Abstract
Melanoma has an extremely poor prognosis due to its rapidly progressive and highly metastatic nature. Several therapeutic drugs have recently become available, but are effective only against melanoma with specific BRAF gene mutation. Thus, there is a need to identify other target molecules. We show here that Transient receptor potential, canonical 3 (TRPC3) is widely expressed in human melanoma. We found that pharmacological inhibition of TRPC3 with a pyrazole compound, Pyr3, decreased melanoma cell proliferation and migration. Similar inhibition was observed when the TRPC3 gene was silenced with short-hairpin RNA (shRNA). Pyr3 induced dephosphorylation of signal transducer and activator of transcription (STAT) 5 and Akt. Administration of Pyr3 (0.05 mg/kg) to mice implanted with human melanoma cells (C8161) significantly inhibited tumor growth. Our findings indicate that TRPC3 plays an important role in melanoma growth, and may be a novel target for treating melanoma in patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s12576-016-0480-1) contains supplementary material, which is available to authorized users.
Keywords: TRPC3, Proliferation, Migration, Melanoma, Metastasis, Pyr3
Introduction
Melanoma has the worst prognosis among skin cancers. Therapy targeting ERK signaling due to mutated and activated BRAF has recently improved both overall and progression-free survival of melanoma patients [1]. Unfortunately, however, this therapy does not work with patients who do not have BRAF mutation. Even among patients with such mutations, some rapidly acquire resistance to BRAF inhibitors [2]. Accordingly, a different strategy that can target key signaling pathways independently of specific gene mutation is needed. So far, however, alterations of cellular signaling associated with melanoma growth remain largely unexplored.
Transient receptor potential, canonical 3 (TRPC3) are Ca2+-permeable cationic channels that regulate Ca2+ influx evoked by G protein-coupled receptor (Gq/Gi-PLCβ) activation and/or by depletion of the Ca2+ store in endoplasmic reticulum (ER). TRP channels are activated by changes of temperature or membrane voltage, regulating adaptive responses to physiological stimuli [3]. TRPC also has a pathological role, being involved in the differentiation of lung cancer cells [4]. TRPC channels, including TRPC3, may also be involved in cancer cell proliferation and tumorigenesis of ovarian cancer [5]. TRPC3 is over-expressed in ovarian and breast cancer tissues, compared with normal tissues [6, 7]. In breast cancer, a highly metastatic cell line expressed TRPC3 at a higher level than a less metastatic cell line [6]. It was also demonstrated that inhibition of TRPC3 decreased cell proliferation and invasion of bladder cancer cells [8]. However, it remains unknown whether TRPC plays a role in other cancer cell types. There is also no report as to whether TRPC channels are expressed in melanoma cells, or whether TRPC3 regulates cell proliferation and migration of human melanoma.
We previously reported that Orai1/STIM1 plays an important role in melanoma cell proliferation and migration via so-called store-operated calcium entry (SOCE) [9]. We further found that YM58483, a pyrazole compound, potently inhibited melanoma cell proliferation by inhibiting SOCE [9]. Because Orai and TRPC complex mediate SOCE [10, 11], we hypothesized that TRPC inhibition might be a useful strategy for melanoma therapy. In this connection, it was recently reported that another pyrazole compound, Pyr3, selectively and directly inhibits TRPC3 [12]. This inhibitor also blocked cardiac hypertrophy in rat via reduced activation of nuclear factor of activated T cells (NFAT), a Ca2+-dependent transcription factor [12].
In the present study, we confirmed that TRPC3 is widely expressed in various melanoma cell types, and we examined the effect of TRPC3 inhibition on melanoma growth. We found that either pharmacological inhibition of TRPC3 or silencing of TRPC3 gene expression effectively inhibited growth of cultured melanoma cells, as well as tumor growth in a melanoma cell-implanted animal model, suggesting TRPC3 is a promising candidate as a target for melanoma therapy.
Materials and methods
Reagents and cell lines
Reagents were purchased from Sigma unless otherwise specified. β-Actin and GAPDH antibodies were purchased from Santa Cruz. STIM1, Signal transducer and activator of transcription (STAT) 5, phospho-STAT5, Akt, and phospho-Akt antibodies were purchased from Cell Signaling. TRPC3 antibody was purchased from Alomone Labs. Second antibodies for mouse and rabbit were purchased from Abcam and Cell Signaling, respectively. C8161 cell line was kindly provided by Dr. Mary J. C. Hendrix. SK-Mel-2 and SK-Mel-24 (human metastatic melanoma) cell lines were obtained from the American Type Culture Collection. HEMA-LP cell line (human melanocyte) was obtained from Invitrogen. C8161 cells were maintained in RPMI-1640 with GlutaMAX (Gibco), with 10 % fetal bovine serum (FBS) and 1 % penicillin–streptomycin. SK-Mel-2 and SK-Mel-24 cells were maintained in MEM containing 10 or 15 % fetal bovine serum (FBS), respectively, and 1 % penicillin–streptomycin. HEMA-LP was maintained in an EndoGRO-VEGF Complete media kit (Millipore). All other melanoma cells were maintained in RPMI (Gibco) containing 10 % FBS and 1 % penicillin–streptomycin.
Immunohistochemistry
Enzymatic immunohistochemical stainings were performed as previously described [9, 13]. Microarray plates (US Biomax Cat. #SK181 and #SK181 s) for melanoma tissues were used for immunohistochemistry with antibodies against melanoma antigen recognized by T-cells 1 (MART1) (Millipore) and TRPC3 (Alomone Labs).
Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Isolation of total RNA and generation of cDNA were performed and RT-PCR analysis was done as described previously [14]. The primer set was as previously described [15]. The PCR cycle consisted of an initial cycle of 95 °C for 4 min, then 40 repeated cycles of 95 °C for 30 s denaturation, then 67 °C annealing for 30 s, and primer extension at 72 °C for 30 s. Melting curve analysis was done from 50 to 95 °C with a heating rate of 0.2 °C per second [15]. The abundance of each gene was determined relative to the 18S transcript [16].
Immunoprecipitation
Dynabeads-protein G for immunoprecipitation (Life Technologies) were incubated with the primary antibodies (anti-TRPC3 and anti-STIM1) and added to the soluble cell lysate fraction. These antibody-coated DynabeadsTM (Life Technologies) bound to the target proteins were separated by the magnet and after repeated washing four times, the isolated protein complexes were subjected to SDS-PAGE and immunoblotting with respective antibodies [17].
Fluorescence imaging of intracellular Ca2+
Measurement of intracellular Ca2+ level was performed as previously described [9, 14]. Cells were incubated with 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) buffer containing 4.0 μmol/l of Fluo-4AM, then washed and incubated with HEPES-buffered saline containing 2.0 mmol/l of CaCl2. An iXon+ 885 charge-coupled-device camera (Andor Technology) was used to monitor fluorescence changes. Full images were collected every 4 s. Fluo-4 fluorescence was excited at 488 nm, and data were expressed as normalized changes in background-corrected fluorescence emission (F/F0). Data were analyzed using Imaging Workbench (INDEC BioSystems). Representative Ca2+ signals averaged from eight to nine individual cells are shown in the figures.
Short-hairpin RNA (shRNA) transduction
C8161 cells were subjected to transduction with TRPC3 and scramble control shRNA. Transductions with lentivirus (Santa Cruz Biotechnology) were performed as previously described [9, 13]. Briefly, cells were incubated with 10 µg/ml of Polybrene (Santa Cruz Biotechnology) and lentiviral particles harboring each shRNA, followed by selection with puromycin dihydrochloride (Santa Cruz Biotechnology) for 1 week.
Cell proliferation assay
Cell proliferation assay was performed using a commercial kit, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium inner salt (XTT) Cell Proliferation Assay Kit (ATCC), as previously reported [9, 18].
Apoptosis assay
Apoptosis assays were performed as described [19, 20]. Cells were seeded on 6 cm dishes, incubated with Pyr3 for 24 h, washed with PBS, and transferred into culture tubes. Annexin V, allophycocyanin conjugate (APC) and 7-amino-actinomycin D (7-AAD) (BD Biosciences) were then added, and the tubes were incubated for 15 min at room temperature in darkness, and then subjected to FACS analysis (Canto™, Japan Becton, Dickinson and Company) within 1 h. Cells that were sorted only with Annexin V were classified as early apoptosis, cells that were sorted with both Annexin V and 7-AAD were classified as late apoptosis, and cells that were sorted only with 7-AAD were classified as apoptosis.
Animal models
C8161 cells were harvested and intradermally grafted (1.0 × 107 cells/serum-free medium 50 μl) into the side chest of Balb/c nu/nu mice (SLC, female, 4 weeks old, n = 4–6). Mice were anesthetized with 250–350 μl/body intraperitoneal Avertin injection. After tumors had formed, 0.005 mg/kg or 0.05 mg/kg Pyr3 or DMSO (control) was intradermally injected around the tumor every day. We measured the diameter of each tumor every 2 days, and calculated the volume as follows: (Tumor volume (mm3) = 4 × π × (major axis)2 × (minor axis)/3).
Time-lapse videomicroscopy
Analysis of cell motility using time-lapse videomicroscopy was performed as described [8]. C8161 cells were subjected to time-lapse video recording. Frames from the recording were digitized at 15-min intervals.
Migration assay
The scratch wound method was performed as described [9, 13]. The cells were plated at a density of 4.0 × 104 cells/500 µl of medium, and incubated for 3 h at 37 °C.
Gelatin zymography
Matrix metalloproteinase 9 (MMP9) activity was examined by gelatin zymography as described [16]. Cells were seeded on a 24-well plate, and incubated in the presence of Pyr3 for 24 h. Supernatants were collected into 1.5-ml tubes and signal intensities were quantified with ImageJ software (NIH).
Western-blot analysis
Western-blot analyses were performed as described [9, 17]. Signal intensities of the bands were quantified with ImageJ software (NIH).
Protein phosphorylation microarray
C8161 cells treated with DMSO or Pyr3 (10 μM) for 15 min was subjected to protein phosphorylation microarray assay using a commercial kit (Cancer Signaling Phospho-Antibody Array; Full Moon BioSystems, Inc.).
Data analysis and statistics
Statistical comparisons among groups were performed using Student’s t test or one-factor analysis of variance (ANOVA) with the Bonferroni post hoc test. The criterion of statistical significance was set as p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns no significant difference.
Ethics statement
The Animal Care and Use Committee at Yokohama City University, School of Medicine, approved all animal studies. (Protocol Number: FA13-014).
Results
TRPC3 is expressed in melanoma
We first examined TRPC3 expression in human primary melanoma tissues, using a tissue microarray. We found that TRPC3 antibody-positive areas, but not -negative areas, coincided immunohistochemically with positivity for melanoma-related antigen (melanoma antigen recognized by T-cells; MART-1) (Fig. 1a), and it appeared that TRPC3 is abundantly expressed in melanoma.
Fig. 1.
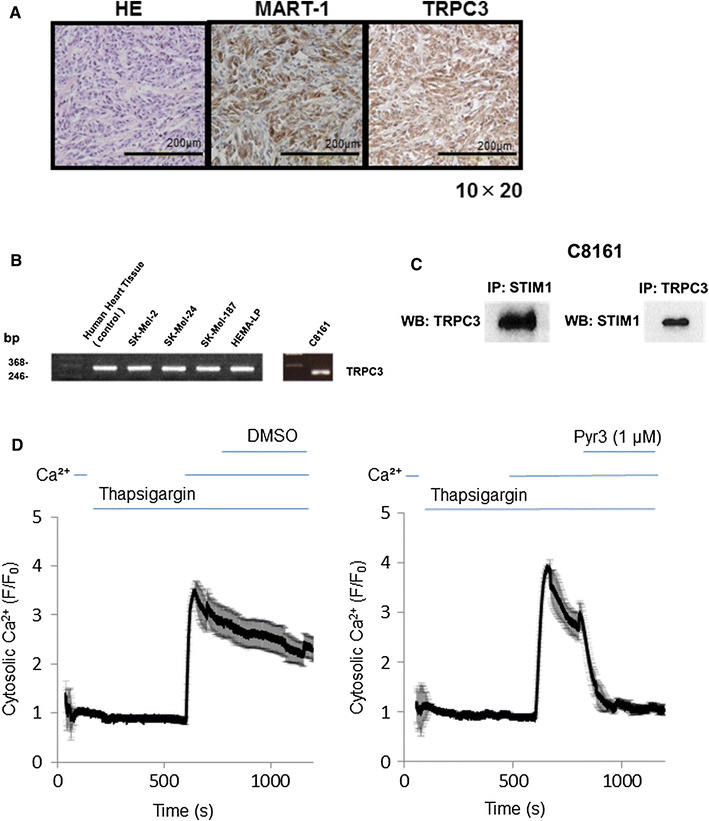
TRPC3 expression and SOCE in melanoma. a Representative images of immunohistochemical staining of HE, MART1, and TRPC3 in a melanoma primary tissue microarray (stage II) (original magnification, b ×200). The calibration bars represent 200 µm. b mRNA expression in various melanoma cell lines. SK-Mel-2 is a skin metastasis melanoma cell line with NRAS mutation, SK-Mel-24 is a lymph node metastasis cell line with BRAFV600E mutation, SK-Mel-187 is also a lymph node metastasis cell line, C8161 is a metastasis cell line with wild-type BRAF, and HEMA-LP is a skin melanocyte cell line. TRPC3 mRNA is widely expressed in human melanoma cell lines, independently of BRAF mutation. c Immunoprecipitation for TRPC3 and STIM1 was performed. d Cytosolic Ca2+ level in C8161 cells is shown as mean ± SD (n = 8–9). SOCE was examined in the presence or absence of DMSO (vehicle control 1 µM) or Pyr3 (1 µM) in C8161. Pyr3 inhibited SOCE in C8161 cells. Pyr3 was added after the addition of Ca2+. The Ca2+ signal was immediately decreased, indicating that Pyr3 inhibits SOCE in melanoma cells. Data in each panel are averages of eight or nine cells
Accordingly, we next examined the expression of TRPC3 in various human melanoma cell lines and melanocytes by means of semi-quantitative PCR (Fig. 1b). TRPC3 mRNA was detected in SK-Mel-2 (NRAS mutated), SK-Mel-187 (BRAF wild-type metastatic human melanoma cell lines), SK-Mel-24 (BRAFV600E mutated), C8161 (BRAF wild-type metastatic human melanoma cell lines), and HEMA-LP (melanocyte cell line). Thus, TRPC3 is widely expressed among human melanoma cell types and melanocyte. It has been reported that TRPC3 is an SOCE component [11], therefore we examined the interaction of TRPC3 and STIM1 in C8161. Immunoprecipitation showed that TRPC interacts directly STIM1 (Fig. 1c).
We further found that the pyrazole compound Pyr3, which is a TRPC3-selective inhibitor [12], suppressed SOCE in C8161 cells (Fig. 1d). Thus, TRPC3 plays a role in SOCE activation, at least in C8161 melanoma cells.
Role of TRPC3 in cellular proliferation and apoptosis
We next examined the effect of depleting TRPC3 expression in C8161 melanoma cells by infecting the cells with lentivirus encoding TRPC3 short-hairpin RNA (shRNA). Knockdown was confirmed by means of real-time PCR and Western-blot analysis (Fig. 2a).
Fig. 2.
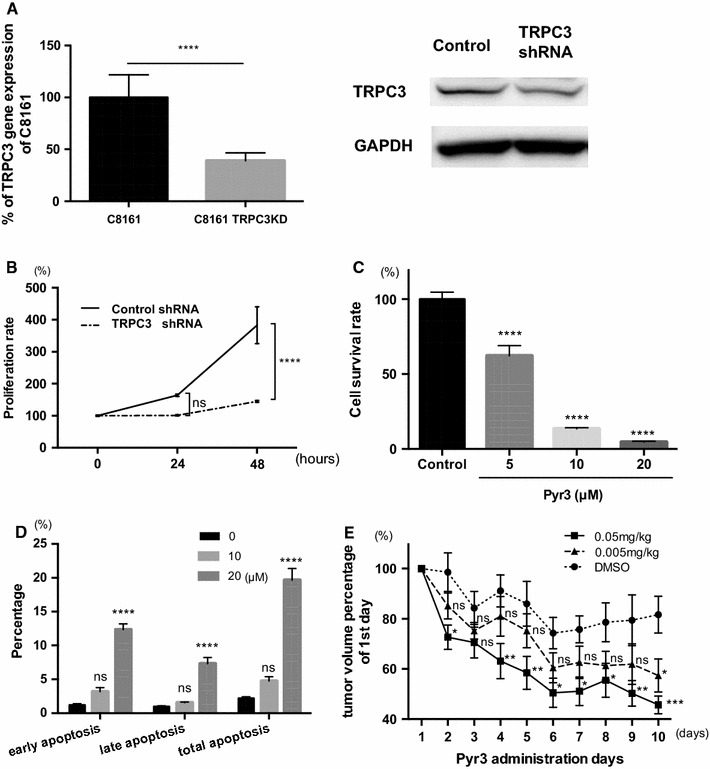
TRPC3 regulates proliferation of melanoma cells and tumor growth in vivo. a Quantitative RT-PCR revealed that TRPC3 was significantly reduced by shRNA transduction with lentivirus (n = 4, **p < 0.001) (left). Western-blot analysis showed that protein expression of TRPC3 was also decreased. b TRPC3-knockdown decreased proliferation of C8161 cells over 48 h (n = 8, ****p < 0.0001). c Pyr3 dose-dependently suppressed proliferation of C8161 cells over 24 h (n = 6, ****p < 0.0001). The IC50 value was 12.99 μM. d Pyr3 (1, 10 and 20 µM) induced apoptosis in melanoma over 24 h (n = 4, ns not significant, ****p < 0.0001). e Pyr3-inhibited tumor growth in vivo. The tumor volume was significantly reduced in the Pyr3-treatment group versus the control after 10 days (n = 4–6, *p < 0.05, ***p < 0.001)
We found that knockdown of TRPC3 reduced proliferation of melanoma cells (Fig. 2b). Therefore, we next examined the effect of pharmacologically inhibiting TRPC3 with a pyrazole compound, Pyr3. As expected, proliferation of C8161 cells was inhibited in a dose-dependent manner; the IC50 value was 12 μM (Fig. 2c). Further, Pyr3 induced apoptosis, as indicated by the increased levels of early, late, and total apoptotic cells (Fig. 2d).
Pyr3 also inhibited proliferation of other melanoma cell lines, such as SK-Mel-2, SK-Mel-24, and HEMA-LP, although the extent of apoptosis induction varied (Fig. S1). We also found that Pyr3 induced cell-cycle blockade of C8161 cells at the G2 phase in a dose-dependent manner over 24 h (Fig. S2).
We therefore examined whether Pyr3 can suppress tumor growth of melanoma in mice. Pyr3 injection (s.c. 0.05 or 0.005 mg/kg) every day for 10 days significantly suppressed tumor growth (Fig. 2e). The effect was greater with a higher dose (0.05 vs. 0.005 mg/kg). These data suggest that the inhibition of TRPC3 suppressed melanoma tumor growth in mice. Importantly, administration of Pyr3 for up to 10 days caused no obvious abnormality or body weight decrease (data not shown) or liver and kidney dysfunction (Fig. S3).
Role of TRPC3 in melanoma migration
We next examined the role of TRPC3 in migration, which is associated with melanoma metastasis. Cell migration determined by total path length of C8161 cells was decreased when TRPC3 was depleted (Fig. 3a). Scratch assay showed that Pyr3 decreased cell migration in a dose-dependent manner (Fig. 3b). These findings support the idea that inhibition and/or depletion of TRPC3 can inhibit melanoma migration.
Fig. 3.

TRPC3 regulates migration of C8161 melanoma cells. a Effect of knockdown of TRPC3 on migration distance of C8161 cells, assessed by means of time-lapse video recording (n = 6, **p < 0.01). b Scratch assay showed that Pyr3 significantly inhibited cell migration in a dose-dependent manner (n = 6, **p < 0.01). c Gelatin zymography confirmed that MMP9 secretion was inhibited by ablation of TRPC3 (n = 4, ***p < 0.001). d Pyr3 also dose-dependently decreased MMP9 secretion (n = 4, **p < 0.01). These data suggest that TRPC3 regulates cell migration, i.e., melanoma hematogenous metastasis, via MMP9 secretion
We also investigated the mechanism of TRPC3-mediated inhibition of migration. It is well known that MMP9, a gelatinase, dissolves type IV collagen in basal membranes, thereby enhancing cellular migration ability [21]. Indeed, MMP9 secretion by C8161 cells was inhibited when TRPC3 expression was blocked with shRNA (Fig. 3c) or pharmacologically inhibited with Pyr3. Inhibition of MMP9 activation by Pyr3 was dose-dependent (Fig. 3d). In contrast, MMP2 secretion was unaffected. These data suggest that TRPC3 plays a role in regulating cell migration by modulating MMP9 activation.
TRPC regulates SOCE, Akt, and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling in melanoma cell lines
We also investigated TRPC3-related cellular signaling in melanoma. The Akt pathway could influence melanoma cell migration and proliferation by coupling SOCE to constitutive activation of PKB/Akt [22]. Therefore, we investigated whether TRPC3 affects Akt signaling in C8161 melanoma cells.
We found that Pyr3 inhibited Akt phosphorylation (Fig. 4a). Protein phosphorylation microarray analysis demonstrated that Pyr3 inhibited phosphorylation of STAT5 as well (Fig. 4b), and we confirmed that this action by using p-STAT antibody (Fig. 4c).
Fig. 4.
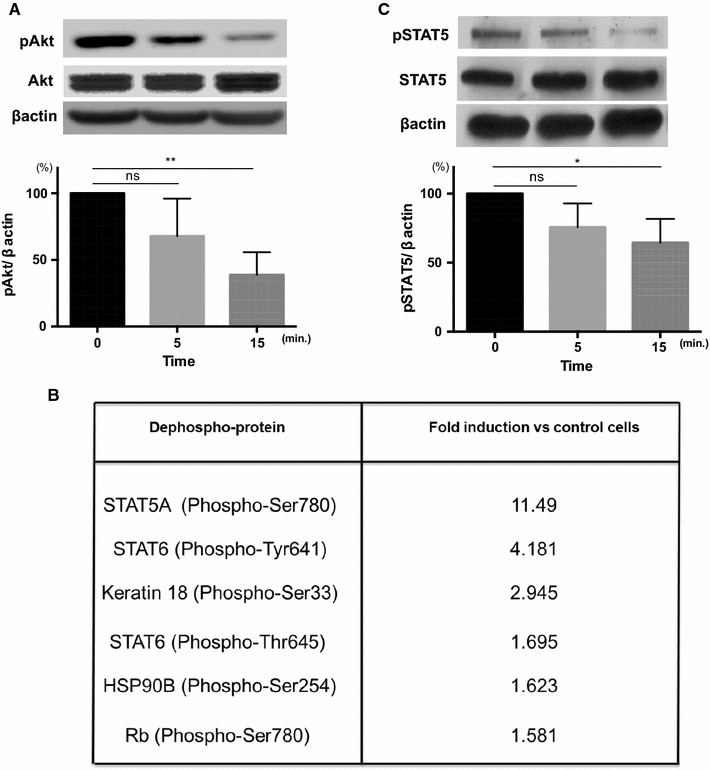
Pyr3 inhibits phosphorylation of STAT5 and Akt. a Representative images of Akt phosphorylation are shown. Densitometric analyses (bar graph) of Western blots showed that phosphorylation of Akt was inhibited by TRPC3 inhibitor, Pyr3 (n = 4, **p < 0.01, ns not significant). b Protein phosphorylation microarray analysis in the presence of Pyr3. C8161 cells were incubated with DMSO (vehicle control) or Pyr3 (10 μM) for 15 min. The Y-axis shows the signal ratio of phosphorylated to non-phosphorylated protein in the presence of Pyr3 as a percentage of that of the DMSO control. c Representative images of STAT5 phosphorylation. Densitometric analyses (bar graph) of Western blots showed that phosphorylation of STAT5 was inhibited by Pyr3 (n = 4, *p < 0.05, ns not significant)
Discussion
Our present findings show that TRPC3 is involved in SOCE and serves to regulate cell proliferation and migration, probably via STAT signaling. TRPC3 are expressed not only in cultured cells, but also in human melanoma tissues. Knockdown or pharmacological inhibition of TRPC3 suppressed both proliferation and cell migration.
We previously showed that SOCE is involved in proliferation and migration of melanoma cells, most probably via ERK signaling. It was also reported that SOCE occurs in lipid rafts, and ablation of the rafts suppresses tumor growth via the Akt pathway [23]. Further, STAT5 phosphorylation in malignant melanoma is mediated through SRC (proto-oncogene tyrosine-protein kinase Src) and Janus kinase (JAK) 1 [24]. It was also demonstrated that STAT5b transcripts were upregulated and phosphorylated in human melanoma metastasis compared to normal human melanocytes and benign nevi. It was recently shown that phosphorylation (upregulation) of STAT5 by JAK 1 kinase is important for melanoma cell survival [23]. Taken together with our findings, these data suggest that TRPC3 may regulate melanoma proliferation and/or migration via Akt and the JAK/STAT5 pathway.
These data, taken together, indicate that SOCE regulate multiple pathways in melanoma, and are consistent with the idea that TRPC3 plays a pivotal role in melanoma progression.
In conclusion, our findings indicate that TRPC3 contributes to melanoma proliferation and migration through activation of Akt and STAT. Therefore we propose that TRPC3 is a promising target for treatment of malignant melanoma, irrespective of whether BRAF mutation is present or not.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by Grants from the Japan Heart Foundation and the Kanagawa Nanbyo Foundation (M.U.). This study was also supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (25670131 to Y.I.); The Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant (22136009 to Y.I.); New Energy and Industrial Technology Development Organization (NEDO) (60890021 to Y.I.); the National Cerebral and Cardiovascular Center (NCVC) (22-2-3 to Y.I.); the Japan Agency for Medical Research and Development (AMED) (66890005, 66890011, 66890001, 66890023 to Y.I.); The Tokyo Biochemical Research Foundation (Y.I.).
Compliance with ethical standards
Conflict of interest
The authors disclose no potential conflicts of interest.
Footnotes
K. Oda and M. Umemura contributed equally to this work.
Contributor Information
Masanari Umemura, Phone: +81-45-787-2575, Email: umemurma@yokohama-cu.ac.jp.
Yoshihiro Ishikawa, Phone: +81-45-787-2575, Email: yishikaw@med.yokohama-cu.ac.jp.
References
- 1.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AMM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen G, Davies MA. Targeted therapy resistance mechanisms and therapeutic implications in melanoma. Hematol Oncol Clin N Am. 2014;28(3):523–536. doi: 10.1016/j.hoc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam K, Montell C. TRP Channels. Annu Rev Biochem. 2007;76(1):387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H-N, Zeng B, Zhang Y, Daskoulidou N, Fan H, Qu J-M, Xu S-Z. Involvement of TRPC channels in lung cancer cell differentiation and the correlation analysis in human non-small cell lung cancer. PLoS One. 2013;8(6):e67637. doi: 10.1371/journal.pone.0067637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng B, Yuan C, Yang X, Latkin S, Xu S-Z. TRPC channels and their splice variants are essential for promoting human ovarian cancer cell proliferation and tumorigenesis. Curr Cancer Drug Tar. 2013;13(1):103–116. doi: 10.2174/156800913804486629. [DOI] [PubMed] [Google Scholar]
- 6.Aydar E, Yeo S, Djamgoz M, Palmer C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 2009;9(1):1–12. doi: 10.1186/1475-2867-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang SL, Cao Q, Zhou KC, Feng YJ, Wang YZ. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009;28(10):1320–1328. doi: 10.1038/onc.2008.475. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Heo K, Choi J, Kim K, An W. The histone variant MacroH2A regulates Ca2+ influx through TRPC3 and TRPC6 channels. Oncogenesis. 2013;2:e77. doi: 10.1038/oncsis.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umemura M, Baljinnyam E, Feske S, De Lorenzo MS, Xie L-H, Feng X, Oda K, Makino A, Fujita T, Yokoyama U, Iwatsubo M, Chen S, Goydos JS, Ishikawa Y, Iwatsubo K. Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS One. 2014;9(2):e89292. doi: 10.1371/journal.pone.0089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC: Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci. 2009;106(9):3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci. 2008;105(8):2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, Ishii M, Takemoto H, Ojida A, Watanabe K, Uemura A, Kurose H, Morii T, Kobayashi T, Sato Y, Sato C, Hamachi I, Mori Y. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci. 2009;106(13):5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baljinnyam E, Umemura M, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS, Iwatsubo K. Epac1 promotes melanoma metastasis via modification of heparan sulfate. Pigment Cell Melanoma Res. 2011;24(4):680–687. doi: 10.1111/j.1755-148X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 14.Baljinnyam E, De Lorenzo MS, Xie L-H, Iwatsubo M, Chen S, Goydos JS, Nowycky MC, Iwatsubo K. Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2+-dependent mechanism. Cancer Res. 2010;70(13):5607–5617. doi: 10.1158/0008-5472.CAN-10-0056. [DOI] [PubMed] [Google Scholar]
- 15.Thilo F, Vorderwülbecke BJ, Marki A, Krueger K, Liu Y, Baumunk D, Zakrzewicz A, Tepel M. Pulsatile atheroprone shear stress affects the expression of transient receptor potential channels in human endothelial cells. Hypertension. 2012;59(6):1232–1240. doi: 10.1161/HYPERTENSIONAHA.111.183608. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama U, Ishiwata R, Jin M-H, Kato Y, Suzuki O, Jin H, Ichikawa Y, Kumagaya S, Katayama Y, Fujita T, Okumura S, Sato M, Sugimoto Y, Aoki H, Suzuki S, Masuda M, Minamisawa S, Ishikawa Y. Inhibition of EP4 signaling attenuates aortic aneurysm formation. PLoS One. 2012;7(5):e36724. doi: 10.1371/journal.pone.0036724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baljinnyam E, Umemura M, Chuang C, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS, Ishikawa Y, Whitelock JM, Iwatsubo K. Epac1 increases migration of endothelial cells and melanoma cells via FGF2-mediated paracrine signaling. Pigment Cell Melanoma Res. 2014;27(4):611–620. doi: 10.1111/pcmr.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eguchi H, Umemura M, Kurotani R, Fukumura H, Sato I, Kim J-H, Hoshino Y, Lee J, Amemiya N, Sato M, Hirata K, Singh DJ, Masuda T, Yamamoto M, Urano T, Yoshida K, Tanigaki K, Yamamoto M, Sato M, Inoue S, Aoki I, Ishikawa Y. A magnetic anti-cancer compound for magnet-guided delivery and magnetic resonance imaging. Sci Rep. 2015;5:9194. doi: 10.1038/srep09194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baljinnyam E, Iwatsubo K, Kurotani R, Wang X, Ulucan C, Iwatsubo M, Lagunoff D, Ishikawa Y. Epac increases melanoma cell migration by a heparan sulfate-related mechanism. Am J Cell Physiol. 2009;297(4):C802–813. doi: 10.1152/ajpcell.00129.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato I, Umemura M, Mitsudo K, Fukumura H, Kim J, Hoshino J, Nakashima H, Kioi M, Nakakaji R, Sato M, Fujita T, Yokoyama U, Okumura S, Oshiro H, Eguchi H, Tohnai I, Ishikawa Y. Simultaneous hyperthermia-chemotherapy with controlled drug delivery using single-drug nanoparticles. Sci Rep. 2016;6:24629. doi: 10.1038/srep24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madhunapantula SV, Robertson GP. Therapeutic implications of targeting AKT signaling in melanoma. Enzyme Research. 2011;2011:327923. doi: 10.4061/2011/327923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedida-Metula S, Feldman B, Koshelev V, Levin-Gromiko U, Voronov E, Fishman D. Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinog. 2012;33(4):740–750. doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- 24.Mirmohammadsadegh A, Hassan M, Bardenheuer W, Marini A, Gustrau A, Nambiar S, Tannapfel A, Bojar H, Ruzicka T, Hengge UR. STAT5 phosphorylation in malignant melanoma is important for survival and is mediated through SRC and JAK1 Kinases. J Invest Dermatol. 2006;126(10):2272–2280. doi: 10.1038/sj.jid.5700385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


