Abstract
Ageing is the primary risk factor for cognitive deterioration. Given that the cerebral blood flow (CBF) or regulation of cerebral circulation is attenuated in the elderly, it could be expected that ageing-induced cognitive deterioration may be affected by a decrease in CBF as a result of brain ischemia and energy depletion. CBF regulation associated with cerebral metabolism thus likely plays an important role in the preservation of cognitive function. However, in some specific conditions (e.g. during exercise), change in CBF does not synchronize with that of cerebral metabolism. Our recent study demonstrated that cognitive function was more strongly affected by changes in cerebral metabolism than by changes in CBF during exercise. Therefore, it remains unclear how an alteration in CBF or its regulation affects cognitive function. In this review, I summarize current knowledge on previous investigations providing the possibility of an interaction between regulation of CBF or cerebral metabolism and cognitive function.
Keywords: Dementia, Cerebral disease, Vascular disease, Vascular risk factors, Autonomic dysfunction, Atherosclerosis, Neurovascular coupling
Introduction
It is generally believed that risk factors for the development of dementia include ageing, family history, low levels of physical activity, education, and the presence of the epsilon 4 allele of the apolipoprotein E (ApoE 4) gene [17, 27, 32, 62, 66, 75]. However, the physiological mechanism of alteration in cognitive function remains unknown.
Recently, some clinical investigations have highlighted the significant contribution of cerebral and vascular diseases in the development of cognitive dysfunction and dementia [62]. It is well known that these diseases also alter cerebral blood flow (CBF) regulation chronically. In addition, the previous studies [1, 9, 30, 55, 69, 73, 74] in healthy subjects demonstrated that several specific conditions (e.g., exercise, hypoxia, heat stress etc.) affect cognitive function with change in CBF. Under these conditions, it could be expected that CBF and its regulation may be a key factor in altering cognitive function. However, it remains unclear how an alteration in CBF and its regulation affects cognitive function. First of all, I summarize recent clinical and physiological investigations, suggesting that change in CBF affects cognitive function.
Cognitive dysfunction occurs with high risk factors for cerebral or vascular diseases
Dementia often occurs together with stroke, and the prevalence of cognitive impairment after stroke is high, particularly after stroke recurrence. However, the mechanism linking stroke to cognitive impairment or dementia remains unclear.
A previous study by Hennerici [20] proposed two different hypotheses for the mechanism of stroke-induced dementia. One hypothesis is that the central role of the stroke itself is related to the development of cognitive impairment, indicating that brain metabolic function associated with stroke itself may interact with cognitive function. The other involves the role of stroke-related deterioration of pre-existing systemic vascular factors, rather than the role of the stroke alone. Indeed, it has been suggested that vascular risk factors for cerebral or vascular disease including hypertension, atherosclerosis, diabetes mellitus, high cholesterol, atrial fibrillation, dyslipidemia, smoking, obesity, and alcohol consumption are associated with cognitive impairment [62]. Interestingly, maintaining ideal vascular health from young adulthood to middle age could be related to better cognitive performance later in life [58]. These findings support the second hypothesis of Hennerici [20] and suggest that cognitive function or the pathogenesis of dementia may be more affected by chronic alteration in vascular risk factors associated with cerebral or vascular disease rather than these diseases alone.
The mechanisms of cerebral and cardiovascular disease risk factor-induced cognitive dysfunction or dementia
Autonomic function could be a predictor of cognitive impairment as it is one of the fundamental homeostatic mechanisms for regulating blood pressure and metabolic rate [8, 61]. Indeed, abnormal autonomic function, including parasympathetic depression and sympathetic exacerbation, has been reported in patients with Alzheimer’s disease [10]. Also, associations between autonomic nervous system dysfunction and dementia [12, 70] or memory impairment [65, 72] have been reported.
Beside autonomic dysfunction, the high risk factors for cerebral or vascular disease also impair regulation of CBF. For example, hypertension results in vascular hypertrophy and remodeling and promotes atherosclerosis in large cerebral arteries and lipohyalinosis in penetrating arterioles [11, 13]. These structural vascular alterations facilitate vascular occlusions and compromise cerebral perfusion as well as the function of cerebral blood vessels, including impairments in endothelium-dependent relaxation [14] and cerebrovascular autoregulation [53]. In addition to abnormal vasculature, resting CBF, metabolism, and cognitive function are reduced in patients with hypertension [16]. On the other hand, atherosclerosis causes irregular vessel lumens, compression or local thickness, which alters smooth blood flow pattern [37]. In addition, the autonomic nervous system affects dynamic CBF regulation [45, 52, 79]. Ogoh et al. [45, 50] have examined CBF responses to acute hypotension with alpha (1)-adrenoreceptor blockade (prazosin) and strongly suggested that in humans sympathetic neural control of the cerebral vasculature provides dynamic functional control of CBF. Considering this evidence, it is highly likely that alteration in vascular risk factor-induced attenuation in CBF or dysfunction of CBF regulation is associated with cognitive function.
An adequate global CBF is needed for an increasing regional CBF for neural coupling to maintain brain function; does global CBF affect regional CBF for neural coupling?
The brain always requires a continuous supply of glucose and oxygen from the cerebral circulation in order to maintain brain function because its energy reserve is relatively small. The close spatial and temporal relationship between neural activity and regional CBF is termed “neurovascular coupling.” During synaptic activity, a sudden increase in the demand for energy could result in a relative lack of oxygen and glucose in the brain. Thus, it is thought that cognitive function is determined by an adequate increase in regional CBF for neural coupling.
It is well established that CBF is altered under a number of conditions [41–43, 47, 49, 63], and the CBF responses to physiological stress may be important to maintain brain metabolism and function [4]. In contrast, disrupted interaction between neural activity and global CBF may contribute to brain dysfunction. Indeed, cerebrovascular dysfunction often precedes the onset of cognitive impairment, suggesting its role in the mechanism of dementia [22].
The increase in regional CBF in the posterior parietal and thalamic areas is attenuated during cognitive tasks in patients with untreated chronic hypertension [26]. Similarly, the increase in regional CBF produced with a simple motor task (hand grip or finger tapping) is attenuated in patients with ischemic stroke [29], lacunar strokes causing limb weakness [54], and extracranial or intracranial artery disease [18]. Neural impairment also corresponds with the severity of cortical ischemia [4]. Interestingly, mechanical occlusion of both intracranial carotid arteries and one vertebral artery decreases regional CBF response to a simple motor task [60]. These findings indicate, thus, that global or focal cerebral ischemia exerts profound influences on the regional CBF regulation associated with neurovascular coupling. In patients with dementia, cerebrovascular structure was shown to be altered; as a result, the number of cerebral microvessels reduced, and flattened endothelial cells and smooth muscle cell degeneration occurred [15]. In addition, resting CBF is reduced, and the increase in regional CBF produced due to several physiological stimuli is attenuated in patients with Alzheimer’s disease [21, 35, 76]. These findings provide the evidence that the regulation of global CBF affects the response of regional CBF to neural coupling.
In patients with Alzheimer’s disease, dysregulation of the cerebral circulation, including endothelium-dependent responses [23], functional hyperemia [40], cerebral autoregulation [38], and responses to vasoconstrictors [39], occurs. The reduction in cerebral perfusion may attenuate cerebral protein synthesis and can thus promote ischemic lesions, which act synergistically with amyloid β-peptide (Aβ) to exacerbate the dementia [22]. An increase in Aβ levels in the neuropil (neuritic plaques) and blood vessels (amyloid angiopathy) is a characteristic of patients with Alzheimer’s disease [64]. Furthermore, insufficient CBF may alter Aβ trafficking across the blood–brain barrier [80], and reduced CBF may slow down Aβ clearance and promote its accumulation in the brain. Altogether, this previous research provides the possibility that structural and functional alterations of the cerebral microvasculature could affect resting CBF or regional CBF regulation and consequently contribute to the mechanisms of brain dysfunction underlying dementia.
Alteration in CBF regulation also affects cognitive function without high risk factors for cerebral or vascular disease
Many previous investigations regarding high risk factors for cerebral or vascular diseases and neural coupling provided the possibility that inadequate global CBF supply appears to cause brain dysfunction, subsequently resulting in dementia. Thus, it is expected that adequate CBF regulation is an important physiological factor for maintaining brain function. However, these clinical investigations could not provide the evidence that change in CBF affects cognitive function because many physiological factors modify cognitive function chronically in patients with cerebral or vascular disease, or dementia. In this section, I would like to discuss the research which investigated the effect on cognitive function of an alteration in CBF or its regulation (i.e., cerebral metabolism) under several conditions without any diseases. I believe that data from this research could provide important information about the relationship between CBF and cognitive function.
The alteration in CBF and its regulation during several physiological stresses likely affects cognitive function
The transient occlusion of CBF in patients with vascular disease was shown to reversibly impair cognition [33]. This finding suggests that an acute change in CBF modifies cognitive function; however, this response may be specific to these patients.
It has been reported that several physiological stresses affect cognitive function with the alteration in CBF and its regulation. For example, heat stress resulted in an expected decrease in anterior CBF [48] and caused cognitive impairment [30, 67, 69]. In addition, an oleuropein (a free radical scavenger)-induced decrease in oxidative stress attenuated cognitive function [1, 55]. Similarly, high-altitude exposure impaired cognitive function [9, 73, 74]. These findings of previous studies likely suggest that decrease in CBF is a key factor in determining cognitive function during these conditions. However, under hypoxic conditions, CBF increases acutely to preserve oxygen delivery to the brain [41] and to modify the systemic vascular system [28]. Hypoxia also affects other physiological factors [41, 42] as is the case with cerebral or vascular disease; thus, the relationship between the CBF response and cognitive function during hypoxia remains controversial. It remains difficult to identify the direct effect of change in CBF on cognitive function because studies on healthy individuals have not manipulated changes in CBF. However, these findings provide the possibility that acute alteration in CBF may affect cognitive function even without risk factors for cerebral or vascular disease.
An isolated change in CBF by manipulating cerebral perfusion does not affect cognitive function
With this in mind, we recently for the first time examined the effect of an isolated change in CBF on cognitive function in humans by manipulating CBF [51]. During dynamic exercise at mild to moderate intensity, increases in cerebral metabolism or cerebral neural activity [24, 42, 43] are paralleled by transient increases in CBF [19, 44, 46, 63]. Indeed, cognitive function improves during a single bout of moderate exercise [3, 34]. In contrast, during prolonged dynamic exercise, CBF gradually decreases toward resting values, and this is associated with hyperventilation [46]. Thus, in this study, we hypothesized that cognitive function is impaired in response to a decrease in CBF during prolonged exercise, and it can be restored with an artificial increase in CBF. However, in contrast to our hypothesis, cognitive function has been found to improve during prolonged exercise despite a decrease in CBF [51]. In addition, unexpectedly, an isolated change (hypercapnia-induced increase) in CBF by manipulating cerebral perfusion did not affect cognitive function at rest or during exercise (Fig. 1). These findings suggest that exercise-induced improvement in cognitive function is not simply due to change in global CBF. Thus, these findings suggest that another factor modified by exercise, rather than change in CBF, affects cognitive function. However, the mechanism of exercise-induced improvement in cognitive function remains unknown.
Fig. 1.
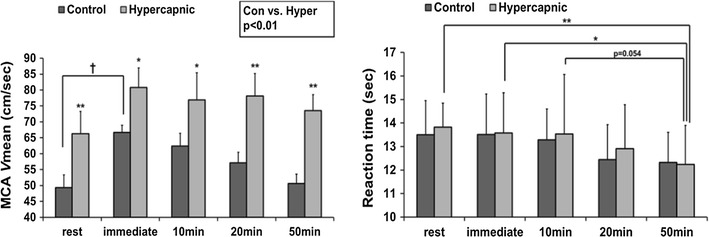
Despite a decrease in the mean blood flow velocity in the middle cerebral artery (MCA V mean), the reaction time of the Stroop test gradually decreased during prolonged exercise (50 min), indicating that cognitive function improves. In addition, the hypercapnia-induced increase in MCA V mean did not alter the reaction time at rest or during exercise [51]
(Modified from Ogoh et al., Physiol Rep, 2014)
Cerebral metabolism may be important for determining cognitive function, rather than change in CBF
Prolonged exercise [49, 51] or exercise under mild normobaric hypoxia induced a decrease in cerebral oxygenation [2] but these conditions did not impair cognitive function in healthy young subjects. These findings suggest that with respect to cognitive function, an alteration in CBF with decreased oxygen delivery to the brain may not correspond to cerebral metabolism or cognitive function. Indeed, previous studies demonstrated that during heavy exercise, compensatory increases in the uptake (arterial-venous difference) of lactate, glucose, and oxygen support the elevation of brain neural activity and metabolism when CBF decreases [24]. Considering that increases in brain neural activity and metabolism are not consistent with increases in CBF [36], cognitive function may be affected by extensive activation of motor and sensory systems due to the higher-order function of the prefrontal cortex, rather than by cerebral perfusion during exercise. On the other hand, more recently, we have demonstrated that cognitive function during exercise may be related to change in blood lactate concentration [71]. In our study, prolonged exercise improved cognitive function, but repeated (2nd) prolonged exercise attenuated this cognitive improvement along with a lack of blood lactate accumulation (Fig. 2). This finding indicates that neuro-humoral and metabolite responses, rather than a change in CBF, are associated with cognitive function during and after exercise.
Fig. 2.
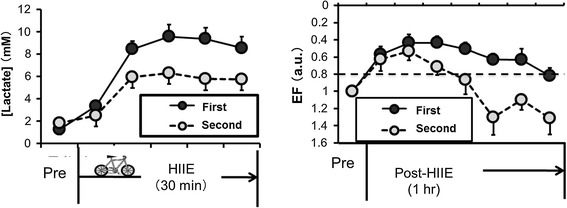
Each subject performed high-intensity interval cycling exercises (HIIE), which consisted of four 4-min bouts at 90% peak oxygen uptake (O2) with a 3-min active recovery period at 60% peak O2. After the first HIIE, executive function (EF) significantly improved and the improved EF was sustained during 40 min of post-exercise recovery. However, repeated bouts of HIIE, which decreased lactate accumulation, dampened the positive effect of exercise on EF during the post-exercise recovery [71]
(Modified from Tsukamoto et al., Physiol & Behav, 2016b)
Moreover, another previous study [25] demonstrated that cognitive function is associated with type of food intake. Cognitive function decline throughout the morning is significantly reduced following intake of a low glycemic index cereal as compared with that of a high glycemic index cereal. These findings suggest that even an acute change in cerebral metabolism associated with food intake may affect cognitive function.
It also has been reported that intravenously or intranasally administered insulin improves cognitive function (memory) in patients with dementia [7, 56, 57]. Insulin resistance is linked to an increased risk for both Alzheimer’s disease [6, 77] and cognitive decline [77, 78]. Although the mechanistic relationship between insulin resistance and cognitive decline remains unclear, it has been reported that insulin signaling is linked to neurotransmission [68] and is impaired in the postmortem brain of individuals with Alzheimer’s disease [31]. However, some previous studies did not show the beneficial effects of insulin on Alzheimer’s disease; insulin’s therapeutic effects may not improve cognitive function in apolipoprotein E (APOE) ε4 carriers [59]. Furthermore, the relationship between circulating insulin, cognition, and brain structure may differ between individuals with normal cognition and Alzheimer’s disease [5]. However, regarding therapy for preventing the acceleration of cognitive dysfunction in patients with dementia, control of their cerebral metabolism (via exercise, diet, administered insulin, and so on) may be important.
Summary
Clinical research provides the possibility that impaired CBF and its regulation causes cognitive dysfunction or dementia. On the other hand, some previous studies in healthy individuals demonstrated that cognitive function is associated with acute change in CBF during some physiological stresses. However, our recent study demonstrated acute change in CBF did not affect cognitive function. Cerebral metabolism may be an important physiological factor that determines cognitive function rather than CBF, although it is also affected by acute or chronic change in CBF. However, we do not have any evidence regarding the physiological mechanism of onset of dementia or cognitive dysfunction from previous investigations. Thus, further studies are needed to identify the relationships between cerebral metabolism, CBF, its regulation, and cognitive function in order to prevent cognitive dysfunction or dementia.
Acknowledgements
I am grateful to Dr. Shoichi Ando and Dr. Takeshi Hashimoto for their comments.
Compliance with ethical standards
Disclosures
No conflicts of interest, financial or otherwise, are declared by the author.
References
- 1.Alirezaei M, Rezaei M, Hajighahramani S, Sookhtehzari A, Kiani K. Oleuropein attenuates cognitive dysfunction and oxidative stress induced by some anesthetic drugs in the hippocampal area of rats. J Physiol Sci. 2017;67(1):131–139. doi: 10.1007/s12576-016-0446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando S, Hatamoto Y, Sudo M, Kiyonaga A, Tanaka H, Higaki Y. The effects of exercise under hypoxia on cognitive function. PLoS One. 2013;8:e63630. doi: 10.1371/journal.pone.0063630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brisswalter J, Collardeau M, Rene A. Effects of acute physical exercise characteristics on cognitive performance. Sports Med. 2002;32:555–566. doi: 10.2165/00007256-200232090-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bundo M, Inao S, Nakamura A, Kato T, Ito K, Tadokoro M, Kabeya R, Sugimoto T, Kajita Y, Yoshida J. Changes of neural activity correlate with the severity of cortical ischemia in patients with unilateral major cerebral artery occlusion. Stroke. 2002;33:61–66. doi: 10.1161/hs0102.101816. [DOI] [PubMed] [Google Scholar]
- 5.Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094–1104. doi: 10.1212/01.wnl.0000276952.91704.af. [DOI] [PubMed] [Google Scholar]
- 6.Cheng D, Noble J, Tang MX, Schupf N, Mayeux R, Luchsinger JA. Type 2 diabetes and late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;31:424–430. doi: 10.1159/000324134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daulatzai MA. Dysfunctional nucleus tractus solitarius: its crucial role in promoting neuropathogenetic cascade of Alzheimer’s dementia—a novel hypothesis. Neurochem Res. 2012;37:846–868. doi: 10.1007/s11064-011-0680-2. [DOI] [PubMed] [Google Scholar]
- 9.de Aquino Lemos V, Antunes HK, dos Santos RV, Lira FS, Tufik S, de Mello MT. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology. 2012;49:1298–1306. doi: 10.1111/j.1469-8986.2012.01411.x. [DOI] [PubMed] [Google Scholar]
- 10.de Vilhena Toledo MA, Junqueira LF., Jr Cardiac sympathovagal modulation evaluated by short-term heart interval variability is subtly impaired in Alzheimer’s disease. Geriatr Gerontol Int. 2008;8:109–118. doi: 10.1111/j.1447-0594.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson CJ. Why are strokes related to hypertension? Classic studies and hypotheses revisited. J Hypertens. 2001;19:1515–1521. doi: 10.1097/00004872-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Duschek S, Muckenthaler M, Werner N, del Paso GA. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol Psychol. 2009;81:110–117. doi: 10.1016/j.biopsycho.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Faraci FM, Baumbach GL, Heistad DD. Cerebral circulation: humoral regulation and effects of chronic hypertension. J Am Soc Nephrol. 1990;1:53–57. doi: 10.1681/ASN.V1153. [DOI] [PubMed] [Google Scholar]
- 14.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- 16.Fujishima M, Ibayashi S, Fujii K, Mori S. Cerebral blood flow and brain function in hypertension. Hypertens Res. 1995;18:111–117. doi: 10.1291/hypres.18.111. [DOI] [PubMed] [Google Scholar]
- 17.Guaita A, Vaccaro R, Davin A, Colombo M, Vitali SF, Polito L, Abbondanza S, Valle E, Forloni G, Ferretti VV, Villani S. Influence of socio-demographic features and apolipoprotein E epsilon 4 expression on the prevalence of dementia and cognitive impairment in a population of 70–74-year olds: the InveCe.Ab study. Arch Gerontol Geriatr. 2015;60:334–343. doi: 10.1016/j.archger.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Hamzei F, Knab R, Weiller C, Rother J. The influence of extra- and intracranial artery disease on the BOLD signal in FMRI. Neuroimage. 2003;20:1393–1399. doi: 10.1016/S1053-8119(03)00384-7. [DOI] [PubMed] [Google Scholar]
- 19.Hellstrom G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. [DOI] [PubMed] [Google Scholar]
- 20.Hennerici MG. What are the mechanisms for post-stroke dementia? Lancet Neurol. 2009;8:973–975. doi: 10.1016/S1474-4422(09)70261-3. [DOI] [PubMed] [Google Scholar]
- 21.Hock C, Villringer K, Muller-Spahn F, Wenzel R, Heekeren H, Schuh-Hofer S, Hofmann M, Minoshima S, Schwaiger M, Dirnagl U, Villringer A. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer’s disease monitored by means of near-infrared spectroscopy (NIRS)–correlation with simultaneous rCBF-PET measurements. Brain Res. 1997;755:293–303. doi: 10.1016/S0006-8993(97)00122-4. [DOI] [PubMed] [Google Scholar]
- 22.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 23.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 24.Ide K, Schmalbruch IK, Quistorff B, Horn A, Secher NH. Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J Physiol. 2000;522(Pt 1):159–164. doi: 10.1111/j.1469-7793.2000.t01-2-00159.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingwersen J, Defeyter MA, Kennedy DO, Wesnes KA, Scholey AB. A low glycaemic index breakfast cereal preferentially prevents children’s cognitive performance from declining throughout the morning. Appetite. 2007;49:240–244. doi: 10.1016/j.appet.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- 27.Jones EL, Kalaria RN, Sharp SI, O’Brien JT, Francis PT, Ballard CG. Genetic associations of autopsy-confirmed vascular dementia subtypes. Dement Geriatr Cogn Disord. 2011;31:247–253. doi: 10.1159/000327171. [DOI] [PubMed] [Google Scholar]
- 28.Katayama K, Ishida K, Saito M, Koike T, Ogoh S. Hypoxia attenuates cardiopulmonary reflex control of sympathetic nerve activity during mild dynamic leg exercise. Exp Physiol. 2016;101:377–386. doi: 10.1113/EP085632. [DOI] [PubMed] [Google Scholar]
- 29.Krainik A, Hund-Georgiadis M, Zysset S, von Cramon DY. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke. 2005;36:1146–1152. doi: 10.1161/01.STR.0000166178.40973.a7. [DOI] [PubMed] [Google Scholar]
- 30.Liu K, Sun G, Li B, Jiang Q, Yang X, Li M, Li L, Qian S, Zhao L, Zhou Z, von Deneen KM, Liu Y. The impact of passive hyperthermia on human attention networks: an fMRI study. Behav Brain Res. 2013;243:220–230. doi: 10.1016/j.bbr.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225:54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag. 2016;12:105–116. doi: 10.2147/VHRM.S75306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall RS, Lazar RM, Pile-Spellman J, Young WL, Duong DH, Joshi S, Ostapkovich N. Recovery of brain function during induced cerebral hypoperfusion. Brain. 2001;124:1208–1217. doi: 10.1093/brain/124.6.1208. [DOI] [PubMed] [Google Scholar]
- 34.McMorris T, Sproule J, Turner A, Hale BJ. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: a meta-analytical comparison of effects. Physiol Behav. 2011;102:421–428. doi: 10.1016/j.physbeh.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Mentis MJ, Horwitz B, Grady CL, Alexander GE, VanMeter JW, Maisog JM, Pietrini P, Schapiro MB, Rapoport SI. Visual cortical dysfunction in Alzheimer’s disease evaluated with a temporally graded “stress test” during PET. Am J Psychiatry. 1996;153:32–40. doi: 10.1176/ajp.153.1.32. [DOI] [PubMed] [Google Scholar]
- 36.Miyazawa T, Horiuchi M, Ichikawa D, Sato K, Tanaka N, Bailey DM, Ogoh S. Kinetics of exercise-induced neural activation; interpretive dilemma of altered cerebral perfusion. Exp Physiol. 2012;97:219–227. doi: 10.1113/expphysiol.2011.061978. [DOI] [PubMed] [Google Scholar]
- 37.Nagata K, Yamazaki T, Takano D, Maeda T, Fujimaki Y, Nakase T, Sato Y. Cerebral circulation in aging. Ageing Res Rev. 2016;30:49–60. doi: 10.1016/j.arr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- 39.Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001;281:H2417–H2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- 40.Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogoh S. Cerebral blood flow regulation during hypoxia. Exp Physiol. 2015;100:109–110. doi: 10.1113/expphysiol.2014.084202. [DOI] [PubMed] [Google Scholar]
- 42.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol. 2009;107:1370–1380. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 43.Ogoh S, Ainslie PN. Regulatory mechanisms of cerebral blood flow during exercise: new concepts. Exerc Sport Sci Rev. 2009;37:123–129. doi: 10.1097/JES.0b013e3181aa64d7. [DOI] [PubMed] [Google Scholar]
- 44.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, OY A, Raven PB. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol. 2005;569:697–704. doi: 10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke. 2008;39:1979–1987. doi: 10.1161/STROKEAHA.107.510008. [DOI] [PubMed] [Google Scholar]
- 46.Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, Secher NH. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1461–H1467. doi: 10.1152/ajpheart.00948.2004. [DOI] [PubMed] [Google Scholar]
- 47.Ogoh S, Nakahara H, Ueda S, Okazaki K, Shibasaki M, Subudhi AW, Miyamoto T. Effects of acute hypoxia on cerebrovascular responses to carbon dioxide. Exp Physiol. 2014;99:849–858. doi: 10.1113/expphysiol.2013.076802. [DOI] [PubMed] [Google Scholar]
- 48.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Morimoto K, Shibasaki M. Blood flow distribution during heat stress: cerebral and systemic blood flow. J Cereb Blood Flow Metab. 2013;33:1915–1920. doi: 10.1038/jcbfm.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Shibasaki M. Hyperthermia modulates regional differences in cerebral blood flow to changes in CO2. J Appl Physiol. 2014;117:46–52. doi: 10.1152/japplphysiol.01078.2013. [DOI] [PubMed] [Google Scholar]
- 50.Ogoh S, Sorensen H, Hirasawa A, Sasaki H, Washio T, Hashimoto T, Bailey DM, Secher NH. Dynamic cerebral autoregulation is unrelated to decrease in external carotid artery blood flow during acute hypotension in healthy young men. Exp Physiol. 2016;101:1040–1049. doi: 10.1113/EP085772. [DOI] [PubMed] [Google Scholar]
- 51.Ogoh S, Tsukamoto H, Hirasawa A, Hasegawa H, Hirose N, Hashimoto T. The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol Rep. 2014;2(9):e12163. doi: 10.14814/phy2.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogoh S, Tzeng YC, Lucas SJ, Galvin SD, Ainslie PN. Influence of baroreflex-mediated tachycardia on the regulation of dynamic cerebral perfusion during acute hypotension in humans. J Physiol. 2010;588:365–371. doi: 10.1113/jphysiol.2009.180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paterno R, Heistad DD, Faraci FM. Potassium channels modulate cerebral autoregulation during acute hypertension. Am J Physiol Heart Circ Physiol. 2000;278:H2003–H2007. doi: 10.1152/ajpheart.2000.278.6.H2003. [DOI] [PubMed] [Google Scholar]
- 54.Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke. 2002;33:103–109. doi: 10.1161/hs0102.100482. [DOI] [PubMed] [Google Scholar]
- 55.Pourkhodadad S, Alirezaei M, Moghaddasi M, Ahmadvand H, Karami M, Delfan B, Khanipour Z. Neuroprotective effects of oleuropein against cognitive dysfunction induced by colchicine in hippocampal CA1 area in rats. J Physiol Sci. 2016;66:397–405. doi: 10.1007/s12576-016-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reger MA, Watson GS, Frey WH, 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 57.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 58.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Jr, Zhu N, Lloyd-Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenbloom MH, Barclay TR, Pyle M, Owens BL, Cagan AB, Anderson CP, Frey WH, Hanson LR. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs. 2014;28:1185–1189. doi: 10.1007/s40263-014-0214-y. [DOI] [PubMed] [Google Scholar]
- 60.Rother J, Knab R, Hamzei F, Fiehler J, Reichenbach JR, Buchel C, Weiller C. Negative dip in BOLD fMRI is caused by blood flow–oxygen consumption uncoupling in humans. Neuroimage. 2002;15:98–102. doi: 10.1006/nimg.2001.0965. [DOI] [PubMed] [Google Scholar]
- 61.Rub U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The autonomic higher order processing nuclei of the lower brain stem are among the early targets of the Alzheimer’s disease-related cytoskeletal pathology. Acta Neuropathol. 2001;101:555–564. doi: 10.1007/s004010000320. [DOI] [PubMed] [Google Scholar]
- 62.Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. Int J Stroke. 2012;7:61–73. doi: 10.1111/j.1747-4949.2011.00731.x. [DOI] [PubMed] [Google Scholar]
- 63.Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol. 2011;589:2847–2856. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 65.Shah AJ, Su S, Veledar E, Bremner JD, Goldstein FC, Lampert R, Goldberg J, Vaccarino V. Is heart rate variability related to memory performance in middle-aged men? Psychosom Med. 2011;73:475–482. doi: 10.1097/PSY.0b013e3182227d6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shibasaki M, Namba M, Oshiro M, Crandall CG, Nakata H. The effect of elevations in internal temperature on event-related potentials during a simple cognitive task in humans. Am J Physiol Regul Integr Comp Physiol. 2016;311:R33–R38. doi: 10.1152/ajpregu.00086.2016. [DOI] [PubMed] [Google Scholar]
- 68.Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-d-aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun G, Yang X, Jiang Q, Liu K, Li B, Li L, Zhao L, Li M. Hyperthermia impairs the executive function using the Attention Network Test. Int J Hyperthermia. 2012;28:621–626. doi: 10.3109/02656736.2012.705217. [DOI] [PubMed] [Google Scholar]
- 70.Toledo MA, Junqueira LF., Jr Cardiac autonomic modulation and cognitive status in Alzheimer’s disease. Clin Auton Res. 2010;20:11–17. doi: 10.1007/s10286-009-0035-0. [DOI] [PubMed] [Google Scholar]
- 71.Tsukamoto H, Suga T, Takenaka S, Tanaka D, Takeuchi T, Hamaoka T, Isaka T, Ogoh S, Hashimoto T. Repeated high-intensity interval exercise shortens the positive effect on executive function during post-exercise recovery in healthy young males. Physiol Behav. 2016;160:26–34. doi: 10.1016/j.physbeh.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 72.Vasudev A, Saxby BK, O’Brien JT, Colloby SJ, Firbank MJ, Brooker H, Wesnes K, Thomas AJ. Relationship between cognition, magnetic resonance white matter hyperintensities, and cardiovascular autonomic changes in late-life depression. Am J Geriatr Psychiatry. 2012;20:691–699. doi: 10.1097/JGP.0b013e31824c0435. [DOI] [PubMed] [Google Scholar]
- 73.Virues-Ortega J, Buela-Casal G, Garrido E, Alcazar B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol Rev. 2004;14:197–224. doi: 10.1007/s11065-004-8159-4. [DOI] [PubMed] [Google Scholar]
- 74.Virues-Ortega J, Garrido E, Javierre C, Kloezeman KC. Human behaviour and development under high-altitude conditions. Dev Sci. 2006;9:400–410. doi: 10.1111/j.1467-7687.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 75.Wallin K, Bostrom G, Kivipelto M, Gustafson Y. Risk factors for incident dementia in the very old. Int Psychogeriatr. 2013;25:1135–1143. doi: 10.1017/S1041610213000409. [DOI] [PubMed] [Google Scholar]
- 76.Warkentin S, Passant U. Functional imaging of the frontal lobes in organic dementia. Regional cerebral blood flow findings in normals, in patients with frontotemporal dementia and in patients with Alzheimer’s disease, performing a word fluency test. Dement Geriatr Cogn Disord. 1997;8:105–109. doi: 10.1159/000106614. [DOI] [PubMed] [Google Scholar]
- 77.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Young SE, Mainous AG, 3rd, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care. 2006;29:2688–2693. doi: 10.2337/dc06-0915. [DOI] [PubMed] [Google Scholar]
- 79.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.CIR.0000031798.07790.FE. [DOI] [PubMed] [Google Scholar]
- 80.Zlokovic BV, Deane R, Sallstrom J, Chow N, Miano JM. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005;15:78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


