Abstract
The present study examined whether touch influences the autonomic responses and subjective pain intensity induced by noxious heat stimulation in humans. Heart rate and digital pulse wave were recorded. Heat stimulation was applied to the right plantar foot before, during, and after touch. Subjective pain intensity was evaluated using a visual analog scale (VAS). Touch was applied over the right medial malleolus for 10 min. Two types of touch were employed in a cross-over double-blinded randomized manner. When touch was applied with a soft elastomer brush, heat-induced autonomic responses attenuated significantly, while VAS scores were unchanged. In contrast, touch with a flat disc was ineffective for any measurement. Participants hardly perceived a difference in the texture of the touching materials. The present study result suggests there are mechanisms in conscious humans where some sort of touch inhibits nociceptive transmission into autonomic reflex pathways independent of sensation and cognition.
Keywords: Touch, Somato-autonomic reflexes, Pain perception, Humans, Cross-over randomized double-blind trial
Introduction
We may all have experienced that pain is sometimes reduced by placing hands on the skin near where the pain is located. In clinical practice, somatosensory stimulation is used as a way to reduce pain. For example, skin-to-skin contact reduced crying and cardiovascular responses due to noxious stimulation (heel stick) for blood sampling of neonates [1–3]. It was also reported that massage applied to the trunk may reduce labor pain [4]. Such responses associated with tactile stimulation may be induced by various factors, including temperature, smell, and even psychological influences [2]. In addition, pain inhibition by somatosensory stimulation is thought to be associated with moving the individual’s attention towards the stimulation [5], and with recognition of the source of the noxious stimulus [6]; however, the mechanism has not yet been identified.
Recently, we examined whether gentle mechanical cutaneous stimulation (touch) influences nociceptive transmission independent of attention and cognition in anesthetized rats, using a model of somato-cardiac sympathetic reflexes [7, 8]. The somato-cardiac sympathetic reflexes consist of two distinct reflex discharges, the A- and C-reflexes, elicited by stimulating myelinated A and unmyelinated C afferent nerve fibers of the hindlimb, respectively. It has been shown that touch using a brush with soft microcones selectively inhibited the somato-cardiac sympathetic C-reflex [7, 8]. Furthermore, C-reflex inhibition by the touch was significantly attenuated [7] or completely eliminated by naloxone [8], suggesting that activation of the endogenous opioid system is involved in a mechanism of the effect of touch. However, touch with a flat disc on skin did not influence the C-reflex [7].
The textures of the skin stimulation tools used in the previous study [7] (Fig. 1a) are hardly distinguishable unless carefully palpated with the tip of the finger. Thus, it may be possible to exclude the influence of perception of cutaneous sensory stimulation from the contributing factor. Of the various somatosensory stimulations, noxious heat stimulation preferentially excites unmyelinated C-fibers. Using two textures of skin stimulation tools and a double-blind randomized study design, the present study examined whether touch would inhibit autonomic responses and influence subjective pain intensity induced by unmyelinated C-fiber stimulation independent of perception and recognition in conscious humans. Parts of the present results have been published in abstract form [9].
Fig. 1.
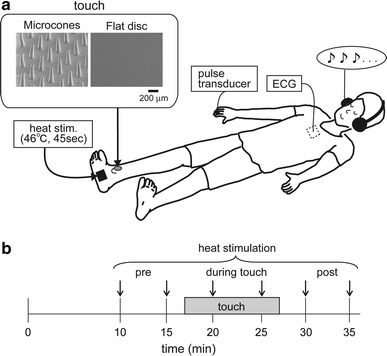
Experimental setting (a) and protocol (b). The frame in the upper left of (a) shows scanning electron microscopic views of the cutaneous stimulation tool surface. The surface was placed on the skin. A horizontal bar located below the right photograph indicates the scale (200 μm) for both pictures (a). Heat stimulation was applied to the plantar foot at a time period indicated by a down arrow (b)
Experimental procedures
Design overview
The present study was designed as a cross-over, double-blind, randomized trial using two different types of skin stimulation tools—an elastomer brush (with approximately 400 microcones on a disc, 11-mm diameter) (Somareson II; Toyoresin, Shizuoka, Japan) and a flat elastomer disc of the same size (custom-made for our research use by Toyoresin). A stimulation tool was placed over the right medial malleolus with an adhesive plaster. The experiment consisted of two 40-min sessions with a 15-min washout period between the sessions. One type of cutaneous stimulation tools was employed in the first session, and the other was used in the second session.
Participants lay supine on the examination table following attachment of the instrumentation (Fig. 1a). During the experiment, participants wore headphones and remained supine with their eyes closed, except during the subjective pain intensity evaluation using a visual analog scale (VAS). Following a 5-min pre-testing rest period, participants were instructed to control their respiration (0.25 Hz, 15 breaths/min) by synchronizing with a metronome rhythm heard through the headphones, and this was continued until the end of the experimental session. Heat stimulation was applied every 5 min, for a total of six repetitions (Fig. 1b). After the end of each heat stimulation, the participant was instructed to complete the VAS. A cutaneous stimulation tool was applied after the second heat stimulation and subsequent VAS recording, and was removed after 10 min.
Participants
Twenty healthy adult males (with no history of cardiovascular disease, diabetes mellitus, or cancer, and with no analgesic drugs taken on the day of the experiment) participated in the present study. The participants were 24.4 ± 6.3 years old and had a body mass index of 22.7 ± 2.1 kg/m2 (mean ± standard deviation). The study was conducted in accordance with the Declaration of Helsinki and approved by the human research ethics committee of our institute. Written informed consent was obtained from all participants before commencement of the experiment. All experiments were conducted in a temperature-controlled (23–26 °C), dimly-lit room.
Blinding and randomization
The cutaneous stimulation tools mentioned above were placed in the same color and shape of cartridge before use in order to disguise their types, and were packaged as a pair and the order of application was written on the cartridge. An examiner arbitrarily chose 1 of 20 pairs of tools for use and followed the order written on the cartridge in order to achieve randomization. The probability of either tool being used first was 50 %. Application and removal of the cutaneous stimulation tool was performed by the experimenter. To ensure the type of a cutaneous stimulation tool was masked to the experimenter, the experimenter used a forceps for handling the stimulation tool and was not allowed to contact the surface.
Before the experiment started, each participant received an explanation on cutaneous stimulation: “there are two kinds of cutaneous stimulation applied to the ankle and these are with and without spikes. The order of use is randomized and no one knows which one is first until this project has been completed.” However, participants were not informed which type of cutaneous stimulation was expected to be effective. After the experiment was complete, the participants were asked to describe freely the perceived differences in the cutaneous stimulation tools, using a written questionnaire: “is there any difference in perception with cutaneous stimulation applied to the leg? If so, please provide details of it”. Four out of 20 participants answered “had a prickling sensation” and “feeling as there are spikes;” however, two of the four participants gained such a sensation from the flat disc. Therefore, it is evident that the double-blind procedure was successful.
Noxious heat stimulation
Noxious heat stimulation was applied with a heat-stimulation device equipped with a Peltier thermode (DPS-777PH; Physio-Tech, Tokyo, Japan). The contact surface of the thermode was 30 mm × 30 mm. The tip of the thermocouple is located just under the surface of the center of the thermode and measures the temperature of the thermode surface (i.e., contacted skin temperature) for feedback control. The thermode was firmly fitted on the center of the right plantar foot using pre-taping underwrap and adhesive tape. The basal thermode temperature was maintained at 33 °C during the experiment. When heat stimulation was applied, the temperature increased to 46 °C at a rate of 1 °C per second, maintained that temperature for 45 s, and subsequently returned to 33 °C. It was found in our pilot study that this form of heat stimulation induced reproducible autonomic responses and pain within tolerable levels.
Explanations were given to participants prior to the experiment that heat stimulation at “a similar temperature” would be applied to the plantar foot. They could terminate the heat stimulation by pressing a hand-switch button if they felt unbearable pain. Before data recording was started, the heat stimulation was applied to the plantar foot once and it was confirmed that the heat stimulation was sufficient to induce pain within tolerable levels.
Measurement of autonomic responses evoked by heat stimulation
To evaluate somato-autonomic responses evoked by noxious heat stimulation, heart rate (HR), and the amplitude of digital pulse wave were measured with an electrocardiogram (ECG) and pulse transducer (MP100; ADInstruments, Bella Vista, Australia), respectively.
The ECG was recorded by attaching disposable silver/silver chloride electrodes (Vitrode Bs-150; Nihon Koden, Tokyo, Japan) on the manubrium of sternum and at the right and left midaxillary lines of the fifth intercostal space. A pulse transducer was wrapped around the right middle finger and the change in circumference of the finger due to the arterial pulse was measured. Since a change in finger circumference indicates a change in finger volume, this recording indirectly reflects a pulsatile change in blood volume in the fingertip.
Signals of the ECG and pulse transducer were fed into a data acquisition system (PowerLab; ADInstruments) with and without amplification, and were then viewed using Chart 7 software (ADInstruments). The digitalized signals were stored on the computer hard disc for later off-line analysis. Data analysis was performed using the Chart 7 software.
From the recorded ECG signals, R–R intervals were measured and the instantaneous HR was calculated. From the pulse transducer waveform, pulse wave amplitude within a cardiac cycle was measured. Heart rate and pulse wave amplitude recorded 60 s before heat stimulation were averaged and were considered baseline values for these measures. The HR and pulse wave amplitude responses to heat stimulation were smoothed by calculating 10-s moving averages (with triangular weighting). The maximal changes during the 46 °C thermal stimulation period (45 s) were determined as differences (Δbpm or ΔV) with respect to HR and pulse wave amplitude baseline values.
Measurement of subjective pain intensity evoked by heat stimulation
Subjective pain intensity induced by heat stimulation was evaluated using a 0–10 VAS. On a 10-cm line drawn on a card, the number “0” was written on one end and “10” was written on the other end. The definitions of 0 and 10 were “no pain” and “the most severe pain imaginable”, respectively. The intensity of pain was marked on this line by participants, and the previous VAS recorded was covered. Subjective pain intensity was evaluated after the thermode temperature had returned to 33 °C. The distance from the number “0” was measured for quantification.
Statistical analysis
The values of all parameters were averaged across two trials (see “Design overview” and Fig. 1b) separately for each set of conditions (pre-, touch, and post-) and each session (microcones and a flat disc). The averages were compared statistically. The autonomic responses were then expressed as % of pre-touch values. For statistical analysis, Prism 5 software (GraphPad Software; La Jolla, CA, USA) was used. All parameters were tested using one-way repeated measures analysis of variance (ANOVA) followed by Dunnett’s test. The statistical significance level was set at 5 %. Data were expressed as mean ± standard error of the mean, unless otherwise stated.
Results
Nineteen of 20 participants completed the experiment. One participant was excluded due to dysfunction of the heat stimulation device temperature control. Analyses were performed on data obtained from the 19 completing study participants.
Control responses to heat stimulation before touch
As shown in Fig. 2a, b, HR and pulse wave amplitude were changed by heat stimulation. The peak of such changes was usually observed during 46 °C heat stimulation.
Fig. 2.
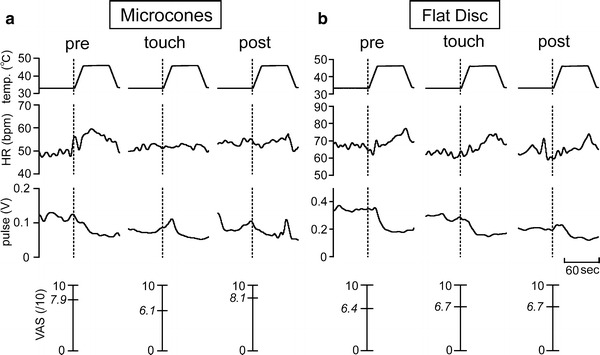
Specimen records of heart rate (HR) and pulse wave amplitude (pulse) changes in response to heat stimulation for touch with microcones (a) and flat disc (b). Values listed between 0 and 10 are actual visual analog scale (VAS) scores recorded following heat stimulation. The data presented in (a) and (b) were obtained from different individuals
When all pre-touch control trials were summarized, of 76 data points (2 trials × 2 sessions × 19 participants), HR increased in 59 observations (range of change: +1 to +22 bpm), decreased in 16 observations (range of change: −1 to −7 bpm), and did not change in 1 observation. Heart rate responses were consistent (increase or decrease) in the consecutive two trials before touch, while in one participant HR decreased in the first trial and increased in the second trial with a similar magnitude of change. The pulse wave amplitude decreased during heat stimulation in 69 trials (range of change: −0.0023 to −0.33 V). In 7 of 76 pre-touch trials, the amplitude increased (range of change: +0.025 to +0.19 V). Changes in the pulse wave amplitude were consistent (decrease or increase) in two trials before touch, except for one participant whose pulse wave amplitude decreased in the first trial and increased in the second trial. Heat stimulation induced pain in all participants (range of VAS scores: 4.3–9.7 on the 0–10 scale), but all participants were able to tolerate the pain.
There were no differences in basal resting HR or pulse wave amplitude (Tables 1, 2), or in heat stimulation-induced changes in HR (Δbpm) and pulse wave amplitude (ΔV) and VAS (Table 3) before application of cutaneous stimulation tools.
Table 1.
Basal heart rate
| Pre-touch | During touch | Post-touch | |
|---|---|---|---|
| Microcones (bpm) | 57.7 ± 1.8 | 57.8 ± 1.8 | 57.5 ± 1.8 |
| Flat disc (bpm) | 58.7 ± 2.0 | 57.6 ± 1.8 | 57.1 ± 1.7** |
n = 19 for each measure. Values are expressed as mean ± standard error of the mean
bpm beat per minute
** p < 0.01 versus pre-touch value; tested by one-way repeated measures ANOVA, followed by Dunnett’s multiple comparison test
Table 2.
Basal amplitude of pulse wave
| Pre-touch | During touch | Post-touch | |
|---|---|---|---|
| Microcones (V) | 0.27 ± 0.05 | 0.26 ± 0.05 | 0.24 ± 0.05 |
| Flat disc (V) | 0.34 ± 0.06 | 0.31 ± 0.06 | 0.29 ± 0.05 |
n = 19 for each measure. Values are expressed as mean ± standard error of the mean
V volt
Table 3.
Heat induced-autonomic responses and subjective pain intensity recorded before touch
| HR (Δbpm) | Pulse (ΔV) | VAS (/10) | |
|---|---|---|---|
| Microcones | 5.8 ± 0.9 | 0.089 ± 0.02 | 6.9 ± 0.3 |
| Flat disc | 5.4 ± 0.8 | 0.080 ± 0.02 | 6.8 ± 0.2 |
n = 19 for each measure. Values are expressed as mean ± standard error of the mean
The effect of touch with the microcone tool
In the example shown in Fig. 2a, heat-induced changes in HR and pulse wave amplitude attenuated by touch with the microcone stimulation tool and the effects continued even after the touch was terminated. The VAS score decreased during the touch but returned to the pre-touch level after the touch was discontinued.
The effects of microcone stimulation tool touch on autonomic responses and VAS are presented for individuals in Fig. 3a–c and summarized in Fig. 4a–c. In the majority of participants, autonomic responses were inhibited during the touch (Fig. 3a, b). The group data showed that the heat-induced HR response was significantly inhibited by 21.6 ± 8.3 % during the touch (p < 0.05) and it tended to remain lower after the touch than the pre-touch level (by 11.8 ± 10.1 %, not significant, n.s.) (Fig. 4a). Heat-induced changes in pulse wave amplitude were also significantly inhibited during the touch (by 27.1 ± 7.5 %, p < 0.01), continuing after the touch was ended (by 28.2 ± 8.3 %, p < 0.01) (Fig. 4b). There were four participants whose VAS scores decreased over 1 cm either during (n = 2) or after (n = 2) the touch (Fig. 3c); however, there was no significant change for the overall population (6.88 ± 0.3 pre-touch; 6.74 ± 0.3 during touch; 6.78 ± 0.3 post-touch, n.s.) (Fig. 4c).
Fig. 3.
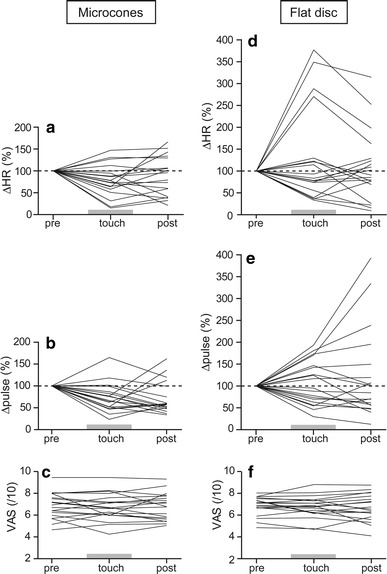
Changes in heart rate (ΔHR) (a, d), pulse wave amplitude (Δpulse) (b, e), and visual analog scale (VAS) (c, f) in response to heat stimulation before, during, and after touch with microcones and flat disc. Each line indicates individual data (n = 19). Data of autonomic responses are expressed as changes with respect to pre-touch values (%)
Fig. 4.
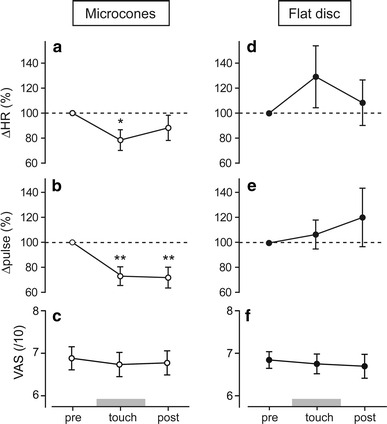
Averages of changes in heart rate (ΔHR) (a, d), pulse wave amplitude (Δpulse) (b, e), and visual analog scale (VAS) (c, f) in response to heat stimulation before, during, and after touch with microcones and flat disc, expressed as mean ± standard error of the mean. *p < 0.05, **p < 0.01 versus pre-touch value, tested by one-way repeated measures ANOVA, followed by Dunnett’s multiple comparison test
The effect of touch with the flat disc tool
In the individual participant’s data shown in Fig. 2b, touch with the flat disc stimulation tool did not influence heat-induced HR change and VAS, while basal levels of HR and pulse wave amplitude decreased and pulse wave amplitude response to heat stimulation was attenuated.
The effects of touch with the flat disc tool on autonomic responses and VAS scores are presented for individual changes in Fig. 3d–f and are summarized for the overall subject population in Fig. 4d–f. There were no constant trends of changes in heat-induced autonomic responses, since an inhibition was observed in half of the subject population during the touch and an exaggeration was seen in the remaining subjects (Fig. 3d, e). In contrast to the effects of microcone touch, touch with the flat disc increased the mean value of heat-induced changes in HR (by 29.3 ± 24.8 % during touch; 8.5 ± 18.2 % post-touch) (Fig. 4d) and pulse wave amplitude (by 6.8 ± 11.6 % during touch; 20.5 ± 23.4 % post-touch) (Fig. 4e). However, these changes were not statistically significant. VAS scores were not influenced by the touch (6.84 ± 0.2 pre-touch; 6.75 ± 0.2 during touch; 6.70 ± 0.3 post-touch, n.s.) (Fig. 4f).
The effect of touch on basal level of heart rate and pulse wave amplitude
As shown in Tables 1 and 2, basal HR and pulse wave amplitude (i.e., before heat stimulation) were not influenced by either type of touch; however, basal HR decreased after touch with the flat disc compared with pre-touch values.
Discussion
The present study examined the effect of gentle mechanical cutaneous stimulation (touch) on autonomic responses and subjective pain intensity induced by a noxious heat stimulus in conscious humans. With two types of cutaneous stimulation tools (microcones and a flat disc), experiments were performed in a cross-over, double-blind, randomized manner. The results showed that there was no difference in perception induced by the cutaneous stimulation tools; however, heat-induced autonomic responses (HR and digital pulse wave) were inhibited by touch with microcones, but not with the flat disc. This result was consistent with a previous study performed on anesthetized rats [7]. On the other hand, there was no significant change in subjective pain intensity in human subjects. The present results suggest that an inhibitory mechanism on nociceptive transmission to evoke autonomic responses in conscious humans can be activated by touch, independent of sensation and cognition.
Autonomic nervous function is significantly influenced by psychological factors. However, noxious somatosensory stimulation induces responses of various organs in animals and humans where psychological influence is excluded [10–13]. For example, it has been demonstrated that noxious heat stimulation induces cardiovascular responses in animals under anesthesia [14–16] and human subjects during sleep [11], indicating that there are neural reflex pathways to evoke autonomic responses to somatosensory stimulation apart from psychological factors. The present cardiovascular responses to heat stimulation applied to the plantar foot are presumably a supraspinal response integrated in the brain stem, based on findings from animal studies [10, 17–19]. As the present study performed experiments on conscious humans, it is not possible to exclude the influence of affective changes due to pain. However, because the intensity of subjective pain sensation did not change with either type of touch, it is thought that autonomic response changes were not mediated by recognition and identification of pain. Dissociation between pain perception and autonomic responses has also been reported, where the perceived intensity of experimental pain reported by male participants was influenced by the experimenter’s gender while the autonomic responses were not affected [20]. Hence, it is assumed that touch with the microcone tool inhibited the nociceptive transmission in somato-cardiovascular reflex pathways and consequently heat-induced cardiovascular responses, similar to that seen in anesthetized animals.
In anesthetized rats, the inhibitory effect on the autonomic reflex was dependent on the location of the touch [7]. The effect of touch with microcones was greatest when the touch was applied to the ipsilateral and closer dermatome to the electrical stimulation. Based on the result of that study, the present study applied the touch on the ipsilateral and closer dermatome to different innervations from the location of heat stimulation. Thus, it is unlikely that nociceptive transmission in the peripheral afferent nerve was blocked by collision. Further similarities to the previous results [7] are that the effect of touch with microcones persisted after the touch was discontinued, the inhibitory effects on autonomic responses were different based on the type of touch, and basal levels of cardiovascular function were not affected by touch with microcones. A characteristic of autonomic nervous function is that efferent nerves have tonic activity. If tonus was reduced by touch and autonomic responses to heat stimulation were consequently attenuated, the basal HR and pulse wave amplitude should have decreased. However, basal values did not significantly change in response to touch with microcones in the present study. Therefore, it was thought that the nociceptive transmission in autonomic reflex ascending pathways was inhibited by microcone touch, and autonomic responses to heat stimulation were subsequently inhibited.
In contrast to touch with microcones, touch with the flat disc tool did not influence heat-induced autonomic responses. This may be attributed to differences in excited sensory receptors and induced sensory afferent responses. In our previous study [7], it was found that touch with microcones excites low threshold cutaneous mechanoreceptors (<4 mN) and induces low frequency activity (<4 Hz) of Aβ, Aδ, and C fibers. Although responses of cutaneous mechanoreceptive units to touch with a flat disc have not been examined, it could be presumed that excited sensory receptors and induced sensory afferent response are different as the texture of the skin contact surface is different. There is supporting evidence to our presumption that responses of mechanoreceptive afferent units are influenced by the texture of skin contact surface [21], and low-threshold mechanoreceptive C-fiber units are particularly responsive to slow stroking on the skin surface [22]. The microcones may vibrate across the skin due to participant’s slight movement (including arterial pulsation) [7]. Thus, it was supposed that a difference in the texture of the touching objects (between microcones and flat disc) resulted in different effects on heat-induced autonomic responses.
Heat-induced autonomic responses were inhibited by touch with microcones, but pain intensity was not significantly influenced in the present study. There are two possible reasons for these findings. Firstly, it was thought that different groups of neurons which send nociceptive information to different brain areas were affected differently by the touch. The neurons in the spinal cord are divided functionally into subpopulations that project nociceptive information to different brain areas related to pain perception, emotion, and autonomic responses [23–26]. Projection of nociceptive information to the thalamus is sent to the cerebral cortex and associated with perception and recognition of pain. However, a group of neurons projecting from the spinal cord to the ventrolateral medulla, where the cardiovascular center is located, is different from those going to the thalamus [24]. Therefore, it was assumed that, since touch with microcones inhibited nociceptive transmission to neurons projecting to brain areas associated with autonomic responses, only autonomic responses were inhibited. Secondly, VAS scoring is a means to evaluate the subjective aspects of pain; however, it has been reported that, by conditioning the participant, belief can influence the perception of pain despite the temperature of heat stimulation [27]. In the present study, because an explanation was given prior to the experiment that heat stimulation at “a similar temperature” would be applied, it was suspected that this might influence the participant’s perception of pain. Thus, autonomic responses might be more sensitive to touch than subjective pain perception under the present experimental conditions.
The present study showed that touch with the microcone tool inhibited the heat-induced autonomic responses by approximately 20 % of pre-touch levels. A similar extent of inhibition of the somato-cardiac sympathetic C-reflex was observed by administration of a chemical substance, and this chemical treatment also reduced chronic pain [28]. Hyperexcitability of sympathetic nerves is thought to be associated with pain chronicity [29]. Therefore, such a form of gentle cutaneous stimulation may be helpful for preventing chronic pain by inhibition of autonomic responses to noxious stimuli and could be useful as a potential therapeutic tool. This presumption is consistent with a clinical finding that the application of the cutaneous stimulation tool with microcones reduces chronic pain (Mukaino, unpublished observation).
Conclusion
The present study demonstrated that gentle mechanical cutaneous stimulation inhibited autonomic responses to heat stimulation, depending on the texture of the surface of the stimulation tools, while a difference in the texture of applied cutaneous stimulation tool was not distinguished. This result, where autonomic responses were inhibited only by the cutaneous stimulation tool with microcones, is consistent with our previous study performed on anesthetized animals. Therefore, it was concluded that an inhibition of autonomic responses to noxious stimulation by gentle mechanical cutaneous stimulation could occur independent of sensation and cognition.
Acknowledgments
This work was partially supported by a Grant from Toyoresin Co.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105:e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 2.Johnston CC, Stevens B, Pinelli J, Gibbins S, Filion F, Jack A, Steele S, Boyer K, Veilleux A. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch Pediatr Adolesc Med. 2003;157:1084–1088. doi: 10.1001/archpedi.157.11.1084. [DOI] [PubMed] [Google Scholar]
- 3.Ludington-Hoe SM, Hosseini RB. Skin-to-skin contact analgesia for preterm infant heel stick. AACN Clin Issues. 2005;16:373–387. doi: 10.1097/00044067-200507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yildirim G, Sahin NH. The effect of breathing and skin stimulation techniques on labour pain perception of Turkish women. Pain Res Manag. 2004;9:183–187. doi: 10.1155/2004/686913. [DOI] [PubMed] [Google Scholar]
- 5.Kakigi R, Shibasaki H. Mechanisms of pain relief by vibration and movement. J Neurol Neurosurg Psychiatry. 1992;55:282–286. doi: 10.1136/jnnp.55.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kammers MPM, de Vignemont F, Haggard P. Cooling the thermal grill illusion through self-touch. Curr Biol. 2010;20:1819–1822. doi: 10.1016/j.cub.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Hotta H, Schmidt RF, Uchida S, Watanabe N. Gentle mechanical skin stimulation inhibits the somatocardiac sympathetic C-reflex elicited by excitation of unmyelinated C-afferent fibers. Eur J Pain. 2010;14:806–813. doi: 10.1016/j.ejpain.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe N, Uchida S, Hotta H. Age-related change in the effect of gentle mechanical cutaneous stimulation on the somato-cardiac sympathetic C-reflex. J Physiol Sci. 2011;61:287–291. doi: 10.1007/s12576-011-0146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe N, Miyazaki S, Uchida S, Hotta H. Effect of gentle cutaneous stimulation on heat-induced autonomic responses and pain intensity in healthy humans. J Physiol Sci. 2012;62(Supp. 1):132. doi: 10.1007/s12576-012-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato A, Sato Y, Schmidt RF. The impact of somatosensory input on autonomic functions. Rev Physiol Biochem Pharmacol. 1997;130:1–328. doi: 10.1007/BFb0046598. [DOI] [PubMed] [Google Scholar]
- 11.Lavigne GJ, Zucconi M, Castronovo V, Manzini C, Veglia F, Smirne S, Ferini-Strambi L. Heart rate changes during sleep in response to experimental thermal (nociceptive) stimulations in healthy subjects. Clin Neurophysiol. 2001;112:532–535. doi: 10.1016/S1388-2457(00)00558-7. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Uchida S, Hotta H. The effects of aging on somatocardiac reflexes in anesthetized rats. Jpn J Physiol. 2004;54:137–141. doi: 10.2170/jjphysiol.54.137. [DOI] [PubMed] [Google Scholar]
- 13.Hotta H, Sato A, Schmidt RF, Suzuki A. Cerebral regional cortical blood flow response during joint stimulation in cats. NeuroReport. 2005;16:1693–1695. doi: 10.1097/01.wnr.0000181584.41507.8e. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman A, Sato A, Sato Y, Sugimoto H. Reflex changes in heart rate after mechanical and thermal stimulation of the skin at various segmental levels in cats. Neuroscience. 1977;2:103–109. doi: 10.1016/0306-4522(77)90071-9. [DOI] [PubMed] [Google Scholar]
- 15.Nagasaka H, Yaksh TL. Effects of intrathecal μ, δ, and κ agonists on thermally evoked cardiovascular and nociceptive reflexes in halothane-anesthetized rats. Anesth Analg. 1995;80:437–443. doi: 10.1097/00000539-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Uchida S, Hotta H, Hanada T, Okuno Y, Aikawa Y. Effects of thermal stimulation, applied to the hindpaw via a hot water bath, upon ovarian blood flow in anesthetized nonpregnant rats. J Physiol Sci. 2007;57:227–233. doi: 10.2170/physiolsci.RP003507. [DOI] [PubMed] [Google Scholar]
- 17.Sato A. Spinal and medullary reflex components of the somatosympathetic reflex discharges evoked by stimulation of the group IV somatic afferents. Brain Res. 1973;51:307–318. doi: 10.1016/0006-8993(73)90381-8. [DOI] [PubMed] [Google Scholar]
- 18.Kimura A, Ohsawa H, Sato A, Sato Y. Somatocardiovascular reflexes in anesthetized rats with the central nervous system intact or acutely spinalized at the cervical level. Neurosci Res. 1995;22:297–305. doi: 10.1016/0168-0102(95)00907-B. [DOI] [PubMed] [Google Scholar]
- 19.Kimura A, Sato A, Sato Y, Suzuki H. A- and C-reflexes elicited in cardiac sympathetic nerves by single shock to a somatic afferent nerve include spinal and supraspinal components in anesthetized rats. Neurosci Res. 1996;25:91–96. doi: 10.1016/0168-0102(96)01031-0. [DOI] [PubMed] [Google Scholar]
- 20.Aslaksen PM, Myrbakk IN, Høifødt RS, Flaten MA. The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain. 2007;129:260–268. doi: 10.1016/j.pain.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Phillips JR, Johansson RS, Johnson KO. Responses of human mechanoreceptive afferents to embossed dot arrays scanned across fingerpad skin. J Neurosci. 1992;12:827–839. doi: 10.1523/JNEUROSCI.12-03-00827.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol. 1990;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 24.Andrew D, Krout KE, Craig AD. Differentiation of lamina I spinomedullary and spinothalamic neurons in the cat. J Comp Neurol. 2003;458:257–271. doi: 10.1002/cne.10592. [DOI] [PubMed] [Google Scholar]
- 25.Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J Comp Neurol. 2009;515:629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakiuchi Y, Nagai J, Gotoh M, Hotta H, Murofushi H, Ogawa T, Ueda H, Murakami-Murofushi K. Antinociceptive effect of cyclic phosphatidic acid and its derivative on animal models of acute and chronic pain. Mol Pain. 2011;7:33. doi: 10.1186/1744-8069-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jänig W. Sympathetic nervous system and pain. In: Robertson D, editor. Primer on the autonomic nervous system. 2. San Diego: Elsevier; 2004. [Google Scholar]


