Abstract
The present study examined the effects of nicotinic acetylcholine receptor activation on the odor-induced blood flow response in the olfactory bulb. In urethane-anesthetized rats, odor stimulation (5% amyl acetate, 30 s) produced an increase in olfactory bulb blood flow (reaching 107% ± 3% of the pre-stimulus basal values), without changes in frontal cortical blood flow or mean arterial pressure. Intravenous injection of nicotine (30 μg/kg), a nicotinic acetylcholine receptor agonist, significantly augmented the odor-induced increase response of olfactory bulb blood flow, without changes in the basal blood flow level. The nicotine-induced augmentation of the olfactory bulb blood flow response to odor was negated by dihydro-β-erythroidine, an α4β2-preferring nicotinic acetylcholine receptor antagonist. Our results suggest that the activation of α4β2-like neuronal nicotinic acetylcholine receptors in the brain potentiates an odor-induced blood flow response in the olfactory bulb.
Keywords: Olfactory bulb, Nicotinic acetylcholine receptor, Regional cerebral blood flow, Odor stimulation, Rat
Introduction
The intracerebral cholinergic system originating in the basal forebrain and projecting to the neocortex, hippocampus, and olfactory bulb contributes to cognition, memory, and olfactory functions, respectively [1–3]. Our research group has previously demonstrated that in the rat, activation of the basal forebrain cholinergic neurons or intravenous injection of nicotine (30 μg/kg) produces an increase in regional blood flow in the neocortex via activation of α4β2-like neuronal nicotinic acetylcholine (ACh) receptors [4–9], but not in the olfactory bulb in rats [10, 11]. In contrast, odor stimulation increases regional blood flow in the rodent olfactory bulb, in association with neuronal activities [12–15]. D’Souza et al. [16] showed that α4β2-like neuronal nicotinic ACh receptors are significant contributors to the excitation of neurons in the mice olfactory bulb in an in vitro electrophysiological study.
Therefore, the present study aimed to clarify, first, the effect of stimulation of nicotinic ACh receptors on the odor-induced increase in regional blood flow in the olfactory bulb, and second, the involvement of α4β2-like nicotinic ACh receptors on its response, in in vivo recording of regional blood flow in the rat olfactory bulb. For comparison, regional blood flow in the neocortex (frontal cortex) and systemic arterial pressure were simultaneously measured.
Materials and methods
The experiments were performed on 10 male adult Wistar rats (body weight, 370–450 g; 5–8 months old), bred at the Tokyo Metropolitan Institute of Gerontology. The study was conducted with the approval of and in accordance with the guidelines for animal experimentation prepared by the Animal Care and Use Committee of Tokyo Metropolitan Institute of Gerontology.
General surgery and anesthesia
The rats were anesthetized with urethane (1.1–1.4 g/kg, subcutaneously). Double tracheotomy surgery was performed. A polyethylene tube [inner diameter (ID) 2.0 mm, outer diameter (OD) 2.7 mm] was inserted 5 mm rostrally into the larynx. A tracheal tube was inserted caudally into the trachea, and respiration was maintained by means of an artificial respirator (model 683, Harvard, MA, USA). The end-tidal CO2 concentration was maintained at 3.0–4.0% by monitoring with a respiratory gas monitor (Microcap, Oridion Medical, Jerusalem, Israel). The arterial blood pressure was measured through a catheter inserted into a femoral artery with a pressure transducer (TP-400T, Nihon Kohden, Tokyo, Japan). Body temperature was measured rectally and continuously using a thermistor and maintained at approximately 37.5 °C by means of a body temperature control system (ATB-1100, Nihon Kohden). The depth of anesthesia was adjusted by additional urethane doses (100 mg/kg, intravenously or subcutaneously via a catheter inserted into a femoral vein) when necessary and by monitoring body movement, blood pressure stability, and respiratory movement.
Measurement of regional blood flow in the olfactory bulb and frontal cortex
Reginal blood flow in the olfactory bulb and the frontal cortex was measured using laser Doppler flowmetry as described previously [10, 17, 18]. The animals were mounted on a stereotaxic instrument (SR-5R-HT, Narishige, Tokyo, Japan) in a prone position. After craniotomy, two recording probes (diameter, 0.8 mm) of the laser Doppler flowmeter (ALF 21D, Advance, Tokyo, Japan) were placed, avoiding the visible blood vessels, on the dorsal surfaces of the olfactory bulb [anteroposterior (AP) = + 7.0 to + 8.2 mm from bregma, lateral (L) = 0.7–1.5 mm to the midline] and the frontal lobe (AP = + 2.0 to + 3.5 mm, L = 2.0–3.5 mm) [19, 20] (Fig. 1). The flowmeter probes were fixed with balancing holders (ALF-B, Advance). The output signal of the laser Doppler flowmeter was expressed in mV and recorded on a recorder as well as on a PC using an A/D converter (Micro1401 mk II, Cambridge Electronic Design, Cambridge, UK) with Spike2 software (Spike2, Cambridge Electronic Design) for offline analyses.
Fig. 1.
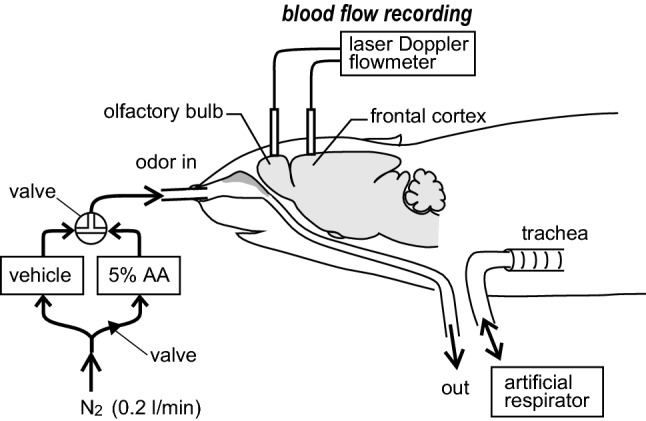
Schematic diagram of the experimental procedures. Regional blood flow in the olfactory bulb and frontal cortex were measured by laser Doppler flowmetry. Odor (5% amyl acetate (AA)] was applied by constant flow of humidified nitrogen into the nose using a custom-built odor delivery system. The flow passes through a tube inserted into the larynx. A tracheal tube was inserted caudally into the trachea, and respiration was artificially maintained using a respirator. Vehicle is 100% propylene glycol
Odor stimulation
Odor [5% amyl acetate (AA)] was applied by continuous flow of humidified medical-grade nitrogen into the nose using a custom-built odor delivery system with a solenoid valve (LHDA12221211H, The Lee Company, Connecticut, USA, set up by Ono-Denki Co., Ltd., Tokyo, Japan) (Fig. 1). The flow rate in this system was set at 0.2 l/min, which is within the flow rate range of inspiratory and expiratory sniffing by awake behaving rats [21]. The solenoid valve diverts flow to one of two 500 ml bottles containing 100% propylene glycol (vehicle) or 5% AA in propylene glycol. This system was constructed with dedicated glass and Teflon tubing (ID 3 mm, OD 4 mm) to minimize contamination and absorption of odor. A one-way check valve (indicated by a triangle arrowhead in Fig. 1, VSCV6733, Tokyo Garasu Kikai, Tokyo, Japan) was placed at the entrance to the odor bottle to avoid contamination. The output line from the solenoid valve was made of a small-diameter polyethylene tube (SP45, ID 0.58 mm, OD 0.96 mm, Natsume, Tokyo, Japan), which then divided into two tubes (SP45, Natsume), reaching into the bilateral nostrils. The two tubes were sealed to the nose tissues using cyanoacrylate glue to prevent flow leakage. The dead volume from the solenoid valve to the bilateral nostrils was approximately 0.35 ml, allowing rapid transition of odor stimulation. The exhaust air was allowed to flow out through a tube inserted into the larynx. The exhaust air was bubbled through distilled water in a flask and then filtered through activated charcoal before release to the atmosphere.
The odor stimulation-induced increase in blood flow in the olfactory bulb was repeatedly measured by delivering odor (5% AA) for 30 s at 5–7 min intervals. This duration and interval of odor stimulations are based on reports by Poplawsky et al. [14, 22], who recorded the blood flow response of the olfactory bulb to odor (5% AA) stimulation for 64 s at 4 min intervals. We confirmed the reproducibility of the odor-induced blood flow responses in the olfactory bulb in our experiment.
Drug administration
(−)Nicotine (Tokyo Kasei Kogyo, Tokyo, Japan) was diluted in saline to final doses of 30 μg/kg body weight (calculated as the free base). Saline or nicotine was injected slowly (duration of about 1 min) through a femoral vein. We chose a dose of 30 μg/kg of nicotine because our previous report showed that this dose is optimal for stimulating nicotinic cholinergic receptors in the brain parenchyma without causing marked changes in systemic arterial pressure [6]. In four rats, the α4β2-preferring nicotinic receptor antagonist dihydro-β-erythroidine hydrobromide (DHβE, Tocris, Bristol, UK, 5 mg/kg) was dissolved in saline and administrated intravenously as reported previously [8].
Statistical analysis
All values are presented as the mean ± standard error of mean (SEM). Statistical comparisons were performed by means of a one-way repeated measures analysis of variance (ANOVA) followed by a Dunnett’s multiple comparison test or paired t-test. A p value < 0.05 was considered statistically significant.
Results
Responses to odor stimulation of blood flow in the olfactory bulb and frontal cortex and mean arterial pressure
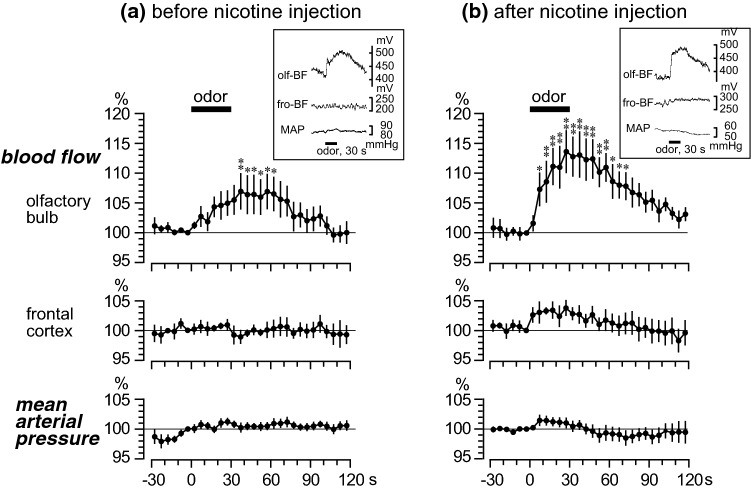
Figure 2a shows the responses of blood flow in the olfactory bulb and frontal cortex and the mean arterial pressure, following odor stimulation. The rate of change of these three parameters were averaged every 5 s in 6 rats. The blood flow in the olfactory bulb gradually increased following the 30-s odor stimulation, reaching a maximum of 107% ± 3% just after the end of the stimulation. The increased response of blood flow in the olfactory bulb was significant for 30 s; then it gradually returned to the pre-stimulus basal level. The blood flow in the frontal cortex and mean arterial pressure exhibited no obvious changes following odor stimulation.
Fig. 2.
Effect of nicotine on the odor-evoked responses in regional blood flow in the olfactory bulb and frontal cortex and mean arterial pressure (MAP). a Before nicotine injection. b After intravenous injection of nicotine (30 μg/kg). Mean values of blood flow (in the olfactory bulb and frontal cortex) and MAP during a 5-s period were plotted every 5 s as percentage of the pre-odor stimulus basal values (−5 to 0 s) (n = 6). (a For each rat, two trials were averaged. b For each rat, a trial obtained 5–10 min after intravenous injection of nicotine was summarized). Each point and vertical bar represent a mean ± SEM. *p < 0.05; **p < 0.01; significantly different from the pre-odor stimulus basal values (−5 to 0 s) using a one-way repeated measures analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Inset in a and b: sample recordings of regional blood flow in the olfactory bulb (olf-BF) and frontal cortex (fro-BF) and MAP to odor stimulation in one rat
Effect of intravenous injection of nicotine on the odor-induced responses
Before the nicotine injection, the basal levels of the blood flow in the olfactory bulb and frontal cortex and the mean arterial pressure were 316 ± 31 mV, 304 ± 30 mV, and 80 ± 4 mmHg, respectively. After the nicotine (30 μg/kg) injection, the basal level of blood flow in the olfactory bulb did not change in 4 of 6 rats, decreased about 10% in other 2 rats, and the total responses in all 6 rats were insignificant (309 ± 30 mV). In contrast, the blood flow in the frontal cortex significantly increased to 462 ± 46 mV (157% ± 8% of the pre-injection basal levels, p < 0.01) (values obtained at 7 min after nicotine injection). This increase in frontal cortical blood flow gradually returned to the pre-injection basal level after more than 15–35 min (varied in each rat). The basal level of the mean arterial pressure was slightly decreased to 71 ± 3 mmHg (88% ± 4% of the pre-injection basal levels, p < 0.05), 7 min after nicotine (30 μg/kg) injection. This depressor response was short-lasting and returned to the pre-injection basal level after another 5 min.
Figure 2b shows the odor-induced responses of blood flow in the olfactory bulb and frontal cortex and the mean arterial pressure, obtained at 5–10 min after nicotine (30 μg/kg) injection. The odor-induced percentage changes were calculated from the new basal levels altered by nicotine injection, although the basal level of the olfactory bulb blood flow was not significantly changed by the nicotine injection. The blood flow in the olfactory bulb increased rapidly after the onset of odor stimulation and reached a maximum of 114% ± 3% at the end of the stimulation. The significant increase in blood flow in the olfactory bulb started at 5 s after the onset of stimulation and lasted for 70 s. The blood flow in the frontal cortex and the mean arterial pressure marginally fluctuated during the odor stimulation, but the responses were not statistically significant.
Focusing on the blood flow response in the olfactory bulb, Fig. 3a summarizes the changes during the 60-s period after the onset of odor stimulation, expressed as percentages of the pre-stimulation values (−30 to 0 s). The increased blood flow response in the olfactory bulb was 104% ± 2% before nicotine injection, and it was significantly potentiated to 110% ± 3% after nicotine injection (obtained at 5–10 min after nicotine injection). The potentiating effect of nicotine on the odor-induced increase in blood flow in the olfactory bulb lasted until 15–35 min after injection (varied in each rat).
Fig. 3.
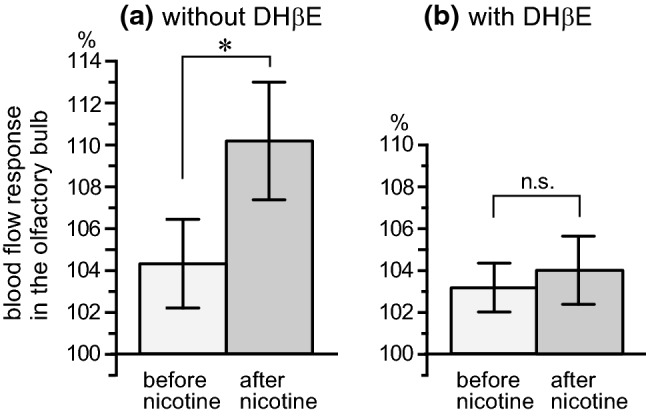
The odor-evoked responses of regional blood flow in the olfactory bulb to intravenous injection of nicotine without (a) and with (b) dihydro-β-erythroidine. The mean values of blood flow in the olfactory bulb during a 60-s period after the onset of odor stimulation were expressed as percentage of the pre-stimulus basal values (−30 to 0 s). In b, nicotine was injected 20–25 min after intravenous administration of dihydro-β-erythroidine (DHβE, 5 mg/kg). Each column and vertical bar represent the mean ± SEM (a: n = 6, b: n = 4). *p < 0.05; significantly different from the response before nicotine injection determined by a paired t-test
Intravenous injection of the same volume of saline instead of nicotine (30 μg/kg) did not change the blood flow (in the olfactory bulb and frontal cortex) or the mean arterial pressure, in both the basal level and the odor-induced olfactory bulb response.
Effect of neuronal α4β2-preference nicotinic receptor antagonist on nicotine-induced potentiation of olfactory bulb response to odor
Figure 3b summarizes the blood flow responses of the olfactory bulb to odor in rats pretreated with α4β2-preference nicotinic receptor antagonist (DHβE). Before the nicotine injection, the blood flow response of the olfactory bulb to odor stimulation in rats pretreated with DHβE (left column of Fig. 3b) was similar to that observed in rats without DHβE (left column of Fig. 3a). In contrast, the potentiation of the olfactory bulb response to odor stimulation after the nicotine (30 μg/kg) injection (right column of Fig. 3a) was negated in rats pretreated with DHβE (right column of Fig. 3b). The nicotine-induced increase in basal blood flow in the frontal cortex and the decrease in basal mean arterial pressure were also negated in rats pretreated with DHβE.
Discussion
This study showed that (1) odor stimulation increases blood flow in the olfactory bulb, without changes in neocortical blood flow and systemic blood pressure; (2) the increased response of the olfactory bulb blood flow is potentiated by intravenous injection of nicotine; and (3) the nicotine-induced augmentation of the olfactory bulb blood flow response to odor is negated by dihydro-β-erythroidine, an α4β2-preferring nicotinic ACh receptor antagonist, in vivo, in anesthetized rats. These results suggest that activation of α4β2-like nicotinic ACh receptors in the brain potentiates odor-induced blood flow response in the olfactory bulb.
It has been reported that odor stimulation increases regional blood flow in the olfactory bulb in association with neuronal activities in anesthetized animals [12, 14, 15, 23]. Consistent with these previous studies, in the present study, odor stimulation (5% AA) produced an increase in olfactory bulb blood flow without changes in systemic arterial pressure. Previous reports observing the odor-induced hemodynamic responses in the olfactory bulb did not measure the blood flow of the other cerebral regions [12, 14, 15]. Our present study measured the regional blood flow of the olfactory bulb and frontal cortex, as well as systemic arterial pressure, and found that odor stimulation increased blood flow in the olfactory bulb without changes in frontal cortical blood flow and systemic arterial pressure.
To our knowledge, this study is the first to demonstrate that activation of the nicotinic ACh receptors by intravenous injection of nicotine (30 μg/kg) potentiates the odor-induced increased response of olfactory bulb blood flow. The basal level of the olfactory bulb blood flow did not change after nicotine injection in most cases (4 of 6 rats), while it decreased slightly after nicotine injection in some cases (2 of 6 rats). Therefore, we cannot deny the possibility that the potentiating effect of nicotine can also be brought about by a relative increase of olfactory bulb blood flow response due to the decrease of its basal level after nicotine injection, in some cases.
Furthermore, in the present study, the α4β2-like neuronal nicotinic ACh receptors were found to be related to the nicotine effect in potentiating the odor-induced increased response of olfactory bulb blood flow. The role of α4β2-like neuronal nicotinic ACh receptors was also found in our previous study [8] observing vasodilation in the neocortex induced by intravenous injection of nicotine (30 μg/kg), which is the same amount used in the present study. Vasodilation in the neocortex following the intravenous injection of nicotine (30 μg/kg) was suggested by Uchida et al. [6] to result from the stimulation of the nicotinic ACh receptors (α4β2-subtype, as mentioned) in the neocortex and in the nucleus basalis of Meynert of the basal forebrain, which provides a major source of cholinergic projection into the neocortex [24, 25]. The olfactory bulb receives cholinergic neural inputs originating in the nucleus of the horizontal limb of the diagonal band of Broca (HDB) in the basal forebrain [24, 26]. In both the olfactory bulb and HDB cholinergic neurons, the expression of α4 and β2 nicotinic ACh receptor subunit mRNAs has been identified in rats [27–29]. Thus, the present findings of the nicotine-induced potentiation of olfactory sensory processing in the olfactory bulb could be due to the activation of the α4β2-like neuronal nicotinic ACh receptors in the olfactory bulb and/or in the HDB cholinergic neurons. Recent studies show that nicotinic acetylcholine receptors are expressed not only on neurons but also on non-neuronal cells (e.g., endothelial cells, glial cells) [30]. Location of nicotinic ACh receptors regarding brain region and cell type that relates to the present findings should be clarified in future studies.
D’Souza et al. [16] reported that activation of α4β2-like nicotinic ACh receptors depolarizes the neurons (external tufted cells) in the olfactory bulb, using in vitro electrophysiological techniques. Our present study showed that in in vivo blood flow recording experiments, activation of α4β2-like nicotinic ACh receptors potentiates the odor-induced blood flow response in the olfactory bulb. From the functional perspective, this potentiation of the odor-induced increase in olfactory bulb blood flow may contribute to the neuronal turnover in the olfactory bulb and the short-term olfactory memory [31–33].
In the present study, changes in olfactory bulb blood flow due to odor (104% before nicotine injection; and additional 6% boost available, that is 110%, after nicotine injection) was much smaller than the changes in the basal level of frontal cortical blood flow due to nicotine (157% of the pre-injection basal levels). A lower distribution density of α4 and β2 subtype nicotinic ACh receptors in the olfactory bulb [29] might be a reason for the varying response levels.
Age-related decline in β2-nicotinic ACh receptor availability in the brain, as well as in the neocortical region, has been reported [34]. In patients with mild Alzheimer’s dementia, compared with age-matched healthy controls, a widespread reduction of cholinergic α4β2-nicotinic ACh receptor availability, especially within the hippocampus, frontotemporal cortices and basal forebrain, has been reported [35]. The decline in α4β2-nicotinic ACh receptor availability, as well as the degeneration of basal forebrain cholinergic neurons [36–38], could explain the olfactory dysfunction in the early stage of Alzheimer’s disease [39–41].
In the present study, we measured the odor-induced olfactory bulb blood flow response which is reported to be associated with neuronal activity [12, 14, 15, 23]. In further experiments, for deeper understanding of cholinergic modulation of olfactory processing, simultaneously to the blood flow measurements, recording of neuronal activity by techniques such as field potential recording [42, 43], optical recording with a voltage-sensitive dye [44], and calcium imaging [45], might be required.
In conclusion, the present study has demonstrated that the activation of α4β2-like neuronal nicotinic ACh receptors potentiates olfactory blood flow response to odor. In a future study, age-related changes in the nicotinic ACh receptor function in potentiating the odor-induced olfactory bulb blood flow response should be clarified.
Author contributions
SU and FK conceived and designed the research; all the authors performed experiments, analyzed data, and interpreted the results of the experiments; SU drafted the manuscript; SU and FK edited and revised the manuscript; and all the authors approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant number 15K08225 to SU) and by the Smoking Research Foundation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies performed by any of the authors with human participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron. 2016;91:1199–1218. doi: 10.1016/j.neuron.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Souza RD, Vijayaraghavan S. Paying attention to smell: cholinergic signaling in the olfactory bulb. Front Synaptic Neurosci. 2014;6:21. doi: 10.3389/fnsyn.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micheau J, Marighetto A. Acetylcholine and memory: a long, complex and chaotic but still living relationship. Behav Brain Res. 2011;221:424–429. doi: 10.1016/j.bbr.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 4.Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- 5.Sato A, Sato Y. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Neurosci Res. 1992;14:242–274. doi: 10.1016/0168-0102(92)90071-J. [DOI] [PubMed] [Google Scholar]
- 6.Uchida S, Kagitani F, Nakayama H, Sato A. Effect of stimulation of nicotinic cholinergic receptors on cortical cerebral blood flow and changes in the effect during aging in anesthetized rats. Neurosci Lett. 1997;228:203–206. doi: 10.1016/S0304-3940(97)00401-1. [DOI] [PubMed] [Google Scholar]
- 7.Uchida S, Suzuki A, Kagitani F, Hotta H. Effects of age on cholinergic vasodilation of cortical cerebral blood vessels in rats. Neurosci Lett. 2000;294:109–112. doi: 10.1016/S0304-3940(00)01556-1. [DOI] [PubMed] [Google Scholar]
- 8.Uchida S, Hotta H, Kawashima K. Long-term nicotine treatment reduces cerebral cortical vasodilation mediated by alpha4beta2-like nicotinic acetylcholine receptors in rats. Eur J Pharmacol. 2009;609:100–104. doi: 10.1016/j.ejphar.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Uchida S, Hotta H, Misawa H, Kawashima K. Sustained subcutaneous infusion of nicotine enhances cholinergic vasodilation in the cerebral cortex induced by stimulation of the nucleus basalis of Meynert in rats. Eur J Pharmacol. 2011;654:235–240. doi: 10.1016/j.ejphar.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Shiba K, Machida T, Uchida S, Hotta H. Effects of nicotine on regional blood flow in the olfactory bulb in rats. Eur J Pharmacol. 2006;546:148–151. doi: 10.1016/j.ejphar.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 11.Uchida S, Kagitani F. Effect of basal forebrain stimulation on extracellular acetylcholine release and blood flow in the olfactory bulb. J Physiol Sci. 2018;68:415–423. doi: 10.1007/s12576-017-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaigneau E, Tiret P, Lecoq J, Ducros M, Knöpfel T, Charpak S. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci. 2007;27:6452–6460. doi: 10.1523/JNEUROSCI.3141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poplawsky AJ, Fukuda M, Murphy M, Kim SG. Layer-specific fMRI responses to excitatory and inhibitory neuronal activities in the olfactory bulb. J Neurosci. 2015;35:15263–15275. doi: 10.1523/JNEUROSCI.1015-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Renken R, Hyder F, Siddeek M, Greer CA, Shepherd GM, Shulman RG. Dynamic mapping at the laminar level of odor-elicited responses in rat olfactory bulb by functional MRI. Proc Natl Acad Sci USA. 1998;95:7715–7720. doi: 10.1073/pnas.95.13.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza RD, Parsa PV, Vijayaraghavan S. Nicotinic receptors modulate olfactory bulb external tufted cells via an excitation-dependent inhibitory mechanism. J Neurophysiol. 2013;110:1544–1553. doi: 10.1152/jn.00865.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiba K, Machida T, Uchida S, Hotta H. Sympathetic neural regulation of olfactory bulb blood flow in adult and aged rats. Auton Neurosci. 2009;147:75–79. doi: 10.1016/j.autneu.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Uchida S, Kagitani F, Suzuki A, Aikawa Y. Effect of acupuncture-like stimulation on cortical cerebral blood flow in anesthetized rats. Jpn J Physiol. 2000;50:495–507. doi: 10.2170/jjphysiol.50.495. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Compact 6. Amsterdam: Academic Press; 2009. [Google Scholar]
- 20.Zilles K. The cortex of the rat. Berlin: Springer; 1985. [Google Scholar]
- 21.Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 22.Poplawsky AJ, Kim SG. Layer-dependent BOLD and CBV-weighted fMRI responses in the rat olfactory bulb. Neuroimage. 2014;91:237–251. doi: 10.1016/j.neuroimage.2013.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiret P, Chaigneau E, Lecoq J, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Methods Mol Biol. 2009;489:81–91. doi: 10.1007/978-1-59745-543-5_4. [DOI] [PubMed] [Google Scholar]
- 24.Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 25.Wenk H, Bigl V, Meyer U. Cholinergic projections from magnocellular nuclei of the basal forebrain to cortical areas in rats. Brain Res Rev. 1980;2:295–316. doi: 10.1016/0165-0173(80)90011-9. [DOI] [PubMed] [Google Scholar]
- 26.Záborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986;243:488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]
- 27.Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–977. doi: 10.1016/S0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 28.Keiger CJ, Walker JC. Individual variation in the expression profiles of nicotinic receptors in the olfactory bulb and trigeminal ganglion and identification of alpha2, alpha6, alpha9, and beta3 transcripts. Biochem Pharmacol. 2000;59:233–240. doi: 10.1016/S0006-2952(99)00326-3. [DOI] [PubMed] [Google Scholar]
- 29.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 30.Zoli M, Pucci S, Vilella A, Gotti C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr Neuropharmacol. 2018;16:338–349. doi: 10.2174/1570159X15666170912110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gengatharan A, Bammann RR, Saghatelyan A. The role of astrocytes in the generation, migration, and integration of new neurons in the adult olfactory bulb. Front Neurosci. 2016;10:149. doi: 10.3389/fnins.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, Lledo PM, Changeux JP. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc Natl Acad Sci USA. 2004;101:9822–9826. doi: 10.1073/pnas.0403361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogino T, Sawada M, Inada H, Nabekura J, Sawamoto K (2017) The role of blood flow in neuronal turnover in the adult olfactory bulbs. JNS meeting planner, Makuhari, Japan. Program no. 2P-012 Neuroscience Society 2017 Online
- 34.Mitsis EM, Cosgrove KP, Staley JK, Bois F, Frohlich EB, Tamagnan GD, Estok KM, Seibyl JP, van Dyck CH. Age-related decline in nicotinic receptor availability with [123I]5-IA-85380 SPECT. Neurobiol Aging. 2009;30:1490–1497. doi: 10.1016/j.neurobiolaging.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabri O, Meyer PM, Gräf S, Hesse S, Wilke S, Becker GA, Rullmann M, Patt M, Luthardt J, Wagenknecht G, Hoepping A, Smits R, Franke A, Sattler B, Tiepolt S, Fischer S, Deuther-Conrad W, Hegerl U, Barthel H, Schönknecht P, Brust P. Cognitive correlates of α4β2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia. Brain. 2018;141:1840–1854. doi: 10.1093/brain/awy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grothe M, Heinsen H, Teipel S. Longitudinal measures of cholinergic forebrain atrophy in the transition from healthy aging to Alzheimer’s disease. Neurobiol Aging. 2013;34:1210–1220. doi: 10.1016/j.neurobiolaging.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T. Aging, Alzheimer’s disease, and the cholinergic system of the basal forebrain. Neurology. 1984;34:741–745. doi: 10.1212/WNL.34.6.741. [DOI] [PubMed] [Google Scholar]
- 38.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, DeLong MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 39.Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol. 1950;2:377–388. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- 43.Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol. 2003;90:3921–3930. doi: 10.1152/jn.00475.2003. [DOI] [PubMed] [Google Scholar]
- 44.Sato K, Momose-Sato Y. Functiogenesis of the embryonic central nervous system revealed by optical recording with a voltage-sensitive dye. J Physiol Sci. 2017;67:107–119. doi: 10.1007/s12576-016-0482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogg MC, Ross JM, Bendahmane M, Fletcher ML. Olfactory bulb acetylcholine release dishabituates odor responses and reinstates odor investigation. Nat Commun. 2018;9:1868. doi: 10.1038/s41467-018-04371-w. [DOI] [PMC free article] [PubMed] [Google Scholar]