Abstract
Using a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Parkinson’s disease mouse model, we investigated protein expression changes associated with the action of electroacupuncture (EA) in the mouse striatum. Twelve-week-old male C57BL/6 mice were injected intraperitoneally with 30 mg/kg of MPTP at 24-h intervals for 5 days, and the 100-Hz EA stimulation was performed at GB34 and GB39 once a day for 12 days consecutively from the first injection. With the EA, the MPTP-induced dopaminergic neuronal destruction was reduced. Of the 13 proteins that were differentially expressed between control and MPTP treated mice, cytosolic malate dehydrogenase, munc18-1, and hydroxyacylglutathione hydrolase, which were increased by MPTP, and cytochrome c oxidase subunit Vb, which was decreased by MPTP, were restored to the level of the saline group after EA treatment. These proteins are likely related to cellular metabolism. Altogether, we propose that the EA may exert neuroprotective effects in mice striatum through reducing MPTP-induced toxicity such as oxidative stress.
Keywords: Acupuncture; Electroacupuncture; Hydroxyacylglutathione hydrolase; 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Parkinson’s disease; Striatum
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that is characterized by tremors, lack of coordination, and difficulties in walking and controlling movements. It is widely accepted that the main behavioral disturbances in PD are the consequence of a substantial loss of dopaminergic (DA) nigral neurons and a depletion of dopamine in the striatum [1]. The striatum, a subcortical part of the telencephalon, is the major input station of the basal ganglia system. With the substantia nigra (SN) pars compacta, it consists of the nigrostriatal pathway and is most affected in early PD [2]. Several agents such as 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and rotenone are widely used in animal models for PD [3]. Especially MPTP causes a dramatic loss of DA neurons, which is accompanied by a loss of DA nerve terminals in the striatum and a dramatic reduction in striatal dopamine levels [4].
Acupuncture, including electroacupuncture (EA), has been reported to have possible therapeutic effectiveness for PD in clinical trials, as manifested by improvement in the clinical symptoms like tremor [5, 6], decrease in the dosage of anti-parkinsonian drugs [6], decrease of the drug side effects [5, 6], and increase of daily living like sleep [5, 7]. In animal studies, acupuncture treatment reduced DA neuronal cell death caused by MPTP [8, 9] or 6-OHDA [10, 11], and GB34 was known to be one of the effective acupoints for protecting DA neurons in the SN and the striatum of PD animal models [8, 10]. Although acupuncture stimulation at GB34 has been shown to exert neuroprotective effects on PD models, the proteins associated with the action of the acupuncture stimulation in these models are still largely unknown.
Two-dimensional electrophoresis (2-DE) is a method for the quantitative analysis of complex protein mixtures extracted from cells, tissues, or other biological samples, and it reflects changes in protein expression level, isoforms, or post-translational modifications [12]. Using this technique, we investigated the EA-associated proteomic profile changes in the striatum in a MPTP-induced mouse model of PD.
Materials and methods
Animals and MPTP administration
This study was approved by the ethics committee of the Acupuncture and Meridian Science Research Center, and all efforts were made to minimize the number of animals and their suffering in accordance with the guidelines of the NIH and Korean Academy of Medical Sciences. Twelve-week-old male C57BL/6 mice (Orient bio, Korea), weighing 24–27 g, were used in all the experiments and were maintained on a 12-h light–dark schedule with free access to food and water. At the beginning of the experiment, the mice were randomly assigned to one of three groups: the saline group (n = 12), which would be injected with normal saline and would not receive acupuncture treatment; the MPTP group (n = 12), which would be injected with MPTP and would not receive acupuncture treatment; and the MPTP + EA group (n = 12), which would be injected with MPTP and would receive EA treatment. Animals received intraperitoneal injections of vehicle (normal saline, in saline group) or MPTP (30 mg/kg, in MPTP and MPTP + EA groups) once daily for five consecutive days [13, 14].
Procedure of electro-acupuncture stimulation
EA treatment was repeated 2 h after MPTP injections and continued once a day until 7 days after the final MPTP injection. Mice were restrained in cylindrical restrainers that were 10 cm long and had small holes in the anterior end for ventilation. The mice were partially immobilized, and the hind legs of the mice were extended out through two rear openings. Each stainless steel acupuncture needle was bent into an ‘L’ shape; the proximal end was soldered to a wire that was connected to one of the output channels of an electric stimulator, HANS LH800 (HANS, China). The bent distal ends were inserted into the GB34 and GB39. GB34 is located in the depression ventral and distal to the head of the fibula, and peroneus longus and extensor digitorum longus muscles are under the acupoint. GB39 is the distal 4/5 point on the line connecting the lateral side of the knee and the lateral malleolus of the tibiofibula, and peroneus brevis and extensor digitorum longus muscles are under the acupoint. After the insertion, the needles were fixed in situ with adhesive tape. EA was applied after the basal threshold determination. The EA parameters were set as follows: constant square wave current output (pulse width: 0.2 ms); 1-mA intensities; a frequency of 100 Hz for 20 min. The saline and MPTP groups were also immobilized for 20 min just as those in the EA group were immobilized.
Immunohistochemistry
The animals were killed by anesthesia with chloral hydrate (300 mg/kg) on day 7 after the last MPTP or saline injection and then perfused transcardially with 4% paraformaldehyde in 0.05 M phosphate buffer. The brains were removed, post-fixed, and cryoprotected. Immunohistochemistry was performed as described previously [15, 16] on free-floating cryomicrotome-cut sections (40 µm thick) that encompassed the entire striatum. After incubation with 3% H2O2 in 0.05 M phosphate-buffered saline (PBS), followed by 0.3% Triton X-100, and 3% bovine serum albumin (BSA) in 0.1 M PBS, the sections were stained overnight at room temperature using the primary antibodies: tyrosine hydroxylase (TH, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) for DA neurons, then incubated with ABC reagent (Vector Laboratories Inc., Burlingame, CA) for 1 h at room temperature, washed in PBS, and incubated for 5 min in 0.02% diaminobenzidine (DAB) and 0.003% hydrogen peroxide in 0.1 M tris–HCl-buffered saline (pH 7.5). After the DAB reaction, the tissues were rinsed with PBS, mounted on gelatin-coated slides, air-dried, dehydrated, and coverslipped. The histological pictures were taken using a bright-field BX51 microscope (Olympus, Japan) and DP70 camera (Olympus, Japan). The survival of DA neurons was quantitated in terms of the number of TH-positive cells in SN and the mean value of optical density (OD) in the striatum using Image-pro plus 6.0 (Media cybernetics, Silver Spring, MD).
Protein extraction procedure
To obtain protein extracts, mice were killed by cervical dislocation 7 days after the last MPTP or saline injection, and the striatums were dissected rapidly and frozen using liquid nitrogen and kept at −80°C until use. The frozen brain tissues were homogenized 4 times with sonication at 70% intensity in 10-s bursts with 1 ml of buffer consisting of 7 M urea, 2 M thiourea, 1% C7BzO, and 40 mM Tris. The homogenates were pelleted at 16000 g for 20 min at 15°C, and the supernatants were reduced and alkylated in 5 mM tributyl phosphine and 10 mM acrylamide monomer at room temperature for 2 h. The reaction was stopped using 10 mM dithiothreitol (DTT). The extract was precipitated using acetone and 20 mg of citric acid. The precipitation was allowed to proceed for 5 min and then centrifuged at 2500×g for 15 min at 15°C. The pellet was air-dried and resuspended in 1 ml of buffer consisting of 7 M urea, 2 M thiourea, and 1% C7BzO. The protein concentration of the final extract solution was determined using the Bradford method.
Two-dimensional gel electrophoresis
IPG dry strips were equilibrated for 12–16 h with 7 M urea, 2 M thiourea containing 2% CHAPS, 1% DTT, and 1% Pharmalyte, and the strips were loaded with 200 µg of sample. IEF was performed at 20°C using a Multiphor II electrophoresis unit and EPS 3500 X L power supply (Amersham Biosciences, Uppsala, Sweden) following the manufacturer’s instruction. For IEF, the voltage was linearly increased from 150 to 3500 V for 3 h for sample entry followed by a constant 3500 V, with the focusing complete after 96 kVh. Prior to the second dimension, strips were incubated for 10 min in equilibration buffer (50 mM Tris–Cl, pH 6.8 containing 6 M urea, 2% SDS, and 30% glycerol), first with 1% DTT and second with 2.5% iodoacetamide. The equilibrated strips were inserted onto SDS–PAGE gels (20–24 cm, 10–16%). SDS–PAGE was performed using a Hoefer DALT 2D system (Amersham Biosciences, Uppsala, Sweden) and by following the manufacturer’s instruction. The 2D gels were run at 20°C for 1700 Vh, and were next silver stained as described by Amersham Biosciences. A quantitative analysis of the digitized images was carried out using the PDQuest software (version 7.0; Bio-Rad, Hercules, CA) according to the protocols provided by the manufacturer. The quantity of each spot was normalized by the total valid spot intensity. Protein spots were selected for the significant expression of variation that deviated over around twofold in its expression level compared with the control or normal sample.
Protein identification
Protein spots showing differential expression on 2-DE gels were excised from gels and enzymatically digested in-gel with sequence grade porcine trypsin (Promega, Madison, WI). Gel pieces were washed with 50% acetonitrile (ACN) to remove SDS, salt, and protein stain. The washed gel pieces were then vacuum-dried to remove solvent, rehydrated with trypsin (8–10 ng/ml), and incubated for 8–10 h at 37°C. Proteolysis was terminated by addition of 5 µl 0.5% trifluoroacetic acid. Tryptic peptides were recovered by combining the aqueous phase from several extractions of gel pieces with 50% aqueous ACN. After concentration, the peptide mixture was desalted using a ZipTip (Millipore, Bedford, MA), and the peptides were eluted in 1–5 µl ACN. An aliquot of this solution was mixed with an equal volume of a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% aqueous ACN, and 1 µl of the mixture was spotted onto the target plate. Protein analysis was performed using an Ettan MALDITOF (Amersham Biosciences, Uppsala, Sweden). Peptides were evaporated with a nitrogen laser at 337 nm using a delayed extraction approach. The peptides were accelerated with a 20-kV injection pulse for TOF analysis. Each spectrum was the cumulative average of 300 laser shots. The search program Pro-Found, developed by Rockefeller University (http://129.85.19.192/profound_bin/WebProFound.exe), was used to search the NCBI databases. Spectra were calibrated with auto-digested trypsin ion peaks (m/z = 842.510 and 2211.1046) as internal standards. The search parameters were set up as follows: the database is NCBI no. 10.21.2003; the mass tolerance was 650 ppm; the number of missed cleavage sites allowed was up to 1; the minimum number of matched peptides was four; selected species was Mus muscus; the monoisotope masses were used; the searching range was within the experimental pI value 62 pH unit.
Western blot
For Western blotting, samples (50 µg protein) were loaded on a 15% sodium dodecyl sulfate–polyacrylamide electrophoresis gel. After separation, the proteins were transferred to a nitrocellulose membrane. The membrane was shaken for 1 h at room temperature in Tris-buffered saline (TBS) containing 0.1% Tween-20, 5% skim milk, and 0.2% BSA. The membrane was incubated for 1 h at room temperature with chicken polyclonal anti-hydroxyacylglutathione hydrolase (abcam, Cambridge, UK; 1:2000) primary antibodies in TBS containing 0.1% Tween-20. The primary antibodies were detected with a horseradish peroxidase conjugated secondary antibody (1:3000, anti-chicken; Pierce, Rockford, IL) and then visualized with enhanced chemiluminescence (Pierce, Rockford, IL). Then, these blots were re-probed with mouse monoclonal anti-β-actin antibody (Sigma, St. Louis, MO; 1:2000). The band intensity of the detected proteins was measured by densitometry.
Statistical analysis
The data were expressed as the mean ± SEM. A statistical analysis was performed with an analysis of variance (ANOVA). The significance was determined through the Scheffe post-hoc test. In all analyses, differences were considered significant at P < 0.05.
Results
The survival of DA neurons in SN and striatum
The immunohistochemistry performed in the present study revealed that MPTP injection produced a significant reduction of the TH-positive neurons in the SN and striatum. The MPTP group had significantly fewer TH-positive cells in the SN (70.86 ± 4.17) compared with the saline control group (129.60 ± 11.69, P < 0.001). However, the MPTP + EA group had significantly more TH-positive cells in the SN (102.00 ± 7.01) compared with the MPTP group (P < 0.05 for each; Fig. 1). In the case of the striatal TH expression, MPTP injections significantly reduced the expression compared to the saline group (P < 0.001). However, the MPTP + EA group (44.53 ± 5.39%, vs. saline group) showed significantly less reduced TH expression compared to the MPTP group (10.08 ± 3.38%, vs. saline group; P < 0.01, Fig. 2), suggesting that 100-Hz EA at GB34 and GB39 protects the loss of TH-positive neuronal fibers in the striatum in the PD mouse model.
Fig. 1.

Tyrosine hydroxylase (TH)-positive neurons in the mouse substantia nigra. Electroacupuncture at GB34 and GB39 alleviated MPTP-induced neuronal destruction in the substantia nigra. Scale bar 200 µm, and data were presented as mean ± SEM. *P < 0.05, compared with the MPTP group
Fig. 2.
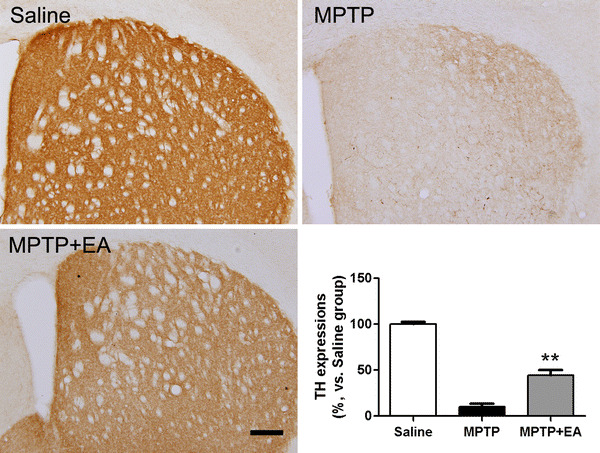
Optical densities after tyrosine hydroxylase (TH)-specific immunohistochemical staining in the striatum. MPTP reduces the optical density in the striatum, whereas electroacupuncture stimulation at GB34 and GB39 prevents this reduction. Scale bar 200 µm, and data were presented as mean ± SEM. **P < 0.01, compared with the MPTP group
Proteins differentially expressed in acupuncture groups compared to MPTP
To obtain the protein profiles of each group, and 2-DE was performed with the protein extracts from the striatum. About 1700 polypeptide spots could be revealed in the pH 3–10 interval with silver staining. After matching the replicated maps, differential changes in intensities between the saline and MPTP mice were limited to 13 proteins; 12 proteins were increased, and one protein was decreased. Pair-wise comparisons with the 13 selected spots were made to assess overall differences in the 2-DE patterns.
Identification of proteins differentially expressed in the acupuncture group compared to MPTP
Of the 13 proteins that were differentially expressed between contol and MPTP treated mice (Table 1), cytosolic malate dehydrogenase (cMDH), munc18-1 and hydroxyacylglutathione hydrolase (HAGH), which were increased by MPTP, and cytochrome c oxidase subunit Vb (COX5b), which was decreased by MPTP, were restored to the level of the saline group after EA treatment (Figs. 3, 4). COX5b, cMDH, and HAGH are involved in cellular metabolism, and munc18-1 is involved in the secretion of synaptic vesicle.
Table 1.
Differentially expressed protein profiles of the protein spots after electroacupuncture (EA) stimulations
| Protein name | Protein ID | MW (kDa) | PI | Sequence coverage (%) | No. of matched peptides | Average ratioa | ||
|---|---|---|---|---|---|---|---|---|
| MPTP/Sal | EA/MPTP | |||||||
| 1 | Cytochrome c oxidase, subunit Vb | CAI25005 | 14.11 | 8.9 | 48 | 7 | −4.6 | 4.1 |
| 2 | Cytosolic malate dehydrogenase | AAA37423 | 36.63 | 6.2 | 31 | 8 | 3.8 | −2.6 |
| 3 | Munc18-1 | BAA32486 | 67.93 | 6.4 | 20 | 11 | 2.7 | −2.9 |
| 4 | Hydroxyacyl glutathione hydrolase | NP_077246 | 29.17 | 6.5 | 28 | 6 | 2.1 | −2.0 |
| 5 | Glial fibrillary acidic protein | AAK56090 | 46.57 | 5.0 | 39 | 14 | 2.9 | −1.2 |
| 6 | Dynein light chain roadblock-type 1 | P62627 | 10.97 | 6.6 | 51 | 4 | 1.7 | −1.4 |
| 7 | NADH dehydrogenase (ubiquinone) Fe–S protein 2 | AAH03898 | 53.71 | 6.4 | 20 | 7 | 11.8 | −1.9 |
| 8 | Dihydropyrimidinase-like 3 | NP_033494 | 62.32 | 6.0 | 36 | 19 | 1.5 | −1.3 |
| 9 | Malate dehydrogenase 1 | AAH50940 | 36.66 | 6.2 | 28 | 7 | 2.0 | −1.9 |
| 10 | mKIAA0106 protein | BAD32166 | 25.17 | 6.0 | 52 | 13 | 1.4 | −1.1 |
| 11 | d-Dopachrome tautomerase | NP_034157 | 13.17 | 6.1 | 64 | 5 | 2.9 | −1.6 |
| 12 | Histidine triad nucleotide binding protein 1 | NP_032274 | 13.87 | 6.4 | 47 | 6 | 1.5 | −1.1 |
| 13 | A Chain A, X-ray structure of nysgrc target T-45 | 1KCX_A | 56.63 | 5.9 | 22 | 8 | 1.7 | 1.3 |
Bold characters represent the proteins showing significant changes compared to the MPTP group
Sal Saline group, MPTP MPTP group, EA MPTP + EA group
aAverage ratio calculated considering 3 replicate gels. A minus means that the protein expression was decreased
Fig. 3.
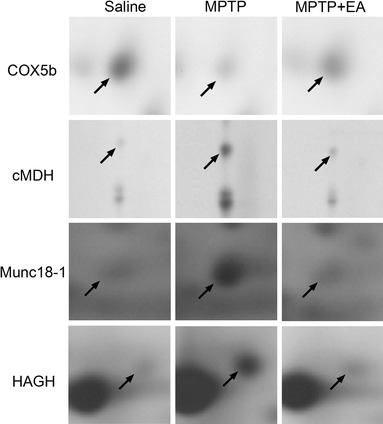
Expression profiles of 4 proteins that showed significantly different changes between the MPTP and MPTP + EA groups after 2-dimensional electrophoresis. MPTP increased the expression of COX5b and decreased the expressions of cMDH, Munc18-1, and HAGH in mouse striatum, but electroacupuncture at GB34 normalized these changes. COX5b cytochrome c oxidase subunit Vb, cMDH cytosolic malate dehydrogenase, HAGH hydroxyacylglutathione hydrolase
Fig. 4.
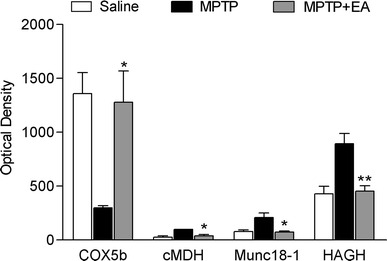
Quantitative analyses of downregulated or upregulated proteins in the MPTP + EA groups compared with the MPTP group (n = 3 for each). The spot intensities were derived from silver-stained 2D gels. Electroacupuncture stimulation at GB34 normalized the decrease of COX5b and increase of cMDH, Munc18-1, and HAGH. COX5b cytochrome c oxidase subunit Vb, cMDH cytosolic malate dehydrogenase, HAGH hydroxyacylglutathione hydrolase. Data are presented as mean ± SEM. *P < 0.05 and **P < 0.01, compared with the MPTP group
Confirmation of altered proteins by western blot analysis
To verify the reliability of proteomics analysis, we carried out a Western blot analysis of a representative protein, HAGH. Anti-actin antibody was used to normalize the optical density values. The analysis was performed with the mice striatum in all groups, and immunoblots were replicated three times. Western blot revealed the same trends in the 2-DE results as shown in Fig. 5.
Fig. 5.
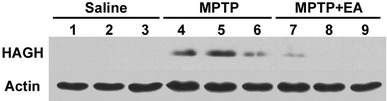
Validation of the proteomic result using Western blot analysis of hydroxyacylglutathione hydrolase protein in the mouse striatum. The same trend as detected in the 2-DE analysis was confirmed. HAGH hydroxyacylglutathione hydrolase
Discussion
Our results demonstrate that 100-Hz EA at GB34 and GB39 significantly protected DA neuronal fibers, and restored the decrease of COX5b and the increase of cMDH, Munc18-1, and HAGH in the MPTP-induced PD model. Using a proteomic analysis, it would be the first study showing that these proteins in the striatum are associated with the neuroprotective actions of EA in the PD mouse model. These findings would be very useful to understand the neurobiological action of acupuncture in PD.
High frequency EA has been known to reduce DA neuronal cell death in the medial forebrain bundle axotomized rat PD model [17], and the effect might originate from the collaboration of its anti-inflammatory and neurotrophic actions [18]. Our previous study also showed that 100-Hz EA at acupoint GB34 changed the expression levels of proteins related to the cell death regulation, inflammation, or restoration from damage, and particularly upregulated cyclophilin A, the neuroprotective protein, in the SN [9]. Acupoint GB34 is widely used for movement disorders such as tremor, walking difficulty, dementia, and stroke, and it is reported that the acupoint protects DA neurons in animal PD models. The present study may suggest the novel direction of neuroprotective mechanisms of 100-Hz EA at acupoint GB34, including possible reduction of oxidative stress in the MPTP-induced PD model.
MPTP-mediated toxicity is induced through conversion to the 1-methyl-4-phenyl-2,3-dihydropyridium ion (MPP+) in astrocytes by monoamine oxidase B [19]. MPP+ is taken up by dopamine transporters and accumulated by the mitochondria, leading to complex I inhibition. MPP+ is also sequestered into synaptic vesicles by the vesicular monoamine transporter, and the sequestration into vesicles decreases MPP+ toxicity by preventing its interaction with mitochondria [20, 21]. Munc18-1, a neuron-specific 67 kDa protein, is independently identified as a syntaxin-binding protein and is essential for the secretion of neurotransmitter-filled synaptic vesicles. In mice, its upregulation results in a robust increase in both large dense core vesicle and synaptic vesicle secretion [22]. We observed the increase of munc18-1 by MPTP, suggesting that Munc18-1 might play a role in secreting MPP+ to the extracellular space for reducing the toxin out of cells.
The present study also demonstrated that MPTP caused the increase of cMDH in the striatum. cMDH is an enzyme in the citric acid cycle that catalyzes the conversion of malate into oxaloacetate. It has two isoforms, a mitochondrial MDH (mMDH) and a cMDH. The function of mMDH is well known, but the functional significance of cMDH is still unclear although it is known to play a role in aerobic energy production for muscle contraction, transmission of neuronal signals, absorption/resorption pathways, as well as the regulation of the nucleic acid-conducting channels [23]. Although there were few studies about the involvement of cMDH in PD, Korolainen et al. [24] showed that the amount of cMDH protein was also increased in Alzheimer's disease (AD) brains, and they suggested that their results might reflected general neurodegeneration related to increased oxidative stress. Given the possible involvement of increased oxidative stress and concomitant neurodegenerative events in MPTP-induced mouse model of PD, it is conceived that the increase of cMDH expression in this study might be due to MPTP-induced neurodegeneration, and EA stimulation prevented such neurodegenerative events.
The glyoxalase system is a metabolic pathway that protects against cellular damage by catalyzing the detoxification of methylglyoxal (MG) to corresponding aldonic acids [25]. The system is present in the cytoplasm of cells and cellular organelles, particularly mitochondria [26], and it consisted of two thiol-dependent enzymes, glyoxalase I (GLX1) and glyoxalase II (GLX2, also known as HAGH). GLX1 catalyzes the isomerization of the spontaneously formed hemithioacetal adduct between glutathione and 2-oxoaldehydes into S-2-hydroxyacylglutathione and decreases the steady-state concentrations of physiological alpha-oxoaldehydes and associated glycation reactions [27]. GLX2/HAGH hydrolyzes these thiolesters and produces d-lactate from S-d-lactoyl-glutathione in the case of methylglyoxal catabolism [28]. HAGH may play a pro-survival role in the metabolic stress response through detoxifying MG [29], and it seems to be the rate-limiting factor within the glyoxalase pathway [30]. It was suggested that MG level could be increased under oxidative stress because of glutathione depletion [31]. The increase of reactive carbonyl compounds such as glyoxal and MG can contribute to pathological changes and dementia progression in AD, and the glyoxalase system prevents cell death through scavenging small dicarbonyl compounds [32]. The glyoxalase system is usually upregulated in pathological conditions such as breast cancer [30], kidney tumor [33], and AD [34]. We found that MPTP caused the increased expression of HAGH, suggesting that MPTP-induced oxidative stress might lead to accumulation of MG, and HAGH was increased for detoxifying MG as a compensatory mechanism. Therefore, our finding may provide intriguing possibilities that MG could be involved not only in AD, but also in PD, and HAGH might be an important factor to control the MG level in PD. In this study, with 100-Hz EA at GB34 and GB39, the increased expression of HAGH was normalized. It has been reported that acupuncture treatment could prevent oxidative stress by increasing the activities of superoxide dismutase and glutathione peroxidase in the hippocampus and ischemic-reperfused rat brains [35, 36]. Taken together, although the exact mechanism of the effect of acupuncture treatment is still elusive, our results may suggest that acupuncture treatment relieves MPTP-induced intracellular oxidative stress in the striatum probably by increasing antioxidant enzyme activities, thereby precluding MG accumulation.
In conclusion, this study showed that 100-Hz EA stimulation at GB34 and GB39 protected DA neuronal fibers and restored proteomic changes in MPTP-induced mouse striatum. MPTP destroyed DA neurons and caused abnormal expressions of the cellular metabolism related proteins. With EA treatment, these MPTP-induced abnormal changes were restored, and the cell destruction was also reduced. Based on our results and other previous studies, we propose that EA may exert neuroprotective effects in MPTP-induced mouse striatum through reducing MPTP-induced toxicity such as oxidative stress.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (R11-2005-014).
References
- 1.Sian J, Gerlach M, Youdim MB, Riederer P. Parkinson’s disease: a major hypokinetic basal ganglia disorder. J Neural Transm. 1999;106:443–476. doi: 10.1007/s007020050171. [DOI] [PubMed] [Google Scholar]
- 2.Cossette M, Lecomte F, Parent A. Morphology and distribution of dopaminergic neurons intrinsic to the human striatum. J Chem Neuroanat. 2005;29:1–11. doi: 10.1016/j.jchemneu.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Shimohama S, Sawada H, Kitamura Y, Taniguchi T. Disease model: Parkinson’s disease. Trends Mol Med. 2003;9:360–365. doi: 10.1016/S1471-4914(03)00117-5. [DOI] [PubMed] [Google Scholar]
- 4.von Bohlen Und Halbach O. Modeling neurodegenerative diseases in vivo review. Neurodegener Dis. 2005;2:313–320. doi: 10.1159/000092318. [DOI] [PubMed] [Google Scholar]
- 5.Ha JY, Lee SH, Yin CS, Park SM, Kang JW, Chang DI, Lee YH. The effect of manual acupuncture therapy on symptoms of the patients with idiopathic Parkinson’s disease. Korean J Orient Med. 2003;24:172–183. [Google Scholar]
- 6.Zhuang X, Wang L. Acupuncture treatment of Parkinson’s disease—a report of 29 cases. J Tradit Chin Med. 2000;20:265–267. [PubMed] [Google Scholar]
- 7.Shulman LM, Wen X, Weiner WJ, Bateman D, Minagar A, Duncan R, Konefal J. Acupuncture therapy for the symptoms of Parkinson’s disease. Mov Disord. 2002;17:799–802. doi: 10.1002/mds.10134. [DOI] [PubMed] [Google Scholar]
- 8.Kang JM, Park HJ, Choi YG, Choe IH, Park JH, Kim YS, Lim S. Acupuncture inhibits microglial activation and inflammatory events in the MPTP-induced mouse model. Brain Res. 2007;1131:211–219. doi: 10.1016/j.brainres.2006.10.089. [DOI] [PubMed] [Google Scholar]
- 9.Jeon S, Kim YJ, Kim ST, Moon W, Chae Y, Kang M, Chung MY, Lee H, Hong MS, Chung JH, Joh TH, Lee H, Park HJ. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson’s disease mouse model. Proteomics. 2008;8:4822–4832. doi: 10.1002/pmic.200700955. [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Lim S, Joo WS, Yin CS, Lee HS, Lee HJ, Seo JC, Leem K, Son YS, Kim YJ, Kim CJ, Kim YS, Chung JH. Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson’s disease model. Exp Neurol. 2003;180:93–98. doi: 10.1016/S0014-4886(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Lim HH, Song YK, Lee HH, Lim S, Han SM, Kim CJ. Effect of acupuncture on 6-hydroxydopamine-induced nigrostratal dopaminergic neuronal cell death in rats. Neurosci Lett. 2005;384:133–138. doi: 10.1016/j.neulet.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 12.Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 13.Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayley S, Crocker SJ, Smith PD, Shree T, Jackson-Lewis V, Przedborski S, Mount M, Slack R, Anisman H, Park DS. Regulation of dopaminergic loss by Fas in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neurosci. 2004;24:2045–2053. doi: 10.1523/JNEUROSCI.4564-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch EC, Faucheux BA. Iron metabolism and Parkinson’s disease. Mov Disord. 1998;13(Suppl 1):39–45. [PubMed] [Google Scholar]
- 16.Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson’s disease: a role in neurodegeneration? Ann Neurol. 1998;44:S115–S120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- 17.Liang XB, Liu XY, Li FQ, Luo Y, Lu J, Zhang WM, Wang XM, Han JS. Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates BDNF mRNA in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Res Mol Brain Res. 2002;108:51–59. doi: 10.1016/S0169-328X(02)00513-2. [DOI] [PubMed] [Google Scholar]
- 18.Liu XY, Zhou HF, Pan YL, Liang XB, Niu DB, Xue B, Li FQ, He QH, Wang XH, Wang XM. Electro-acupuncture stimulation protects dopaminergic neurons from inflammation-mediated damage in medial forebrain bundle-transected rats. Exp Neurol. 2004;189:189–196. doi: 10.1016/j.expneurol.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 19.Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-C. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci USA. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toonen RF. Role of Munc18-1 in synaptic vesicle and large dense-core vesicle secretion. Biochem Soc Trans. 2003;31:848–850. doi: 10.1042/BST0310848. [DOI] [PubMed] [Google Scholar]
- 23.Hanss B, Leal-Pinto E, Teixeira A, Christian RE, Shabanowitz J, Hunt DF, Klotman PE. Cytosolic malate dehydrogenase confers selectivity of the nucleic acid-conducting channel. Proc Natl Acad Sci USA. 2002;99:1707–1712. doi: 10.1073/pnas.022355499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korolainen MA, Goldsteins G, Nyman TA, Alafuzoff I, Koistinaho J, Pirttila T. Oxidative modification of proteins in the frontal cortex of Alzheimer’s disease brain. Neurobiol Aging. 2006;27:42–53. doi: 10.1016/j.neurobiolaging.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Thornalley PJ. Glutathione-dependent detoxification of alpha-oxoaldehydes by the glyoxalase system: involvement in disease mechanisms and antiproliferative activity of glyoxalase I inhibitors. Chem Biol Interact. 1998;111–112:137–151. doi: 10.1016/S0009-2797(97)00157-9. [DOI] [PubMed] [Google Scholar]
- 26.Padmanabhan PK, Mukherjee A, Madhubala R. Characterization of the gene encoding glyoxalase II from Leishmania donovani: a potential target for anti-parasite drugs. Biochem J. 2006;393:227–234. doi: 10.1042/BJ20050948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornalley PJ. Glyoxalase I––structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;31:1343–1348. doi: 10.1042/BST0311343. [DOI] [PubMed] [Google Scholar]
- 28.Vander Jagt DL. Glyoxalase II: molecular characteristics, kinetics and mechanism. Biochem Soc Trans. 1993;21:522–527. doi: 10.1042/bst0210522. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Chen X. Glyoxalase II, a detoxifying enzyme of glycolysis byproduct methylglyoxal and a target of p63 and p73, is a pro-survival factor of the p53 family. J Biol Chem. 2006;281:26702–26713. doi: 10.1074/jbc.M604758200. [DOI] [PubMed] [Google Scholar]
- 30.Rulli A, Carli L, Romani R, Baroni T, Giovannini E, Rosi G, Talesa V. Expression of glyoxalase I and II in normal and breast cancer tissues. Breast Cancer Res Treat. 2001;66:67–72. doi: 10.1023/A:1010632919129. [DOI] [PubMed] [Google Scholar]
- 31.Gnerer JP, Kreber RA, Ganetzky B. Wasted away, a Drosophila mutation in triosephosphate isomerase, causes paralysis, neurodegeneration, and early death. Proc Natl Acad Sci USA. 2006;103:14987–14993. doi: 10.1073/pnas.0606887103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munch G, Kuhla B, Luth HJ, Arendt T, Robinson SR. Anti-AGEing defences against Alzheimer’s disease. Biochem Soc Trans. 2003;31:1397–1399. doi: 10.1042/BST0311397. [DOI] [PubMed] [Google Scholar]
- 33.Antognelli C, Baldracchini F, Talesa VN, Costantini E, Zucchi A, Mearini E. Overexpression of glyoxalase system enzymes in human kidney tumor. Cancer J. 2006;12:222–228. doi: 10.1097/00130404-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Kuhla B, Boeck K, Schmidt A, Ogunlade V, Arendt T, Munch G, Luth HJ. Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer’s disease brains. Neurobiol Aging. 2007;28:29–41. doi: 10.1016/j.neurobiolaging.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Liu CZ, Yu JC, Zhang XZ, Fu WW, Wang T, Han JX. Acupuncture prevents cognitive deficits and oxidative stress in cerebral multi-infarction rats. Neurosci Lett. 2006;393:45–50. doi: 10.1016/j.neulet.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 36.Siu FK, Lo SC, Leung MC. Electroacupuncture reduces the extent of lipid peroxidation by increasing superoxide dismutase and glutathione peroxidase activities in ischemic-reperfused rat brains. Neurosci Lett. 2004;354:158–162. doi: 10.1016/j.neulet.2003.10.009. [DOI] [PubMed] [Google Scholar]


