Abstract
Peroxiredoxins (PRDXs) are multifunctional proteins that have recently received much attention. They are part of the endogenous antioxidative capacity and function as efficient scavengers, especially for hydrogen peroxides. Studies show that physical training can induce an upregulation of PRDX isoform contents in the long term. This might help counteract chronic diseases that are causally linked to a high amount of free radicals, e.g., diabetes mellitus. Furthermore, it has been demonstrated that PRDX can overoxidize under pathological conditions during acute exercise. Overoxidized PRDXs could be useful because they act as protective chaperones. Taken together, it can be speculated that physical activity has a positive effect on the PRDX system and thereby prevents cells from free radical-induced damage.
Keywords: Peroxiredoxins, Antioxidative defense, Sports, Physical activity
Peroxiredoxins as antioxidative proteins
The biomedical research community has increasingly focused its attention on peroxiredoxins as effective antioxidative proteins. While only 48 publications with the keyword “peroxiredoxins” were published in PubMed between 1990 and 1995, the number of new publications increased to 1,382 articles between 2005 and 2010. While a total of 2,292 publications on peroxiredoxins (PRDXs) had been published by August 2011, 46,208 publications on other antioxidative enzymes such as superoxide dismutase (SOD), 37,585 on catalase (CAT) and 19,267 on glutathione peroxidase (GPX) were available. Accordingly, the current level of knowledge about PRDX is significantly lower than that about the other antioxidative proteins mentioned [1].
PRDXs have a molecular size of 20–30 kDa [2, 3] and are found in practically all organisms [1]. They make up approximately 0.1–1 % of soluble proteins in the majority of human cells [3, 4] and constitute the third highest protein in the cytosol of erythrocytes [5]. In humans, six PRDX isoforms with different cellular localizations can be distinguished (Table 1).
Table 1.
Cellular localization of peroxiredoxin (PRDX) isoforms (information from Wood et al. [6])
| Peroxiredoxin isoform | Cellular localization |
|---|---|
| PRDX1 | Cytosol, nucleus |
| PRDX2 | Cytosol, membrane |
| PRDX3 | Mitochondria |
| PRDX4 | Cytosol, Golgi apparatus |
| PRDX5 | Cytosol, mitochondria, peroxisomes |
| PRDX6 | Cytosol |
PRDXs can reduce peroxynitrate (ONOO*−) and hydroperoxide (ROOH) [6, 7]. Moreover, next to the enzymes GPX and CAT, they are involved in the decomposition of H2O2 to water [8, 9]. Hydrogen peroxide is not particularly reactive, but can, inter alia, react to the highly reactive hydroxyl radical (*OH) via the Haber-Weiss reaction. A large amount of H2O2 thus leads to radical-induced damage.
Catalytic mechanism
Peroxiredoxins are not classical enzymes because they contain a co-substrate. All PRDXs share the same catalytic mechanism: A peroxide-specific cysteine residue (-Cys-SPH) is oxidized through the peroxide substrate [6]. PRDX molecules can be divided into three classes [typical 2-Cys-PRDX (PRDX1-4), atypical 2-Cys-PRDX (PRDX5) and 1-Cys-PRDX (PRDX6)] according to the number of cysteine residues and their way of recovery. Oxidized typical 2-Cys-PRDXs form an intermolecular disulfide bridge with a thiol of another PRDX molecule, oxidized atypical 2-Cys-PRDXs form an intramolecular disulfide bridge with a thiol of the same molecule, and 1-Cys-PRDXs probably do not form a disulfide bridge. Nearly all PRDX molecules are finally converted into the initial molecules through the thioredoxin system, although the hydrogen donor of PRDX6 has not yet been clearly identified [10, 11] (Fig. 1).
Fig. 1.
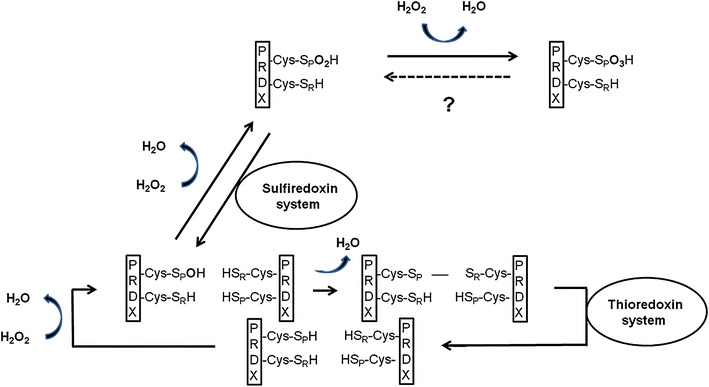
Catalytic mechanism of typical 2-Cys-peroxiredoxins, -Cys-SPH, peroxide-specific cysteine residue; -Cys-SPO2H, overoxidized cysteine residue; -Cys-SPO3H, double overoxidized cysteine residue; -Cys-SRH, resolving cysteine residue; H2O, water molecule; H2O2, hydrogen peroxide molecule
In vitro experiments suggest that typical 2-Cys-PRDX and 1-Cys-PRDX can overoxidize in case of high exposure to peroxides [12–14]. The oxidized peroxide-specific cysteine residue of the PRDX molecule reacts with another peroxide to form sulfinic acid (-Cys-SpO2H). This overoxidation process seems to be reversible—the conversion into the initial molecule can be achieved through a sulfiredoxin-mediated process [13, 14].
It has been shown for the first time that high concentrations of peroxides can in fact result in a double overoxidation of Tsa1p (yeast PRDX, orthologous to PRDX1) through the creation of sulfonic acid (-Cys-SpO3H) [15]. An intracellular reduction system has, however, not yet been identified. Because no reduction of double overoxidized PRDX takes place—even after a 12-h regeneration period [16]—it is assumed that double overoxidation is irreversible. In contrast to PRDX1, it seems that an N-terminal acetylation in PRDX2 plays a role in protecting the protein from a possibly irreversible double overoxidation [17]. Thus far, there is no evidence of double overoxidation of the other PRDX isoforms.
Function of overoxidized peroxiredoxins
With regard to the significance of overoxidation, both “loss of function” and “gain of function” possibilities are being discussed [1]. The speculative “floodgate” model entails a loss of function when PRDXs are exposed to higher concentrations of peroxides because of overoxidation and hence lose their function as radical blockers, which enables a local accumulation of hydrogen peroxides that induce signal cascades as second messengers [18]. A gain of function has been described in studies that conclude that overoxidized PRDXs may lose their catalytic function, yet create large molecular compounds, and, like molecular complexes, can exhibit chaperone activity to protect proteins from denaturation [19].
Effects of physical training on peroxiredoxins
Several working groups recently examined the effects of physical training on the protein synthesis of peroxiredoxins (Table 2). The studies mentioned indicate that regular physical activity with submaximal intensity mainly causes an upregulation of the PRDX system in the long term. However, it is also noteworthy that the regulation of PRDX isoforms by physical training can obviously differ between cells (e.g., PRDX2 is upregulated in erythrocytes, while there is no change of PRDX2 in skeletal muscle cells and a decrease in heart muscle cells [20, 21, 23]). Furthermore, the regulation of PRDX isoform contents can even differ within a cell (PRDX3 is upregulated in subsarcolemmal mitochondria of heart muscle cells, but does not change in intermyofibrillar mitochondria after training [22]). Further studies should clarify the related complex mechanisms underlying the specific regulation of PRDX isoform contents within a cell and between different cells.
Table 2.
Influence of (a) physical training and (b) acute physical exercise on peroxiredoxin (PRDX) contents
| Study | Training program/ physical activity | Subjects | Cells/ cellular compartments | PRDX system |
|---|---|---|---|---|
| Physical training | ||||
| Moghaddam et al. [20] | 3 months, 3 times a week, cycling (25–50 min, 75 % HRmax) | Type 2 diabetic men (n = 6) | Erythrocytes |
PRDX1 ↔ PRDX2 ↑ |
| Brinkmann et al. [21] | 3 months, twice a week, cycling (25–50 min, HR: corresponding to 2 mmol/l blood lactate concentration) or whole body strength training (30–75 % 1-RM) | Type 2 diabetic men (n = 16) | Skeletal muscle cells, musculus vastus lateralis |
PRDX1 ↔ PRDX2 ↔ PRDX3 ↔ PRDX4 ↔ PRDX5 ↑ PRDX6 ↔ |
| Kavazis et al. [22] | 10 days, daily, treadmill running (10–60 min, 30 m/min, 0° inclination, 70 % maximal oxygen consumption) | Male Sprague–Dawley rats (n = 8) | Isolated mitochondria from heart muscle cells | |
| subsarcolemmal | PRDX3 ↑ | |||
| intermyofibrillar | PRDX3 ↔ | |||
| Richters et al. [23] | 2 months, 5 times a week, treadmill running (1 h, 15 m/min, 5° inclination) | Male SOD2-knock-out-mices (n = 14) | Heart muscle cells |
PRDX1 ↑ PRDX2 ↓ PRDX3 ↑ PRDX4 ↑ PRDX5 ↔ PRDX6 ↔ |
| Acute physical exercise | ||||
| Brinkmann et al. [24] | WHO cycling step test to physical exhaustion (25 W + 25 W every 2 min) | Type 2 diabetic men (n = 15) | Erythrocytes | PRDX-SO2–3 ↑ |
| Non-diabetic men (n = 12) | PRDX-SO2–3 ↔ | |||
HR max maximal heart rate, 1-RM 1-repetition maximum, PRDX-SO 2–3 overoxidized peroxiredoxins, SOD superoxide dismutase, WHO World Health Organization
Additional results suggest that training can modify PRDX contents. A study on erythrocytes determined the extent to which the level of fitness per se affects the PRDX system in type 2 diabetic men (n = 22). Regression analyses reveal a positive correlation between the erythrocyte contents of PRDX2 plotted against the VO2peak/workload at 4 mmol/l lactate concentration [based on the World Health Organization (WHO) step test on a bicycle ergometer] [25].
An exercise-induced improvement of the antioxidative defense capacity may be particularly relevant for patients who suffer from diseases associated with an increased level of radicals, such as diabetes mellitus [20, 21].
Evidently, the PRDX isoforms in skeletal and heart muscle cells—in particular, which are located in the mitochondria—are upregulated through exercise [21–23]. This is an indication that many free radicals (and thus also hydrogen peroxides) are generated in the mitochondria during exercise [26], and antioxidative protection is particularly useful in this case.
Effect of acute exercise on peroxiredoxins
Since the literature suggests that peroxiredoxins that are exposed to a high amount of free radicals can overoxidize, our working group recently examined whether an overoxidation of PRDX occurs during acute exercise, that is, in a state in which an increasing number of free radicals develop in vivo [27].
An increase in the contents of overoxidized peroxiredoxins (PRDX-SO2–3) was only apparent in the erythrocytes of type 2 diabetic men during the WHO endurance step test on a bicycle ergometer, but not in non-diabetic men (Table 2). The amount of overoxidized PRDX in diabetic men had not decreased significantly up to 30 min following acute exercise [24].
The fact that PRDX under increased oxidative stress overoxidize in the erythrocytes of only type 2 diabetic men during exercise indicates that the level of activity of PRDX differs in diabetic patients. Whether an irreversible overoxidation of PRDX occurs in diabetic men during acute exercise or whether 30 min are insufficient for reducing the amount of overoxidized PRDX cannot yet be adequately answered.
Conclusion and outlook
In addition to the “classical” radical buffers, PRDXs have been receiving increasing attention—particularly because of their comparatively high abundance in human cells. Their ability in an overoxidized state to act as chaperones emphasizes their potential in preventing radical-induced damages on different levels. Future studies should focus on their role/positioning in the integrated antioxidative system, especially under physical exercise.
Regular physical training can cause a partial upregulation of the PRDX system and thus presumably contribute to a reduction in oxidative stress. This could be particularly interesting for patients who suffer from diseases related to increased levels of free radicals.
Future studies shall elucidate in more detail which exercise programs are most suitable for achieving an upregulation of PRDX isoforms. The question arises, for example, about what effect high-intensity training in addition to submaximal intensity training has on PRDX contents.
An overoxidation of PRDX and thus a change in the protein's functioning may occur under pathological conditions during acute physical exercise.
The implications of overoxidation of PRDX during acute exercise could also be further substantiated. It is not clear which specific (patho-)physiological parameters are responsible for causing overoxidation during acute exercise.
To date, the effect of sports on PRDX has only been examined in a few tissues. This area also clearly requires further research in order to shed more light on these important antioxidative proteins.
Footnotes
This mini-review elucidates the significance of peroxiredoxins in the defense against free radicals and provides an overview of the most recent studies on the influence of sports on peroxiredoxins.
An erratum to this article is available at http://dx.doi.org/10.1007/s12576-017-0551-y.
References
- 1.Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins–structures, mechanisms and functions. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim K, Kim IH, Lee KY, Rhee SG, Stadtman ER. The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem. 1988;263:4704–4711. [PubMed] [Google Scholar]
- 3.Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 4.Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999;300:219–226. doi: 10.1016/S0076-6879(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 5.Low FM, Hampton MB, Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–1630. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- 6.Wood ZA, Schröder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 7.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 8.Aran M, Ferrero DS, Pagano E, Wolosiuk RA. Typical 2-Cys peroxiredoxins-modulation by covalent transformations and noncovalent interactions. FEBS J. 2009;276:2478–2493. doi: 10.1111/j.1742-4658.2009.06984.x. [DOI] [PubMed] [Google Scholar]
- 9.Chae HZ, Chung SJ, Rhee SG. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 10.Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The high reactivity of peroxiredoxin 2 with H(2)O(2) is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 11.Kang SW, Baines IC, Rhee SG. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J Biol Chem. 1998;273:6303–6311. doi: 10.1074/jbc.273.11.6303. [DOI] [PubMed] [Google Scholar]
- 12.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Cho CS, Lee S, Lee GT, Woo HA, Choi EJ, Rhee SG. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid Redox Signal. 2010;12:1235–1246. doi: 10.1089/ars.2009.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee SG, Jeong W, Chang TS, Woo HA (2007) Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int (106): Suppl S3–8 [DOI] [PubMed]
- 15.Yang KS, Kang SW, Woo HA, Hwang SC, Chae HZ, Kim K, Rhee SG. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J Biol Chem. 2002;277:38029–38036. doi: 10.1074/jbc.M206626200. [DOI] [PubMed] [Google Scholar]
- 16.Lim JC, Choi HI, Park YS, Nam HW, Woo HA, Kwon KS, Kim YS, Rhee SG, Kim K, Chae HZ. Irreversible oxidation of the active-site cysteine of peroxiredoxin to cysteine sulfonic acid for enhanced molecular chaperone activity. J Biol Chem. 2008;283:28873–28880. doi: 10.1074/jbc.M804087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo JH, Lim JC, Lee DY, Kim KS, Piszczek G, Nam HW, Kim YS, Ahn T, Yun CH, Kim K, Chock PB, Chae HZ. Novel protective mechanism against irreversible hyperoxidation of peroxiredoxin: Nalpha-terminal acetylation of human peroxiredoxin II. J Biol Chem. 2009;284:13455–13465. doi: 10.1074/jbc.M900641200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozonet SM, Findlay VJ, Day AM, Cameron J, Veal EA, Morgan BA. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J Biol Chem. 2005;280:23319–23327. doi: 10.1074/jbc.M502757200. [DOI] [PubMed] [Google Scholar]
- 19.Jang HH, Lee KO, Chi YH, Jung BG, Park SK, Park JH, Lee JR, Lee SS, Moon JC, Yun JW, Choi YO, Kim WY, Kang JS, Cheong GW, Yun DJ, Rhee SG, Cho MJ, Lee SY. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Moghaddam DA, Heber A, Capin D, Kreutz T, Opitz D, Lenzen E, Bloch W, Brixius K, Brinkmann C. Training increases peroxiredoxin 2 contents in the erythrocytes of overweight/obese men suffering from type 2 diabetes. Wien Med Wochenschr. 2011;161:511–518. doi: 10.1007/s10354-011-0037-0. [DOI] [PubMed] [Google Scholar]
- 21.Brinkmann C, Chung N, Schmidt U, Kreutz T, Lenzen E, Schiffer T, Geisler S, Graf C, Montiel-Garcia G, Renner R, Bloch W, Brixius K. Training alters the skeletal muscle antioxidative capacity in non-insulin-dependent type 2 diabetic men. Scand J Med Sci Sports. 2012;22:462–470. doi: 10.1111/j.1600-0838.2010.01273.x. [DOI] [PubMed] [Google Scholar]
- 22.Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol. 2009;297:H144–H152. doi: 10.1152/ajpheart.01278.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richters L, Lange N, Renner R, Treiber N, Ghanem A, Tiemann K, Scharffetter-Kochanek K, Bloch W, Brixius K. Exercise-induced adaptations of cardiac redox homeostasis and remodeling in heterozygous SOD2-knockout mice. J Appl Physiol. 2011;111:1431–1440. doi: 10.1152/japplphysiol.01392.2010. [DOI] [PubMed] [Google Scholar]
- 24.Brinkmann C, Blossfeld J, Pesch M, Krone B, Wiesiollek K, Capin D, Montiel G, Hellmich M, Bloch W, Brixius K. Lipid-peroxidation and peroxiredoxin-overoxidation in the erythrocytes of non-insulin-dependent type 2 diabetic men during acute exercise. Eur J Appl Physiol. 2012;112:2277–2787. doi: 10.1007/s00421-011-2203-x. [DOI] [PubMed] [Google Scholar]
- 25.Brinkmann C, Neumann E, Blossfeld J, Frese S, Orthmann P, Montiel G, Bloch W, Brixius K. Influence of glycemic status and physical fitness on oxidative stress and the peroxiredoxin system in the erythrocytes of non-insulin-dependent type 2 diabetic men. Exp Clin Endocrinol Diabetes. 2011;119:559–564. doi: 10.1055/s-0031-1279712. [DOI] [PubMed] [Google Scholar]
- 26.Sahlin K, Shabalina IG, Mattsson CM, Bakkman L, Fernström M, Rozhdestvenskaya Z, Enqvist JK, Nedergaard J, Ekblom B, Tonkonogi M. Ultraendurance exercise increases the production of reactive oxygen species in isolated mitochondria from human skeletal muscle. J Appl Physiol. 2010;108:780–787. doi: 10.1152/japplphysiol.00966.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashton T, Rowlands CC, Jones E, Young IS, Jackson SK, Davies B, Peters JR. Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J Appl Physiol Occup Physiol. 1998;77:498–502. doi: 10.1007/s004210050366. [DOI] [PubMed] [Google Scholar]


