Abstract
The aim of this study was to explore the mechanism underlying the cardioprotection bestowed by chronic intermittent hypobaric hypoxia (CIHH) against ischemia/reperfusion (I/R) injury in developing rats. Neonatal male rats were subjected to CIHH treatments that simulated an altitude of 3000 m a.s.l. for 28 days (CIHH28) and 42 days (CIHH42), respectively, or no treatment (control). The left ventricular function of isolated hearts was evaluated. The ultra-microstructure, superoxide dismutase (SOD) activity and total anti-oxidation capacity (TAC) of the myocardium were determined. The basic left ventricular function remained unchanged in CIHH rats, except for an increased coronary flow. The recovery of cardiac function from I/R, however, was much better in CIHH rats than in control rats. Compared to control rats, CIHH rats had much higher SOD levels and TAC, and the ultra-microstructure damage to mitochondria was considerably less. The cardiac protection of CIHH was canceled out by glibenclamide, an inhibitor of the ATP-sensitive potassium (KATP) channel, 5-hydroxydecanoate, an inhibitor of mitochondrial KATP (mitoKATP), and atractyloside, an opener of the mitochondrial permeability transition pore (MPTP). To the contrary, diazoxide, an opener of mitoKATP, and cyclosporin A, a blocker of MPTP opening, induced cardioprotection in control rats. These results suggest that CIHH protects the heart against I/R injury in developing rats through opening of the KATP channel and inhibiting of opening of the MPTP.
Keywords: Cardioprotection, Chronic intermittent hypobaric hypoxia, Ischemia/reperfusion, ATP sensitive potassium channels, Mitochondrial permeability transition pore, Rat
Introduction
It is well-known that ischemia/reperfusion (I/R) has harmful effects on the body, tissue and cells. Heart diseases induced by I/R, such as necrosis, heart failure and arrhythmia, are common diseases and leading causes of morbidity and mortality clinically. To date, only limited success has been achieved in preventing and treating ischemic heart diseases [1].
There is strong experimental evidence showing that chronic intermittent hypobaric hypoxia (CIHH) can effectively protect the heart against I/R injury [2–4]. Various studies and our own investigations have shown that adult rats exposed to hypobaric hypoxic conditions which mimic high-altitude conditions of 5000 m a.s.l. [P B (barometric pressure) = 404 mmHg; (partial pressure of oxygen) = 84 mmHg] for 6 h/day for 28 days develop significant cardioprotection against I/R injury, promoting the recovery of cardiac systolic function from I/R, limiting cardiac infarction and reducing arrhythmia [5–7]. The proposed mechanisms include enhancement of myocardial antioxidant capacity, increase in myocardial capillary density and coronary blood flow, attenuation of beta-adrenergic receptor activity, increase in the expression of the heat shock protein 70 family, activation of protein kinase C, decrease of I/R-induced apoptosis, opening of ATP-dependent (sensitive) potassium channels (KATP channel) in both the cell and mitochondrial membranes and inhibition of the opening of the mitochondrial permeability transition pore (MPTP) [8–14].
Most studies on the cardioprotective effects of CIHH have been carried out in adult animals and, consequently, the effect of CIHH on the developing heart is not well defined. It is well known that the developing and adult heart differ greatly in terms of morphology, function, metabolism and blood supply; for example, the healthy immature myocardium is more tolerant to ischemia than the adult heart [15]. In an earlier study [16], we showed that CIHH simulating an altitude of 5000 m a.s.l. for 6 h/day for 28 day induces cardioprotection in adult rats but has harmful effects in developing rats. However, in the same study, we found that mild CIHH, which simulates an altitude of 3000 m a.s.l. for 5 h/day for 28 days also induces cardioprotection in developing rats [16]. Despite this result, little is still known about the underlying mechanism of cardioprotection in the developing rat heart. We recently demonstrated that CIHH protects developing rat hearts during I/R by enhancing the resistance against calcium overload and by preserving normal INa/Ca and Sodium Calcium Exchanger 1 (NCX1) protein levels [17]. The aim of the study reported here was to examine the cardiac protection of CIHH against I/R injury provided by the opening of KATP channels and by the inhibition of MPTP opening in the myocardium in developing rats.
Materials and methods
Drugs
Cyclosporin A (CSA), diazoxide (DIAZ), 5-hydroxydecanoate (5-HD), glibenclamide (GLI) and atractyloside (ATRA) were purchased from the Sigma-Aldrich Corp. (St. Louis, MO). The analysis kits for superoxide dismutase (SOD) and total antioxidation capacity (TAC) were provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Animal groups and hypoxia treatment
Postnatal male Sprague–Dawley rats (n = 84), provided by the Experimental Animal Center of Hebei Province, were divided randomly into four groups: (1) the CIHH 28-day treatment group (CIHH28); (2) the CIHH 42-day treatment group (CIHH42); (3) matched control group for CIHH28 (CON28); (4) matched control group for CIHH42 (CON42). All animal experiments were conducted in compliance with the 1996 Guide for the Care and Use of Laboratory Animals (National Research Council, Bethesda, MD). For the CIHH groups, neonatal rats with maternal rats were exposed to CIHH in a hypobaric chamber for 2–3 days before birth and thereafter to CIHH for 28 days (CIHH28 treatment) or 42 days (CIHH42 treatment) mimicking an altitude of 3000 m a.s.l. (P B = 525 mmHg; = 108.8 mmHg) for 5 h/day. The control animals were kept under the same environmental conditions as the CIHH rats, with free access to water and food, but without exposure to hypoxia. Both CIHH and CON rats were raised at room temperature under a 12/12-h light/dark photoperiod. Body weight and physical activity were recorded at the same time each week. All experiments were carried out on the day following the end of the CIHH treatment in order to avoid the negative effects of acute hypoxia on the animals.
Measurement of ventricular function in isolated hearts
Rats were anesthetized with sodium pentobarbital (45 mg/kg, interperitoneal), following which the hearts removed and perfused immediately through the aorta with Krebs–Henseleit (K–H) buffer (in mM: NaCl 118.0, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25.0, KH2PO4 1.2, glucose 11.0, pH 7.4; gassed with 5 % CO2 and 95 % O2; 37 °C). Perfusion occurred in a non-circulating Langendorff apparatus at a constant pressure of 80 mmHg. A saline-filled latex balloon-tipped catheter was placed into the left ventricle through the left atrium and adjusted to a left ventricular end diastolic pressure (LVEDP) of 5–8 mmHg during the initial equilibration. The distal end of the catheter was connected to a PowerLab system via a pressure transducer (model Gould P23Db; ADInstrument Ltd., Bella Vista, Australia) for measurement of left ventricular pressure. Left ventricular developing pressure (LVDP), LVEDP, maximal positive velocity of left ventricular pressure (LVdp/dt max), maximal negative velocity of left ventricular pressure (LVdp/dt min) and coronary flow (CF) were continuously recorded. After 20 min of stabilization, the heart was subjected to 30 min of global no-flow ischemia, followed by 60 min of reperfusion (I/R). The data were analyzed using Chart software (ADInstrument Ltd.). At the end of the experiment, the heart was rapidly removed from the Langendorff apparatus to measure heart weight and for morphological examination. A number of hearts were frozen in liquid nitrogen and stored at −70 °C for biochemical assay.
Experiment protocols
Six experimental protocols were followed. These were:
- Protocol 1 (I/R):
The hearts of rats were perfused with K–H buffer for 20 min, then subjected to 30 min of global no-flow ischemia followed by 60 min of reperfusion with K–H buffer.
- Protocol 2 (CON + DIAZ + I/R):
The hearts of control rats were perfused with K–H buffer for 10 min and with K–H buffer containing DIAZ for another 10 min, then subjected to 30 min of global no-flow ischemia followed by 60 min of reperfusion with K–H buffer.
- Protocol 3 (CON + CSA + I/R):
The hearts of control rats were perfused with K–H buffer for 20 min and then subjected to 30 min of global no-flow ischemia, followed sequentially by 15 min of reperfusion with K–H buffer containing CSA and another 45 min of reperfusion with K–H buffer.
- Protocol 4 (CIHH + 5-HD + I/R):
The hearts of CIHH rats were perfused with K–H buffer for 10 min and with K–H buffer containing 5-HD for another 10 min, then subjected to 30 min of global no-flow ischemia followed by 60 min of reperfusion with K–H buffer.
- Protocol 5 (CIHH + GLI + I/R):
The hearts of CIHH rats were perfused with K–H buffer for 10 min and with K–H buffer containing GLI for another 10 min, then subjected to 30 min of global no-flow ischemia, followed by 60 min of reperfusion with K–H buffer.
- Protocol 6 (CIHH + ATRA + I/R):
The hearts of CIHH rats were perfused with K–H buffer for 20 min, and then subjected to 30 min of global no-flow ischemia, followed sequentially by 15 min of reperfusion with K–H buffer containing ATRA and another 45 min of reperfusion with K–H buffer.
SOD and TAC biochemical assays
The free walls of the left ventricle were weighed and then homogenized in 9 volumes of ice-cooled 100 mM K-phosphate buffer (pH 7.4). The homogenate was then centrifuged at 1000 g for 10 min at 4 °C to remove nuclei and tissue debris, and protein content was estimated by the Bradford method using bovine serum albumin as a standard.
The antioxidant defense system consists of enzymatic and non-enzymatic antioxidants which are able to reduce Fe3+ to Fe2+. TAC was measured by the reaction of phenanthroline, and Fe2+ was measured using a spectrophotometer at 520 nm. At 37 °C, a TAC unit is defined as the amount of antioxidant required to produce an absorbance increase of 0.01 in 1 ml of homogenate. Total SOD activity was determined by inhibition of pyrogallol antioxidation. One unit of the enzyme is generally defined as the amount of enzyme that inhibits the reaction by 50 %.
Morphological examination
At the end of each experiment, the apical part of the hearts was removed in ice and immediately put into 4 % glutaraldehyde fixation solution. The tissue was cut into 1.0-mm pieces and fixed with 4 % glutaraldehyde fixation solution for 24 h, following which sections were dehydrated, embedded, cut and stained. The sections were observed by transmission electron microscopy (TEM; model H 7500; Hitachi, Tokyo, Japan).
Statistics
All data were expressed as the mean ± standard error of the mean. Statistical significance for comparison among multiple groups was estimated by one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test or two-way ANOVA followed by Tukey’s post hoc test. The comparison between two groups was performed with the t test. P < 0.05 was considered to be statistically significant.
Results
Effects of CIHH on body and heart weight
There were no significant differences in body weight between CIHH and CON rats during the entire experimental period (P > 0.05, Table 1). Also, there was no difference in the ventricle-to-body weight, right ventricle-to-body weight and right ventricle-to-left ventricle + inter-ventricular septum weight ratios between CIHH and CON rats (P > 0.05; Table 2), which indicates that no hypertrophy occurred in the CIHH rats.
Table 1.
Effect of chronic intermittent hypobaric hypoxia on body weight of developing rats
| Treatment groupsa | Body weight of developing rats at different experimental time-points | ||||
|---|---|---|---|---|---|
| 14 day (g) | 21 day (g) | 28 day (g) | 35 day (g) | 42 day (g) | |
| CON | 29.50 ± 0.78 | 45.90 ± 0.86 | 99.30 ± 3.31 | 149.90 ± 1.35 | 199.00 ± 4.33 |
| CIHH | 28.80 ± 0.65 | 45.10 ± 0.65 | 98.90 ± 2.16 | 148.00 ± 1.27 | 198.40 ± 3.13 |
All data expressed as the mean ± standard error of the mean (SEM) (n = 6 rats per group)
aCIHH, Chronic intermittent hypobaric hypoxia; CON, control; for more detail see section “Animal groups and hypoxia treatment”
Table 2.
Effect of chronic intermittent hypobaric hypoxia on the heart weight of developing rats
| Treatment groupsa | Heart weight parameters | ||
|---|---|---|---|
| VW/BW (g/kg) | RVW/BW (g/kg) | RVW/(LVW-ISW) (g/g) | |
| CON28 | 4.22 ± 0.09 | 0.77 ± 0.02 | 0.25 ± 0.02 |
| CON42 | 3.60 ± 0.06 | 0.71 ± 0.01 | 0.26 ± 0.02 |
| CIHH28 | 4.17 ± 0.07 | 0.78 ± 0.02 | 0.26 ± 0.01 |
| CIHH42 | 3.55 ± 0.07 | 0.74 ± 0.01 | 0.30 ± 0.02 |
All data expressed as mean ± SEM (n = 6 per group)
BW body weight, VW ventricular weight, RVW right ventricular weight, LVW-ISW left ventricular + inter-ventricular septum weight
aCON28, Control 28-day group; CON42, control 42-day group; CIHH28, CIHH 28-day group; CIHH42, CIHH 42-day group; for more detail see section “Animal groups and hypoxia treatment”
Effect of CIHH on cardiac function
The values of cardiac function, including LVDP, LVEDP, LVdp/dt max, LVdp/dt min and CF, at baseline, ischemia and different times of reperfusion are shown in Table 3. There was no significant difference in LVDP, LVEDP, LVdp/dt max and LVdp/dt min at baseline (pre-ischemia) among the groups (P > 0.05). During ischemia, the LVDP, LVdp/dt max and LVdp/dt min decreased, while LVEDP increased. The decreased cardiac function parameters recovered gradually during reperfusion—relative to the ischemic values—but the recovery of cardiac parameters was much better in CIHH rats than in CON rats (P < 0.05–0.01), and much better in CIHH42 rats than in CIHH28 rats (P < 0.05–0.01). The recovery of cardiac parameters during reperfusion in CON28 rats was comparable to that in CON42 rats (P > 0.05). These results demonstrate that CIHH bestowed protective effects on the heart against I/R injury and that this protective effect was correlated with the length of the CIHH treatment: the longer the CIHH treatment, the stronger the cardioprotective effect.
Table 3.
Effect of chronic intermittent hypobaric hypoxia on cardiac function in isolated rat hearts subjected to 30 min of global ischemia followed by 60 min of reperfusion
| Ventricular function parameters | Treatment | |||||
|---|---|---|---|---|---|---|
| Pre-ischemia | Ischemia | Reperfusion–10 min | Reperfusion–20 min | Reperfusion–30 min | Reperfusion–60 min | |
| LVDP (mmHg) | ||||||
| CON28 | 96.9 ± 3.8 | 0++ | 4.5 ± 1.3++ | 4.0 ± 1.1++ | 9.5 ± 1.6++ | 12.6 ± 3.3++ |
| CIHH28 | 96.2 ± 0.6 | 0++ | 12.1 ± 5.1++ | 18.2 ± 6.0++,&&,** | 24.5 ± 6.3++,&&,** | 25.6 ± 3.3++,&&,$,** |
| CON42 | 101.5 ± 8.7 | 0++ | 5.6 ± 2.4++ | 7.2 ± 2.6++ | 12.8 ± 2.4++ | 16.6 ± 3.1++ |
| CIHH42 | 98.0 ± 4.3 | 0++ | 18.3 ± 4.7++,&,* | 32.5 ± 5.2++,&&,*,# | 41.9 ± 7.4++,&&,$,$,**,## | 46.2 ± 6.6++,&&,$,$,**,## |
| LVEDP (mmHg) | ||||||
| CON28 | 7.5 ± 0.7 | 72.8 ± 6.6++ | 68.5 ± 4.5++ | 77.2 ± 2.8++ | 76.7 ± 3.5++ | 76.7 ± 3.9++ |
| CIHH28 | 5.6 ± 1.0 | 59.2 ± 8.2++ | 67.5 ± 7.2++ | 60.1 ± 7.0++,* | 55.8 ± 6.8++,** | 51.5 ± 6.7++ ** |
| CON42 | 7.0 ± 1.2 | 77.3 ± 9.7++ | 78.4 ± 9.2++ | 79.6 ± 6.7++ | 75.3 ± 6.6++ | 76.2 ± 6.4++ |
| CIHH42 | 6.2 ± 2.3 | 55.6 ± 6.5++,* | 64.7 ± 4.2++ | 54.5 ± 7.1++,* | 49.4 ± 7.1++,* | 43.3 ± 6.9++,** |
| LVdp/dt max (mmHg/s) | ||||||
| CON28 | 3478.2 ± 149.7 | 0++ | 234.1 ± 15.1++ | 241.7 ± 20.0++ | 240.0 ± 24.8++ | 246.9 ± 36.9++ |
| CIHH28 | 3583.0 ± 205.3 | 0++ | 343.5 ± 45.7++,& | 465.7 ± 38.9++,&&,* | 565.7 ± 36.5++,&&,** | 708.3 ± 57.0++,&&,$,$,** |
| CON42 | 3458.3 ± 179.9 | 0++ | 296.3 ± 57.8++ | 353.5 ± 71.2++ | 433.6 ± 81.7++ | 486.4 ± 103.3++,# |
| CIHH42 | 3326.9 ± 195.9 | 0++ | 698.5 ± 156.7++,&&,*,# | 841.2 ± 135.7++,&&,*,# | 1180.8 ± 234.6++,&&,**,## | 1383.6 ± 228.4++,&&,$,**,## |
| LVdp/dt min (mmHg/s) | ||||||
| CON28 | −2270.8 ± 155.7 | 0++ | −221.3 ± 20.5++ | −247.6 ± 20.8++ | −254.5 ± 16.1++ | −242.1 ± 34.1++ |
| CIHH28 | −2171.4 ± 103.0 | 0++ | −423.5 ± 114.8++,& | −496.4 ± 161.9++,&&,* | −598.5 ± 145.0++,&&,** | −643.8 ± 111.9++,&&,** |
| CON42 | −2156.9 ± 123.3 | 0++ | −284.6 ± 57.4++ | −322.5 ± 59.0++ | −392.8 ± 66.3++,$ | −439.4 ± 92.0++,$ |
| CIHH42 | −1992.7 ± 170.6 | 0++ | −242.0 ± 43.4++,& | −542.4 ± 72.2++,&&,* | −699.6 ± 115.6++,&&,$,$,** | −878.7 ± 99.4++,&&,$,$,** |
| CF (ml/min) | ||||||
| CON28 | 5.8 ± 0.3 | 0++ | 3.4 ± 0.2++,&& | 2.8 ± 0.3++,&& | 2.5 ± 0.3++,&& | 2.1 ± 0.3++,&&,$,$ |
| CIHH28 | 7.6 ± 0.2** | 0++ | 4.8 ± 0.3++,&&,** | 4.3 ± 0.4++,&&,** | 3.3 ± 0.2++,&&,$,$,@,* | 3.6 ± 0.4++,&&,$,$,** |
| CON42 | 6.4 ± 0.8 | 0++ | 5.0 ± 0.2&&,## | 4.5 ± 0.2++,&&,## | 4.1 ± 0.2++,&&,## | 3.4 ± 0.2++,&&,$,## |
| CIHH42 | 11.3 ± 0.4**,## | 0++ | 6.8 ± 0.5++,&&**,## | 6.3 ± 0.5++,&&,**,## | 5.6 ± 0.5++,&&,**,## | 5.0 ± 0.5++,&&,$,$,**,## |
* P < 0.05, ** P < 0.01 vs. corresponding CON group; # P < 0.05, ## P < 0.01 vs. CIHH28 or CON28; ++ P < 0.01 vs. corresponding pre-ischemia; & P < 0.05, && P < 0.01 vs. corresponding ischemia–30 min; $ P < 0.05, $ P < 0.01 vs. corresponding reperfusion–10 min; @ P < 0.05 vs. corresponding reperfusion–20 min
Data are expressed as the mean ± SEM (n = 6 per group)
LVDP Left ventricular developing pressure, LVEDP left ventricular end-diastolic pressure, LVdP/dt max maximum rate of rise of left ventricular developed pressure, LVdP/dt min maximum rate of decline of left ventricular developed pressure. CF coronary flow
At baseline, CF was higher in CIHH28 and CIHH42 rats than in CON28 and CON42 rats, respectively (P < 0.01), and was higher in CIHH42 rats than in CIHH28 rats (P < 0.01). The recovery of CF during reperfusion was better in CIHH42 rats than in CIHH28 rats and better in CON42 rats than in CON28 rats (P < 0.05–0.01). These results demonstrate that CF increased together with CIHH treatment and animal growth.
Effects of CIHH on myocardial ultrastructure
Transmission electron microscopy revealed that the myocardium without I/R in the CON rats had a normal ultrastructure: the myocardiac filaments were ordered with distinct Z-lines, and the mitochondria retained their normal shape and compact cristae (Fig. 1a). After I/R, severe damage to the ultrastructure of the myocardium in the CON rats was clearly visible by TEM: the mitochondria showed diffuse swelling with vacuoles and the cristae were ruptured and lysed (Fig. 1b). Damage to myocardial ultrastructure with I/R was much less in CIHH rats than in CON rats: in the former, the mitochondria were swollen, but the cristae were only slightly damaged and there were fewer vacuoles (Fig. 1c, d).
Fig. 1.

Effects of chronic intermittent hypobaric hypoxia (CIHH) on mitochondrial ultrastructure of cardiomyocytes after ischemia/reperfusion (I/R), revealed by transmission electron microscopy (×20,000). a Control heart without I/R with normal structure of mitochondria. b control heart with I/R: mitochondria are markedly swollen, and cristae are disrupted, dissolved or have disappeared, and are markedly reduced in number. c, d The mitochondria of the hearts of CIHH28 (28-day CIHH treatment) and CIHH42 (42-day CIHH treatment) rats showed much less I/R injury
The role of the KATP channel on cardioprotection by CIHH
Pretreatment with an non-specific blocker of KATP (GLI; 10 μM) or a mitochondria KATP channel blocker (5-HD; 100 μM) for 10 min before ischemia canceled out the protective effect of CIHH against I/R injury on the hearts of CIHH rats. However, pretreatment with a mitochondria KATP opener (DIAZ; 100 μM) before ischemia provided a protective effect against I/R injury on the hearts of CON rats, such as promoting cardiac function recovery from I/R (Fig. 2). GLI and 5-HD also had an inhibitory effect on the recovery of CF (LVdp/dt max) from I/R in CON rats, but DIAZ had no effect on the recovery of cardiac function from I/R in CIHH rats (Table 4). These results suggest that KATP channels in both the cell membrane and mitochondrial membrane play an important role in the protective effect of CIHH on the heart against I/R injury in the developing rat.
Fig. 2.
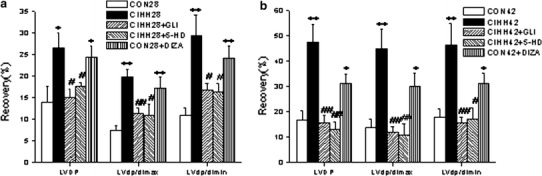
Effects of glibenclamide (GLI), diazoxide (DIAZ) and 5-hydroxydecanoate (5-HD) on the recovery of cardiac function after 30 min ischemia and 60 min reperfusion in CON28 and CIHH28 rats (a), or CON42 and CIHH42 rats (b). LVDP Left ventricular developing pressure, ± LVdP/dt maximal positive and negative velocity of left ventricular pressure. All data are presented as the mean ± standard error of the mean (SEM) (n = 6 per group). *P < 0.05, **P < 0.01 vs. corresponding CON group; # P < 0.05, ## P < 0.01 vs. corresponding CIHH group
Table 4.
Effects of glibenclamide, 5-hydroxydecanoate, atractyloside, cyclosporin A and diazoxide on cardiac function at pre-ischemia and at 60-min reperfusion in the developing rat
| Ventricular function parameters | CON28 | CON28 + GLI | CON28 + 5-HD | CON28 + ATRA | CIHH28 | CIHH28 + CSA | CIHH28 + DIAZ |
|---|---|---|---|---|---|---|---|
| LVDP (mmHg) | |||||||
| Pre-ischemia | 94.4 ± 2.7 | 96.1 ± 1.0 | 93.2 ± 2.5 | 93.6 ± 1.2 | 96.5 ± 1.1 | 96.1 ± 3.4 | 95.8 ± 4.2 |
| Reperfusion | 14.6 ± 2.5 (15.5 ± 2.7) | 12.3 ± 2.7 (12.85 ± 2.9) | 12.1 ± 2.7 (13.0 ± 3.0) | 13.9 ± 2.3 (14.7 ± 2.3) | 26.2 ± 2.6 (27.2 ± 2.7) | 24.6 ± 2.7 (25.9 ± 3.3) | 26.8 ± 2.3 (28.3 ± 3.1) |
| LVdp/dt max (mmHg/s) | |||||||
| Pre-ischemia | 3322.4 ± 283.1 | 3217.0 ± 131.7 | 3293.9 ± 197.6 | 3248.7 ± 230.7 | 3555.6 ± 324.3 | 3322.3 ± 92.1 | 3434.2 ± 85.1 |
| Reperfusion | 288.3 ± 45.2 (8.5 ± 0.7) | 166.0 ± 31.8 (5.2 ± 1.0)* | 203.8 ± 24.0 (6.2 ± 0.6)* | 327.3 ± 45.4 (10.1 ± 1.4) | 655.0 ± 53.1 (18.7 ± 1.9) | 717.9 ± 89.6 (21.5 ± 2.1) | 698.9 ± 63.3 (20.4 ± 2.0) |
| Lvdp/dt min (mmHg/s) | |||||||
| Pre-ischemia | 2078.4 ± 110.1 | 2168.0 ± 120.0 | 2239.8 ± 242.9 | 2013.9 ± 110.0 | 2375.7 ± 248.2 | 2347.0 ± 214.0 | 2105.9 ± 92.2 |
| Reperfusion | 243.9 ± 45.6 (11.7 ± 2.1) | 166.1 ± 39.0 (7.6 ± 1.7) | 208.7 ± 34.4 (9.6 ± 1.7) | 216.5 ± 47.0 (10.7 ± 2.1) | 663.7 ± 115.5 (27.8 ± 3.6) | 606.7 ± 39.9 (26.7 ± 3.6) | 560.7 ± 64.0 (26.7 ± 2.8) |
*P < 0.05 vs. CON28
All data are presented as the mean ± SEM (n = 4 per group). Values given in parenthesis are percentage values (reperfusion/pre-ischemia) and used for reperfusion comparison
GLI Glibenclamide, 5-HD 5-hydroxydecanoate, ATRA atractyloside, CSA Cyclosporin A, DIAZ diazoxide
The role of MPTP on the cardioprotection bestowed by CIHH
The protective effect of CIHH on cardiac function against I/R injury in CIHH rats was canceled out following pretreatment with an opener of MPTP (ATRA; 20 μM) after ischemia. Conversely, treatment with a blocker of MPTP opening (CSA; 0.2 μM) after ischemia enhanced the recovery of cardiac function from I/R in CON rats (Fig. 3). Neither ATRA nor CSA had an effect on cardiac function during reperfusion in CON rats and CIHH rats, respectively (Table 4). These results suggest that inhibition of MPTP opening plays an important role in the protective effect of CIHH on the heart against I/R injury in the developing rat.
Fig. 3.

Effects of atractyloside (ATRA) and cyclosporin A (CSA) on the recovery of cardiac function after 30 min of ischemia and 60 min of reperfusion in CON28 and CIHH28 rats (a) and in CON42 and CIHH42 rats (b). LVdP/dt max Maximal positive velocity of left ventricular pressure, LVdP/dt min maximal negative velocity of left ventricular pressure. All data are presented as the mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01 vss corresponding CON group; # P < 0.05, ## P < 0.01 vs. corresponding CIHH group
Effect of CIHH on SOD and TAC
After 30 min of ischemia and 60 min of reperfusion, myocardial SOD activity and TAC had increased significantly in CIHH28 and CIHH42 rats compared with the corresponding CON rats (P < 0.05; Table 5), suggesting that CIHH enhanced the anti-oxidation of myocardia in developing rats.
Table 5.
Effect of chronic intermittent hypobaric hypoxia on superoxide dismutase activity and total antioxidation capacity of the myocardium in developing rats
| SOD activity/TAC capacity | CON28 | CON42 | CIHH28 | CIHH42 |
|---|---|---|---|---|
| SOD | 5.0 ± 0.6 | 4.6 ± 0.5 | 7.0 ± 0.9* | 6.5 ± 0.4* |
| TAC | 8.6 ± 0.6 | 8.4 ± 1.7 | 17.5 ± 2.2** | 16.7 ± 1.1** |
* P < 0.05, ** P < 0.01 vs corresponding CON rats (CON28 or CON42, respectively)
All data are presented as the mean ± SEM (n = 6 per group)
SOD superoxide dismutase, TAC total antioxidation capacity
Discussion
The aim of this study was to investigate the role of the KATP channel and MPTP in the cardiac protection against I/R injury bestowed by CIHH treatment in developing rats. Our results show that CIHH treatment had no effect on the basal cardiac function, with the exception of increasing of CF. However, CIHH treatment did provide effective cardiac protection against I/R injury in developing rats by promoting the recovery of left ventricular function and diminishing ultrastructural damage to the myocardium. This cardiac protection was canceled out by treatment with GLI, 5-HD and ATRA, which suggests that the cardioprotective effect of CIHH treatment in developing rats may be related to the activation of KATP in the cell membrane and mitochondrial membrane, as well as to the inhibition of MPTP opening.
The ATP-sensitive potassium channel (KATP), which is activated by intra-cellular low-level [ATP]I, is an important K+ channel in the myocardium. KATP can be categorized into two types, namely, the membrane K+ channel (non-selective K+ channel) and the mitochondrion K+ channel (mitoKATP), according to their location. KATP, especially mitoKATP, has been proven to participate in short-term cardiac protection, such as ischemia preconditioning, hypoxia preconditioning and Ca2+ preconditioning [18–20]. The mitoKATP channel may also take part in the diosgenin-induced anti-arrhythmic effect [21]. In our study, the non-specific blocker of KATP GLI, the mitochondria KATP channel blocker 5-HD and the mitochondria KATP opener DIAZ were applied to observe the effect of KATP on the cardioprotection bestowed by CIHH. GLI and 5-HD abolished the cardioprotective effect of CIHH in CIHH rats, while DIAZ induced a cardiac protective effect in CON rats. These results suggest that the opening of KATP channels in both the cell membrane and mitochondrial membrane may be one of the mechanisms of CIHH cardioprotection in the developing rat. GLI and 5-HD were also found to have no effect on the basic cardiac function but they did contribute to a worsening of the I/R-induced injury on hearts in control rats. These results suggest that some KATP channels in the cell membrane or mitochondrial membrane can open as a self-protective mechanism during I/R.
The MPTP, a non-specific large pore in the inner mitochondrial membrane, plays a pivotal role in mitochondrial information exchange. It forms at the site of contact between the mitochondrial inner and outer membranes and allows for the flux of solutes with a molecular weight of <1.5 kDa. Depeding on the cellular environment, MPTP appears in three states. The first of these is the completely closed state in which the normal mitochondrial transmembrane potential is sustained [22]. The second is the reversible low-level open state in which molecules of <300 Da are allowed to pass through. In this state, the membrane potential decreases reversibly, and the functioning of MPTP is related with conduction of the electrical signal and Ca2+ entering or leaving the mitochondrion. The third state is the irreversible high-level open state in which molecules of <1500 Da are allowed through the membrane and the membrane potential decreases irreversibly [23]. When the permeability transition pore is open as a result of exposure to high calcium or inorganic phosphate levels, depletion of NAD (P)H, alkaline pH or reactive oxygen species (ROS), low-molecular weight substrates can freely penetrate the mitochondrial matrix, carrying along with them water. This results in mitochondrial swelling and the release of cytochrome c into the cytosol. The release of cytochrome c triggers a cascade of events that will lead to either apoptosis (in ATP-replete cells) or necrosis (in ATP-depleted cells) [24, 25]. Recent studies have shown that the opening of MPTP is an important cause of I/R injury [26] and that inhibition of MPTP opening participates in providing cardioprotection by theaflavin against I/R injury [20]. In our study, the MPTP opening inhibitors CSA and ATRA were applied to determine their effect on the cardioprotection provided by CIHH. In accordance with the results of an earlier study in adult rats [14], our findings showed that cardioprotection was abolished by ATRA in CIHH rats and was induced by CSA in CON rats, suggesting that the inhibition of MPTP opening is yet another mechanism for the cardioprotection providing by CIHH in the developing rat.
It is well known that the increase of ROS production during I/R is a major cause of the cardiac damage. Both SOD and TAC are important enzymes in the antioxidant system, and increases in SOD activity and TAC reflect increased antioxidative capability. Consistent with the results of our previous study showing that antioxidation of the myocardium was improved in CIHH-treated adult rats [5], the finding of the present study demonstrated that antioxidative capability of the myocardium was enhanced in CIHH-treated developing rats. These results imply that the enhancement of antioxidative capability plays an important role in the cardioprotection of CIHH against I/R injury in developing rats.
The functional characteristics of cardiac blood supply include plentiful blood, high oxygen consumption and a higher oxygen uptake rate. During arterial blood flows throughout the heart, about 65–70 % of the oxygen is taken up by cardiac muscle, which means that the latent capacity of increasing cardiac muscle oxygen uptake through increasing the oxygen uptake rate is limited. The increase in oxygen supply to the myocardium, such as during physical exercise, is accomplished mainly through the increase of CF. Therefore, an increase in CF means an enhancement of cardiac function. Our previous study showed that CIHH increased CF and myocardial capillary angiogenesis in adult rats [9]. In the present study, we observed that CF increased in CIHH-treated young rats, which suggests that a well-preserved cardiac function during I/R is related to the increase of CF.
Our previous study showed that CIHH simulating an altitude of 5000 m a.s.l. for 6 h/day for 28 days induces cardioprotection in adult rats but has harmful effects in developing rats. However, mild CIHH, which simulates an altitude of 3000 m a.s.l. for 5 h/day for 28 days produces cardiac protection in developing rats [16, 17]. In the present study, the same protective result was confirmed by using the 3000-m a.s.l. protocol in developing rats. However, there has been a report that CIHH simulating an altitude of 5000 m a.s.l. for 6 h/day for 28 days induces cardioprotection in developing rats [27]. It is interesting to note that the difference between these two experiments with opposite results is the initial time of hypoxia exposure. The protective effect was produced when the neonate rats began CIHH treatment after birth [27] and the damaging effect was produced when the neonate rats began the CIHH treatment before birth [16]. These results suggest that effect of CIHH on the developing heart is complex and depends not only on the level and duration of hypoxia, but also on the age of the animal and the timing of first exposure to CIHH.
In conclusion, this study demonstrated for the first time that CIHH protects the heart of developing rats against I/R injury through opening KATP channels, especially the mitoKATP channel, and inhibiting MPTP opening. The cardiac protection bestowed by CIHH may also be related to the enhancement of myocardial antioxidation and increased CF.
Acknowledgments
This work was supported by the National Basic Research Development Program of China (No.2006CB504100, No.2012CB518200), the National Natural Science Foundation of China (No.30393130, No.30572086).
Conflict of interest
None.
Footnotes
H. Bu and C. Yang contributed equally to this study.
C. Yang is deceased.
References
- 1.Madonna R, Cevik C, Nasser ME. Electrical plasticity and cardioprotection in myocardial ischemia-role of selective sodium channel blockers. Clin Cardiol. 2013;36(5):255–261. doi: 10.1002/clc.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou JJ, Wei Y, Zhang L, Zhang J, Guo LY, Gao C, Li DP, Zhang Y. Chronic intermittent hypobaric hypoxia prevents cardiac dysfunction through enhancing antioxidation in fructose-fed rats. Can J Physiol Pharmacol. 2013;91(5):332–337. doi: 10.1139/cjpp-2012-0059. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Yang HT, Zhou ZN. The cardioprotection of intermittent hypoxia adaptation. Sheng Li Xue Bao. 2007;59(5):601–613. [PubMed] [Google Scholar]
- 4.Zhang Y, Zhong N, Zhou ZN. Effects of chronic intermittent hypobaric hypoxia on the L-type calcium current in rat ventricular myocytes. High Alt Med Biol. 2010;11(1):61–67. doi: 10.1089/ham.2009.1011. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhong N, Zhou ZN. Antiarrhythmic and antioxidative effects of intermittent hypoxia exposure on rat myocardium. Sheng Li Xue Bao. 2000;52(2):89–92. [PubMed] [Google Scholar]
- 6.Zhang Y, Zhong N, Zhou ZN. Effects of intermittent hypoxia on action potential and contraction in non-ischemic and ischemic rat papillary muscle. Life Sci. 2000;67(20):2465–2471. doi: 10.1016/S0024-3205(00)00832-8. [DOI] [PubMed] [Google Scholar]
- 7.Zong P, Setty S, Sun W, Martinez R, Tune JD, Ehrenburg IV, Tkatchouk EN, Mallet RT, Downey HF. Intermittent hypoxic training protects canine myocardium from infarction. Exp Biol Med (Maywood) 2004;229(8):806–812. doi: 10.1177/153537020422900813. [DOI] [PubMed] [Google Scholar]
- 8.Zhong N, Zhang Y, Fang QZ, Zhou ZN. Intermittent hypoxia exposure-induced heat-shock protein 70 expression increases resistance of rat heart to ischemic injury. Acta Pharmacol Sin. 2000;21(5):467–472. [PubMed] [Google Scholar]
- 9.Zhong N, Zhang Y, Zhu HF, Wang JC, Fang QZ, Zhou ZN. Myocardial capillary angiogenesis and coronary flow in ischemia tolerance rat by adaptation to intermittent high altitude hypoxia. Acta Pharmacol Sin. 2002;23(4):305–310. [PubMed] [Google Scholar]
- 10.Guan Y, Gao L, Ma HJ, Li Q, Zhang H, Yuan F, Zhou ZN, Zhang Y. Chronic intermittent hypobaric hypoxia decreases β-adrenoceptor activity in right ventricular papillary muscle. Am J Physiol Heart Circ Physiol. 2010;298(4):H1267–H1272. doi: 10.1152/ajpheart.00410.2009. [DOI] [PubMed] [Google Scholar]
- 11.Ding HL, Zhu HF, Dong JW, Zhu WZ, Zhou ZN. Intermittent hypoxia protects the rat heart against ischemia/reperfusion injury by activating protein kinase C. Life Sci. 2004;75(21):2587–2603. doi: 10.1016/j.lfs.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res. 2003;13(5):385–391. doi: 10.1038/sj.cr.7290184. [DOI] [PubMed] [Google Scholar]
- 13.Zhu HF, Dong JW, Zhu WZ, Ding HL, Zhou ZN. ATP-dependent potassium channels involved in the cardiac protection induced by intermittent hypoxia against ischemia/reperfusion injury. Life Sci. 2003;73(10):1275–1287. doi: 10.1016/S0024-3205(03)00429-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhu WZ, Xie Y, Chen L, Yang HT, Zhou ZN. Intermittent high altitude hypoxia inhibits opening of mitochondrial permeability transition pores against reperfusion injury. J Mol Cell Cardiol. 2006;40(1):96–106. doi: 10.1016/j.yjmcc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Calmettes G, John SA, Weiss JN, Ribalet B. Hexokinase–mitochondrial interactions regulate glucose metabolism differentially in adult and neonatal cardiac myocytes. J Gen Physiol. 2013;142(4):425–436. doi: 10.1085/jgp.201310968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Yang ChY, Wang YP, Wang X, Cui F, Zhou ZhN, Zhang Y. Effects of different modes of intermittent hypobaric hypoxia on ischemia/reperfusioninjury in developing rat hearts. Sheng Li Xue Bao. 2007;59(5):660–666. [PubMed] [Google Scholar]
- 17.Ma HJ, Li Q, Ma HJ, Guan Y, Shi M, Yang J, Li DP, Zhang Y. Chronic intermittent hypobaric hypoxia ameliorates ischemia/reperfusion-induced calcium overload in heart via Na+/Ca2+ exchanger in developing rats. Cell Physiol Biochem. 2014;34:313–324. doi: 10.1159/000363001. [DOI] [PubMed] [Google Scholar]
- 18.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767(8):1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sukhodub A, Jovanović S, Du Q, Budas G, Clelland AK, Shen M, Sakamoto K, Tian R, Jovanović A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol. 2007;210(1):224–236. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma HJ, Huang XL, Li Q, Guan Y, Yuan F, Zhang Y. ATP-sensitive potassium channels and mitochondrial permeability transition pores play roles in the cardioprotection of theaflavin in young rat. J Physiol Sci. 2011;61(4):337–342. doi: 10.1007/s12576-011-0148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badalzadeh R, Yousefi B, Majidinia M, Ebrahimi H. Anti-arrhythmic effect of diosgenin in reperfusion-induced myocardial injury in a rat model: activation of nitric oxide system and mitochondrial KATP channel. J Physiol Sci. 2014;64(6):393–400. doi: 10.1007/s12576-014-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanchet L, Grefte S, Smeitink JA, Willems PH, Koopman WJ. Photo-induction and automated quantification of reversible mitochondrial permeability transition pore opening in primary mouse myotubes. PLoS ONE. 2014;9(11):e114090. doi: 10.1371/journal.pone.0114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70(2):191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Scatena R, Bottoni P, Botta G, Martorana GE, Giardina B. The role of mitochondria in pharmacotoxicology: a reevaluation of an old, newly emerging topic. Am J Physiol Cell Physiol. 2007;293(1):C12–C21. doi: 10.1152/ajpcell.00314.2006. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi A, Kim B, Matsuoka S. The destiny of Ca2+ released by mitochondria. J Physiol Sci. 2015;65(1):11–24. doi: 10.1007/s12576-014-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baines CP. The mitochondrial permeability transition pore and ischemia–reperfusion injury. Basic Res Cardiol. 2009;104(2):181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu WZ, Dong JW, Ding HL, Yang HT, Zhou ZN. Postnatal development in intermittent hypoxia enhances resistance to myocardial ischemia/reperfusion in male rats. Eur J Appl Physiol. 2004;91(5–6):716–722. doi: 10.1007/s00421-003-0939-7. [DOI] [PubMed] [Google Scholar]


