Abstract
Rodents show grooming, a typical self-care behavior, under stress and non-stress conditions. Previous studies revealed that grooming under stress conditions such as the open-field test (OFT) or the elevated plus-maze test (EPM) is associated with anxiety, but the roles of grooming under non-stress conditions are not well understood. Here, we examined spray-induced grooming as a model of grooming under a non-stress condition to investigate the relationship between this grooming and depression-like behavior in the forced swim test (FST) and tail suspension test, and we compared spray-induced grooming with OFT- and EPM-induced grooming. The main finding was that the duration of spray-induced grooming, but not that of OFT/EPM-induced grooming, was negatively correlated with the duration of immobility in the FST, an index of depression-like behavior. The results suggest that spray-induced grooming is functionally different from the grooming in the OFT and EPM and is related to reduction of depressive behavior.
Keywords: Grooming, Self-care, Depression, Anxiety, Immobility
Introduction
Self-grooming behavior, an innate stereotyped behavior for the cleaning of skin and fur, is a typical self-care behavior under normal/healthy conditions as well as under various stress conditions [1–4]. This behavior is common in rodents, representing up to 30–50 % of their waking time [1, 2]. It has been shown that rats perform different types of grooming depending on environmental conditions. Under non-stress conditions such as in a home cage, rats show spontaneous self-paced grooming characterized by a stereotyped cephalocaudal sequence [1, 2]. Under stress conditions such as the open-field test (OFT) or the elevated plus-maze test (EPM), rats express grooming characterized by an interrupted and disorganized sequence that depends on anxiety levels [3–5].
Previous studies revealed that stress-induced grooming is associated with high anxiety levels [6–9] and related to depression [10–14]. Depressive rats after chronic mild stress or chronic forced swim stress show longer EPM- or OFT-induced grooming than control rats [10–14]. Interestingly, it was reported that depression-inducing treatments suppressed spray-induced grooming in a familiarized place [13, 15–17]. The opposite associations between depression and OFT/EPM-induced grooming vs spray-induced grooming led us to hypothesize that grooming under stress and non-stress conditions may have different relationships with and functions in depression.
In the present study, we examined spray-induced grooming by rats under familiarized conditions and compared it with the OFT- and EPM-induced grooming in relation to depressive behavior in the forced swim test (FST) and tail suspension test (TST) to elucidate the emotional significance of self-care behavior under less stressful, healthier conditions, and to see how that differs from such behavior under stressful conditions.
Materials and methods
Animals
Seven-week-old male Wistar rats were purchased from Kyudo Corp. (Tosu, Japan). The experiments were carried out using 62 male Wistar rats (260–280 g) after 1 week of habituation. All animals used in this study were experimentally naive, housed two per cage and kept in a controlled environment maintained at a constant temperature (23 ± 1 °C) and humidity (50 ± 5 %), with free access to laboratory chow (CE-2, CLEA Japan, Tokyo) and tap water. The animals were maintained on a 12:12-h light/dark cycle (lights on at 08:00 h and off at 20:00 h). All animal care procedures and experiments were conducted in accord with the guidelines set by the Kyushu Institute of Technology Directives on the use and care of laboratory animals and were approved by the Ethical Committee of the Kyushu Institute of Technology.
Behavioral tests
The following behavioral tests were performed using 22 rats: spray test (at 8 weeks of age), OFT (9 weeks), EPM (10 weeks), and FST (11 weeks). Another 20 rats were tested by spray test (8 weeks) and FST (9 weeks) to avoid influence of OFT and EPM on results of FST. In addition, spray test (8 weeks) and TST (9 weeks), another animal model to evaluate depressive behavior, was performed using a different group of rats (n = 20). All behavioral tests were conducted between 20:00 and 24:00 h. The spray test was conducted in the dark to reduce stress (22:00–24:00), while the OFT, EPM, FST and TST were conventionally conducted in the light (20:00–23:00). The spray test and TST sessions were manually recorded and analyzed. All of the OFT, EPM and FST sessions were manually or automatically recorded with a computer-based video tracking system (Ethovision v1.96, Noldus Information Technology, the Netherlands).
Spray test
The rats’ spray-induced grooming was observed in the dark. For the spray test, the rat was put in a plastic test chamber (30 × 30 × 50 cm) and was familiarized with the box for 2 h (21:00–23:00). At 23:00–23:40, to induce grooming, the rat was misted in the chamber with water (23 °C) from a hand spray: the rat was placed toward the spray nozzle (20–30 cm away) and lightly sprayed from above eight times to adequately coat the rat’s dorsal surface with mist. The rat’s behavior was then recorded for 20 min by a video camera (Sony Digital Handycam, XR550 V, in the dark, using the ‘nightshot’ mode). Using the videos, we determined the number and duration of grooming bouts—which was characterized as continuous self-grooming without interruption—and the portion of each bout in which the subject was grooming a specific body region (i.e., the face area including nose, the head and neck, or the body area including the tail and genital area).
Open-field test (OFT)
The open-field apparatus was a square field (80 × 56 × 40 cm) with an object (12 × 9 × 7 cm) placed at the center [18, 19]. For the OFT, the rat was placed inside the apparatus at one side and then allowed to move freely for 10 min. The frequency, duration and body regions of self-grooming, total distance moved, frequency and duration of rearing (standing on hind legs), and the time spent in the center area were recorded and analyzed by the computer-based video tracking system. The total distance moved was evaluated as an index of locomotor activity. The rearing behavior and the time spent in the center area were evaluated as an index of exploratory behavior.
Elevated plus-maze test (EPM)
The elevated plus-maze was made of translucent brown acrylic material and consisted of two open arms (50 × 10 cm) and two closed arms (50 × 10 cm), each of which had 39-cm-high walls [18–21]. The arms extended from a center square platform (10 × 10 cm) and were arranged so that the arms of the same type (open vs closed) were opposite each other. The apparatus was 50 cm above the floor. The rat being tested was placed on the center square platform and allowed to move freely for 5 min. We recorded and analyzed the frequency, duration and body regions of self-grooming, the total distance moved, the time spent in the open arms, and the numbers of entries into the open and closed arms by the computer-based video tracking system. We also calculated the number of total entries (open and closed arm entries) and the percentage: (number of open arm entries)/(number of total entries)*100 (%). The total time spent and the percentage of time spent in the open arms were evaluated as an index of anxiety.
Forced swimming test (FST)
This test was used to evaluate the depressive state of rats, as described [19, 22, 23]. The water tank, a cylindrical acrylic container (50 cm high, 30 cm dia.), was filled 35 cm deep with tap water (24 ± 1 °C). The rat was placed in the water for 15 min on the first day and for 5 min on the second day. After testing, the rat was dried with a towel and returned to its home cage. The duration of the periods of struggling and that of immobility were recorded, and the data obtained on the second day were used for correlation analyses. A rat’s behavior was characterized as struggling when the rat vigorously broke the water surface with its head and forepaws or actively tried to climb the wall of the tank with its paws. Immobility was defined as floating motionlessly on the surface of the water.
Tail suspension test (TST)
The rats were individually suspended by the tail with an adhesive tape placed 2 cm away from the tip of the tail [24]. Duration of immobility was recorded for 6 min from side view using video camera. Rats were considered immobile only when they hang passively and were completely motionless.
Statistical analyses
All data were analyzed using SPSS software (ver. 22, IBM, Tokyo), and the results are presented as the mean ± standard error of the mean (SEM). The results of the behavioral tests were analyzed by Kruskal–Wallis test because some data did not show normal distribution. Differences in grooming patterns (face grooming vs body grooming) were analyzed by a χ 2 test. The relationships between spray-induced grooming and the following four parameters were explored at the individual level by determining the Spearman rank correlation between them: (1) locomotor activity (distance moved in the OFT), (2) exploratory behavior (rearing and time spent staying in the center area in the OFT), (3) anxiety level (time spent staying in the open arms in the EPM) and (4) depression-like behavior (duration of immobility in the FST and TST). Correlation coefficients between OFT/EPM-induced grooming and the other behavioral parameters mentioned above were also evaluated. Statistical differences were considered significant when the P value was below 0.05.
Results
Relationship between the spray-induced grooming and the OFT/EPM-induced grooming
The rats (n = 22) performed grooming behavior for 230.7 ± 26.5 s (19.2 ± 2.2 %) in the spray test (20 min), 9.9 ± 2.1 s (1.6 ± 0.4 %) in the OFT (10 min) and 12.0 ± 3.1 s (4.0 ± 1.0 %) in the EPM (5 min). In the spray test, the rats groomed for a greater percentage of time than in the OFT or EPM (Kruskal–Wallis test, P < 0.001).
In the spray test, body grooming was predominant (75.6 ± 8.5 %), while face grooming was mainly observed in the OFT (93.8 ± 21.8 %) and the EPM (94.4 ± 25.0 %) (Fig. 1). This difference in grooming patterns was significant (χ 2 = 157.6, P < 0.001).
Fig. 1.
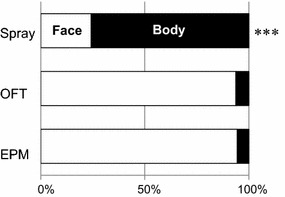
Percentage of the time spent on grooming behaviors in the face area (open column) or body area including genital and leg region (black column) in the spray test, OFT and EPM. ***P < 0.001, χ 2 test. For all results in all figures, n = 22
The total duration of spray-induced grooming was not correlated with that of OFT-induced grooming (r s = −0.18, P = 0.42) but it was correlated with the time spent on EPM-induced grooming (r s = 0.44, P = 0.04) (Fig. 2). The total duration of OFT-induced grooming was also not correlated with that of EPM-induced grooming (r s = −0.25, P = 0.27).
Fig. 2.
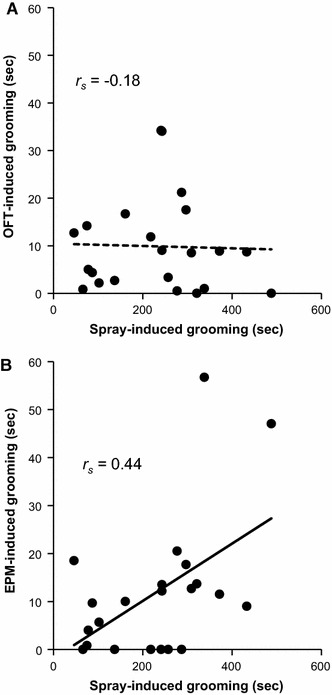
Correlations between the duration of spray-induced grooming and that of OFT-induced grooming (a) or that of EPM-induced grooming (b). A significant negative correlation was found between spray-induced grooming and EMP-induced grooming using the Spearman rank correlation test. Each point represents an individual animal
Relationships between spray-induced grooming and behaviors in the OFT and EPM
In the OFT, the rats moved 2816 ± 213 cm, performed rearing behavior for 75.5 ± 8.2 s and spent time in the center area for 81.9 ± 14.2 s. In the EPM, the rats spent 31.6 ± 6.2 s in the open arms and 194.3 ± 12.6 s in the closed arms.
Spray-induced grooming was not correlated with the time spent in the center area in the OFT (an index of exploratory behavior, r s = −0.15, P = 0.51) or with the time spent in the open arms in the EPM (an index of anxiety level, r s = 0.084, P = 0.71) (Fig. 3) The time spent in the closed arms in the EPM was also not correlated with spray-induced grooming (r s = 0.057, P = 0.80). Other behavioral parameters in the OFT, such as distance moved (locomotor activity, r s = −0.032, P = 0.89) and rearing behavior (exploratory behavior, r s = −0.08, P = 0.72) did not show a correlation with spray-induced grooming.
Fig. 3.
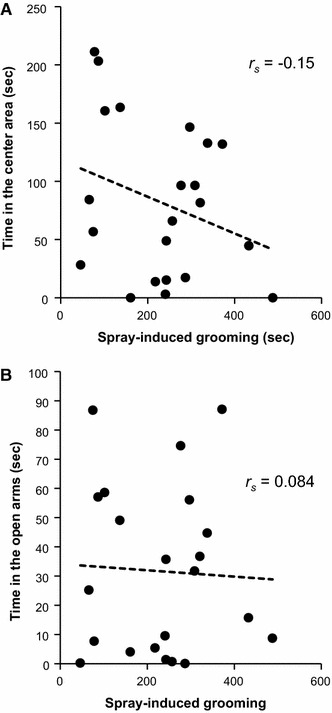
Correlations between the duration of spray-induced grooming and the time spent in the center area in the OFT (exploratory behavior) (a) or the time spent in the open arms in the EPM (an index of anti-anxiety level) (b). No correlation was found using the Spearman rank correlation test. Each point represents an individual animal
Relationships between OFT- and EPM-induced grooming and behaviors in the OFT and EPM
OFT-induced grooming tended to be negatively correlated with the time spent in the center area (r s = −0.41, P = 0.055, Fig. 4a), distance moved (r s = −0.37, P = 0.093) and rearing behavior (r s = −0.39, P = 0.070) in the OFT, whereas OFT-induced grooming was not correlated with the time spent in the open arms (r s = −0.34, P = 0.12, Fig. 4b) or the time spent in the closed arms (r s = 0.18, P = 0.41) in the EPM.
Fig. 4.
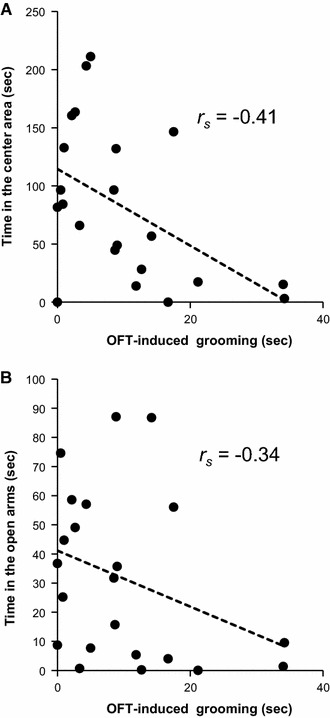
Correlations between the duration of OFT-induced grooming and the time spent in the center area in the OFT (exploratory behavior) (a) or the time spent in the open arms in the EPM (an index of anti-anxiety level) (b). A tendency of negative correlation was found between OFT-induced grooming and the time spent in the center area in the OFT, using the Spearman rank correlation test. Each point represents an individual animal
EPM-induced grooming was not correlated with the time spent in the center area (r s = 0.027; P = 0.90, Fig. 5a), distance moved (r s = −0.25, P = 0.26), or rearing behavior (r s = −0.12, P = 0.60) in the OFT. The time spent in the open arms (r s = 0.20; P = 0.37, Fig. 5b) or closed arms in the EPM (r s = 0.20, P = 0.38) was also not correlated with EPM-induced grooming.
Fig. 5.
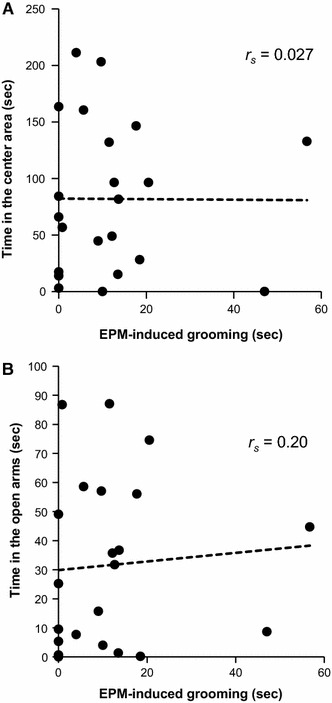
Correlations between the duration of EPM-induced grooming and the time spent in the center area in the OFT (exploratory behavior) (a) or the time spent in the open arms in the EPM (an index of anti-anxiety level) (b). No correlation was found using the Spearman rank correlation test. Each point represents an individual animal
Relationships between spray-, OFT- and EPM-induced grooming and depressive behavior in the FST
In the FST (the second day, 5 min), the rats showed struggling behavior for 81.1 ± 7.7 s and showed immobility (an index of depression-like behavior) for 12.9 ± 3.0 s. Although there were no clear relationships between grooming behaviors and behaviors in the OFT and EPM as mentioned above, a specific relationship between spray-induced grooming and depressive behavior in the FST was observed. The total duration of immobility in the FST was negatively correlated with spray-induced grooming (r s = −0.50; P = 0.018), but not with the OFT-induced grooming (r s = −0.034; P = 0.88) or EPM-induced grooming (r s = 0.051 P = 0.82) (Fig. 6). Struggling behavior in the FST was not correlated with spray- (r s = 0.24, P = 0.29), OFT- (r s = 0.30, P = 0.17) or EPM-induced grooming (r s = −0.23, P = 0.32).
Fig. 6.
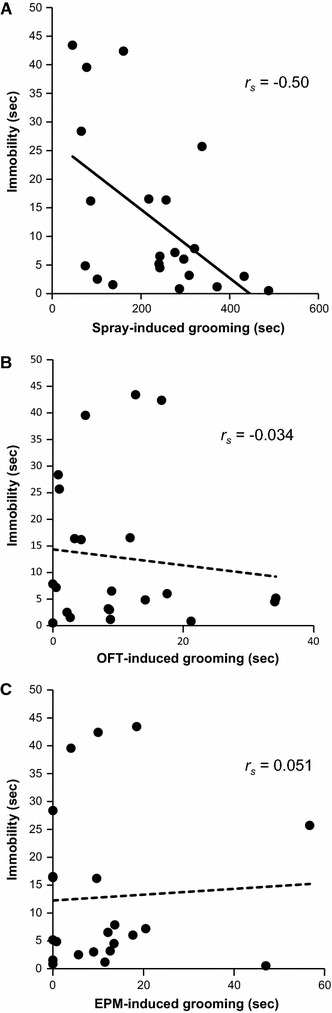
Correlations between the durations of spray-induced grooming (a), OFT-induced grooming (b) or EPM-induced grooming (c) and the immobility time in the FST (depression-like behavior). A significant negative correlation was found between spray-induced grooming and the immobility time in the FST, using the Spearman rank correlation test. Each point represents an individual animal
In additional studies using different groups of rats (n = 20, each), a negative correlation between spray-induced grooming and immobility in the FST was also found in the group tested only with the spray test and FST without testing the OFT and EPM (r s = −0.59; P = 0.006). In contrast, immobility time in the TST was not correlated with spray-induced grooming (r s = 0.029; P = 0.91).
Discussion
Our study revealed that immobility in the FST was negatively correlated with spray-induced grooming but not with OFT- or EPM-induced grooming. The spray-induced grooming is predominantly composed of body grooming as previously reported [25, 26], and different from the grooming seen in OFT or EPM, in which self-grooming behavior was interrupted and disorganized, showing face-dominant grooming as described in detail in previous studies [3, 4, 27]. The present results suggest that spray-induced grooming is behaviorally and functionally different from the grooming in the OFT and EPM, and that individual differences in spray-induced grooming predict individual degrees of depression-like behavior in the FST but not TST. Spray-induced grooming in rats may be a good animal model for studying self-care functions under non-stress conditions which enhance resistance to depression under certain conditions.
Spray-induced grooming and OFT/EPM-induced grooming
Spray-induced grooming has been used for neurobehavioral analyses under both high and low stress conditions [13, 15–17]. Kalueff and colleagues compared rats’ spray-induced grooming after the rats stayed in their home cages and after exposure to stress conditions such as OFT, EPM and social stress (with an unfamiliar conspecific male) and found that spray-induced grooming showed both cephalocaudal/“non-stress” and interrupted/“stress” patterns of grooming depending on environmental conditions [3, 4]. In the present study, we induced grooming by spraying room-temperature water (23 °C) in a dark environment after 2-h habituation to the measurement chamber, which corresponds to grooming under non-stress conditions.
High percentages of grooming of a body area may reflect the high occurrence of stereotyped cephalocaudal (from face to body) grooming and indicate that rats engaged in such grooming are in a non-stressed state. This is consistent with the present results that the duration of spray-induced grooming was not correlated with anxiety or other stress-related activities evaluated in the OFT and EPM (except for EPM-induced grooming). In contrast, grooming in the OFT and EPM may correspond to that in a stress state and thus this grooming has characteristics of an incomplete or interrupted pattern, which may result in high percentages of facial grooming due to interruptions of cephalocaudal sequences.
Although the present grooming pattern results suggest that spray-induced grooming is different from OFT- and EPM-induced grooming, our correlation analysis of these three types of grooming revealed that spray-induced grooming has a positive correlation with EPM-induced grooming but not OFT-induced grooming, and there was no correlation between OFT-induced grooming and EPM-induced grooming. These differences suggest that the behavioral significance of each of these three types of grooming is different, even though both OFT- and EPM-induced grooming are categorized as stress-induced grooming.
We observed that OFT-induced grooming tended to be negatively correlated with the time spent in the center area and with rearing behavior in the OFT, which are indices of exploratory behavior and of distance moved (an index of locomotor activity). These major behaviors in the OFT are mutually exclusive, and therefore a tendency toward a negative correlation was observed. Another possibility that has been suggested is that OFT/EPM-induced grooming is a de-arousal process after environmental change [2], whereas exploratory behavior in novel situations is an arousal response that gradually decreases through habituation. The de-arousal process of grooming and the arousal process of exploratory behavior in the OFT may counteract each other, causing the negative-correlation tendency between them.
Spray-induced grooming and depressive behavior in the FST and TST
The results of the present study are the first to demonstrate that rats showing longer spray-induced grooming under non-stress conditions spend shorter immobility times in the FST. These results imply that rats with higher self-care function under normal conditions have higher resistance to depressive stimuli. In contrast, the OFT- or EPM-induced grooming was not related to immobility as reported in a study in which no difference in OFT-induced grooming was observed between high- and low-immobility groups [28]. It is known that chronic mild stress or chronic forced swim stress (which induce depressive symptoms in rodents) suppress spray-induced grooming [15–17]. Those studies showed effects of depression on self-grooming, but the present results suggest the existence of an opposite association from self-grooming to depression. It is also known that depression reduces self-care behavior in humans [29, 30], whereas increases in self-care behavior ameliorates depressive symptoms in humans [31], as indicated in the present study of rodents. We suggest that spray-induced grooming under non-stress conditions can be an animal model for studying self-care functions that promote tolerance to a depressive condition.
Immobility in the TST, another model of depression-like behavior, was not correlated with spray-induced grooming. It has been reported that there is no or little correlation between the two types of immobility [32, 33] and their genetic background, and the neurochemical pathways involved are not identical [33–35]. Monoaminergic and thermoregulatory pathways are involved in neural controls of both grooming and FST-induced behaviors but not of TST-induced ones [2, 34, 36–38]. These findings with present results suggest that spray-induced grooming is related to depressive components in the FST but not TST in which monoamine and/or thermoregulatory responses under wet conditions may play some roles.
The physiological mechanisms underlying the negative correlation between self-grooming and depressive behavior are as yet unknown. One possible neural substrate is reward circuits, as self-grooming under non-stress conditions has been shown to induce endocrine and neural responses related to a rewarding effect [25]. Experiencing a reward has been shown to ameliorate depressive symptoms and increase resilience [39, 40], and the removal of rewards promotes depressive reactions [41–43]. In addition, depressive conditions have been shown to increase the reward threshold [44, 45]. These findings suggest that neural substrates used to control self-care behavior such as spray-induced grooming share reward pathways, at least in part, which promote resistance to depression. This hypothesis remains to be tested.
Acknowledgments
We thank Dr. Masaharu Mizuno for his help with the experiments. This work was partly supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Bolles RC. Grooming behavior in the rat. J Comp Physiol Psychol. 1960;53:306–310. doi: 10.1037/h0045421. [DOI] [PubMed] [Google Scholar]
- 2.Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- 3.Kalueff AV, Tuohimaa P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res Protoc. 2004;13:151–158. doi: 10.1016/j.brainresprot.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007;2:2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- 5.Kalueff AV, Tuohimaa P. Experimental modeling of anxiety and depression. Acta Neurobiol Exp. 2004;64:439–448. doi: 10.55782/ane-2004-1526. [DOI] [PubMed] [Google Scholar]
- 6.Homberg JR, van den Akker M, Raasø HS, Wardeh G, Binnekade R, Schoffelmeer ANM, de Vries TJ. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur J Neurosci. 2002;15:1542–1550. doi: 10.1046/j.1460-9568.2002.01976.x. [DOI] [PubMed] [Google Scholar]
- 7.Homberg JR, Arends B, Wardeh G, Raasø HS, Schoffelmeer ANM, de Vries TJ. Individual differences in the effects of serotonergic anxiolytic drugs on the motivation to self-administer cocaine. Neuroscience. 2004;128:121–130. doi: 10.1016/j.neuroscience.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Homberg JR, Wardeh G, Raasø HS, Schoffelmeer ANM, de Vries TJ. Neuroadaptive changes in mesocorticolimbic dopamine and acetylcholine neurons following cocaine or saline self-administration are dependent on pre-existing individual differences. Neuroscience. 2003;121:829–836. doi: 10.1016/j.neuroscience.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Estanislau C, Ramos AC, Ferraresi PD, Costa NF, de Carvalho HM, Batistela S. Individual differences in the elevated plus-maze and the forced swim test. Behav Process. 2011;86:46–51. doi: 10.1016/j.beproc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Gao D, Zheng Z, Han M, Tang X, Sun X. Findings of P300-like and CNV-like potentials in rat model of depression following repeatedly forced swim stress. Int J Psychophysiol. 2009;72:160–165. doi: 10.1016/j.ijpsycho.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Hu H, Su L, Xu YQ, Zhang H, Wang LW. Behavioral and [F-18] fluorodeoxyglucose micro positron emission tomography imaging study in a rat chronic mild stress model of depression. Neuroscience. 2010;169:171–181. doi: 10.1016/j.neuroscience.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 12.Kompagne H, Bárdos G, Szénási G, Gacsályi I, Hársing LG, Lévay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008;193:311–314. doi: 10.1016/j.bbr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Liang X, Huang YN, Chen SW, Wang WJ, Xu N, Cui S, Liu XH, Zhang H, Liu Yue Nan, Liu S, Yang M, Dong Y. Antidepressant-like effect of asiaticoside in mice. Pharmacol Biochem Behav. 2008;89:444–449. doi: 10.1016/j.pbb.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Qi X, Lin W, Li J, Pan Y, Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behav Brain Res. 2006;175:233–240. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Ducottet C, Belzung C. Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiol Behav. 2004;81:417–426. doi: 10.1016/j.physbeh.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Ducottet C, Belzung C. Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res. 2005;156:153–162. doi: 10.1016/j.bbr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Ducottet C, Aubert A, Belzung C. Susceptibility to subchronic unpredictable stress is related to individual reactivity to threat stimuli in mice. Behav Brain Res. 2004;155:291–299. doi: 10.1016/j.bbr.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–356. doi: 10.1016/S0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 21.Someya N, Narikiyo K, Masuda A, Aou S. Stress-induced food intake is related to anxiety-like behavior but not to daily food intake in rats. J Physiol Sci. 2011;61(Suppl 1):S153. [Google Scholar]
- 22.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats:a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1977;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 23.Aou S, Ma J, Shiramine K, Hori T. The stomach is the etiologic organ for immobilization-induced hypocalcemia in rats. Am J Physiol. 1993;265:R1376–R1379. doi: 10.1152/ajpregu.1993.265.6.R1376. [DOI] [PubMed] [Google Scholar]
- 24.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 25.van Erp AM, Kruk MR, Meelis W, Willekens-Bramer DC. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behav Brain Res. 1994;65:47–55. doi: 10.1016/0166-4328(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 26.Hartley JE, Montgomery AMJ. 8-OH-DPAT inhibits both prandial and waterspray-induced grooming. J Psychopharmacol. 2008;22:746–752. doi: 10.1177/0269881107082903. [DOI] [PubMed] [Google Scholar]
- 27.Kalueff AV, Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods. 2005;143:169–177. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Sequeira-Cordero A, Mora-Gallegos A, Cuenca-Berger P, Fornaguera-Trías J. Individual differences in the immobility behavior in juvenile and adult rats are associated with monoaminergic neurotransmission and with the expression of corticotropin-releasing factor receptor 1 in the nucleus accumbens. Behav Brain Res. 2013;252:77–87. doi: 10.1016/j.bbr.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 29.Egede LE, Ellis C, Grubaugh AL. The effect of depression on self-care behaviors and quality of care in a national sample of adults with diabetes. Gen Hosp Psychiatry. 2009;31:422–427. doi: 10.1016/j.genhosppsych.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 31.Ory MG, Ahn S, Jiang L, Smith ML, Ritter PL, Whitelaw N, Lorig K. Successes of a national study of the chronic disease self-management program: meeting the triple aim of health care reform. Med Care. 2013;51:992–998. doi: 10.1097/MLR.0b013e3182a95dd1. [DOI] [PubMed] [Google Scholar]
- 32.El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci USA. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshikawa T, Watanabe A, Ishitsuka Y, Nakaya A, Nakatani N. Identification of multiple genetic loci linked to the propensity for “behavioral despair” in mice. Genome Res. 2002;12:357–366. doi: 10.1101/gr.222602. [DOI] [PubMed] [Google Scholar]
- 34.Renard CE, Dailly E, David DJP, Hascoet M, Bourin M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam Clin Pharmacol. 2003;17:449–455. doi: 10.1046/j.1472-8206.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 35.Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/S0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- 36.Thiessen DD. Body temperature and grooming in the Mongolian gerbil. Ann N Y Acad Sci. 1988;525:27–39. doi: 10.1111/j.1749-6632.1988.tb38593.x. [DOI] [PubMed] [Google Scholar]
- 37.Abdelhamid RE, Kovács KJ, Nunez MG, Larson AA. Depressive behavior in the forced swim test can be induced by TRPV1 receptor activity and is dependent on NMDA receptors. Pharmacol Res. 2014;79:21–27. doi: 10.1016/j.phrs.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 39.Lambert KG, Hyer MM, Rzucidlo AA, Bergeron T, Landis T, Bardi M. Contingency-based emotional resilience: effort-based reward training and flexible coping lead to adaptive responses to uncertainty in male rats. Front Behav Neurosci. 2014;8:124. doi: 10.3389/fnbeh.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wichers M, Peeters F, Geschwind N, Jacobs N, Simons CJP, Derom C, Thiery E, Delespaul PH, van Os J. Unveiling patterns of affective responses in daily life may improve outcome prediction in depression: a momentary assessment study. J Affect Disord. 2010;124:191–195. doi: 10.1016/j.jad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Huston JP, Silva MA, Komorowski M, Schulz D, Topic B. Animal models of extinction-induced depression: loss of reward and its consequences. Neurosci Biobehav Rev. 2013;37:2059–2070. doi: 10.1016/j.neubiorev.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- 43.Topic B, Kröger I, Vildirasova PG, Huston JP. Indices of extinction-induced “depression” after operant learning using a runway vs. a cued free-reward delivery schedule. Neurobiol Learn Mem. 2012;98:329–340. doi: 10.1016/j.nlm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Sepulveda JA, Flagel SB, Garcia-Fuster MJ, Slusky RJ, Aldridge JW, Watson S, Akil H. Differential impact of a complex environment on positive affect in an animal model of individual differences in emotionality. Neuroscience. 2013;248:436–447. doi: 10.1016/j.neuroscience.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


