. doi: 10.1046/j.1432-1327.2000.01197.x. [DOI] [PubMed] [Google Scholar]
- 100.Chickarmane V, Kholodenko BN, Sauro HM. Oscillatory dynamics arising from competitive inhibition and multisite phosphorylation. J Theor Biol. 2007;244(1):68–76. doi: 10.1016/j.jtbi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 101.Nakayama K, Satoh T, Igari A, Kageyama R, Nishida E. FGF induces oscillations of Hes1 expression and Ras/ERK activation. Curr Biol. 2008;18(8):R332–R334. doi: 10.1016/j.cub.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 102.Hilioti Z, Sabbagh W, Jr, Paliwal S, Bergmann A, Goncalves MD, Bardwell L, Levchenko A. Oscillatory phosphorylation of yeast Fus3 MAP kinase controls periodic gene expression and morphogenesis. Curr Biol. 2008;18(21):1700–1706. doi: 10.1016/j.cub.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shankaran H, Ippolito DL, Chrisler WB, Resat H, Bollinger N, Opresko LK, Wiley HS. Rapid and sustained nuclear-cytoplasmic ERK oscillations induced by epidermal growth factor. Mol Syst Biol. 2009;5:332. doi: 10.1038/msb.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell. 2013;49(2):249–261. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aoki K, Kumagai Y, Sakurai A, Komatsu N, Fujita Y, Shionyu C, Matsuda M. Stochastic ERK activation induced by noise and cell-to-cell propagation regulates cell density-dependent proliferation. Mol Cell. 2013;52(4):529–540. doi: 10.1016/j.molcel.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 106.Buday L, Warne PH, Downward J. Downregulation of the Ras activation pathway by MAP kinase phosphorylation of Sos. Oncogene. 1995;11(7):1327–1331. [PubMed] [Google Scholar]
- 107.Novak B, Tyson JJ. Design principles of biochemical oscillators. Nat Rev Mol Cell Biol. 2008;9(12):981–991. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Regot S, Hughey JJ, Bajar BT, Carrasco S, Covert MW. High-sensitivity measurements of multiple kinase activities in live single cells. Cell. 2014;157(7):1724–1734. doi: 10.1016/j.cell.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, Edwards SW, McDowell HP, Unitt JF, Sullivan E, Grimley R, Benson N, Broomhead D, Kell DB, White MR. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science (New York) 2004;306(5696):704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 110.Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. Single-cell NF-kappaB dynamics reveal digital activation and analogue information processing. Nature. 2010;466(7303):267–271. doi: 10.1038/nature09145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet. 2004;36(2):147–150. doi: 10.1038/ng1293. [DOI] [PubMed] [Google Scholar]
- 112.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161(5):899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, Ryan S, Spiller DG, Unitt JF, Broomhead DS, Kell DB, Rand DA, See V, White MR. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science (New York) 2009;324(5924):242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]


