Abstract
Remote ischemic preconditioning (RIPC) is an intriguing approach which exposes a remote organ/tissue to a non-lethal transient ischemia/reperfusion (I/R) in order to potentiate the resistance of the desired organ/tissue against the next unwanted I/R. It has been suggested that RIPC exerts its effect through neuronal and hormonal pathways. The underlying mechanisms of RIPC are obscure and should be elucidated. In this study, we induced RIPC in mice using 3 cycles of 5 min ischemia alternating with 5 min reperfusion of the left renal artery. Renal failure was induced in mice by intra-peritoneal (i.p.) injection of 200 mg/kg body weight of gentamicin twice per day for 4 consecutive days. Global hippocampal ischemia reperfusion (I/R) was performed by bilateral carotid artery occlusion for 20 min followed by reperfusion for 72 h. Moreover, the retention trial of passive avoidance test was determined 72 h after global ischemia. Histopathological changes of hippocampus neurons were observed using Nissl staining to detect neuronal loss. Finally, terminal deoxynucleotidyl transferase mediated dUTP nick end-labeling (TUNEL) was performed to assess the status of apoptotic cells in the hippocampus. The results of this study suggest that renal ischemic preconditioning is a good candidate for prevention of I/R-induced hippocampal injury. However, RRPC (remote renal preconditioning) failed to exert a neuroprotective effect in mice with renal failure (RF), indicating the probable role of a humoral factor which is released from kidneys in response to ischemia. In agreement with this hypothesis, treatment of mice with rhEPO (5000 IU/kg intraperitoneal) before induction of RRPC restored the neuroprotective effects of RRPC in RF mice. Accordingly, it is plausible to expect that erythropoietin is released from kidneys to act as a mediator for RRPC-induced neuroprotective effects. Renal ischemic preconditioning prevents I/R-induced hippocampal injury. In contrast, renal failure hampers protective effects of RRPC, while exogenous administration of erythropoietin (EPO) significantly prevents the inhibiting effects of renal failure.
Keywords: Hippocampus, Remote ischemic preconditioning, Renal failure, Erythropoietin
Introduction
Global cerebral ischemia leads to significant hippocampal neuronal damage, and learning and memory impairment [1, 2]. Many parts of the brain, such as the CA1 area of the hippocampus, can be affected by global cerebral ischemia; a main cause of brain strokes [1, 3].
In spite of various methods and remedies for treatment of strokes, the mortality and morbidity rates are high, and brain strokes have become an important concern for health care providers [4].
In addition to several side effects, current treatments for brain strokes are not effective and the outcomes are disappointing.
Remote ischemic preconditioning (RIPC) has been considered as an appropriate alternative therapy for different types of ischemia [5, 6]. Interestingly, this novel approach exposes an organ/tissue from a distant site to a non-lethal transient ischemia/reperfusion (I/R) in order to augment the resistance of the desired organ/tissue against the probable next unwanted I/R. The underlying mechanisms of RIPC are very complicated and not yet fully understood. Reasonably, numerous studies have indicated that humoral and neurogenic pathways contribute to the RIPC approach. The preconditioned organ will synthesize and release the humoral substance into the blood stream, and consequently a remote region or organ will be protected. Adenosine, bradykinin, angiotensin-1 and erythropoietin are prominent and well-known examples for humoral compounds [7–9]. An intriguing study conducted by McClanahan et al. revealed that remote renal preconditioning (RRPC), as a short period of renal ischemia and reperfusion, significantly alleviated myocardial infarct size in rabbits [10]. Transient renal ischemia is able to induce several, probably signaling, pathways such as NFκB [11], hypoxia-inducible factor [8], PPARα and γ [12], angiotensin A1 receptors [13], erythropoietin [11] etc. Erythropoietin (EPO), a growth factor, is a member of the type 1 cytokine family, which contributes to the process of hematopoiesis [14]. Of note, there are many initiating factors like hypoxia, injury and metabolic stress which trigger EPO biosynthesis [15, 16]. EPO receptors are expressed on the surface of neural cells, endothelial cells and astrocytes [17]. Furthermore, approximately 90 % of circulating EPO in blood originates from the kidneys [18]. It is also worth mentioning that EPO is mainly considered to be a hematopoietic factor, but it is a multi-functional protein with prominent impact on recuperation from diseases such as cancer, stroke, and I/R renal injury [19–21]. Previous studies indicated that short-term induction of renal ischemia by artery occlusion may lead to biosynthesis of some transcription factors like HIF-1, which in turn is able to induce the transcription of the EPO gene [22].
Our recent studies showed that RRPC could prevent ischemia/reperfusion injuries in the hippocampus by activating signaling pathways, including mammalian target of rapamycin (MTOR) [2] and kATP channels [23]. Accordingly, this study was designed to evaluate the probable neuroprotective effect of erythropoietin which is released from preconditioned kidney.
Materials and methods
Experimental groups
Adult male BALB/C mice weighing 35–40 g were used for this experiment. The animals were housed in cages under standard environmental conditions at a temperature of 21 ± 1 °C, relative humidity 50 ± 10 %, on a 12 h light/12 h dark cycle with free access to water and food.
All experimental stages were confirmed by the ethics committee of Yazd University of Medical Science and were in accordance with the NIH (US National Institutes of Health) guide for the care and use of laboratory animals. Animals were acclimated to the cage for a minimum 15 days before surgery.
Sixty-three mice were divided randomly into nine experimental groups (n = 7) as follows: group I, sham operated group; group II IPC (ischemia preconditioning), underwent a renal IPC by 3 cycles of 5 min occlusion of left renal artery; group III (I/R), the mice were exposed to 20 min global cerebral ischemia followed by 72 h reperfusion; group IV RRPC (IPC + I/R), underwent a renal IPC 24 h prior to I/R; group V [renal failure (RF) + I/R], renal failure was induced by administering gentamicin (200 mg/kg twice daily, i.p. for 4 days) before renal sham surgery (24 h before I/R); group VI (RF + RRPC), RF was induced before renal surgery; group VII (EPO + I/R), EPO (5000 IU/kg, intraperitoneal) was administered 40 min prior to I/R; group VIII (RF + EPO + I/R), EPO was injected before I/R in group V; group IX (RF + EPO + RRPC), EPO was induced before I/R in up VI.
Remote renal preconditioning (RRPC)
The mice were subjected RRPC surgery as described previously [2]. Briefly, mice were anesthetized using intraperitoneal injection of a solution (0.01 ml/g) containing ketamine (10 mg/ml) and xylazine (2 mg/ml). It is noteworthy that their body temperature was maintained at nearly 37 °C during the surgical process. An incision 1.5–2 cm long was made on the left side of the abdomen. The left renal pedicle, consisting of the renal artery and vein was separated from surrounding tissues and was clamped using a non-traumatic vascular clamp for 3 cycles of 5 min ischemia followed by 5 min reperfusion. Twenty-four hours after renal transient ischemia, animals were anesthetized with the same dose of ketamine and xylazine, and global cerebral ischemia (I/R) was performed. I/R was performed by common carotid arteries occlusion for 20 min. Then the clamp was released for reperfusion for 72 h.
Renal failure (RF)
RF was created by administration of gentamicin at a dose of 200 mg/kg i.p. twice per day for 4 repeated days [24] before RRPC. RF was confirmed by measurement of blood urea nitrogen and serum creatinine level on the 5th day.
Biochemical assessment
The blood sampling was done before transient renal ischemia and transient renal ischemia was done 24 h before I/R. Plasma concentrations of blood urea nitrogen (BUN) and creatinine were evaluated by spectrophotometric methods using commercially available kits [25].
Behavioral experiment
The passive avoidance task was performed using the shuttle box to study the learning and short-term memory status in mice. The shuttle box consisted of a two-compartment light/dark box with identical dimensions and grid floors. The two compartments were separated by a guillotine door. Because mice prefer a dark place, they move into the dark box when they are placed in the light box. This experiment was performed in 3 steps: habituation, acquisition and retention. On the 1st and 2nd day, each mouse was allowed a 3-min adaptation period to the shuttle box, in which it could explore the light compartment and move about freely. If the animal did not move to the dark compartment after 100 s, it was removed from study. This adaptation was repeated 30 min later on the 2nd day. In the acquisition step on the 3rd day, 1 h before global brain ischemia, the mouse was placed in the bright compartment, and after 5 s the door was opened and the mouse was allowed to move freely into the dark chamber. Upon entry into the dark chamber, the door was closed and an electrical foot shock (50 Hz, 1 mA and 1 s) was delivered through the stainless steel rods. In the retention step that was performed 72 h after global cerebral ischemia, the mice were placed in the bright compartment, then the guillotine door was opened and the time to enter the dark part as latency time was recorded and was compared between groups [2].
Histopathology assessment
Mice were deeply anesthetized 72 h after I/R (n = 4 per group). All animals were sacrificed and were transcardially perfused with saline (50 ml) followed by 50 ml 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4).Then the brain was removed and was kept immersed for 2 days at 4 °C in a fixative solution containing 4 % paraformaldehyde. After dehydration in graded concentrations of ethanol and butanol, brains were embedded in paraffin. Selection of anatomical levels for sectioning was conducted with reference to illustrations from Paxinos and Watson. Consecutive sectioning at a thickness of 7 μm was then performed at the level of 2.7 mm of bregma. Three section slides of brain from each mouse were subjected to Nissl and TUNEL staining for evaluation of neuronal loss and cell apoptosis in the hippocampus [26, 27]. For Nissl staining, sections were deparaffinized in xylene and hydrated through a series of alcohols, rinsed in distilled water and were incubated with 0.1 % cresyl violet solution for 3–10 min. Then slides were dehydrated in a series of alcohols, cleared in xylene, and finally intact pyramidal neurons in the CA1 region were counted under a light microscope using 400× magnification. TUNEL staining using an in situ cell death detection kit, POD (Roche Molecular Biochemicals kit, Germany) was performed according to the manufacturer’s instructions to investigate the apoptosis. Paraffin-embedded tissue was deparaffinized in xylene and hydrated with an ethanol series (absolute, 95, 90, 80, 70 %, diluted in double distilled water). The endogenous peroxidase activity was blocked by using 0.3 % H2O2 in methanol for 10 min, after this stage and all subsequent stages, the slides were rinsed twice with PBS. In a later step, the sections were permeabilized with proteinase K solution for 30 min. Then, the sections were re-incubated in TUNEL reaction mixture (5 ml TUNEL-enzyme solution to 45 ml TUNEL-label solution) for 60 min at 37 °C in a humidified chamber. After washing, the sections were incubated with fluorescent antibody conjugated with horseradish peroxidase for 30 min and visualized by diaminobenzidine (DAB) substrate solution. After a final wash, slides were dehydrated in a series of alcohols, cleared in xylene and were viewed using a light microscope at 400× magnification. Apoptotic neurons were diagnosed by existence of different forms of chromatin condensation or apoptotic bodies [28].
Results
Gentamicin-induced renal failure
As shown in Fig. 1, administration of gentamicin led to renal failure. In contrast to the I/R group, the gentamicin-treated mice significantly showed increased serum concentration of renal function markers including blood urea nitrogen and serum creatinine (P < 0.01). In addition, the results indicated that RRPC and EPO failed to decrease the creatinine and blood urea nitrogen level in RF groups (Fig. 1).
Fig. 1.
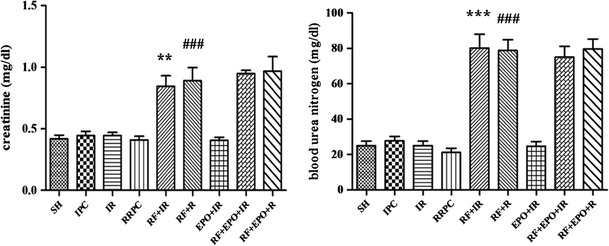
Serum levels of creatinine and urea nitrogen in different groups (mean ± SE, n = 7). Statistical analysis was done using one-way ANOVA followed by post hoc Tukey test. **P < 0.01 vs I/R group, ### P < 0.001 vs RRPC group, ***P < 0.001 vs I/R group, ### P < 0.001 vs sham group and RRPC group
Effect of EPO on passive avoidance memory
The passive avoidance memory test was performed in different groups and the results were analyzed 24 h after the training session. Data analysis using one-way ANOVA revealed that there were statistically significant differences among experimental groups (P < 0.01).
.The sham group demonstrated increased latency after electric shock training, indicating that the mice had acquired memory of the aversive stimulation associated with the darkened compartment. The comparison between each pair of groups showed that the ischemia group decreased the latency (62.71 ± 16.18) as compared to the sham group (227.4 ± 21.74), (P < 0.001). In addition, RRPC-induced rats which experienced RRPC surgery, indicated an increased latency (189.3 ± 27.73) relative to the I/R group (P < 0.001), while the RF group showed significantly decreased latency time (71.7 ± 14.51, P < 0.05). Also, ischemic mice treated with EPO had increased latency (182.6 ± 28.18) in comparison to the ischemia + RF group (71.7 ± 14.5, P < 0.05) (Fig. 2).
Fig. 2.
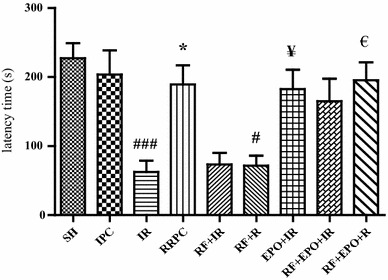
Latency time in passive avoidance test in different groups. The latency was the time spent in crossing from the illuminated compartment (electric shock-free part) to the darkened compartment (shock part) in the retention session (mean ± SE, n = 7). Statistical analysis was done using one-way ANOVA followed by post hoc Tukey test. ### P < 0.001 vs SH group, *P < 0.05 vs I/R group, # P < 0.05 vs RRPC group. ¥ P < 0.05 vs I/R group, € P < 0.05 vs RF + R group
EPO inhibits ischemia/reperfusion–induced reduction in density of live neurons of hippocampal CA1 cells
The results of Nissl staining showed that induction of cerebral ischemia in mice caused a decrease in the density of live neurons of the hippocampus in the CA1 area (58 ± 7.43) when compared to the sham group (132.3 ± 5.02, P < 0.001). RRPC elevated the density of live neurons (103.3 ± 5.69) compared to the I/R group (P < 0.001), while RF significantly decreased the density of live neurons in groups which experienced RRPC surgery (60 ± 3.76 % and P < 0.01). EPO-treated ischemic mice also showed increased density of live neurons (90 ± 3.93) when compared to ischemia + RF group (59 ± 5.78, P < 0.05, Figs. 3, 4).
Fig. 3.
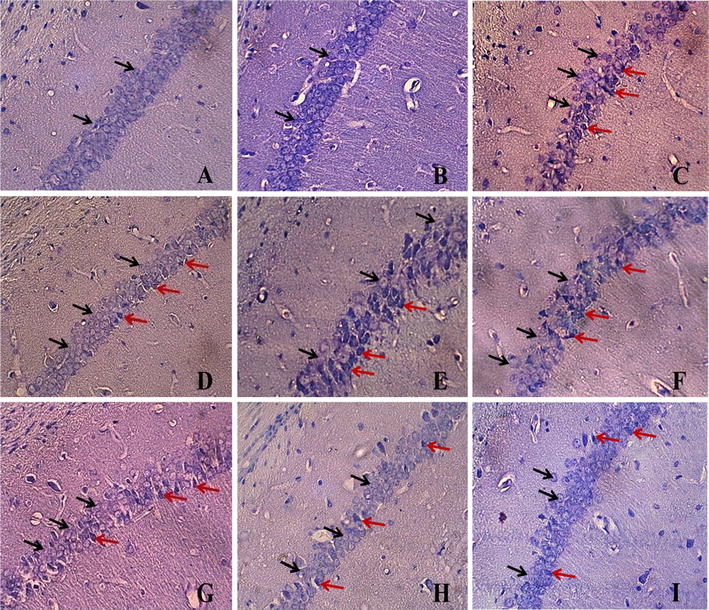
Nissl staining of hippocampal CA1 subregion (magnification ×400). a Sham group; b IPC group; c I/R group; d RRPC (IPC + I/R) group; e RF + I/R group; f RF + RRPC group; g EPO + I/R group; h RF + EPO + I/R group; i RF + EPO + R group. Black arrows indicate intact cells and red arrows indicate necrotic cells
Fig. 4.
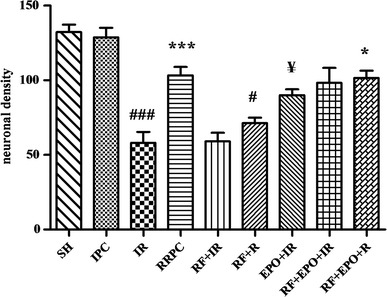
The graph shows density of live neurons which were counted in hippocampal CA1 subregion (mean ± SE, n = 4). Statistical analysis was done using one-way ANOVA followed by post hoc Tukey test. ### P < 0.001 vs SH group, ***P < 0.001 vs I/R group, # P < 0.05 vs RRPC group, ¥ P < 0.05 vs I/R group, *P < 0.05 vs RF + R group
EPO inhibits ischemia/reperfusion–induced apoptotic cell death of hippocampus neurons
As is obvious in Figs. 5 and 6, neuronal cell apoptosis had been induced in the CA1 subregion of the hippocampus of mice which were under global brain ischemia (I/R). Regarding the percentage of apoptotic cells/total cells, statistical analysis showed a significant difference between the I/R group (55.75 ± 5.82) and the sham group (1.5 ± .64, P < 0.001). Moreover, the induction of RRPC reduced the number of apoptotic cells in the I/R group (19.25 ± 1.75) as compared to the I/R group without RRPC induction (P < 0.001). According to the obtained data, RF significantly caused an increase in the total percentage of apoptotic cells (58.25 ± 2.46). A similar result was obtained in RF rats which experienced RRPC surgery (41.75 ± 6.31, P < 0.05). In contrast, ischemic mice treated with EPO showed that the apoptosis had been modulated (22.00 ± 3.76) compared to ischemia + RF group (41.75 ± 6.31 P < 0.05). Furthermore, RRPC significantly decreased apoptotic cells when compared to RF + EPO +I/R (P < 0.05, Figs. 5, 6).
Fig. 5.
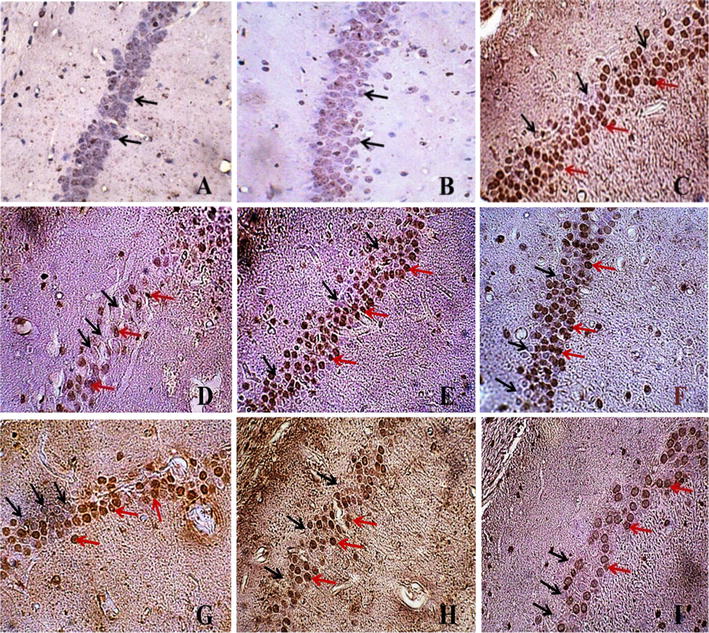
TUNEL staining of hippocampal CA1 region (magnification ×400). a Sham group; b IPC group; c I/R group; d RRPC (IPC + I/R) group; e RF + I/R group; f RF + RRPC group; g EPO + I/R group; h RF + EPO + I/R group; i RF + EPO + R group. Black arrows indicate intact cells and red arrows indicate necrotic cells
Fig. 6.
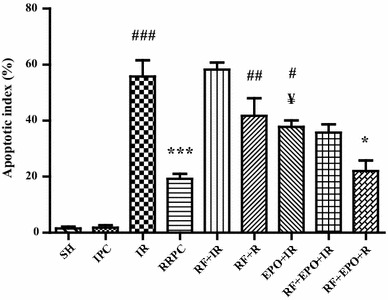
The graph shows the percentage of TUNEL-positive cells that was explained as percent of apoptotic cells in each field (mean ± SE, n = 4). Statistical analysis was done using one-way ANOVA followed by post hoc Tukey test. ### P < 0.001 vs SH group; ***P < 0.001 vs I/R group; ## P < 0.01, # P < 0.05 vs RRPC group; ¥ P < 0.05 vs I/R group; *P < 0.05 vs RF + R group
Discussion
This study was conducted to find out whether the RIPC approach is able to protect the hippocampus against global cerebral ischemia. In addition, the results of this study suggested that the mechanism of action of RRPC might be at least in part due to biosynthesis and secretion of EPO from kidneys. Interestingly, the protective effects of RRPC on hippocampal cells were halted in rats which were treated by gentamicin, as a toxic compound with ability to induce renal failure.
In the present study, we showed that hippocampal injury and subsequently memory impairment in mice with induced global cerebral ischemia can be modulated by exogenous administration of EPO. On the other hand, we found that RRPC significantly reduced ischemia/reperfusion-induced hippocampal injury and memory impairment. Importantly, our data revealed that intraperitoneal administration of EPO dramatically prevented the adverse effects of renal failure against the neuroprotective action of RRPC. Therefore, we hypothesized that EPO may be released from preconditioned kidneys and it possibly plays an important role in protection of neural cells against global brain ischemia. To the best of our knowledge, this is the first study which has evaluated the probable role of EPO in RRPC-induced neuroprotective effects in hippocampal cells. However, this study has also shown that RRPC may be a more effective neuroprotective factor than EPO. This is probably due to other factors, in addition to EPO, being released from the kidney during preconditioning.
The dosage used in this study (5000 U/kg i.p.) is similar to other investigations showing beneficial effects in different pathophysiological settings of ischemia [29].
In the current study, we found that RRPC attenuated I/R-induced hippocampal injury. This finding was corroborated by the results of our previous studies. The protective effect of RRPC on brain ischemia was proved by behavioral and histological studies. In our previous study, we explored the probable signaling pathways in which RRPC could exert its beneficial effects. Interestingly, we found that RRPC induction would induce MTOR phosphorylation and kATP channel activation [23]. In the present study, we aimed to explore the neuroprotective factor which is released from the kidney as a result of RRPC. In another words, we wanted to find out what factor released from kidneys ameliorates brain ischemia induced-neural injury in the RRPC strategy. One of the most important factors that is released from the kidney under conditions of hypoxia is erythropoietin [8].
Many studies have claimed that erythropoietin treatment might have a neuroprotective effect [30]. TNF-α is a pro-inflammatory cytokine [31, 32], and its concentration elevates in patients with strokes and traumatic brain injury, which leads to exacerbation of inflammatory responses, production of reactive oxygen species and also an increase in the number of apoptotic cells [33]. EPO is considered to be a protein with an ability to act against the inflammatory condition. For example, EPO is able to inhibit tumor necrosis factor (TNF)-α, a major deleterious pro-inflammatory cytokine [34]. Previous studies showed that EPO has anti-inflammatory action via inhibition of inflammatory cytokine TNF [34, 35].
Heat shock protein (HSP) is responsible for the appropriate folding of proteins during normal and stressful conditions such as ischemia, oxidative stress, glucose deprivation and exposure to toxins (Liebelt et al. 2010). It has been indicated that HSPs might overexpress in response to ischemia/reperfusion injuries and exert neuroprotective effects [36]. A recent study showed that EPO modulated the occurrence of apoptosis in neural cells, probably due to the up-regulation of HSP-27. Extracellular signal-regulated kinase 1/2 (ERK1/2) is involved in mitogen-activated protein kinase pathways and is constitutively expressed in the adult brain [37]. These ERK1/2-regulated pathways are involved in signal transduction and protect neural cells against ischemia/reperfusion injury. The aforementioned pathways activate during ischemia/reperfusion injury to enhance the ability of neural tissues to repair by inducing cell division and decreasing the rate of cell death [38]. Furthermore, it appears that EPO is able to up-regulate ERK1/2 [39].
Another explanation for the observed neuroprotective effect of EPO would be the inhibition of free radical formation. In normal physiological situations, there is a balance between the production of reactive oxygen species (ROS) and the anti-oxidant system. Both forms of reactive oxygen and nitrogen elements might be produced in greater quantities in pathophysiological conditions, which in the case of brain strokes leads to increased neuronal damage by protein degradation, DNA damage and elevated levels of lipid peroxidation products in cellular membranes [40]. Moreover, Zhai et al. reported that EPO induces the enzymatic activity of selected anti-oxidative enzymes catalase (CAT) and glutathione peroxidase (GSH-Px) in brain tissues [41].
Caspase-3 has been identified as a key mediator of apoptosis in animal models of ischemic stroke [42]. Asahi and co-workers demonstrated upregulation of caspase-3 mRNA in rat brain 1 h after the onset of focal ischemia. Both genetic disruption and pharmacological inhibition of caspases have been found to have a strong neuroprotective effect in experimental strokes [43]. A previous study demonstrated EPO inhibition of caspase activation [44].
Conclusion
Brain ischemia/reperfusion causes hippocampal cell death and memory impairment which can be attenuated by renal ischemic preconditioning. In contrast, renal failure hampers the protective effects of RRPC while exogenous administration of EPO significantly prevents the inhibiting effects of renal failure. Therefore, we suggest that EPO might be a neuroprotective agent that is released from the kidney during preconditioning.
References
- 1.Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F et al (2015) Visfatin reduces hippocampal CA1 cells death and improves learning and memory deficits after transient global ischemia/reperfusion. Neuropeptides [DOI] [PubMed]
- 2.Mehrjerdi FZ, Aboutaleb N, Habibey R, Ajami M, Soleimani M, Arabian M, et al. Increased phosphorylation of mTOR is involved in remote ischemic preconditioning of hippocampus in mice. Brain Res. 2013;1526:94–101. doi: 10.1016/j.brainres.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Atlasi MA, Naderian H, Noureddini M, Fakharian E, Azami A. Morphology of rat hippocampal CA1 neurons following modified two and four-vessels global ischemia models. Arch Trauma Res. 2013;2(3):124. doi: 10.5812/atr.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnamurthi RV, deVeber G, Feigin V, Barker-Collo S, Fullerton H, Mackay M, et al. Stroke prevalence, mortality and disability-adjusted life years in children and youth aged 0–19 years: data from the global and regional burden of stroke 2013. Neuroepidemiology. 2015;45(3):177–189. doi: 10.1159/000441087. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Yellon DM (2008) Remote ischemic preconditioning: underlying mechanisms and clinical application. Cardiovascular Research [DOI] [PubMed]
- 6.Schmidt MR, Støttrup NB, Michelsen MM, Contractor H, Sørensen KE, Kharbanda RK, et al. Remote ischemic preconditioning impairs ventricular function and increases infarct size after prolonged ischemia in the isolated neonatal rabbit heart. J Thorac Cardiovasc Surg. 2014;147(3):1049–1055. doi: 10.1016/j.jtcvs.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Hu S, Dong H, Zhang H, Wang S, Hou L, Chen S, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012;1459:81–90. doi: 10.1016/j.brainres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Diwan V, Jaggi AS, Singh M, Singh N, Singh D. Possible involvement of erythropoietin in remote renal preconditioning-induced cardioprotection in rats. J Cardiovasc Pharmacol. 2008;51(2):126–130. doi: 10.1097/FJC.0b013e31815d88c9. [DOI] [PubMed] [Google Scholar]
- 9.Kloner RA. Clinical application of remote ischemic preconditioning. Circulation. 2009;119(6):776–778. doi: 10.1161/CIRCULATIONAHA.108.832832. [DOI] [PubMed] [Google Scholar]
- 10.McClanahan T, Nao B, Wolke L, Martin B, Mertz T, Gallagher K, editors. Brief renal occlusion and reperfusion reduces myocardial infarct size in rabbits. FASEB JOURNAL; 1993: FEDERATION AMER SOC EXP BIOL 9650 ROCKVILLE PIKE, BETHESDA, MD 20814-3998 USA
- 11.Diwan V, Kant R, Jaggi AS, Singh N, Singh D. Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem. 2008;315(1–2):195–201. doi: 10.1007/s11010-008-9808-3. [DOI] [PubMed] [Google Scholar]
- 12.Lotz C, Lazariotto M, Redel A, Smul TM, Stumpner J, Blomeyer C, et al. Activation of peroxisome-proliferator-activated receptors α and γ mediates remote ischemic preconditioning against myocardial infarction in vivo. Exp Biol Med. 2011;236(1):113–122. doi: 10.1258/ebm.2010.010210. [DOI] [PubMed] [Google Scholar]
- 13.Singh D, Chopra K. Evidence of the role of angiotensin AT (1) receptors in remote renal preconditioning of myocardium. Methods Find Exp Clin Pharmacol. 2004;26(2):117–122. doi: 10.1358/mf.2004.26.2.800064. [DOI] [PubMed] [Google Scholar]
- 14.Genc S, Koroglu TF, Genc K. Erythropoietin and the nervous system. Brain Res. 2004;1000(1):19–31. doi: 10.1016/j.brainres.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Ghezzi P, Bernaudin M, Bianchi R, Blomgren K. Erythropoietin: not just about erythropoiesis. Lancet. 2010;375(9732):2142. doi: 10.1016/S0140-6736(10)60992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velly L, Pellegrini L, Guillet B, Bruder N, Pisano P. Erythropoietin 2nd cerebral protection after acute injuries: a double-edged sword? Pharmacol Ther. 2010;128(3):445–459. doi: 10.1016/j.pharmthera.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Brines ML, Ghezzi P, Keenan S, Agnello D, De Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci. 2000;97(19):10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souma T, Suzuki N, Yamamoto M. Renal erythropoietin-producing cells in health and disease. Name Front in Physiol. 2015;6:167. doi: 10.3389/fphys.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaapil MC, Reinbothe S, Larsson A-M, Wigerup C, Sun J, Jögi A et al (2014) The effect of EPO-receptor in estrogen receptor positive breast cancer. Cancer Research 74(19 Supplement):3464
- 20.Souvenir R, Doycheva D, Zhang HJ, Tang J. Erythropoietin in stroke therapy: friend or foe. Curr Med Chem. 2015;22(10):1205–1213. doi: 10.2174/0929867322666150114152134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loeffler I, Wolf G. The role of hypoxia and Morg1 in renal injury. Eur J Clin Invest. 2015;45(3):294–302. doi: 10.1111/eci.12405. [DOI] [PubMed] [Google Scholar]
- 22.Akhtar M, Sutherland A, Huang H, Ploeg R, Pugh C. The role of hypoxia-inducible factors in organ donation and transplantation: the current perspective and future opportunities. Am J Transplant. 2014;14(7):1481–1487. doi: 10.1111/ajt.12737. [DOI] [PubMed] [Google Scholar]
- 23.Mehrjerdi FZ, Aboutaleb N, Pazoki-Toroudi H, Soleimani M, Ajami M, Khaksari M et al (2015) The protective effect of remote renal preconditioning against hippocampal Ischemia reperfusion injury: role of kATP channels. Journal of Molecular Neuroscience 1–7 [DOI] [PubMed]
- 24.Ortega A, Rámila D, Izquierdo A, González L, Barat A, Gazapo R, et al. Role of the renin-angiotensin system on the parathyroid hormone-related protein overexpression induced by nephrotoxic acute renal failure in the rat. J Am Soc Nephrol. 2005;16(4):939–949. doi: 10.1681/ASN.2004040328. [DOI] [PubMed] [Google Scholar]
- 25.Khastar H, Kadkhodaee M, Sadeghipour HR, Seifi B, Hadjati J, Delavari F, et al. Leukocyte involvement in renal reperfusion-induced liver damage. Ren Fail. 2011;33(1):79–83. doi: 10.3109/0886022X.2010.541585. [DOI] [PubMed] [Google Scholar]
- 26.Aboutaleb N, Shamsaei N, Khaksari M, Erfani S, Rajabi H, Nikbakht F (2015) Pre-ischemic exercise reduces apoptosis in hippocampal CA3 cells after cerebral ischemia by modulation of the Bax/Bcl-2 proteins ratio and prevention of caspase-3 activation. The Journal of Physiological Sciences 1–9 [DOI] [PMC free article] [PubMed]
- 27.Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F. Nampt/PBEF/visfatin exerts neuroprotective effects against ischemia/reperfusion injury via modulation of Bax/Bcl-2 ratio and prevention of caspase-3 activation. J Mol Neurosci. 2015;56(1):237–243. doi: 10.1007/s12031-014-0486-1. [DOI] [PubMed] [Google Scholar]
- 28.Gheibi S, Aboutaleb N, Khaksari M, Kalalian-Moghaddam H, Vakili A, Asadi Y, et al. Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci. 2014;54(2):264–270. doi: 10.1007/s12031-014-0284-9. [DOI] [PubMed] [Google Scholar]
- 29.Sirén A-L, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci. 2001;98(7):4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rangarajan V, Juul SE. Erythropoietin: emerging role of erythropoietin in neonatal neuroprotection. Pediatr Neurol. 2014;51(4):481–488. doi: 10.1016/j.pediatrneurol.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y-R, Wang D, Liu Y, Shan L, Zhou J-L (2015) The PI3 K/Akt, p38MAPK, and JAK2/STAT3 signaling pathways mediate the protection of SO2 against acute lung injury induced by limb ischemia/reperfusion in rats. The Journal of Physiological Sciences 1–11 [DOI] [PMC free article] [PubMed]
- 32.Ahmad Z, Ng CT, Fong LY, Bakar NAA, Hussain NHM, Ang KP et al (2016) Cryptotanshinone inhibits TNF-α-induced early atherogenic events in vitro. The Journal of Physiological Sciences 1–8 [DOI] [PMC free article] [PubMed]
- 33.Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10(1):95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198(6):971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen AQ, Cherry BH, Scott GF, Ryou M-G, Mallet RT (2014) Erythropoietin: powerful protection of ischemic and post-ischemic brain. Experimental Biology and medicine 1535370214523703 [DOI] [PMC free article] [PubMed]
- 36.Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16(1):53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Sharony R, Pintucci G, Saunders PC, Grossi EA, Baumann FG, Galloway AC, et al. Matrix metalloproteinase expression in vein grafts: role of inflammatory mediators and extracellular signal-regulated kinases-1 and-2. Am J Physiol Heart Circ Physiol. 2006;290(4):H1651–H1659. doi: 10.1152/ajpheart.00530.2005. [DOI] [PubMed] [Google Scholar]
- 38.Cavanaugh JE. Role of extracellular signal regulated kinase 5 in neuronal survival. Eur J Biochem. 2004;271(11):2056–2059. doi: 10.1111/j.1432-1033.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- 39.Jones NM, Bergeron M. Hypoxia-induced ischemic tolerance in neonatal rat brain involves enhanced ERK1/2 signaling. J Neurochem. 2004;89(1):157–167. doi: 10.1111/j.1471-4159.2004.02324.x. [DOI] [PubMed] [Google Scholar]
- 40.Moghaddasi M, Javanmard SH, Reisi P, Tajadini M, Taati M. The effect of regular exercise on antioxidant enzyme activities and lipid peroxidation levels in both hippocampi after occluding one carotid in rat. The Journal of Physiological Sciences. 2014;64(5):325–332. doi: 10.1007/s12576-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhai Y, Wang H, Sun H, Zhang G, Wu H. [Effect of erythropoietin on activities of antioxidant enzymes in the brain tissue of aged rats]. Nan fang yi ke da xue xue bao= Journal of Southern Medical University. 2013;33(9):1332–1335. [PubMed] [Google Scholar]
- 42.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 43.Asahi M, Hoshimaru M, Uemura Y, Tokime T, Kojima M, Ohtsuka T, et al. Expression of interleukin-1β converting enzyme gene family and bcl-2 gene family in the rat brain following permanent occlusion of the middle cerebral artery. J Cereb Blood Flow Metab. 1997;17(1):11–18. doi: 10.1097/00004647-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Popp E, Vogel P, Teschendorf P, Böttiger BW. Effects of the application of erythropoietin on cerebral recovery after cardiac arrest in rats. Resuscitation. 2007;74(2):344–351. doi: 10.1016/j.resuscitation.2007.01.019. [DOI] [PubMed] [Google Scholar]


